Abstract
Androgens regulate broad physiologic and pathologic processes, including external genitalia development, prostate cancer progression, and anti-inflammatory effects in both cancer and asthma. In prostate cancer, several lines of evidence have implicated dietary and endogenous fatty acids in cell invasion, angiogenesis, and treatment resistance. However, the role of fatty acids in steroidogenesis and the mechanisms by which alterations in this pathway occur are not well understood. Here, we show that, of a panel of fatty acids tested, arachidonic acid and its specific metabolite 5-hydroxyeicosatetraenoic acid (5-HETE) regulate androgen metabolism. Arachidonic acid is metabolized to 5-HETE and reduces androgens by inducing aldo-keto reductase (AKR) family members AKR1C2 and AKR1C3 expression in human prostate, breast, and lung epithelial cells. Finally, we provide evidence that these effects require the expression of the antioxidant response sensor, nuclear factor erythroid 2-related factor 2 (Nrf2). Our findings identify an interconnection between conventional fatty acid metabolism and steroid metabolism that has broad relevance to androgen physiology and inflammatory regulation.
Keywords: androgens, metabolism, enzymes, fatty acids, arachidonic acid
Androgens are usually thought of in the context of male hormones that drive the development and maintenance of male sexual characteristics. However, our knowledge concerning the diverse roles of sex steroids in normal organ function and disease has evolved beyond this focus. Androgens have broad roles in physiology and pathophysiology, including in prostate cancer (1), development (2, 3), asthma (4), and sex-associated differences in multiple and diverse disease processes (5, 6). In males, gonadal testosterone (T) is peripherally converted to 5α-dihydrotestosterone (DHT). Both males and females produce T and DHT from the adrenal precursor steroid dehydroepiandrosterone (DHEA) via the rate-limiting enzyme 3β-hydroxysteroid dehydrogenase-1 (3βHSD1) (7–9). Once produced, T and DHT bind and activate the androgen receptor (AR), a nuclear receptor expressed in a variety of cells and tissues, including adipose, prostate, breast, and lung tissues, as well as in the immune system (10). Androgen activation of AR results in the transcriptional activation of androgen-responsive genes involved in various cellular processes, including cell survival, differentiation, glucocorticoid regulation, and immune modulation (8, 11–16).
Metabolic enzymes are necessary to regulate the balance between generation of biologically active androgens and inactive steroids (Supplementary Fig. S1 (17)). Two members of the reductive aldo-keto reductase (AKR) family, AKR1C2 and AKR1C3, maintain that balance by inactivating DHT and producing inactive 5α-reduced androgens. These enzymes differ in their preference for 3-keto vs 17-keto reduction of androgens. AKR1C2 is the predominant isoform that inactivates DHT by (3-keto to 3α-OH), catalyzing it to weaker AR ligands such as 5α-androstane-3α,17β-diol (5α-diol), whereas AKR1C3 has a preference for 17-keto reduction to the 17β-OH of T and DHT (18). However, both enzymes can use both 3-keto and 17-keto androgen substrates (19). Clinical and preclinical data implicate AKR1C3 overexpression in development of castration-resistant prostate cancer (20). Importantly, however, adrenal androgen metabolism occurs in both males and females. Beyond prostate cancer, adrenal androgens also play important roles in breast cancer, endometrial cancer, anti-inflammatory responses, and other physiologic processes (4, 21–24).
How do other metabolites control endogenous regulation of androgen metabolism in peripheral tissues? Because of their possible roles in regulating inflammatory processes and cancer (25, 26), we sought to determine the effect of selected fatty acids on androgen metabolism for this study. Moreover, fatty acids are integral to cell signaling, chemotaxis, and phospholipid membrane structure and are rich sources of energy. For example, arachidonic acid is a crucial component of the cell membrane and can enzymatically be converted to several downstream metabolites involved in a range of physiological processes, including inflammatory responses, bronchoconstriction, detoxification, tissue regeneration, and stress responses (27). Because fatty acid accumulation in cells can have major effects on cellular processes, intracellular levels are normally tightly regulated (28). Cellular utilization of some fatty acids and their derivatives can modulate cancer growth (26, 29) and inflammation (30).
In this study, we show that, of a panel of selected fatty acids tested for their effects on androgen metabolism, arachidonic acid promotes inactivation of potent androgens by upregulating the expression of AKR1C2 and AKR1C3. Moreover, in addition to induction of this pathway in prostate cancer cell lines, AKR1C2 and AKR1C3 expression is increased in normal bronchial epithelial and in breast cancer cell lines in response to arachidonic acid treatment. We also demonstrate that these effects are mediated largely by the enzymatic conversion of arachidonic acid to 5-HETE. Finally, we provide evidence that induction of AKR1C2 and AKR1C3 is regulated by nuclear factor erythroid 2-related factor 2 (Nrf2), following 5-HETE treatment. These data demonstrate how 5-HETE broadly regulates the balance of active vs inactive androgens, thereby directing their multiple physiologic effects.
Materials and Methods
Chemicals
Arachidonic acid was purchased from MP Biomedicals (#02150384-CF). All other fatty acids and inhibitors were purchased from Cayman Chemicals. Fatty acids and inhibitors were dried under a gentle stream of nitrogen gas and resuspended in anhydrous DMSO (Millipore #MX14577), aliquoted, purged with argon gas to prevent oxidation, and stored at −80 °C. Opti-MEM I Reduced Serum Medium was purchased from Gibco (31985-070). All secondary antibodies were purchased from Thermo Fisher Scientific. The cDNA synthesis and reverse transcription polymerase chain reaction (RT-PCR) reagents were purchased from BioRad. All tissue culture plates and plastics were purchased from Eppendorf. Tissue culture reagents were from Thermo Fisher. Chemicals for high-performance liquid chromatography (HPLC) and untargeted metabolomics were purchased from Thermo Fisher and were of the highest possible purity.
Cells
LAPC4 prostate cancer cells were a generous gift from Dr. Charles Sawyers (Memorial Sloan Kettering Cancer, New York, NY, USA) and were maintained in Iscove's Modified Dulbecco's Medium with 10% fetal bovine serum (Gemini). LNCaP and C4-2 prostate cancer cells, BT474, HCC70, MCF7, T47D, and ZR474 breast cell lines, and BET1A and BEAS2B bronchial epithelial cell lines were purchased from ATCC (Manassas, VA, USA). Prostate and breast cell lines were cultured in Roswell Park Memorial Institute (RPMI) medium (Sigma) with 10% fetal bovine serum (Gemini). Cells were maintained in media supplemented with 1% penicillin–streptomycin (pen/strep) and 1% L-glutamine (L-glut) and incubated at 37 °C in a CO2 humidified incubator. RWPE1 benign prostate cells (ATCC) were cultured in keratinocyte serum-free media containing 5 ng/mL human recombinant epithelial growth factor and 0.05 mg/mL bovine pituitary extract (Thermo Fisher # 17005042). BET1A and BEAS2B bronchial epithelial cells were cultured in serum-free Lechner and LaVeck medium (LHC-8, Biofluids, Inc., Rockville, MD, USA) supplemented with 0.33 nM retinoic acid, 2.75 mM epinephrine, and 1% pen–strep on flasks precoated with media containing 0.01 mg/mL bovine fibronectin (Thermo Fisher Scientific), 0.03 mg/mL bovine collagen type I (Sigma-Aldrich) and 0.01 mg/mL bovine serum albumin (BSA; Sigma-Aldrich). Cells were maintained in a 5% CO2 humidified incubator. VCaP prostate cancer cells were purchased from ATCC, maintained in Dulbecco's Modified Eagle Medium containing 10% fetal bovine serum, and incubated in an 8% CO2 humidified incubator. Cells were routinely tested for Mycoplasma infection and cell authenticity.
shRNA Lentiviral Knockdown
Lentiviral shRNA plasmids for NFE2L2 (shNFE2L2 1: NM_006164.2-1144s1c1, shNFE2L2 2: NM_006164.2-1987s1c1) and PLK0.1 non-targeting control (SHC002), glycerol stocks, and plasmid were purchased from Sigma-Aldrich. The day before transduction, 293 T cells were seeded in 100-mm dishes at 60% to 70% confluency. Lentiviral packaging of shRNA was performed by cotransfecting 1.25 µg psPAX2 (Addgene #12261) and pMD2.G (#12259) plasmids along with 2.5 µg shRNA vectors using Fugene 6 transfection reagent (Promega) per the manufacturer's instructions. Lentiviral particles were collected at 24 and 48 hours, passed through 0.45 µM filters and concentrated 100 times using PEG-it virus precipitation solution (System Bioscience, #LV825A-1) per the manufacturer's instructions. Viral particles were aliquoted and stored at −80 °C for future use.
The day before transduction, 300 000 C4-2 cells/well were plated on 6-well dishes and allowed to settle overnight. The next day, 20 µL concentrated virus was added to the cells and incubated for 24 hours and then the media was changed every 2 to 3 days for 1 week. Lentiviral-infected cells were selected by culturing in 1 µg/mL puromycin for 2 weeks before checking for knockdown efficiency.
Tritiated Steroid Metabolism Experiments
C4-2 (4 × 105) and LNCaP (5 × 105) cells were plated in 12-well plates coated with poly-L-ornithine (Sigma, St. Louis, MO, USA) for 24 hours. Cells were washed once with phosphate-buffered saline (PBS), and 1 mL phenol red-free RPMI medium containing 1% pen/strep, 1% L-glut, and 5 g/L D-(+)-glucose (Sigma #7021) was added for 16 hours. Cells were treated with [3H]-labeled DHEA or [3H]-androstenedione (AD) (300 000-600 000 cpm) purchased from Perkin Elmer (Waltham, MA) and 100 nM unlabeled DHEA or AD (Steraloids, Newport, RI) along with dimethyl sulfoxide (DMSO) or 50 μM of the following fatty acids: arachidonic acid oleic acid (#90260), palmitic acid (#10006627), palmitoleic acid (#10009871), linoleic acid (#90150), or steric acid (#10011298). For experiments examining the effects of HETE metabolites, cells were treated with 25 μM 5-HETE (#34210) and 15-HETE (#34700). Cells were incubated at 37 °C and 250 μL aliquots of medium were collected for up to 48 hours and stored at −20 °C. For intracellular steroid analysis, 8 × 105 C4-2 and 1 × 106 LNCaP cells were plated in 12-well poly-L-ornithine-coated plates overnight. Cells were washed with PBS and changed to serum-free medium for 16 hours and then treated with 1 000 000 cpm [3H]-AD, cold AD (100 nM final concentration), and fatty acids. After each time point, 250 μL medium was collected, and cells were scraped in 500 μL PBS and stored at −20 °C until steroids were extracted. Cell pellets were lysed by 4 freeze–thaw cycles on dry ice.
Media and lysed LNCaP cells were incubated with 300 units β-glucuronidase (E. coli; Novoprotein Scientific, #CH85) at 37 °C for 2 hours. C4-2 cells have no glucuronidase activity, so this step was omitted. Steroids were extracted by adding 2 volumes of 1:1 ethyl acetate:isooctane and dried under a steam of nitrogen gas. Dried samples were dissolved in 120 μL 50% methanol, microcentrifuged at maximum speed for 10 minutes, and injected on a Breeze 1525 HPLC system (Waters Corp., Milford, MA, USA) according to methods previously published (31). All metabolism studies were performed in duplicate and repeated in at least 3 biological replicates.
Mass Spectrometry Studies
For time course studies, C4-2 cells (1 × 106) were plated in triplicate for each time point in 6-well dishes coated with poly-L-ornithine in complete RPMI medium and allowed to settle overnight. Cells were washed once with PBS and changed to phenol red-free RPMI medium containing 1% pen/strep, 1% L-glut, and 5 g/L D-(+)-glucose for 16 hours followed by treatment with 100 nM AD and DMSO or 50 µM arachidonic acid. Cell pellets were weighed in preweighed tubes and stored at −80 °C until analysis. For analysis, pellets were suspended in 500 µL liquid chromatography-mass spectrometry (LC-MS) grade water (Fisher) and freeze–thawed on dry ice for 4 cycles. The cell suspension was centrifuged at high speed for 20 minutes at 4 °C. Samples were spiked with 10 μL internal standard mix (25 ng/mL; 13C3-AD and DHT-d3) and transferred to a glass tube. Steroids were extracted by adding 2 mL methyl tert–butyl ether (Across), vortexed for 5 minutes and centrifuged for 2 minutes at 3000g. Samples were evaporated to dryness under nitrogen gas and reconstituted with 120 μL 50% methanol. Steroid metabolites were separated as previously described (31, 32).
