Abstract
Autosomal dominant tubulointerstitial kidney disease (ADTKD) is a clinical entity defined by interstitial fibrosis with tubular damage, bland urinalysis and progressive kidney disease. Mutations in UMOD and MUC1 are the most common causes of ADTKD but other rarer (REN, SEC61A1), atypical (DNAJB11) or heterogeneous (HNF1B) subtypes have been described. Raised awareness, as well as the implementation of next-generation sequencing approaches, have led to a sharp increase in reported cases. ADTKD is now believed to be one of the most common monogenic forms of kidney disease and overall it probably accounts for ∼5% of all monogenic causes of chronic kidney disease. Through international efforts and systematic analyses of patient cohorts, critical insights into clinical and genetic spectra of ADTKD, genotype–phenotype correlations as well as innovative diagnostic approaches have been amassed during recent years. In addition, intense research efforts are addressed towards deciphering and rescuing the cellular pathways activated in ADTKD. A better understanding of these diseases and of possible commonalities with more common causes of kidney disease may be relevant to understand and target mechanisms leading to fibrotic kidney disease in general. Here we highlight recent advances in our understanding of the different subtypes of ADTKD with an emphasis on the molecular underpinnings and its clinical presentations.
Keywords: cohort studies, inherited kidney diseases, interstitial fibrosis, monogenic kidney disease, rare diseases
AUTOSOMAL DOMINANT TUBULOINTERSTITIAL KIDNEY DISEASE: COMMON FEATURES
Autosomal dominant tubulointerstitial kidney disease (ADTKD) is a genetically heterogeneous disease entity increasingly recognized in the era of next-generation sequencing (NGS) as a cause of familial kidney disease. The unifying features of autosomal dominant inheritance of (often isolated) kidney interstitial fibrosis with tubular atrophy (IF/TA) prompted the reclassification of diseases formerly known as ‘medullary cystic kidney disease’ or ‘familial juvenile hyperuricemic nephropathy’ under the umbrella term ADTKD [1, 2]. Given their non-specific presentations, molecular genetics play an important role in the diagnosis and classification of ADTKD. This is also reflected in the proposed terminology, where the identified causal gene is appended (e.g. ADTKD-UMOD) or cases are labelled ADTKD-NOS (not otherwise specified) if no genetic testing has occurred or no mutation has been identified in the known genes [1]. It is currently difficult to estimate their overall prevalence due to still limited clinical recognition and the difficulties of diagnosing one of the more common subtypes (ADTKD-MUC1), but overall, ADTKD is probably among the three most prevalent genetic kidney disease entities in adults (with ADPKD and Alport syndrome) [3, 4].
In 2015, a Kidney Disease: Improving Global Outcomes consensus report defined diagnostic criteria and outlined testing and management strategies in ADTKD [1]. Minimal criteria for a clinical suspicion of ADTKD should be a positive family history of progressive chronic kidney disease (CKD) with bland urine sediment, absence of significant proteinuria and normal or small-sized kidneys. Histology findings are non-specific, including IF/TA, thickening and lamellation of the tubular basement membranes and tubular dilatations, however, kidney biopsies should be avoided [1, 5, 6]. Genetic testing should be a first-line diagnostic approach and a definitive diagnosis requires identification of a mutation in one of the known genes [1]. Conversely, absence of a mutation does not exclude ADTKD, as additional loci still require identification [7]. The rate of kidney function decline is highly variable in most ADTKD subtypes and environmental or genetic factors contributing to this variability are largely unknown [8–10].
Currently no mechanistic therapies are available and patient care generally follows recommendations for CKD [1] with a few exceptions as seen below and the caveat that recommendations in this setting are based on limited evidence [1, 7]. Genetic counselling should be offered as the risk of disease transmission to offspring is 50%. No recurrence of ADTKD in the renal transplant is expected and kidney transplantation is currently the preferred therapeutic option for patients with end-stage kidney disease (ESKD) [1, 7]. As for most rare diseases, clinical and genetic characterization of ADTKD has been hampered in the past by low patient numbers and clustering of cohorts. However, large international studies have contributed significant novel insights into several of the ADTKD subtypes during the last year [8, 9, 11–13].
ADTKD DUE TO MUTATIONS IN UMOD (ADTKD-UMOD)
UMOD encodes uromodulin (UMOD, Tamm-Horsfall protein), the most abundant protein excreted in mammalian urine in physiological conditions and is only expressed in kidney distal tubular cells. UMOD is heavily glycosylated and contains four epidermal growth factor (EGF)-like domains, a cysteine-rich domain (D8C) and a bipartite zona pellucida domain, allowing protein polymerization. UMOD enters the secretory pathway where endoplasmic reticulum (ER) exit requires engagement of all 48 conserved cysteine residues in the formation of 24 intramolecular disulphide bonds. Urinary release and polymerization is mediated by the serine protease hepsin. UMOD’s functions include roles in the thick ascending limb of the loop of Henle (TAL)/distal convoluted tubule (DCT) electrolyte transport, protection from urinary tract infections (UTIs) and calcium (Ca2+)-containing nephrolithiasis and a role in local and systemic immunomodulation (Table 1) [14]. Common regulatory SNPs at the UMOD locus have been consistently and robustly associated with the risk of CKD and other complex diseases such as hypertension and kidney stones in the general population [14].
Table 1.
Genes associated with typical and atypical ADTKD
| Genes causing typical and atypical ADTKD (OMIM number) | Encoded protein | Protein functions/KO phenotypes |
|---|---|---|
| UMOD (*191845) | Uromodulin |
|
| MUC1 (*158340) | Mucin 1 |
|
| REN (*179820) | Preprorenin |
|
| HNF1B (*189907) | Hepatocyte nuclear factor 1β |
|
| SEC61A1 (*609213) | α1 subunit of SEC61 translocon |
|
| DNAJB11 (*611341) | DNAJ/HSP40 homolog, subfamily B, member 11 (cofactor of BiP) |
|
IMPC, International Mouse Phenotyping Consortium.
Genetics
Heterozygous (het.) mutations in UMOD [Online Mendelian Inheritance in Man (OMIM #162000)] were first reported in 2002 in four families presenting with an autosomal dominant pattern of kidney failure, adolescent hyperuricaemia and gout [15]. Since then, 135 mutations [9, 14] have been reported, the large majority of them missense variants. There is a certain geographical clustering, with variants more frequently reported in the UK (see below) or in the USA (p.H177_R185del) [8, 16]. About 95% of mutations map to exons 3 and 4, which correspond to the N-terminal portion of the protein and coding for the four EGF-like domains and the D8C [9]. About 60% of mutations delete, insert or replace a cysteine residue that is crucial for the complex tertiary folding of UMOD. Penetrance is believed to be ~100% and only a few cases of unaffected heterozygotes have been reported [17].
