Abstract
Genetic syndromes resulting from molecular alterations of the RAS-MAPK signaling cascade have become the focus of heightened interest among behavioral scientists due to discoveries that proteins within this pathway play an important role in memory formation and consolidation. Individuals with Noonan syndrome (NS), caused by germline mutations in the RAS-MAPK pathway, exhibit wide variability in cognitive and memory skills. The current study aimed to characterize memory deficits that occur in some affected individuals as a key step toward understanding the neurocognitive effects of dysregulated Ras signaling. Learning and memory skills were assessed among twenty-nine children and adolescents with NS using the Wide Range Assessment of Memory and Learning, Second Edition. Performance across subdomains (verbal memory, visual memory and working memory) was compared, as well as the effect of response type (free recall vs. recognition). For immediate memory, children with NS performed significantly better on verbal memory tasks than on visual memory or working memory tasks. For delayed memory, verbal free recall tasks that depend heavily on prefrontal-hippocampal networks were more challenging than recognition tasks that rely on more distributed temporal cortical regions. Additionally, verbal information presented in context was more easily retained than that presented in a rote format. The current study contributes to our knowledge of the effects of dysregulated RAS-MAPK signaling on the brain and behavior. Continued research on neurocognitive skills in NS has the potential to generate a novel conceptualization of how learning disabilities can arise from altered molecular processes within a specific biological pathway.
Keywords: Noonan syndrome, learning, memory, hippocampus, RAS-MAPK pathway, PTPN11, SOS1, BRAF
INTRODUCTION
Noonan syndrome (NS) is a multiple congenital anomaly syndrome characterized by short stature, cardiac disease, distinctive facial features, and musculoskeletal abnormalities. NS is fairly common, with an incidence estimated at 1/1000 to 1/ 2500 live births and a presumed higher rate of milder expression [Allanson, 2007]. The syndrome is caused by germline mutations in genes coding for molecules in the RAS- MAPK signaling cascade. Mutations in the PTPN11, SOS1, KRAS, NRAS, RAF1, SHOC2, CBL, MEK1, and BRAF genes account for 60–80% of cases of clinically diagnosed NS [Tartaglia et al., 2010; Zenker, 2011], while the underlying genetic etiologies for the remaining cases have yet to be determined. Additional genes within the RAS-MAPK pathway are associated with clinically related disorders (“RASopathies”), including neurofibromatosis type 1 (NF1), cardiofaciocutaneous syndrome, multiple lentigenes syndrome and Costello syndrome [Tidyman and Rauen, 2009]. Variable neurocognitive impairments are observed in all of the RASopathies, with effects ranging from absent or mild learning problems to severe intellectual disability [Zenker, 2011].
In NS, the majority of individuals perform in the low average to average range on IQ tests, although intellectual functioning tends to be reduced relative to the general population [Pierpont et al., 2009]. An elevated frequency of deficits in specific neuropsychological domains has been reported. These domains include visual processing [Alfieri et al., 2011a], verbal long-term memory [Alfieri et al., 2011b], language development [Pierpont et al., 2010a], motor skills [Lee et al., 2005] and social-emotional functioning [Wingbermuhle et al., 2011].
The exact neurobiological mechanism(s) that cause cognitive and learning disabilities in some individuals with NS are not yet known. However, experimental animal models have demonstrated that RAS-MAPK pathway proteins may play a key role in the process of memory formation and consolidation [e.g., Costa et al., 2002; Pagani et al., 2009], suggesting a plausible molecular explanation for the observed deficits. Only one previous study has investigated memory functions in individuals with NS. The authors reported that 50% of their cohort of children with confirmed mutations in PTPN11, SHOC2, RAF1 or SOS1 had impaired performance on a verbal free recall task, but only 5–20% demonstrated impairments on visual and spatial recognition memory tasks [Alfieri et al., 2011b]. While results of this study suggest that some aspects of memory may be disproportionately affected in NS, the exact source of the greater difficulty on the verbal task is not clear. One possible explanation is that individuals with NS could have greater difficulty remembering information in the verbal domain than the visual domain. If this were true, it would suggest potential hemispheric differences in brain function, as studies of individuals with temporal lobe epilepsy and other brain anomalies have reported at least partial differentiation of verbal and visual memory in the left and right hemispheres respectively [e.g., Ariza et al., 2006; Lee et al., 2002].
