Abstract
BACKGROUND AND OBJECTIVES:
Preterm and former preterm children frequently require sedation/anesthesia for diagnostic and therapeutic procedures. Our objective was to determine the age at which children who are born <37 weeks gestational age are no longer at increased risk for sedation/anesthesia adverse events. Our secondary objective was to describe the nature and incidence of adverse events.
METHODS:
This is a prospective observational study of children receiving sedation/anesthesia for diagnostic and/or therapeutic procedures outside of the operating room by the Pediatric Sedation Research Consortium. A total of 57 227 patients 0 to 22 years of age were eligible for this study. All adverse events and descriptive terms were predefined. Logistic regression and locally weighted scatterplot regression were used for analysis.
RESULTS:
Preterm and former preterm children had higher adverse event rates (14.7% vs 8.5%) compared with children born at term. Our analysis revealed a biphasic pattern for the development of adverse sedation/anesthesia events. Airway and respiratory adverse events were most commonly reported. MRI scans were the most commonly performed procedures in both categories of patients.
CONCLUSIONS:
Patients born preterm are nearly twice as likely to develop sedation/anesthesia adverse events, and this risk continues up to 23 years of age. We recommend obtaining birth history during the formulation of an anesthetic/sedation plan, with heightened awareness that preterm and former preterm children may be at increased risk. Further prospective studies focusing on the etiology and prevention of adverse events in former preterm patients are warranted.
What’s Known on This Subject:
Preterm infants and children <3 years of age receiving sedation/anesthesia are more likely to develop adverse events related to the airway and pulmonary systems.
What This Study Adds:
Children born preterm receiving sedation/anesthesia for nonoperating room procedures are nearly twice as likely to develop adverse events compared with children born at term. The longitudinal risk analysis suggests that children born preterm remain at increased risk into adulthood.
According to the Centers for Disease Control and Prevention, ∼450 000 children are born prematurely in the United States each year.1 Preterm children present with complex medical issues including neurologic disabilities, respiratory issues, feeding dysfunction, and developmental delay. These comorbidities frequently require diagnostic tests and surgical procedures that cannot be completed without the administration of sedation/anesthesia. Previous publications have demonstrated that preterm children, children under the age of 3 years, and those with significant comorbidities are at increased risk for the development of adverse events related to sedation/anesthesia.2–11 Moreover, preterm children <60 weeks postgestational age who receive anesthesia are at increased risk for the development of apnea after anesthesia.12–14 It is unknown if preterm and former preterm children receiving sedation/anesthesia for diagnostic or therapeutic procedures outside of the operating room experience the same adverse sedation/anesthesia events as children born at term. In order to evaluate the current rate of sedation/anesthesia adverse events in this population, we utilized a large database of prospectively collected data on procedural sedation/anesthesia encounters from the Pediatric Sedation Research Consortium (PSRC), which has been compiling information on sedation/anesthesia safety and effectiveness for more than 10 years. We hypothesized that children born preterm would have a higher rate of adverse events, and that this difference in adverse event rate would decrease with increasing age.
Methods
This is a prospective observational study of children receiving sedation/anesthesia for diagnostic or therapeutic procedures outside of the operating room with data collected by the PSRC. Sedation/anesthesia services were provided by a variety of specialists including anesthesiologists, pediatric anesthesiologists, pediatric emergency department physicians, pediatric critical care physicians, advanced nurse practitioners, physician assistants, nurses, pediatricians, radiologists, fellows, and dentists. The data collection methodology used by the PSRC has been described in previous studies from this consortium, including a report on the first 30 000 sedations/anesthetics that were performed.15 Forty-one locations participated in this study, including large children’s hospitals, children’s hospitals within hospitals, and community hospitals (Supplemental Information). The participating institutions were self-selected for involvement in the PSRC data sharing group. There were no specific selection criteria for participation in the consortium; however, any interested institutions were required to obtain institutional review board approval for data collection, identify a primary investigator, and agree to a standardized methodology for data collection. The study Web site and data entry portal are secured using Secure Sockets Layer, and each study participant is authenticated through the Web site and authorized to access only the parts of the Web site relevant to his or her institution. All data collected in this study met Health Insurance Portability and Accountability Act of 1996 requirements for deidentification. No patient-identifiable data were transmitted during this study.
All participating institutions and primary investigators are blinded to the data submitted from any individual institution other than their own. Study authors are blinded to referring institutions. All primary investigators are required to perform data audits on 10 charts every 6 months and report accuracy of transmitted data. Investigators are required to review total counts of sedations/anesthetics performed in their institution (independently recorded) versus that of the number of records submitted to the PSRC. Any discrepancies in numbers require a complete review of the data-gathering methodology at the institution.
Data collected on each patient include age, gender, weight, American Society of Anesthesiology Physical Status (PS), procedure type, location, primary diagnosis, comorbidities, and adverse events (Supplemental Table 7). All adverse events and descriptive terms were predefined.
For the purpose of this study, children born at <37 weeks’ gestational age were categorized as preterm children, including preterm and former preterm children. Children born at ≥37 weeks’ gestational age were categorized as term children. Patients older than 22 years were excluded from this study. Common descriptive statistics were used to portray characteristics of both groups. Means and medians were used to measure central values in continuous variables. Pearson χ2 and Fisher exact tests were used to compare adverse events. Unadjusted and adjusted logistic regression and locally weighted scatterplot regression (lowess) were used to analyze the occurrence of adverse events between the 2 groups. To determine the effect of postgestational age on outcome, a sensitivity analysis was performed in children 0 to 36 months of age. This analysis was conducted by subtracting the assumed degree of prematurity from the preterm category by using 1, 2, and 3 months as possible values and modeling postgestational age as the independent variable. Stata 13 (Stata Corp, College Station, TX) was used for analysis.