Pathophysiology
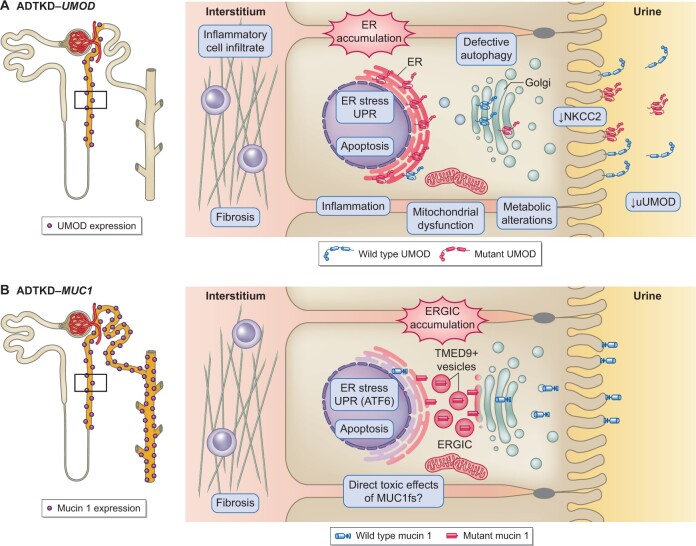
Disease manifestations are secondary to toxic gain-of-function effects as Umod-deficient mice do not develop tubulointerstitial kidney disease and the UMOD gene is not under apparent constraint for predicted loss-of-function variants [7]. Evidence obtained in different cell lines and mouse models establishes impaired trafficking of mutant UMOD, with ER accumulation and reduced urinary levels as the primary molecular defect [18–20]. In addition, mutant UMOD that escapes ER quality control forms extracellular aggregates at the plasma membrane instead of wild-type polymers [21]. The links between ER accumulation and tubulointerstitial fibrosis are less well understood. ER stress induces the unfolded protein response (UPR) and different branches have been implicated in vivo and in vitro [19, 20, 22]. ER stress has also been shown on human kidneys [9]. Some in vitro studies show a link to increased apoptosis, but in vivo studies are conflicting on this point [19, 20]. Induction of inflammation is an early event preceding fibrosis [23] and secondary mitochondrial dysfunctions as well as impaired energy homoeostasis have been shown in vivo [23, 24]. Using mouse and human tissue, general TAL dysfunction including reduced expression of sodium–potassium–chloride (Na+–K+–2Cl) cotransporter (NKCC2) has been evidenced [18, 25, 26] (Figure 1). Compensatory upregulation of the Na+-coupled urate transporters at the proximal tubule in response to volume contraction is the hypothesized mechanism of hyperuricaemia in ADTKD-UMOD [26].
FIGURE 1.
Pathophysiology of ADTKD-UMOD and ADTKD-MUC1. (A) Left panel: UMOD expression is restricted to the thick ascending limb and the early DCT. Right panel: Cellular pathways activated by the expression of mutant UMOD (in cellular and murine studies as well as patient biopsies) and its toxic accumulation in the ER. (B) Left panel: Mucin 1 expression along the entire distal tubule. Right panel: Cellular pathways activated by the expression of mutant mucin 1 (in cellular and murine studies as well as patient biopsies) and its toxic accumulation in the ERGIC.
Clinical presentation
In a UK single-centre study, ADTKD-UMOD was the most common form of inherited CKD after ADPKD, with a prevalence of 1% in CKD Stages 3–5 and 2% in patients with ESKD [3]. Whole-exome sequencing (WES) in a cohort of ˃3000 patients with mostly ESKD found a UMOD mutation in ∼0.3% of the whole cohort and 3% of all patients with a monogenic diagnosis [4]. Affected individuals present with progressive CKD, bland urinalysis, normal or slightly elevated blood pressure and normal (or small)-sized kidneys. Clinical presentation, especially in advanced disease, might be misleading, as cases with secondary FSGS and subnephrotic proteinuria have been described [27]. An autosomal dominant inheritance is the general rule, but de novo UMOD mutations have been reported [9]. CKD progression is highly variable, both inside and between families. In general, ESKD is reached at a median age of 47 years (range 18–87) [8, 9] (Table 2). The only extrarenal manifestation is hypouricosuric hyperuricaemia with gout, typically preceding CKD onset. In a European cohort, gout was present in 50% of women and 75% of men with a median age of onset of 21 years and hyperuricaemia >75th percentile [corrected for glomerular filtration rate (GFR)] was observed in 71% of patients [33]. Early-onset gout is specifically associated with ADTKD-UMOD (and ADTKD-REN) but not with ADTKD-MUC1 [9]. Only one-third of patients have renal cysts on ultrasound, and those are virtually never medullary [2, 9, 33]. An association between the age of onset of ESKD, the underlying UMOD mutation and the UMOD domain in which the mutation is mapped has been reported [35]. Somewhat counterintuitively, UMOD mutations involving cysteine residues seem to have no adverse effect or are even associated with a slightly better outcome [9, 17]. A recent large study [8] showed that male gender was a significant predictor of worse renal outcomes. In multivariate analyses, the combination of gender and the family mean age of ESKD were the best predictors of kidney survival [8]. Finally, rare patients with homozygous UMOD mutations tend to present with more severe clinical disease, in line with a dosage-dependent toxic gain-of-function effect [36, 37].
Table 2.
Clinical presentation of ADTKD subtypes
| Characteristics | UMOD | MUC1 | REN (het.) | HNF1B | DNAJB11 (het.) | SEC61A1 |
|---|---|---|---|---|---|---|
| Estimated prevalence/reported cases | Around 30 families described globally [11] | ∼19% in selected patient cohorts, ranging from 5% to 31% and highest in children with CAKUT [29] |
|
|
||
| ESKD onset and renal survival |
|
|
Median renal survival: 75 years, 95% CI 72.5–77.5 years [12] | Unknown | ||
| ESKD onset: age distribution | 18–87 years [8] | 16–>80 years [10] | 15 years to >80 years | Highly variable, ESKD can occur before age 2 years [31] | 55–89 years | Infantile onset of CKD, one patient with ESKD ∼70 years and one patient with CKD G4 at 53 years |
| Extrarenal clinical features | Prevalent (∼80%) and early-onset gout (median: 27 years) [9] | Gout less prevalent (∼25%) and later onset (median: 45 years) [9] |
|
|
|
|
| Laboratory features |
|
– |
|
|
– |
|
| Kidney imaging features |
|
Small-to-normal size kidneys [32] | Wide spectrum of renal appearances including pre-natal hyperechogenic kidneys, cysts, renal hypo/dysplasia, horseshoe kidney |
|
small dysplastic kidneys (± cysts) [34] |
FEuric acid, fractional excretion of uric acid.
