Abstract
BACKGROUND AND OBJECTIVES:
Parent-child reading is widely advocated to promote cognitive development, including in recommendations from the American Academy of Pediatrics to begin this practice at birth. Although parent-child reading has been shown in behavioral studies to improve oral language and print concepts, quantifiable effects on the brain have not been previously studied. Our study used blood oxygen level–dependent functional magnetic resonance imaging to examine the relationship between home reading environment and brain activity during a story listening task in a sample of preschool-age children. We hypothesized that while listening to stories, children with greater home reading exposure would exhibit higher activation of left-sided brain regions involved with semantic processing (extraction of meaning).
METHODS:
Nineteen 3- to 5-year-old children were selected from a longitudinal study of normal brain development. All completed blood oxygen level–dependent functional magnetic resonance imaging using an age-appropriate story listening task, where narrative alternated with tones. We performed a series of whole-brain regression analyses applying composite, subscale, and individual reading-related items from the validated StimQ-P measure of home cognitive environment as explanatory variables for neural activation.
RESULTS:
Higher reading exposure (StimQ-P Reading subscale score) was positively correlated (P < .05, corrected) with neural activation in the left-sided parietal-temporal-occipital association cortex, a “hub” region supporting semantic language processing, controlling for household income.
CONCLUSIONS:
In preschool children listening to stories, greater home reading exposure is positively associated with activation of brain areas supporting mental imagery and narrative comprehension, controlling for household income. These neural biomarkers may help inform eco-bio-developmental models of emergent literacy.
What’s Known on This Subject:
The American Academy of Pediatrics recommends parent-child reading from infancy through at least kindergarten, the span of maximal brain growth. Home literacy environment, including reading behaviors and access to books, has been shown to promote oral language and print concepts.
What This Study Adds:
Home reading environment is positively associated with activation of brain areas supporting narrative comprehension and mental imagery in preschool children. This offers novel insight into the neurobiological foundations of emergent literacy and potential effect of shared reading during early childhood.
Emergent literacy is defined as the skills, knowledge, and attitudes supporting reading and writing that accrue from infancy. 1 Although organic reading disability (dyslexia) affects an estimated 5% to 12% of US children, 2 the majority of illiteracy is preventable, attributable to inadequate resources, motivation, and/or stimulation required to learn to read. 3 As parents are “a child’s first and most important teachers,” 4 the quality of cognitive stimulation in the home, especially before school entry, strongly influences achievement and health outcomes. 5 – 8 Children’s books are catalysts for parent-child engagement during sensitive developmental stages when brain growth and plasticity are maximal. 9 , 10 They provide broader, more grammatically correct vocabulary and range of subject matter than everyday conversation, especially in low-socioeconomic status (SES) households. 11 , 12 Given these factors, the American Academy of Pediatrics (AAP) recommends shared reading beginning at birth, citing direct, lasting benefits for the developing brain, 13 a claim echoed by many advocacy groups. 14
While behavioral evidence affirms moderate to large benefits of shared reading on a subset of emergent literacy skills (oral language and print concepts) through kindergarten, 5 , 15 quantifiable effects on the brain have not been previously studied. Similarly, interventions improving home literacy environment, a variably defined measure of reading behaviors and access to books, have been shown to improve oral language and school readiness, 16 – 20 although neurobiological mechanisms have yet to be described. Neuroimaging offers a means to address these knowledge gaps, informing an eco-bio-developmental model of emergent literacy incorporating genetic, environmental, and neurobiological factors. 21 – 25 Such models have been advocated by the AAP and National Institutes of Health 25 and are especially valuable for young children, where behavioral measures can underestimate the effects of learning and experience on the developing brain. 10 , 26 Neuroimaging has been extensively applied in dyslexia research (albeit in older children and adults), identifying activation patterns associated with reading difficulty and response to intervention, 2 , 27 – 29 as well as helping define the mature reading network. 30 , 31 Only recently has high-resolution neuroimaging been applied in younger, preliterate children, 32 most often in the context of normal language development. 33 , 34 How language networks become “ready” for reading and to what extent they are influenced by home literacy environment or interventions during the critical pre-kindergarten period, however, are unclear.
For our study, a sample of 3- to 5-year-old children underwent blood oxygen level dependent functional magnetic resonance imaging (BOLD fMRI) using a story listening task. 35 , 36 This task requires the application of emergent literacy skills supporting semantic processing (extraction of meaning), including vocabulary and listening comprehension. 37 – 40 Given behavioral evidence, 1 , 5 we hypothesized that children with more stimulating home environments, particularly shared reading exposure, would show more robust activation in brain areas supporting these skills. The semantic network includes left-sided inferior frontal, middle temporal, inferior parietal, and lateral occipital lobes. 35 , 39 , 41 We predicted that differential activation within this network would remain significant after controlling for household income, a common confounder in studies of cognitive ability. 42 – 44
Methods
Participants
All participants in this analysis were enrolled in a longitudinal study of normal brain development at our institution (Cincinnati MR Imaging of NeuroDevelopment; C-MIND). 45 Inclusion criteria for C-MIND are as follows: full-term gestation, healthy, right-handed, native English speakers, and no standard contraindications to MRI. By design, the C-MIND cohort is demographically diverse (38% nonwhite, 55% female, median household income $42 500), intended to reflect the US population. At the time of our study, 23 children between 3 and 5 years of age had completed BOLD fMRI while performing a story listening task, in accordance with the C-MIND protocol. Of these, we were able to contact 19 families (82.6%) for enrollment and survey administration. Despite multiple attempts, we were unable to contact the other 4 families, who were excluded. Informed consent was obtained from each child’s custodial parent, families were compensated for time and travel, and our study was approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board.
