Abstract
Background
To date, research on adverse drug reactions (ADRs) has focused on secondary care, and there is a paucity of studies that have prospectively examined ADRs affecting older adults in general practice.
Aim
To examine the cumulative incidence and severity of ADRs and associated patient characteristics in a sample of community-dwelling older adults.
Design and setting
Prospective cohort study of older adults (aged ≥70 years, N = 592) recruited from 15 general practices in the Republic of Ireland.
Method
Manual review of the participant’s general practice electronic medical record, linked to the national dispensed prescription medicine database, and a detailed, self-reported patient postal questionnaire. The primary outcomes were ADR occurrence and severity over a 6-year period (2010–2016). Unadjusted and adjusted logistic regression models examined potential associations between patient characteristics and ADR occurrence.
Results
A total of 211 ADRs were recorded for 159 participants, resulting in a cumulative incidence of 26.9% over 6 years. The majority of ADRs detected were mild (89.1%), with the remainder classified as moderate (10.9%). Eight moderate ADRs, representing 34.8% of moderate ADRs and 3.8% of all ADRs, required an emergency hospital admission. ADRs were independently associated with female sex (adjusted odds ratio [OR] 1.83, 95% confidence interval [CI] = 1.17 to 2.85; P = 0.008), polypharmacy (5–9 drug classes) (adjusted OR 1.81, 95% CI = 1.17 to 2.82; P = 0.008), and major polypharmacy (≥10 drug classes) (adjusted OR = 3.33, 95% CI = 1.62 to 6.85; P = 0.001).
Conclusion
This prospective cohort study of ADRs in general practice shows that over one-quarter of older adults experienced an ADR over a 6-year period. Polypharmacy is independently associated with ADR risk in general practice and older adults on ≥10 drug classes should be prioritised for regular medication review.
Keywords: adverse drug reaction, drug-related side effects and adverse reactions, electronic health records, general practice, older adults, polypharmacy
INTRODUCTION
As older people are living longer, often with multimorbidity, caring for this population is becoming increasingly complex, and much of this care occurs in primary care.1,2 For the GP, estimating the benefits and harms of a medication in an older person is particularly challenging as comorbidities, concurrent medications, pharmacokinetics, and pharmacodynamics can all impact the clinical outcome.3,4 The prevalence of polypharmacy, defined as ≥5 regular prescribed medications, is increasing, particularly among older people.5,6 Polypharmacy is the primary risk factor for adverse drug reactions (ADRs), one type of medication-related harm.5–7 The World Health Organization (WHO) defines an ADR as any noxious, unintended, and undesired effect of a drug, excluding therapeutic failures, intentional and accidental poisoning, and drug abuse.8
Despite limited research to date, ADRs reported incidence in primary care ranges from 6%–80%, reflecting variation in study design, populations, and measurement periods utilised.9 Older people are especially vulnerable to ADRs and related adverse outcomes such as emergency admission, drug-related morbidity, and mortality.4,10–13 ADRs are the cause of nearly 10% of hospitalisations of older adults,14 and contribute considerable additional costs to healthcare systems.15,16 A retrospective population cohort study found that ADRs accounted for 9.5% of all direct healthcare costs,17 and ADR-related hospitalisations have been estimated to cost the NHS £466 million per annum.13
ADRs are heterogeneous by nature, and developing methods to identify those at high risk has proved challenging.18 To date, research has focused on secondary care, and there is a paucity of studies that have prospectively examined ADRs in older adults in general practice. In recent systematic reviews of ADRs in primary care (n = 33 studies), only two included studies were prospective cohort studies, neither of which were conducted in general practice nor examined older adults specifically.19–21 Furthermore, neither examined ADR prevalence beyond 3 months.20,21 The majority of general practice ADR studies were cross-sectional, with approximately half conducted by screening administrative databases for ADRs recorded during routine care.19,22–24 Only two studies conducted a medication/medical record review in combination with a patient survey.25,26 This study aimed to examine the cumulative incidence and severity of ADRs and associated patient characteristics in older community-dwelling adults attending general practice.
How this fits in
| To the authors’ knowledge, no prospective studies have examined adverse drug reaction (ADR) occurrence among older adults attending general practice. In this study, ADRs were found to occur for approximately one in four older adults over a 6-year period. Cardiovascular, nervous system, and anti-infective drugs for systemic use were the most commonly implicated drug classes. Approximately one in four ADRs rated as moderate resulted in additional healthcare utilisation. Female sex, polypharmacy (5–9 drug classes), and major polypharmacy (≥10 drug classes) increased the likelihood of ADRs. |
METHOD
The Strengthening and Reporting of Observational Studies in Epidemiology (STROBE) guidelines were adhered to in the conduct and reporting of this study.27 A more detailed description of the methods is presented in Supplementary Appendix S1.
Study design and population
This is a 6-year (2010–2016) prospective cohort study of older patients (aged ≥70 years) recruited from 15 general practices in Leinster, Republic of Ireland.28,29 A proportionate stratified random sampling approach was used to recruit patients.30,31 Each general practice contributed a number of participants proportionate to the size of the practice. A random sample of patients from each of the 15 participating general practices was invited to take part in the study. The sample was calculated using proportionate stratified random sampling based on the overall sample required, the total number of eligible patients aged ≥70 years in all 15 practices, and assuming a 50% response rate.