Diagnosis
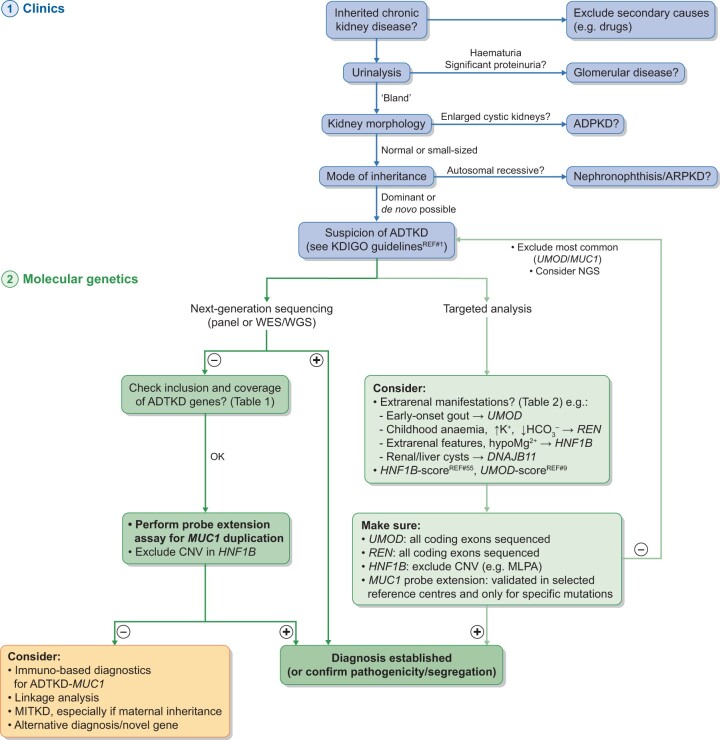
UMOD immunostaining on kidney biopsy can be informative; however, this staining is not available in most patients, is operator dependent and requires adequate controls and therefore its value in clinical decision making remains unclear. Aggregated UMOD accumulations are detectable in 85% of ADTKD-UMOD patients usingperiodic acid–Schiff staining under light microscopy examination [5]. In advanced disease, biopsy findings can present as secondary FSGS with partial podocyte foot process effacement [27]. Genetic testing by conventional Sanger sequencing or NGS-based approaches (gene panel analysis or WES) is now readily available and affordable (Figure 2). A genetic result allows a definitive diagnosis and is a likely prerequisite for inclusion in future therapeutic trials. In addition, it allows the testing of family members for the specific mutation and for live-related kidney donor eligibility. The assessment of urinary UMOD levels might offer a non-invasive tool to differentiate patients with UMOD mutations from other ADTKD patients and could be integrated into clinical diagnostic algorithms [9].
FIGURE 2.
Diagnostic algorithm in ADTKD. ADTKD should be suspected based on a series of clinical findings (or the absence thereof) and typically a positive family history (in blue) [1]. A firm diagnosis in suspected cases can only be established by molecular genetics (in green). Diagnostic approaches are likely to change with evolving molecular methodologies. Targeted analysis should take into consideration typical extrarenal features where present and may also take into account clinical scores developed for the rational prioritization of genetic tests. NGS-based approaches will likely become the preferred technology for detecting ADTKD with the limitations that variants in MUC1 VNTR are not detectable by NGS and require tailored approaches. +, positive result; –, negative result; ARPKD, autosomal recessive polycystic kidney disease; CNV, copy number variation; MLPA, multiplex ligation-dependent probe amplification.;Adapted from Devuyst O, Olinger E, Weber S et al. Autosomal dominant tubulointerstitial kidney disease. Nat Rev Dis Primer 2019; 5: 60.
Management
There are currently no mechanistic therapies for ADTKD-UMOD and general recommendations for management of CKD apply [1]. Allopurinol (or alternative urate-lowering therapy) will help prevent gout attacks, but whether this, or a low purine diet, slows CKD progression is debated. If renin–angiotensin–aldosterone system (RAAS) blockade is indicated, losartan is the preferred option for its uricosuric effect. Diuretics should be used with caution, as they might lead to symptomatic volume contraction [25]. In patients with ESKD, transplantation is the preferred therapeutic option, with outcomes that are comparable with the general transplant population and no evidence of disease recurrence [38]. In line with early involvement of inflammatory pathways, in vivo tumour necrosis factor α blockade slowed disease progression in a mouse model of ADTKD-UMOD [20]. While the chemical chaperone sodium 4-phenylbutyrate showed promising effects on mutant UMOD maturation in vitro, it failed to reproduce these effects in vivo [39]. Recently the small molecule BRD4780, successfully tested for ADTKD-MUC1 in vivo, showed effects on mutant UMOD accumulation in vitro, but preclinical data have not yet been reported [40].
ADTKD-UMOD in the UK
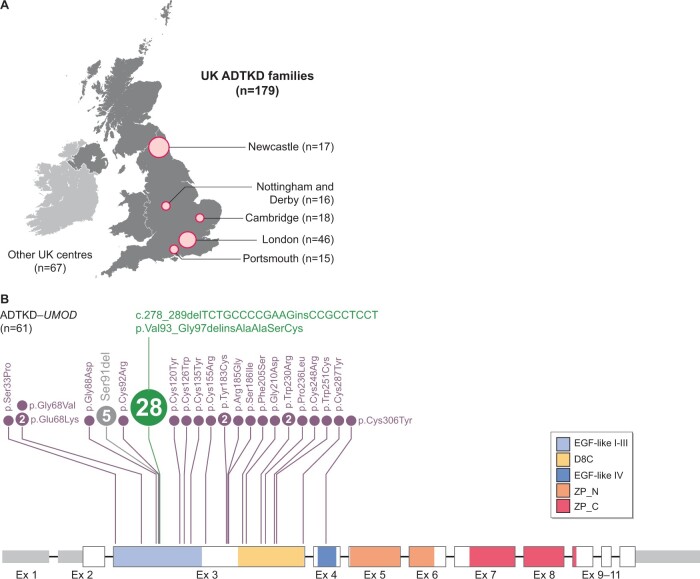
By establishing nationwide patient registries, population biobanks and integrating whole-genome sequencing into their national health system [41], the UK has pioneered research in rare genetic diseases. The National Registry of Rare Kidney Disease (RaDaR) is a clinician-led centralized and regularly updated web-based registry where longitudinal datasets are designed using accepted ontology in a prospective manner and allow secondary audit [42]. Recruited patients allow their information to be automatically linked with biochemical, imaging and kidney pathology data. Another example is the Genomics England 100 000 Genomes project [41], where ~35 000 probands with rare diseases have been recruited, often together with their parents, and have their whole genome sequenced. Results are then reported to recruiting clinicians and directly impact patient management. Currently there are 179 families with ADTKD recruited into RaDaR and the 100 000 Genomes Project from all over the UK. Among them, 61 families have a genetic diagnosis of ADTKD-UMOD. As reported before, the UMOD mutations cluster in exons 3 and 4 [9]. Strikingly, almost half of these families carry the in-frame indel mutation p.Val93_Gly97delinsAlaAlaSerCys (Figure 3) that has been associated with a milder in vitro phenotype, a slower decline in kidney function and virtual absence of gout [16]. Surprisingly, a previous study in four extensive indel families did not find a shared haplotype as indication of a founder effect [16]. Embedded within longitudinal registries, this genetically less-heterogeneous ADTKD population will provide important information to understand environmental and genetic factors of disease progression.
FIGURE 3.
ADTKD in the UK. (A) Major UK centres (with family numbers) from which ADTKD families have been recruited into the RaDaR and Genomics England 100 000 Genomes Project. (B) UMOD mRNA (NM_003361) and exon structure with UTR in grey. Protein domains are shown in colour code. UMOD mutations detected in the recruited families are presented above the mRNA structure with respect to their location and with disc size reflecting family numbers. For five families, the exact UMOD mutations were not recorded.
