Abstract
OBJECTIVE:
Compare the risk of harm from pharmacologic interventions in pediatric versus adult randomized controlled trials (RCTs).
METHODS:
We used systematic reviews from the Cochrane Database of Systematic Reviews. We considered separately 7 categories of harms/harm-related end points: severe harms, withdrawals due to harms, any harm, organ system–level harms, specific harms, withdrawals for any reason, and mortality. Systematic reviews with quantitative synthesis from at least 1 adult and 1 pediatric RCT for any of those end points were eligible. We calculated the summary odds ratio (experimental versus control intervention) in adult and pediatric trials/meta-analysis; the relative odds ratio (ROR) in adults versus children per meta-analysis; and the summary ROR (sROR) across all meta-analyses for each end point. ROR <1 means that the experimental intervention fared worse in children than adults.
RESULTS:
We identified 176 meta-analyses for 52 types of harms/harm-related end points with 669 adult and 184 pediatric RCTs. Of those, 165 had sufficient data for ROR estimation. sRORs showed statistically significant discrepancy between adults and children only for headache (sROR 0.82; 95% confidence interval 0.70–0.96). Nominally significant discrepancies for specific harms were identified in 12 of 165 meta-analyses (RORs <1 in 7, ROR >1 in 5). In 36% of meta-analyses, the ROR estimates suggested twofold or greater differences between children and adults, and the 95% confidence intervals could exclude twofold differences only in 18% of meta-analyses.
CONCLUSIONS:
Available evidence on harms/harm-related end points from pharmacologic interventions has large uncertainty. Extrapolation of evidence from adults to children may be tenuous. Some clinically important discrepancies were identified.
Keywords: comparative safety, harms, withdrawals, mortality, children, adults, pharmacologic interventions
What’s Known on This Subject:
Drug use in pediatrics is often based on adult efficacy data. Clinically significant discrepancies between adults and children may exist. To our knowledge, there is no large-scale evaluation of evidence comparing rates of adverse events between adults and children.
What This Study Adds:
Available evidence on the comparative safety of pharmacologic interventions in adults versus children is inconclusive. In a third of meta-analyses, twofold or greater differences were identified between adults and children, and some clinically important discrepancies were also found.
Adverse events from pharmacologic interventions are common among children and adult patients both in hospital and ambulatory settings. 1 – 3 For most of these harms, it is unknown whether their frequency and profile differs between children and adults. Differences between these 2 age groups 4 , 5 may be due to unique pharmacokinetic and pharmacodynamic properties of drugs in children. 6 Children could be more resilient to drug adverse events due to better organ function or more vulnerable due to higher tissue sensitivity. There is also evidence that adverse drug reactions can lead more often to hospital admissions in children than in adults, 7 and certain pediatric populations may be at even higher risk for hospitalization. 8 , 9 Pediatric use of drugs often depends on adult efficacy data because of the limited amount of data from randomized controlled trials (RCTs) in children. However, clinically significant discrepancies between adults and children may occur. 10 To our knowledge, there is no large-scale evaluation of the evidence on the comparative rates of adverse events between adults and children. Meta-analysis can be used as a tool to improve the power to detect clinically significant differences in harms.
We set out to perform a large-scale empirical evaluation, across diverse topics, of the relative risk of harms and related outcomes between adults and children by focusing on outcomes reported in meta-analyses of pediatric and adult RCTs. We wanted to study whether differences exist in the relative risks of harms and other harm-related outcomes (such as withdrawals and mortality) between adults and children.
Methods
Eligible Systematic Reviews and Trials
We perused a sample of 106 systematic reviews previously identified in the Cochrane Database of Systematic Reviews (CDSR) 10 as part of an empirical evaluation comparing primary effectiveness outcomes in pediatric versus adult RCTs. The eligibility criteria of these reviews have been described in detail elsewhere. 10
We screened reviews in the 2011 (issue 1) of the CDSR (except for mortality, for which we used the CDSR 2013, issue 1 vol) to identify meta-analyses on pharmacologic interventions (drugs, biologics, vaccines, parenteral solutions) with a quantitative synthesis for binary harms and harm-related outcomes from ≥1 pediatric RCT and ≥1 RCT in adults. For the characterization of trials as adult or pediatric RCTs, we used the same rules as previously reported 10 (Supplemental Appendix 1).
The types of end points considered were grouped in the following 7 categories: severe harm, withdrawals due to harm, any harm (without further specification), organ system–level harm (eg, gastrointestinal adverse events), specific harm (eg, headache, nausea), withdrawals for any reason, and mortality. The last 2 categories combine both effectiveness and harms.