Results
A total of 57 628 cases were entered into the research database from November 2012 to April 2014, of which 401 cases were excluded because of incomplete patient or adverse event information. Patient characteristics are described in Table 1. In this cohort, 8.6% of all children experienced an adverse sedation/anesthesia event (Table 2). The greatest frequency of adverse events occurred in infants <6 months of age. The most common adverse events reported included airway obstruction (2.0%), coughing (2.0%), snoring (1.7%), and oxygen desaturation (1.8%), defined as oxygen saturation <90% for >30 seconds. There was no significant difference in the frequency of emergency intubation between the 2 categories (P = .7). The unadjusted and adjusted odds ratios (ORs) for adverse events reported to the PSRC are listed in Table 3. The OR for the development of any adverse event adjusted for age and gender in the preterm category was 1.93. There were no deaths reported in this study.
TABLE 1.
Characteristics of Patients Participating in the PSRC, November 2012 to April 2014
| Characteristic | Term | Preterm | All Children |
|---|---|---|---|
| Patients | 56 542 (98.8) | 685 (1.2) | 57 227 (100) |
| Gender | |||
| Male | 31 077 (55) | 386 (56) | 31 463 (55) |
| Female | 25 465 (45) | 299 (44) | 25 764 (45) |
| Age, y | |||
| Mean | 6.26 | 3.75 | 6.23 |
| Median | 5 | 2.33 | 5 |
| Range | 0–22 | 0–22 | 0–22 |
| Weight, kg | |||
| Mean | 26.6 | 16.0 | 26.5 |
| Median | 19.7 | 12.2 | 19.6 |
| Range | 1.7–263.2 | 2.3–99.1 | 1.7–263.2 |
| PS | |||
| 1 | 11 220 (19.4) | 108 (15.8) | 11 328 (19.8) |
| 2 | 35 427 (62.7) | 445 (65.0) | 35 872 (62.7) |
| 3 | 9704 (17.16) | 127 (18.5) | 9831 (17.2) |
| 4 | 108 (0.19) | 2 (0.29) | 110 (0.2) |
| 1E | 40 (0.07) | 2 (0.29) | 42 (0.07) |
| 2E | 12 (0.02) | 1 (0.15) | 13 (0.02) |
| 3E | 26 (0.05) | 0 | 26 (0.05) |
| 4E | 5 (0.01) | 0 | 5 (0.01) |
Values are expressed as n (%) unless noted otherwise.
TABLE 2.
Comparison of the Frequency of Adverse Events in Term and Preterm Patients
| Adverse Event | Term | Preterm | All Children | P a |
|---|---|---|---|---|
| n | 56 542 | 685 | 57 227 | |
| None | 51 747 (91.5) | 584 (85.3) | 52 331 (91.4) | <.001 |
| Any complication | 4795 (8.5) | 101 (14.7) | 4896 (8.6) | <.001 |
| Airway obstruction | 1138 (2.0) | 20 (2.9) | 1158 (2.0) | .094 |
| Oxygen desaturation | 999 (1.8) | 31 (4.5) | 1030 (1.8) | <.001 |
| Coughing | 1092 (1.9) | 23 (3.4) | 1115 (2.0) | .007 |
| Secretions | 544 (1.0) | 18 (2.6) | 562 (1.0) | <.001 |
| Agitation | 263 (0.5) | 2 (0.3) | 265 (0.5) | .78b |
| Apnea | 470 (0.8) | 14 (2) | 484 (0.9) | .001 |
| IV related | 249 (0.4) | 5 (0.7) | 254 (0.4) | .24b |
| Hemodynamic changec | 160 (0.3) | 3 (0.4) | 163 (0.3) | .45b |
| Laryngospasm | 184 (0.3) | 1 (0.2) | 185 (0.3) | .73b |
| Vomiting | 137 (0.2) | 1 (0.2) | 138 (0.2) | 1.0b |
| Aspiration | 7 (0.01) | 1 (0.2) | 8 (0.01) | .09b |
| Stridor | 89 (0.2) | 1 (0.2) | 90 (0.2) | 1.0b |
| Unplanned admission | 17 (0.03) | 0 (0.0) | 17 (0.03) | 1.0b |
| Bronchospasm | 41 (0.1) | 1 (0.2) | 42 (0.1) | .40b |
| Allergic reaction | 11 (0.02) | 0 (0.0) | 11 (0.02) | 1.0b |
| Failed sedation | 93 (0.16) | 0 (0.0) | 93 (0.16) | .63b |
| Procedure not completed | 151 (0.3) | 1 (0.2) | 152 (0.3) | 1.0b |
| Emergent airway | 89 (0.2) | 1 (0.2) | 90 (0.2) | 1.0b |
| Emergency anesthesia consult | 12 (0.02) | 0 (0.0) | 12 (0.02) | 1.0b |
| Hypothermia | 5 (0.01) | 2 (0.3) | 7 (0.01) | .003b |
| Myoclonus | 54 (0.1) | 0 (0.0) | 54 (0.1) | 1.0b |
| Seizure | 16 (0.03) | 0 (0.0) | 16 (0.03) | 1.0b |
| Reversal agent | 2 (0.0) | 1 (0.2) | 3 (0.01) | .04b |
| Cardiac event | 4 (0.01) | 0 (0.0) | 4 (0.01) | 1.0b |
| Hiccups | 78 (0.1) | 1 (0.1) | 79 (0.1) | .6b |
| Snoring | 930 (1.6) | 19 (2.8) | 949 (1.7) | .02 |
| Death | 0 (0.0) | 0 (0.0) | 0 (0.0) | — |
Values are expressed as n (%) unless noted otherwise. P values <.05 were considered significant between the 2 groups. IV, intravenous line.