ADTKD DUE TO MUTATIONS IN MUC1 (ADTKD-MUC1)
Mutations in UMOD or MUC1 are consistently reported as the leading causes for ADTKD [6, 9] and together they explain ∼50% of all cases [9]. MUC1 encodes for mucin 1, a heavily glycosylated transmembrane protein that forms a protective mucous barrier on the apical side of epithelial cells and may be involved in cellular signalling, cell–cell interactions, modulation of immune functions and sensing of the extracellular environment (Table 1). MUC1 expression is widespread, including epithelia from the stomach, pancreas, lung, trachea, kidney, salivary and mammary glands [7, 43]. In human kidney, mucin 1 protein expression from the TAL down to the collecting duct has been reported [44]. In the distal tubule, mucin 1 stabilizes Ca2+-channel, transient receptor potential cation channel, subfamily V, member 5 (TRPV5) and increases urinary Ca2+ reabsorption. In a physiopathological context, protective upregulation of mucin 1 in the proximal tubule was shown after acute kidney injury [7]. In addition, MUC1 upregulation in different carcinomas and metastatic lesions suggests a role in tumour progression and led to its use as a prognostic marker in breast cancer [45].
Genetics
A common feature of MUC genes consists of GC-rich variable number of tandem repeats (VNTRs) of a defined number of nucleotides (the VNTR is 60-nt long for MUC1), coding for an extracellular domain rich in threonines and/or serines with heavy O-glycosylation [7, 46]. In MUC1, the number of 60-nt repeats is highly variable and may be between 20 and 125 copies [47]. The consequences for molecular diagnosis are at least 2-fold: the long GC-rich sequence leads to underrepresentation in polymerase chain reaction (PCR)-based sequencing approaches (including NGS) and the skewed ratio of 1 mutant repeat to numerous wild-type copies imposes a high wild-type background [48]. This explains why, despite consistent linkage to a locus at 1q21, it took more than a decade to identify MUC1 as the causative gene [47]. The mutation detected originally in six ADTKD families consisted in a cytosine (C) duplication within a stretch of seven Cs in a single copy of the canonical 60-mer repeat. Subsequently, long-range PCR with sequencing of the entire VNTR confirmed this mutational mechanism, i.e. a C duplication at the same location within the 60-mer repeat but affecting a different repeat in most of the families [46]. Given the resistance to endonuclease cleavage conferred by the C duplication, approaches based on mutant repeat enrichment and probe extension coupled with mass spectrometry or capillary electrophoresis have been developed as screening approaches for this particular mutation [2, 47, 48]. Živná et al. reported the C duplication in 183 of 191 ADTKD-MUC1 families [49]. A few cases of novel frameshifting variants inside the VNTR domain have been identified using long-range PCR [9, 49], but only 1 ADTKD-MUC1 family harboured a frameshifting mutation located before the VNTR [50]. Importantly, all these mutations in MUC1 are predicted to lead to the same neo-protein [frameshifted/mutant mucin 1 (MUC1fs)] that contains many copies of a novel frameshifted repeat sequence with a premature stop codon shortly after the VNTR, thus lacking both the transmembrane and intracellular domains [47]. MUC1fs VNTR domains lose the characteristic enrichment for serine and threonine residues with the introduction of one cysteine and six basic amino acid residues per mutated VNTR unit, conferring a high isoelectric point and potential cell toxicity [49].
Pathophysiology
Muc1 knockout (KO) mice showed no obvious histopathological abnormalities in the kidneys, therefore a toxic gain-of-function effect of MUC1fs is likely [7]. Using antibodies raised against MUC1fs, a distinct intracellular staining was detected in patient distal kidney tubules, contrasting with strictly apical staining for wild-type mucin 1 [40, 44, 47]. Mucin 1 normally enters the secretory pathway and is trafficked from the ER to the Golgi apparatus via coat protein II vesicles to be delivered to the apical plasma membrane. A recent comprehensive study identified the exact location of MUC1fs entrapment in mouse models and patient-derived cells and tissues to be cargo receptor TMED9-positive vesicles of the ER–Golgi intermediate compartment (ERGIC) [40] (Figure 1). There, MUC1fs upregulates the UPR with specific activation of the ATF6 branch in vitro and in vivo. Apoptosis was only observed in patient cells after inhibition of ATF6, suggesting a cytoprotective effect in this setting and that MUC1fs accumulation alone is not sufficient to drive cell death. Indeed, apoptosis was only detected in aged mutant mice, and it has been hypothesized that additional stressors are needed to activate cytotoxic pathways. It is currently not understood why phenotypes in ADTKD-MUC1 are limited to the kidney while MUC1fs is accumulating in other organs as well [49]. A possible interaction with UMOD is unlikely given that urinary UMOD levels in ADTKD-MUC1 are not altered [9].
Clinical presentation
No extrarenal presentations, other than gout, have been reported in ADTKD-MUC1 (OMIM 174000). In the first larger cohort, Bleyer et al. [10] described 24 ADTKD-MUC1 families with 206 genetically confirmed or inferred het. patients. A notable feature was progressive CKD, with a mean age at ESKD of ∼45 years but a striking variability in ESKD onset ranging from 16 to >80 years with large inter- and intrafamilial variability (Table 2). Furthermore, the authors noted a more rapid decline of renal function once eGFR decreased to ˂50 mL/min/1.73 m2 and described a trend for anticipation, i.e. earlier onset of ESKD in the offspring [10]. In the most recent analysis, the median age for ESKD for ADTKD-MUC1 was found to be significantly younger than for ADTKD-UMOD [36 years (IQR 30–46) versus 46 years (IQR 39–57)], with a median renal survival of 46 years (95% CI 39.3–52.7) in ADTKD-MUC1 [9]. All studies concur that hyperuricaemia and gout are less prevalent in ADTKD-MUC1 compared with ADTKD-UMOD and of later onset [6, 9, 10]. Microhaematuria and proteinuria are usually absent in ADTKD-MUC1, although a few instances of proteinuria >1 g/day have been described [6, 9]. Kidneys are normal in size or atrophic as CKD progresses. Despite its former descriptive name, ‘medullary cystic kidney disease type 1’, most individuals do not have kidney cysts and no medullary cysts have been identified in ADTKD-MUC1 patients by ultrasound or magnetic resonance imaging [2, 6, 10]. There are no overt genotype–phenotype correlations with respect to the type of mutation, position of affected repeat inside the VNTR or overall number of VNTRs [46, 49].
Diagnosis
A diagnosis of ADTKD-MUC1 can only be established with molecular diagnostics and kidney biopsy findings are unspecific [2, 6, 10]. The initial approach (usually after ruling out at least UMOD) is currently a probe extension coupled either with mass spectrometry [47, 48] or capillary electrophoresis (SNaPshot minisequencing) [2] (Figure 2). These techniques rely on pre-analytical enrichment based on acquired endonuclease resistance and have only been systematically reported for the most common C duplication. The interpretation of these tests, including appropriate controls, remains difficult and they are only available in a few laboratories (the Broad Institute, Cambridge, MA, USA and a few academic centres in Europe; see, for instance, Ekici et al. [2] and Ayasreh et al. [6]. Long-range PCR-based techniques allow assembly of the VNTR and exact positioning of causative mutations and are not restricted to the C duplication and therefore are suitable (in a research context) for all patients with strong suspicion of ADTKD-MUC1 but negative on probe-extension assay [46, 49]. Such strong suspicion could arise from previous linkage to 1q21 or antibody-based diagnostic approaches that rely on the specific detection of MUC1fs. Applied to patient kidney tissue, sensitivities and specificities of at least 91.7 and 81.8% have been reported for these immunostains [49], but these could be potentially higher after refinements [44]. Interestingly, non-kidney tissue stains produce a much less reliable result [44, 49]. Applied to urinary cell smears, MUC1fs immunostaining yielded a sensitivity of 94.2% and a specificity of 88.6%, with the advantage of being non-invasive and applicable to transplanted patients (staining of non-kidney-derived cells) [49]. Very recently, the plasma mucin 1 (CA15-3) levels in ADTKD-MUC1 patients were shown to be ∼40% lower than in controls. However, considerable overlap between groups urges further studies to establish its utility in diagnosis or as a disease biomarker [45].
