Abstract
BACKGROUND AND OBJECTIVE:
Complications after adenotonsillectomy (AT) in children have been extensively studied, but differences between children with and without obstructive sleep apnea (OSA) have not been systematically reported. Our objective was to identify the most frequent complications after AT, and evaluate if differences between children with and without OSA exist.
METHODS:
Several electronic databases were searched. A partial gray literature search was undertaken by using Google Scholar. Experts were consulted to identify any missing publications. Studies assessing complications after AT in otherwise healthy children were included. One author collected the required information from the selected articles. A second author crosschecked the collected information and confirmed its accuracy. Most of the selected studies collected information from medical charts.
RESULTS:
A total of 1254 studies were initially identified. Only 23 articles remained after a 2-step selection process. The most frequent complication was respiratory compromise (9.4%), followed by secondary hemorrhage (2.6%). Four studies compared postoperative complications in children with and without OSA, and revealed that children with OSA have nearly 5 times more respiratory complications after AT than children without OSA (odds ratio = 4.90; 95% confidence interval: 2.38–10.10). In contrast, children with OSA are less likely to have postoperative bleeding when compared with children without OSA (odds ratio = 0.41; 95% confidence interval: 0.23–0.74).
CONCLUSIONS:
The most frequent early complications after AT are respiratory compromise and secondary hemorrhage. Based on the current limited evidence, children with OSA appear to have more respiratory complications. Conversely, hemorrhage appears to be more frequent in children without OSA.
Obstructive sleep apnea (OSA) is widely recognized as a potential cause of significant morbidity in children. 1 , 2 OSA symptoms include habitual snoring and reporting of disturbed unrefreshing sleep, frequently accompanied by excessive daytime sleepiness, and daytime neurobehavioral and mood problems. 3 The prevalence of OSA is markedly variable during childhood (1%–5%), with age, gender, and ethnicity as major contributors. 2 , 4 – 6 However, independently of the lowest or the highest reported prevalence, OSA is a relatively frequent condition that imposes a high degree of disease burden, thereby requiring timely diagnosis and effective treatment.
The first-line treatment of pediatric OSA is provided through either medical or surgical procedures on the basis of the underlying severity of the condition as defined by overnight polysomnography (PSG). 3 Medical treatments in mild cases may include weight loss management in overweight children, intranasal steroids, leukotriene modifiers, and oral or topical descongestants. 3 However, in the majority of cases, adenoidectomy and tonsillectomy (AT) remains the most common surgical procedure performed for OSA in children, 7 with more than 530 000 of these procedures being performed annually in the United States alone. 8 Similarly, AT is traditionally performed in cases fulfilling the criteria of recurrent tonsillitis, which in the past accounted for the majority of AT surgeries, before being surpassed by OSA indications. 9 As with most surgical procedures, AT involves potential intraoperative risks and postoperative complications. Among the latter, minor complications include pain, nausea, vomiting, and dehydration. 10 However, more serious complications may occur, such as hemorrhage, respiratory decompensation, velopharyngeal incompetence, subglottic stenosis, and rarely death. 11
Some authors 12 – 16 have suggested that differences in the frequency of these complications could be present after AT in children with OSA and children without OSA. Because these complications can have a significant impact on the burden of care, the purpose of this systematic review was (1) to identify the most frequent postoperative complication during the first 3 weeks after AT, and (2) to critically evaluate the differences comparing children with OSA and children without OSA. These findings should help in making physicians aware of potential complications after this type of surgical procedures in specific target populations.
Methods
This systematic review was done adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist. 17
Diagnostic Terminology
All terms that indicated OSA, 1 including sleep disordered breathing (SDB), sleep-related breathing disorder, and OSA syndrome, were all considered synonymous of OSA for this submission.
Protocol and Registration
The systematic review protocol was registered at the International Prospective Register of Systematic Reviews 18 under number CRD42015016102.
Study Design
A systematic review that evaluated (within 3 weeks) postoperative complications after AT in children with OSA and children without OSA aimed at answering 2 specific questions:
What are the most frequent immediate postoperative complications after AT?
Do children with OSA and children without OSA differ in the frequency of immediate postoperative complications after AT?
Eligibility Criteria
Inclusion Criteria
Clinical studies that evaluated (within 3 weeks) postoperative complications after AT in children with OSA and children without OSA (0–18 years old).
No language restriction was set.
Complications were defined as any deviation from the usual postoperative recovery course that required intervention. All reported complications were reviewed to determine type, occurrence time, and need for an extended hospital stay and/or intensive care unit (ICU) monitoring. 19
Exclusion Criteria
The studies were excluded in 2 phases.
In phase 1 (titles and abstracts) the following exclusion criteria were applied:
-
1
Studies that targeted a different condition.
-
2
Studies in adults.
-
3
Reviews, letters, conference abstracts, editorials, case-series studies.
-
4
Studies in which the cohort sample included subjects previously diagnosed with genetic syndromic patients (eg, Down syndrome, craniofacial anomalies, neuromuscular disorders, chromosomal abnormality, etc), coagulation disorders, or cerebral palsy.
In phase 2 (full-text) these additional exclusion criteria were applied:
-
5
Studies that did not reveal complications during the first 3 weeks of follow-up.
Information Sources
Detailed individual search strategies for each of the following bibliographic databases were developed: Cochrane, Embase, Medline, PubMed, Web of Science, and Literatura Latino-americana e do Caribe em Ciências da Saúde. A partial gray literature search was taken by using Google Scholar. The end search date was January 3, 2015, across all databases. The references cited in the selected articles were also checked for any additional references that could have been inadvertently omitted during the electronic database searches. In addition, experts in the field of sleep, otolaryngology, and/or respiratory medicine were approached to identify any missing important publication. Experts were identified based on the main reference list, such that authors who had (as a first/senior author) > 6 publications in this main search were contacted, and were asked to identify the 10 most important publications regarding AT postoperative complications.
Search
Appropriate truncation and word combinations were selected and adapted for each database search (Supplemental Table 4). All references were managed by reference manager software (RefWorks-COS, ProQuest, Bethesda, MD) and duplicate hits were removed.
Study Selection
The selection was completed in 2 phases. In phase 1, 2 reviewers (Drs De Luca Canto and Pachêco-Pereira) independently reviewed the titles and abstracts of all identified electronic database citations. A third author (Dr Aydinoz) read all abstracts selected to reach the final decision. This was important when disagreements emerged between the 2 initial evaluators. Any studies that did not fulfill the inclusion criteria were discarded.
In phase 2, the same 2 reviewers (Drs De Luca Canto and Pachêco-Pereira) independently participated in phase 2. The reference lists of all included articles were critically assessed by 1 examiner (Dr De Luca Canto). Selected studies were read by both examiners (Drs De Luca Canto and Pachêco-Pereira). Any disagreement in either phase was resolved by discussion and mutual agreement between the 3 reviewers (Drs De Luca Canto, Pachêco-Pereira, and Aydinoz).
A fourth author (Dr Gozal) was involved when controversy arose in the process of reaching a final decision either in phase 1 or 2. Final selection was always on the basis of the full-text of the publication.