For the categorization of medical interventions into experimental and control interventions, we used the same criteria previously described. 10 We excluded systematic reviews with no quantitative synthesis for any study end point and those without data available for both age groups. The screening was done in duplicate by 2 independent investigators (DL, MB) and disagreements were discussed with a third investigator (DCI) to reach consensus.
Data Extraction
From each eligible meta-analysis, we extracted the following information: title, experimental intervention, control intervention, outcome, and trials per age group (author, year, 2×2 table-data for outcomes per trial). Meta-analyses for different end points in the same systematic review were considered separately.
Primary and Secondary End Points
We considered the following 2 primary end points: severe harms and withdrawals due to harms. As secondary end points we considered any harm, organ system–level harms, specific harms, withdrawals due to any reason, and mortality.
Quantitative Data Synthesis
Odds ratio (OR) was used as the metric of relative risk for each harm or harm-related end point. All interventions and end points were coined so that the compared arms always referred to the experimental versus the control intervention and the calculated OR always referred to an adverse outcome (ie, OR <1.00 means that the experimental intervention fared better than the control).
When >1 trial per age group was available in each meta-analysis, we estimated the OR for the experimental versus the control intervention separately for the adult and the pediatric trials combining the ORs within each age group by fixed and random effects models. 11 , 12 Between-study heterogeneity was evaluated with the I 2 metric and the corresponding 95% confidence intervals (CI). 13 Moreover, each end point was considered separately.
For each meta-analysis, we then calculated the relative odds ratio (ROR) and the corresponding 95% CI of adult versus pediatric trials per meta-analysis by dividing the OR in adults by the OR in children. A topic with ROR <1.00 means that the experimental intervention (versus the control intervention) fared worse in children compared with adults. Finally, when ≥4 meta-analyses were available for an end point, we calculated the summary ROR (sROR) in adults versus children, across all topics, by combining the natural logarithms of all individual RORs per random effects meta-analysis. 12 We calculated the between-topic heterogeneity for the sROR estimates by using I 2 and the 95% CIs thereof. 13 – 15
We identified the topics for which the results in children versus adults differed beyond chance; those where the point estimate of the ROR suggested differences ≥20% in the OR between children and adults; those where the point estimate of the ROR suggested differences twofold or greater in the OR between children and adults; and those in which the 95% CIs of the estimated ROR excluded twofold difference in the OR between children and adults. Calculations were performed in Stata 12 (Stata Corp, College Station, TX) using the metan module. The CIs for I 2 were obtained by using the heterogi module. 13 P values are 2-tailed.
Results
Eligible Topics
Of the previously identified systematic reviews, 10 we considered as eligible 55 reviews (Supplemental Appendix 2) corresponding to 176 individual meta-analyses, pertaining to 113 comparisons of experimental versus control interventions and targeting 52 types of harm and harm-related end points. The total number of meta-analyses for each type of harms and harm-related end point is shown in Fig 1. After excluding 75 mixed age group and 69 unspecified age group studies, we included 669 adult RCTs and 184 pediatric RCTs across all 176 meta-analyses (Fig 1). RORs were calculated in 165 of 176 meta-analyses. In the remaining 11 meta-analyses, RORs could not be calculated because there were no harm or harm-related events in all adult or all pediatric trials or in both.
FIGURE 1.
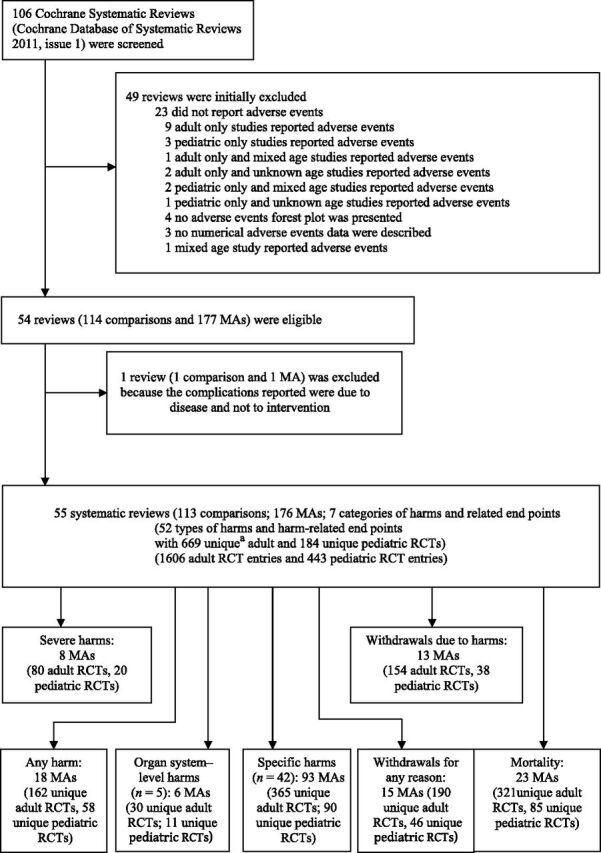
Flow chart: total number of meta-analyses for each type of harm and harm-related end point. a Each RCT could have reported >1 harm or harm-related end points. MA, meta-analysis.