Management and novel therapeutic leads
A recent high-content screen using an immunofluorescence cell-based assay for clearing MUC1fs from intracellular stores identified a lead compound (BRD4780) with drug-like properties including excellent oral bioavailability. BRD4780 was able to specifically remove MUC1fs from the ERGIC compartment, without affecting MUC1 transcriptional levels, and subsequently rescuing UPR levels in the cell and murine studies. The mechanism of action for BRD4780 includes physical binding to cargo receptor TMED9, thereby releasing trapped MUC1fs and promoting its anterograde trafficking and lysosomal degradation [40]. These findings suggest BRD4780 as a promising candidate for the first mechanistic therapy in ADTKD and possibly other toxic proteinopathies of membrane-associated proteins.
ADTKD DUE TO MUTATIONS IN REN (ADTKD-REN)
Mutations in REN are a much rarer cause of ADTKD. Nevertheless, recent international efforts helped better define the genetic and clinical spectra of this disease. REN encodes preprorenin, a 406 amino acid protein mainly expressed in granular cells of the juxtaglomerular apparatus. During cellular processing, the N-terminal signal peptide is cleaved off in the ER, leading to prorenin. Prorenin can then be secreted via a constitutive pathway or, alternatively, the N-terminal pro-segment is cleaved off to form renin that is stored in dense core vesicles for regulated exocytosis. Renin is an aspartic protease that catalyses the first step of the RAAS, a hormone system that has critical roles in the regulation of blood pressure, fluid balance and nephrogenesis. In addition, renin acts through local renin–angiotensin systems in many organs [7, 51] (Table 1).
Genetics
Rare biallelic loss-of-function mutations in components of the RAAS, including REN, have been linked to autosomal recessive renal tubular dysgenesis, a severe disorder of renal tubular development characterized by persistent foetal anuria and pulmonary hypoplasia [52]. A few years later, three families were identified segregating a het. in-frame deletion or a missense in the signal peptide of REN with early-onset anaemia, hyperuricaemia and progressive kidney disease, i.e. ADTKD-REN (OMIM 613092) [32]. Since then, 14 further mutations, all missenses, have been described [11, 53]. Mutations in the signal peptide (amino acids 1–23) are the most frequent (70% of the families), while more recently described mutations in the pro-segment and mature part of the protein have similar frequencies (13 and 17%, respectively) [11].
Pathophysiology
The location of the affected amino acid associates with the primary molecular defect. Signal peptide mutations p.(Leu16del) and p.(Leu16Arg) decrease signal sequence hydrophobicity with impairment of ER translocation and induction of mild ER stress. In concordance with this, expression of renin was decreased in kidney biopsy specimens [32] and accumulation of non-glycosylated preprorenin in the cytoplasm has been described for a signal peptide mutation [54]. In contrast, mature protein mutation p.(Leu381Pro) is translated but mutant protein accumulates in the ER, where it also induces ER stress and activation of the UPR [53]. In a recent systematic analysis, Živná et al. [11] studied 10 representative mutations in transfected cells and confirmed these primary defects. Moreover, they unravelled a third cellular phenotype for mutations in the pro-segment that lead to mutant prorenin accumulation in the ERGIC. Virtually all mutations, however, are associated with reduced renin activity in the cell medium and affect renin and/or prorenin secretion [11, 51]. While the aggregate of these experiments suggests a predominant gain-of-function effect, the exact toxic pathways causing the manifestations of ADTKD-REN, the contribution of UPR to the disease and possible dominant-negative effects on the wild-type renin allele require further investigations [51].
Clinical presentation
In addition to the classical renal features of ADTKD, early reports noted hypoproliferative transient infantile/childhood anaemia, mild hyperkalaemia, mild hypotension due to hyporeninaemic hypoaldosteronism as well as hyperuricaemia with gout in patients with REN signal peptide mutations [7, 32]. Detailed characterization of an international cohort of 111 individuals from 30 ADTKD-REN families very recently refined the typical clinical features and delineated genotype–phenotype associations. A childhood presentation of anaemia or anaemia with acidosis and kidney failure was common in ADTKD-REN. The mean haemoglobin levels in children were ~10 g/dL and levels tended to increase in men after puberty. Common features of all ADTKD-REN patients were high or normal–high serum potassium (K+) levels and generally low serum bicarbonate levels [11]. Interestingly, mutations in the signal peptide and the pro-segment present with similar clinical features as those classically assigned to ADTKD-REN: very early onset (approximately half of them presented before 10 years of age) and slowly progressive CKD with a median renal survival of ∼60 years, early-onset gout and transient (in men) childhood anaemia in the majority of cases. In contrast, mutations in the mature part of the protein present with adult-onset CKD and gout and reach ESKD in their late 60s, resembling ADTKD-UMOD [11, 51] (Table 2).
Diagnosis
The typical constellation includes anaemia, hyperkalaemia and acidosis, in addition to CKD, and this should prompt the appropriate diagnostic workup for ADTKD-REN. The value of plasma renin levels and activity or renin immunostaining in guiding the diagnosis has not been systematically assessed. Molecular approaches are required for a diagnosis and are first-line in patients with suspected ADTKD-REN (Figure 2). Thus far ADTKD-REN is caused by single-nucleotide variants, except for one in-frame deletion of one amino acid. Therefore standard methods of genetic testing, including Sanger sequencing and NGS, are appropriate if ADTKD-REN is suspected. If targeted analyses are employed, those should not be restricted to exon 1 (encoding the signal peptide and formerly believed to be the mutation hot spot [1]).
Management
Given the early manifestations in patients with signal peptide and pro-segment mutations, children with ADTKD-REN are likely to benefit from paediatric nephrology care [7]. The use of fludrocortisone appears safe in these patients and was associated with increased eGFR, lower serum K+ and improved serum bicarbonate levels as well as increased blood pressure in the few reported cases [11, 54]. Anaemia is usually responsive to erythropoiesis-stimulating agents [32]. As for ADTKD-UMOD, the use of uric acid–lowering treatments to prevent gout should be considered. Given the primary defect and the reported predisposition to the development of acute kidney injury, diuretics or other volume-depleting drugs, salt-restricted diets, non-steroidal anti-inflammatory drugs or drugs interfering with the RAAS should be avoided in ADTKD-REN patients [7, 25, 54].
RARE AND ATYPICAL FORMS OF ADTKD
Several other genes lead to clinical presentations reminiscent of ADTKD and should not be overlooked, especially when UMOD and MUC1 testing is negative or if additional clinical features are present.