Behavioral Measures
Cognitive stimulation in the home was assessed using the preschool version of the StimQ (StimQ-P), 46 which was administered to a custodial parent via telephone or during C-MIND follow-up visits by a trained clinical research coordinator. Time elapsed between fMRI scan and StimQ administration ranged from 0 to 20 months (10 ± 8.8). The StimQ-P is validated for ages 36 to 72 months 47 and involves mostly “yes/no” questions. Three subscales were used: (1) Reading, reflecting access to books, frequency of shared reading, and variety of books read; (2) Parental Involvement in Developmental Advance (PIDA), reflecting the teaching of specific concepts such as letters; and (3) Parental Verbal Responsivity (PVR), reflecting verbal interaction. Parents were also asked to report the age of initiation of reading to their child, which is not included in the StimQ-P.
fMRI Acquisition Specifications and Preliminary Analyses
Details of techniques used to acclimatize children to the MRI acquisition process are described by Vannest et al. 32 Details of BOLD MRI acquisition specifications used in the C-MIND study are described in Schmithorst et al. 35 , 48 Details of data preprocessing for the C-MIND study are described in Sroka et al. 49 All children were awake and nonsedated during MRI scans. Voxel size used for acquisition and analysis was 3 × 3 × 4 mm. We used the FEAT (fMRI Expert Analysis Tool) modality of FSL (fMRI-Brain Software Library, Oxford, United Kingdom) for all group mean and higher-level regression analyses. 50
fMRI Story Listening Task and Group Mean Analysis
The story listening task consists of 10 alternating blocks of active and control conditions (5 each) of 64 seconds’ duration. During the “active” condition, a series of 5 recorded stories of 9 to 10 sentences each read in a female voice was presented via headphones. The stories were designed by a speech pathologist with consistent vocabulary and syntax appropriate for young children (download: https://www.irc.cchmc.org/software/pedaudio.php). The control condition consisted of nonspeech tones in a range of frequencies simulating human speech to control for baseline acoustic processing. Subjects closed their eyes or saw a blank screen during scanning.
During task presentation, the MRI scanner continuously acquired BOLD-weighted scans, covering the entire brain with 24 slices at 4-second intervals. Image time series data were entered into a general linear model for “first-level,” voxelwise analysis, using the story and tone intervals as the regressor of interest. Contrast maps (stories > tones activation) were converted to z score maps with statistical threshold of P < .05, applying a false discovery rate (FDR) correction for multiple voxel comparisons across the brain. Using these, we obtained a whole-brain, group mean activation map for our 19 subjects, representing mean neural activation listening to stories, minus activation listening to tones (ie, activation attributable to the story task, excluding general acoustic processing).
Regression With StimQ-P and Other Predictors
For each subject, the z score map representing the contrast of (stories > tones) was used as the dependent variable in a series of “higher-level” regression analyses, applying StimQ-P scores (Reading, PIDA, PVR, Composite) or responses to individual questions as the explanatory variable. Income category (low/not low) was applied as a binary covariate when significant neural activation was found, with household income <200% of the 2015 Federal Poverty Guidelines, 51 adjusted for household size, defined as low income (see Table 1). 52 Subject age and gender were considered as covariates but excluded because no significant correlation was found between neural activation and either variable. Regression maps of neural activation (stories > tones), along with summary statistics for size, intensity, and location of activation clusters, were reported for all significant results, using a threshold for statistical significance of P < .05 applying FDR correction. The FSLView 50 package was used to identify brain areas corresponding to active clusters in normalized, three-dimensional, Montreal Neurologic Institute (MNI) coordinate space, 53 using the Harvard-Oxford Cortical Structural Atlas (2-mm scale).
TABLE 1.
Demographic Characteristics of C-MIND Sample Subjects
| Characteristic | n | % |
|---|---|---|
| Sample | 19 | 100 |
| Age (y) | ||
| 3+ | 10 | 52 |
| 4+ | 6 | 32 |
| 5+ | 3 | 16 |
| Gender | ||
| Male | 8 | 42 |
| Female | 11 | 58 |
| Annual household income (US$) | ||
| <5000 | 0 | 0 |
| 5000–10 000 | 1 | 5 |
| 10–15 000 | 1 | 5 |
| 15 000–25 000 | 2 | 11 |
| 25 000–35 000 | 1 | 5 |
| 35 000–50 000 | 2 | 11 |
| 50 000–75 000 | 4 | 21 |
| 75 000–100 000 | 4 | 21 |
| 100 000–150 000 | 2 | 11 |
| >150 000 | 2 | 11 |
| Household income level | ||
| Below 200% poverty (low) | 7 | 37 |
| Above 200% poverty | 12 | 63 |
| Children in the household | ||
| 1 | 3 | 16 |
| 2–3 | 12 | 63 |
| 4–5 | 3 | 16 |
| 6 | 1 | 5 |
Results
Demographic characteristics for our sample are described in Table 1.
StimQ-P and Other Behavioral Predictors
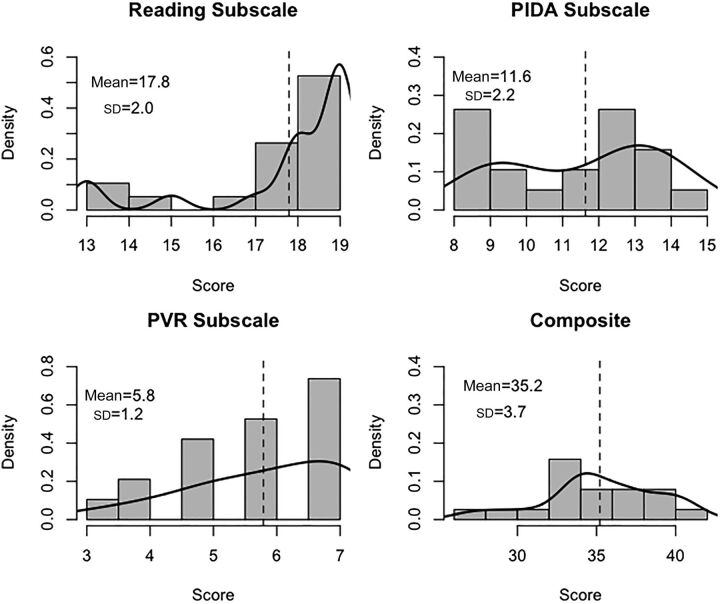
A summary of StimQ-P subscale and composite scores, and reported age of initiation of shared reading are described in Table 2 and Fig 1.
TABLE 2.
StimQ-P and Specific Reading-Related Item Scores
| StimQ-P | Possible | Mean | SD | Min | Max |
|---|---|---|---|---|---|
| Reading | 19 | 17.8 | 2.0 | 13 | 19 |
| PIDA | 15 | 11.6 | 2.2 | 8 | 15 |
| PVR | 7 | 5.8 | 1.2 | 3 | 7 |
| Composite | 41 | 35.2 | 3.7 | 27 | 41 |
| Specific Items | |||||
| Age (mo) initiation of reading | n/a | 5.0 | 5.5 | 0 | 24 |
| Months of reading exposure | n/a | 43.0 | 9.5 | 30 | 63 |
| Children’s books in the home | n/a | 162.0 | 112.8 | 10 | 400 |
| Reading nights/wk | 7 | 6.0 | 1.9 | 2 | 7 |
Summary of StimQ-P subscale, Composite, and reading-related item scores. Total possible score (where applicable), mean, SD, minimum (Min), and maximum (Max) are presented. Individual questions other than age of initiation of reading are part of the StimQ-P Reading subscale.