Study inclusion criteria were:
aged ≥70 years on 1 January 2010;
in receipt of a valid General Medical Services card; and
in receipt of at least one drug.
Exclusion criteria were:
receiving palliative care;
cognitive impairment at the level that would impact ability to complete the outcome measure (defined as Mini-Mental State Examination ≤20);
significant hearing/speech/visual impairment;
currently experiencing a psychotic episode;
hospitalised long-term, in a nursing home, homeless, or in sheltered accommodation; and
recent (<1 month) bereavement.
Each participant’s GP applied the exclusion criteria at baseline to assess eligibility for participation in this cohort study.
Baseline data collection took place in 2010, with follow-up data collection conducted in 2012 and 2016. Data collection involved review of the participant’s general practice electronic medical record and a detailed, self-reported patient postal questionnaire. Participant consent was obtained to link their medical record and questionnaire data with their prescription dispensing information from the national Health Service Executive-Primary Care Reimbursement Service (HSE-PCRS) database.
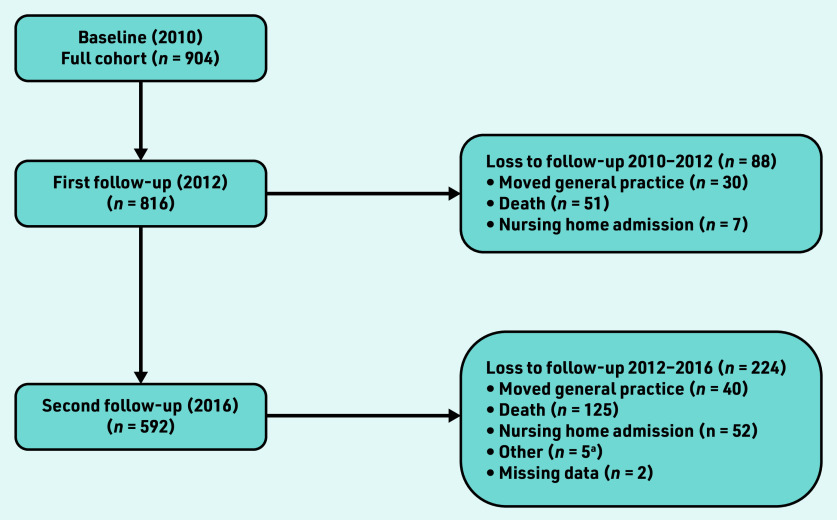
At baseline, 1487 patients met inclusion criteria and were invited to participate. Of these, 904 participated, representing a response rate of 61%. A total of 592 participants completed three waves of data collection. Losses to follow-up are presented in Figure 1. Descriptive statistics for those who completed study follow-up and those excluded are reported in Supplementary Tables S1–S3. Ethical approval for this study was obtained from the Royal College of Surgeons Ireland University of Medicine and Health Science’s Human Research Ethics Committee.
Figure 1.
Study flow diagram (2010–2016) describing losses to follow-up. aHospice, n = 2; long-stay inpatient, n = 3.
Primary outcomes
The primary outcomes were ADR occurrence and severity. ADRs were recorded by manual review of the general practice electronic medical record (see Supplementary Appendix S1). Detecting ADRs using this method involved reviewing each participant’s individual GP consultations and their hospital and other correspondence over the outcome measurement period. Manual chart review, albeit not without its limitations, is considered the gold standard method for the detection of ADRs.32,33 Drug classes were classified using the WHO Anatomical Therapeutic Code (ATC) classification.
ADR causality was assessed using the EU pharmacovigilance working group classification system.34 ADR severity was assessed using a previously validated severity classification system: mild, moderate, or severe (see Supplementary Appendix S1).35 ADRs were independently rated in terms of severity by an academic pharmacist and an academic GP. Disagreements were resolved by consensus.
Explanatory variables/patient characteristics
Patient characteristics were selected a priori and recorded at baseline from the general practice electronic medical record (age, sex, deprivation, and multimorbidity according to the Charlson comorbidity index); through linkage to the HSE-PCRS pharmacy claims database (number of drug classes and medication possession ratio [MPR]); and from the patient questionnaire (marital status, private health insurance, and vulnerability using the Vulnerable Elders Survey [VES]-13).36 The MPR is a measure of prescription refill and was calculated using the pharmacy claims linked data.37 The measure is calculated as the sum of days supplied for all medications (that is, the medication quantity supplied) divided by the time period. The average MPR rate for medication classes, categorised according to the WHO-ATC classification system, was determined for each patient.
Statistical analysis
Descriptive statistics were utilised to describe the study population and the primary outcomes. The cumulative incidence of ADRs was expressed as the proportion of participants who experienced at least one ADR over the study period (2010–2016). Differences in participant characteristics at baseline were explored. Unadjusted and adjusted logistic regression analyses were used to investigate the association between patient characteristics at baseline and the primary outcome of ADR occurrence. Unadjusted and adjusted odds ratios (ORs), 95% confidence intervals (CIs), and P-values were calculated and reported. Stata (version 15) was used for all analyses.
Sensitivity analysis
A sensitivity analysis was conducted by examining ADR occurrence among participants with at least 2 years of follow-up data (see Supplementary Appendix S1). As the follow-up duration varied for participants in the sensitivity sample, the cumulative incidence of ADRs over 6 years could not be determined. Baseline descriptive statistics are presented for those included in and those excluded from the sensitivity analysis in Supplementary Table S4. For this sample, the proportion of participants who experienced at least one ADR is reported, using the total number of participants in the sensitivity sample (n = 816) as the denominator. Both the unadjusted and adjusted logistic regression models controlled for length of follow-up (years).