Data Collection Process
One author (Dr De Luca Canto) collected the required information from the selected articles. A second author (Dr Pachêco-Pereira) crosschecked all the collected information and confirmed its accuracy. Again, any disagreement in either phase was resolved by discussion and mutual agreement between the 3 reviewers (Drs De Luca Canto, Pachêco-Pereira, and Aydinoz). The fourth author was involved as required, to enable formulation of the final decision (Dr Gozal).
Data Items
The following information was recorded: author(s), year of publication, country, study design, recruitment method, follow-up length, sample size, participant’s age, complication type, surgery type, results, and main conclusion. If the required data were not complete, attempts were made to contact the authors to retrieve the missing information.
Risk of Bias in Individual Studies
The methodology of selected studies was evaluated by using the Meta-Analysis of Statistics Assessment and Review Instrument. 20 Two reviewers (Drs De Luca Canto and Pachêco-Pereira) scored each item with “yes,” “no,” “unclear,” or “not applicable,” and assessed independently the quality of each included study. Disagreements between both reviewers were resolved by a third reviewer (Dr Aydinoz).
Summary Measures
Early (within 3 weeks) postoperative complications after AT were considered as the main outcome. Any type of outcome measurement was considered (categorical and continuous variables).
Synthesis of Results
Studies that revealed postoperative prevalence but did not directly compare OSA and non-OSA groups were analyzed through a meta-analysis performed with MedCalc (MedCalc Software, Ostend, Belgium). Studies that directly compared children with OSA and children without OSA were analyzed through a meta-analysis by using Review Manager 5.2 (RevMan, The Nordic Cochrane Centre, Copenhagen, Denmark).
Risk of Bias Across Studies
We assessed the clinical heterogeneity (by comparing variability among the participant’s characteristics, type of interventions and outcomes studied), methodological heterogeneity (by comparing the variability in study design and risk of bias), and statistical heterogeneity (by comparing variability in the intervention effects in the different included studies).
Results
Study Selection
During the initial search (phase 1), 1254 different citations were identified across the 6 electronic databases. Then, after a comprehensive evaluation of the abstracts, only 92 articles were deemed potentially relevant, and were selected for phase 2 assessment. An additional 60 citations from Google Scholar, and another 48 citations from content experts were also considered. Of these 108 articles (not initially included among those from the electronic databases), only 10 were deemed appropriate for phase 2 assessments. No additional study that might have been inadvertently missed by the search procedures was identified after further reviewing the reference list of the 102 studies. From these remaining studies, 79 were subsequently excluded (Supplemental Table 5). Thus, only 23 studies were retained for the final meta-analysis aimed at answering the first question. From these 23 studies, only 4 studies differentiated between OSA and non-OSA groups, and were used in the meta-analysis aiming to answer the second question. All of these articles were identified from the main electronic search. A flowchart of the process of identification, inclusion, and exclusion of studies is shown in Fig 1.
FIGURE 1.
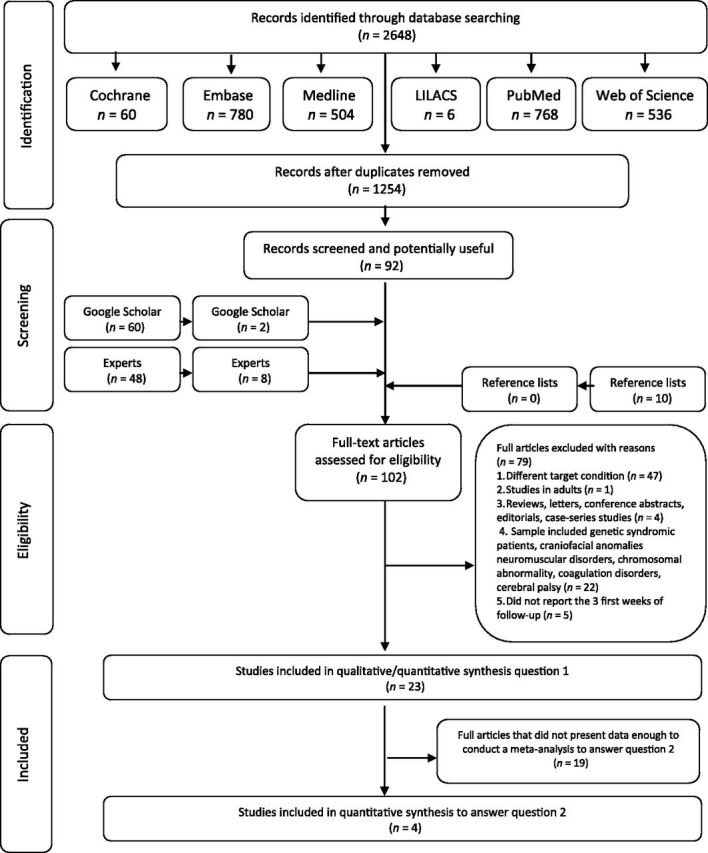
Flow diagram of literature search and selection criteria. Adapted from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. 17
Study Characteristics
The 23 studies that were retained to address question 1 revealed the following postoperative complications: respiratory complications, 12 , 13 , 16 , 19 , 21 – 33 hemorrhage, 15 , 21 , 25 , 26 , 28 , 30 , 34 – 38 pain, 27 , 28 nausea, 27 , 34 vomiting, 27 , 35 refuse to drink, 35 inadequate oral intake, 21 dehydratation, 28 , 29 , 34 fever, 24 , 29 dysphagia, 28 , 34 and cardiac complications. 35
The sample size ranged widely from 102 3 to 9023 subjects. 15 A summary of the study descriptive characteristics can be found in Table 1. The characteristics from the 4 studies selected to answer question 2 can be found in Table 2.
TABLE 1.