FIGURE 1.
StimQ-P Subscale and Composite scores. Histograms and density curves for StimQ-P scores. Mean and SD are provided, with a dashed vertical line for each mean. The Reading subscale reflects parent-child reading materials and behaviors (maximum score 19); PIDA measures parental involvement teaching specific skills (maximum 15); PVR indicates parent-child verbal interaction (maximum 7).
Group Mean Activation for the Narrative Comprehension Task
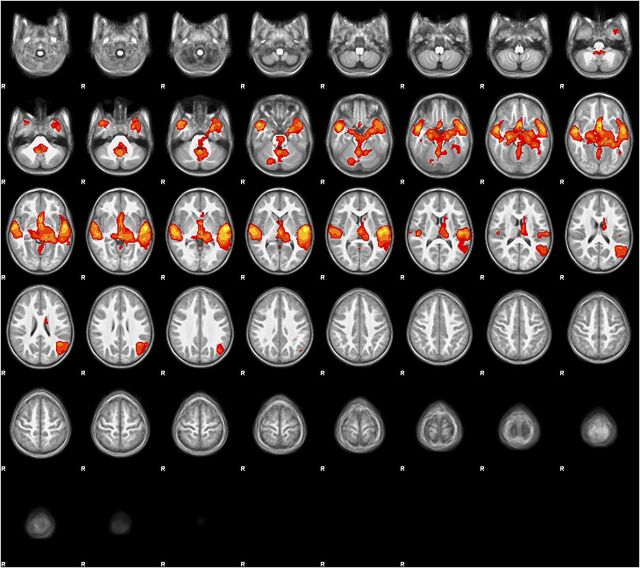
Group mean activation for the narrative condition compared with baseline tones (all voxels P < .05, FDR correction) involved bilateral, left-lateralized cortical and subcortical regions involved with acoustic, phonological, and semantic language processing (see Fig 2), as described by Karunanayaka et al. 54
FIGURE 2.
Group mean activation map for the story listening task. Group mean BOLD fMRI activation map (stories > tones) in 3- to 5-year-old children (N = 19). All voxels significant at P < .05 (FDR corrected), slice thickness 5 mm for contiguous slices. Slices range from z = –28 to z = 74 in MNI coordinate space. Color scale ranges from t = 1.25 (cooler) to 4 (hotter). Radiologic orientation, left = right, right = left.
Regression of Neural Activation With StimQ-P Scores and Other Predictors
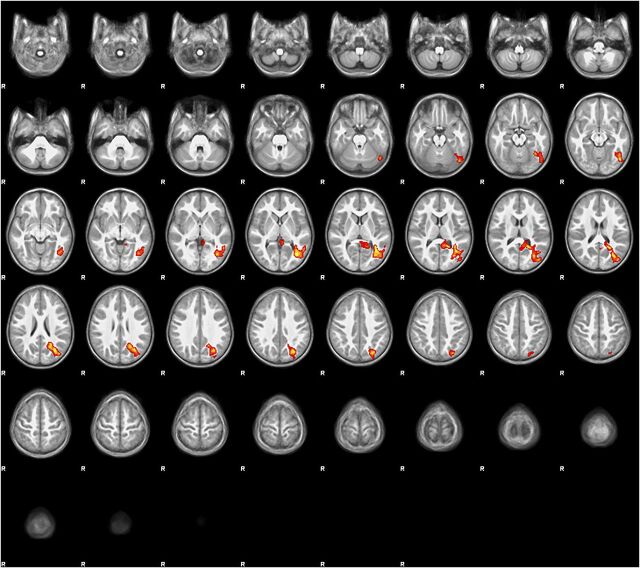
Applying linear regression, StimQ-P Reading subscale scores were positively correlated with higher activation in a confluent region of left-sided, posterior cortex involving the occipital fusiform, lateral occipital, posterior inferior temporal, posterior middle temporal, posterior cingulate, and angular gyri, and left precuneus, as illustrated in Fig 3 (all voxels P < .05, FDR correction). Collectively, these areas reside within the parietal-temporal-occipital (PTO) association cortex, which supports multimodal semantic processing, especially for language. 41 , 55 An exception is the posterior cingulate gyrus, which plays a role in semantic processing and other functions, including memory encoding 41 and visual attention. 56
FIGURE 3.
Regression map (stories > tones activation) with StimQ-P Reading subscale score as explanatory variable. Regression map for the story listening task (stories > tones) in 3- to 5-year-old children (N = 19), with StimQ-P Reading score as explanatory variable. Cluster size 4087 voxels significant at P < .05 (FDR corrected), z score local maxima 3.25 to 3.44. Five-millimeter slices from z = –28 to z = 74 in MNI coordinate space. Color scale from t = 1.25 (cooler) to 4 (hotter). Radiologic orientation, left = right, right = left.
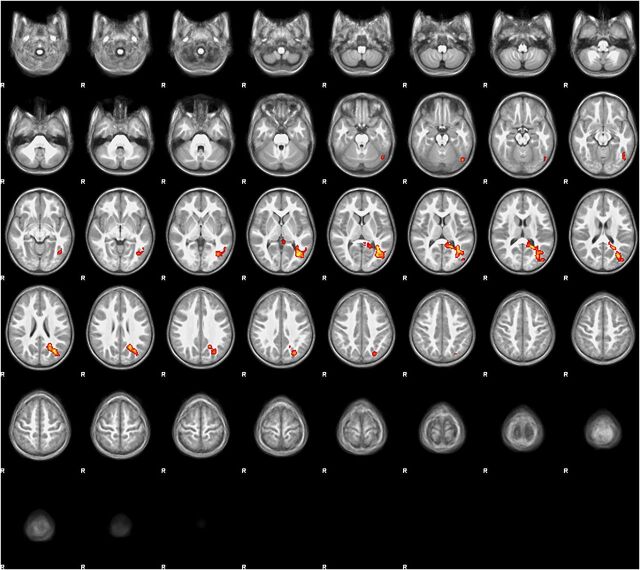
The correlation between StimQ-P Reading subscale score and neural activation within the left PTO cortex remained consistent and significant when expanding the statistical model to control for household income as a binary covariate (low/not low). Activation clusters were of similar intensity, with slight to moderate decreases in size, as shown in Fig 4 (all voxels P < .05, FDR correction). The largest decreases were in posterior cingulate, inferior temporal, occipital fusiform, and the most superior lateral occipital areas. Fig 5 displays orthogonal sagittal, coronal, and axial slice views (origin x = –34 y = –66, z = 14, MNI coordinate space) to more clearly illustrate the anatomic extent of this PTO activation cluster.
