Abstract
Background
Frailty has emerged as one of the major risk factors of loss of autonomy and it can be reverted through early and appropriate interventions. A wide range of available frailty screening tools are administered, mainly in clinical settings. However, few frailty instruments are self-administered.
Objectives
The aim of this study was to determine the diagnostic test accuracy of a modified self-administered questionnaire derived from the Study of Osteoporotic Fractures (SOF) index against the Fried frailty phenotype in identifying frailty.
Design
Observational, multicenter, diagnostic test accuracy study.
Participants
Participants aged 70 and over, living at home or in community-dwelling (n=5134) in two centers in France were contacted.
Measurements
Participants were mailed self-administered questionnaires derived from the SOF index. Responders who accepted the home evaluation were assessed by trained nurses, blinded to results of the questionnaire, using the Fried frailty phenotype as the reference method.
Results
The questionnaire was sent to 5134 participants, of which 1878 (36.6%) met inclusion criteria and returned the questionnaire. Fried frailty assessments were obtained in 691 (35.4%) participants. A total of 639 subjects had a complete evaluation on both the self-administered questionnaire and the Fried phenotype. Mean age was 78.9 (standard deviation [SD]: 5.95) years and 359 (56.2%) participants were women. According to the questionnaire, 159 (24.9%) subjects were considered frail, 172 (26.9%) pre-frail, and 308 (48.2) robust. With the home evaluation, Fried frailty phenotype results were respectively, 114 (17.8%), 295 (46.2%) and 230 (36%). The self-administered questionnaire presented a sensitivity of 66.6% (95% CI: 57.2–75.2) and a specificity of 84.2% (95% CI: 80.8–87.2).
Conclusions
A self-administered questionnaire can be used in elders and represents an opportunity for empowering them in the management of their health in the context of frailty
Electronic Supplementary Material
Supplementary material is available in the online version of this article at 10.14283/jfa.2023.11.
Key words: Frailty, self-assessment, diagnostic accuracy
Introduction
The physical frailty phenotype, first described by Fried and colleagues (1) has been described as a state of increased vulnerability to stressor events, resulting from the accumulation with age of deficits in multiple physiological systems. These deficits cause a decrease in the body’s functional reserves and in its ability to cope with a stressful situation (1–5). Worldwide yearly incidence of frailty among community dwelling adults above 60 years old is estimated to be 4.3% (3). Frailty syndrome has severe consequences, with several prospective studies and metaanalyses showing an increased risk of adverse health outcomes, such as falls, disability, hospitalization, institutionalization, and death (6–13). A few studies have also indicated that frailty is also associated with increased healthcare costs (14–15). However, recent clinical trials have shown that frailty syndrome is potentially reversible if early preventive and rehabilitative interventions are implemented (2).
Being common, costly, severe and potentially reversible with an appropriate early intervention makes the frailty syndrome an almost perfect candidate for a population screening according to the World Health Organization classical criterias (31). However, to the best of our knowledge only England has implemented a kind of systematic frailty screening (32). A recent qualitative meta-synthesis showed that some of the main reasons caregivers, healthcare providers and elderly adults give for the absence of screening are the lack of a proper tool and of a screening pathway. Indeed among the self-screening tools developed very little have correct diagnostic properties evaluated against an appropriate reference standard (17, 23).
The Study of Osteoporotic Fractures (SOF) index is a rapid and validated easy-to-apply screening tool to assess frailty phenotype in the older population in clinical practice. It includes 3 items: weight loss, inability to rise from a chair five times without using the arms and reduced energy. It is established as a predictor of adverse health outcomes based on assessments conducted in clinical settings (7, 18). Ruiz and colleagues have recommended the SOF scale to be incorporated in primary care settings for the identification of patients who may require referral for comprehensive geriatric assessment (19). Because of its simplicity and proximity with the original items of the Fried criteria, and its shortness, we believed the SOF to be a good candidate for an adaptation destined for self-screening by postal mail. Indeed postal mail is the favorite way of communication reported by older adults and would enable medico-social organizations to have a simple, and reliable self-administered screening tool to detect frailty in the elderly people. This pragmatic approach would promote subjects’ empowerment in the management of their health, hence complementing the treating physician’s role in primary prevention.