RESULTS
Study population
Baseline descriptive statistics are presented for those with and without the primary outcome of ADR in Table 1 (n = 592), and for the full study sample (n = 904) in Supplementary Table S1. The median age was 75 years (interquartile range [IQR] 73–79) and 125 (21.1%) participants had multimorbidity (Charlson comorbidity index score ≥2) (see Supplementary Table S1). The median number of drug classes was 5 (IQR 3–7). Overall, 287 (48.5%) participants experienced polypharmacy (5–9 drug classes) and 53 (9.0%) experienced major polypharmacy (≥10 drug classes).
Table 1.
Descriptive characteristics at baseline for those with and without an ADR over 6 years
| Characteristic | Without ADR, n = 433, median (IQR) | With ADR, n = 159, median (IQR) | P-value |
|---|---|---|---|
| Age, years | 75 (73 to 79) | 76 (73 to 80) | 0.11 |
|
| |||
| Deprivation, patient | 1.08 (−0.64 to 2.88) | 1.75 (−0.45 to 2.88) | 0.18 |
|
| |||
| Number of drug classes | 5 (3 to 7) | 6 (4 to 8) | <0.001d |
|
| |||
| n (%) | n (%) | P-value | |
|
| |||
| Sex | |||
| Female | 212 (49.0) | 110 (69.2) | <0.001d |
| Male | 221 (51.0) | 49 (30.8) | |
|
| |||
| Private health insurance | 203 (46.9) | 73 (45.9) | 0.83 |
|
| |||
| Marital statusa | |||
| Married | 226 (52.2) | 66 (41.5) | 0.03c |
| Separated/divorced | 24 (5.5) | 6 (3.8) | |
| Widowed | 114 (26.3) | 61 (38.4) | |
| Never married/single | 68 (15.7) | 26 (16.4) | |
|
| |||
| Charlson comorbidity indexa | |||
| 0 | 237 (54.7) | 78 (49.1) | 0.27 |
| 1 | 103 (23.8) | 48 (30.2) | |
| ≥2 | 92 (21.2) | 33 (20.8) | |
|
| |||
| Medication adherence, MPR ≥80%b | 273 (63.0) | 101 (63.5) | 0.54 |
|
| |||
| VES ≥3 | 112 (25.9) | 60 (37.7) | 0.005c |
|
| |||
| Polypharmacy | |||
| 1–4 drug classes | 207 (47.8) | 45 (28.3) | <0.001d |
| 5–9 drug classes | 200 (46.2) | 87 (54.7) | |
| ≥10 drug classes | 26 (6.0) | 27 (17.0) | |
Missing data (n= 1).
Missing data (n= 35).
P<0.05.
P<0.001.
P-values obtained from Mann–Whitney U test (continuous variables with non-normal distribution) and χ2 tests of independence for categorical variables. ADR = adverse drug reaction. IQR = interquartile range. MPR = medication possession ratio. VES = Vulnerable Elders Scale.
Primary outcome: ADRs
A total of 211 ADRs were recorded in 159 participants, indicating a cumulative incidence of 26.9% over the 6-year period (2010–2016) (Table 1). Overall, 25 (4.2%) participants experienced two ADRs, seven (1.2%) experienced three ADRs, three (0.5%) experienced four ADRs, and one (0.2%) experienced five ADRs (data not shown). Cardiovascular, nervous system, and anti-infective drugs were most commonly implicated in ADRs (Table 2). Regarding ADR severity, 188 (89.1%) ADRs were classified as mild and 23 (10.9%) as moderate, with no ADRs categorised as severe (data not shown). A total of 10 moderate ADRs (4.7% of all ADRs) resulted in additional healthcare utilisation. Two ADRs resulted in an outpatient appointment and eight ADRs in emergency hospital admission. Thus, 34.8% of moderate ADRs (representing 3.8% of all ADRs) resulted in an emergency admission. No ADRs resulting in death were detected. Examples of the different types of ADRs experienced by degree of severity are presented in Box 1.
Table 2.