The topics for those systematic reviews are shown in Supplemental Table 3. We performed quantitative synthesis for sROR estimation for the following 12 unique harms and related end points with ≥4 pertinent meta-analyses: severe harms, withdrawals due to harms, any harm, 7 specific harms (headache, drowsiness, nausea, fatigue, dizziness, tremor, and infections; from a total of 42 specific harms), withdrawals for any reason, and mortality. Additionally, there were 5 organ system–level harms and 35 specific harms for which only individual RORs were calculated because there were not ≥4 pertinent meta-analyses for each.
Frequency of Differences Between Children and Adults
Nominally significant discrepancies for harm risks in children versus adults were identified in 12 of 165 (7%) meta-analyses (ROR <1 in 7, ROR >1 in 5).
In 38% (63 of 165) of the studied meta-analyses, the point estimates of the RORs were ≤0.83, and in 19% (32 of 165), they were ≤0.5. In another 36% (59 of 165) of the meta-analyses, the point estimates of the RORs were ≥1.20, and in 16% (27 of 165) they were ≥2.0. Thus the point estimates suggested ≥20% differences in the OR between children and adults in 74% of the meta-analyses and twofold or greater differences in the OR in 36% of the meta-analyses.
The lower 95% CI of the estimated RORs were ≤0.5 in 73% of the meta-analyses (120 of 165 for which RORs were calculated). The upper 95% CI of RORs were ≥2.0 in 76% (124 of 165) of the meta-analyses. Only in 18% (30 of 165) of the meta-analyses could twofold differences in the OR between children and adults be excluded in both directions based on the 95% CIs. The characteristics of included meta-analyses for each harm and harm-related end point and the respective quantitative synthesis results are shown in Supplemental Tables 3 and 4.
sRORs
Across the 12 types of harms and harm-related end points for which quantitative synthesis was performed (Table 1), nominally statistically significant discrepancies between adults and children were identified for only 1, headache. The sROR for headache was 0.82 (95% CI 0.70–0.96), indicating that children fared worse than adults for this adverse event, although none of the individual meta-analyses on headache had nominally statistically significant RORs. For the other 11 categories of harm, the differences were not beyond chance, and sROR estimates were relatively close to 1.00 (range 0.88–1.15) for the end points that had the largest number of trials (192–406) and the largest number of meta-analyses (13–23): that is, mortality, withdrawals due to harms, withdrawals due to any reason, and any harm.
TABLE 1.
sRORs for Harms and Harms-Related Endpoints in Adult Versus Pediatric Studies
| Harms and Harm-Related Endpoints | N (MA) | RCTs (Adults/Peds) | sROR (95% CI) | I 2 (95% CI) | RCT per MA (Median Adults/Peds) | Sample Size per MA (Median Adult/Peds) | Sample Size per RCT (Median Adult/Peds) |
|---|---|---|---|---|---|---|---|
| Severe harms | 8 | 80/20 | 1.25 (0.73–2.15) | 0 (0–79) | 4/3 | 947/362 | 143/119 |
| Withdrawals due to harms | 13 | 154/38 | 1.11 (0.78–1.57) | 0 (0–57) | 10/1 | 1483/329 | 202/186 |
| Any harm | 18 | 162/58 | 1.03 (0.82–1.30) | 57 (26–75) | 4/2 | 1132/335 | 185/139 |
| Specific harms | |||||||
| Headache | 15 | 164/41 | 0.82 (0.70–0.96) | 0 (0–54) | 4/1 | 1958/269 | 231/206 |
| Drowsiness | 8 | 66/9 | 1.02 (0.58–1.79) | 34 (0–71) | 7/1 | 995/93 | 53/60 |
| Nausea | 8 | 33/9 | 0.69 (0.40–1.21) | 0 (0–68) | 2/1 | 769/223 | 272/199 |
| Fatigue | 6 | 24/6 | 0.81(0.48–1.35) | 0 (0–75) | 3/1 | 854/224 | 127/224 |
| Dizziness | 5 | 21/5 | 0.69 (0.37–1.29) | 0 (0–85) | 4/1 | 735/199 | 105/199 |
| Tremor | 4 | 28/10 | 0.88 (0.68–1.13) | 0 (0–85) | 7/2 | 1503/338 | 195/131 |
| Infections | 4 | 42/7 | 0.62 (0.27–1.43) | 0 (0–90) | 10/2 | 1839/113 | 75/60 |
| Withdrawals for any reason | 15 | 190/46 | 1.15 (0.88–1.49) | 38 (0–66) | 9/1 | 1287/193 | 184/174 |
| Mortality | 23 | 321/85 | 0.88 (0.70–1.11) | 32 (0–62) | 7/2 | 1414/222 | 90/75 |
MA, meta-analyses; Peds, pediatrics.