Human Prostate Tissue Explant Steroid Metabolism
Fresh prostate tissue cores from the transitional zone (TZ; benign) and peripheral zone (PZ; gross malignant) were obtained from 10 de-identified patients who underwent radical prostatectomy at Cleveland Clinic in accordance with institutional review board-approved protocols. Paired transitional zone and peripheral zone cores were collected in phenol red-free RPMI containing 1% pen/strep, 1% L-glut, 5 g/L D-(+)-glucose, 10 μg/mL hydrocortisone (Sigma #H0888), and 10 ug/mL recombinant human insulin (Thermo Fisher #12585014). Tissue was divided into 1-mm3 cubes, and 4 pieces were cultured on Vetspon dental sponges (Patterson Veterinary, Loveland, CO #07-849-4032) presoaked in serum-free RPMI on a 12-well plate. Tissues were cultured in 1 mL media containing [3H]-labeled AD and 100 nM unlabeled AD along with DMSO or 50 μM arachidonic acid for up to 72 hours. After each time point, 200 μL medium was collected and stored at −20 °C. Medium was treated with 300 units β-glucuronidase for 2 hours, and steroids were extracted as described above.
Western Blot Analysis and RT-PCR
Cells were washed once in PBS, and whole-cell lysate was collected in RIPA buffer (Sigma #R0278) containing a protease/phosphatase inhibitor cocktail (Thermo Fisher #78441). Nuclear and cytoplasmic protein fractions were isolated using the NE-PER nuclear and cytoplasmic extraction kit from Thermo Fisher (#78833). Tissue explants were frozen at −80 °C and pulverized using a mortar and pestle and suspended in RIPA buffer. Protein concentration was measured using a BCA Protein Assay Kit (Thermo Fisher #PI23227), and 50 μg protein was separated on 12% SDS PAGE precast gels (BioRad #4561044). Protein was transferred to a PVDF membrane (Amersham #10600023), blocked with 5% nonfat milk (Fisher Scientific #NC9121673), and probed using primary antibodies for AKR1C2 (Catalog #13035S, AB_2798094), AKR1C3 (Catalog #MA5-35413, CAB_2849314), Nrf2 (Catalog #12721, AB_2715528), and β actin (Catalog #A2228, AB_476697) at 1:1000 dilution. Membranes were probed with anti-rabbit and anti-mouse secondary antibodies at 1:10 000 dilution and visualized by SuperSignal West Pico PLUS (Thermo Scientific #34580) and ECL Western Blotting Detecting Reagents (Amersham #RPN2209) and developed on x-ray film (Genesee, San Diego, CA, USA). Blots were scanned, and densitometry was performed using ImageJ software.
For in vitro gene expression, RNA was extracted from cells using the GenElute Total RNA Miniprep Kit (Sigma #RTN350-1KT) according to the manufacturer's instructions. Tissue explants were pulverized as above, and RNA was extracted using Trizol, chloroform, and ethanol, followed by purification using the RNeasy Mini Kit (Qiagen) according to the manufacturer's protocol. One microgram RNA was reverse transcribed using the iScript cDNA Synthesis Kit (#1708891), and iTaq Fast SYBR Green Supermix with ROX kit (#1725125) was used for the thermocycling reaction in an ABI-7500 Real-Time PCR instrument (Applied Biosystems). Expression of AKR1C2 (forward, 5′-AAG TAA AGC TCT AGA GGC CGT-3′; reverse, 5′-GCT CCT CAT TAT TGT AAA CAT GT-3′), AKR1C3 (forward, 5′-GAG AAG TAA AGC TTT GGA GGT GAC A-3′; reverse, 5′-CAA CCT GCT CCT CAT TAT TGT ATA AAT GA-3′), and NFE2L2 (forward, 5′-CGG TAT GCA ACA GGA CAT TG-3′; reverse, 5′-ACT GGT TGG GGT CTT CTG TG-3′) were analyzed using human gene-specific primer sets. RPLP0 (forward, 5′-CAC ATT CCC CCG GAT ATG A; reverse, 5′ CGA GGG CAC CTG GAA AAC) was used as control. Quantitative PCR analysis was performed in triplicate.
Metabolomics
Untargeted metabolomics via liquid chromatography-mass spectrometry
C4-2 cells were seeded at a density of 5 × 106 per 100 mm dish and allowed to attach overnight. Cells were washed twice with PBS, and medium was changed to phenol red-free RPMI containing 1% pen/strep, 1% L-glut, and 5 g/L D-(+)-glucose for 16 hours, followed by treatment with DMSO or 100 μM arachidonic acid for 24 hours. Cells were washed twice with PBS and collected, centrifuged at 6000g, and stored at −80 °C. For analysis, pellets were resuspended in 150 μL prechilled Optima LC grade water and sonicated, and 10 μL lysate was reserved for protein measurement using a BCA assay. The remaining lysate was suspended in 150 μL prechilled Optima acetonitrile along with internal standards containing betadine-d9, carnitine-d9, ornithine-d6, valine-13C3, tyrosine-13C, 15N, estrone-13C3, and cholesterol-13C3 totaling 50 μM. Lysed cells were microcentrifuged at high speed at 4 °C for 20 minutes, and the supernatant was collected and dried under a nitrogen gas stream until less than 10 μL remained. Dried samples were resuspended in 300 μL 2% acetonitrile in water. One microliter was taken from each sample and pooled for a quality control standard that was injected every tenth injection. Metabolomics was performed by injecting 5 μL of each sample onto a 10-cm C18 column (Thermo Fisher) coupled to a Vanquish ultra-performance liquid chromatograph running at 0.2 mL/min using water and 0.1% formic acid (solvent A) and acetonitrile and 0.1% formic acid (solvent B). An Oribitrap Q-Exactive HF was operated in positive and negative electrospray ionization modes over a mass range of 56 to 850 Da using MS full scans taken at a resolution of 120 000 and MS/MS collected on the top 5 ions in data-dependent acquisition mode at a resolution of 30 000. Six biological replicates were used for this analysis.
Targeted LC-MS
C4-2 cells were seeded at 5 × 106 per 100-mm dish and allowed to attach overnight. Cells were washed twice with PBS and changed to phenol-red-free RPMI containing 1% pen/strep, 1% L-glut, and 5 g/L D-(+)-glucose for 16 hours followed by treatment with DMSO or 100 μM arachidonic acid for 24 hours. Cells were washed twice with PBS and collected, centrifuged at 6000g, and stored at −80 °C. Pellets were resuspended in 500 μL cold PBS, freeze–thawed for 6 cycles, and 10 μL lysate was reserved for protein measurement. To the remaining lysate, 1 mL 80% methanol was added and freeze–thaw cycles were repeated to release all HETEs from organelles and phospholipid membranes. The suspension was centrifuged at high speed for 12 minutes and transferred to a glass tube. The supernatant was dried under a gentle nitrogen gas stream and resuspended in 100 μL 50% methanol. Metabolomics was performed by injecting 10 μL of each sample onto a 3-μm C18 column (Phenomenex) to detect arachidonic acid and 5-, 8-, 9, 11-, 12-, and 15-HETE as previously described (33).
LC-MS data analysis
XCMS was used to deconvolute data using 2.5 consecutive scan ppm, S/N threshold of 10 and minimum of 6 and maximum of 45 seconds peak width. The resulting peaks were further analyzed via MetaboLyzer (34). Briefly, the ion presence threshold was set at 0.7 in each study group, and data were log-transformed and analyzed for significance with a parametric Mann–Whitney U test (P < 0.05). All P values were corrected via the Benjamini–Hochberg step-up procedure for false discovery rate correction (0.2). The data were used for putative identification assignment and pathway enrichment analysis via KEGG.
Statistical Analyses and Figure Production
All graphing and data analysis were performed using GraphPad Prism (version 9.0.0, San Diego, CA, USA). Differences in gene expression, cell viability, Western blot quantitation, and targeted metabolic analysis of 5- and 15-HETE were assessed by the Student t test. HPLC analyses were assessed by two-way ANOVA comparing control vs treatment at each time point. Differences were considered significant at P < 0.05 compared with control, and all data are shown as mean ± SD. The androgen metabolism pathway was created using BioRender.
Results
Arachidonic Acid Alters Adrenal Androgen Metabolism in Prostate Cancer Cells
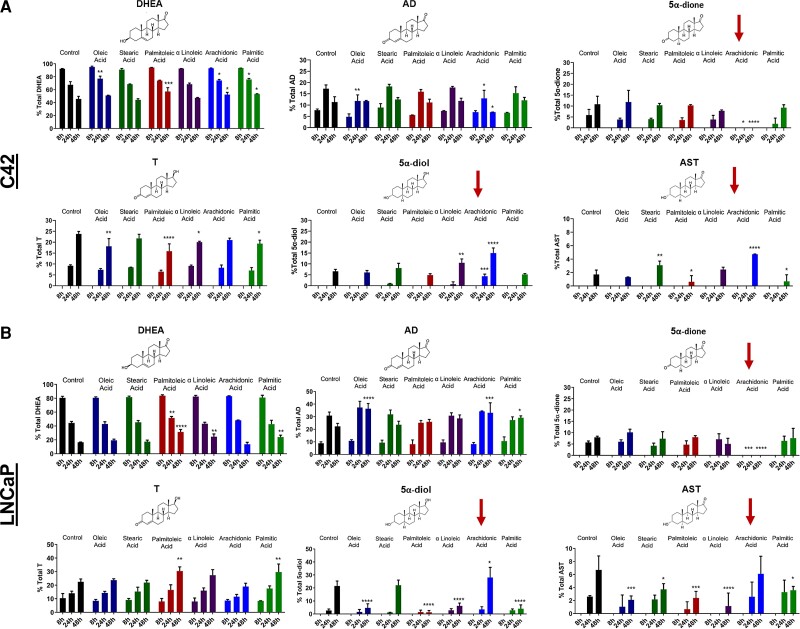
DHEA and DHEA-sulfate are together the most abundant adrenal androgens found in human male and female circulation. To explore the effects of fatty acids on androgen metabolism, we treated C4-2 and LNCaP prostate cancer cells with [3H]-DHEA along with vehicle, or 50 μM oleic acid (18:1), stearic acid (18:0), palmitoleic acid (16:1), linoleic acid (18:2), arachidonic acid (20:4), or palmitic acid (16:0); the concentration and fatty acids were selected based on prior clinical reports and in vitro reports showing that elevated fatty acids might have a role in cancer progression or inflammatory signaling (25, 35). [3H]-DHEA metabolism was assessed after steroid extraction and separation by HPLC. Strikingly, 5α-dione production, which has both 3-keto and 17-keto groups, was suppressed in C4-2 cells (Fig. 1A). Levels of the androgen precursors DHEA and AD were not affected, whereas there was an increase in downstream 5α-diol and androsterone (AST). Arachidonic acid treatment also had no significant effect on T although T is one the precursors of 5α-diol. This finding supports our previous reports that AD is preferentially converted to 5α-dione over T to produce the 3-OH and 17-OH hydroxysteroids (36).
Figure 1.
Arachidonic acid specifically promotes the loss of 5α-androstanedione (5α-dione). (A) C4-2 and (B) LNCaP cells were treated with [3H]-DHEA (100 nM) along with DMSO (control) or 50 μM oleic acid, steric acid, palmitoleic acid, α-linoleic acid, arachidonic acid, or palmitic acid. Medium was collected at the indicated time points, and steroids were extracted, separated, and quantified by HPLC. Loss of 5α-dione co-occurred with increased conversion to 5α-diol, AST, or both (red arrows). Note differences in panels for Y axis ranges. Samples were analyzed in triplicate, and error bars show the SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
We also investigated the effects of long-chain fatty acids on androgen metabolism in LNCaP cells, from which C4-2 cells are derived. Both express a mutant form of 3βHSD1 that results in rapid metabolism of DHEA (32). Like C4-2 cells, arachidonic acid treatment resulted in reduced levels of 5α-dione and increased levels of AST and 5α-diol (Fig. 1B). Again, levels of T were not significantly changed by arachidonic acid treatment; DHT was not detected. Interestingly, AD levels also remained high in LNCaP cells at 48 hours, suggesting increased metabolism of DHEA in LNCaP cells results in accumulation of AD and possible saturation of other steroidogenic enzymes. Together, instead of inhibition of 5α-dione production, this suggested hastened loss and reduction of 5α-dione to the 3α-OH androgens 5α-diol or AST (Supplementary Fig. S1 (17)).