The main objective of this study is therefore to assess in elderly subjects aged 70 years and over, the diagnostic test accuracy in detecting frailty of a modified self-administered SOF index, sent by postal mail against the Fried frailty phenotype evaluated by a healthcare professional. Another objective is to assess the diagnostic accuracy of the self-administered questionnaire against the Fried phenotype in detecting both frailty and pre-frailty.
Methods
Study design, setting and participants
We conducted a multicenter, observational, diagnostic cross sectional study. It included 5134 participants aged 70 and over, living at home or in community-dwelling, from Paris and Toulouse areas. Participants were randomly selected from the French National Old-Age-Insurance Fund (Caisse Nationale d’Assurance Vieillesse “CNAV”). The CNAV is the main public pension fund in France covering more than 80 percent of pensioners. Using their records, the CNAV produced a sample representative of the general French population in terms of age and sex.
Participants were excluded from the study if they were institutionalized, received the personalized allowance for autonomy or had been previously enrolled in other frailty studies. Excluding participants receiving the personal allowance for autonomy allowed us to exclude older adults with identified dependence, thus excluding subjects with identified cognitive impairments.
In September 2019, 5134 participants received a postal letter from the CNAV explaining the study objectives and a separate modified self-administered questionnaire derived from the SOF index to be filled in and sent back in a pre-paid envelope to the International Longevity Centre France (ILC-France), the coordinating site. After 4 weeks, a reminder was sent to the 3866 non-responders. A pilot study, initially conducted in 300 subjects from Paris, provided a response rate of 46% with the mailing approach (unpublished data).
Participants who accepted the home assessment were examined by trained nurses, blinded to the results of the self-administered questionnaire, using the Fried frailty phenotype as the reference measure of frailty. Participants also received information related to preventive actions (individual or collective sessions) organized as part of the healthy ageing promotion programs (eg: physical activities, cognitive training, nutritional advice etc…).
When a frail or pre-frail individual was identified by the nurse and with the participant’s consent, ILC-France liaised directly with the geriatric platform of Paris or Toulouse to ensure a tailored loss of autonomy prevention plan was implemented in collaboration with the subject’s treating physician.
All participants gave written informed consent prior to conducting the Fried frailty phenotype assessment. This study was approved by the independent ethics committee, “Comité de protection des personnes Ile de France 7”, Approval Number: 19.01.21.51235.
Measurements
Sociodemographic characteristics
Age, sex, marital status, and educational level were collected.
Frailty self-administered questionnaire derived from the SOF index
The SOF index was adapted to be used for self-diagnosis rather than clinical use, therefore items were transformed into simple questions. Some examples of what a 5% percent weight loss would represent depending on the person’s original weight were added.
The adapted SOF consisted of three items (Table 1): weight loss, inability to rise from a chair five times without using the arms and exhaustion.
Table 1.
Questionnaire derived from the SOF index
| Items | Questions | Answer | Score |
|---|---|---|---|
| Weight loss | In the past year, have you lost 5% or more of your usual weight? | Yes | 1 |
| Non | 0 | ||
| Inability to rise from a chair five times without using the arms | Can you rise from a chair five times without using the arms? | Yes | 0 |
| No | 1 | ||
| Exhaustion | Do you feel without energy, much more tired than usual? | Yes | 1 |
| Non | 0 |
Participants were considered “Robust” if none of the above criteria were found, “Pre-frail” when one criterion was reported and “Frail” if at least two criteria were met.
Fried frailty phenotype assessment
Frailty defined by the Fried frailty phenotype was based on the presence of 3 or more of the following 5 criteria: unintentional weight loss, low physical activity level, self-reported exhaustion, muscle weakness and slow walking speed (Table 2).
Table 2.
Fried frailty phenotype
| Items | Questions | Answers | Score |
|---|---|---|---|
| Weight loss | In the past two years, have you lost 5% or more of your usual weight? | ||
| Exhaustion | How often over the last week, you felt that everything you did was an effort, or you could not get going*: — often (more than 3 times a week) or most of the time? | Yes | 1 |
| No | 0 | ||
| Low physical activity level | What is your physical activity level? No physical activity or rather sedentary: short walks or physical activities of light intensity? | Yes | 1 |
| No | 0 | ||
| Muscle weakness | Dominant hand grip strength measurement in kilograms using a calibrated hydraulic hand dynamometer (Jamar Hand Dynamometer), categorizations were stratified by sex (≤ 29 kg for males and ≤ 17 kg for females)- hand grip strength measurements below the threshold? | Yes | 1 |
| No | 0 |
*: Using the Center for Epidemiological Studies-Depression scale 20; **: The pedestrian crossing is calculated for a walking speed of 1m/sec.