Pearson χ2.
Fisher exact tests reported.
Unexpected change in heart rate or blood pressure >30%.
TABLE 3.
Unadjusted and Adjusted ORs for the Development of Sedation/Anesthesia Adverse Events in the Preterm Category
| Event | Unadjusted OR | OR Adjusted for Age and Gender | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR 95% CI | P | |
| Any adverse event | 1.87 (1.51–2.31) | <.001 | 1.93 (1.56–2.39) | <.001 |
| No adverse event | 0.54 (0.43–0.66) | <.001 | 0.52 (0.42–0.64) | <.001 |
| Airway obstruction | 1.46 (0.93 -2.3) | .10 | 1.58 (1.01–2.49) | .045 |
| Oxygen desaturation | 2.64 (1.83-3.80) | <.001 | 2.61 (1.81–3.76) | <.001 |
| Coughing | 1.76 (1.16-2.69) | .01 | 1.59 (1.04–2.43) | .03 |
| Secretions | 2.78 (1.73–4.48) | <.001 | 2.45 (1.52–3.95) | <.001 |
| Agitation | 0.63 (0.16–2.53) | .48 | 0.63 (0.16–2.54) | .52 |
| Apnea | 2.50 (1.46–4.28) | .001 | 3.08 (1.79–5.24) | <.001 |
| IV related | 1.66 (0.68–4.05) | .26 | 1.36 (0.56–3.30) | .51 |
| Hemodynamic change | 1.55 (0.49–4.87) | .43 | 1.98 (0.62–6.21) | .24 |
| Laryngospasm | 0.45 (0.63–3.20) | .42 | 0.44 (0.06–3.12) | .41 |
| Vomiting | 0.60 (0.08–4.27) | .61 | 0.71 (0.10–5.08) | .74 |
| Aspiration | 11.8 (1.5–96.1) | .02 | 13.2 (1.57–111.4) | .02 |
| Stridor | 0.93 (0.13–6.70) | .94 | 0.86 (0.12–6.06) | .26 |
| Bronchospasm | 2.03 (0.28–14.7) | .49 | 1.57 (0.21–11.5) | .66 |
| Emergent airway | 0.93 (0.13–6.70) | .94 | 0.95 (0.13–6.81) | .95 |
| Hypothermia | 33.1 (6.41–170.9) | <.001 | 20.7 (3.90–109.7) | <.001 |
| Reversal agent | 41.3 (3.74–456.3) | .002 | 46.8 (3.87–565.4) | .002 |
| Hiccups | 1.06 (0.15–7.62) | .96 | 1.03 (0.14–7.50) | .97 |
| Snoring | 1.71 (1.08–2.71) | .02 | 1.90 (1.21–3.02) | .01 |
Variables that predicted failure in the model were omitted. P values <0.05 for preterm variable were considered significant. IV, intravenous line.
The sedations/anesthetics were administered by a variety of providers, including pediatric intensive care physicians (59.2%), pediatric emergency department physicians (18.3%), and pediatric anesthesiologists (8.7%). In children 0 to 36 months of age, there was no statistical difference in the type of provider between the 2 groups (P = .12). Analysis of children in the preterm category demonstrated no significant differences in the development of adverse events among different providers (OR 0.96; P = .4).
The most common procedures requiring sedation/anesthesia in the preterm group were MRI scans (57.5%), auditory brainstem response (ABR) testing (7.7%), and upper endoscopy (7.5%). Logistic regression analysis of adverse events by procedure type in the preterm group is presented in Table 4. In the term group, the most common procedures performed included MRI scans (41.7%), lumbar puncture for chemotherapy administration (14.7%), and upper endoscopy (6.6%). Propofol as a single agent or combined with a benzodiazepine or narcotic was administered in the majority of cases. Children in the preterm category were at significantly increased risk for the development of apnea (defined as complete lack of respiratory effort >15 seconds with or without bradycardia), oxygen desaturation, coughing, secretions, and snoring but not for the development of airway obstruction or a >30% change in hemodynamics.
TABLE 4.
