Abstract
OBJECTIVES:
Chest pain is a common reason for referral to pediatric cardiologists and often leads to an extensive cardiac evaluation. The objective of this study is to describe current management practices in the assessment of pediatric chest pain and to determine whether a standardized care approach could reduce unnecessary testing.
PATIENTS AND METHODS:
We reviewed all patients, aged 7 to 21 years, presenting to our outpatient pediatric cardiology division in 2009 for evaluation of chest pain. Demographics, clinical characteristics, patient outcomes, and resource use were analyzed.
RESULTS:
Testing included electrocardiography (ECG) in all 406 patients, echocardiography in 175 (43%), exercise stress testing in 114 (28%), event monitoring in 40 (10%), and Holter monitoring in 30 (7%). A total of 44 (11%) patients had a clinically significant medical or family history, an abnormal cardiac examination, and/or an abnormal ECG. Exertional chest pain was present in 150 (37%) patients. In the entire cohort, a cardiac etiology for chest pain was found in only 5 of 406 (1.2%) patients. Two patients had pericarditits, and 3 had arrhythmias. We developed an algorithm using pertinent history, physical examination, and ECG findings to suggest when additional testing is indicated. Applying the algorithm to this cohort could lead to an ∼20% reduction in echocardiogram and outpatient rhythm monitor use and elimination of exercise stress testing while still capturing all cardiac diagnoses.
CONCLUSIONS:
Evaluation of pediatric chest pain is often extensive and rarely yields a cardiac etiology. Practice variation and unnecessary resource use remain concerns. Targeted testing can reduce resource use and lead to more cost-effective care.
Keywords: chest pain, congenital heart disease, resource utilization, SCAMP
WHAT'S KNOWN ON THIS SUBJECT:
Chest pain is common in children and is a frequent reason for referral to pediatric cardiologists. Despite the benign nature of the vast majority of pediatric chest pain, extensive and costly cardiac evaluation is common in these patients.
WHAT THIS STUDY ADDS:
Described here is an approach to pediatric chest pain that will reduce unnecessary resource use while maintaining high-quality care.
Chest pain is a common presenting complaint to pediatricians, pediatric cardiologists, and pediatric emergency departments. The overwhelming majority of chest pain in otherwise healthy children has a noncardiac etiology. 1 – 4 In contrast to adults, in whom chest pain often signals a significant cardiac problem, the most common etiologies in children are benign and include musculoskeletal, gastrointestinal, pulmonary, idiopathic, and psychogenic causes. 5 – 11 Despite the low prevalence of serious cardiac pathology in children, extensive and costly cardiac evaluation is common. Identifying current management practices and factors leading to increased resource use is the first step toward delivering more cost-effective care.
Quality-improvement studies frequently have shown that reducing practice variation leads to better patient outcomes, decreased patient care costs, and improved efficiency. 12 Few studies have evaluated practice variation and resource use in the management of pediatric chest pain. 13,14 A standardized approach to pediatric chest pain currently is being implemented in our pediatric cardiology department as part of a broader quality-improvement initiative that we have named Standardized Clinical Assessment and Management Plans (SCAMP). 15,16 The goals of the SCAMP initiative, including the chest-pain SCAMP, are to decrease practice variation, improve patient care, and reduce unnecessary resource use. The objectives of this study are to describe the current practice trends in the evaluation of chest pain by pediatric cardiologists and to evaluate whether a mechanism to standardize care will lead to a reduction in unnecessary resource use while still capturing all important cardiac etiologies of chest pain.
PATIENTS AND METHODS
Patient Selection
We reviewed the records of all patients, aged 7 to 21 years, presenting to our outpatient division of pediatric cardiology in 2009 for initial evaluation of chest pain. We identified patients on the basis of the International Classification of Diseases, Ninth Revision billing codes for chest pain. Patients were excluded if they had a known history of heart disease or had been previously evaluated by a pediatric cardiologist for chest pain. The institutional review board for clinical research at Children's Hospital Boston approved the use of patient medical records for this retrospective review.