Nominally Significant Differences in Children Versus Adults for Specific Topics
There were 12 meta-analyses for which the RORs on specific harms or harm-related end points were nominally statistically significant (Supplemental Table 4). On the basis of random effects calculations of RORs, children fared worse than adults in 7 of those cases (ROR <1), whereas the opposite occurred in 5 cases. Results are summarized in Table 2, and we present these 12 topics in more detail next.
TABLE 2.
Individual Meta-Analyses With Statistically Significant RORs
| CD Number | Condition | Comparisons | Harms and Harm–Related Endpoints | N Adult Studies (Events/Total Sample Size)/N Pediatric Studies (Events/Total Sample Size) | ROR (95% CI) | OR Adults (95% CI) | OR Children (95% CI) |
|---|---|---|---|---|---|---|---|
| Any harm | |||||||
| CD000247 | Common cold and acute purulent rhinitis | Antibiotic versus placebo | Adverse events | 4 (169/1267)/2 (43/228) | 2.67 (1.19–5.99) | 2.42 (1.68–3.48) | 0.91 (0.44–1.86) |
| CD001954 | Acute lower respiratory tract infections | Azithromycin versus amoxycillin or amoxycillin–clavulanate | Adverse events | 10 (368/2071)/2 (122/335) | 2.5 (1.43–4.38) | 0.75 (0.59–0.95) | 0.30 (0.18–0.49) |
| CD002109 | Community acquired pneumonia | Clarithromycin versus erythromycin | Adverse events | 2 (162/476)/1 (61/260) | 0.28 (0.14–0.57) | 0.30 (0.20–0.45) | 1.07 (0.60–1.9) |
| Specific harms | |||||||
| CD000059 | Schizophrenia | Clozapine versus typical antipsychotics | Drowsiness | 14 (681/1415)/1 (12/21) | 0.073 (0.006–0.87) | 1.76 (1.33–2.31) | 24 (2.05–279.5) |
| CD001417 | Drug resistant partial epilepsy | Topiramate versus placebo | Drowsiness | 9 (236/1226)/1 (25/86) | 2.76 (1.009–7.55) | 2.81 (1.92–4.11) | 1.02 (0.40–2.58) |
| CD004125 | Prevention of post-operative nausea and vomiting | Hyoscine versus placebo | Dry mouth | 9 (364/766)/1 (28/40) | 0.08 (0.009–0.79) | 1.97 (1.34–2.89) | 23.2 (2.58–208.6) |
| CD006198 | Malaria prevention | RTS,S vaccine versus control | Injection site pain | 2 (644/1003)/3 (578/6499) | 3.23 (1.86–5.62) | 5.31 (3.86–7.30) | 1.64 (1.05–2.57) |
| CD006198 | Malaria prevention | RTS,S vaccine versus control | Swelling | 1 (1/865)/3 (327/6464) | 0.32 (0.12–0.80) | 0.33 (0.13–8.09) | 10.38 (6.67–16.16) |
| Withdrawals for any reason | |||||||
| CD002217 | Partial onset seizures and generalized onset tonic/clonic seizures | Phenytoin versus phenobarbitone | Withdrawal | 2 (186/436)/1 (25/63) | 0.18 (0.33–0.99) | 1.54 (1.04–2.26) | 8.47 (1.62–44.25) |
| CD005161 | Liver transplantation | Tacrolimus versus cyclosporine | Withdrawals | 11 (395/2446)/1 (64/181) | 2.16 (1.09–4.29) | 0.71 (0.55–0.91) | 0.33 (0.17–0.62) |
| Mortality | |||||||
| CD000527 | Severe malaria | Any artemisinin drug versus quinine | Mortality | 11(236/1414)/8 (232/1492) | 0.51 (0.34–0.78) | 0.49 (0.36–0.65) | 0.94 (0.71–1.26) |
| CD002152 | Cerebral malaria | Phenobarbitone versus placebo or nothing | Death within 6 mo | 1 (62/185)/1 (44/340) | 0.25 (0.10–0.63) | 0.60 (0.32–1.11) | 2.39 (1.20–4.68) |
CD, Cochrane Database; MA, meta-analysis.