Subjects were scored “Robust” when none of the criteria was fulfilled, “Pre-frail” if they met 1 to 2 criteria and “Frail” if at least 3 criteria were present.
Outcome assessment
Measures of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) were used to determine the ability of the self-administered questionnaire to identify frail subjects compared to the Fried frailty phenotype.
Postal response as well as the home assessment acceptance’s rates were evaluated.
Statistical analysis
Sample size and confidence intervals calculations were done using the Clopper-Pearson exact formula for proportion confidence intervals (21, 22). The pilot study estimated a sensitivity of 85% and a specificity of 60% for the self-administered questionnaire. Aiming at a 95% confidence interval with a width equal or inferior to 0.15 and assuming the frailty prevalence is 20% according to Fried criteria, the estimated number of required participants is 495 for sensitivity and 388 for specificity. We therefore used the highest of the two numbers (i.e 495 for sensitivity) as the number we aimed to recruit. Results from the pilot study indicated that about 50% of participants would return the self-completed questionnaire. And that among responders, only 20% would accept the home assessment by the nurse. Therefore, we sent questionnaires to more than 4950 candidates (5134) aiming at recruiting 495 participants with both self-screening and home assessment.”
Quantitative variables were described as mean and standard deviation or median and interquartile ranges (IQR), qualitative variables were described as number and percentage.
Sensitivity, specificity, PPV and NPV were calculated using a contingency table with frailty defined by the Fried criteria as the gold standard and frailty defined by the self-administered questionnaire as the candidate values. We also estimated frailty diagnosis rates both on all invited subjects to participate and responders. For the secondary outcome, the same procedure was applied to identify both frailty and pre-frailty defined with Fried criteria as the gold standard and frailty, or prefrailty defined with the self-administered questionnaire as the candidate values.
Attrition bias was assessed by comparing subjects who only completed the self-administered questionnaire and those who had both evaluations according to sex, age stratification and each modified SOF criterion using Chi2 tests.
To assess whether there were some predictors associated with the diagnostic questionnaire performance, a multivariate regression was performed on frail subjects identified by the Fried criteria, using frailty identified by the self-administered questionnaire as outcome and sex, age, marital status, educational level (high school level or above versus others) and duration time between the self-assessment and Fried evaluations as explaining variables.
A sensitivity analysis was conducted on subjects evaluated before the first Covid-19 lockdown implementation in France in March 2020 as it might have modified the way of evaluating patients as well as delayed the nurses evaluation.
Finally, due to the potential impact of the delay between the two evaluations we calculated NPV, PPV, sensitivity and specificity separately for participants above or below the median delay and above or under third quartile delay.
Results
Of the 5134 participants, 1950 (37.9%) completed the self-administered questionnaire and 1878 (36.6%) responders fulfilled the study selection criteria. Among responders, 1745 (92.9%) participants completed all 3 questions, 1824 (97.1%), 1854 (98.7%), and 1806 (95.9%) participants replied respectively to the questions related to weight loss, subject’s inability to rise from a chair, and reduced energy level.
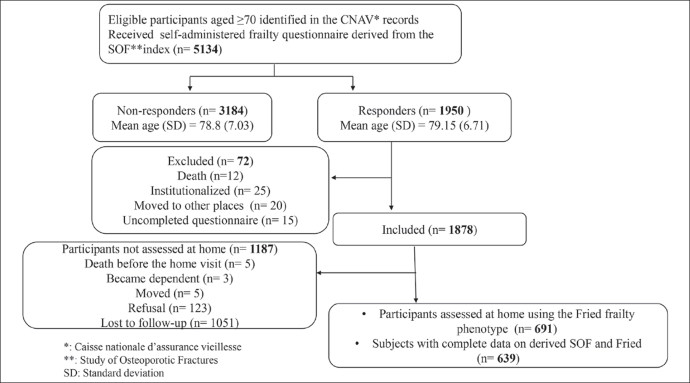
Trained nurses conducted frailty assessments at home for 691 participants using the Fried frailty phenotype (figure 1), representing 35.4% of all responders and 13.5% of the initial sample. A total of 639 subjects had a complete evaluation on both the self-administered questionnaire and the Fried phenotype, corresponding to 12.4% of the initial sample. Characteristics of these participants are reported in table 3.