Demographic and clinical characteristics were collected for each patient. Past medical history, family history, and presenting symptoms, including chest-pain characteristics and associated symptoms, were retrospectively collected from the cardiologist's clinic note produced at the time of the visit. We specifically categorized patients as having exertional versus nonexertional chest pain and recorded the presence of associated symptoms, including syncope, palpitations, and dyspnea. Past medical history was considered positive if any diagnoses that could lead to increased risk of cardiac chest pain were present (ie, systemic inflammatory diseases, malignancy, thrombophilia, myopathies). Family history was considered positive if any of the following were present in a first-degree relative: sudden or unexplained death; aborted sudden death; cardiomyopathy; severe familial hyperlipidemia; or pulmonary hypertension. Abnormalities on physical examination that were considered pertinent positives included pathologic murmur, gallop, pericardial rub, abnormal second heart sound, distant heart sounds, hepatomegaly, decreased fermoral or peripheral pulses, peripheral edema, painful or swollen extremities, tachypnea, and fever (oral temperature > 38.4°C).
Test Interpretation
Electrocardiogram (ECG) interpretation was based on documented findings in the cardiologist's clinic note. Ventricular hypertrophy, atrial enlargement, pathologic ST-segment or T-wave changes, high-grade atrioventricular block, ventricular or atrial ectopy, axis deviation, and other miscellaneous findings (eg, ventricular preexcitation) were classified as abnormalities. Incomplete right-bundle branch block and early repolarization were considered to be normal variants.
Echocardiogram, cardiac MRI, and exercise stress test (EST) results were obtained from reports generated at the time of the study. Diagnoses that were considered potential cardiac causes of chest pain included coronary anomalies, cardiomyopathies, myocarditis, pericarditis, pulmonary hypertension, aortic dissection, arrhythmia, pulmonary embolus, and moderate or greater left ventricular outflow tract obstruction. Findings considered to be positive on the EST included ST-segment or T-wave changes concerning ischemia or tachyarrhythmias. Likewise, tachyarrhythmias or bradyarrhythmias on the Holter or event monitor were considered to be positive findings.
Calculating Charges
The number and charges for cardiac tests obtained in conjunction with the clinic visit were analyzed. All patients in this cohort had at least 1 cardiology clinic visit. An ECG was performed at all clinic visits. The number of echocardiogram, EST, Holter monitor, loop-event monitor, and cardiac MRI studies performed were tabulated. Testing and visit charges were calculated on the basis of medium-level services obtained from our 2009 billing data. The number of follow-up cardiology visits, emergency-department visits, and hospital admissions for chest pain in 2009 were collected for each patient. The comprehensive, estimated charges for chest-pain assessment were calculated for each patient on the basis of the above components.
Practice variation and resource use were evaluated on the basis of provider experience and clinical volume. Providers were divided into groups on the basis of the number of years of practice after training (≤5, 6–14, and ≥15 years) and their outpatient-clinic volume (number of clinic patients seen during the 2009 calendar year). The number of echocardiograms and ESTs were compared between groups.