RORs <1
For 7 topics, children had more unfavorable outcomes than adults. Clarithromycin had fewer adverse events than erythromycin for community acquired pneumonia in adults, whereas there was no difference between the 2 medications in children. The RTS,S malaria vaccine caused more swelling than the control in children, but this was not documented in adults; of note, there was only 1 adult RCT (n = 865 patients) versus 3 substantially larger pediatric RCTs (n = 6464 children). Clozapine caused more drowsiness than typical neuroleptics in both adults and children with schizophrenia, but in children, it was much more prominent, and a 24-fold increase was documented in the single small pediatric RCT with 22 children. Hyoscine for prevention of postoperative nausea/vomiting caused dry mouth more frequently than placebo in both adults and children, but in children, it was more prominent, with a 23-fold increase documented in the single pediatric RCT of 40 children. Children had relatively more withdrawals for any reason with phenytoin versus phenobarbitone for partial or generalized seizures. Phenytoin was associated with an increase in discontinuation rates in both adults and children, but children had significantly more withdrawals in the single pediatric RCT of 63 children. Artemisin drugs offered a survival benefit versus quinine for severe malaria only in adults, not in children (11 adult RCTs, n = 1414 patients; 8 pediatric RCTs, n = 1492 children). Furthermore, children had increased mortality with routine use of phenobarbitone versus placebo for treating cerebral malaria (single large pediatric RCT, n = 340 children), while such an increase was not seen in adults.
RORs >1
For 5 topics, children had less unfavorable outcomes than adults. Azithromycin caused fewer harms than amoxicillin/amoxicillin-clavulanate in both adults and children with lower respiratory infections, but the decrease was even more prominent in children than in adults. Adults experienced a statistically significant increase in adverse events, mostly gastrointestinal, from antibiotics versus placebo when they were given for common cold and acute purulent rhinitis, whereas children did not show such a pattern; however, this difference should be interpreted with caution, because 5 additional pediatric trials had not reported results for adverse events.
For specific harms, topiramate caused increased drowsiness versus placebo in adults treated for partial seizure, but this was not documented in children. The RTS,S malaria vaccine caused injection-site pain in both adults and children; however, the increase in pain in children was relatively less.
Finally, in liver transplantation, tacrolimus was associated with fewer withdrawals due to harm than cyclosporine in both adults and children; however, the benefit was more prominent in children.
Discussion
In this large-scale meta-epidemiologic evaluation of the relative risks of harms in adults versus children, we identified a number of topics in which harms differed nominally beyond chance in the 2 populations. In a third of the meta-analyses, the point estimates of the ROR suggested twofold or greater differences in the estimated OR for harms and harm-related outcomes between adults and children. In the majority of topics, evidence was limited, and thus twofold differences could be excluded only in 18% of cases.
We targeted for convenience a selected group of systematic reviews 10 that had already included both adult and pediatric RCTs in their primary efficacy outcome analyses. Still, we were able to identify pertinent meta-analyses with some data on harm or harm-related end points from both adults and children in only half of those topics. Furthermore, there was a relative paucity of data on some clinically important types of adverse events, such as severe harm. Moreover, across the compiled database of 176 meta-analyses, the total number of identified pediatric trials reporting harms and harm-related end points was less than a third of the total number of adult trials. The dearth of pediatric evidence suggests that extrapolations from adults to children regarding the harm of drugs may be tenuous.
Clinical investigators should systematically collect and report information on harms and harm-related end points in pediatric RCT. Pharmaceutical companies do not typically evaluate most new agents in children because children represent a small market, and most pediatric trials are not funded by industry. 16 Pediatric trials often rely on governmental and nonprofit organization funding sources. 16 Moreover, drugs tested in children under the Pediatric Exclusivity law often do not reflect true priorities in children, and pediatric trials are often performed for blockbusters in the adult market. 17 Pediatric information on drug labeling is lacking in most drugs licensed in the United States, 18 and many children, in both inpatient and outpatient settings worldwide, are prescribed off-label drugs. 19 – 21 Even for conditions with high disease burden in children, only a small percentage of clinical trials have included children 16 ; the problem is even larger for conditions with high disease burden in developing countries.