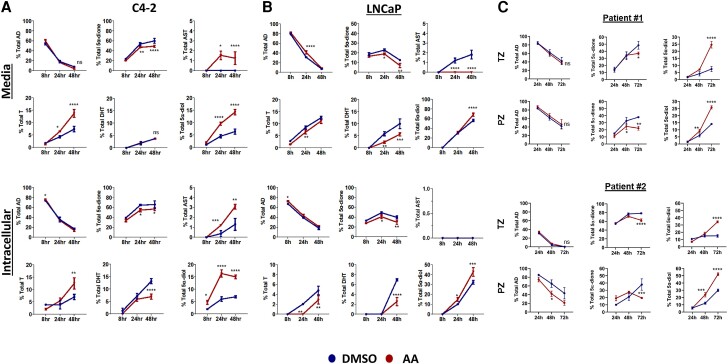
To further investigate these findings concerning loss vs generation of 5α-dione, we treated C4-2 (Fig. 2A) and LNCaP (Fig. 2B) cells with DMSO or arachidonic acid along with the more distal androgen metabolite [3H]-AD because levels of DHEA or AD were not changed in control or fatty acid treated cells and AD is one step upstream of 5α-dione. We analyzed steroid metabolites in intracellular and media samples to ensure detection of all possible steroids, in particular DHT and AST, which were lower in media samples (Fig. 1). C4-2 and LNCaP cells treated with arachidonic acid and [3H]-AD preferentially converted 5α-dione to 3α-OH hydroxysteroids as compared with the 17-keto reduction to DHT. Unlike DHEA treatment where 5α-dione is rapidly metabolized, levels of 5α-dione were not as clearly diminished with arachidonic acid treatment due to possible substrate saturation in the conversion to downstream inactive metabolites, consistent with our previous findings (36).
Figure 2.
Arachidonic acid accelerates metabolism of 5α-dione to 5α-diol and AST in prostate cancer cells and tissue explants. (A) C4-2 cells, (B) LNCaP cells, and (C) prostate tissue explants were treated with DMSO or 50 μM arachidonic acid (AA) along with 100 nM [3H]-AD. Steroids were extracted from medium (top panels) and cells (bottom panels) for the indicated times and quantified by HPLC. Samples were analyzed in triplicate, and error bars represent the SD. Cell experiments are representative of at least 3 biological replicates. Patient prostate tissue experiments are representative of explants from 10 patients. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To validate our findings and ensure that low levels of androgens would be detected, we performed LC-MS on intracellular contents of C4-2 cells treated with AD and arachidonic acid (Supplementary Fig. S2 (17)). Concentrations of T were moderately increased while DHT was reduced in response to arachidonic acid treatment, validating our hypothesis that T is not as readily metabolized to DHT. As expected, levels of 5α-diol were significantly increased in intracellular samples treated with arachidonic acid. We also assessed intracellular concentrations of the inactive 3-keto and 17-keto reduced androgens 3-OH steroids, 3α-AST (androsterone) and 3β-AST (epiandrosterone), isomers that are indistinguishable by HPLC and are both inactive analytes. Arachidonic acid–induced inactivation of both stereoisomers was observed, with the 3α-AST concentration being significantly higher. Together, these data suggest the strongest metabolic effect induced by arachidonic acid was reduction of 3-keto to 3α-OH androgens.
To investigate whether these findings also occur in patient prostate tissues, we obtained freshly collected tissue punches from the transitional zone and the peripheral zone from the prostates of men undergoing radical prostatectomy for prostate cancer and cultured them ex vivo with arachidonic acid and [3H]-AD for up to 72 hours. Our previous work demonstrates that ex vivo patient tumor cells can remain enzymatically active even after 1 week in culture (15, 37). 5α-diol and 5α-dione were the only metabolites detected by HPLC, which is consistent with our previous studies of patient tissue explants cultured with [3H]-AD (Fig. 2C) (38). Nonetheless, the effects of arachidonic acid on androgen metabolism in the transitional and peripheral zone tissues were similar to our in vitro findings, particularly compared to C4-2 cells. Collectively, these data suggest that arachidonic acid treatment can promote androgen reduction in clinical prostate tissues.
Arachidonic Acid Induces Expression of AKR1C2 and AKR1C3
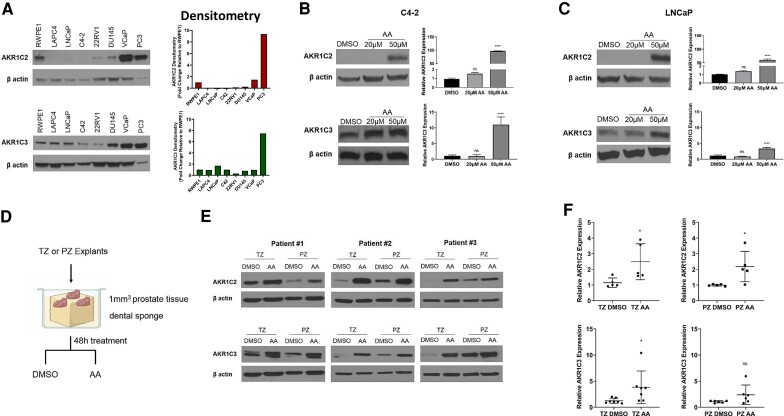
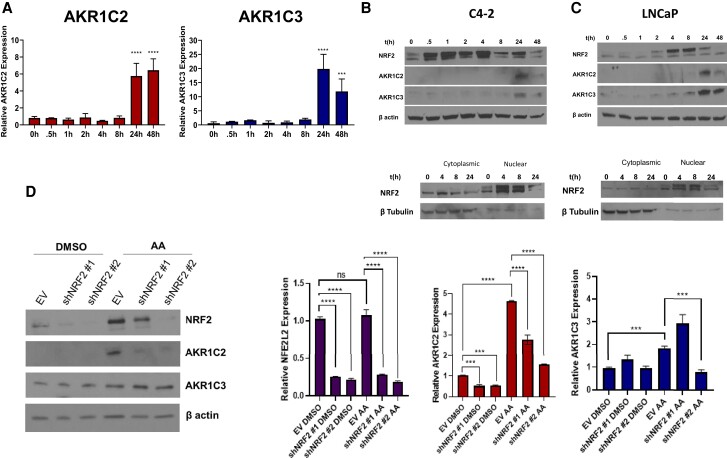
To determine how arachidonic acid exerts its reductive effects on androgen metabolism, we characterized the effects on regulation of 2 of the major aldo-keto reductase (AKR) enzymes, AKR1C2 and AKR1C3 (39). Expression of endogenous AKR1C2 protein is higher in the non-cancer prostate cell line RWPE1 and the DU145, VCaP, and PC3 prostate cancer cell lines (Fig. 3A, top panel). Conversely, the LAPC4, LNCaP, C4-2, and 22Rv1 prostate cancer cell lines have lower expression. Expression of endogenous AKR1C3 was robust in many of the prostate cell lines compared with AKR1C2, with PC3 cells again having the most abundant expression (Fig. 3A, bottom panel). Next, we tested the effects of increasing concentrations of arachidonic acid on AKR1C2 and AKR1C3 expression in C4-2 and LNCaP cells. After 24 hours, there was significant induction of protein and mRNA expression of AKR1C2 and AKR1C3 in both cell lines in response to arachidonic acid treatment (Fig. 3B and 3C). Induction of expression was generally greater for AKR1C2 compared with AKR1C3. We also tested the effects of arachidonic acid in transitional zone and peripheral zone prostate tissue explants. After 48-hour treatment with arachidonic acid (Fig. 3D), protein and mRNA expression of AKR1C2 and AKR1C3 were increased in transitional zone (TZ) and peripheral zone (PZ) prostate tissues, validating our in vitro findings (Fig. 3E and 3F). Together, these results support the role of arachidonic acid as a stimulus to induce androgen metabolic enzyme expression and alter metabolism of androgens in prostate cells.
Figure 3.
Arachidonic acid (AA) induces expression of AKR1C2 and AKR1C3. (A) Endogenous protein expression and quantification of AKR1C2 (top panel) and AKR1C3 (bottom panel). (B, C) Protein and transcript expression of AKR1C2 and AKR1C3 in C4-2 and LNCaP cells treated with DMSO (control) or AA (20 μM and 50 μM) for 24 hours. (D) Schematic of ex vivo culture of prostate tissue explants from the transitional zone (TZ) and peripheral zone (PZ) treated with DMSO (control) or AA (50 μM). (E) Protein and transcript (F) expression of TZ and PZ tissue explants treated with DMSO or AA for 48 hours. Western blots were quantified with ImageJ and are representative of at least 5 biological replicates. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns = not significant.
Arachidonic acid is a key precursor of inflammatory chemokines in various tissues. Bronchial epithelial cells and fibroblasts use fatty acid precursors to produce potent inflammatory cytokines that mediate vasoconstriction and symptoms associated with asthma and respiratory infections (40, 41). Accelerated fatty acid oxidation and lipid metabolism are key sources of energy, which aids in breast cancer cell growth, survival, inflammation, and contributes to poor overall survival (42). To determine the broader effects of arachidonic acid on AKR enzyme expression in cells derived from other tissues, we treated breast cancer cell lines with arachidonic acid and found that treatment also increased AKR1C2 and AKR1C3 transcripts (Supplementary Fig. S3A (17)). AKR1C2 and AKR1C3 were also induced in BET1A and BEAS2B bronchial epithelial cells treated with 10 µM and 5 µM arachidonic acid, respectively, but not with 1 µM treatment (Supplementary Fig. S3B (17)). Together, these results suggest an expansive role for arachidonic acid in fine-tuning androgen metabolism beyond prostatic tissues including, physiologically, multiple human tissue compartments.
5-HETE Induces Expression of AKR1C2 to Suppress Androgen Production
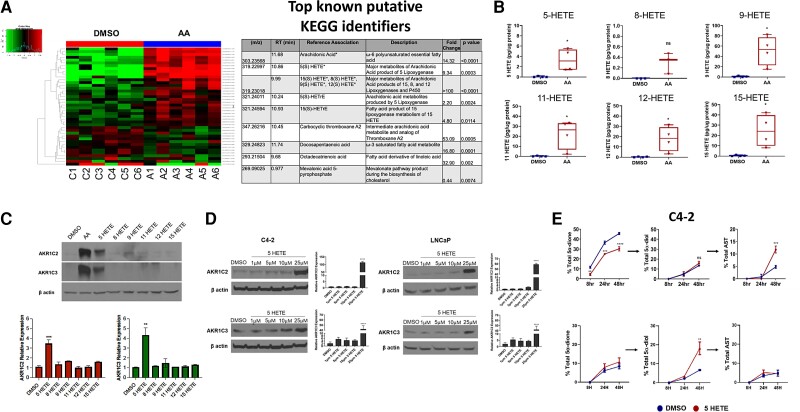
Enzymatic oxidation of arachidonic acid produces multiple metabolites that are important in cell signaling, inflammatory regulation, and other cellular processes (43, 44). To determine the effects of arachidonic acid on the prostate cancer metabolome and to determine the identity of the specific signaling mediator for AKR1C2/3 using an unbiased approach, we cultured C4-2 cells with 100 μM arachidonic acid for 24 hours and performed LC-MS-based untargeted metabolomics. Statistically significant changes with a cutoff of 0.05 occurred in the relative abundances of 216 putative metabolites, matched against the NIST 17.0 spectral library, which resulted from arachidonic acid treatment (Fig. 4A) and Table 1. Of the 216 putative metabolites, we chose to focus on validating the metabolites produced by lipoxygenase (LOX), cyclooxygenase (COX), and P450 enzymes because these enzymes are largely responsible for metabolizing arachidonic acid (Table 1 (17)) (45). We validated the chemical identities of several of these metabolites against their pure standards, including 5-HETE, 15-HETE, and 12-HETE, which are produced by 5-, 15-, and 12-lipoxygenase, respectively. Additional saturated fatty acids were found to be increased, including docosapentaenoic acid, octadecatrienoic acid (a derivative of linoleic acid), and the potent inflammatory mediator carboxylic thromboxane A2, after arachidonic acid treatment. However, validation studies did not show altered androgen metabolism when LNCaP and C4-2 cells were treated with the individual fatty acids (data not shown).