Algorithm
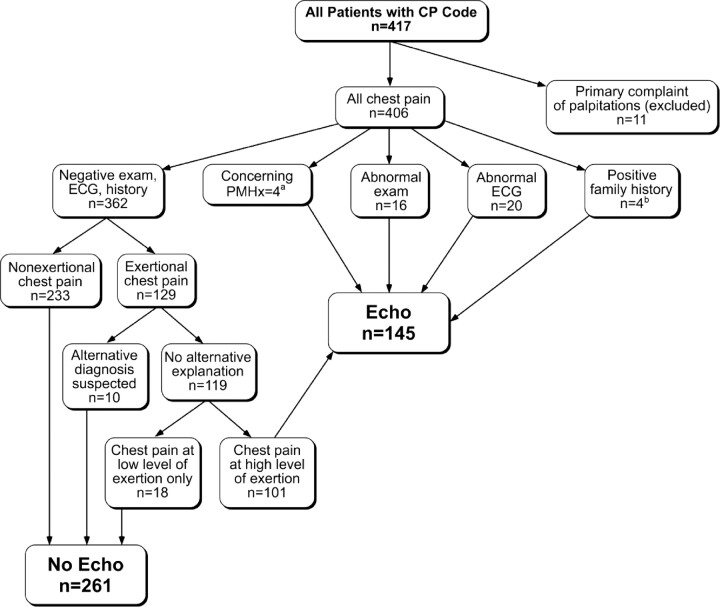
We developed an algorithm (Fig 1), which forms the basis of the chest pain SCAMP, that uses history, physical examination, and ECG to suggest when additional testing, specifically an echocardiogram, is indicated. This algorithm is based on previous work from our institution 17 and was targeted at identifying the relevant causes of cardiac chest pain listed above. Using the clinical information collected from a retrospective chart review of the 406 patients seen for chest pain in 2009, we evaluated what type of testing would be obtained for each patient using the algorithm. We then compared resource use and charges from the 2009 cohort to the predicted charges and resource use on the basis of the algorithm.
FIGURE 1.
SCAMP algorithm to guide testing in patients with chest pain. a Diagnoses that lead to increased risk of cardiac chest pain (ie, inflammatory disorders, malignancy, thrombophilia). b Family history was considered positive if any of the following were present in a first-degree relative: sudden or unexplained death; aborted sudden death; cardiomyopathy; or pulmonary hypertension. Six patients had an abnormal ECG result and an abnormal past medical history, family history, or physical examination. Patients with more than 1 abnormality (ie, ECG, past medical history, family history, and/or physical examination) were counted in only 1 category in this figure. CP indicates chest pain; PMHx, past medical history; echo, echocardiogram.
Patients were identified by the International Classification of Diseases, Ninth Revision code for chest pain, which leads to a potential ascertainment bias because patients found to have significant heart disease are likely to be coded based on the disease code rather than the presenting symptom code. To account for this potential bias, the algorithm was validated against a previously reported cohort of patients who presented to our outpatient division with a primary complaint of chest pain and were found to have significant cardiac pathology.
Statistical Analysis
Clinical symptoms and the number of cardiac tests were expressed as counts and percentages. Charges for testing and visits were summed for each patient and are expressed as medians and ranges. Analysis of resource use on the basis of provider experience and volume were performed using the χ2 test. All statistical analysis were 2-sided, and type I error was controlled at a level of 0.05. Analyses were performed with SPSS 16.0 (SPSS Inc, Chicago, IL).
RESULTS
In 2009, 417 patients between the ages of 7 and 21 years presented to our outpatient division for a first-time evaluation with chest pain as the major diagnosis code. Review of records revealed that 11 patients were incorrectly coded and presented with palpitations without chest pain, leaving the remaining 406 patients as the cohort for this study. Patient demographics and clinical data are summarized in Table 1. Chest pain was reported as exertional in 150 (37%) patients, of whom 46 (31%) had exertional dyspnea and 21 (14%) had associated dizziness or lightheadedness. No patient had syncope associated with exertional chest pain. In the entire cohort, 66 (16%) patients reported palpitations associated with chest pain.
TABLE 1.