In the absence of pediatric RCTs for drugs widely used in routine pediatric clinical practice, safety information from postmarketing surveillance studies might fill this knowledge gap. 22 , 23 Nevertheless, postmarketing surveillance studies have their own challenges 23 and exhibit variable accuracy and completeness. Moreover, most postmarketing studies that pharmaceutical companies commit to are never completed. 22 Rigorous prospective postmarketing safety surveillance studies should be systematically performed and reported. 24 This would be particularly important for rare adverse events that can occur in children and that could not be detected in even large pediatric RCTs. 25
Unfortunately, robust studies targeting harms in children are often never performed, and the documentation of true differences between adults and children will remain elusive. Availability of information on adverse events in RCTs remains suboptimal 26 – 31 despite the existence of specific standards for reporting of harms. 32 , 33 Problems include deficiencies in the study design phase to capture adverse events; neglected collection of adverse events during the trial conduct; lack of reporting or restricted reporting of adverse events; and occasionally even silencing of evidence on harms. 26 Long-term adverse events might even not be detected within the time frame of the RCTs and safety data for vulnerable populations at risk for adverse events may be lacking because these populations are usually excluded. Furthermore, reporting of the severity of clinical adverse events, laboratory-determined toxicities, and patient withdrawals due to adverse events is often inadequate. 29 – 31 , 34 , 35 Individual trials may also be underpowered to provide conclusive evidence for rare but clinically significant adverse events. 36 Even when pediatric trials are performed, they often yield small data sets that do not allow robust inferences for safety. Meta-analyses on harms may increase the power to detect clinically significant differences but also have their own methodologic challenges 37 – 39 because they depend on the presence of high-quality data in individual studies on collected and reported harms. With our meta-epidemiologic approach, we increased our power to detect significant discrepancies in harms between the 2 age groups and across several diverse topics. However, even meta-analyses may not have adequate power to detect modest differences. Selective reporting may also cause some spurious differences if it happens to affect more RCTs of 1 age group than another. For example, although the reported evidence suggests that antibiotics cause adverse events only in adults when given for common cold or purulent rhinitis (an inappropriate indication), many trials in children do not even report the respective information on adverse events.
We should acknowledge that observational studies may be more suitable to detect rare or late adverse effects of treatments; however, analysis of the evidence from observational studies was beyond the scope of this project. It would be interesting to compile observational data on medication-related adverse events from pediatric populations for comparative analyses against adult data. However, to our knowledge, such a collection of data does not exist yet, in contrast to the more routine availability of compiled RCT data in CDSRs.
There are some limitations that we should acknowledge in our study. First, in our analysis, we limited our search to topics previously identified as having comparative effectiveness results from both adults and children. It is possible that if we had screened the whole CDSR only for harms, we could have identified some additional pertinent meta-analyses. However, the scenario in which comparative safety data would be available without having any comparative efficacy data is likely to be uncommon. Second, by considering only meta-analyses that had included both pediatric and adult RCTs, it is possible that medical interventions for conditions that pertained exclusively to pediatric populations would not have been captured. Third, in our analyses we considered all pediatric RCTs together. Given the paucity of data, any age subgroup analyses (eg, infants, preschoolers) would be unable to detect any clinically significant differences. 40 , 41 Fourth, for some subjective harms (eg, pain), it is possible that children might underreport them, and this may affect the frequency of estimated discrepancies between adults and children for such end points. Fifth, we also considered mortality among the eligible end points, although death is often considered an efficacy outcome. However, one cannot exclude that death could also be caused by treatment-related harms.
Acknowledging these limitations, we were able to document several cases in which significant discrepancies existed in harms between adults and children. However, in the majority of cases, our analysis suggests that evidence about the comparative harms of drugs in these 2 populations is inconclusive. Even large differences cannot be excluded. Pediatric drug therapy should certainly take into account the physiologic differences between children and adults, 42 but making guesses about toxicity in the absence of evidence is not easy. In the absence of sufficient evidence, extrapolation to children of safety information for pharmacologic interventions from adults is likely to be tenuous. 43
Supplementary Material
Glossary
- CDSR
Cochrane Database of Systematic Reviews
- CI
confidence interval
- OR
odds ratio
- RCT
randomized controlled trial
- ROR
relative odds ratio
- sROR
summary relative odds ratio
Footnotes
Dr Lathyris contributed to the study design and generation of the methodologic plan for data extraction and analysis, performed data extraction, coordinated and supervised data collection, and reviewed and revised the manuscript; Dr Panagiotou contributed to the study design and generation of the methodologic plan for data analysis, performed statistical analyses, and reviewed and revised the manuscript; Dr Baltogianni contributed to the study design, performed data extraction, and reviewed and revised the manuscript; Dr Ioannidis contributed to the study design, generated the methodologic and statistical analysis plans, and reviewed and revised the manuscript; Dr Contopoulos-Ioannidis conceptualized and designed the study methodology, coordinated and supervised data collection, performed statistical analyses, and drafted the initial manuscript; and all authors approved the final manuscript as submitted.