An alternative explanation for the results of Alfieri et al. [2011b] is that individuals with NS could have disproportionate difficulty with free recall tasks compared with recognition tasks. Recall tasks require conscious recollection and retrieval of recently presented items, while recognition tasks are thought to depend on familiarity-based judgments [Yonelinas et al., 2002]. Neuroimaging and patient studies indicate that the hippocampus, parahippocampal regions and prefrontal cortex are recruited during recall/recollection, whereas perirhinal regions of the temporal lobe support familiarity judgments [Davachi et al., 2003; Eichenbaum et al., 2007]. Accordingly, in patients with damage restricted to the hippocampus, item recognition ability tends to be spared relative to recall; in contrast, patients with more diffuse temporal lobe damage tend to demonstrate deficits in both recall and recognition [Vargha-Khadem et al., 1997; Yonelinas et al., 2002]. Thus, a deficit in recall but not recognition in NS could potentially arise due to functional problems within the hippocampus and/or prefrontal networks.
The present study aimed to distinguish among these possibilities by further investigating memory abilities in children and adolescents with NS. Based on the findings by Alfieri and colleagues [2011b], it was hypothesized that either verbal memory would constitute a weaker aspect of memory among individuals with NS than the other domains, or that tests of free recall would be weaker than recognition within the verbal domain. In addition, the current study also included tests working memory (WM). WM, defined as the short-term storage and maintenance of task-relevant information [Baddeley, 1992], is particularly dependent on prefrontal brain regions as well as additional content-specific frontal cortices [D'Esposito, 2007]. A recent study demonstrated that Ras-dependent increases in GABA release in the medial prefrontal cortex and striatum of an NF1 mouse model led to decreases in WM function [Shilyansky et al., 2010a]. Thus, we hypothesized that aberrant RAS-MAPK signaling in NS could also lead to deficits in WM tasks.
MATERIALS AND METHODS
Participants
The participant group included 29 children and adolescents with NS (17 boys, 12 girls), with a mean age of 11.43 years (SD = 3.04; range = 6.00 to 16.58 years). Participants were recruited from a cohort of families who were previously enrolled in a genotype-phenotype correlation study directed by the final author and had provided permission to be contacted for future research. Diagnostic criteria for NS, based on a NS scoring system by van der Burgt et al. [1994], were confirmed by a medical examination and/or review of medical records. Additionally, all participants underwent molecular PTPN11 testing (and additional genotyping, when indicated). The sample contained 19 individuals with confirmed gene mutations, including 14 individuals with PTPN11 mutations and 5 individuals with SOS1 mutations. The remaining 10 individuals (34%) had unknown mutations. Among those individuals with unknown mutations, all had tested negative for at least PTPN11/SOS1/KRAS mutations except one participant who had only received PTPN11 testing. Thus, the “unknown” mutation group is expected to be heterogeneous with respect to the causative gene. In the analyses below, data were excluded for one child who met the clinical criteria for NS and was tested for this study, but was found to have a mutation in the BRAF gene. There is some debate regarding whether individuals with BRAF mutations can be considered to have NS or may in fact actually have mild CFC syndrome [Neri et al., 2008].
Families were enrolled in the study at Boston Children’s Hospital (n = 22), the 2011 meeting of The Noonan Syndrome Support Group (n = 2) or by arrangement to be tested in Wisconsin or Minnesota (n = 5). Participants and their parent(s) provided written informed consent prior to enrollment. The study was approved by the Boston Children’s Hospital Institutional Review Board.
Procedures
Children participated in a behavioral testing session with the first author. The core battery of the Wide Range Assessment of Memory and Learning, Second Edition [Sheslow and Adams, 2003] was administered to evaluate memory in three domains: verbal memory, visual memory and WM. Descriptions of individual WRAML2 subtests are provided in Table I. Note that despite use of the term “Attention/Concentration Index” by the test makers, it was deemed appropriate to use the term WM to describe subtests in this index. These tasks are highly correlated with other tests of WM, including subtests from the Wechsler Memory Scale-III Working Memory Index (r = .65) [Sheslow and Adams, 2003], and it has been suggested that this index measures a similar construct as other WM scales [Strauss et al., 2006].