Demographics and Clinical Data
| Patients With Chest Pain (N = 406) | |
|---|---|
| Male, n (%) | 207 (51) |
| Age, median (range), y | 13.7 (7.1–20.9) |
| Age, n (%) | |
| 7–11 y | 118 (29) |
| 12–16 y | 184 (45) |
| 17–21 y | 104 (26) |
| Exertional chest pain, n (%) | 150 (37) |
| Associated palpitations, n (%) | 66 (16) |
| Positive past medical history, n (%) | 4 (1) |
| Positive family history, n (%) | 4 (1) |
| Abnormal physical exam, n (%) | 16 (4) |
| Abnormal electrocardiogram, n (%) | 25 (6) |
| Emergency-department visit before cardiology visit, n (%) | 21 (5) |
A total of 44 of 406 (11%) patients had pertinent positive findings on either past medical history, family history, ECG, and/or physical examination (Table 1). Sixteen (4%) patients had an abnormal cardiovascular examination with the following findings: 6 patients had a pathologic murmur; 4 had a systolic click; 3 had a friction rub; 1 had a gallop; and 1 had an abnormal second-heart sound. Past medical history was notable for a single patient with each of the following: systemic lupus erythematous; juvenile rheumatoid arthritis; carnitine deficiency; and congenital adrenal insufficiency. Four patients had a positive family history, including the following: a father who died suddenly at the age of 29 years; a brother with cardiac arrest at the age of 28 years; a younger sibling with resuscitated cardiac arrest; and a father with hypertrophic cardiomyopathy. Twenty-five (6%) patients had an abnormal ECG; the most common abnormalities were increased left ventricular forces (n = 15) and pathologic ST-segment or T-wave abnormalities (n = 6). Other ECG abnormalities included axis deviation (n = 2), frequent premature ventricular contractions (n = 1), and Wolff-Parkinson-White syndrome (n = 1).
There were a total of 461 cardiology clinic visits. Thirty-eight patients had 2 or more clinic visits, accounting for a total of 55 repeat visits, with the remaining 368 patients having a single clinic visit. All patients had at least 1 ECG. Thirty-eight patients had 2 or more ECGs, leading to a total of 457 ECGs. A total of 175 (43%) patients had echocardiograms, 114 (28%) had ESTs, 40 (10%) had event monitors, and 30 (7%) had Holter monitors. Seven (2%) patients had cardiac MRI performed; the indication in each case was the evaluation of coronary origins in patients whose coronaries were not well visualized on by transthoracic echocardiography.
Four patients were admitted for inpatient evaluation of chest pain. Two patients, 1 of whom was admitted twice, were hospitalized secondary to pericardial effusion. Another patient was admitted with exertional chest pain and dyspnea. This patient underwent an echocardiogram and EST, both of which were normal. The patient was discharged with a diagnosis of noncardiac chest pain. Another patient was admitted with chest pain with associated palpitations and underwent an echocardiogram, EST, and telemetry monitoring, all of which were normal. Fifteen (4%) patients also were seen by a pulmonologist and 9 (2%) patients by a gastroenterologist for chest-pain evaluation.
Subgroup analysis on the basis of symptomatology showed that of 150 patients with exertional chest pain, 100 (67%) patients had an echocardiogram and 92 (61%) had an EST. No cardiac etiologies for exertional chest pain were found by EST in this group. Seventy patients were prescribed a heart-rhythm monitor, of whom 55 had palpitations in addition to chest pain, whereas the remaining 15 (21%) had chest pain alone.
A cardiac etiology for chest pain was found in 5 of 406 patients (1%) Two of 5 patients had pericarditits. Both of these patients presented with friction rub on examination, positional chest pain, ST-segment and T-wave changes on ECG consistent with pericardial disease, and echocardiograms showing small pericardial effusion. Two patients had supraventricular tachycardia, and 1 patient had short runs of nonsustained ventricular tachycardia, all of which were identified on outpatient rhythm monitors. All 3 patients with arrhythmia presented with palpitations as a significant complaint in addition to chest pain. The remaining 401 (99%) patients were felt to have noncardiac chest pain, most commonly nonspecific musculoskeletal pain, costochondritis, or respiratory/asthma related. Several patients had cardiac diagnoses discovered during the chest-pain evaluation that were unrelated to the presenting complaint of chest pain. Incidental diagnoses included Wolff-Parkinson-White syndrome (n = 1), mild subaortic stenosis (n = 1), small atrial septal defect (n = 1), small muscular ventricular septal defect (n = 1), mitral valve prolapse and dilated aortic root (n = 1), and small coronary artery to pulmonary artery fistula (n = 3).