Logistic Regression Analysis of Sedation/Anesthesia Adverse Events by the Most Commonly Performed Procedures in the Preterm Group
| Procedure | Percentage | Unadjusted OR (95% CI) | P | Adjusted OR (95% CI) | P |
|---|---|---|---|---|---|
| MRI | 57.5 | 1.50 (1.13–1.99) | .005a | 1.39 (1.04–1.85) | .024a |
| ABR | 7.74 | 2.72 (1.37–5.41) | .004a | 2.11 (1.04–4.27) | .038a |
| Upper endoscopy (GI) | 7.45 | 1.73 (0.68–4.40) | .3 | 1.23 (0.48–3.19) | .664 |
| Computed tomography scan | 5.55 | 0.91 (0.28–2.99) | .9 | 0.88 (0.27–2.92) | .835 |
| PICC line placement | 3.65 | 3.21 (1.32–7.80) | .01a | 3.02 (1.22–7.51) | .017a |
| Total | 81.9 |
ORs were adjusted for age, gender, PS, and responsible provider. GI, gastrointestinal; PICC, peripherally inserted central catheter.
Procedures longer in duration demonstrated a significant difference between the 2 groups.
Preterm and former preterm children had a higher frequency of adverse events compared with term children (14.7% vs 8.5%, respectively) and were at higher risk to develop any adverse event regardless of age (Figs 1 and 2). The most common adverse sedation/anesthesia events reported in the preterm group were oxygen desaturation (4.5%), coughing (3.4%), and airway obstruction (2.9%). In the term group, airway obstruction was the most commonly reported sedation adverse event (2.0%), followed by coughing (1.9%) and oxygen desaturation (1.8%).
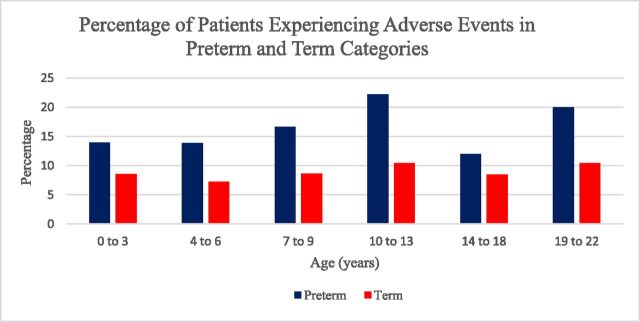
FIGURE 1.
Percentage of patients in term and preterm categories that developed sedation/anesthesia adverse events by age group. Children in the preterm category experienced a higher frequency of adverse events.
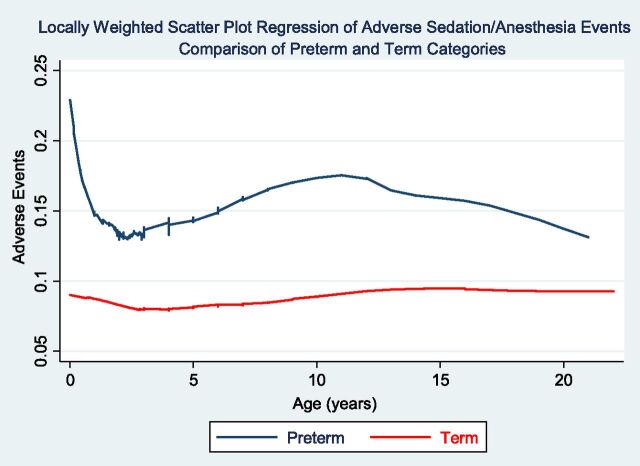
FIGURE 2.
Locally weighted scatter plot regression of sedation/anesthesia adverse events comparing preterm and term patient categories. Preterm children <1 year of age were at higher risk for developing sedation/anesthesia adverse events. Patients in the preterm category were at higher risk regardless of age at the time of the procedure.
Analysis of adverse events by age categories demonstrated that a higher percentage of adverse events occurred in the preterm group (Fig 1). In the preterm category, the highest percentage of adverse events occurred in the 10- to 13-year age range. Airway obstruction was the predominant event reported in this age group. Locally weighted scatterplot regression analysis demonstrated that patients in the preterm category had a higher frequency of adverse events compared with the term group regardless of age at the time of the procedure (Fig 2). A subanalysis of adverse events in children 0 to 36 months was performed (Table 5). The development of apnea and oxygen desaturation was significantly different between the preterm and term groups.
TABLE 5.
Comparison of ORs for Developing Overall and Airway Adverse Events in Preterm and Term Patients in Patients 0 to 36 Months of Age
| Event | 0 to 6 mo | 7 to 18 mo | 19 to 36 mo | |||
|---|---|---|---|---|---|---|
| Preterm | Term | Preterm | Term | Preterm | Term | |
| Any adverse event | 2.63 | 0.38a | 1.71a | 0.59a | 1.30 | 0.77 |
| Apnea | 5.26a | 0.19a | 2.37 | 0.42 | 5.19a | 0.19a |
| Oxygen desaturation | 1.33 | 0.75 | 2.77a | 0.36a | 1.98 | 0.51 |
| Airway obstruction | 1.56 | 0.64 | 1.29 | 0.77 | 2.22 | 0.45 |
| Cough | 1.46 | 0.68 | 0.94 | 1.06 | 1.41 | 0.71 |
| Secretions | 3.15 | 0.32 | 1.98 | 0.51 | 1.40 | 0.71 |
Patients in the 0- to 6-mo age category were at highest risk for developing adverse events. The occurrence of apnea was dramatically higher in preterm infants. The odds of preterm children developing oxygen desaturation increased after 6 months of age, suggesting that this group has decreased reserve compared with term children of similar age. There were no incidents of laryngospasm, bronchospasm, or snoring in the preterm group 0 to 6 mo of age.
Statistical significance (P < .05).