Drug classes implicated in ADRs according to the WHO-ATC classification system (n = 159 study participants)
| WHO-ATC class | ADRs, n | % all ADRs | |
|---|---|---|---|
| A | Alimentary tract and metabolism | 18 | 8.53 |
| A02 | Drugs for acid-related disorders | 10 | 4.74 |
| A07 | Antidiarrhoeal, intestinal anti-inflammatory/anti-infective agents | 1 | 0.47 |
| A10 | Drugs used in diabetes | 5 | 2.37 |
| A11 | Vitamins | 1 | 0.47 |
| A12 | Mineral supplements | 1 | 0.47 |
|
| |||
| B | Blood and blood forming organs | 4 | 1.90 |
| B01 | Antithrombotic agents | 3 | 1.42 |
| B03 | Antianaemic preparations | 1 | 0.47 |
|
| |||
| C | Cardiovascular system | 69 | 32.70 |
| C01 | Cardiac therapy | 5 | 2.37 |
| C02 | Antihypertensives | 2 | 0.95 |
| C03 | Diuretics | 13 | 6.16 |
| C07 | Beta blocking agents | 4 | 1.90 |
| C08 | Calcium channel blockers | 17 | 8.06 |
| C09 | Agents acting on the renin-angiotensin system | 19 | 9.00 |
| C10 | Lipid-modifying agents | 9 | 4.27 |
|
| |||
| G | Genito-urinary system and sex hormones | 10 | 4.74 |
| G04 | Urologicals | 10 | 4.74 |
|
| |||
| H | Systemic hormonal preparations, excluding sex hormones and insulins | 4 | 1.90 |
| H03 | Thyroid therapy | 3 | 1.42 |
| H05 | Calcium homeostasis | 1 | 0.47 |
|
| |||
| J | Anti-infectives for systemic use | 26 | 12.32 |
| J01 | Antibacterials for systemic use | 26 | 12.32 |
|
| |||
| L | Antineoplastic and immunomodulating agents | 1 | 0.47 |
| L04 | Immunosuppressants | 1 | 0.47 |
|
| |||
| M | Musculoskeletal system | 14 | 6.64 |
| M01 | Anti-inflammatory and antirheumatic products | 10 | 4.74 |
| M05 | Drugs for treatment of bone diseases | 4 | 1.90 |
|
| |||
| N | Nervous system | 61 | 28.91 |
| N01 | Anaesthetics | 4 | 1.90 |
| N02 | Analgesics | 28 | 13.27 |
| N03 | Antiepileptics | 7 | 3.32 |
| N04 | Anti-Parkinson’s drugs | 1 | 0.47 |
| N05 | Psycholeptics | 4 | 1.90 |
| N06 | Psychoanaleptics | 16 | 7.58 |
| N07 | Other nervous system drugs | 1 | 0.47 |
|
| |||
| R | Respiratory system | 4 | 1.90 |
| R03 | Drugs for obstructive airway diseases | 2 | 0.95 |
| R06 | Cough and cold preparations | 2 | 0.95 |
ADR = adverse drug reaction. WHO-ATC = World Health Organization Anatomical Therapeutic Code.
Box 1.
Examples of ADRs by severity
| ADR Severity | ADR details | WHO-ATC code(s) |
|---|---|---|
| Mild | Gastrointestinal upset; nausea; vomiting; constipation; and diarrhoea | A02, A10, B03, C03, C09, G04, H05, J01, M01, M05, N01, N02, N06 |
| Headaches | A02, C01, C08, N02, N03, R06 | |
| Dry mouth | C03, G04, N02, N06 | |
| Dizziness | A10, C02, C03, C07, C08, C09, N02, N06 | |
| Sedation | N02, N03, N06, N07, R06 | |
| Oedema | C03, C08, M01 | |
|
| ||
| Moderate | Gastrointestinal upset, resulting in hospitalisation | B01 |
| Confusion, hallucinations | N02 | |
| Hyponatraemia | N06 | |
| Dystonic reaction | N05 | |
| High INR, resulting in hospitalisation | B01 | |
ADR = adverse drug reaction. INR = international normalised ratio. WHO-ATC = World Health Organization Anatomical Therapeutic Code.
Associations between patient characteristics and ADRs
Unadjusted associations (Table 3) for ADRs were observed for female sex, marital status, VES-13 score, polypharmacy (5–9 drug classes), and major polypharmacy (≥10 drug classes). In the adjusted model, independent associations remained for female sex (OR 1.83, 95% CI = 1.17 to 2.85, P = 0.008), with a dose-response relationship observed for polypharmacy (5–9 drug classes) (OR 1.81, 95% CI = 1.17 to 2.82, P = 0.008), and major polypharmacy (≥10 drug classes) (OR 3.33, 95% CI = 1.62 to 6.85, P = 0.001).
Table 3.
Unadjusted (n= 592) and adjusted (n = 555) logistic regression for at least one ADR over 6 years (2010–2016)
| Characteristic | Unadjusted | Adjusted | ||
|---|---|---|---|---|
|
| ||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age | 1.04 (0.99 to 1.08) | 0.09 | 1.00 (0.95 to 1.05) | 0.87 |
|
| ||||
| Deprivation | 1.05 (0.98 to 1.13) | 0.17 | 1.07 (0.98 to 1.16) | 0.15 |
|
| ||||
| Sex, female | 2.34 (1.59 to 3.44) | <0.001a | 1.83 (1.17 to 2.85) | 0.008b |
|
| ||||
| Private health insurance | 0.96 (0.67 to 1.38) | 0.83 | 1.33 (0.86 to 2.07) | 0.20 |
|
| ||||
| Medication adherence, MPR ≥80% c | 0.88 (0.60 to 1.31) | 0.54 | 0.81 (0.53 to 1.22) | 0.31 |
|
| ||||
| Marital statusd | 0.03b | |||
| Separated/divorced | 0.86 (0.34 to 2.18) | 0.75 | 0.76 (0.29 to 1.99) | 0.57 |
| Widowed | 1.83 (1.21 to 2.77) | 0.004b | 1.36 (0.84 to 2.20) | 0.22 |
| Never married/single | 1.31 (0.77 to 2.22) | 0.32 | 1.21 (0.67 to 2.17) | 0.52 |
|
| ||||
| Charlson comorbiditye | 0.28 | |||
| 1 | 1.42 (0.92 to 2.17) | 0.11 | 1.22 (0.76 to 1.95) | 0.41 |
| ≥2 | 1.09 (0.68 to 1.75) | 0.72 | 0.97 (0.57 to 1.65) | 0.90 |
|
| ||||
| VES ≥3 | 1.74 (1.18 to 2.56) | 0.005b | 1.22 (0.77 to 1.96) | 0.40 |
|
| ||||
| Polypharmacyf | <0.001a | |||
| 5–9 drug classes | 2.00 (1.33 to 3.01) | 0.001b | 1.81 (1.17 to 2.82) | 0.008b |
| ≥10 drug classes | 4.78 (2.55 to 8.95) | <0.001a | 3.33 (1.62 to 6.85) | 0.001b |
P<0.001.