Resource Utilization Based on Provider Volume and Experience
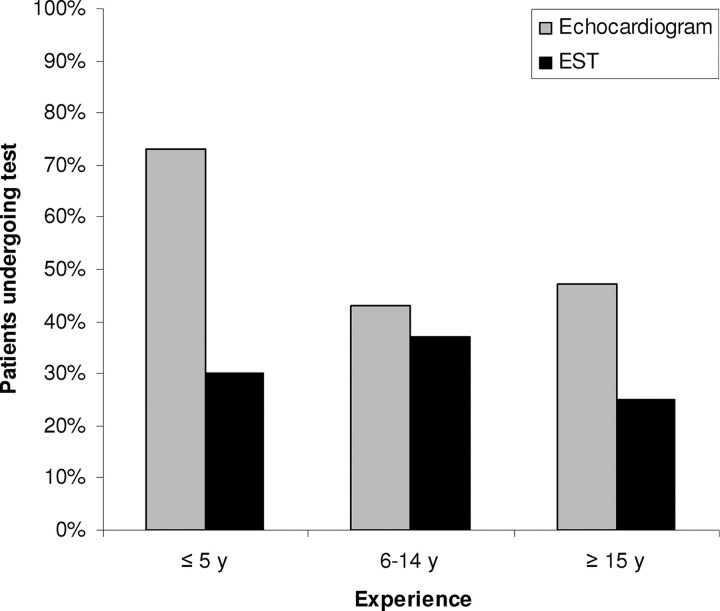
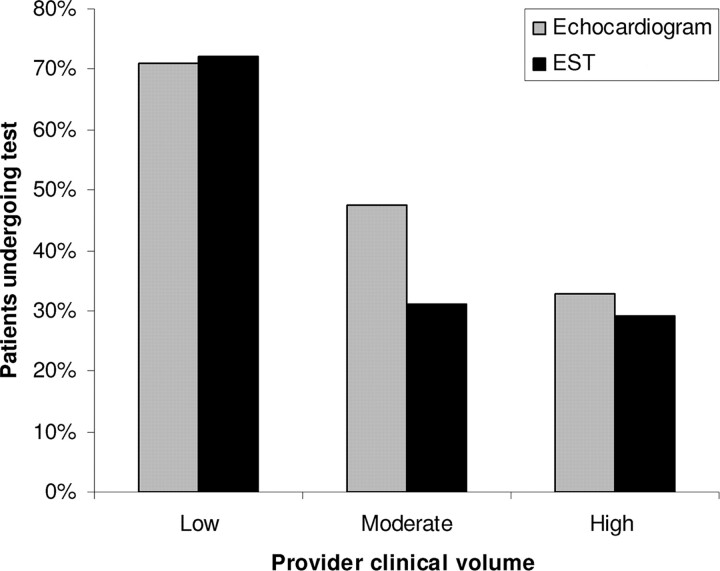
Cardiologists with more than 5 years of posttraining clinical experience ordered fewer echocardiograms than those with less clinical experience (P = .003) (Fig 2). Cardiologists with higher annual clinic-visit volumes ordered fewer echocardiograms than those who see fewer clinic patients (P < .001) (Fig 3). The number of ESTs performed did not significantly differ on the basis of the provider's experience or clinical volume.
FIGURE 2.
Cardiac testing according to provider experience.
FIGURE 3.
Cardiac testing according to clinical volume.
Algorithm Validation
The algorithm was validated against a previously reported cohort of patients who presented to our outpatient division with a primary complaint of chest pain and were found to have a cardiac etiology for chest pain. In a study by Kane et al 17 , patients were initially identified on the basis of disease code for potential causes of cardiac chest pain and were included if they presented to our outpatient division with a primary complaint of chest pain. Over a 10-year period, 41 patients were found to have a cardiac etiology for chest pain, including the following: anomalous coronary artery origin (n = 32); pericarditis (n = 4); hypertrophic cardiomyopathy (n = 3); myocarditis (n = 1); and pulmonary hypertension (n = 1). The algorithm we describe would identify all 41 patients as needing an echocardiogram on the basis of presenting complaints, positive past medical and family history, physical examination findings, and/or ECGs.