Figure 4.
Multiple HETE metabolites are produced from arachidonic acid, and 5-HETE specifically induces expression of AKR1C2 and AKR1C3 and results in androgen reduction. (A) Hierarchical clustering heat map (left) and table (right) shows the top intracellular metabolites altered in C4-2 cells treated with DMSO (control) or AA (100 μM) for 24 hours as measured by untargeted metabolomics. Data are representative of 6 biological replicates. *Represents compounds further validated by targeted mass spectrometry. (B) Absolute concentrations of arachidonic acid and the major HETE metabolites in C4-2 cells treated with DMSO (vehicle) or 100 μM arachidonic acid. (C) Protein (top panel) and transcript (bottom panel) levels of AKR1C2 and AKR1C3 in C4-2 cells treated with DMSO or 25 μM of the indicated HETEs. (D) AKR1C2 and AKR1C3 protein and transcript expression in C4-2 cells (left panels) and LNCaP cells (right panels) treated with increasing concentrations of 5-HETE for 24 hours. (E) HPLC analysis of 5α-dione, 5α-diol, and AST in C4-2 (top) and LNCaP (bottom) cells treated with DMSO (blue) or 25 μM 5-HETE (red) along with 100 nM [3H]-AD. Experiments were performed in triplicate and represent at least 3 biological replicates. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns = not significant.
Table 1.
Putative metabolites detected by LC-MS analysis of C4-2 cells treated with arachidonic acid
| m/z | RT (min) | HMDB identification | P value (Mann–Whitney U test) | Fold change | ESI mode |
|---|---|---|---|---|---|
| 371.2237 | 11.83 | Leukotriene B4; 5(S)-Hydroperoxyeicosatetraenoic acid; 8-iso-PGA1; Prostaglandin A1; Prostaglandin B1; 12(S)-HPETE; 15(S)-HPETE; Hepoxilin A3; Hepoxilin B3; 12(R)-HPETE; 11H-14,15-EETA; 11(R)-HPETE; 8(S)-HPETE; 15H-11,12-EETA; 6-trans-Leukotriene B4; 6-trans-12-epi-Leukotriene B4; 12(S)-Leukotriene B4; 14,15-DiHETE; 17,18-DiHETE; 5,15-DiHETE; 8,15-DiHETE; 5-HPETE; 15-Deoxy-16;12,14-Prostaglandin J2-2-glycerol ester | 2.16E−03 | 100 | NEG |
| 303.23579 | 11.83 | Arachidonic acid; Cis-8,11,14,17-Eicosatetraenoic acid; Mesterolone; 15(S)-Hydroxyeicosatrienoic acid | 4.33E−03 | 5.64 | NEG |
| 329.25167 | 12.08 | Docosapentaenoic acid (22n-6); Docosapentaenoic acid; 4,7,10,13,16-Docosapentaenoic acid | 1.52E−02 | 26.62 | NEG |
| 319.23078 | 11.17 | 5,6-Epoxy-8,11,14-eicosatrienoic acid; 8,9-Epoxyeicosatrienoic acid; 14R,15S-EpETrE; Hydroxyeicosatetraenoic acid; 15(S)-HETE; 14,15-Epoxy-5,8,11-eicosatrienoic acid; 11,12-Epoxyeicosatrienoic acid; 8-HETE; 16(R)-HETE; 11(R)-HETE; 20-Hydroxyeicosatetraenoic acid; 12-HETE; 18-Hydroxyarachidonic acid; 9-HETE; 11,12-EpETrE; 5-HETE; 19(S)-HETE; 14,15-DiHETrE; 8,9-DiHETrE; 11,12-DiHETrE; 5,6-DHET; 12-Keto-tetrahydro-leukotriene B4 | 2.60E−02 | 3.51 | NEG |
| 249.11537 | 7.05 | Ubiquinone Q1; 3-Carboxy-4-methyl-5-pentyl-2-furanpropionic acid | 6.49E−02 | 1.16 | NEG |
| 369.17709 | 12.84 | Androsterone sulfate; 5a-Dihydrotestosterone sulfate; Etiocholanolone sulfate | 1.32E−01 | 1.27 | NEG |
| 464.28062 | 8.24 | LysoPC(14:1(9Z)); Lithocholyltaurine | 1.32E−01 | 0.68 | NEG |
| 364.12188 | 1.33 | 2-(acetylamino)-1,5-anhydro-2-deoxy-3-O-b-D-galactopyranosyl-D-arabino-Hex-1-enitol; 2-(acetylamino)-1,5-anhydro-2-deoxy-4-O-b-D-galactopyranosyl-D-arabino-Hex-1-enitol; 1-(1,2,3,4,5-pentahydroxypent-1-yl)-1,2,3,4-tetrahydro-beta-carboline-3-carboxylate; N-Acetyllactosamine; Beta-1,4-mannose-N-acetylglucosamine; Lacto-N-biose I; Poly-N-acetyllactosamine | 1.82E−01 | 1.78 | NEG |
| 299.16401 | 5.31 | 2-Methoxyestrone; 19-Oxoandrost-4-ene-3,17-dione; Adrenosterone; 2-Hydroxy-3-methoxyestrone; Ubiquinone Q2 | 1.82E−01 | 0.4 | NEG |
| 531.29452 | 10.59 | 5b-Cyprinol sulfate; Pregnanediol-3-glucuronide; 3-alpha,20-alpha-dihydroxy-5-beta-pregnane 3-glucuronide | 1.82E−01 | 0.63 | NEG |
| 277.14304 | 3.92 | Alpha-CEHC; Monoethylhexyl phthalic acid; diisobutyl phthalate | 1.82E−01 | 1.9 | NEG |
| 464.30916 | 10.59 | Glycocholic acid; 3a,7b,12a-Trihydroxyoxocholanyl-Glycine; Hexadecanedioic acid mono-L-carnitine ester | 1.82E−01 | 0.36 | NEG |
| 365.23413 | 10.14 | 3b-Allotetrahydrocortisol; 5a-Tetrahydrocortisol; Tetrahydrocortisol; Cortolone; Beta-Cortolone; 9,12,13-TriHOME; 9,10,13-TriHOME | 2.40E−01 | 0.76 | NEG |
| 167.04703 | 2.2 | 2,3-Diaminosalicylic acid; Ureidopropionic acid; L-Asparagine; Glycyl-glycine; N-Carbamoylsarcosine | 2.40E−01 | 0.85 | NEG |
| 196.0628 | 2.92 | L-Dopa; DL-Dopa; Aminoadipic acid; Kinetin | 2.40E−01 | 1.13 | NEG |
| 98.0246 | 1.19 | Acetylglycine; L-2-Amino-3-oxobutanoic acid; L-Aspartate-semialdehyde | 2.40E−01 | 1.22 | NEG |
| 361.16658 | 7.88 | Thyrotropin-releasing factor; 12-Oxo-20-carboxy-leukotriene B4; Secoisolariciresinol | 2.40E−01 | 1.03 | NEG |
| 366.13388 | 2.18 | N-Acetyl-6-O-L-fucosyl-D-glucosamine; 2-Acetamido-2-deoxy-6-O-a-L-fucopyranosyl-D-glucose; 3-O-fucopyranosyl-2-acetamido-2-deoxyglucopyranose | 2.40E−01 | 0.85 | NEG |
| 71.01339 | 1.2 | Pyruvaldehyde; Malondialdehyde; L-Lactic acid; Hydroxypropionic acid; Glyceraldehyde; D-Lactic acid; Dihydroxyacetone | 3.10E−01 | 0.82 | NEG |
| 243.06433 | 1.22 | Uridine; Pseudouridine; 3,3′,4′5-Tetrahydroxystilbene; (R)-2-Benzylsuccinate; L-beta-aspartyl-L-glutamic acid | 3.10E−01 | 1.24 | NEG |
| 133.01452 | 0.93 | L-Malic acid; Malic acid; Xanthine; 6,8-Dihydroxypurine | 3.10E−01 | 0.85 | NEG |
| 350.10595 | 0.81 | N-Acetyl-7-O-acetylneuraminic acid; N-Acetyl-9-O-acetylneuraminic acid; N-Acetyl-4-O-acetylneuraminic acid | 3.10E−01 | 1.33 | NEG |
| 240.99249 | 7 | 5-Methylthioribose 1-phosphate; 5-Methylthioribulose 1-phosphate | 3.10E−01 | 0.52 | NEG |
| 257.0802 | 1.17 | Ribothymidine; Imidazoleacetic acid riboside; O-Desmethylangolensin; 3-Methyluridine; Monoisobutyl phthalic acid; Monobutylphthalate; Gamma Glutamylglutamic acid | 3.94E−01 | 1.13 | NEG |
| 132.03051 | 0.75 | L-Aspartic acid; D-Aspartic acid; Iminodiacetate; Guanine; 2-Hydroxyadenine; 8-Hydroxyadenine | 3.94E−01 | 0.87 | NEG |
| 89.02415 | 1.03 | L-Lactic acid; Hydroxypropionic acid; Glyceraldehyde; D-Lactic acid; Dihydroxyacetone | 3.94E−01 | 0.8 | NEG |
| 184.98678 | 0.79 | 2-Phosphoglyceric acid; 3-Phosphoglyceric acid; 2-Phospho-D-glyceric acid; 8-Chloroxanthine | 3.94E−01 | 0.64 | NEG |
| 193.05356 | 6.03 | Succinylacetone; Isopropylmaleate; Vanillactic acid | 3.94E−01 | 0.77 | NEG |
| 181.03958 | 0.75 | 3-Methyluric acid; 9-Methyluric acid; 7-Methyluric acid | 3.94E−01 | 1.11 | NEG |
| 405.1776 | 12.29 | Androsterone sulfate; 5a-Dihydrotestosterone sulfate; Etiocholanolone sulfate | 3.94E−01 | 1.12 | NEG |
| 401.08248 | 6.03 | 4-Phosphopantothenoylcysteine; Xanthurenate-8-O-beta-D-glucoside; Mercaptoacetate | 3.94E−01 | 0.76 | NEG |
| 333.21019 | 10.07 | 15-Keto-13,14-dihydroprostaglandin A2; Prostaglandin J2; Prostaglandin A2; 12-Keto-leukotriene B4; Prostaglandin B2; Delta-12-Prostaglandin J2; Leukotriene B5; Prostaglandin E2; (13E)-11a-Hydroxy-9,15-dioxoprost-13-enoic acid; Prostaglandin I2; Prostaglandin H2; Prostaglandin D2; Thromboxane A2; 20-Hydroxy-leukotriene B4; Prostaglandin F3a; 8-iso-PGF3a; Levuglandin E2; Levuglandin D2; 13,14-Dihydro-15-keto-PGE2; 15-Keto-prostaglandin F2a; Lipoxin A4; 8-iso-15-keto-PGF2a; Lipoxin B4; 8-isoprostaglandin E2; (5Z)-(15S)-11alpha-Hydroxy-9,15-dioxoprostanoate | 3.94E−01 | 1.3 | NEG |
| 299.10821 | 0.83 | Acetyl-N-formyl-5-methoxykynurenamine; Alpha-N-Phenylacetyl-L-glutamine | 3.94E−01 | 0.87 | NEG |
| 249.04022 | 2.82 | DL-Homocystine; L-Homocystine | 3.94E−01 | 1.31 | NEG |
| 259.02477 | 0.78 | Fructose 6-phosphate; Myo-inositol 1-phosphate; Galactose 1-phosphate; Dolichyl phosphate D-mannose; Fructose 1-phosphate; Mannose 6-phosphate; D-Myo-inositol 4-phosphate; Glucose 6-phosphate; Glucose 1-phosphate; Inositol phosphate; Beta-D-Glucose 6-phosphate; Beta-D-Fructose 6-phosphate; D-Tagatose 1-phosphate; D-Mannose 1-phosphate; Beta-D-Fructose 2-phosphate; 1D-myo-Inositol 3-phosphate; D-Tagatose 6-phosphate | 3.94E−01 | 0.75 | NEG |
| 181.05167 | 2.82 | Homovanillic acid; Isohomovanillic acid; 3,4-Dihydroxyhydrocinnamic acid; Hydroxyphenyllactic acid; 3-(3-hydroxyphenyl)-3-hydroxypropanoic acid; 3-Methoxy-4-hydroxyphenylglycolaldehyde; 2-Methylglutaric acid; Adipic acid; Methylglutaric acid; Monomethyl glutaric acid; 2,2-Dimethylsuccinic acid; (S)-2-Aceto-2-hydroxybutanoic acid | 3.94E−01 | 1.29 | NEG |
| 193.0252 | 1.05 | 4,5-Dihydroorotic acid; L-Dihydroorotic acid; Urolithin B | 4.85E−01 | 0.47 | NEG |