Summary of Descriptive Characteristics of Included Studies to Answer Question 1 (n = 23)
| Source | Country | Sample | Results | Additional Data | ||||
|---|---|---|---|---|---|---|---|---|
| n | Characteristics | Overall, % a | Hemorrhage, % b | Respiratory Complications, % | Other, % | |||
| Ahmad et al 2010 25 | Malaysia | 267 | OSA or RT or other (unclear if PSG was performed) | 7.2 | Primary: 1.9 | Extubation failure: 0.4 | Infection: 1.1 | Increase in risk with every 1 min increase in duration of surgery |
| Secondary: 1.9 | Other: 1.9 | Primary hemorrhage (OR: 1.05; 95% CI: 1.01–1.09 min; P = .020) | ||||||
| Respiratory complications (OR: 1.08; 95% CI: 1.01–1.16 min; P = .024) | ||||||||
| Baguley et al 2014 31 | Australia | 100 | OSA (confirmed by PSG) | 11.0 | NA | Left recovery with oxygen prescribed: 11.0 | Major complications: zero | apnea/hypopneia index (AHI) cutoff above which oxygen supplementation was given (OR: 1.135; 95% CI: 0.99–1.3; P = .07) |
| Brown 2006 23 | Canada | 54 | OSA (confirmed by PSG or cardiorespiratory sleep studies or oximetry or awake capillary carbon dioxide tension) | 61.1 | NA | Overall: 61.1 a (reintubation, ventilation, and/or administration of racemic epinephrine or salbutamol, oxygen administration, reintubation in the recovery room for respiratory compromise) | NA | Risk factors: Associated medical condition or 8.15; 95% CI: 1.81–36.73 |
| Preoperative saturation nadir less than 80% | ||||||||
| OR: 5.54; 95% CI: 1.15–26.72 | ||||||||
| Atropine administration at induction decreased the risk of postoperative respiratory complications (OR: 0.18; 95% CI: 0.11–1.050) | ||||||||
| Del-Rio Camacho et al 2014 16 | Spain | 229 | OSA (confirmed by PSG) and RT | 3.5 | Primary: 1.3 | 2.2 | NA | OSA group (3.23%) vs non-OSA group (1.47%), presented a higher incidence of respiratory complications (not statistically significant, P = .39) |
| All respiratory complications took place in the immediate postoperative period. | ||||||||
| Hadden et al 2011 27 | United States | 102 | OSA and others (unclear if PSG was performed) | 100 | NA | Respiratory events: 26.5 a | Pain: 66.7 a Nausea and vomiting: 6.9 a | — |
| Hamada et al 2015 37 | Japan | 147 | OSA (confirmed by home-PSG) | 7.4 | Primary: 5.4 | NA | NA | AT can be performed without major complications. |
| Lalakea et al 1999 21 | United States | 134 | OSA (unclear if PSG was performed) and RT | 5.3 | Primary: 1.6 | Zero | Vomiting and or inadequate oral intake: 3.7 | The complication rate did not vary significantly with the duration of postoperative observation (P = .71) |
| Ma et al 32 | China | 86 | OSA (confirmed by PSG) | 6.8 | Zero | Postoperative desaturation: 6.8 | NA | Children with desaturation after tonsilletomy and adenotonsillectomy had significant higher mean BMI z score than children without desaturation (P = .014) |
| Muninnobpamasa et al 2012 28 | Thailand | 481 | OSA (unclear if PSG was performed), RT, peritonsillar abscess and others | 91.3 | Primary: 4.1 | Anesthetic (partial airway obstruction that needed airway intervention): 1.6 | Dysphagia: 29.0 | The average length of hospital stay was 3.6 d and readmissions 3.7%. |
| Secondary: 3.9 | Dehydration: 4.6 | |||||||
| Pain: 48.1 | ||||||||
| Nixon et al 2005 22 | Canada | 10 | OSA (confirmed by PSG) | 10.0 | NA | Intervention for respiratory compromise: 10 | NA | — |
| Onotai and Lilly-Tariah 2013 30 | Nigeria | 100 | OSA (confirmed by PSG), RT, and recurrent ear infection | 9.0 | Primary: 4.0 | 2.0 | Cardiac: 1.0 | Mortality was recorded in 3% because of severe respiratory distress and cardiac arrest. |
| Secondary: 2.0 | ||||||||
| Perkins et al 2012 15 | United States | 9023 | OSA (confirmed by PSG), chronic tonsillitis, and others | 2.3 | Primary: 0.5 | NA | NA | Children with OSA were half as likely to hemorrhage compared with chronic tonsillitis patients (P = .04) |
| Secondary: 1.8 | ||||||||
| Rakover et al 1997 35 | Israel | 363 | OSA (unclear if PSG was performed), RT, and others | 9.9 | Primary: 3.9 | NA | NA | Three was no increase in the rate of postoperative hemorrhage and of readmissions among the children discharged from the hospital after the operation, compared with those hospitalized. There was no correlation between type of procedure: tonsillectomy or AT and postoperative complications. |
| Secondary: 1.9 | ||||||||
| Riaz et al 2009 13 | Saudi Arabia | 60 | OSA (confirmed by PSG) and RT | 50.0 | NA | Desaturation <95%, cough, stridor, laryngospasm: 41.7 | Nausea and vomiting: 8.3 | The complications at extubation (cough, laryngospasm, postoperative nausea, and vomiting) were higher in OSA group, but not statistically significant; P > .05). |
| At the time of extubation, desaturation was significantly higher in OSA group (43.3% vs 6.6%, P = .002, OR = 10.70). | ||||||||
| More patients of OSA group required oxygen (63.3% vs 10%, P < .001, OR = 15.54). | ||||||||
| Six children from OSA group required insertion of an oropharyngeal airway. No child from the non-OSA group required it (P = .023). | ||||||||
| Ruboyianes 1996 19 | United States | 44 | OSA (confirmed by PSG) | 32.0 | Zero | Intermittent obstruction: 2.3 | NA | Factors associated with development of complications included age < 3 (P = .10), thin body habitus (P = .027), and acute airway obstruction (P < .001). |
| Upper airway obstruction, pulmonary edema: 4.5 | ||||||||
| Upper airway obstruction, pneumonia: 2.3 | ||||||||
| Stridor, atelectasis: 2.3 | ||||||||
| Upper airway edema: 4.5 | ||||||||
| Laryngospasm: 4.5 | ||||||||
| Transient upper airway obstruction: 2.3 | ||||||||
| Central apnea: 2.3 | ||||||||
| Viral bronchitis: 2.3 | ||||||||
| Sanders et al 2006 12 | Mexico | 82 | OSA (confirmed by PSG) and RT | 78.0 | NA | Medical intervention (the need for oral airway, oxygen to keep O2 saturations >92%, assisted ventilation and reintubation in recovery: 22.0 | Pain: 56.0 | OSA group had more respiratory complications than non-OSA (5.7 vs 2.9, P = .0001). |
| Shakeel et al 2012 38 | United Kingdom | 106 | OSA (unclear if PSG was performed) and RT | 7.6 | Primary: 0.9 | NA | NA | Secondary hemorrhage versus: |
| Secondary: 6.7 | Indication for surgery OSA versus recurrent tonsillitis (OR: 0.180; 95% CI: 0.02–1.82) | |||||||
| Obstructive symptoms to recurrent tonsillitis (OR: 1.004; 95% CI: 0.09–11.03) | ||||||||
| Shott et al 1987 34 | United States | 421 | OSA (confirmed by PSG) | 3.8 | Secondary: 2.1 | NA | Nausea and vomiting: 0.5 | — |
| Dehydration: 0.7 | ||||||||
| Dysphagia: 0.5 | ||||||||
| Spencer and Jones 2012 29 | United States | 86 | OSA (unclear if PSG was performed), RT, and chronic tonsillitis | 7.0 | NA | Reactive airway disease: 1.2 | Dehydration: 4.7 | — |
| Fever: 1.2 | ||||||||
| Vlastos et al 2010 26 | Greece | 910 | OSA (unclear if PSG was performed) | 2.8 | Primary: 1.4 | 1.1 | 0.3 | — |
| Wang et al 2009 24 | China | 82 | OSA (confirmed by PSG) | 21.5 | Zero | Airway edema with increased snoring, mouth breathing, and apneas in the first night: 17.1; required CPAP in the first 3 d: 3.7 | Fever: 0.7 | |
| Wong et al 2007 36 | China | 329 | OSA (unclear if PSG was performed) and RT | 2.4 | Secondary: 2.4 | NA | Mortality zero | — |
| Ye et al 2009 33 | China | 321 | OSA (confirmed by PSG) | 11.2 | Zero | Overall: 11.2 (required an oropharyngeal or nasopharyngeal airway, had multiple episodes of desaturation, and other respiratory complications) | NA | — |
CPAP, continuous positive airway pressure; NA, not applicable, when the item was not evaluated by the study; RT, recurrent tonsillitis.
Data were calculated by authors.
We considered primary hemorrhage when it occurs in the first 24 h, and secondary hemorrhage when it occurs after 24 h.
TABLE 2.