Hepatocyte nuclear factor 1 homeobox β (HNF1β)
HNF1β is a transcription factor expressed in the developing kidney and in several adult organs with roles in both nephrogenesis and maintenance of tubular function. It plays crucial functions during mouse early embryogenesis and is suggested to exert direct transcriptional control on cystic disease genes (Pkhd1, Pkd2) and Umod in the kidney. HNF1β has also been shown to regulate transcription of FXYD2 (γ subunit of Na+-K+-ATPase) in the distal tubule, thereby indirectly affecting electrolyte handling [7] (Table 1). Het. variants in HNF1B are associated with highly variable renal phenotypes such as congenital anomalies of the kidney and urinary tract (CAKUT), Gitelman-like tubular disorders, cystic kidney disease and chronic tubulointerstitial disease. Extrarenal features include maturity-onset diabetes of the young type 5 (MODY5), pancreatic hypoplasia, genital tract malformations and abnormal serum liver enzymes. Neurological features have been described in cases of chromosome 17q12 deletion encompassing HNF1B [29]. Recently a mnemonic (MAGIC LUCID) has been suggested for the varied clinical manifestations associated with HNF1B mutations [43]. The term ADTKD-HNF1B should only be reserved for those cases in which kidney tubulointerstitial fibrosis is the leading manifestation [1]. The exact proportion of cases that indeed present as ADTKD and mimic ADTKD-UMOD is unknown. Hyperechogenic kidneys in children, cysts and CAKUT appeared to be the predominant renal manifestations and hyperuricaemia was more frequently reported in infants [31, 55] (Table 2). Recently, chronic tubulointerstitial nephritis had been described in 9 of 13 patients with HNF1B mutations. However, several of these patients had extrarenal features or presented with enlarged cystic kidneys diverging from the classical ADTKD presentation [13]. In ∼50% of patients, the molecular defect is a whole HNF1B gene deletion, occurring in the context of the 17q12 recurrent deletion syndrome. In the remaining cases, het. HNF1B variants are detected that can be missense (∼50%), frameshift, splice-affecting or nonsense variants [7, 13]. In up to 50% of cases, HNF1B variants occur de novo and a positive family history is missing [7]. A preponderance of 81% dominant maternal inheritance suggests epigenetic modifiers or defects in male fertility [31]. Regarding genotype–phenotype correlations, HNF1B whole gene deletions have a more favourable kidney prognosis, but the underlying mechanisms are unclear [7]. Due to highly variable clinical presentations and overlap with other entities, genetic testing is the gold standard to establish a diagnosis. It is important to choose an appropriate test able to detect large deletions (Figure 2). An HNF1B score based on clinical, imaging and biological variables has been developed as a simple tool to select patients for HNF1B screening [55]. Rarely, patients with HNF1B mutations present with very-early-onset disease and can develop ESKD before the age of 2 years, and those cases require specialized therapeutic approaches [31]. For the other cases, CKD is slowly progressive and morbidity arises largely from extrarenal disease. Although MODY5 is usually responsive to oral hypoglycaemic agents, most patients eventually require insulin treatment and combined kidney–pancreas transplantation should be considered in patients with diabetes mellitus and ESKD [1].
DNAJ/HSP40 homolog, subfamily B, member 11 (DNAJB11)
DNAJB11 is a cofactor of BiP (GRP78), an ER chaperone controlling protein folding, trafficking and degradation (Table 1). Het., mostly truncating, variants in DNAJB11 (OMIM 618061), have been recently associated with a clinical presentation described as a hybrid of ADTKD and ADPKD. Typically, patients progress to ESKD at a median age of 75 years, suggesting a better renal outcome compared with ADTKD-UMOD and ADTKD-MUC1. The disease hallmark is bilateral multiple small kidney cysts in the absence of significant kidney enlargement and liver cysts in ∼50% of cases. Tubulointerstitial fibrosis was described in a few kidney biopsies. Early-onset gout has not been described in these patients that may, however, display vascular phenotypes [12, 56] (Table 2). A recent case report described bi-allelic pathogenic variants in DNAJB11 associated with a severe foetal presentation of renal–hepatic–pancreatic dysplasia and ciliopathy features [57].
SEC61A1
Het. missense variants in SEC61A1, encoding the α1 subunit of the SEC61 translocon, have also been associated with ADTKD (OMIM 617056). This subunit is part of the ER translocon that allows newly synthesized proteins to be transported into the ER (Table 1). The altered structure of SEC61 translocon may lead to changes in post-translational modifications, folding and sorting of transmembrane proteins and may also alter Ca2+ homeostasis, both of which can trigger ER stress. Clinical features include early-onset but slowly progressive CKD with early-onset gout (in one family). On ultrasound, the kidneys appear small, dysplastic or normal sized with multiple bilateral cysts. Kidney biopsy is compatible with IF/TA. However, it should be noted that several extrarenal features are present, most notably haematological abnormalities such as congenital anaemia, neutropaenia and hypogammaglobulinaemia, and that SEC61A1 variants have also been described in patients with haematological disorders in the absence of overt kidney disease. Additional syndromic features including growth retardation, cleft palate, bifid uvula, mild cognitive impairment and polydactyly have been described (Table 2) [34].
Mitochondrially inherited tubulointerstitial kidney disease
A homoplasmic substitution in the control region of the mitochondrial genome (m.547A>T) has been detected in a large family with maternally inherited tubulointerstitial kidney disease. Also, two kindreds with tubulointerstitial kidney disease were identified carrying a homoplasmic mutation in mitochondrial tRNAPhe (m.616T>C) [58]. Carriers showed full penetrance of significant renal disease by the fourth decade of life and kidney biopsies were consistent with tubulointerstitial disease. Mutations in mitochondrial DNA (mtDNA), including m.616T>C, have been previously associated with kidney disease, most often with severe encephalopathy or other features of mitochondrial disease [59]. Strikingly, however, these more recently reported cases did not show overt extrarenal disease, suggesting that mtDNA mutations may present as isolated tubulointerstitial kidney disease. Kidney damage is likely mediated through reduced function of mitochondrial tRNAPhe and the kidney may be susceptible to specific alterations in tRNAPhe. The terminology mitochondrially inherited tubulointerstitial kidney disease (MITKD) should be used to complement ADTKD for those cases in which mtDNA mutation causes a predominant picture of tubulointerstitial kidney disease and MITKD should be screened for in unexplained familial tubulointerstitial kidney disease with compatible patterns of inheritance [58] (Figure 2).
CONCLUSION AND FUTURE PERSPECTIVES
The definition of ADTKD as a disease entity has led to increased awareness and has boosted its clinical recognition as a major cause of monogenic kidney disease. Identification of genetically defined disease subtypes enabled international collaborations during the last year that have gathered a critical amount of patient data and facilitated novel insights into the genetic and phenotypical spectra, genotype–phenotype correlations as well as novel diagnostic approaches. In parallel, intense research efforts are aimed at understanding the disease mechanisms driving IF and to rescue defects in proteostasis. The hope is that disease pathways operating in ADTKD are also relevant for more common forms of CKD sharing IF as an endpoint. More generally, these disorders also raise important questions regarding the role of the distal tubule, ER proteostasis and energy metabolism in the development of renal fibrosis. For the first time, mechanistic therapies are on the horizon for ADTKD-MUC1 and will hopefully be available soon for clinical testing. Given the generally slow progression of CKD, there is an urgent need for the identification and validation of disease-specific biomarkers to track disease activity and allow future therapeutic trials in patients. In addition, identification of further loci associated with ADTKD as well as genetic and environmental factors influencing disease progression will add to our understanding of ADTKD and fibrotic diseases.