| 266.0906 | 0.85 | Adenosine; Deoxyguanosine; Neuraminic acid | 4.85E−01 | 0.96 | NEG |
| 297.1111 | 0.82 | 7-Methylguanosine; 7C-aglycone; Enterolactone | 4.85E−01 | 0.87 | NEG |
| 281.07576 | 0.68 | 5,6-Dihydrouridine; L-alpha-Aspartyl-L-hydroxyproline | 4.85E−01 | 1.11 | NEG |
| 274.10711 | 0.85 | Norophthalmic acid; Gamma-Glutamylglutamine | 4.85E−01 | 1.44 | NEG |
| 283.07108 | 1.97 | Xanthosine; 5-Hydroxyindoleacetylglycine | 4.85E-01 | 1.7 | NEG |
| 187.1351 | 7.17 | 3-Hydroxycapric acid; (R)-3-Hydroxydecanoic acid | 4.85E-01 | 1.11 | NEG |
| 334.0657 | 0.78 | S-Formylglutathione; D-4'-Phosphopantothenate | 4.85E−01 | 0.74 | NEG |
| 147.03042 | 1.07 | Citramalic acid; 3-Hydroxyglutaric acid; D-2-Hydroxyglutaric acid; L-2-Hydroxyglutaric acid; Ribonolactone; D-Xylono-1,5-lactone; 2-Hydroxy-2-methylsuccinic acid; Ethylsuccinic acid; arabonolactone; Arabinonic acid; Ribonic acid; 3-Methylxanthine; 7-Methylxanthine; 1-Methylxanthine; Noradrenochrome o-semiquinone | 4.85E−01 | 1.09 | NEG |
| 165.0565 | 7.25 | L-3-Phenyllactic acid; Phenyllactic acid; 4-Methoxyphenylacetic acid; Desaminotyrosine; Homovanillin; 2-Methyl-3-ketovaleric acid; 3-Methyl-2-oxovaleric acid; Ketoleucine; 2-Ketohexanoic acid; Mevalonolactone; 3-Oxohexanoic acid; Adipate semialdehyde; Vanylglycol; Phosphorylcholine | 4.85E−01 | 1.03 | NEG |
| 192.06777 | 3.77 | Phenylacetylglycine; Methylhippuric acid; 2-Methylhippuric acid; m-Methylhippuric acid; p-Methylhippuric acid; N-Methylhippuric acid; 3-Methylcrotonylglycine; Tiglylglycine; N-acetylproline; 3-Methoxytyrosine; Methyldopa | 4.85E−01 | 1.19 | NEG |
| 327.23597 | 11.67 | Docosahexaenoic acid; 3b,17b-Dihydroxyetiocholane; 3a,17a-Dihydroxy-5b-androstane; 3b,17a-Dihydroxy-5a-androstane; 5a-Androstane-3a,17a-diol; 5a-Androstane-3b,17b-diol; Androstanediol; Etiocholanediol; Dihydroandrosterone | 5.89E−01 | 1.2 | NEG |
| 149.04609 | 0.85 | D-Xylose; D-Ribose; 2-Deoxyribonic acid; L-Arabinose; L-Threo-2-pentulose; D-Xylulose; Beta-D-ribopyranose; Arabinofuranose; 1-Methylhypoxanthine | 5.89E−01 | 1.08 | NEG |
| 197.04677 | 4.51 | Vanillylmandelic acid; 3-(3,4-Dihydroxyphenyl)lactic acid; 2-Hydroxyadipic acid; 3-Hydroxyadipic acid; 3-Hydroxymethylglutaric acid; 2(R)-Hydroxyadipic acid; Glucosan | 5.89E−01 | 0.97 | NEG |
| 239.06936 | 8.4 | L-beta-aspartyl-L-alanine; 5-L-Glutamylglycine; Ribothymidine; Imidazoleacetic acid riboside; O-Desmethylangolensin; 3-Methyluridine | 5.89E−01 | 0.99 | NEG |
| 175.02578 | 1.44 | D-Glucurono-6,3-lactone; D-Glucuronic acid; Galacturonic acid; Iduronic acid; 5-Keto-D-gluconate; 2-Keto-L-gluconate | 5.89E−01 | 1.1 | NEG |
| 168.02865 | 0.74 | 2-Furoylglycine; L-2,3-Dihydrodipicolinate; L-Aspartic acid; D-Aspartic acid; Iminodiacetate | 5.89E−01 | 1.03 | NEG |
| 145.09872 | 0.72 | L-Lysine; D-Lysine; (3S)-3,6-Diaminohexanoate; (3S,5S)-3,5-Diaminohexanoate; 4-(1,1,3,3-tetramethylbutyl)-phenol | 5.89E−01 | 1.12 | NEG |
| 163.11281 | 1.37 | 4-(1,1,3,3-tetramethylbutyl)-phenol; Octanal; Nonanal; 2-nonanone; 8-pentadecanone | 5.89E−01 | 1.05 | NEG |
| 116.07178 | 1.03 | Betaine; L-Valine; N-Methyl-a-aminoisobutyric acid; Norvaline | 5.89E−01 | 1.07 | NEG |
| 267.07463 | 0.77 | Inosine; 3-Deoxy-D-glycero-D-galacto-2-nonulosonic acid; Allopurinol riboside; Arabinosylhypoxanthine | 5.89E−01 | 0.89 | NEG |
| 478.29809 | 10.29 | LysoPE(0:0/18:1(11Z)); LysoPE(0:0/18:1(9Z)); LysoPE(18:1(11Z)/0:0); LysoPE(18:1(9Z)/0:0) | 5.89E−01 | 0.85 | NEG |
| 227.14192 | 2.6 | L-isoleucyl-L-proline; L-leucyl-L-proline; Vitispirane I; Vitispirane II | 5.89E−01 | 0.82 | NEG |
| 203.06875 | 0.82 | L-beta-aspartyl-L-alanine; 5-L-Glutamylglycine; Monoisobutyl phthalic acid; Monobutylphthalate | 5.89E−01 | 0.8 | NEG |
| 188.05763 | 1.21 | Glutarylglycine; N-Acetylglutamic acid; FAPy-adenine; Dihydrolipoamide | 5.89E−01 | 1.19 | NEG |
| 195.06505 | 4.11 | Homoveratric acid; 3-Methyladipic acid; Pimelic acid; 3,3-Dimethylglutaric acid | 5.89E−01 | 1.1 | NEG |
| 148.06528 | 6.37 | 6-Methyladenine; 1-Methyladenine; 3-Methyladenine; 7-Methyladenine | 5.89E−01 | 1.03 | NEG |
| 242.082 | 0.77 | Cytidine; N-Acetyl-L-phenylalanine; Phenylpropionylglycine; 3-Phenylpropionylglycine | 5.89E−01 | 0.99 | NEG |
| 115.00375 | 1.2 | Fumaric acid; Maleic acid; L-Malic acid; Malic acid | 5.89E−01 | 0.81 | NEG |
| 239.06167 | 7.16 | Ribothymidine; Imidazoleacetic acid riboside; 3-Methyluridine | 5.89E−01 | 1.06 | NEG |
| 277.14709 | 9.5 | Alpha-CEHC; Monoethylhexyl phthalic acid; diisobutyl phthalate | 5.89E−01 | 1.05 | NEG |
| 202.01925 | 2.03 | Cystathionine ketimine; Homocysteinesulfinic acid; S-(3-oxo-3-carboxy-n-propyl)cysteine | 5.89E−01 | 1.19 | NEG |
| 326.12757 | 2.18 | S-(3-Methylbutanoyl)-dihydrolipoamide-E; S-(2-Methylbutanoyl)-dihydrolipoamide | 5.89E−01 | 1.15 | NEG |
| 285.20983 | 8.6 | Hexadecanedioic acid; 22,4-trimethyl-3-carboxyisopropylpentanoic acid, isobutyl ester | 5.89E−01 | 1.02 | NEG |
| 177.04305 | 2.97 | Gluconolactone; 2-Keto-3-deoxy-D-gluconic acid; 3-Keto-b-D-galactose; Galactonolactone; L-Gulonolactone; galactono-1,4-lactone; Galactonic acid; Gluconic acid; 1,3-Dimethyluric acid; 3,7-Dimethyluric acid; 1,9-Dimethyluric acid; Gulonic acid; 7,9-Dimethyluric acid; 1,7-Dimethyluric acid; Mannonate | 5.89E−01 | 0.9 | NEG |
| 130.0512 | 1.29 | Hydroxyproline; N-Acetyl-L-alanine; Propionylglycine; 5-Aminolevulinic acid; L-Glutamic-gamma-semialdehyde; 3-Hydroxy-L-proline; 4-Hydroxy-L-proline; 5-Amino-2-oxopentanoic acid; 3-hydroxyproline; 6-Methyladenine; 1-Methyladenine; 3-Methyladenine; 7-Methyladenine | 5.89E−01 | 1.08 | NEG |
| 220.0844 | 0.84 | N-Acetylgalactosamine; N-Acetyl-D-glucosamine; 2'-Deoxysepiapterin; Beta-N-Acetylglucosamine; N-Acetyl-b-D-galactosamine; N-Acetylmannosamine; N-Acetyl-D-mannosamine; Dihydrobiopterin; 6-Lactoyltetrahydropterin; 4a-Carbinolamine tetrahydrobiopterin; 1-hydroxy-2-Oxopropyl tetrahydropterin | 5.89E−01 | 0.97 | NEG |
| 397.31079 | 10.92 | 24,25-Dihydroxyvitamin D; 25,26-dihydroxyvitamin D; Calcitriol; 7a,12a-Dihydroxy-cholestene-3-one; 24R,25-Dihydroxyvitamin D3; 23S,25-dihydroxyvitamin D3; 3 beta-Hydroxy-5-cholestenoate; 7 alpha,24-Dihydroxy-4-cholesten-3-one; 7 alpha,26-Dihydroxy-4-cholesten-3-one | 6.99E−01 | 1.3 | NEG |
| 126.05628 | 1.19 | D-1-Piperideine-2-carboxylic acid; (S)-2,3,4,5-Tetrahydropiperidine-2-carboxylate; 4-methyleneproline; Isobutyrylglycine; N-Butyrylglycine; Allysine; 4-Acetamidobutanoic acid; (S)-5-Amino-3-oxohexanoate; 2-Keto-6-aminocaproate | 6.99E−01 | 1.22 | NEG |
| 403.21225 | 4.97 | 19-Hydroxy-PGE2; 6,15-Diketo,13,14-dihydro-PGF1a; Prostaglandin G2; 20-Hydroxy-PGE2; 6-Ketoprostaglandin E1; 11-Dehydro-thromboxane B2; Thromboxane B3; 5(6)-Epoxy Prostaglandin E1 | 6.99E−01 | 0.95 | NEG |
| 229.01376 | 0.78 | D-Ribulose 5-phosphate; Xylulose 5-phosphate; Ribose 1-phosphate; D-Ribose 5-phosphate; D-Xylulose 1-phosphate; D-Arabinose 5-phosphate; Beta-L-arabinose 1-phosphate | 6.99E−01 | 0.99 | NEG |
| 269.15316 | 5.24 | Estrone; Estriol; 2-Hydroxyestradiol; 16b-Hydroxyestradiol; 17-Epiestriol; 16,17-Epiestriol; 4-hydroxyestradiol | 6.99E−01 | 1.01 | NEG |
| 217.0501 | 0.76 | Homovanillic acid; Isohomovanillic acid; 3,4-Dihydroxyhydrocinnamic acid; Hydroxyphenyllactic acid; 3-(3-hydroxyphenyl)-3-hydroxypropanoic acid; 3-Methoxy-4-hydroxyphenylglycolaldehyde | 6.99E−01 | 0.94 | NEG |
| 158.08292 | 2.88 | 2-Methylbutyrylglycine; Isovalerylglycine; Valerylglycine; N-Acetylvaline; 3-Dehydrocarnitine; 5-Acetamidovalerate | 6.99E−01 | 1.05 | NEG |
| 138.02002 | 5.1 | 4-Nitrophenol; 6-Hydroxynicotinic acid; 3-Hydroxypicolinic acid; Pyruvatoxime; 3-Oxoalanine; 2-Aminomuconic acid | 6.99E−01 | 1.03 | NEG |
| 282.03868 | 0.72 | N-Acetyl-D-Glucosamine 6-Phosphate; N-Acetyl-D-mannosamine 6-phosphate; N-Acetyl-glucosamine 1-phosphate; N-Acetylglucosamine 6-phosphate; N-Acetyl-D-galactosamine 1-phosphate | 6.99E−01 | 0.88 | NEG |
| 137.03453 | 0.77 | Urocanic acid; Nicotinamide N-oxide; 5-Hydroxymethyl-4-methyluracil; 4-Imidazolone-5-propionic acid; Imidazolelactic acid | 6.99E−01 | 0.92 | NEG |
| 159.10333 | 5.41 | 7-Hydroxyoctanoic acid; Hydroxyoctanoic acid; 3-Hydroxyoctanoic acid; (R)-2-Hydroxycaprylic acid; (R)-3-Hydroxyoctanoic acid | 6.99E−01 | 0.99 | NEG |
| 461.21823 | 5.03 | 6-Dehydrotestosterone glucuronide; 11-Oxo-androsterone glucuronide | 8.18E−01 | 0.96 | NEG |