P<0.05.
Missing data (n = 35 cases).
Referent married, missing data (n = 1 case).
Referent 0, missing data (n = 1 case).
Referent 0–4 drug classes. ADR = adverse drug reaction. MPR = medication possession ratio. OR = odds ratio. VES = Vulnerable Elders Scale.
Sensitivity analysis
A sensitivity analysis examined ADR occurrence for participants with at least 2 years of follow-up data (n = 816). Baseline descriptive statistics are presented for those with and without the primary outcome of ADR in Supplementary Table S5. A total of 259 ADRs relating to 199 participants were included; thus, 24.4% of participants experienced at least one ADR. In the adjusted model, female sex (OR 1.68, 95% CI = 1.14 to 2.47, P = 0.009), deprivation (OR 1.09, 95% CI = 1.01 to 1.17, P = 0.03), polypharmacy (5–9 drug classes: OR 1.87; 95% CI = 1.24 to 2.82, P = 0.003) and major polypharmacy (≥10 drug classes: OR 2.72, 95% CI = 1.50 to 4.93, P = 0.001) were associated with an increased likelihood of experiencing an ADR (see SupplementaryTable S6).
DISCUSSION
Summary
This prospective cohort study over 6 years shows that ADRs are common in older people attending general practice, with approximately one in four (26.9%) experiencing at least one ADR over the period. While the majority of ADRs are mild (89.1%), approximately one-third (34.8%) of moderate ADRs result in an emergency admission. In total, 10 ADRs (4.7%) resulted in additional healthcare utilisation (outpatient appointment or hospitalisation). Drug classes most commonly implicated include: cardiovascular system drugs (for example, amlodipine and furosemide), nervous system drugs (for example, citalopram, mirtazapine, and tramadol), and anti-infectives for systemic use (for example, amoxicillin and co-amoxiclav). ADRs were independently associated with female sex, polypharmacy (5–9 drug classes), and major polypharmacy (≥10 drug classes), while the likelihood for ADR increased more than threefold for those with major polypharmacy.
Strengths and limitations
Strengths of this study include the manual review of general practice electronic medical records, considered the gold standard for ADR detection.32 Previous reviews of ADRs in general practice have reported a small number of studies, with inconsistent methodology.9,19 This study extends the evidence base by reporting ADR cumulative incidence and severity over 6 years. Furthermore, the data collected allowed for the inclusion of several confounding variables (for example, multimorbidity, medication adherence, and functional status) in the statistical analysis. The robustness of the study findings is supported by the sensitivity analysis. In terms of study limitations, ADRs (mild, moderate, or severe) could have accounted for admission to a care home and/or death among this older cohort. Over the course of 6 years of follow-up, death occurred in 176 (19.5%) participants, while 59 (6.5%) were admitted to a care home. Caution is required in interpreting overall incidence of ADRs for this reason. A recent retrospective analysis of VigiBase, the WHO’s pharmacovigilance database, investigated fatal adult ADRs (2010–2019) reported by physicians.38 Of >3.2 million included ADRs, just over 1% were fatal, with males, patients aged >65 years, and those taking antineoplastic/immunomodulating drugs at higher risk. There was also significant variation in fatal ADR rates across different countries and continents. Future studies need to obtain ADR data when patients die or transition to care home settings. Another limitation to this study is that it was not possible to classify ADRs by type or preventability, nor was it possible to look at ADR annual incidence. Lastly, recruitment is limited to a regional health area and potentially limits the generalisability of the study findings.
Comparison with existing literature
Understanding of the impact of ADRs in general practice has been limited by the lack of research conducted to date. A systematic review by Insani et al of 33 primary care studies reported 10 general practice studies (nine cross-sectional and one retrospective cohort study).19 Only two general practice studies used medical record and patient survey methodology, whereas five studies screened administrative databases for ADRs recorded during routine care. Furthermore, only two studies examined ADRs in older adults specifically.26,39 Two prospective studies have been conducted in primary care internal medicine settings; however, neither examined ADR occurrence beyond 3 months, nor examined older adults specifically.20,21 To the best of the authors’ knowledge, this is the first prospective general practice study examining ADRs among older adults.