Figure 1.
Flowchart of subjects selection and frailty assessment
Table 3.
Characteristics of 639 participants
| Variables | Participants, Mean (SD) or n (%) |
|---|---|
| Age | 78.9 (5.95) |
| Sex, Female | 359 (56.2) |
| Median time in days between the SOF and Fried frailty evaluations (IQR) | 106 (103) |
| Marital status | |
| Single | 97 (15.3) |
| Married | 274 (43.3) |
| Divorced | 120 (19) |
| Widowed | 132 (20.9) |
| Cohabitation | 10 (1.6) |
| Educational level | |
| Illiterate | 14 (2.2) |
| Primary school | 36 (5.7) |
| Lower secondary | 161 (25.4) |
| Upper secondary | 107 (16.9) |
| University | 317 (49.9) |
| Individual self-administered questionnaire items | |
| Unintentional weight loss | 155 (24.3) |
| Inability to rise from a chair 5 times without using arms | 130 (20.3) |
| Reduced energy | 256 (40.1) |
| Individual Fried frailty items | |
| Unintentional weight loss | 122 (19.1) |
| Exhaustion | 184 (28.8) |
| Low physical activity level | 228 (35.7) |
| Slowness | 86 (13.5) |
| Weakness | 220 (34.4) |
| Frailty status by self-administered questionnaire | |
| Frail | 159 (24.9) |
| Prefrail | 172 (26.9) |
| Robust | 308 (48.2) |
| Frailty status by Fried frailty phenotype | |
| Frail | 114 (17.8) |
| Prefrail | 295 (46.2) |
| Robust | 230 (36) |
IQR: interquartile range
Mean age was 78.9 (standard deviation [SD]: 5.95) years, 359 (56.2%) were women, 317 (49.9%) participants had a university educational level, 107 (16.9%) had a high school level and 161 (25.5%) reached the lower secondary level.
According to the self-administered questionnaire, 159 (24.9%) participants were considered frail, 172 (26.9 %) pre-frail, and 308 (48.2%) robust. The most frequently reported item was exhaustion, encountered in 256 (40.1%) participants.
According to the Fried frailty phenotype, 114 (17.8%) participants were frail, 295 (46.2%) prefrail and 230 (36%) robust. The most frequently reported frailty items were low physical activity (35.7%), muscle weakness (34.4%), and exhaustion (28.8%).
Comparison between participants who accepted both evaluations (n=691) and those who only completed the self-administered questionnaire (n=1187) indicated that women, participants aged between 80–84 and those reporting a weight loss were more likely to be evaluated at home by the nurses (p-values <0.05). Results are shown in table 4.
Table 4.
Comparison between participants who only completed the questionnaire and those assessed at home
| Completed SOF only, n (%) N = 1187 | Assessed at home by the nurse n (%) N = 691 | p-value | |
|---|---|---|---|
| Sexe Female | 734 (61.8) | 395 (57.2) | 0.038 |
| Age, N | 1187 | 691 | 0.001 |
| 70–74 | 380 (32) | 227 (32.9) | |
| 75–79 | 303 (25.5) | 181 (26.2) | |
| 80–84 | 225 (18.9) | 157 (22.7) | |
| 85–89 | 167 (14.1) | 95 (13.7) | |
| > 90 | 112 (9.5) | 31 (4.5) | |
| Weight loss per questionnaire | 228 (19.8) | 162 (24.1) | 0.035 |
| Inability to rise from a chair per questionnaire | 262 (22.4) | 141 (20.7) | 0.431 |
| Exhaustion per questionnaire | 437 (38.2) | 272 (41) | 0.262 |
Table 5 displays the comparison of results from the self-administered questionnaire and the Fried frailty phenotype. The self-administered questionnaire presented a sensitivity of 66.6% (95% CI: 57.2–75.2), a specificity of 84.2% (95% CI: 80.8–87.2), a PPV of 47.8% (95% CI: 39.8–55.9) and a NPV of 92.1% (95% CI: 89.3–94.3) in identifying frail participants. The detection rate of frailty was 4.04% (95% CI: 3.2–5) in included responders (n=1878), and 1.48% (95% CI: 1.17–1.84) in the overall sample (n=5134), respectively. Using logistic regression analyses, we observed that age, gender, marital status, educational level, or duration time between the self-administered questionnaire and Fried evaluation did not affect the self-administered questionnaire in identifying frail participants.