Analysis of adverse events based on the invasiveness of the procedure was conducted. When adjusted for age, gender, and responsible provider, patients in the preterm category demonstrated a higher frequency of adverse events in both the noninvasive (OR 1.69, P < .001) and invasive (OR 2.02, P < .001) categories compared with patients in the term category.
A sensitivity analysis was conducted to assess the impact of reduced postgestational age in preterm children 0 to 36 months of age. This model was created to equalize postgestational age in the preterm group. The analysis revealed that the OR in the preterm group was significantly higher than in the term group (Table 6).
TABLE 6.
Sensitivity Analysis of the Impact of Reduced Postgestational Age in Preterm Group 0 to 36 Months of Age
| Assumed Prematurity | OR | 95% CI | P |
|---|---|---|---|
| 3 mo | 2.76 | 1.32–5.80 | .007 |
| 2 mo | 2.83 | 1.31–6.13 | .008 |
| 1 mo | 2.91 | 1.30–6.49 | .009 |
| Term | 2.98 | 1.30–6.87 | .010 |
This analysis was performed by subtracting an assumed degree of prematurity from all premature infants, using 1, 2, and 3 mo as possible values and modeling postgestational age as the independent variable. The OR for prematurity changed little and was significant in all cases, suggesting that postgestational age alone cannot account for increased odds of developing an adverse event. Models were adjusted for age and gender of the patient.
Discussion
In this prospective analysis of patients 0 to 22 years of age undergoing procedural sedation/anesthesia, we determined that a considerable percentage (8.6%) of all children experienced an adverse event, with the most common adverse events associated with the airway and respiratory systems. Our data confirmed our primary hypothesis that preterm and formerly preterm patients would be at higher risk for adverse events during sedation/anesthesia. Our sensitivity analysis conducted in children 0 to 36 months of age suggests that postgestational age alone cannot explain the differences in frequency of adverse events. The small changes between the groups in this subanalysis suggest that there is an additive effect of preterm birth that cannot be solely explained by postgestational age. This implies there is a factor inherent to preterm children that places them at increased risk for the development of adverse events related to sedation/anesthesia. Our analysis suggests that preterm and formerly preterm patients undergoing sedation/anesthesia for nonoperating room procedures should be considered a population at risk for the development of adverse events.
Both categorical and the locally weighted regression analysis suggest that this risk continues into adulthood; however, the exact reason is not clear. It is known that lung maturation is incomplete at birth and continues until 20 years of age with a further increase in alveolarization.16,17 Even moderate prematurity may adversely affect the maturation process, resulting in an increased incidence of respiratory disease later in childhood.18,19 Sedation/anesthesia may unmask subclinical pulmonary dysfunction, resulting in an increased likelihood of oxygen desaturation. Neurologic dysfunction commonly seen in preterm and former preterm infants may also play a role in the development of adverse events, including apnea and airway obstruction.
It is not surprising that the highest risk group included infants 0 to 6 months of age. Our subanalysis revealed that this age group was more than twice as likely to develop an adverse sedation/anesthesia event (OR 2.6, P = .001). Apnea was one of the most notable adverse events in this age group, particularly in preterm and formerly preterm infants. Formerly preterm and preterm infants 0 to 6 months of age were 5 times as likely to experience apnea, and this elevated risk was also demonstrated in the 24- to 36-month age group. Approximately 78% of infants in the 0- to 6-month age category received propofol, which is known to cause apnea. There is considerable variability in both the pharmacokinetics and pharmacodynamics of this drug, especially in preterm neonates, which may in part explain the occurrence of apnea.20,21 In addition, abnormal respiratory function frequently observed in the preterm population is complex and not completely understood. The immaturity of the respiratory and central nervous systems, altered carotid chemoreceptor responses to hypoxia and hyperoxia, numerous neurotransmitters, genetic predisposition, and laryngeal chemoreflexes are a few of the mechanisms that have been implicated in the development of apnea and respiratory abnormalities in the preterm infant.22–25 These factors may contribute to the development of airway and respiratory adverse events related to sedation/anesthesia.
Oxygen desaturation is one of the more common adverse sedation/anesthesia events that can result in significant morbidity or mortality. In our study, infants 0 to 6 months of age experienced the highest frequency of oxygen desaturation; however, no significant difference was noted between preterm and term groups (4.1% vs 3.1%; P = .63). We postulate that this is due to the higher oxygen consumption and limited physiologic reserve in all children <6 months of age. It is interesting to note a significant difference in oxygen desaturation developed in formerly preterm patients 7 to 18 months of age (OR 2.8; P = .003), suggesting there is an underlying pathology in the preterm group that predisposes them to hypoxia. The effect of age on the development of oxygen desaturation has been previously described by Coté et al.26 These investigators reported that children undergoing inhalational general anesthesia, by either mask or endotracheal tube, were more likely to experience a major desaturation event (defined as oxygen saturation ≤85% for >30 seconds). This occurred in ∼28% of children 0 to 6 months of age, with a marked decrease in the 7- to 24-month age group (8.0%). They also examined the difference in oxygen desaturation between children 7 to 24 months of age and those >24 months and found no significant difference between the 2 groups. Although their study had a mean age less than ours, we found similar results. In our study, infants 0 to 6 months of age were significantly more likely to experience oxygen desaturation than older children, and no significant difference was found between the 2 older categories of children ages 7 to 24 months and 25 to 36 months (P = .5). This affirms that patients <7 months of age are at increased risk for the development of oxygen desaturation.