Summary of Descriptive Characteristics of Included Studies to Answer Question 2 (n = 4)
| Source | Country | Place of Recruitment | Follow Up | OSA, n | Non-OSA With Tonsillitis, n | Mean Age or Range | Surgery Procedure/Complication Assessment Location | Results | Main Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Del-Rio Camacho et al 2014 16 | Spain | Hospital pediatric department | Once the surgery was finished | 93 | 136 | 4.97 | AT by curettage and tonsillectomy by dissection | Hemorrhage: 1.3% | Children who undergo AT and have an immediate postoperative period without complications do not need to be admitted to an ICU, even though they present severe OSA. |
| Respiratory: 2.2% | |||||||||
| OSA group (3.23%) vs non-OSA group (1.47%), presented a higher incidence of respiratory complications (not statistically significant, P = .39). | |||||||||
| Complication during anesthetic recovery and while the patients was in the ward or ICU. | All respiratory complications took place in the immediate postoperative period. | ||||||||
| Perkins et al 2012 15 | United States | Pediatric hospital | 48 h–18 d | 267 | 158 | 6.90 | Tonsillectomy by electrocautery or coblation | Children with OSA were half as likely to hemorrhage compared with chronic tonsillitis patients (P = .04). | Children with OSA may be less likely to have postoperative hemorrhage than children without OSA with chronic tonsillitis. |
| Riaz et al 2009 13 | Saudi Arabia | Hospital anesthesiology department | Once the surgery was finished | 30 | 30 | 6.60 | AT | The complications at extubation (cough, laryngospasm, postoperative nausea, and vomiting) were higher in OSA group, but not statistically significant; P > .05). | Children with OSA operated for AT are at significant risk of certain life-threatening perioperative anesthetic complications. |
| At the time of extubation, desaturation was significantly higher in OSA group (43.3% vs 6.6%, P = .002, OR = 10.70). | |||||||||
| Recovery room | More patients of OSA group required oxygen (63.3% vs 10%, P < .001, OR = 15.54). | ||||||||
| Six children from OSA group required insertion of an oropharyngeal airway. No child from non-OSA group required it (P = .023). | |||||||||
| Sanders et al 2006 12 | Mexico | Pediatric otolaryngology service from university’s hospital | Until discharge of recovery room | 61 | 21 | 2–16 | AT by electrocautery dissection | OSA group had more respiratory complications than non-OSA (5.7 vs 2.9, P = .0001). | Children with OSA are at risk for respiratory complications after AT. These complications do not prolong the time to discharge. |
| Supraglottic obstruction, breath holding, and desaturation on anesthetic induction and emergence were the most common complications. | |||||||||
| Recovery room | Increased severity of OSA, low weight, and young age are correlated with an increased rate of complications. | ||||||||
| Medical intervention was more necessary in OSA group during recovery and emergence than in the non-OSA group (17/61 vs 1/21, P = .05). |
Risk of Bias Within Studies
The studies selected to answer question 1 were very heterogeneous, 6 of them had high risk of bias, 6 had moderate risk, and only 7 had low risk of bias. None of the studies fulfilled all methodological quality criteria. The studies selected to answer question 2 were more homogeneous, 2 had moderate risk of bias and 1 had low risk. More information about the risk of bias of included studies can be found in Table 3 (summarized assessment) and Supplemental Tables 6 and 7 (detailed assessment).
TABLE 3.
Risk of Bias Summarized Assessment
| Source | Risk of Bias |
|---|---|
| Ahmad et al 25 | Moderate |
| Baguley et al 31 | Moderate |
| Brown 23 | Low |
| Del-Rio Camacho et al 16 | Moderate |
| Hadden et al 27 | Low |
| Hamada et al 37 | Low |
| Lalakea et al 21 | Low |
| Ma et al 32 | Low |
| Muninnobpamasa et al 28 | Moderate |
| Nixon et al 22 | Moderate |
| Onotai and Lilly-Tariah 30 | High |
| Perkins et al 15 | Low |
| Rakover et al 35 | High |
| Riaz et al 13 | Moderate |
| Ruboyianes 19 | High |
| Sanders et al 12 | Low |
| Shakeel et al 38 | Low |
| Shott et al 34 | High |
| Spencer and Jones 29 | Moderate |
| Vlastos et al 26 | Low |
| Wang et al 24 | High |
| Wong et al 36 | High |
| Ye et al 33 | Moderate |
Results of Individual Studies
The results of studies selected to answer question 1 are reported in Table 1. The results of the studies selected to answer question 2 are synthesized below (with additional information in Table 2).
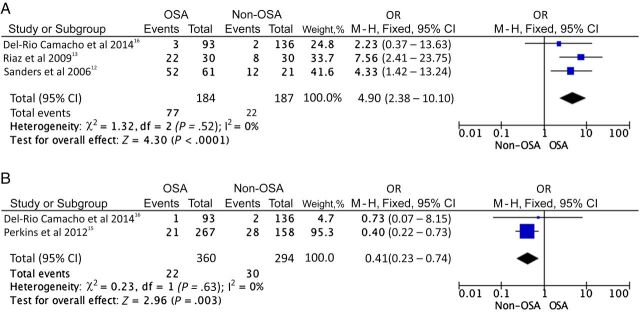
Riaz et al 13 reported that at the time of extubation, desaturation was significantly more frequent in the OSA group (43.3% vs 6.6%, P = .002, odds ratio [OR] = 10.70). Other complications at extubation (ie, cough, laryngospasm, and postoperative nausea and vomiting) were also more frequent in the OSA group, but were not statistically significant (P = .999). In the postanesthesia care unit, the frequency of complications and medical interventions was also higher in OSA group. More patients in the OSA group required oxygen supplementation (63.3% vs 10%, P < .001, OR = 15.54). They concluded that children with OSA undergoing AT are at significant risk of certain life-threatening perioperative anesthetic complications.
Similarly, Sanders et al 12 evaluated the rate of complications experienced by children who undergo AT for OSA, the safety of a standard anesthetic protocol for these children, and preoperative predictors of complications. They found that children with OSA had more respiratory complications than children without OSA (5.7 vs 2.9, P < .0001). Supraglottic obstruction, breath holding, and desaturation on anesthetic induction and emergence from anesthesia were the most common complications. Increased severity of OSA, low body weight, and young age were associated with an increased rate of complications. Medical intervention was necessary in a higher proportion of children with OSA during recovery and emergence from anesthesia than in the non-OSA group (17/61 vs 1/21, P < .05). However, both groups of children had similar opioid requirements, and the time to discharge from the recovery room was also similar. They concluded that children with OSA are at risk for respiratory complications after AT, but that these complications do not appear to prolong the time to discharge.
Del-Rio Camacho et al 16 found that when considering all children, complications occurred in only 3.5% of children, with 2.2% corresponding to respiratory complications. Children with mild to moderate OSA (3.23% vs 1.47%, P = .39) and children with severe OSA (3.77% vs 1.70%, P = .32) presented a higher incidence of respiratory complications, which did not achieve statistical significance. All respiratory complications took place in the immediate postoperative period.