Charges for Chest-Pain Evaluation and Effect of SCAMP Use
Total estimated charges for chest-pain evaluation in the entire cohort were $1 166 465. Clinic visits alone accounted for approximately one-quarter of all charges. Cardiac testing accounted for almost 70% of the total patient-care charges, with echocardiograms accounting for approximately one-half of the cardiac-testing charges. ESTs accounted for ∼15% of testing charges. No cardiac testing other than ECGs was performed in 172 patients (43%), all of whom were felt to have noncardiac chest pain. Despite having no adjunctive testing, this subgroup still accounted for nearly $150 000 in charges.
The SCAMP algorithm in Fig 1 uses pertinent history, physical examination findings, and ECG to suggest when additional testing, specifically an echocardiogram, is indicated. Applying the algorithm to the patients seen in 2009, use of echocardiography, Holter monitors, and event monitors would all decrease by ∼20% (Table 2). The algorithm eliminates ESTs for the evaluation of pediatric chest pain and suggests Holter and event monitors only for patients with palpitations as a significant complaint. Altogether, charges for cardiac evaluation could be reduced by over $245 000 per year (21%) at our institution with use of the SCAMP. Additional reductions in cost of care could be made with a reduction in the number of repeat clinic visits and emergency-department visits.
TABLE 2.
Change in Resource Use When Using Chest-Pain SCAMP
| Study Type | Studies in 2009 Patients (N = 406), n | Studies Using the SCAMP (N = 406), n | Change in Resource Use, N (%) |
|---|---|---|---|
| Echocardiogram | 175 | 145 | −30 (18) |
| EST | 114 | 0 | −114 (100) |
| Holter monitoring | 30 | 24 | −6 (20) |
| Loop-event monitoring | 40 | 32 | −8 (20) |
DISCUSSION
In this study, we report resource use, patient-care charges, and results of cardiac evaluation in a large cohort of children seen at a single institution for chest pain in 2009. Our results emphasize the findings of previous reports showing that cardiac etiologies for pediatric chest pain are rare. 2 – 5,8 Nonetheless, extensive and costly cardiac testing is common. We show that implementation of a pediatric chest pain SCAMP could lead to a 21% reduction in charges; at a blended cost-to-charge ratio of 60%, the savings would approach $150 000 per year at our institution alone. Considering the looming crisis in health care finance and the likely impending changes in the US health care–delivery system, methods to reduce unnecessary resource use are critical. Understanding current resource use and the impact of interventions to reduce health care expenditures is critical to delivering more cost-effective care. In this study, we suggest that careful history, physical examination, and screening ECGs can detect essentially all cardiac causes of chest pain, and we propose that a standardized approach to pediatric chest pain evaluation could lead to a reduction in resource use and cost of care while maintaining or even improving quality of care.
Reducing the number of unnecessary echocardiograms, ESTs, and outpatient rhythm monitors are the main source of projected savings. The serious cardiac causes of pediatric chest pain, including anomalous coronary origins, cardiomyopathy, pulmonary hypertension, myocarditis, and pericarditis, are readily diagnosed by suggestive history, cardiac examination, ECG, and selective use of echocardiography. Therefore, echocardiography is our diagnostic test of choice for patients with concerning past medical or family histories or abnormal EKG or physical examination findings. In our study, 50 (33%) patients with exertional chest pain did not have echocardiograms and 75 (29%) patients without exertional chest pain did have echocardiograms. The chest-pain SCAMP algorithm would increase appropriate use of testing and reduce inappropriate use, leading to improved care.
We propose eliminating ESTs in the routine evaluation of pediatric chest pain. Previous studies have shown ESTs add little to the evaluation of pediatric chest pain. In a study assessing the utility of ESTs in 263 children complaining of chest pain, no evidence for cardiac disease was discovered in any patient. 18 Basso et al 19 report on 27 young athletes who died suddenly and were found at autopsy to have an anomalous coronary origin. Six of 27 patients previously had an EST, all which were normal. These findings are confirmed in our study, in which 114 ESTs were performed and no cardiac abnormalities were identified.