Table 5.
Comparison of results from the self-administered questionnaire and the Fried phenotype in detecting frailty
| Frail per Fried phenotype | Robust or Prefrail per Fried phenotype | |
|---|---|---|
| Frail per self-administered questionnaire | 76 | 83 |
| Robust or Prefrail per self-administered questionnaire | 38 | 442 |
Sensitivity: 66.6% (95% CI: 57.2–75.2); Specificity: 84.2% (95% CI: 80.8–87.2); PPV: 47.8% (95% CI: 39.8–55.9); NPV: 92.1% (95% CI: 89.3–94.3)
We conducted a separate analysis among 493 subjects assessed at home prior to the first COVID-19 lockdown implementation in France between March 17 and May 11, 2020, to avoid measuring variability secondary to the lockdown. During that period, all home visits were put on hold, however nurses maintained participant contact through telephone calls. Similar results as the primary analysis were found with a sensitivity and a specificity of 64.4% (95% CI: 53.7–74.3), and 85.4% (95%CI: 81.5–88.7), respectively (table 6).
Table 6.
Comparison of results from the self-administered questionnaire and the Fried phenotype in detecting frailty prior to the first COVID-19 lockdown.
| Frail per Fried phenotype | Robust or Prefrail per Fried phenotype | |
|---|---|---|
| Frail per self-administered questionnaire | 58 | 59 |
| Robust or Prefrail per self-administered questionnaire | 32 | 344 |
Sensitivity: 64.4% (95% CI: 53.7–74.3); Specificity: 85.4% (95% CI: 81.5–88.7); PPV: 49.6% (95% CI: 40.2–58.9); NPV: 91.5% (95% CI: 88.2–94.1)
Sensitivity, specificity, NPV and PPV were very similar in patients above or below the median or third quarter delay between evaluations (Supplementary Table 1 and 2).
The self-administered questionnaire presented a sensitivity of 65.5% (95% CI: 60.7–70.1), a specificity of 72.6% (95% CI: 66.4–78.3), a PPV of 80.9% (95% CI: 76.3–85.1) and a NPV of 54.2% (95% CI: 48.5–59.9) in identifying frail or prefrail subjects (secondary endpoint) as reflected in table 7.
Table 7.
Comparison of results from the self-administered questionnaire and the Fried phenotype in detecting frailty or pre-frailty
| Frail or Pre-frail per Fried phenotype | Robust per Fried phenotype | |
|---|---|---|
| Frail or Pre-frail per self-administered questionnaire | 268 | 63 |
| Robust per self-administered questionnaire | 141 | 167 |
Sensitivity: 65.5% (95% CI: 60.7–70.1); Specificity: 72.6% (95% CI: 66.4–78.3); PPV: 80.9% (95% CI: 76.3–85.1); NPV: 54.2% (95% CI: 48.5–59.9)
Discussion
In the present study, the self-administered questionnaire derived from the SOF index provided a sensitivity of 66.6% and a specificity of 84.2% in detecting frailty against the Fried frailty phenotype.
Our results are within the ranges described in a recent systematic review on the diagnostic accuracy of self-reported/administered screening instruments to identify frailty in community-dwelling (17).
Sensitivity values were between 20.3–100% while specificity ranges varied from 60.5 to 95.6%. When comparing self-administered frailty instruments against the Fried phenotype, the authors indicated a sensitivity of 100% (95% CI: 34–100) and a specificity of 80% (95% CI: 67–89) for the Groningen Frailty Indicator (GFI), however the sample size was small (n=52) with wide confidence intervals. Hoogendijk and colleagues (23) reported a sensitivity of 57% and a specificity of 72% for the GFI, PRISMA-7 displayed a sensitivity of 86% and a specificity of 83%, and Self-rated health showed a sensitivity of 85% and a specificity of 73% (n=102). The Tilburg Frailty Indicator presented a sensitivity of 79.4% and a specificity of 60.5% (n=267) whereas the Frailty Postal Questionnaire showed a sensitivity of 75% and a specificity of 69% (n=1037) (24–26).