FUNDING: No external funding.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1. Cohen AL , Budnitz DS , Weidenbach KN , et al. National surveillance of emergency department visits for outpatient adverse drug events in children and adolescents. J Pediatr. 2008;152(3):416–421 [DOI] [PubMed] [Google Scholar]
- 2. Holdsworth MT , Fichtl RE , Behta M , et al. Incidence and impact of adverse drug events in pediatric inpatients. Arch Pediatr Adolesc Med. 2003;157(1):60–65 [DOI] [PubMed] [Google Scholar]
- 3. Taché SV , Sönnichsen A , Ashcroft DM . Prevalence of adverse drug events in ambulatory care: a systematic review. Ann Pharmacother. 2011;45(7–8):977–989 [DOI] [PubMed] [Google Scholar]
- 4. Choonara I . Safety of new medicines in young children. Arch Dis Child. 2011;96(9):872–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tullus K . Safety concerns of angiotensin II receptor blockers in preschool children. Arch Dis Child. 2011;96(9):881–882 [DOI] [PubMed] [Google Scholar]
- 6. Kearns GL , Abdel-Rahman SM , Alander SW , Blowey DL , Leeder JS , Kauffman RE . Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167 [DOI] [PubMed] [Google Scholar]
- 7.Posthumus AA, Alingh CC, Zwaan CC, van Grootheest KK, Hanff LL, Witjes BB, et al. Adverse drug reaction-related admissions in paediatrics, a prospective single-centre study. BMJ Open. 2012;2(4):e000934 [DOI] [PMC free article] [PubMed]
- 8. Le J , Nguyen T , Law AV , Hodding J . Adverse drug reactions among children over a 10-year period. Pediatrics. 2006;118(2):555–562 [DOI] [PubMed] [Google Scholar]
- 9. Gallagher RM , Bird KA , Mason JR , et al. Adverse drug reactions causing admission to a paediatric hospital: a pilot study. J Clin Pharm Ther. 2011;36(2):194–199 [DOI] [PubMed] [Google Scholar]
- 10.Contopoulos-Ioannidis DG, Baltogianni MS, Ioannidis JP. Comparative effectiveness of medical interventions in adults versus children. J Pediatr. 2010;157(2):322–330.e317 [DOI] [PubMed]
- 11. DerSimonian R , Laird N . Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188 [DOI] [PubMed] [Google Scholar]
- 12. Lau J , Ioannidis JP , Schmid CH . Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826 [DOI] [PubMed] [Google Scholar]
- 13.Orsini N, Bottai M, Higgins J, Buchan I. HETEROGI: Stata module to quantify herterogeneity in a meta-analysis. EconPapers. Available at: http://econpapers.repec.org/software/bocbocode/s449201.htm Accessed January 2013
- 14. Ioannidis JP , Patsopoulos NA , Evangelou E . Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335(7626):914–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JP , Thompson SG . Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558 [DOI] [PubMed] [Google Scholar]
- 16. Bourgeois FT , Murthy S , Pinto C , Olson KL , Ioannidis JP , Mandl KD . Pediatric versus adult drug trials for conditions with high pediatric disease burden. Pediatrics. 2012;130(2):285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boots I , Sukhai RN , Klein RH , et al. Stimulation programs for pediatric drug research—do children really benefit? Eur J Pediatr. 2007;166(8):849–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sachs AN , Avant D , Lee CS , Rodriguez W , Murphy MD . Pediatric information in drug product labeling. JAMA. 2012;307(18):1914–1915 [DOI] [PubMed] [Google Scholar]
- 19. Shah SS , Hall M , Goodman DM , et al. Off-label drug use in hospitalized children. Arch Pediatr Adolesc Med. 2007;161(3):282–290 [DOI] [PubMed] [Google Scholar]
- 20. Ballard CD , Peterson GM , Thompson AJ , Beggs SA . Off-label use of medicines in paediatric inpatients at an Australian teaching hospital. J Paediatr Child Health. 2013;49(1):38–42 [DOI] [PubMed] [Google Scholar]
- 21. Pandolfini C , Bonati M . A literature review on off-label drug use in children. Eur J Pediatr. 2005;164(9):552–558 [DOI] [PubMed] [Google Scholar]
- 22. Fontanarosa PB , Rennie D , DeAngelis CD . Postmarketing surveillance—lack of vigilance, lack of trust. JAMA. 2004;292(21):2647–2650 [DOI] [PubMed] [Google Scholar]
- 23. Lloyd-Johnsen C , Justice F , Donath S , Bines JE . Retrospective hospital based surveillance of intussusception in children in a sentinel paediatric hospital: benefits and pitfalls for use in post-marketing surveillance of rotavirus vaccines. Vaccine. 2012;30(suppl 1):A190–A195 [DOI] [PubMed] [Google Scholar]