FIGURE 4.
Regression map (stories > tones activation) with StimQ-P Reading subscale score as explanatory variable, controlling for household income. Regression map for the story listening task (stories > tones) in 3- to 5-year-old children (N = 19), with StimQ-P Reading score as explanatory variable, controlling for household income. Cluster size 2467 voxels significant at P < .05 (FDR corrected), z score local maxima 3.15 to 3.38. Five-millimeter slices from z = –28 to z = 74 in MNI coordinate space. Color scale from t = 1.25 (cooler) to 4 (hotter). Radiologic orientation, left = right, right = left.
FIGURE 5.
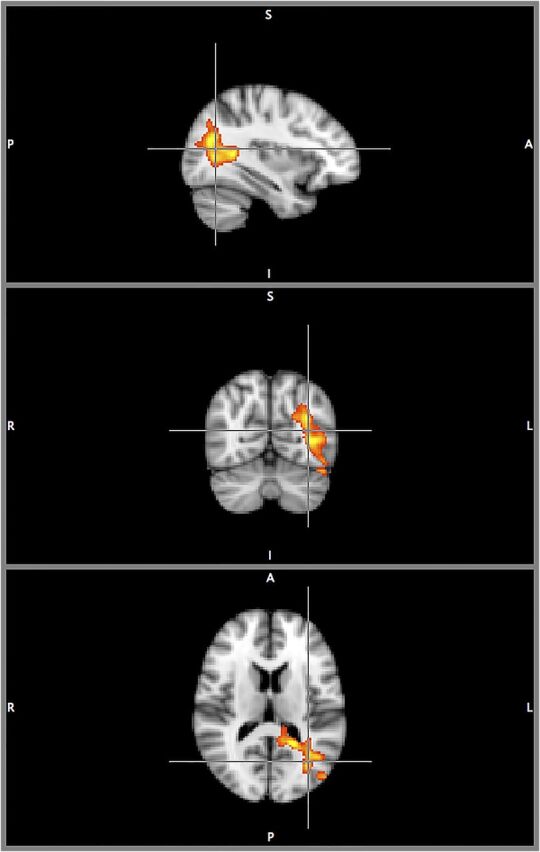
Triplanar view of neural activation (stories > tones) with StimQ-P Reading subscale score as explanatory variable, controlling for household income. Orthogonal triplanar view (origin x = –34, y = –66, z = 14, MNI coordinate space) of activation for the story listening task (stories > tones), with StimQ-P Reading score as explanatory variable, controlling for household income. Cluster size 2467 voxels significant at P < .05 (FDR corrected). Color scale ranges from t = 1.25 (cooler) to 4 (hotter). All views in radiologic orientation, left = right, right = left, with sagittal plane viewed from the right.
No significant correlation was found between brain activation during the story listening task and other StimQ-P subscales, StimQ-P composite, age of initiation of reading, or months of reading exposure (initiation to scan).
Discussion
“Biological embedding” describes the long-term impact on brain development resulting from the quality of cognitive stimulation and nurturing during early childhood. 6 , 57 Learning to read involves the integration of a formidable array of skills sequentially and efficiently, 5 supported by language, visual, and association brain networks, the growth and plasticity of which peak in the first few years of life. 58 , 59 During this critical prekindergarten period, children are highly vulnerable to disparities in cognitive stimulation, especially spoken language, as well as toys and books promoting constructive parent-child engagement. 12 , 58 , 60 Many children arrive at school at a significant disadvantage in reading readiness, and it is clear that those who are poor readers in first grade 61 are unlikely to catch up with peers, at great societal cost. 62 This underscores the need for effective interventions applied as early as possible, when brain networks are most amenable to change. 10 , 58 , 62
Our findings support our hypothesis that while listening to stories, young children from more stimulating home reading environments more robustly engage neural circuitry supporting narrative comprehension, a foundational component of emergent literacy. 63 Specifically, children in our study with higher StimQ-P Reading scores showed greater activation in the left parietal-temporal-occipital (PTO) association cortex, a “hub” region facilitating semantic processing. 41 , 64 Outbound PTO connections include limbic areas involved with long-term memory (eg, hippocampus) and assigning emotional value to experiences, and prefrontal executive function areas, each integral for learning. 65 “Recycling” their role in oral language, areas within the PTO are recruited for reading, facilitating efficient assignment of meaning to letters and words. 41 , 66 , 67 The angular gyrus (located in the inferior parietal lobe) at the core of the PTO is particularly noteworthy and plays an integral role in this process. 23 , 41 , 68 Although not observed in our subjects, hypoactivation of the angular gyrus during reading tasks has been cited as a biomarker for dyslexia, with potential application for early identification and remediation. 27 , 69
Importantly, PTO activation in our subjects associated with home reading environment reflects recruitment of oral language skills supporting context and comprehension (semantics), not word-level decoding. This is consistent with behavioral evidence for the influence of parent-child reading exclusively on “outside-in” oral language skills (understanding outside of the word itself) described by Whitehurst et al. 1 , 5 Vocabulary is among the most important of these skills, 70 shown to be influenced by home reading environment 17 and recently found to be positively associated with left angular gyrus activation during our story listening task in young children. 49 Thus, PTO activation may offer potential as a biomarker of oral language ability (the outside-in domain of emergent literacy), although further studies are needed to clarify how the PTO is integrated into the reading network. That home reading environment was not associated with activation of brain areas supporting phonological processing (“inside-out” decoding skills) in our study reinforces behavioral evidence 5 that these skills seem largely dependent on explicit instruction. 15 Additional research in this area is also needed.
Higher StimQ Reading scores were associated with particularly robust activation in occipital areas within the PTO cortex, notably lateral occipital gyrus and precuneus. Schmithorst et al attributed activation in these areas during the story listening task (when no visual stimulus is presented) to mental imagery. 35 The ability to “see” what is being heard has been extensively shown in behavioral studies to improve narrative comprehension and recall. 71 This was affirmed in a recent, imaging-based study that found positive association between greater activation of lateral occipital cortex during the story listening task in 5- to 7-year-old children and higher reading scores at age 11. 40 Recruitment of left-sided PTO areas during high-imagery tasks has also been described in adults. 72 Thus, our results provide a neurobiological correlate to the enchantment often seen at preschool story time, especially in children with greater practice at home: activation of PTO circuits to visualize and understand what is happening. It is intriguing to infer that children better able to recruit these circuits and apply mental imagery may better manage the transition from picture- to text-based books as they advance in school. Conversely, those with less practice seeing and understanding, with consequently underdeveloped visual-semantic neural infrastructure, may be more likely to struggle.