In contrast, Perkins et al 15 hypothesized that a diagnosis of OSA may be protective against postoperative hemorrhage. A total of 9023 tonsillectomy patients were identified. Of these, only 2.4% (n = 212) presented with hemorrhage. There were 48 patients (22.6%) with primary, and 164 patients (77.4%) with secondary hemorrhage, with the majority occurring among children with recurrent tonsillitis. The authors concluded that children with OSA were half as likely to manifest bleeding as a complication after AT when compared with children with chronic tonsillitis (P = .04).
Synthesis of Results
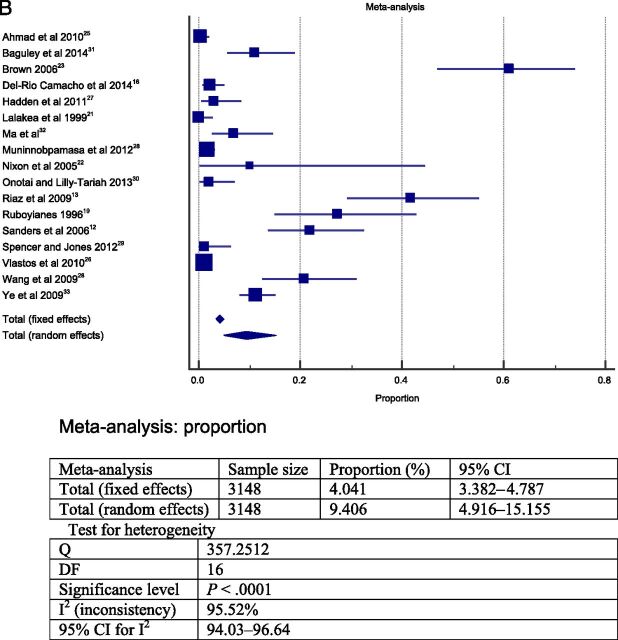
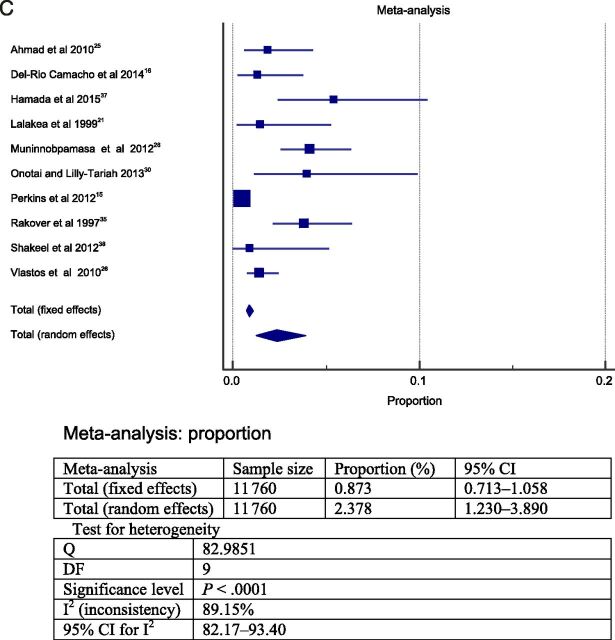
The meta-analysis was performed in 2 steps. To answer question 1, the 23 studies selected were grouped and a meta-analysis was performed. The heterogeneity between the studies found in the meta-analysis was high; therefore, a random model was choosen. 39 The results from this meta-analysis revealed that the overall frequency of postoperative complications was ∼19% (total sample = 13 537; Fig 2A). The most frequent postoperative complications were respiratory compromise (9.4% in sample = 3148 cases; Fig 2B) followed by secondary hemorrhage (2.6% in sample = 11 090 cases; Fig 2D) and primary hemorrhage (2.4% in sample = 11 760; Fig 2C).
FIGURE 2.
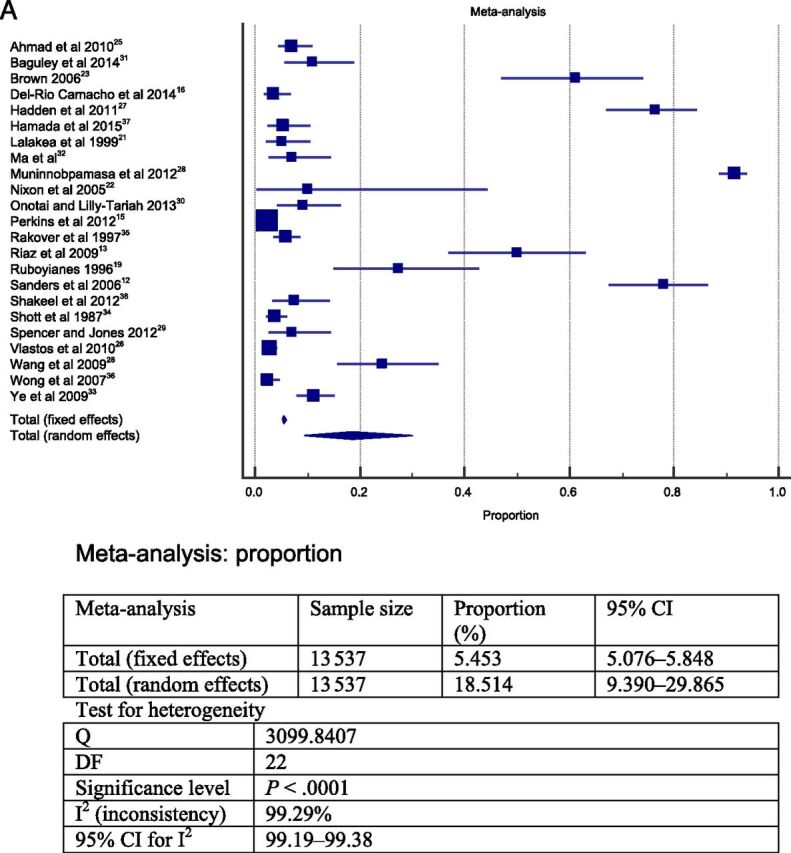
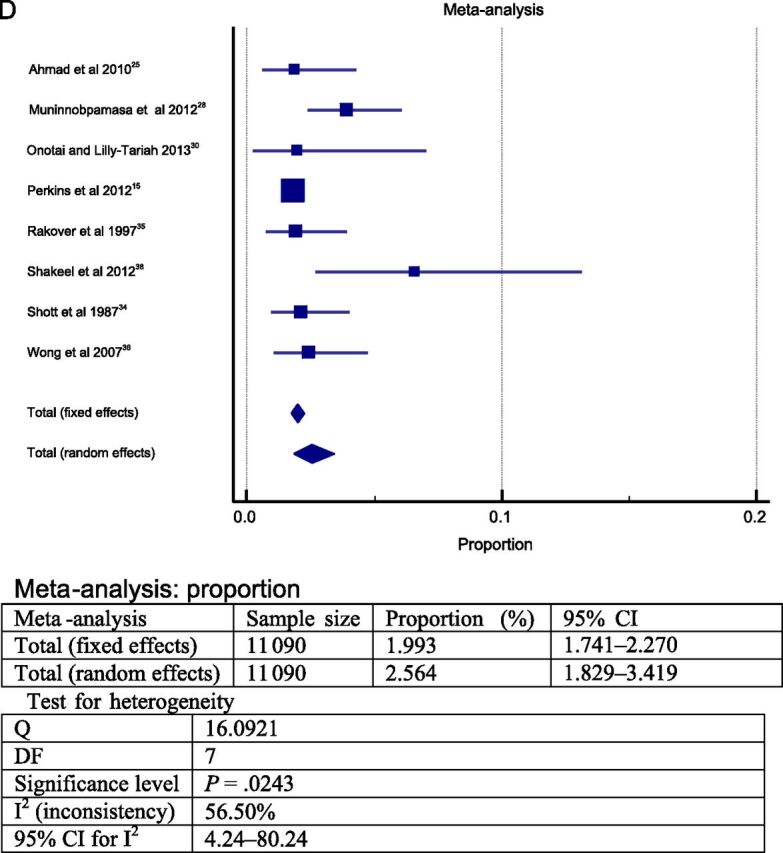
Forest plot question 1. Frequency of complications after AT in children (question 1). Results from 2 types of meta-analysis: fixed and random effects. A, Forest plot for all postoperative complications. Sample = 13 537. B, Forest plot for respiratory complications. Sample = 3148. C, Forest plot for primary hemorrhage. Sample = 11 760. D, Forest plot for secondary hemorrhage. Sample = 11 090.