We investigated the possibility that preterm and former preterm patients required more invasive procedures than term children, requiring deeper levels of sedation or the administration of general anesthesia; however, our data suggest there was not a substantial difference between the 2 groups. Diagnostic procedures such as MRI scans and ABRs accounted for >60% of procedures experienced by preterm and former children, whereas MRI scans, upper endoscopies, and lumbar punctures accounted for >60% of procedures requiring sedation/anesthesia in term children. In light of the fact that esophageal endoscopy and lumbar punctures are more stimulating procedures, which could result in increased airway and respiratory adverse events, we could not conclude that preterm children experienced a higher percentage of adverse events solely owing to more invasive or painful procedures. Diagnostic procedures such as MRI scans may require a deep level of sedation or general anesthesia to obtain complete motion control that is necessary for adequate studies, in contrast to the variable level of sedation required for esophageal endoscopy and lumbar punctures, since the amount of movement that is tolerated during these procedures is not consistent. There were no significant differences in the occurrence of adverse events between the 2 groups undergoing computed tomography scans or upper endoscopies. In this instance, we believe the brief duration of the procedures decreases the exposure to risk. In addition, in patients undergoing upper endoscopy, the procedure itself may adversely impact the airway and respiratory systems, resulting in both groups experiencing a high rate of adverse events. Our findings suggest that the duration and level of sedation or administration of general anesthesia may be more important than the amount of stimulation.
It is well known that age is a predictor of adverse events during sedation and general anesthesia.5,8–10,27–32 This fact has been attributed to the variation in pharmacokinetics and pharmacodynamics of sedative and anesthetic drugs and to the limited physiologic reserve in children <1 year of age.20,21,32,33 We postulated that preterm children were more susceptible to experiencing adverse events at a younger age, but that the risk would decrease as they grew older owing to the increase in physiologic reserve that occurs with increasing age. The finding that a decrease in frequency of adverse events did not occur in formerly preterm patients as they reached adulthood was unexpected. The examination of the data by both categorical methods and nonparametric regression analysis revealed the same result; preterm and former preterm children are at higher risk for the development of adverse sedation/anesthesia events regardless of age at the time of the procedure. We postulated several possibilities for this finding, but as in any observational study, we cannot determine the cause. We suspect that the degree of prematurity may influence this outcome; however, we were unable to evaluate that possibility since the database does not collect information on the exact postgestational age at birth.
As previously noted, our analysis by age revealed a biphasic distribution in the frequency of adverse events. The increase in adverse events in preadolescent children has been previously reported in the sedation literature.27,34 Srinivasan et al reported an increase in adverse sedation events with the use of propofol in patients >12 years of age.34 Green et al reported an unexpected increase in adverse sedation events with the use of ketamine in teenagers.27 It is noteworthy that the increase in incidence of adverse events that occurs in infants and preteenage children corresponds to the well-described increase in minimum alveolar concentration in these age groups.35–37 It is possible that these children require higher doses of sedative/anesthetics agents, thereby decreasing the margin of safety. Furthermore, older patients who require sedation for these procedures may represent a selected group of individuals with behavioral or developmental pathology that places them at greater risk, since the ability to sedate this population is frequently more difficult.
Our findings were similar to those published by other investigators in several ways. Our analysis revealed that sedation/anesthesia adverse events were primarily related to airway and respiratory systems, the highest frequency occurred in infants <6 months, and an increase in the frequency of adverse events was noted in the preadolescent population. On the other hand, our longitudinal risk analysis demonstrated that preterm and former preterm children remain at increased risk into adulthood. To our knowledge, this has not been previously reported. This may be due to the fact that a history of prematurity may not be routinely obtained, since it has not been considered a risk factor for the development of an adverse event.
This study has several limitations, primarily related to its observational nature. These data were prospectively collected and retrospectively analyzed. As with all observational studies, observer bias may be present. The small number of patients who experienced uncommon adverse events, such as hypothermia, makes it challenging to draw any inferences from the data. Although it is plausible that hypothermia occurs more frequently in the preterm group receiving sedation/anesthesia in a non–thermal-controlled environment, the small number of children experiencing hypothermia in the entire database precludes any conclusion. Furthermore, organizations participating in this registry are primarily children’s hospitals that are motivated to improve the quality of sedation/anesthesia care to children. The reporting institutions have sedation systems that are highly organized, have been in existence for more than 5 to 10 years, and practice under controlled circumstances. We also recognize that the sedations/anesthetics reported are almost entirely elective in nature. Data from emergency procedures could be significantly different. These are a selected group of providers and a limited range of procedures, and as such the data do not reflect pediatric sedation or anesthesia as it occurs randomly across the United States. Most importantly, we note that we do not know the degree of prematurity present in children classified in the preterm group. It is plausible that children born at 36 weeks’ gestation have adverse event rates similar to those born at 37 weeks. If this is true, the risk of adverse sedation/anesthetic events would be higher in very premature (28–32 weeks’ gestation) and extremely premature (<28 weeks’ gestation) children. In addition, institutions involved in the research consortium are more likely to provide care to very and extremely premature children, resulting in the increased incidence of adverse events.