The systematic review by Insani et al (total population >1.5 million participants) further reported a pooled ADR prevalence rate of 8.32%.19 Notably, this pooled estimate is predicated mostly on cross-sectional studies and also includes paediatric populations. Subgroup analysis found prevalence estimates varied according to age, ADR detection method, setting, and sample size. In the present study, the cumulative incidence of ADRs over 6 years (26.9%) is congruent with the pooled prevalence of ADRs among those aged ≥65 years (28.43%) identified in their subgroup analysis.19
The majority of ADRs detected were rated as mild (89.1%), with the remainder (10.9%) rated as moderate. Several primary care studies report the proportion of mild ADRs to range from 2.2%–45.9% and moderate ADRs to range from 42.2%–96.4%.40–42 Ten ADRs (4.7%) resulted in either an outpatient appointment or hospital admission, which is comparable to the 1.3%–9.1% of ADRs reported to require an emergency department visit and/or hospital admission.19
The most commonly identified drug classes (diuretics, calcium channel blockers, angiotensin-converting enzyme inhibitors, opioid analgesics, and antidepressants) are consistent with higher-risk drug classes reported previously and represent those most commonly prescribed in primary care.9,19,20,22,42–45 Two general practice studies found that cardiovascular drugs were implicated in approximately 18% of ADRs.22,42 In the present study, cardiovascular drugs were implicated in 32.7% of all ADRs, which is comparable to rates identified in primary care internal medicine settings (23.7%–31.0%).20,44 A sizeable proportion of ADRs (28.9%) were attributed to nervous system drugs. The systematic review by Insani et al reported a median ADR rate of 13.4% for nervous system drugs across eight studies (range: 3.5%–39.6%).19
Female sex and polypharmacy (both 5–9 drug classes and ≥10 drug classes) are associated with experiencing an ADR in multivariable analyses, which aligns with findings consistently reported in the literature.4,20,23,46–49 Polypharmacy may serve as a modifiable target for reducing ADR risk in the context of deprescribing initiatives for potentially inappropriate medications and those no longer clinically indicated.
Implications for research and practice
The findings may inform future initiatives, including structured medication reviews (SMRs) in general practice, by highlighting several intervention targets. Cardiovascular, nervous system, and anti-infective drugs were identified as the higher-risk drug classes and represent the most commonly prescribed medications in general practice.45 Through a shared decision-making approach, GPs and their patients need to balance the benefits and risks of these agents. The potential for ADRs, which are often difficult to diagnose in older adults,4,50 should form part of every differential diagnosis for older patients, especially those who have recently started a new medication or experienced a dose adjustment. ADRs can be difficult to identify in medically complex older adults as they often present as non-specific symptoms such as delirium, drowsiness, falls, fatigue, and constipation, all of which have several potential causes.4,47 ADR symptoms may be mistaken as the onset of a new medical problem or related to an existing diagnosis, rather than being secondary to medication.47 The failure to recognise an ADR may result in a prescribing cascade, where a new medication is initiated to treat the ADR, thereby exposing the older adult to additional risk.51,52
The findings also indicate that the greater the medication burden the greater the likelihood for medication-related harm. Those prescribed ≥10 drug classes were over three times more likely to experience an ADR, and therefore may receive the optimum benefit from SMRs. Existing guidance, such as the Scottish Polypharmacy Guidance, has emphasised the prioritisation of SMRs for those prescribed high-risk drug classes and ≥10 medications.51 The findings provide additional evidence to support such guidance where case finding indicators have previously been developed by clinical consensus. From a policy perspective, SMRs have been identified as a strategic intervention to address the estimated 10% overprescribing of medications in primary care.52,53 This study shows that addressing polypharmacy is a critical component in reducing medication burden and lessening the likelihood of ADRs for vulnerable patients.
In conclusion, ADRs are common among older adults in general practice, with females and those with major polypharmacy at highest risk. While the majority of ADRs identified were mild, a considerable proportion of moderate ADRs resulted in additional healthcare utilisation. ADRs can be difficult to identify in medically complex older adults as they often present as non-specific symptoms. GPs are well placed to detect the occurrence of ADRs from drugs prescribed in primary care as well as in other care settings.54 Deprescribing of ineffective medications and those no longer clinically indicated is one approach to reducing the risk of ADRs in older patients.
Acknowledgments
The authors would like to thank Caitriona Cahir and Norah Murphy for their work in baseline and follow-up data collection, respectively.
Funding
This work was supported by the Health Research Board (HRB) of Ireland through the HRB Centre for Primary Care Research (grant number: HRC/2007/1) and the HRB Emerging Clinician Scientist Award (grant number: ECSA/2020/002). The funders had no role in study design, data collection and analysis, manuscript preparation, or the decision to submit for publication.
Ethical approval
Ethical approval was received from the Royal College of Surgeons Ireland University of Medicine and Health Science’s Human Research Ethics Committee (reference numbers: REC462b; REC462bb; REC462bbb; REC1277; REC1277b; and REC127bbb).