To answer the question 2, the 4 studies 12 , 13 , 15 , 16 that directly compared an OSA and a non-OSA group, as defined by nocturnal PSG were grouped, and a meta-analysis was performed. Only a meta-analysis with a fixed effect model was performed by using the method of Mantel-Haenszel. 39 In this meta-analysis, only data relying on PSG-confirmed OSA and non-OSA were considered. Because of these criteria, different sample sizes emerge in Tables 1 and 2 for the same studies. To perform this meta-analysis, the studies were grouped in 2 groups, A (respiratory complications) and B (bleeding complications). The heterogeneity between the studies found in the meta-analysis was 0% indicating high homogeneity (confirming that we can analyze these data with fixed effect). The meta-analysis of group A (respiratory complications) included 371 patients (184 with OSA and 187 without OSA) and confirmed that children with OSA appear to have more frequent postoperative respiratory complications after AT than children without OSA (OR = 4.90; 95% confidence interval (CI): 2.38–10.10). In group B (bleeding complications), 360 children with OSA and 294 children without OSA were included. The meta-analysis confirmed that bleeding is more likely to occur among children without OSA (OR = 0.41; 95% CI: 0.23–0.74; Fig 3).
FIGURE 3.
Forest plot question 2. Postoperative respiratory and bleeding complications after AT in children with OSA and children without OSA. Children with OSA were at significantly higher odds for respiratory complications (A), and conversely children without OSA were at increased odds for hemorrhage after surgery (B). A, Forest plot for post-AT respiratory complications. B, Forest plot for post-AT bleeding complications.
Risk of Bias Across Studies
Although the studies had different study design (experimental and descriptive), the main methodological problem concerns the actual subject sample. Most of the studies selected to answer question 1 used a convenience sample from a hospital. Even the experimental studies did not randomly assign participants. Besides this particular issue, most of the observational studies did not perform appropriate statistical analyses.
Discussion
AT is a relatively common procedure in clinical otolaryngology practice and usually follows referrals from primary care physicians. 25 It is the current treatment of choice for treatment of OSA in children, when indicated, due to its perceived efficacy and cost effectiveness. 40
Currently guidelines used by clinicians to identify children who are appropriate candidates for AT have indications based primarily on obstructive and infectious causes. 41 Before 1995, the major indication for AT was recurrent tonsillitis, with OSA being a distant second. However, in the past 10 years the number of children referred for OSA-related AT surgery has increased markedly, reflecting an increased awareness and improved diagnosis of OSA by pediatricians and other primary care physicians. 36
This systematic review investigated the available evidence about the most frequent early postoperative complications after AT, and also evaluated potential differences in the associated complications between children with OSA and those without OSA. Regarding the most frequent early postoperative complications, respiratory compromise and hemorrhage were the most frequent, followed by pain, fever, nausea and vomiting, and dehydration. Infection and cardiac complications were rare. These results are in agreement with the available literature. 3 , 21 , 29 , 35 , 37 Mortality rates of AT have been estimated between 1 in 16 000 and 1 in 35 000 cases. 42
Nixon et al 22 demonstrated that sleep-related airway obstruction is a common postoperative complication leading to significant oxyhemoglobin desaturations after AT in children with severe OSA. Episodes of desaturation occurred during the first postoperative night in all 10 children studied, and these desaturation episodes were predominantly caused by upper airway obstruction. Several mechanisms may explain ongoing upper airway obstruction after AT, including copious nasal secretions after surgery, and reactive, postsurgical edema in the adenoid and tonsillar beds. Thus, the first night after AT will usually manifest marked sleep disturbance, airway obstruction, and desaturation. 22
In this systematic review, we found a very high frequency of pain as the major complication of AT, with some of the included studies revealing exceedingly high rates of pain, 12 , 27 , 28 such that the frequency of overall postoperative complications when pain was included in the meta-analysis reached the vicinity of 20%, even after random effects were accounted for. Under these circumstances, the frequency of respiratory compromise after AT was 9.4% and primary and secondary hemorrhage prevalence was 2.4% and 2.6%, respectively. Of note, although postoperative bleeding can be a serious complication after AT, 21 it rarely requires transfusion. 30 Thus, AT has substantial postoperative morbidity, and identification of children at risk for either respiratory compromise or bleeding would be obviously important, particularly because the majority of AT surgeries are conducted in outpatient settings.
In this context, our meta-analysis highlights 2 relevant clinical practice points. The presence of OSA significantly increases the odds for postoperative respiratory complications, with children with OSA exhibiting a nearly fivefold increase in the odds of developing respiratory complications when compared with children without OSA. These findings concur with previous studies that suggested that as the severity of OSA increases, the probability of respiratory complications after AT increases as well. 43 – 45 Children with OSA not only had more frequent complications during induction and emergence from anesthesia, they were also more likely to require supplemental oxygen, oral or nasal airway insertion, or assisted ventilation in the immediate postoperative period. 12 Thus, identification of children at higher risk for respiratory complications before AT can help in formulating a safe anesthetic strategy for children with OSA, and should be pursued in future prospective large-scale studies. 13 In contrast, our meta-analysis indicated that the presence of tonsillitis in children without OSA increased the odds for hemorrhagic complications. Children without OSA with tonsillitis appear to have 2.5-fold increases in the odds for bleeding complications when compared with children with OSA. Perkins et al 15 suggested that either OSA is protective against postoperative hemorrhage or that recurrent tonsil infection increases the risk for bleeding through undefined mechanisms, most likely involving increased vascularity of tonsillar and surrounding upper airway tissues.
Despite the current guidelines from the American Academy of Pediatrics, several important obstacles have thus far precluded widespread implementation of PSG for OSA, such as cost and reduced availability. 3 However, a definitive diagnosis of OSA requires a PSG, and our current findings further suggest that among children with OSA, the risk of postoperative respiratory complications is high. Children with OSA are clearly at higher anesthetic risk than are patients with normal upper airway function. Anesthesiologists should routinely screen patients for snoring, airway dysfunction, airway anatomic disorders, and other coexisting diseases that can increase risk from OSA in the postoperative period. Despite the pressure to reduce costs, both surgeons and anesthesiologists should improve screening procedures, perhaps develop alternate surgical approaches, to decrease the risks. 46
Thus, systematic implementation of PSG-based OSA diagnosis could potentially enable delineation of PSG-based criteria that would inform ear, nose and throat (ENT) surgeons on the presence of specific children with higher respiratory risk, and thus improve peri- and postoperative phase planning, while ascertaining that high-risk patients undergo surgery in a medical center capable of monitoring and treating more complex pediatric patients postoperatively. 3
Raman et al 47 established guidelines for patients undergoing AT resulting in an overall reduction in unanticipated admissions. The authors emphasize that guidelines could be universally applied in an outpatient screening process for identification of at risk surgical patients. This would aid in identifying those patients who may not be ideal candidates for outpatient surgical facilities.