- 24. Benjamin DK Jr , Smith PB , Murphy MD , et al. Peer-reviewed publication of clinical trials completed for pediatric exclusivity. JAMA. 2006;296(10):1266–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buttery JP , Danchin MH , Lee KJ , et al. PAEDS/APSU Study Group . Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine. 2011;29(16):3061–3066 [DOI] [PubMed] [Google Scholar]
- 26. Ioannidis JP . Adverse events in randomized trials: neglected, restricted, distorted, and silenced. Arch Intern Med. 2009;169(19):1737–1739 [DOI] [PubMed] [Google Scholar]
- 27. Ioannidis JP , Contopoulos-Ioannidis DG . Reporting of safety data from randomised trials. Lancet. 1998;352(9142):1752–1753 [DOI] [PubMed] [Google Scholar]
- 28. Nuovo J , Sather C . Reporting adverse events in randomized controlled trials. Pharmacoepidemiol Drug Saf. 2007;16(3):349–351 [DOI] [PubMed] [Google Scholar]
- 29. Anderson M , Choonara I . A systematic review of safety monitoring and drug toxicity in published randomised controlled trials of antiepileptic drugs in children over a 10-year period. Arch Dis Child. 2010;95(9):731–738 [DOI] [PubMed] [Google Scholar]
- 30. Sammons HM , Gray C , Hudson H , Cherrill J , Choonara I . Safety in paediatric clinical trials—a 7-year review. Acta Paediatr. 2008;97(4):474–477 [DOI] [PubMed] [Google Scholar]
- 31. Nor Aripin KN , Choonara I , Sammons HM . Systematic review of safety in paediatric drug trials published in 2007. Eur J Clin Pharmacol. 2012;68(2):189–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ioannidis JP , Evans SJ , Gøtzsche PC , et al. CONSORT Group . Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141(10):781–788 [DOI] [PubMed] [Google Scholar]
- 33. Barbour V , Clark J , Jones S , Norton M , Simpson P , Veitch E . Why drug safety should not take a back seat to efficacy. PLoS Med. 2011;8(9):e1001097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ioannidis JP , Lau J . Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. JAMA. 2001;285(4):437–443 [DOI] [PubMed] [Google Scholar]
- 35. Pitrou I , Boutron I , Ahmad N , Ravaud P . Reporting of safety results in published reports of randomized controlled trials. Arch Intern Med. 2009;169(19):1756–1761 [DOI] [PubMed] [Google Scholar]
- 36. Tsang R , Colley L , Lynd LD . Inadequate statistical power to detect clinically significant differences in adverse event rates in randomized controlled trials. J Clin Epidemiol. 2009;62(6):609–616 [DOI] [PubMed] [Google Scholar]
- 37. Golder S , Loke YK , Bland M . Meta-analyses of adverse effects data derived from randomised controlled trials as compared to observational studies: methodological overview. PLoS Med. 2011;8(5):e1001026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hernandez AV , Walker E , Ioannidis JP , Kattan MW . Challenges in meta-analysis of randomized clinical trials for rare harmful cardiovascular events: the case of rosiglitazone. Am Heart J. 2008;156(1):23–30 [DOI] [PubMed] [Google Scholar]
- 39. Papanikolaou PN , Ioannidis JP . Availability of large-scale evidence on specific harms from systematic reviews of randomized trials. Am J Med. 2004;117(8):582–589 [DOI] [PubMed] [Google Scholar]
- 40. Contopoulos-Ioannidis DG , Seto I , Hamm MP , et al. Empirical evaluation of age groups and age-subgroup analyses in pediatric randomized trials and pediatric meta-analyses. Pediatrics. 2012;129(suppl 3):S161–S184 [DOI] [PubMed] [Google Scholar]
- 41. Williams K , Thomson D , Seto I , et al. StaR Child Health Group . Standard 6: age groups for pediatric trials. Pediatrics. 2012;129(suppl 3):S153–S160 [DOI] [PubMed] [Google Scholar]
- 42. Anderson BJ . Developmental pharmacology; filling one knowledge gap in pediatric anesthesiology. Paediatr Anaesth. 2011;21(3):179–182 [DOI] [PubMed] [Google Scholar]
- 43. Klassen TP , Hartling L , Craig JC , Offringa M . Children are not just small adults: the urgent need for high-quality trial evidence in children. PLoS Med. 2008;5(8):e172 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