Data
The dataset analysed during the current study is not publicly available in accordance with the consent provided by participants. The participants of this study did not give written consent for their data to be shared publicly. Data may be made available following a reasonable request and ethical approval.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Cassell A, Edwards D, Harshfield A, et al. The epidemiology of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract. 2018. DOI: . [DOI] [PMC free article] [PubMed]
- 2.Violan C, Foguet-Boreu Q, Flores-Mateo G, et al. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS One. 2014;9(7):e102149. doi: 10.1371/journal.pone.0102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peeters LEJ, Kester MP, Feyz L, et al. Pharmacokinetic and pharmacodynamic considerations in the treatment of the elderly patient with hypertension. Expert Opin Drug Metab Toxicol. 2019;15(4):287–297. doi: 10.1080/17425255.2019.1588249. [DOI] [PubMed] [Google Scholar]
- 4.Lavan AH, Gallagher P. Predicting risk of adverse drug reactions in older adults. Ther Adv Drug Saf. 2016;7(1):11–22. doi: 10.1177/2042098615615472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moriarty F, Hardy C, Bennett K, et al. Trends and interaction of polypharmacy and potentially inappropriate prescribing in primary care over 15 years in Ireland: a repeated cross-sectional study. BMJ Open. 2015;5(9):e008656. doi: 10.1136/bmjopen-2015-008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. doi: 10.1186/s12877-017-0621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saedder EA, Lisby M, Nielsen LP, et al. Number of drugs most frequently found to be independent risk factors for serious adverse reactions: a systematic literature review. Br J Clin Pharmacol. 2015;80(4):808–817. doi: 10.1111/bcp.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards IR, Ar onson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356(9237):1255–1259. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 9.Khalil H, Huang C. Adverse drug reactions in primary care: a scoping review. BMC Health Serv Res. 2020;20(1):5. doi: 10.1186/s12913-019-4651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother. 2008;42(7):1017–1025. doi: 10.1345/aph.1L037. [DOI] [PubMed] [Google Scholar]
- 11.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 12.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279(15):1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 13.Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oscanoa TJ, Lizaraso F, Carvajal A. Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur J Clin Pharm. 2017;73(6):759–770. doi: 10.1007/s00228-017-2225-3. [DOI] [PubMed] [Google Scholar]
- 15.Batel Marques F, Penedones A, Mendes D, Alves C. A systematic review of observational studies evaluating costs of adverse drug reactions. Clinicoecon Outcomes Res. 2016;8:413–426. doi: 10.2147/CEOR.S115689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sultana J, Cutroneo P, Trifirò G. Clinical and economic burden of adverse drug reactions. J Pharmacol Pharmacother. 2013;4(Suppl 1):S73–S77. doi: 10.4103/0976-500X.120957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gyllensten H, Hakkarainen KM, Hägg S, et al. Economic impact of adverse drug events – a retrospective population-based cohort study of 4970 adults. PLoS One. 2014;9(3):e92061. doi: 10.1371/journal.pone.0092061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevenson JM, Williams JL, Burnham TG, et al. Predicting adverse drug reactions in older adults; a systematic review of the risk prediction models. Clin Interv Aging. 2014;9:1581–1593. doi: 10.2147/CIA.S65475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Insani WN, Whittlesea C, Alwafi H, et al. Prevalence of adverse drug reactions in the primary care setting: a systematic review and meta-analysis. PLoS One. 2021;16(5):e0252161. doi: 10.1371/journal.pone.0252161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care. N Engl J Med. 2003;348(16):1556–1564. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- 21.Trinkley KE, Weed HG, Beatty SJ, et al. Identification and characterization of adverse drug events in primary care. Am J Med Qual. 2017;32(5):518–525. doi: 10.1177/1062860616665695. [DOI] [PubMed] [Google Scholar]
- 22.Veehof LJG, Stewart RE, Meyboom-de Jong B, Haaijer-Ruskamp FM. Adverse drug reactions and polypharmacy in the elderly in general practice. Eur J Clin Pharmacol. 1999;55(7):533–536. doi: 10.1007/s002280050669. [DOI] [PubMed] [Google Scholar]
- 23.Calderon-Larranaga A, Poblador-Plou B, Gonzalez-Rubio F, et al. Multimorbidity, polypharmacy, referrals, and adverse drug events: are we doing things well? Br J Gen Pract. DOI: . [DOI] [PMC free article] [PubMed]
- 24.Eguale T, Buckeridge DL, Verma A, et al. Association of off-label drug use and adverse drug events in an adult population. JAMA Intern Med. 2016;176(1):55–63. doi: 10.1001/jamainternmed.2015.6058. [DOI] [PubMed] [Google Scholar]
- 25.Benson H, Lucas C, Kmet W, et al. Pharmacists in general practice: a focus on drug-related problems. Int J Clin Pharm. 2018;40(3):566–572. doi: 10.1007/s11096-018-0617-9. [DOI] [PubMed] [Google Scholar]
- 26.Cahir C, Wallace E, Cummins A, et al. Identifying adverse drug events in older community-dwelling patients. Ann Fam Med. 2019;17(2):133–140. doi: 10.1370/afm.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cahir C, Moriarty F, Teljeur C, et al. Potentially inappropriate prescribing and vulnerability and hospitalization in older community-dwelling patients. Ann Pharmacother. 2014;48(12):1546–1554. doi: 10.1177/1060028014552821. [DOI] [PubMed] [Google Scholar]