Surprisingly, we did not find significant association between neural activation and PIDA, PVR, or Composite StimQ-P scores. We view this as likely a byproduct of subscale themes. The StimQ-P Reading subscale measures reading-specific practices, assessing frequency, access to books, and variety of subject matter. As these opportunities and experiences are directly related to story listening, small variations, even with scores skewed toward the maximum, seem adequate to differentiate neural activation in subjects performing this task. By contrast, PIDA measures the teaching of specific cognitive skills and PVR assesses parent-child conversation, each possibly more applicable to abilities other than narrative comprehension. Any composite effect was likely diluted by PIDA and PVR scores.
Contrary to our hypothesis, age of initiation of shared reading and months of reading exposure were not associated with neural activation, although behavioral studies have associated these with home literacy orientation. 9 , 73 This may be attributable to responses skewed by social desirability and/or recall bias or, more likely, greater predictive power of the validated StimQ-P measure. The Reading subscale captures 3 aspects of home reading environment: frequency (4 points, including for days/week), access to books (5 points, including for number of books in the home), and variety of content (10 points, for different types of books, eg concepts, beliefs, relationships). The relative influence of each of these factors on neural activation supporting narrative processing is complex, likely involving behaviors and proclivities that are more difficult to capture, and merits further study. For example, greater variety may reflect differences in how books are shared, in addition to how many and how often. This qualitative aspect of reading aloud (notably, dialogic reading in which the child actively participates) has been shown to provide a disproportionate share of its benefits, behaviorally 74 , 75 and possibly in terms of neurobiological effect.
Our study has several important strengths. Our sample of 3- to 5-year-old children is considerably younger than most neuroimaging-based studies of emergent literacy, 27 with ample sample size 76 drawn from a diverse cohort, applying an established fMRI paradigm and validated measure of home cognitive environment. Our findings are consistent with current models of language and reading brain networks, 23 complementary with behavioral models of emergent literacy, 15 and robust in controlling for household income, a common confounder in studies of cognitive development. 77 Using an innovative approach, our results also inform clinical practice during a foundational stage of development in which “preventative medicine” may offer maximal benefit. For example, because there is evidence that the Reach Out and Read intervention advocated in AAP recommendations 13 improves home reading environment, 13 , 18 and we have found that home reading environment is positively associated with activation of brain circuits supporting semantic processing, logical inference leads us to speculate that early home literacy intervention such as Reach Out and Read, consistently applied, has the potential to enhance the development of these brain circuits.
Our study also has several limitations. Although it used existing imaging and behavioral data, the StimQ-P was retrospectively administered, with a variable time from fMRI acquisition. Thus, recall and social desirability bias are possible, with parents overreporting reading practices. That said, household reading behaviors have been shown to be stable during the preschool period, tempering such recall effects. 78 Families agreeing to participate in our study may be more likely to constructively engage in their child’s development (participation bias); although C-MIND is not advertised in the context of reading, its demographic mix is diverse by design, and all subjects who were able to be contacted agreed to participate, minimizing the prospect of self-selection. The exclusion of four low-SES families was a consequence of unreliable contact information (ie, phone out of service), shifting our demographic profile toward higher SES, although 37% of our sample was low-income. Our high reported StimQ-P subscale scores suggest potential ceiling effects, although the Reading subscale provided sensitivity ideal for our task. Finally, whereas our results show robust association between home reading environment and neural activation, our cross-sectional design cannot establish causation. Longitudinal studies are needed to discern the influence of shared reading on emergent literacy skills beginning in infancy, especially in low-SES populations. Such studies may help us better understand how the developing brain responds to various platforms, styles (notably dialogic reading), and interventions at different developmental stages, as well as identify children at-risk as early as possible to ensure the best possible outcome for all.
Conclusions
Our study used fMRI to for the first time demonstrate an association between home reading environment and activation of specific brain regions supporting emergent literacy during the prekindergarten period. While listening to stories, children with greater home reading exposure showed significantly higher activation in areas within the left-sided, multimodal association cortex, which facilitates mental imagery and extraction of meaning (semantic processing). Critical for oral language, this region is later integrated into the reading network, 35 , 54 with hypoactivation, a biomarker of reading disability. 27 This study suggests a novel, neurobiological correlate to oral language skills fostered by parent-child reading in early childhood, offering insight into how this practice may shape the developing brain, and informing an eco-bio-developmental model of emergent literacy and its promotion.
Acknowledgments
The authors thank Claire Sroka and Sarah Finucane for their invaluable help conducting the StimQ surveys and organizing behavioral data. We also thank Molly Grainger for her assistance with the FSL fMRI analysis package.
The C-MIND database used for this study can be accessed free of charge at https://research.cchmc.org/c-mind. The authors thank the C-MIND Authorship Consortium:
Scott K. Holland, PhD, Pediatric Neuroimaging Research Consortium, Department of Radiology, Department of Otolaryngology, Communication Sciences Research Center, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, Ohio; Jennifer Vannest, PhD, Pediatric Neuroimaging Research Consortium and Division of Neurology, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, Ohio; Vincent J. Schmithorst, PhD, Pediatric Neuroimaging Research Consortium, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, Ohio, and Pediatric Imaging Research Center, Department of Radiology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, Pennsylvania; Mekibib Altaye, PhD, Pediatric Neuroimaging Research Consortium and Division of Biostatistics and Epidemiology, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, Ohio; Gregory Lee, PhD, Pediatric Neuroimaging Research Consortium and Department of Radiology, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, Ohio; Luis Hernandez-Garcia, PhD, Functional MRI Laboratory, Department of Biomedical Engineering, University of Michigan, Ann Arbor, Michigan; Michael Wagner, PhD, Pediatric Neuroimaging Research Consortium and Division of Biomedical Informatics, Deparment of Pediatrics, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, Ohio; Arthur Toga, PhD, Laboratory of Neuroimaging and Departments of Ophthalmology, Neurology, Psychiatry, and the Behavioral Sciences, Radiology and Engineering, Keck School of Medicine of USC, Los Angeles, California; Jennifer Levitt, MD, Psychiatry and Biobehavioral Sciences, UCLA, Los Angeles, California; Anna W. Byars, PhD, Pediatric Neuroimaging Research Consortium and Division of Neurology, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, Ohio; Andrew Dimitrijevic, PhD, Department of Otolaryngology and Communication Sciences Research Center, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, Ohio; Nicolas Felicelli, Division of Biomedical Informatics, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, Ohio; Darren Kadis, PhD, Pediatric Neuroimaging Research Consortium and Division of Neurology, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, Ohio; James Leach, MD, Department of Radiology, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, Ohio; Katrina Peariso, MD, PhD, Division of Neurology, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, Ohio; Elena Plante, PhD, Deptartment of Speech, Language, and Hearing Sciences, University of Arizona, Tucson, Arizona; Akila Rajagopal, MS, Pediatric Neuroimaging Research Consortium, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, Ohio; Andrew Rupert, MS, Division of Biomedical Informatics, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, Ohio; Mark Schapiro, MD, Pediatric Neuroimaging Research Consortium and Division of Neurology, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, Ohio; Karen Crawford, Laboratory of Neuroimaging, Keck School of Medicine of USC, Los Angeles, California; Ronald Ly, Psychiatry and Biobehavioral Sciences, UCLA, Los Angeles, California; Katherine Narr, PhD, Department of Neurology, UCLA, Los Angeles, California; Petros Petrosyan, Laboratory of Neuroimaging, Keck School of Medicine of USC, Los Angeles, California; J.J. Wang, PhD, Department of Neurology, UCLA, Los Angeles, California; and Lisa Freund, PhD, Eunice Kennedy Shriver National Institute of Child Health and Human Development Bethesda, Maryland.