Holter and event monitors are unlikely to be helpful in the evaluation of chest pain in the absence of palpitations or syncope. A study analyzing recordings from 495 rhythm monitors in children performed for palpitations, chest pain, or syncopal symptoms showed that the only diagnostic rhythm recorded was supraventricular tachycardia, a generally benign rhythm. 20 In addition, no patient with chest pain alone had supraventricular tachycardia or other arrhythmia documented during outpatient rhythm monitoring. Of note, 20% of patients in our cohort who had outpatient rhythm monitoring had no palpitations or syncopal symptoms. We propose limiting rhythm monitors to those patients with chest pain and palpitations.
Nearly one-half (43%) of the cohort in this study had no cardiac testing apart from an ECG at the cardiology visit, yet this group still accumulated approximately $150 000 in charges. This finding highlights 3 important points. First, our providers already are relatively selective and judicious in the use of cardiac testing. Second, there continues to be wide practice variation in evaluation of pediatric chest pain, with less-experienced and lower-volume providers generally ordering more tests. Finally, our results suggest that many patients in this cohort could have been adequately evaluated by their primary care provider using careful history, physical examination, and ECG. A SCAMP targeting chest-pain evaluation in the pediatrician's office is currently being developed and could help limit unnecessary referrals and lead to further reduction in cost of care in this population.
There are several important limitations to this study. Our analysis relied entirely on the clinic note to record patient symptoms, history, and examination. In addition, the patient's diagnosis was determined on the basis of a retrospective review of the clinic note, testing results, and any subsequent clinical contacts. Because not all patients underwent extensive testing and follow-up is limited, we cannot account for any potential missed cardiac diagnoses. We were only able to capture events that occurred at our institution; thus, the charges for repeat primary care physician visits, visits to other specialists, outside emergency-department visits, and testing that was not conducted through the primary cardiologist are not included in this analysis.
A significant limitation of our analyses of charges and resource use is that we assume 100% compliance to the SCAMP. For multiple reasons, universal adherence will not be the case, which likely leads to an overestimate of the effect of the SCAMP on resource use and charges. Prospective results from the chest-pain SCAMP, including information regarding diagnostic yield, practitioner compliance, and changes in resource use will become available in the near future.
CONCLUSIONS
Practice variation, resource use, and charges for pediatric chest-pain evaluation remain high. We show that use of a standard management approach to pediatric chest pain could lead to a reduction in resource use and charges while maintaining quality of care.
ACKNOWLEDGMENTS
This research was supported by the Boston Children's Heart Foundation, the Provider-Payor Quality Initiative, the Program for Patient Safety and Quality, and the Hinden Family Fund.
Dr Friedman contributed to the study design, data collection, data analysis, manuscript preparation, and approval of the final version; Dr Kane contributed to the study design, data analysis, manuscript preparation, and approval of the final version; Dr Rathod contributed to the study design, manuscript preparation, and approval of the final version; Ms Renaud contributed to the data collection, manuscript preparation, and approval of the final version; Dr Farias contributed to the data collection, study design, manuscript preparation, and approval of the final version; Dr Geggel contributed to the study design, manuscript preparation, and approval of the final version; Dr Fulton contributed to the study design, manuscript preparation, and approval of the final version; Dr Lock contributed to the study design, manuscript preparation, and approval of the final version; and Dr Saleeb contributed to the study design, data collection, data analysis, manuscript preparation, and approval of the final version.