| 179.05711 | 0.72 | D-Glucose; D-Galactose; D-Mannose; Myo-inositol; 3-Deoxyarabinohexonic acid; Beta-D-Glucose; D-Fructose; L-Sorbose; Paraxanthine; Alpha-D-Glucose; D-Tagatose; Beta-D-Galactose; L-Gulose; Adrenochrome o-semiquinone; D-chiro-inositol; 5-Acetylamino-6-amino-3-methyluracil | 8.18E−01 | 0.96 | NEG |
| 165.0565 | 4.19 | L-3-Phenyllactic acid; Phenyllactic acid; 4-Methoxyphenylacetic acid; Desaminotyrosine; Homovanillin; 2-Methyl-3-ketovaleric acid; 3-Methyl-2-oxovaleric acid; Ketoleucine; 2-Ketohexanoic acid; Mevalonolactone; 3-Oxohexanoic acid; Adipate semialdehyde; Vanylglycol; Phosphorylcholine | 8.18E−01 | 1.02 | NEG |
| 165.05649 | 6.73 | L-3-Phenyllactic acid; Phenyllactic acid; 4-Methoxyphenylacetic acid; Desaminotyrosine; Homovanillin; 2-Methyl-3-ketovaleric acid; 3-Methyl-2-oxovaleric acid; Ketoleucine; 2-Ketohexanoic acid; Mevalonolactone; 3-Oxohexanoic acid; Adipate semialdehyde; Vanylglycol; Phosphorylcholine | 8.18E−01 | 1 | NEG |
| 153.05629 | 4.89 | 3-Hydroxy-2-methyl-R-(R,S)-butanoic acid; 2-Methyl-3-hydroxybutyric acid; 2-Ethylhydracrylic acid; 2-Hydroxy-3-methylbutyric acid; 3-Hydroxy-2-methyl-S-(R,R)-butanoic acid; 3-Hydroxyvaleric acid; Erythronilic acid; 3-Hydroxyisovaleric acid; 2-Hydroxyvaleric acid; 2-Hydroxy-2-methylbutyric acid; 4-Hydroxyisovaleric acid; 2-Octenedioic acid; cis-4-Octenedioic acid; trans-3-Octenedioic acid | 8.18E−01 | 0.99 | NEG |
| 345.20617 | 4.88 | Cortexolone; Corticosterone; 21-Deoxycortisol; 21-Hydroxy-5b-pregnane-3,11,20-trione; 19-Hydroxydeoxycorticosterone; 12(13)Ep-9-KODE; Tetrahydrocortisone; Dihydrocortisol; 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al; 11b,21-Dihydroxy-3,20-oxo-5b-pregnan-18-al; 11b,17a,21-Trihydroxypreg-nenolone; 18-Hydroxy-11-dehydrotetrahydrocorticosterone | 8.18E−01 | 0.98 | NEG |
| 128.03553 | 1.17 | Pyroglutamic acid; Pyrrolidonecarboxylic acid; Pyrroline hydroxycarboxylic acid; N-Acryloylglycine; 1-Pyrroline-4-hydroxy-2-carboxylate; 4-Oxoproline; L-Glutamic acid; N-Methyl-D-aspartic acid; N-Acetylserine; D-Glutamic acid; L-4-Hydroxyglutamate semialdehyde | 8.18E−01 | 1.01 | NEG |
| 311.20461 | 10.74 | 16-Dehydroprogesterone; Tetrahydrogestrinone; 19-Norandrosterone; 19-Nor-5-androstenediol; 19-Noretiocholanolone; Stearidonic acid; Deoxycorticosterone; 17-Hydroxyprogesterone; 11a-Hydroxyprogesterone | 9.37E−01 | 0.98 | NEG |
| 195.05228 | 0.76 | Galactonic acid; Gluconic acid; 1,3-Dimethyluric acid; 3,7-Dimethyluric acid; 1,9-Dimethyluric acid; Gulonic acid; 7,9-Dimethyluric acid; 1,7-Dimethyluric acid; Mannonate | 9.37E−01 | 1 | NEG |
| 110.02473 | 1.23 | Pyrrole-2-carboxylic acid; Pyrrole-3-carboxylic acid; Glycine; Pyroglutamic acid; Pyrrolidonecarboxylic acid; Pyrroline hydroxycarboxylic acid; N-Acryloylglycine; 1-Pyrroline-4-hydroxy-2-carboxylate; 4-Oxoproline | 9.37E−01 | 1 | NEG |
| 185.04427 | 2.02 | D-Xylose; D-Ribose; 2-Deoxyribonic acid; L-Arabinose; L-Threo-2-pentulose; D-Xylulose; Beta-D-ribopyranose; Arabinofuranose; 1-Methylhypoxanthine | 9.37E−01 | 1.04 | NEG |
| 165.02013 | 3.36 | Benzoquinoneacetic acid; Terephthalic acid; Glutaconic acid; Citraconic acid; Mesaconic acid; Itaconic acid; Gamma-delta-Dioxovaleric acid; 3,4-Dihydroxymandelic acid; 4-O-Methylgallic acid | 9.37E−01 | 1.09 | NEG |
| 218.06869 | 1.3 | 2,6-Diamino-4-hydroxy-5-N-methylformamidopyrimidine; Sepiapterin; Biopterin; D-Biopterin; Orinapterin; Dyspropterin; Primapterin; 8-(aminomethyl)sulfanyl-6-sulfanyloctanoic acid | 9.37E−01 | 1 | NEG |
| 204.068 | 4.48 | Indolelactic acid; 5-Methoxyindoleacetate; Cinnamoylglycine; Norepinephrine; Pyridoxine; 6-Hydroxydopamine; 5-Hydroxydopamine; N-Acetyl-L-tyrosine | 9.37E−01 | 1.08 | NEG |
| 197.04678 | 3.53 | Vanillylmandelic acid; 3-(3,4-Dihydroxyphenyl)lactic acid; 2-Hydroxyadipic acid; 3-Hydroxyadipic acid; 3-Hydroxymethylglutaric acid; 2(R)-Hydroxyadipic acid; Glucosan | 9.37E−01 | 0.97 | NEG |
| 191.0209 | 1.05 | Citric acid; Isocitric acid; D-threo-Isocitric acid; Diketogulonic acid; Tricarballylic; Galactaric acid; Glucaric acid | 9.37E−01 | 0.96 | NEG |
| 135.0455 | 3.73 | Phenylacetic acid; 4-Hydroxyphenylacetaldehyde; 4-Methylbenzoic acid; Senecioic acid; Tiglic acid; 2-Ethylacrylic acid; 3-Methylbutyrolactone | 9.37E−01 | 0.96 | NEG |
| 272.07595 | 2.78 | Sepiapterin; Biopterin; D-Biopterin; Orinapterin; Dyspropterin; Primapterin; 8-(aminomethyl)sulfanyl-6-sulfanyloctanoic acid | 9.37E−01 | 0.99 | NEG |
Abbreviations: ESI, electrospray ionization; HMDB, Human Metabolome Database; RT, retention time.
We then performed targeted mass spectrometry analysis using high-performance liquid chromatography (HPLC) with on-line electrospray ionization tandem mass spectrometry (LC/ESI/MS/MS) to quantify levels of oxidation products of arachidonic acid so as to validate those metabolites identified by untargeted mass spectrometry, namely 5-HETE, 8-HETE, 9-HETE, 11-HETE, 12-HETE, and 15-HETE (Fig. 4B). As expected, many of the oxidized fatty acids interrogated were increased in C4-2 cells due to arachidonic acid treatment, except for 8-HETE. Next, we sought to determine whether the androgen metabolic phenotype and concordant enzyme expression induced by arachidonic acid are recapitulated by any of the specific metabolites derived from arachidonic acid. Of the HETEs assessed, only 5-HETE induced AKR1C2 and AKR1C3 transcript and protein expression in C4-2 cells (Fig. 4C; Supplementary Fig. S4A, S4B (17)). There was also a concentration-dependent increase in AKR1C2 and AKR1C3 expression in response to 5-HETE treatment (Fig. 4D), similar to effects we observed with arachidonic acid (Fig. 3B).
To investigate whether 5-HETE can recapitulate the metabolic phenotype induced by arachidonic acid and accelerate the conversion from (3-keto) 5α-dione to produce 3-OH and 17-OH androgens, C4-2 and LNCaP cells were treated with 5-HETE, and conversion of [3H]-AD to downstream 5α-reduced metabolites was assessed by HPLC. Similar to arachidonic acid treatment, C4-2 cells treated with 5-HETE exhibited hastened metabolism of 5α-dione and increased levels of AST whereas 5α-diol was unaltered (Fig. 4E, top panel). Levels of 5α-diol were significantly increased in LNCaP cells treated with 5-HETE whereas no significant differences in 5α-dione or AST were observed. Together, these data suggest that while 5-HETE can elicit the expression of AKR1C2 and AKR1C3, there may be other analytes of arachidonic acid that may work in concert to elicit a stronger metabolic phenotype than was observed with 5-HETE alone.
AKR1C2 and AKR1C3 Expression Is Regulated by Nrf2 Activation
Finally, we wanted to understand the mechanism of arachidonic acid-dependent induction of AKR1C2 and AKR1C3. One of the byproducts of arachidonic acid oxidation is the accumulation of reactive oxygen species, which activates downstream antioxidant responses. A key transcription factor that mediates this process is nuclear factor erythroid 2 related factor 2 (Nrf2; encoded by the gene NFE2L2) (46). Previous work demonstrated that Nrf2 regulates several AKR family members, including AKR1C2 and AKR1C3 (47).
To determine the timing of Nrf2 induction with respect to AKR1C2 and AKR1C3 expression, we performed a time course study of AKR1C2 and AKR1C3 expression. Transcript levels of both AKR1C2 and AKR1C3 enzymes were increased 24 hours after treatment (Fig. 5A). However, total Nrf2 protein was activated as early as 30 minutes after treatment with arachidonic acid in C4-2 and LNCaP cells and remained elevated after 4 hours but began to decline after 8 hours, indicating upstream induction of Nrf2 occurs prior to induction of AKR transcripts (Fig. 5B and 5C, top panels). Analysis over time of Nrf2 nuclear translocation in C4-2 and LNCaP cells demonstrated a significant increase at 4 hours in response to arachidonic acid treatment with elevated AKR1C2 and AKR1C3 protein levels observed at 24 hours (Fig. 5B and 5C, bottom panels). To determine the effects of Nrf2 loss on AKR1C2 and AKR1C3 expression and steroid metabolism, we stably knocked down the NFE2L2 gene in C4-2 cells using lentiviral shRNA constructs. Knockdown of NFE2L2 abrogated the induction of AKR1C2, which is the dominantly induced AKR enzyme, in C4-2 cells treated with arachidonic acid (Fig. 5D). However, reduced Nrf2 expression did not result in a significant reduction of AKR1C3 protein levels. Taken together, these data demonstrate that effects of fatty acid–induced expression of AKR1C2 can be reversed by targeting Nrf2.