Glossary
- AAP
American Academy of Pediatrics
- BOLD
blood oxygen level–dependent
- C-MIND
Cincinnati MR Imaging of NeuroDevelopment
- FDR
false discovery rate
- fMRI
functional magnetic resonance imaging
- MNI
Montreal Neurologic Institute
- PIDA
Parental Involvement in Developmental Advance
- PVR
Parental Verbal Responsivity
- PTO
parietal-temporal-occipital
- SES
socioeconomic status
Footnotes
Dr Hutton conceptualized and designed the study, performed all data analysis, and drafted the initial manuscript and subsequent revisions; Dr Horowitz-Kraus provided guidance on study design and analysis, assisted with coordination of data collection, and reviewed and revised the manuscript; Dr Mendelsohn served as national outside facilitator and mentor for this project, provided advice on the use of the StimQ measure, and reviewed and revised the manuscript; Dr DeWitt provided guidance on the study design and reviewed and revised the manuscript; Dr Holland designed and secured funding for the parent neuroimaging study and acquired imaging and behavioral data in all participants; he was also responsible for image and behavioral data quality control, database archiving and first-level analysis of fMRI data and participated in the study design for addition of reading measures and supervised collection of the StimQ questionnaire; and all authors approved the final manuscript as submitted.
FUNDING: All phases of this study were supported by National Institutes of Health contract HHSN275200900018C to the Pediatric Functional Neuroimaging Research Network, a Ruth L Kirschstein National Research Service Award, and an Academic Pediatric Association Young Investigator Award (YIA) for Primary Care Strategies for the Promotion of Early Literacy and School Readiness Supported by Reach Out and Read.
POTENTIAL CONFLICT OF INTEREST: John Hutton received an Academic Pediatric Association Young Investigator Award supported by Reach out and Read in support of this project, although this award provided no direct financial or salary compensation. Tom DeWitt has served on the board of directors of Reach Out and Read from 2009-present but receives no financial compensation. Alan Mendelsohn serves as Senior Research Advisor for Child Development and School Readiness for Reach Out and Read but receives no financial compensation. The other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1. Whitehurst GJ , Lonigan CJ . Child development and emergent literacy. Child Dev. 1998;69(3):848–872 [PubMed] [Google Scholar]
- 2. Norton ES , Beach SD , Gabrieli JD . Neurobiology of dyslexia. Curr Opin Neurobiol. 2015;30:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cree A. The Economic and Social Cost of Illiteracy: A Snapshot of Illiteracy in a Global Context. World Literacy Foundation; April 2012
- 4. Ramey CT , Ramey SL . Early intervention and early experience. Am Psychol. 1998;53(2):109–120 [DOI] [PubMed] [Google Scholar]
- 5. National Early Literacy Panel . Developing Early Literacy: Report of the National Early Literacy Panel. Washington, DC: National Institute for Literacy; 2008. [Google Scholar]
- 6. Bradley RH , Corwyn RF . Socioeconomic status and child development. Annu Rev Psychol. 2002;53:371–399 [DOI] [PubMed] [Google Scholar]
- 7. Cates CB , Dreyer BP , Berkule SB , White LJ , Arevalo JA , Mendelsohn AL . Infant communication and subsequent language development in children from low-income families: the role of early cognitive stimulation. J Dev Behav Pediatr. 2012;33(7):577–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanders LM , Federico S , Klass P , Abrams MA , Dreyer B . Literacy and child health: a systematic review. Arch Pediatr Adolesc Med. 2009;163(2):131–140 [DOI] [PubMed] [Google Scholar]
- 9. Zuckerman B , Augustyn M . Books and reading: evidence-based standard of care whose time has come. Acad Pediatr. 2011;11(1):11–17 [DOI] [PubMed] [Google Scholar]
- 10. Knudsen EI . Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16(8):1412–1425 [DOI] [PubMed] [Google Scholar]
- 11. Duursma E , Augustyn M , Zuckerman B . Reading aloud to children: the evidence. Arch Dis Child. 2008;93(7):554–557 [DOI] [PubMed] [Google Scholar]
- 12. Hart B , Risley T . Meaningful Differences in the Everyday Experience of Young American Children. Baltimore, MD: Paul Brookes Publishing Company; 1995. [Google Scholar]
- 13. High PC , Klass P Council on Early Childhood . Literacy promotion: an essential component of primary care pediatric practice. Pediatrics. 2014;134(2):404–409 [DOI] [PubMed] [Google Scholar]
- 14.Too STF. Hillary Rodham Clinton to Unveil Early Literacy Toolkit for Pediatricians and Parents at American Academy of Pediatrics National Conference. 2014. Available at: http://toosmall.org/news/press-releases. Accessed February 1, 2015
- 15. Storch SA , Whitehurst GJ . Oral language and code-related precursors to reading: evidence from a longitudinal structural model. Dev Psychol. 2002;38(6):934–947 [PubMed] [Google Scholar]
- 16. Golova N , Alario AJ , Vivier PM , Rodriguez M , High PC . Literacy promotion for Hispanic families in a primary care setting: a randomized, controlled trial. Pediatrics. 1999;103(5 pt 1):993–997 [DOI] [PubMed] [Google Scholar]
- 17. Mendelsohn AL , Mogilner LN , Dreyer BP , et al. The impact of a clinic-based literacy intervention on language development in inner-city preschool children. Pediatrics. 2001;107(1):130–134 [DOI] [PubMed] [Google Scholar]
- 18. Needlman R , Toker KH , Dreyer BP , Klass P , Mendelsohn AL . Effectiveness of a primary care intervention to support reading aloud: a multicenter evaluation. Ambul Pediatr. 2005;5(4):209–215 [DOI] [PubMed] [Google Scholar]