Observational studies such as this are essential tools in quality improvement initiatives. The advantage of studies conducted on large databases such as one developed by the PSRC includes the description of current practice at the national level, benchmarking, and the identification of trends and populations at risk. In addition, observational studies provide the ability to generate hypotheses for future randomized controlled studies. For example, it is known that children <60 weeks’ postconception age receiving general anesthesia for surgical procedures are at increased risk for developing apnea perioperatively; however, the body of evidence supporting similar conclusions in children receiving propofol sedation is limited. Our data suggest that children receiving propofol for sedation/anesthesia and those born preterm are at increased risk for the development of apnea; however, the severity and duration of the apnea for diagnostic procedures is unknown.
Although the majority of patients in our study did not experience a serious adverse event with untoward outcomes, pediatricians and other health care professionals should be aware that diagnostic procedures frequently require sedation/anesthesia and expose children to the development of potentially serious adverse events. A risk/benefit assessment should be conducted before ordering elective studies, with patient age, birth history, and comorbidities taken into consideration.
Conclusions
Our longitudinal risk analysis of patients 0 to 22 years of age suggests that preterm and formerly preterm children are at increased risk for developing adverse sedation/anesthesia events. The highest risk group for the development of serious adverse events consisted of patients <6 months of age; however, an increase in the rate of adverse events was noted in the 10- to 13-year-old age group. These data suggest that age and history of prematurity should be considered during the development and implementation of a sedation/anesthetic plan.
Supplementary Material
Acknowledgments
The authors thank Susan Gallagher, BS, in the Department of Bioinformatics Service Center at Geisel School of Medicine at Dartmouth, Lebanon, NH, and Angela J. Myers, MBA, Pittsburgh, PA, for their assistance in the preparation of this manuscript.
Glossary
- ABR
auditory brainstem response
- OR
odds ratio
- PS
American Society of Anesthesiology Physical Status
- PSRC
Pediatric Sedation Research Consortium
Footnotes
Dr Havidich conceptualized and designed the study, drafted the initial manuscript, and carried out the initial analysis; Dr Beach assisted with the analysis; Drs Suresh, Dierdorf, Onega, and Cravero critically reviewed and revised the manuscript; Drs Dierdorf and Onega contributed to the manuscript; Dr Cravero assisted with conceptualization and study design; and all authors approved the final manuscript.
Dr Dierdorf's current affiliation is Medical University of South Carolina, Charleston, SC; Dr Suresh's current affiliation is Texas Children's Hospital, Houston, TX.
FUNDING: No external funding.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2015-4579.
References
- 1.Centers for Disease Control and Prevention;Preterm birth . Available at: www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm. Accessed April 16, 2014
- 2.Cohen MM, Cameron CB, Duncan PG. Pediatric anesthesia morbidity and mortality in the perioperative period. Anesth Analg. 1990;70(2):160–167 [DOI] [PubMed] [Google Scholar]
- 3.Keenan RL, Shapiro JH, Dawson K. Frequency of anesthetic cardiac arrests in infants: effect of pediatric anesthesiologists. J Clin Anesth. 1991;3(6):433–437 [DOI] [PubMed] [Google Scholar]
- 4.Lee C, Mason L. Complications in paediatric anaesthesia. Curr Opin Anaesthesiol. 2006;19(3):262–267 [DOI] [PubMed] [Google Scholar]
- 5.Litman RS, Soin K, Salam A. Chloral hydrate sedation in term and preterm infants: an analysis of efficacy and complications. Anesth Analg. 2010;110(3):739–746 [DOI] [PubMed] [Google Scholar]
- 6.Mason KP, Fontaine PJ, Robinson F, Zgleszewski S. Pediatric sedation in a community hospital-based outpatient MRI center. AJR Am J Roentgenol. 2012;198(2):448–452 [DOI] [PubMed] [Google Scholar]
- 7.Litman R, Soin K, Salam A. Chloral hydrate sedation in term and preterm infants: an analysis of efficacy and complications. Anesth Analg. 2010;110:739–746 [DOI] [PubMed] [Google Scholar]
- 8.Morray JP, Geiduschek JM, Caplan RA, Posner KL, Gild WM, Cheney FW. A comparison of pediatric and adult anesthesia closed malpractice claims. Anesthesiology. 1993;78(3):461–467 [DOI] [PubMed] [Google Scholar]
- 9.Morray JP, Geiduschek JM, Ramamoorthy C, et al. Anesthesia-related cardiac arrest in children: initial findings of the Pediatric Perioperative Cardiac Arrest (POCA) Registry. Anesthesiology. 2000;93(1):6–14 [DOI] [PubMed] [Google Scholar]
- 10.Murat I, Constant I, Maud’huy H. Perioperative anaesthetic morbidity in children: a database of 24,165 anaesthetics over a 30-month period. Paediatr Anaesth. 2004;14(2):158–166 [DOI] [PubMed] [Google Scholar]