FUNDING
E.O. is supported by an Early Post-doc Mobility Stipendium of the Swiss National Science Foundation (P2ZHP3_195181) and Kidney Research UK (Paed_RP_001_20180925). H.M. is a Medical Research Council Clinical Doctoral Training Fellow. J.A.S. is supported by Kidney Research UK and the Northern Counties Kidney Research Fund. This research was made possible through access to the data and findings generated by the 100 000 Genomes Project that is managed by Genomics England Limited (a wholly owned company of the Department of Health and Social Care). The 100 000 Genomes Project is funded by the National Institute for Health Research and NHS England. The Wellcome Trust, Cancer Research UK and the Medical Research Council have also funded research infrastructure. The 100 000 Genomes Project uses data provided by patients and collected by the National Health Service as part of their care and support. The RaDaR [42] is a Renal Association initiative designed to pull together information from patients with certain rare kidney diseases, including ADTKD, co-led by Prof. Fiona Karet and Prof. John Sayer (https://renal.org/rare-renal/patient/autosomal-dominant-tubulointerstitial-kidney-disease-0).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
REFERENCES
- 1. Eckardt KU, Alper SL, Antignac C. et al. Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management—a KDIGO consensus report. Kidney Int 2015; 88: 676–683 [DOI] [PubMed] [Google Scholar]
- 2. Ekici AB, Hackenbeck T, Morinière V. et al. Renal fibrosis is the common feature of autosomal dominant tubulointerstitial kidney diseases caused by mutations in mucin 1 or uromodulin. Kidney Int 2014; 86: 589–599 [DOI] [PubMed] [Google Scholar]
- 3. Gast C, Marinaki A, Arenas-Hernandez M. et al. Autosomal dominant tubulointerstitial kidney disease-UMOD is the most frequent non polycystic genetic kidney disease. BMC Nephrol 2018; 19: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Groopman EE, Marasa M, Cameron-Christie S. et al. Diagnostic utility of exome sequencing for kidney disease. N Engl J Med 2019; 380: 142–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Onoe T, Hara S, Yamada K. et al. Significance of kidney biopsy in autosomal dominant tubulointerstitial kidney disease-UMOD: is kidney biopsy truly nonspecific? BMC Nephrol 2021; 22: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ayasreh N, Bullich G, Miquel R. et al. Autosomal dominant tubulointerstitial kidney disease: clinical presentation of patients with ADTKD-UMOD and ADTKD-MUC1. Am J Kidney Dis off Dis 2018; 72: 411–418 [DOI] [PubMed] [Google Scholar]
- 7. Devuyst O, Olinger E, Weber S. et al. Autosomal dominant tubulointerstitial kidney disease. Nat Rev Dis Primers 2019; 5: 60. [DOI] [PubMed] [Google Scholar]
- 8. Kidd K, Vylet’al P, Schaeffer C. et al. Genetic and clinical predictors of age of ESKD in individuals with autosomal dominant tubulointerstitial kidney disease due to UMOD mutations. Kidney Int Rep 2020; 5: 1472–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olinger E, Hofmann P, Kidd K. et al. Clinical and genetic spectra of autosomal dominant tubulointerstitial kidney disease due to mutations in UMOD and MUC1. Kidney Int 2020; 98: 717–731 [DOI] [PubMed] [Google Scholar]
- 10. Bleyer AJ, Kmoch S, Antignac C. et al. Variable clinical presentation of an MUC1 mutation causing medullary cystic kidney disease type 1. Clin J Am Soc Nephrol 2014; 9: 527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Živná M, Kidd K, Zaidan M. et al. An international cohort study of autosomal dominant tubulointerstitial kidney disease due to REN mutations identifies distinct clinical subtypes. Kidney Int 2020; 98: 1589–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huynh VT, Audrézet MP, Sayer JA. et al. Clinical spectrum, prognosis and estimated prevalence of DNAJB11-kidney disease. Kidney Int 2020; 98: 476–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Izzi C, Dordoni C, Econimo L. et al. Variable expressivity of HNF1B nephropathy, from renal cysts and diabetes to medullary sponge kidney through tubulo-interstitial kidney disease. Kidney Int Rep 2020; 5: 2341–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schaeffer C, Devuyst O, Rampoldi L.. Uromodulin: roles in health and disease. Annu Rev Physiol 2021; 83: 477–501 [DOI] [PubMed] [Google Scholar]
- 15. Hart TC, Gorry MC, Hart PS. et al. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet 2002; 39: 882–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith GD, Robinson C, Stewart AP. et al. Characterization of a recurrent in-frame UMOD indel mutation causing late-onset autosomal dominant end-stage renal failure. Clin J Am Soc Nephrol 2011; 6: 2766–2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gong K, Xia M, Wang Y. et al. Autosomal dominant tubulointerstitial kidney disease genotype and phenotype correlation in a Chinese cohort. Sci Rep 2021; 11: 3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bernascone I, Vavassori S, Di Pentima A. et al. Defective intracellular trafficking of uromodulin mutant isoforms. Traffic 2006; 7: 1567–1579 [DOI] [PubMed] [Google Scholar]
- 19. Piret SE, Olinger E, Reed AAC. et al. A mouse model for inherited renal fibrosis associated with endoplasmic reticulum stress. Dis Model Mech 2017; 10: 773–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson BG, Dang LT, Marsh G. et al. Uromodulin p.Cys147Trp mutation drives kidney disease by activating ER stress and apoptosis. J Clin Invest 2017; 127: 3954–3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schaeffer C, Cattaneo A, Trudu M. et al. Urinary secretion and extracellular aggregation of mutant uromodulin isoforms. Kidney Int 2012; 81: 769–778 [DOI] [PubMed] [Google Scholar]
- 22. Schaeffer C, Merella S, Pasqualetto E. et al. Mutant uromodulin expression leads to altered homeostasis of the endoplasmic reticulum and activates the unfolded protein response. PLoS One 2017; 12: e0175970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trudu M, Schaeffer C, Riba M. et al. Early involvement of cellular stress and inflammatory signals in the pathogenesis of tubulointerstitial kidney disease due to UMOD mutations. Sci Rep 2017; 7: 7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kemter E, Fröhlich T, Arnold GJ. et al. Mitochondrial dysregulation secondary to endoplasmic reticulum stress in autosomal dominant tubulointerstitial kidney disease–UMOD (ADTKD-UMOD). Sci Rep 2017; 7: 42970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Labriola L, Olinger E, Belge H. et al. Paradoxical response to furosemide in uromodulin-associated kidney disease. Nephrol Dial Transplant 2015; 30: 330–335 [DOI] [PubMed] [Google Scholar]
- 26. Ma L, Liu Y, Landry NK. et al. Point mutation in D8C domain of Tamm-Horsfall protein/uromodulin in transgenic mice causes progressive renal damage and hyperuricemia. PLoS One 2017; 12: e0186769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chun J, Wang M, Wilkins MS. et al. Autosomal dominant tubulointerstitial kidney disease-uromodulin misclassified as focal segmental glomerulosclerosis or hereditary glomerular disease. Kidney Int Rep 2020; 5: 519–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cormican S, Connaughton DM, Kennedy C. et al. Autosomal dominant tubulointerstitial kidney disease (ADTKD) in Ireland. Ren Fail 2019; 41: 832–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clissold RL, Hamilton AJ, Hattersley AT. et al. HNF1B-associated renal and extra-renal disease-an expanding clinical spectrum. Nat Rev Nephrol 2015; 11: 102–112 [DOI] [PubMed] [Google Scholar]