- 19. Sharif I , Rieber S , Ozuah PO . Exposure to Reach Out and Read and vocabulary outcomes in inner city preschoolers. J Natl Med Assoc. 2002;94(3):171–177 [PMC free article] [PubMed] [Google Scholar]
- 20. Whaley SE , Jiang L , Gomez J , Jenks E . Literacy promotion for families participating in the women, infants and children program. Pediatrics. 2011;127(3):454–461 [DOI] [PubMed] [Google Scholar]
- 21.National Scientific Council on the Developing Child. Working Paper #5: The Timing and Quality of Early Experiences Combine to Shape Brain Architecture. Cambridge, MA: Harvard University Center on the Developing Child; 2008
- 22. Carreiras M , Seghier ML , Baquero S , et al. An anatomical signature for literacy. Nature. Oct 15 2009;461(7266):983–986 [DOI] [PubMed] [Google Scholar]
- 23. Dehaene S . Reading in the Brain: The New Science of How We Read. New York, NY: Penguin Books; 2010. [Google Scholar]
- 24. Shonkoff JP . Building a new biodevelopmental framework to guide the future of early childhood policy. Child Dev. 2010;81(1):357–367 [DOI] [PubMed] [Google Scholar]
- 25.American Academy of Pediatrics Council on Early Brain and Child Development. Eco-Bio-Developmental Model of Human Health and Disease. 2014. Available at: http://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/EBCD/Pages/Eco-Bio-Developmental.aspx. Accessed February 1, 2015
- 26. Hoeft F , Ueno T , Reiss AL , et al. Prediction of children’s reading skills using behavioral, functional, and structural neuroimaging measures. Behav Neurosci. 2007;121(3):602–613 [DOI] [PubMed] [Google Scholar]
- 27. Shaywitz SE , Shaywitz BA . Paying attention to reading: the neurobiology of reading and dyslexia. Dev Psychopathol. 2008;20(4):1329–1349 [DOI] [PubMed] [Google Scholar]
- 28. Blau V , Reithler J , van Atteveldt N , et al. Deviant processing of letters and speech sounds as proximate cause of reading failure: a functional magnetic resonance imaging study of dyslexic children. Brain. 2010;133(pt 3):868–879 [DOI] [PubMed] [Google Scholar]
- 29. Maisog JM , Einbinder ER , Flowers DL , Turkeltaub PE , Eden GF . A meta-analysis of functional neuroimaging studies of dyslexia. Ann N Y Acad Sci. 2008;1145:237–259 [DOI] [PubMed] [Google Scholar]
- 30. Dehaene S , Pegado F , Braga LW , et al. How learning to read changes the cortical networks for vision and language. Science. 2010;330(6009):1359–1364 [DOI] [PubMed] [Google Scholar]
- 31. Brem S , Bach S , Kucian K , et al. Brain sensitivity to print emerges when children learn letter-speech sound correspondences. Proc Natl Acad Sci USA. 2010;107(17):7939–7944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vannest J , Rajagopal A , Cicchino ND , et al. CMIND Authorship Consortium . Factors determining success of awake and asleep magnetic resonance imaging scans in nonsedated children. Neuropediatrics. 2014;45(6):370–377 [DOI] [PubMed] [Google Scholar]
- 33. Szaflarski JP , Schmithorst VJ , Altaye M , et al. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann Neurol. 2006;59(5):796–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuhl PK . Early Language Learning and Literacy: Neuroscience Implications for Education. Mind Brain Educ. 2011;5(3):128–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schmithorst VJ , Holland SK , Plante E . Cognitive modules utilized for narrative comprehension in children: a functional magnetic resonance imaging study. Neuroimage. 2006;29(1):254–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vannest JJ , Karunanayaka PR , Altaye M , et al. Comparison of fMRI data from passive listening and active-response story processing tasks in children. J Magn Reson Imaging. 2009;29(4):971–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Holland SK , Vannest J , Mecoli M , et al. Functional MRI of language lateralization during development in children. Int J Audiol. 2007;46(9):533–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pugh KR , Landi N , Preston JL , et al. The relationship between phonological and auditory processing and brain organization in beginning readers. Brain Lang. 2013;125(2):173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Berl MM , Duke ES , Mayo J , et al. Functional anatomy of listening and reading comprehension during development. Brain Lang. 2010;114(2):115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Horowitz-Kraus T , Vannest JJ , Holland SK . Overlapping neural circuitry for narrative comprehension and proficient reading in children and adolescents. Neuropsychologia. 2013;51(13):2651–2662 [DOI] [PubMed] [Google Scholar]
- 41. Binder JR , Desai RH , Graves WW , Conant LL . Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19(12):2767–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noble KG, Tottenham N, Casey BJ. Neuroscience perspectives on disparities in school readiness and cognitive achievement. The Future of Children. 2005;15(1):71–89 [DOI] [PubMed]
- 43. Noble KG , McCandliss BD , Farah MJ . Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev Sci. 2007;10(4):464–480 [DOI] [PubMed] [Google Scholar]
- 44. Noble KG , McCandliss BD . Reading development and impairment: behavioral, social, and neurobiological factors. J Dev Behav Pediatr. 2005;26(5):370–378 [DOI] [PubMed] [Google Scholar]
- 45.Holland SKVJ, Schmithorst VJ, Wagner M. Overview of the Pediatric Functional Neuroimaging Research Network Project, Methods, Tools and Database: a.k.a CMIND. Paper presented at New Horizons in Human Brain Imaging: A Focus on the Neuroimaging of Brain Development; May 5–7, 2014; Turtle Bay, HI [Google Scholar]
- 46. The Bellevue Project for Early Language L. Education Success (BELLE), STIMQ Cognitive Home Environment . http://pediatrics.med.nyu.edu/developmental/research/the-belle-project/stimq-cognitive-home-environment, 2014.