- 29.Wallace E, McDowell R, Bennett K, et al. Impact of potentially inappropriate prescribing on adverse drug events, health related quality of life and emergency hospital attendance in older people attending general practice: a prospective cohort study. J Gerontol A Biol Sci Med Sci. 2017;72(2):271–277. doi: 10.1093/gerona/glw140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cahir C, Bennett K, Teljeur C, Fahey T. Potentially inappropriate prescribing and adverse health outcomes in community dwelling older patients. Br J Clin Pharmacol. 2014;77(1):201–210. doi: 10.1111/bcp.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallace E, McDowell R, Bennett K, et al. External validation of the Vulnerable Elder’s Survey for predicting mortality and emergency admission in older community-dwelling people: a prospective cohort study. BMC Geriatr. 2017;17(1):69. doi: 10.1186/s12877-017-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murff HJ, Patel VL, Hripcsak G, Bates DW. Detecting adverse events for patient safety research: a review of current methodologies. J Biomed Inform. 2003;36:1–2. 131–143. doi: 10.1016/j.jbi.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Shojania KG, Marang-van de Mheen PJ. Identifying adverse events: reflections on an imperfect gold standard after 20 years of patient safety research. BMJ Qual Saf. 2020;29(4):265–270. doi: 10.1136/bmjqs-2019-009731. [DOI] [PubMed] [Google Scholar]
- 34.Meyboom RHB, Royer RJ. Causality classification at pharmacovigilance centres in the european community. Pharmacoepidem Drug Safe. 1992;1(2):87–97. [Google Scholar]
- 35.Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274(1):29–34. [PubMed] [Google Scholar]
- 36.Saliba D, Elliott M, Rubenstein LZ, et al. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49(12):1691–1699. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 37.Steiner JF, Prochazla The assessment of refill compliance using pharmacy records: methods, validity and applications. J Clin Epidemiol. 1997;50(1):105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 38.Montastruc J-L, Lafaurie M, de Canecaude C, et al. Fatal adverse drug reactions: a worldwide perspective in the World Health Organization pharmacovigilance database. Br J Clin Pharmacol. 2021;87(11):4334–4340. doi: 10.1111/bcp.14851. [DOI] [PubMed] [Google Scholar]
- 39.Veehof LJ, Stewart RE, Meyboom-de Jong B, Haaijer-Ruskamp FM. Adverse drug reactions and polypharmacy in the elderly in general practice. Eur J Clin Pharmacol. 1999;55(7):533–536. doi: 10.1007/s002280050669. [DOI] [PubMed] [Google Scholar]
- 40.Cooper JW. Probable adverse drug reactions in a rural geriatric nursing home population: a four-year study. J Am Geriatr Soc. 1996;44(2):194–197. doi: 10.1111/j.1532-5415.1996.tb02439.x. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen JK, Fouts MM, Kotabe SE, Lo E. Polypharmacy as a risk factor for adverse drug reactions in geriatric nursing home residents. Am J Geriatr Pharmacother. 2006;4(1):36–41. doi: 10.1016/j.amjopharm.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Miller GC, Valenti L, Britt H, et al. Drugs causing adverse events in patients aged 45 or older: a randomised survey of Australian general practice patients. BMJ Open. 2013;3(10):e003701. doi: 10.1136/bmjopen-2013-003701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hakkarainen KM, Gyllensten H, Jönsson AK, et al. Prevalence, nature and potential preventability of adverse drug events — a population-based medical record study of 4970 adults. Br J Clin Pharmacol. 2014;78(1):170–183. doi: 10.1111/bcp.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneider JK, Mion LC, Frengley JD. Adverse drug reactions in an elderly outpatient population. Am J Hosp Pharm. 1992;49(1):90–96. [PubMed] [Google Scholar]
- 45.Audi S, Burrage DR, Lonsdale DO, et al. The ‘top 100’ drugs and classes in England: an updated ‘starter formulary’ for trainee prescribers. Br J Clin Pharmacol. 2018;84(11):2562–2571. doi: 10.1111/bcp.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bourgeois FT, Shannon MW, Valim C, Mandl KD. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf. 2010;19(9):901–910. doi: 10.1002/pds.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zopf Y, Rabe C, Neubert A, et al. Risk factors associated with adverse drug reactions following hospital admission: a prospective analysis of 907 patients in two German university hospitals. Drug Saf. 2008;31(9):789–798. doi: 10.2165/00002018-200831090-00007. [DOI] [PubMed] [Google Scholar]
- 48.Saheb Sharif-Askari F, Syed Sulaiman SA, Saheb Sharif-Askari N, Al Sayed Hussain A. Development of an adverse drug reaction risk assessment score among hospitalized patients with chronic kidney disease. PLoS One. 2014;9(4):e95991. doi: 10.1371/journal.pone.0095991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rademaker M. Do women have more adverse drug reactions? Am J Clin Dermatol. 2001;2(6):349–351. doi: 10.2165/00128071-200102060-00001. [DOI] [PubMed] [Google Scholar]
- 50.Petrovic M, van der Cammen T, Onder G. Adverse drug reactions in older people: detection and prevention. Drugs Aging. 2012;29(6):453–462. doi: 10.2165/11631760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 51.Scottish Government Polypharmacy Model of Care Group Polypharmacy Guidance: Realistic Prescribing. (3rd Edition) 2018 2018. https://www.therapeutics.scot.nhs.uk/wp-content/uploads/2018/09/Polypharmacy-Guidance-2018.pdf (accessed 13 Dec 2022). [Google Scholar]
- 52.Department of Health and Social Care Good for you, good for us, good for everybody A plan to reduce overprescribing to make patient care better and safer, support the NHS, and reduce cardbon emissions. 2021 https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1019475/good-for-you-good-for-us-good-for-everybody.pdf (accessed 13 Dec 2022). [Google Scholar]
- 53.Stewart D, Madden M, Davies P, et al. Structured medication reviews: origins, implementation, evidence, and prospects. Br J Gen Pract. 2021. DOI: . [DOI] [PMC free article] [PubMed]
- 54.Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83(3):457–502. doi: 10.1111/j.1468-0009.2005.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]