Future prospective studies may address the development of screening tools for patients in need of additional education in normal and abnormal postoperative symptomatology or health care support. This may ultimately lower postoperative emergency department visits and overall health care cost associated with this procedure. 48
Limitations
Most of the selected studies were retrospective. Documentation of a respiratory complication was collected from medical charts. It is likely that minor complications that did not require sentinel event reporting, hospital readmission, or operative intervention were not taken into account in the published literature. In most studies, the criteria used to establish the diagnosis and severity of OSA relied on clinical assessment, rather than PSG. Therefore, accurate stratification of OSA severity is not possible to enable aforementioned OSA severity stratification of postoperative risk.
Notwithstanding that our findings emanate from a meta-analysis of the pertinent published studies, caution should be exercised because the sample size was relatively small, and thus does not enable irrefutable evidence to unequivocally answer the 2 questions from this systematic review. This is even more noticeable for the second question.
Conclusions
The most frequent complications after AT include respiratory compromise and secondary hemorrhage. Based on the current limited evidence, children with OSA appear to have more respiratory complications after AT than children without OSA. In contrast, hemorrhage appears to be more frequent in children without OSA.
Supplementary Material
Acknowledgments
We thank the experts who kindly responded to specific questions during the conduct of this research: Dr Karen A. Brown at McGill University Health Centre and The Montreal Children’s Hospital; Dr Ronald D. Chervin at the University of Michigan; Dr Ron B. Mitchell at the University of Texas Southwestern and Children’s Medical Center Dallas; and Dr Hawley E. Montgomery-Downs at West Virginia University.
Glossary
- AT
adenoidectomy and tonsillectomy
- CI
confidence interval
- ICU
intensive care unit
- OR
odds ratio
- OSA
obstructive sleep apnea
- PSG
polysomnography
- SDB
sleep disordered breathing
Footnotes
Dr De Luca Canto worked on study conceptualization, design, data collection, data analysis, drafted the initial manuscript, and critically reviewed the manuscript; Dr Pachêco-Pereira worked on study conceptualization, design, data collection, data analysis, and manuscript preparation; Dr Aydinoz worked on data collection, data analysis, and manuscript preparation; Drs Bhattacharjee, Tan, and Kheirandish-Gozal worked on data analysis and manuscript preparation; Drs Flores-Mir and Gozal worked on study conceptualization, design, data analysis, and critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
FUNDING: Drs Gozal and Kheirandish-Gozal are supported by National Institutes of Health (NIH) grant HL-65270. Dr Bhattacharjee is supported by a scientist development grant from the American Heart Association (3SDG14780079).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1. Jurado-Gamez B , Bujalance Cabrera C , Caballero Ballesteros L , et al. Association of cellular adhesion molecules and oxidative stress with endothelial function in obstructive sleep apnea. Intern Med. 2012;51(4):363–368 [DOI] [PubMed] [Google Scholar]
- 2. Lumeng JC , Chervin RD . Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):242–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marcus CL , Brooks LJ , Draper KA , et al. American Academy of Pediatrics . Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576–584 [DOI] [PubMed] [Google Scholar]
- 4. Bixler EO , Vgontzas AN , Lin HM , et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32(6):731–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamagishi K , Ohira T , Nakano H , et al. Cross-cultural comparison of the sleep-disordered breathing prevalence among Americans and Japanese. Eur Respir J. 2010;36(2):379–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ralls FM , Grigg-Damberger M . Roles of gender, age, race/ethnicity, and residential socioeconomics in obstructive sleep apnea syndromes. Curr Opin Pulm Med. 2012;18(6):568–573 [DOI] [PubMed] [Google Scholar]
- 7. Sundaram S , Bridgman SA , Lim J , Lasserson TJ . Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005; (4):CD001004 [DOI] [PubMed] [Google Scholar]
- 8. Traeger N , Schultz B , Pollock AN , Mason T , Marcus CL , Arens R . Polysomnographic values in children 2-9 years old: additional data and review of the literature. Pediatr Pulmonol. 2005;40(1):22–30 [DOI] [PubMed] [Google Scholar]
- 9. Mitchell RB , Pereira KD , Friedman NR . Sleep-disordered breathing in children: survey of current practice. Laryngoscope. 2006;116(6):956–958 [DOI] [PubMed] [Google Scholar]
- 10. Brigger MT , Brietzke SE . Outpatient tonsillectomy in children: a systematic review. Otolaryngol Head Neck Surg. 2006;135(1):1–7 [DOI] [PubMed] [Google Scholar]
- 11. Pratt LW , Gallagher RA . Tonsillectomy and adenoidectomy: incidence and mortality, 1968–1972. Otolaryngol Head Neck Surg (1979). 1979;87(2):159–166 [DOI] [PubMed] [Google Scholar]
- 12. Sanders JC , King MA , Mitchell RB , Kelly JP . Perioperative complications of adenotonsillectomy in children with obstructive sleep apnea syndrome. Anesth Analg. 2006;103(5):1115–1121 [DOI] [PubMed] [Google Scholar]
- 13. Riaz A , Malik HS , Fazal N , Saeed M , Naeem S . Anaesthetic risks in children with obstructive sleep apnea syndrome undergoing adenotonsillectomy. J Coll Physicians Surg Pak. 2009;19(2):73–76 [PubMed] [Google Scholar]
- 14. Achar P , Sharma RK , De S , Donne AJ . Does primary indication for tonsillectomy influence post-tonsillectomy haemorrhage rates in children? Int J Pediatr Otorhinolaryngol. 2015;79(2):246–250 [DOI] [PubMed] [Google Scholar]
- 15. Perkins JN , Liang C , Gao D , Shultz L , Friedman NR . Risk of post-tonsillectomy hemorrhage by clinical diagnosis. Laryngoscope. 2012;122(10):2311–2315 [DOI] [PubMed] [Google Scholar]
- 16. del-Río Camacho G , Martínez González M , Sanabria Brossart J , Gutiérrez Moreno E , Gómez García T , Troncoso Acevedo F . Post-operative complications following adenotonsillectomy in children with severe sleep apnea-hypopnea syndrome. Do they need to be admitted to an intensive care unit? Acta Otorrinolaringol Esp. 2014;65(5):302–307 [English Edition] [DOI] [PubMed] [Google Scholar]