- 30. Dubois-Laforgue D, Cornu E, Saint-Martin C. et al. Diabetes, associated clinical spectrum, long-term prognosis, and genotype/phenotype correlations in 201 adult patients with hepatocyte nuclear factor 1B (HNF1B) molecular defects. Diabetes Care 2017; 40: 1436–1443 [DOI] [PubMed] [Google Scholar]
- 31. Okorn C, Goertz A, Vester U. et al. HNF1B nephropathy has a slow-progressive phenotype in childhood-with the exception of very early onset cases: results of the German Multicenter HNF1B Childhood Registry. Pediatr Nephrol 2019; 34: 1065–1075 [DOI] [PubMed] [Google Scholar]
- 32. Zivná M, Hůlková H, Matignon M. et al. Dominant renin gene mutations associated with early-onset hyperuricemia, anemia, and chronic kidney failure. Am J Hum Genet 2009; 85: 204–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bollée G, Dahan K, Flamant M. et al. Phenotype and outcome in hereditary tubulointerstitial nephritis secondary to UMOD mutations. Clin J Am Soc Nephrol 2011; 6: 2429–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bolar NA, Golzio C, Živná M. et al. Heterozygous loss-of-function SEC61A1 mutations cause autosomal-dominant tubulo-interstitial and glomerulocystic kidney disease with anemia. Am J Hum Genet 2016; 99: 174–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moskowitz JL, Piret SE, Lhotta K. et al. Association between genotype and phenotype in uromodulin-associated kidney disease. Clin J Am Soc Nephrol 2013; 8: 1349–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Edwards N, Olinger E, Adam J. et al. A novel homozygous UMOD mutation reveals gene dosage effects on uromodulin processing and urinary excretion. Nephrol Dial Transplant 2017; 32: 1994–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rezende-Lima W, Parreira KS, García-González M. et al. Homozygosity for uromodulin disorders: FJHN and MCKD-type 2. Kidney Int 2004; 66: 558–563 [DOI] [PubMed] [Google Scholar]
- 38. Cormican S, Kennedy C, Connaughton DM. et al. Renal transplant outcomes in patients with autosomal dominant tubulointerstitial kidney disease. Clin Transplant 2020; 34: e13783. [DOI] [PubMed] [Google Scholar]
- 39. Kemter E, Sklenak S, Rathkolb B. et al. No amelioration of uromodulin maturation and trafficking defect by sodium 4-phenylbutyrate in vivo: studies in mouse models of uromodulin-associated kidney disease. J Biol Chem 2014; 289: 10715–10726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dvela-Levitt M, Kost-Alimova M, Emani M. et al. Small molecule targets TMED9 and promotes lysosomal degradation to reverse proteinopathy. Cell 2019; 178: 521–535.e23 [DOI] [PubMed] [Google Scholar]
- 41. Turro E, Astle WJ, Megy K. et al. Whole-genome sequencing of patients with rare diseases in a national health system. Nature 2020; 583: 96–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. RaDaR Registry. The Renal Association. https://renal.org/rare-renal/radar (7 August 2021, date last accessed)
- 43. Bleyer AJ, Wolf MT, Kidd KO. et al. Autosomal dominant tubulointerstitial kidney disease: more than just HNF1β. Pediatr Nephrol 2021; doi: 10.1007/s00467-021-05118-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Knaup KX, Hackenbeck T, Popp B. et al. Biallelic expression of mucin-1 in autosomal dominant tubulointerstitial kidney disease: implications for nongenetic disease recognition. J Am Soc Nephrol 2018; 29: 2298–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vylet’al P, Kidd K, Ainsworth HC. et al. Plasma mucin-1 (CA15-3) levels in autosomal dominant tubulointerstitial kidney disease due to MUC1 mutations. Am J Nephrol 2021; 52: 378–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wenzel A, Altmueller J, Ekici AB. et al. Single molecule real time sequencing in ADTKD-MUC1 allows complete assembly of the VNTR and exact positioning of causative mutations. Sci Rep 2018; 8: 4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kirby A, Gnirke A, Jaffe DB. et al. Mutations causing medullary cystic kidney disease type 1 lie in a large VNTR in MUC1 missed by massively parallel sequencing. Nat Genet 2013; 45: 299–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Blumenstiel B, DeFelice M, Birsoy O. et al. Development and validation of a mass spectrometry-based assay for the molecular diagnosis of mucin-1 kidney disease. J Mol Diagn 2016; 18: 566–571 [DOI] [PubMed] [Google Scholar]
- 49. Živná M, Kidd K, Přistoupilová A. et al. Noninvasive immunohistochemical diagnosis and novel MUC1 mutations causing autosomal dominant tubulointerstitial kidney disease. J Am Soc Nephrol 2018; 29: 2418–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yamamoto S, Kaimori JY, Yoshimura T. et al. Analysis of an ADTKD family with a novel frameshift mutation in MUC1 reveals characteristic features of mutant MUC1 protein. Nephrol Dial Transplant 2017; 32: 2010–2017 [DOI] [PubMed] [Google Scholar]
- 51. Schaeffer C, Olinger E.. Clinical and genetic spectra of kidney disease caused by REN mutations. Kidney Int 2020; 98: 1397–1400 [DOI] [PubMed] [Google Scholar]
- 52. Gribouval O, Gonzales M, Neuhaus T. et al. Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet 2005; 37: 964–968 [DOI] [PubMed] [Google Scholar]
- 53. Schaeffer C, Izzi C, Vettori A. et al. Autosomal dominant tubulointerstitial kidney disease with adult onset due to a novel renin mutation mapping in the mature protein. Sci Rep 2019; 9: 11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bleyer AJ, Zivná M, Hulková H. et al. Clinical and molecular characterization of a family with a dominant renin gene mutation and response to treatment with fludrocortisone. Clin Nephrol 2010; 74: 411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Faguer S, Chassaing N, Bandin F. et al. The HNF1B score is a simple tool to select patients for HNF1B gene analysis. Kidney Int 2014; 86: 1007–1015 [DOI] [PubMed] [Google Scholar]
- 56. Cornec-Le Gall E, Olson RJ, Besse W. et al. Monoallelic mutations to DNAJB11 cause atypical autosomal-dominant polycystic kidney disease. Am J Hum Genet 2018; 102: 832–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jordan P, Arrondel C, Bessières B. et al. Bi-allelic pathogenic variations in DNAJB11 cause Ivemark II syndrome, a renal-hepatic-pancreatic dysplasia. Kidney Int 2021; 99: 405–409 [DOI] [PubMed] [Google Scholar]
- 58. Connor TM, Hoer S, Mallett A. et al. Mutations in mitochondrial DNA causing tubulointerstitial kidney disease. PLoS Genet 2017; 13: e1006620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lorenz R, Ahting U, Betzler C. et al. Homoplasmy of the mitochondrial DNA mutation m.616T>C leads to mitochondrial tubulointerstitial kidney disease and encephalopathia. Nephron 2020; 144: 156–160 [DOI] [PubMed] [Google Scholar]