These results illustrate high heterogeneity related to the diagnostic test accuracy of the frailty tools, the study designs, the sample sizes, the frailty prevalence, and settings.
A separate analysis was also conducted among participants evaluated prior to the first Covid-19 lockdown to avoid measuring variability attributable to the lockdown effect. Results showed similar findings as the primary analysis in terms of sensitivity and specificity.
Clegg and colleagues (28) suggested a two-step assessment of frailty using first a score with high sensitivity and then a score with high specificity. Because of its relatively low sensibility but high specificity the modified SOF that we used would be better suited used as a second step following the Frailty Postal Questionnaire allowing for a full postal diagnosis.
The screening procedure through the postal questionnaire enabled participants to use a simple, and quick self-administered tool to detect frailty themselves at home, hence empowering the participants in the management of their health. A response rate of 38% in our study population can be considered satisfactory compared to previously reported response rates (29). Such approach addresses the request from the patient group consumer outlined in the clinical practice guidelines for identification and management of physical frailty issued by the task force of the International Conference of Frailty and Sarcopenia (27).
This study has some limitations. Like the home evaluations, items included in the self-administered questionnaire assessed weight loss, muscle weakness and exhaustion which are part of the Fried criteria. However, there is evidence to indicate that these 3 components reflect impairment in physiologic domains most frequently mentioned in the frailty literature (7). In order to reduce bias, nurses were blinded to the results of the self-administered questionnaire and were instructed not to discuss with the participants of these results during the Fried evaluations.
There was a relatively long median delay of 106 days between both evaluations. However there was no difference in sensitivity or specificity between participants evaluated with a delay over or under that threshold. Participants who accepted the Fried evaluations at home belonged to people with a high level of education (49.9% with a university level and 16.9% with an upper secondary school level). As a result, there is an under-representation of subjects with low educational level and these data cannot be used to draw a conclusion in this population. This raised the question about how low educated participants could be reached for frailty assessment as some evidence indicates that higher frailty prevalence is observed among them (30). Future studies are needed to get more insights on potential motivators, barriers, and optimal strategies to engage this population in frailty detection and early intervention programs.
In addition, the attrition bias indicated that women, participants aged between 80–84 and those reporting a weight loss on the self-administered questionnaire were more likely to be evaluated at home by the trained nurses.
In conclusion, the present study confirms that the self-administered questionnaire adapted from the SOF index can be easily used in subjects who might benefit from the healthy ageing promotion and prevention programs.
Comprehensive geriatric assessments are time-consuming and are not available in all parts of our and other high income country and in low-income countries. Given resource scarcity and the busy workload in clinical and primary care settings, the self-administered questionnaire seems suitable for old persons’ self-evaluation of frailty and would benefit for countries with limited healthcare resources.
This simple and easy to apply tool may represent, as well, an opportunity for empowering elders to identify frailty themselves and raising frailty awareness.
Electronic Supplementary Material
Supplementary material, approximately 14.7 KB.
Acknowledgments
The authors would like to thank all the participants, Caroline Berbon, Magali Poly and Sandrine Vaysset from Toulouse University Hospital.
Contributors
PL: contributed to the study design; study supervision and coordination; data acquisition and verification, analysis, and interpretation of the data; and wrote the first and successive drafts of the manuscript. JS: accessed and verified data, carried out the study analysis and interpretation of the data as well as drafting and revision of the first draft of the manuscript and rewriting the drafts of the manuscript for revision.NT: contributed to the study conduct and coordination, collection of data, interpretation of the data, and drafting and/or revision of the manuscript. MRL: contributed to the study conduct, collection of data, interpretation of the data, and drafting and/or revision of the manuscript. MLS: contributed to the study design, interpretation of the data, and drafting and/or revision of the manuscript. OH: contributed to the study design, and interpretation of data as well as drafting and/or revision of the manuscript. SA: contributed to the study design, the statistical analysis plan and interpretation of the data, as well as drafting and/or revision of the manuscript. BV: contributed to the study design and interpretation of data as well as drafting and/or revision of the manuscript. FF: contributed to the study design; acquisition, verification, analysis, and interpretation of the data; and drafting and/or revision of the manuscript. All authors read and approved the final manuscript for submission.