Figure 5.
Arachidonic acid (AA) induces Nrf2 transcription of AKR1C2. (A) Time course experiment of transcriptional upregulation of AKR1C2 (left panel) and AKR1C3 (right panel) after treatment of C4-2 cells with 50 μM AA for the indicated times. (B, C) Top panels: Western blot analysis of Nrf2 in whole-cell lysate (top panel) of C4-2 and LNCaP cells treated with AA. Bottom panels: Nrf2 protein translocation in nuclear and cytoplasmic fractions in C4-2 and LNCaP cells treated with AA. (D) Protein (left panel) and mRNA levels (right panel) of Nrf2, AKR1C2, and AKR1C3 in C4-2 cells stably expressing shCTRL or 2 different shRNAs targeting NFE2L2 (shNRF2 #1 and shNRF2 #2). ***P < 0.001, ****P < 0.0001, ns not significant.
Discussion
Androgens are critical hormones that have broad roles in endocrine-related malignancies, lipid metabolism, asthma, inflammatory regulation, and many other aspects of physiology (4, 48–50). Understanding the effects of lipids and fatty acid products on steroid metabolic pathways is important in understanding drivers of disease and how they can be therapeutically targeted. Previous studies have shown that loss of AKR1C2 prevents DHT inactivation in primary omental and subcutaneous pre-adipocytes, resulting in reduced lipid accumulation and adipocyte maturation (51). Moreover, interrogation of dietary interventions and the effects of fatty acids in endocrine-driven cancers have underscored the pivotal and diverse roles of lipid mediators in cellular function, signaling, and disease progression (26, 52).
In the present study, we investigated the functional implications of exposure to and signaling from several fatty acids in cancer, using an unbiased approach. Testing of a fatty acid panel revealed varying effects on androgen metabolism in prostate cancer cells. The strongest androgen metabolic phenotype we identified led us to explore the effects of arachidonic acid and its downstream fatty acid metabolites. We provide evidence that arachidonic acid promotes the production of the 3α-OH androgens, 5α-diol and AST, in both prostate cancer cell lines and tissue explants from men with prostate cancer (Fig. 2). Interestingly, downstream metabolites were similar in the C4-2 cells and prostate tissue explants, particularly the predominant production of 5α-diol. We speculate this is because both tissue explants and C4-2 cells lack expression and activity of androgen glucuronidation enzymes as compared with LNCaP cells (53). We also observed increased production of T in response to arachidonic acid treatment in both C4-2 and LNCaP cells (Fig. 2A and 2B; Supplementary Fig. S2 (17)).
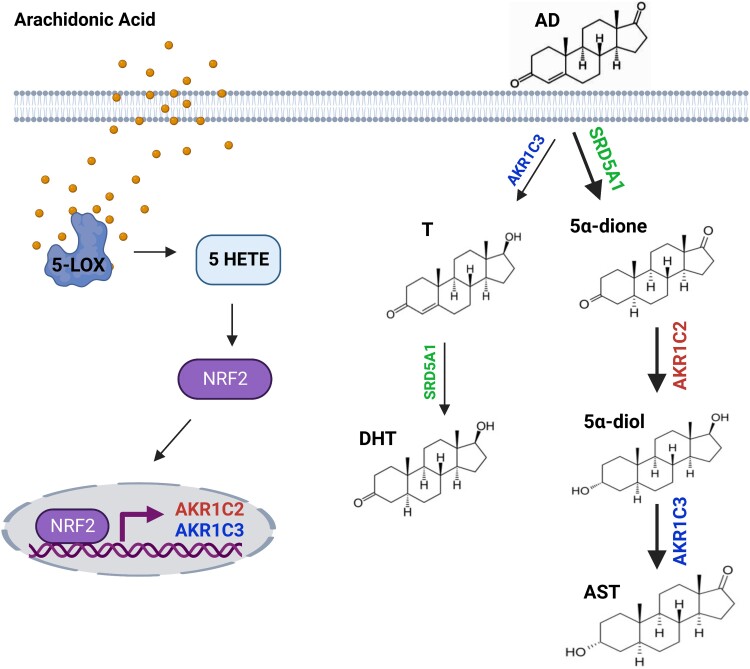
This study demonstrates these effects are the result of induction of expression of the AKR enzymes, largely AKR1C2 and perhaps to a lesser extent, AKR1C3. Moreover, arachidonic acid–induced AKR expression in breast cancer and lung epithelial cells, suggesting that this mechanism is not just limited to prostatic tissues but rather occurs more broadly in epithelial cells derived from various human tissues. Untargeted metabolomics examination identified 216 putative IDs in C4-2 cells treated with arachidonic acid (Supplementary Table S1 (17)). Several of the analytes detected were products of the 3 major classes of enzymes that metabolize arachidonic acid, namely lipoxygenase, cyclooxygenase, and P450. We validated several metabolites that previous studies had linked with increased tumor growth, invasion, and oncogenic signaling and identified 5-HETE as a metabolite that induces expression of AKRs and promotes androgen reduction and inactivation (Fig. 4). We also show that the antioxidant sensor Nrf2 regulates transcriptional activation of AKRs in response to fatty acid treatment. Together, our data highlight the direct impact of fatty acid oxidation on the expression of androgen-inactivating enzymes (Fig. 6).
Figure 6.
Proposed mechanism of arachidonic acid treatment on AKR1C-mediated androgen reduction. Arachidonic acid metabolites such as 5-HETE result in nuclear translocation of Nrf2, increasing transcription of AKR1C2 expression. AKR1C2 induction accelerates the metabolism of 5α-dione to inactive metabolites 5α-diol and AST.
The conversion of arachidonic acid to 5-HETE and inflammatory prostaglandins has significant roles in asthma, cancer, neutrophil and macrophage function, atherosclerosis, and Alzheimer disease (54). Interestingly, other studies have shown that the sex discordance of inflammatory processes, including asthma (55) and multiple sclerosis (56), is mediated in part by androgens and their anti-inflammatory properties. Conversely, androgen depletion in prostate cancer removes the anti-inflammatory effects of androgens and is immunostimulatory (14, 57). Our study suggests that the pro-inflammatory 5-HETE counters the effects of active androgens in part by generating inactive androgens through induction of AKR1C2 expression. This might be physiologically important for ablating the anti-inflammatory effects of androgens and maintaining a sustained inflammatory response. Notably, androgens also play an essential role in the response to therapeutic interventions in malignancies not generally recognized to be endocrine-responsive, such as melanoma. A recent report found that androgen signaling mediates melanoma drug resistance. Male patients with metastatic melanoma had greater AR expression after treatment with BRAF/MEK inhibitors, resulting in reduced overall response rate and relapse-free survival compared with women receiving the same treatment (5); this study provides additional evidence for sex-specific differences in disease and response to therapeutic interventions. Our data suggest that androgen reduction might be important in these newly identified pathophysiologic processes.
Our work has implicated arachidonic acid metabolism to 5-HETE in AKR1C2 and AKR1C3 inactivation of androgens; however, we cannot discount the effects of other metabolites of arachidonic acid on the steroidogenic pathway. It is possible that other fatty acids in addition to or in combination with 5-HETE also contribute to the changes observed in steroid metabolism and expression of AKRs as demonstrated by the partial metabolic response observed with 5-HETE treatment (Fig. 4E). However, investigating the roles of individual fatty acids in steroidogenesis may prove to be challenging due to de novo production of arachidonic acid and other fatty acids, which may contribute to androgen production. In vivo studies could provide further insight concerning the effects of dietary arachidonic acid on androgen inactivation in endocrine organs. Although we observed increased levels of 5α-diol in ex vivo tissue cultures (Fig. 2C), downstream metabolites including T, DHT, and AST were not detected, limiting our ability to understand the total effect of fatty acids in androgen metabolism. Also, batch-to-batch variability in arachidonic acid resulted in variable responses in downstream production of 3-OH and 17-OH metabolites.
The functional role of Nrf2 in cancer is evolving, as reports show that it can act as either a tumor suppressor or an oncogene (58). A study investigating the effects of Nrf2 expression in prostate cancer cells reported that overexpression of Nrf2 in LNCaP and C4-2 cells resulted in reduced levels androgen receptor activation in response to DHT treatment (59). Our findings show that knockdown of NFE2L2 inhibited arachidonic acid induction of AKR1C2 gene and protein expression whereas it had no significant effect on AKR1C3 protein expression (Fig. 5D). Nrf2 loss significantly affected AKR1C2 expression, and additional studies are needed to determine the downstream effects of Nrf2 and AKR1C2 loss on androgen metabolism. However, previous studies provide key clues to suggest NRF2 loss may be a driver of castrate-resistant disease. Reports have shown that Nrf2 expression is gradually lost in tumor tissue and cell lines with tumor progression (60).
The evolving connection between lipid metabolism and androgens may have important clinical implications. Cyclooxygenase-2 is an inducible enzymatic driver of inflammatory events that converts arachidonic acid to produce prostaglandins and thromboxane, which can promote cardiovascular disease, tumorigenesis, arthritis, and other inflammatory diseases (61, 62). Cyclooxygenase-2 inhibition with nonsteroidal anti-inflammatory drugs (NSAIDs) has been extensively investigated in various clinical contexts, including cancer. A potential implication of our work is that NSAIDs might affect arachidonic acid substrate availability for 5-lipoxygenase, generation of 5-HETE, and downstream androgen inactivation. A recent randomized phase 3 clinical trial assessing the efficacy of combining androgen-deprivation therapy (ADT) with the adrenal androgen synthesis inhibitor abiraterone reported that the metastasis-free survival benefit from adrenal androgen ablation was strongly associated with NSAID use, with an approximately 2-fold difference in hazard ratios (63). Whether the observed benefits are directly attributable to altered arachidonic acid metabolism and substrate availability for the generation and effects of 5-HETE on androgen metabolism is a possibility that warrants investigation. Nonetheless, this striking finding provides evidence that lipid modulation could significantly alter the response to adrenal androgen ablation and the efficacy of drugs currently used in the clinic.
In summary, our findings show that the generation of 5-HETE from arachidonic acid is a potent modulator of AKR expression in epithelial cells from multiple organs, including breast, prostate, and lung epithelial cells. 5-HETE alters the balance of potent vs inactive androgens in a wide array of tissue types. Taken together, this study highlights a novel functional role of arachidonic acid metabolism and 5-HETE in androgen metabolism and physiology.
Acknowledgments
We thank Cassandra Talerico, Ph.D., a salaried employee of the Cleveland Clinic, for help with manuscript editing.
Abbreviations
- 3βHSD1
3β-hydroxysteroid dehydrogenase-1
- AD
androstenedione
- AKR1C2
aldo-keto reductase family 1 member C2
- AR
androgen receptor
- AST
androsterone
- DHEA
dehydroepiandrosterone
- DHT
5α-dihydrotestosterone
- DMSO
dimethyl sulfoxide
- HETE
hydroxyeicosatetraenoic acid
- HPLC
high-performance liquid chromatography
- LC-MS
liquid chromatography-mass spectrometry
- Nrf2
nuclear factor erythroid 2-related factor 2
- NSAID
nonsteroidal anti-inflammatory drug
- PBS
phosphate-buffered saline
- pen/strep
penicillin–streptomycin
- RPMI
Roswell Park Memorial Institute (medium)
- RT-PCR
reverse transcription polymerase chain reaction
- T
testosterone
Contributor Information
Aimalie L Hardaway, Genitourinary Malignancies Research Center, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44195, USA.
Maryam Goudarzi, Proteomics and Metabolomics Core, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44195, USA.
Michael Berk, Genitourinary Malignancies Research Center, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44195, USA.
Yoon-Mi Chung, Genitourinary Malignancies Research Center, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44195, USA.
Renliang Zhang, Proteomics and Metabolomics Core, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44195, USA.
Jianneng Li, Genitourinary Malignancies Research Center, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44195, USA.
Eric Klein, Department of Urology, Glickman Urological and Kidney Institute, Cleveland Clinic, Cleveland, OH 44195, USA.
Nima Sharifi, Genitourinary Malignancies Research Center, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44195, USA; Department of Urology, Glickman Urological and Kidney Institute, Cleveland Clinic, Cleveland, OH 44195, USA; Department of Hematology and Oncology, Taussig Cancer Institute, Cleveland Clinic, Cleveland, OH 44195, USA.