- 47. Dreyer BPMA , Tamis-LeMonda CS . Assessing the child’s cognitive home environment through parental report: reliability and validity. Early Dev Parent. 1996;5:271–287 [Google Scholar]
- 48. Schmithorst VJ , Vannest J , Lee G , et al. CMIND Authorship Consortium . Evidence that neurovascular coupling underlying the BOLD effect increases with age during childhood. Hum Brain Mapp. 2015;36(1):1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sroka MC , Vannest J , Maloney TC , Horowitz-Kraus T , Byars AW , Holland SK CMIND Authorship Consortium . Relationship between receptive vocabulary and the neural substrates for story processing in preschoolers. Brain Imaging Behav. 2015;9(1):43–55 [DOI] [PubMed] [Google Scholar]
- 50. Smith SM , Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(S1):208–219 [DOI] [PubMed] [Google Scholar]
- 51.Office of the Secretary. Annual Update of the HHS Poverty Guidelines In: Department of Health and Human Services, ed. Vol 80. Washington, DC: Federal Register; 2015 [Google Scholar]
- 52.The Henry J. Kaiser Family Foundation. The Kaiser Family Foundation State Health Facts. 2015. Available at: http://kff.org/statedata. Accessed June 9, 2015
- 53. Brett MCK , Cusack R , Lancaster J . Using the Talairach Atlas with the MNI template. Neuroimage. 2001;13(6):85 [Google Scholar]
- 54. Karunanayaka PR , Holland SK , Schmithorst VJ , et al. Age-related connectivity changes in fMRI data from children listening to stories. Neuroimage. 2007;34(1):349–360 [DOI] [PubMed] [Google Scholar]
- 55. Sabsevitz DS , Medler DA , Seidenberg M , Binder JR . Modulation of the semantic system by word imageability. Neuroimage. 2005;27(1):188–200 [DOI] [PubMed] [Google Scholar]
- 56. Leech R , Sharp DJ . The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(pt 1):12–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hertzman C . The biological embedding of early experience and its effects on health in adulthood. Ann N Y Acad Sci. 1999;896:85–95 [DOI] [PubMed] [Google Scholar]
- 58. National Research Council and Institute of Medicine Committee on Integrating the Science of Early Childhood Development . From Neurons to Neighborhoods: The Science of Early Childhood Development. Washington, DC: National Academy Press; 2000. [Google Scholar]
- 59. Redcay E , Haist F , Courchesne E . Functional neuroimaging of speech perception during a pivotal period in language acquisition. Dev Sci. 2008;11(2):237–252 [DOI] [PubMed] [Google Scholar]
- 60. Tomopoulos S , Dreyer BP , Tamis-LeMonda C , et al. Books, toys, parent-child interaction, and development in young Latino children. Ambul Pediatr. 2006;6(2):72–78 [DOI] [PubMed] [Google Scholar]
- 61. Gabrieli JD . Dyslexia: a new synergy between education and cognitive neuroscience. Science. 2009;325(5938):280–283 [DOI] [PubMed] [Google Scholar]
- 62. The Annie E. Casey Foundation . Double Jeopardy: How Third Grade Reading Skills and Poverty Influence High School Graduation. Baltimore, MD: The Annie E. Casey Foundation; 2012. [Google Scholar]
- 63.Cornelissen P, Hansen P, Kringelbach M, Pugh K. The Neural Basis of Reading. New York, NY: Oxford University Press; 2010 [Google Scholar]
- 64. Ganis G , Thompson WL , Kosslyn SM . Brain areas underlying visual mental imagery and visual perception: an fMRI study. Brain Res Cogn Brain Res. 2004;20(2):226–241 [DOI] [PubMed] [Google Scholar]
- 65. Wright A . Higher Cortical Functions: Association and Executive Processing. Neuroscience Online: An Electronic Textbook for the Neurosciences. Houston, TX: Department of Neurobiology and Anatomy at University of Texas Medical School at Houston; 2014. [Google Scholar]
- 66. Dehaene S . Inside the letterbox: how literacy transforms the human brain. Cerebrum. 2013;2013:7 [PMC free article] [PubMed] [Google Scholar]
- 67. Shankweiler D , Mencl WE , Braze D , Tabor W , Pugh KR , Fulbright RK . Reading differences and brain: cortical integration of speech and print in sentence processing varies with reader skill. Dev Neuropsychol. 2008;33(6):745–775 [DOI] [PubMed] [Google Scholar]
- 68. Pugh KR , Mencl WE , Shaywitz BA , et al. The angular gyrus in developmental dyslexia: task-specific differences in functional connectivity within posterior cortex. Psychol Sci. 2000;11(1):51–56 [DOI] [PubMed] [Google Scholar]
- 69. Hoeft F , Meyler A , Hernandez A , et al. Functional and morphometric brain dissociation between dyslexia and reading ability. Proc Natl Acad Sci USA. 2007;104(10):4234–4239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Duff FJ , Reen G , Plunkett K , Nation K . Do infant vocabulary skills predict school-age language and literacy outcomes? J Child Psychol Psychiatry. 2015; (Jan):4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. National Reading Panel . Report of the National Reading Panel: Teaching Children to Read: An Evidence-Based Assessment of the Scientific Research Literature on Reading and Its Implications for Reading Instruction. Washington, DC: Eunice Kennedy Shriver National Institute of Child Health and Human Development; 2000. [Google Scholar]
- 72. Just MA , Newman SD , Keller TA , McEleney A , Carpenter PA . Imagery in sentence comprehension: an fMRI study. Neuroimage. 2004;21(1):112–124 [DOI] [PubMed] [Google Scholar]
- 73. Needlman R , Silverstein M . Pediatric interventions to support reading aloud: how good is the evidence? J Dev Behav Pediatr. 2004;25(5):352–363 [DOI] [PubMed] [Google Scholar]
- 74.Whitehurst GJ. Dialogic reading: an effective way to read to preschoolers. 2013. Available at: http://www.readingrockets.org/article/400. Accessed February 1, 2015
- 75. Swanson E , Wanzek J , Petscher Y , et al. A synthesis of read-aloud interventions on early reading outcomes among preschool through third graders at risk for reading difficulties. J Learn Disabil. 2011;44(3):258–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Desmond JE , Glover GH . Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J Neurosci Methods. 2002;118(2):115–128 [DOI] [PubMed] [Google Scholar]
- 77. Raizada RD , Kishiyama MM . Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to levelling the playing field. Front Hum Neurosci. 2010;4:3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. National Household Education Survey. Children’s School Readiness Skills: 1993 and 2007. Washington, DC: US Census Bureau Statistical Abstract of the United States; 2012. [Google Scholar]