Funding
This work was supported by Caisse Nationale d’Assurance Vieillesse, Agences Régionales de Santé Ile de France and Occitanie, Malakoff Humanis and Nutricia. The funding body had no role in the study design, data collection, data analysis and interpretation, presentation of results, decision to publish, or preparation of the manuscript.
Footnotes
Declaration of interests
We declare no competing interests.
References
- 1.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in Older Adults: Evidence for a Phenotype. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2001;56(3):M146–57. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 2.Puts MTE, Toubasi S, Andrew MK, Ashe MC, Ploeg J, Atkinson E, et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing. 2017 Jan 6;ageing;afw247v1. Doi: 10.1093/ageing/afw247 [DOI] [PMC free article] [PubMed]
- 3.Ofori-Asenso R, Chin KL, Mazidi M, et al. Global Incidence of Frailty and Prefrailty Among Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. JAMA Netw Open. 2019;2(8):e198398. doi: 10.1001/jamanetworkopen.2019.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xue Q-L. The Frailty Syndrome: Definition and Natural History. Clinics in Geriatric Medicine. 2011;27(1):1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. The Lancet. 2013;381(9868):752–62. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-Term Risks of Death and Institutionalization of Elderly People in Relation to Deficit Accumulation at Age 70: LONG-TERM RISK OF DEATH DEFINED BY AGE 70. Journal of the American Geriatrics Society. 2006;54(6):975–9. doi: 10.1111/j.1532-5415.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 7.Ensrud KE. Comparison of 2 Frailty Indexes for Prediction of Falls, Disability, Fractures, and Death in Older Women. Arch Intern Med. 2008;168(4):382. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 8.Shamliyan T, Talley KMC, Ramakrishnan R, Kane RL. Association of frailty with survival: A systematic literature review. Ageing Research Reviews. 2013;12(2):719–36. doi: 10.1016/j.arr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Lahousse L, Maes B, Ziere G, Loth DW, Verlinden VJA, Zillikens MC, et al. Adverse outcomes of frailty in the elderly: the Rotterdam Study. Eur J Epidemiol. 2014;29(6):419–27. doi: 10.1007/s10654-014-9924-1. [DOI] [PubMed] [Google Scholar]
- 10.Kojima G. Frailty as a predictor of hospitalisation among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Community Health. 2016;70(7):722–9. doi: 10.1136/jech-2015-206978. [DOI] [PubMed] [Google Scholar]
- 11.Vermeiren S, Vella-Azzopardi R, Beckwée D, Habbig A-K, Scafoglieri A, Jansen B, et al. Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. Journal of the American Medical Directors Association. 2016;17(12):1163.e1–1163.e17. doi: 10.1016/j.jamda.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Kojima G, Iliffe S, Walters K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age and Ageing. 2018;47(2):193–200. doi: 10.1093/ageing/afx162. [DOI] [PubMed] [Google Scholar]
- 13.Aw D, Woodrow L, Ogliari G, Harwood R. Association of frailty with mortality in older inpatients with Covid-19: a cohort study. Age and Ageing. 2020 Aug 10;afaa184. Doi: 10.1093/ageing/afaa184 [DOI] [PMC free article] [PubMed]
- 14.Bock J-O, König H-H, Brenner H, Haefeli WE, Quinzler R, Matschinger H, et al. Associations of frailty with health care costs — results of the ESTHER cohort study. BMC Health Serv Res. 2016;16(1):128. doi: 10.1186/s12913-016-1360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kojima G. Increased healthcare costs associated with frailty among community-dwelling older people: A systematic review and meta-analysis. Archives of Gerontology and Geriatrics. 2019;84:103898. doi: 10.1016/j.archger.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 17.Ambagtsheer RC, Thompson MQ, Archibald MM, Casey MG, Schultz TJ. Diagnostic test accuracy of self-reported screening instruments in identifying frailty in community-dwelling older people: A systematic review. Geriatr Gerontol Int. 2020;20(1):14–24. doi: 10.1111/ggi.13810. [DOI] [PubMed] [Google Scholar]