Abbreviations:
- SCAMP
- Standardized Clinical Assessment and Management Plans
- ECG
- electrocardiogram
- EST
- exercise stress test
REFERENCES
- 1. Balfour IC , Rao PS . Chest pain in children. Indian J Pediatr. 1998;65(1):21–26 [DOI] [PubMed] [Google Scholar]
- 2. Evangelista JA , Parsons M , Renneburg AK . Chest pain in children: diagnosis through history and physical examination. J Pediatr Health Care. 2000;14(1):3–8 [DOI] [PubMed] [Google Scholar]
- 3. Tunaoglu FS , Olgunturk R , Akcabay S , Oguz D , Gucuyener K , Demirsoy S . Chest pain in children referred to a cardiology clinic. Pediatr Cardiol. 1995;16(2):69–72 [DOI] [PubMed] [Google Scholar]
- 4. Zavaras-Angelidou KA , Weinhouse E , Nelson DB . Review of 180 episodes of chest pain in 134 children. Pediatr Emerg Care. 1992;8(4):189–193 [DOI] [PubMed] [Google Scholar]
- 5. Cava JR , Sayger PL . Chest pain in children and adolescents. Pediatr Clin North Am. 2004;51(6):1553–1568, viii [DOI] [PubMed] [Google Scholar]
- 6. Danduran MJ , Earing MG , Sheridan DC , Ewalt LA , Frommelt PC . Chest pain: characteristics of children/adolescents. Pediatr Cardiol. 2008;29(4):775–781 [DOI] [PubMed] [Google Scholar]
- 7. Diehl AM . Chest pain in children: tip-offs to cause. Postgrad Med. 1983;73(6):335–332 [DOI] [PubMed] [Google Scholar]
- 8. Fukushige J , Tsuchihashi K , Harada T , Ueda K . Chest pain in pediatric patients. Acta Paediatr Jpn. 1988;30(5):604–607 [DOI] [PubMed] [Google Scholar]
- 9. Leung AK , Giuffre RM . Pediatric chest pain. Clin Pediatr (Phila). 2004;43(9):863 [DOI] [PubMed] [Google Scholar]
- 10. Talner NS , Carboni MP . Chest pain in the adolescent and young adult. Cardiol Rev. 2000;8(1):49–56 [DOI] [PubMed] [Google Scholar]
- 11. Thull-Freedman J . Evaluation of chest pain in the pediatric patient. Med Clin North Am. 2010;94(2):327–347 [DOI] [PubMed] [Google Scholar]
- 12. O'Connor GT , Plume SK , Olmstead EM , et al. A regional intervention to improve the hospital mortality associated with coronary artery bypass graft surgery: the Northern New England Cardiovascular Disease Study Group. JAMA. 1996;275(11):841–846 [PubMed] [Google Scholar]
- 13. Hambrook JT , Kimball TR , Khoury P , Cnota J . Disparities exist in the emergency department evaluation of pediatric chest pain. Congenit Heart Dis. 2010;5(3):285–291 [DOI] [PubMed] [Google Scholar]
- 14. Selbst SM , Ruddy RM , Clark BJ , Henretig FM , Santulli T Jr . Pediatric chest pain: a prospective study. Pediatrics. 1988;82(3):319–323 [PubMed] [Google Scholar]
- 15. Friedman KG , Rathod RH , Farias M , et al. Resource utilization after introduction of a standardized clinical assessment and management plan. Congenit Heart Dis. 2010;5(4):374–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rathod RH , Farias M , Friedman KG , et al. A novel approach to gathering and acting on relevant clinical information: SCAMPs. Congenit Heart Dis. 2010;5(4):343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kane DA , Fulton DR , Saleeb S , Zhou J , Lock JE , Geggel RL . Needles in hay: chest pain as the presenting symptom in children with serious underlying cardiac pathology. Congenit Heart Dis. 2010;5(4):366–373 [DOI] [PubMed] [Google Scholar]
- 18. Danduran MJ , Earing MG , Sheridan DC , Ewalt lA , Frommelt PC . Chest pain: characteristics of children/adolescents. Pediatr Cardiol. 2008;29(4):775–781 [DOI] [PubMed] [Google Scholar]
- 19. Basso C , Maron BJ , Corrado D , Thiene G . Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol. 2000;35(6):1493–1501 [DOI] [PubMed] [Google Scholar]
- 20. Saarek EV , Stefanelli CB , Fischbach PS , Serwer GA , Rosenthal A , Dick M . Transtelephonic electrocardiographic monitors for evaluation of children and adolescents with suspected arrhythmias. Pediatrics. 2004;113(2):248–251 [DOI] [PubMed] [Google Scholar]