- 17. Moher D , Liberati A , Tetzlaff J , Altman DG PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341 [DOI] [PubMed] [Google Scholar]
- 18.PROSPERO. University of York. Available at www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015016102. Accessed January 22, 2015
- 19. Ruboyianes JM , Cruz RM . Pediatric adenotonsillectomy for obstructive sleep apnea. Ear Nose Throat J. 1996;75(7):430–433 [PubMed] [Google Scholar]
- 20. Joanna Briggs Institute Reviewers Manual. Adelaide, Australia: The Joanna Briggs Institute; 2014. [Google Scholar]
- 21. Lalakea ML , Marquez-Biggs I , Messner AH . Safety of pediatric short-stay tonsillectomy. Arch Otolaryngol Head Neck Surg. 1999;125(7):749–752 [DOI] [PubMed] [Google Scholar]
- 22. Nixon GM , Kermack AS , McGregor CD , et al. Sleep and breathing on the first night after adenotonsillectomy for obstructive sleep apnea. Pediatr Pulmonol. 2005;39(4):332–338 [DOI] [PubMed] [Google Scholar]
- 23. Brown PM . How safe is paediatric tonsillectomy? Int J Pediatr Otorhinolaryngol. 2006;70(4):575–577 [DOI] [PubMed] [Google Scholar]
- 24. Wang JL , Huang ZY , Zong JW , Tan ZY , Chou SY . The clinical observation of coblation-assisted adenotonsillectomy for treatment of children with obstructive sleep apnea hypopnea syndrome. Chinese J Evid Med. 2009;9:709–712 [Google Scholar]
- 25. Ahmad R , Abdullah K , Amin Z , Rahman JA . Predicting safe tonsillectomy for ambulatory surgery. Auris Nasus Larynx. 2010;37(2):185–189 [DOI] [PubMed] [Google Scholar]
- 26. Vlastos IM , Athanasopoulos I , Economides J , Parpounas K , Houlakis M . Outpatient cold knife tonsillectomy in toddlers with sleep disordered breathing. B-ENT. 2010;6(4):245–250 [PubMed] [Google Scholar]
- 27. Hadden SM , Burke CN , Skotcher S , Voepel-Lewis T . Early postoperative outcomes in children after adenotonsillectomy. J Perianesth Nurs. 2011;26(2):89–95 [DOI] [PubMed] [Google Scholar]
- 28. Muninnobpamasa T , Khamproh K , Moungthong G . Prevalence of tonsillectomy and adenoidectomy complication at Phramongkutklao Hospital. J Med Assoc Thai. 2012;95(suppl 5):S69–S74 [PubMed] [Google Scholar]
- 29. Spencer DJ , Jones JE . Complications of adenotonsillectomy in patients younger than 3 years. Arch Otolaryngol Head Neck Surg. 2012;138(4):335–339 [DOI] [PubMed] [Google Scholar]
- 30. Onotai L , Lilly-Tariah O . Adenoid and tonsil surgeries in children: how relevant is pre-operative blood grouping and cross-matching? Afr J Paediatr Surg. 2013;10(3):231–234 [DOI] [PubMed] [Google Scholar]
- 31. Baguley KE , Cheng AT , Castro C , Wainbergas N , Waters KA . Is day stay adenotonsillectomy safe in children with mild to moderate obstructive sleep apnoea? A retrospective review of 100 patients. Int J Pediatr Otorhinolaryngol. 2014;78(1):71–74 [DOI] [PubMed] [Google Scholar]
- 32. Ma AL , Lam YY , Wong SF , Ng DK , Chan CH . Risk factors for post-operative complications in Chinese children with tonsillectomy and adenoidectomy for obstructive sleep apnea syndrome. Sleep Breath. 2012;16(3):909–911 [DOI] [PubMed] [Google Scholar]
- 33. Ye J , Liu H , Zhang G , Huang Z , Huang P , Li Y . Postoperative respiratory complications of adenotonsillectomy for obstructive sleep apnea syndrome in older children: prevalence, risk factors, and impact on clinical outcome. J Otolaryngol Head Neck Surg. 2009;38(1):49–58 [PubMed] [Google Scholar]
- 34. Shott SR , Myer CM III , Cotton RT . Efficacy of tonsillectomy and adenoidectomy as an outpatient procedure: a preliminary report. Int J Pediatr Otorhinolaryngol. 1987;13(2):157–163 [DOI] [PubMed] [Google Scholar]
- 35. Rakover Y , Almog R , Rosen G . The risk of postoperative haemorrhage in tonsillectomy as an outpatient procedure in children. Int J Pediatr Otorhinolaryngol. 1997;41(1):29–36 [DOI] [PubMed] [Google Scholar]
- 36. Wong BYH , Ng YW , Hui Y . A 10-year review of tonsillectomy in a tertiary centre. HK J Paediatr. 2007;12:297–299 [Google Scholar]
- 37. Hamada M , Iida M , Nota J , et al. Safety and efficacy of adenotonsillectomy for obstructive sleep apnea in infants, toddlers and preschool children. Auris Nasus Larynx. 2015;42(3):208–212 [DOI] [PubMed] [Google Scholar]
- 38. Shakeel M , Trinidade A , Al-Adhami A , Supriya M , Kubba H . Coblation adenotonsillectomy in children. J Coll Physicians Surg Pak. 2012;22(9):579–581 [PubMed] [Google Scholar]
- 39.Deeks JJHJ, Altman DG. Analyzing data and undertaking meta-analyses. In: Higgins JPTGS, ed. London, UK: The Cochrane Collaboration; 2008:243–293 [Google Scholar]
- 40. Lim J , McKean M . Adenotonsillectomy for obstructive sleep apnoea in children. Cochrane Database Syst Rev. 2003; (1):CD003136 [DOI] [PubMed] [Google Scholar]
- 41. Ramos SD , Mukerji S , Pine HS . Tonsillectomy and adenoidectomy. Pediatr Clin North Am. 2013;60(4):793–807 [DOI] [PubMed] [Google Scholar]
- 42. Stevenson AN , Myer CM III , Shuler MD , Singer PS . Complications and legal outcomes of tonsillectomy malpractice claims. Laryngoscope. 2012;122(1):71–74 [DOI] [PubMed] [Google Scholar]
- 43. McColley SA , April MM , Carroll JL , Naclerio RM , Loughlin GM . Respiratory compromise after adenotonsillectomy in children with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 1992;118(9):940–943 [DOI] [PubMed] [Google Scholar]
- 44. Rosen GM , Muckle RP , Mahowald MW , Goding GS , Ullevig C . Postoperative respiratory compromise in children with obstructive sleep apnea syndrome: can it be anticipated? Pediatrics. 1994;93(5):784–788 [PubMed] [Google Scholar]
- 45. Wilson K , Lakheeram I , Morielli A , Brouillette R , Brown K . Can assessment for obstructive sleep apnea help predict postadenotonsillectomy respiratory complications? Anesthesiology. 2002;96(2):313–322 [DOI] [PubMed] [Google Scholar]
- 46. Schwengel DA , Sterni LM , Tunkel DE , Heitmiller ES . Perioperative management of children with obstructive sleep apnea. Anesth Analg. 2009;109(1):60–75 [DOI] [PubMed] [Google Scholar]
- 47. Raman VT , Jatana KR , Elmaraghy CA , Tobias JD . Guidelines to decrease unanticipated hospital admission following adenotonsillectomy in the pediatric population. Int J Pediatr Otorhinolaryngol. 2014;78(1):19–22 [DOI] [PubMed] [Google Scholar]
- 48. Bangiyev JN , Thottam PJ , Christenson JR , Metz CM , Haupert MS . The association between pediatric general emergency department visits and post operative adenotonsillectomy hospital return. Int J Pediatr Otorhinolaryngol. 2015;79(2):105–107 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.