- 18.Bilotta C. Frailty syndrome diagnosed according to the Study of Osteoporotic Fractures (SOF) criteria and adverse health outcomes among community-dwelling older outpatients in Italy. A one-year prospective cohort study. Archives of Gerontology and Geriatrics. 2012;6. Doi: 10.1016/j.archger.2011.06.037 [DOI] [PubMed]
- 19.Ruiz JG, Dent E, Morley JE, Merchant RA, Beilby J, Beard J, Tripathy C, Sorin M, Andrieu S, Aprahamian I, Arai H, Aubertin-Leheudre M, Bauer JM, Cesari M, Chen LK, Cruz-Jentoft AJ, De Souto Barreto P, Dong B, Ferrucci L, Fielding R, Flicker L, Lundy J, Reginster JY, Rodriguez-Mañas L, Rolland Y, Sanford AM, Sinclair AJ, Viña J, Waters DL, Won Won C, Woo J, Vellas B. Screening for and Managing the Person with Frailty in Primary Care: ICFSR Consensus Guidelines. J Nutr Health Aging. 2020;24(9):920–927. doi: 10.1007/s12603-020-1498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 21.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. 3rd edn. New York: John Wiley & Sons; 2003. [Google Scholar]
- 22.Newcombe R G. Two-Sided Confidence Intervals for the Single Proportion: Comparison of Seven Methods. Statistics in Medicine. 1998;17:857–872. doi: 10.1002/(SICI)1097-0258(19980430)17:8<857::AID-SIM777>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 23.Hoogendijk EO, van der Horst HE, Deeg DJH, Frijters DHM, Prins BAH, Jansen APD, et al. The identification of frail older adults in primary care: comparing the accuracy of five simple instruments. Age and Ageing. 2013;42(2):262–5. doi: 10.1093/ageing/afs163. [DOI] [PubMed] [Google Scholar]
- 24.Braun T, Grüneberg C, Thiel C. German translation, cross-cultural adaptation and diagnostic test accuracy of three frailty screening tools: PRISMA-7, FRAIL scale and Groningen Frailty Indicator. Z Gerontol Geriat. 2018;51(3):282–92. doi: 10.1007/s00391-017-1295-2. [DOI] [PubMed] [Google Scholar]
- 25.Mossello E, Profili F, Di Bari M, Bandinelli S, Razzanelli M, Salvioni A, et al. Postal screening can identify frailty and predict poor outcomes in older adults: longitudinal data from INTER-FRAIL study. Age Ageing. 2016;45(4):469–74. doi: 10.1093/ageing/afw048. [DOI] [PubMed] [Google Scholar]
- 26.Roppolo M, Mulasso A, Gobbens R, Mosso C, Rabaglietti E. A comparison between uni- and multidimensional frailty measures: prevalence, functional status, and relationships with disability. CIA. 2015 Oct;1669. Doi: 10.2147/CIA.S92328 [DOI] [PMC free article] [PubMed]
- 27.Dent E, Morley JE, Cruz-Jentoft AJ, Woodhouse L, Rodríguez-Mañas L, Fried LP, et al. Physical Frailty: ICFSR International Clinical Practice Guidelines for Identification and Management. J Nutr Health Aging. 2019;23(9):771–87. doi: 10.1007/s12603-019-1273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clegg A, Rogers L, Young J. Diagnostic test accuracy of simple instruments for identifying frailty in community-dwelling older people: a systematic review. Age and Ageing. 2015;44:148–152. doi: 10.1093/ageing/afu157. [DOI] [PubMed] [Google Scholar]
- 29.Sinclair M, O’Toole J, Malawaraarachchi M, Leder K. Comparison of response rates and cost-effectiveness for a community-based survey: postal, internet and telephone modes with generic or personalised recruitment approaches. BMC Med Res Methodol. 2012;12(1):132. doi: 10.1186/1471-2288-12-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoogendijk EO, van Hout HPJ, Heymans MW, van der Horst HE, Frijters DHM, Broese van Groenou MI, et al. Explaining the association between educational level and frailty in older adults: results from a 13-year longitudinal study in the Netherlands. Annals of Epidemiology. 2014;24(7):538–544.e2. doi: 10.1016/j.annepidem.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 31.J.M.G. Wilson et G. Jungner, Principles and practice of screening for disease, Genève, World Health Organization, coll. « Public health papers » (no 34) (1re éd. 1968)
- 32.Updated guidance on supporting routine frailty identification and frailty care through the GP Contract 2017/2018 [https://intranet.eastberkshireccg.nhs.uk/wp-content/uploads/2018/11/NHSE-Frailty-Guidance-GP-Contract-2017-18-Sep-2017.pdf]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material, approximately 14.7 KB.