- 11.Tiret L, Nivoche Y, Hatton F, Desmonts JM, Vourc’h G. Complications related to anaesthesia in infants and children. A prospective survey of 40240 anaesthetics. Br J Anaesth. 1988;61(3):263–269 [DOI] [PubMed] [Google Scholar]
- 12.Coté CJ, Zaslavsky A, Downes JJ, et al. Postoperative apnea in former preterm infants after inguinal herniorrhaphy. A combined analysis. Anesthesiology. 1995;82(4):809–822 [DOI] [PubMed] [Google Scholar]
- 13.Welborn LG, Hannallah RS, Luban NL, Fink R, Ruttimann UE. Anemia and postoperative apnea in former preterm infants. Anesthesiology. 1991;74(6):1003–1006 [DOI] [PubMed] [Google Scholar]
- 14.Welborn LG, Rice LJ, Hannallah RS, Broadman LM, Ruttimann UE, Fink R. Postoperative apnea in former preterm infants: prospective comparison of spinal and general anesthesia. Anesthesiology. 1990;72(5):838–842 [DOI] [PubMed] [Google Scholar]
- 15.Cravero JP, Blike GT, Beach M, et al. ; Pediatric Sedation Research Consortium . Incidence and nature of adverse events during pediatric sedation/anesthesia for procedures outside the operating room: report from the Pediatric Sedation Research Consortium. Pediatrics. 2006;118(3):1087–1096 [DOI] [PubMed] [Google Scholar]
- 16.Narayanan M, Owers-Bradley J, Beardsmore CS, et al. Alveolarization continues during childhood and adolescence: new evidence from helium-3 magnetic resonance. Am J Respir Crit Care Med. 2012;185(2):186–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weibel ER. It takes more than cells to make a good lung. Am J Respir Crit Care Med. 2013;187(4):342–346 [DOI] [PubMed] [Google Scholar]
- 18.Carraro S, Filippone M, Da Dalt L, et al. Bronchopulmonary dysplasia: the earliest and perhaps the longest lasting obstructive lung disease in humans. Early Hum Dev. 2013;89(suppl 3):S3–S5 [DOI] [PubMed] [Google Scholar]
- 19.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729 [DOI] [PubMed] [Google Scholar]
- 20.Allegaert K, Peeters MY, Verbesselt R, et al. Inter-individual variability in propofol pharmacokinetics in preterm and term neonates. Br J Anaesth. 2007;99(6):864–870 [DOI] [PubMed] [Google Scholar]
- 21.Schüttler J, Ihmsen H. Population pharmacokinetics of propofol: a multicenter study. Anesthesiology. 2000;92(3):727–738 [DOI] [PubMed] [Google Scholar]
- 22.Martin RJ, Abu-Shaweesh JM. Control of breathing and neonatal apnea. Biol Neonate. 2005;87(4):288–295 [DOI] [PubMed] [Google Scholar]
- 23.Abu-Shaweesh JM, Martin RJ. Neonatal apnea: what’s new? Pediatr Pulmonol. 2008;43(10):937–944 [DOI] [PubMed] [Google Scholar]
- 24.Katz ES, Mitchell RB, D’Ambrosio CM. Obstructive sleep apnea in infants. Am J Respir Crit Care Med. 2012;185(8):805–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Praud J-P. Larynx and neonatal apneas. Pediatr Pulmonol Suppl. 1999;18:190–193 [PubMed] [Google Scholar]
- 26.Coté CJ, Rolf N, Liu LM, et al. A single-blind study of combined pulse oximetry and capnography in children. Anesthesiology. 1991;74(6):980–987 [DOI] [PubMed] [Google Scholar]
- 27.Green SM, Roback MG, Krauss B, et al. Predictors of airway and respiratory adverse events with ketamine sedation in the emergency department: an individual-patient data meta-analysis of 8,282 children. Ann Emerg Med. 2009;54(2):158–168 [DOI] [PubMed] [Google Scholar]
- 28.Krauss B, Green SM. Procedural sedation and analgesia in children. Lancet. 2006;367(9512):766–780 [DOI] [PubMed] [Google Scholar]
- 29.Mason KP, Lerman J. Dexmedetomidine in children: current knowledge and future applications. Anesth Analg. 2011;113(5):1129–1142 [DOI] [PubMed] [Google Scholar]
- 30.Malviya S, Voepel-Lewis T, Tait AR. Adverse events and risk factors associated with the sedation of children by nonanesthesiologists. Anesth Analg. 1997;85(6):1207–1213 [DOI] [PubMed] [Google Scholar]
- 31.Coté CJ, Karl HW, Notterman DA, Weinberg JA, McCloskey C. Adverse sedation events in pediatrics: analysis of medications used for sedation. Pediatrics. 2000;106(4):633–644 [DOI] [PubMed] [Google Scholar]
- 32.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167 [DOI] [PubMed] [Google Scholar]
- 33.Bartelink IH, Rademaker CM, Schobben AF, van den Anker JN. Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin Pharmacokinet. 2006;45(11):1077–1097 [DOI] [PubMed] [Google Scholar]
- 34.Srinivasan M, Turmelle M, DePalma LM, Mao J, Carlson DW. Procedural sedation for diagnostic imaging in children by pediatric hospitalists using propofol: analysis of the nature, frequency, and predictors of adverse events and interventions. J Pediatr 2012;160(5):801–806 [DOI] [PubMed] [Google Scholar]
- 35.Lerman J, Sikich N, Kleinman S, Yentis S. The pharmacology of sevoflurane in infants and children. Anesthesiology. 1994;80(4):814–824 [DOI] [PubMed] [Google Scholar]
- 36.Katoh T, Ikeda K. Minimum alveolar concentration of sevoflurane in children. Br J Anaesth. 1992;68(2):139–141 [DOI] [PubMed] [Google Scholar]
- 37.Gregory GA, Eger EI II, Munson ES. The relationship between age and halothane requirement in man. Anesthesiology. 1969;30(5):488–491 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.