Funding
This work was supported in part by grants from the National Cancer Institute (R01CA172382, R01CA236780, R01CA261995, and R01CA249279 to N.S. and R50CA251961 to M.B.), the Prostate Cancer Foundation (to N.S.), the U.S. Army Medical Research and Development Command (W81XWH2010137 and W81XWH-22-1-0082 to N.S.).
Disclosures
None.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on request.
References
- 1. Desai K, McManus JM, Sharifi N. Hormonal therapy for prostate cancer. Endocr Rev. 2021;42(3):354–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moghrabi N, Hughes IA, Dunaif A, Andersson S. Deleterious missense mutations and silent polymorphism in the human 17beta-hydroxysteroid dehydrogenase 3 gene (HSD17B3). J Clin Endocrinol Metab. 1998;83(8):2855–2860. [DOI] [PubMed] [Google Scholar]
- 3. Wilson JD. The role of 5alpha-reduction in steroid hormone physiology. Reprod Fertil Dev. 2001;13(8):673–678. [DOI] [PubMed] [Google Scholar]
- 4. Zein J, Gaston B, Bazeley P, et al. HSD3B1 Genotype identifies glucocorticoid responsiveness in severe asthma. Proc Natl Acad Sci U S A 2020;117(4):2187–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vellano CP, White MG, Andrews MC, et al. Androgen receptor blockade promotes response to BRAF/MEK-targeted therapy. Nature. 2022;606(7915):797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. [DOI] [PubMed] [Google Scholar]
- 7. Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dai C, Heemers H, Sharifi N. Androgen signaling in prostate cancer. Cold Spring Harb Perspect Med. 2017;7(9):a030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naelitz BD, Sharifi N. Through the looking-glass: reevaluating DHEA metabolism through HSD3B1 genetics. Trends Endocrinol Metab. 2020;31(9):680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davey RA, Grossmann M. Androgen receptor structure, function and biology: from bench to bedside. Clin Biochem Rev. 2016;37(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- 11. Spaanderman DCE, Nixon M, Buurstede JC, et al. Androgens modulate glucocorticoid receptor activity in adipose tissue and liver. J Endocrinol. 2019;240(1):51–63. [DOI] [PubMed] [Google Scholar]
- 12. Bereshchenko O, Bruscoli S, Riccardi C. Glucocorticoids, sex hormones, and immunity. Front Immunol. 2018;9:1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alyamani M, Li J, Patel M, et al. Deep androgen receptor suppression in prostate cancer exploits sexually dimorphic renal expression for systemic glucocorticoid exposure. Ann Oncol. 2020;31(3):369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guan X, Polesso F, Wang C, et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature. 2022;606(7915):791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li J, Berk M, Alyamani M, et al. Hexose-6-phosphate dehydrogenase blockade reverses prostate cancer drug resistance in xenograft models by glucocorticoid inactivation. Sci Transl Med. 2021;13(595):eabe8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Valle S, Sharifi N. Targeting glucocorticoid metabolism in prostate cancer. Endocrinology. 2021;162(9):bqab132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hardaway AG, Berk M, Chung Y-M, et al. 5-hydroxyeicosatetraenoic acid controls androgen reduction in diverse types of human epithelial cells. Supplementary data. BioStudies ELIXIER Deposition Database.November 14,2022. https://www.ebi.ac.uk/biostudies/studies/S-BSST941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poutanen M, Penning TM. Biology and clinical relevance of hydroxysteroid (17beta) dehydrogenase enzymes. Mol Cell Endocrinol. 2019;489:1–2. [DOI] [PubMed] [Google Scholar]
- 19. Adeniji AO, Chen M, Penning TM. AKR1C3 As a target in castrate resistant prostate cancer. J Steroid Biochem Mol Biol. 2013;137:136–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Penning TM, Wangtrakuldee P, Auchus RJ. Structural and functional biology of Aldo-Keto reductase steroid-transforming enzymes. Endocr Rev. 2019;40(2):447–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kruse ML, Patel M, McManus J, et al. Adrenal-permissive HSD3B1 genetic inheritance and risk of estrogen-driven postmenopausal breast cancer. JCI Insight. 2021;6(20):e150403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang X, Simpson ER, Brown KA. Aromatase overexpression in dysfunctional adipose tissue links obesity to postmenopausal breast cancer. J Steroid Biochem Mol Biol. 2015;153:35–44. [DOI] [PubMed] [Google Scholar]
- 23. Flanagan MR, Doody DR, Voutsinas J, et al. Association of HSD3B1 genotype and clinical outcomes in postmenopausal estrogen-receptor-positive breast cancer. Ann Surg Oncol. 2022;29(11):7194–7201. [DOI] [PubMed] [Google Scholar]
- 24. McManus JM, Vargas R, Bazeley PS, Schumacher FR, Sharifi N. Association between adrenal-restrictive HSD3B1 inheritance and hormone independent subtypes of endometrial and breast cancer. JNCI Cancer Spectr. 2022;6(5):pkac061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Calder PC. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot Essen Fat Acids. 2006;75(3):197–202. [DOI] [PubMed] [Google Scholar]
- 26. Munir R, Lisec J, Swinnen JV, Zaidi N. Too complex to fail? Targeting fatty acid metabolism for cancer therapy. Prog Lipid Res. 2022;85:101143. [DOI] [PubMed] [Google Scholar]
- 27. Sonnweber T, Pizzini A, Nairz M, Weiss G, Tancevski I. Arachidonic acid metabolites in cardiovascular and metabolic diseases. Int J Mol Sci. 2018;19(11):3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tallima H, El Ridi R. Arachidonic acid: physiological roles and potential health benefits—a review. J Adv Res. 2018;11:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chaudry AA, Wahle K, McClinton S, Moffat L. Arachidonic acid metabolism in benign and malignant prostatic tissue in vitro: effects of fatty acids and cyclooxygenase inhibitors. Int J Cancer. 1994;57(2):176–180. [DOI] [PubMed] [Google Scholar]
- 30. Luo Y, Jin M, Lou L, et al. Role of arachidonic acid lipoxygenase pathway in asthma. Prostaglandins Other Lipid Mediat. 2022;158:106609. [DOI] [PubMed] [Google Scholar]
- 31. Dai C, Chung YM, Kovac E, et al. Direct metabolic interrogation of dihydrotestosterone biosynthesis from adrenal precursors in primary prostatectomy tissues. Clin Cancer Res. 2017;23(20):6351–6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang KH, Li R, Kuri B, et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154(5):1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shishehbor MH, Zhang R, Medina H, et al. Systemic elevations of free radical oxidation products of arachidonic acid are associated with angiographic evidence of coronary artery disease. Free Radic Biol Med. 2006;41(11):1678–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mak TD, Laiakis EC, Goudarzi M, Fornace AJ. Selective paired ion contrast analysis: a novel algorithm for analyzing postprocessed LC-MS metabolomics data possessing high experimental noise. Anal Chem. 2015;87(6):3177–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Giovannucci E, Rimm EB, Colditz GA, et al. A prospective study of dietary fat and risk of prostate cancer. J Natl Cancer Inst. 1993;85(19):1571–1579. [DOI] [PubMed] [Google Scholar]
- 36. Chang KH, Li R, Papari-Zareei M, et al. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci U S A. 2011;108(33):13728–13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li J, Alyamani M, Zhang A, et al. Aberrant corticosteroid metabolism in tumor cells enables GR takeover in enzalutamide resistant prostate cancer. Elife. 2017;6:e20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sharifi N. The 5alpha-androstanedione pathway to dihydrotestosterone in castration-resistant prostate cancer. J Investig Med. 2012;60(2):504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Penning TM, Jin Y, Rizner TL, Bauman DR. Pre-receptor regulation of the androgen receptor. Mol Cell Endocrinol. 2008;281(1-2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rutting S, Horvat J, Wood L, Hansbro P, Oliver B. The effect of dietary fatty acids on respiratory infection in human lung cells. Eur Respir J. 2018;52:PA4983. [Google Scholar]
- 41. Rutting S, Zakarya R, Bozier J, et al. Dietary fatty acids amplify inflammatory responses to infection through p38 MAPK signaling. Am J Respir Cell Mol Biol. 2019;60(5):554–568. [DOI] [PubMed] [Google Scholar]
- 42. Monaco ME. Fatty acid metabolism in breast cancer subtypes. Oncotarget. 2017;8(17):29487–29500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Holtzman MJ. Arachidonic acid metabolism. Implications of biological chemistry for lung function and disease. Am Rev Respir Dis. 1991;143(1):188–203. [DOI] [PubMed] [Google Scholar]
- 44. Wang B, Wu L, Chen J, et al. Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets. Signal Transduct Target Ther. 2021;6(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Powell WS, Rokach J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim Biophys Acta. 2015;1851(4):340–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shaw P, Chattopadhyay A. Nrf2-ARE signaling in cellular protection: mechanism of action and the regulatory mechanisms. J Cell Physiol. 2020;235(4):3119–3130. [DOI] [PubMed] [Google Scholar]
- 47. MacLeod AK, McMahon M, Plummer SM, et al. Characterization of the cancer chemopreventive NRF2-dependent gene battery in human keratinocytes: demonstration that the KEAP1–NRF2 pathway, and not the BACH1–NRF2 pathway, controls cytoprotection against electrophiles as well as redox-cycling compounds. Carcinogenesis. 2009;30(9):1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Beggs LA, Yarrow JF, Conover CF, et al. Testosterone alters iron metabolism and stimulates red blood cell production independently of dihydrotestosterone. Am J Physiol Endocrinol Metab. 2014;307(5):E456–E461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jordan CL, Doncarlos L. Androgens in health and disease: an overview. Horm Behav. 2008;53(5):589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Auchus RJ, Sharifi N. Sex hormones and prostate cancer. Annu Rev Med. 2020;71(1):33–45. [DOI] [PubMed] [Google Scholar]
- 51. Veilleux A, Côté J-A, Blouin K, et al. Glucocorticoid-induced androgen inactivation by aldo-keto reductase 1C2 promotes adipogenesis in human preadipocytes. Am J Physiol-Endocrinol Metab. 2012;302(8):E941–E949. [DOI] [PubMed] [Google Scholar]
- 52. McEntee MF, Ziegler C, Reel D, et al. Dietary n-3 polyunsaturated fatty acids enhance hormone ablation therapy in androgen-dependent prostate cancer. Am J Pathol. 2008;173(1):229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhu Z, Chung YM, Sergeeva O, et al. Loss of dihydrotestosterone-inactivation activity promotes prostate cancer castration resistance detectable by functional imaging. J Biol Chem. 2018;293(46):17829–17837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sun Q-Y, Zhou H-H, Mao X-Y. Emerging roles of 5-lipoxygenase phosphorylation in inflammation and cell death. Oxid Med Cell Longev. 2019;2019:2749173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pace S, Pergola C, Dehm F, et al. Androgen-mediated sex bias impairs efficiency of leukotriene biosynthesis inhibitors in males. J Clin Invest. 2017;127(8):3167–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhu ML, Bakhru P, Conley B, et al. Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator. Nat Commun. 2016;7(1):11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Drake CG, Doody AD, Mihalyo MA, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7(3):239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jeddi F, Soozangar N, Sadeghi MR, Somi MH, Samadi N. Contradictory roles of Nrf2/Keap1 signaling pathway in cancer prevention/promotion and chemoresistance. DNA Repair (Amst). 2017;54:13–21. [DOI] [PubMed] [Google Scholar]
- 59. Schultz MA, Hagan SS, Datta A, et al. Nrf1 and Nrf2 transcription factors regulate androgen receptor transactivation in prostate cancer cells. PLoS One. 2014;9(1):e87204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Frohlich DA, McCabe MT, Arnold RS, Day ML. The role of Nrf2 in increased reactive oxygen species and DNA damage in prostate tumorigenesis. Oncogene. 2008;27(31):4353–4362. [DOI] [PubMed] [Google Scholar]
- 61. Vecchio AJ, Simmons DM, Malkowski MG. Structural basis of fatty acid substrate binding to cyclooxygenase-2. J Biol Chem. 2010;285(29):22152–22163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Alexanian A, Sorokin A. Cyclooxygenase 2: protein-protein interactions and posttranslational modifications. Physiol Genomics. 2017;49(11):667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Attard G, Murphy L, Clarke NW, et al. Abiraterone Acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet. 2022;399(10323):447–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on request.