Table I.
Description of WRAML2 subtests
| Verbal Index | Description | |
|---|---|---|
| Story Memory | Immediate Recall | The examiner reads two short stories to the participants, who must repeat each immediately from memory. Scores are based on the number of specific story themes and details correctly recalled. |
| Delayed Recall | Participants must recount the stories after a 15 minute time delay, with no additional exposure to the stories. | |
| Delayed Recognition | A multiple-choice format is used to evaluate recognition of specific story details after a delay. | |
|
| ||
| Verbal Learning | Immediate Recall | A list comprised of either 13 words (ages 6–8) or 16 words (ages 9+) is read by the examiner. Across four learning trials, participants must recall as many words from the list as can be remembered. |
| Delayed Recall | Free recall of the list is assessed after a 15 minute time delay. | |
| Delayed Recognition | The participant is read randomly ordered words from the original Verbal Learning list as well as words not encountered previously. Participants must respond yes/no to indicate whether the word is recognized as an item from the original list. | |
|
| ||
| Visual Index | ||
|
| ||
| Design Memory | Immediate Recall | Participants are shown five stimulus cards containing geometric forms, each for 5 seconds. After a 10-second delay, the participant is asked to draw on a response form what is remembered from each card. A score is awarded for inclusion of specific design components. |
| Delayed Recognition | Participants are shown set of geometric shapes, half of which were previously seen on the cards presented on the Design Memory test. Participants must respond yes/no to indicate whether the shape was contained on any of the original five stimulus cards. | |
|
| ||
| Picture Memory | Immediate Recall | Participants are shown four common but visually complex scenes (e.g., a classroom, a zoo), each for 10 seconds. An alternate scene is subsequently presented and participants are asked to identify elements that have been “changed, moved or added.” |
| Delayed Recognition | Participants are shown a set of picture elements, half of which were seen on the Picture Memory subtest and half of which are new. Participants must respond yes/no to indicate whether each element was encountered previously. | |
|
| ||
| Attention/Concentration Index | ||
|
| ||
| Finger Windows | Participants are shown a vertically presented card containing asymmetrically located holes. The examiner indicates patterns of gradually increasing length by pointing through the holes in a specified sequence; the participant must attempt to duplicate each sequence by pointing through the holes in the same pattern. | |
|
| ||
| Number Letter | Sequences comprised of single digits and letters are orally presented by the examiner and the participant must repeat each sequence; number-letter strings of increasing length are presented. | |
Statistical analysis
All statistical analyses were conducted by using the PASW Statistics package version 17.0. Unless otherwise noted, statistical significance was set at a p-value of less than 0.05.
RESULTS
Descriptive statistics for the performance of children with NS on the WRAML2 memory indexes are reported in Table II. As a full group, individuals with NS scored within the average range, with a mean standard score of approximately 90 for the General Memory Index (GMI). Girls with NS achieved higher GMI scores (M = 98.42, SD = 16.65) than boys (M = 84.24, SD = 16.75), F(1,27) = 5.07, p = .033, partial η2 = .158. WRAML2 performance of individuals with mutations in the two most common NS genes was compared; no significant difference was found between individuals with PTPN11 mutations (n = 14) and those with SOS1 mutations (n = 5) on the GMI or any of the WRAML2 memory subdomains. However, caution must be taken on interpreting this finding due to the small sample size. In addition, it was noted that the three individuals with mutations in p.N308D and p.N308S, which have previously been associated with unimpaired intellectual functioning and regular education placement [e.g., Pierpont et al., 2009; Tartaglia et al., 2002], all performed in the average to above average range on the memory tasks (GMI range: 94–117).
Table II.
Performance of 29 children with NS on the WRAML2
| Genotype |
|||||
|---|---|---|---|---|---|
| Measure | Statistic |
PTPN11 n = 14 |
SOS1 n = 5 |
Unknown n = 10 |
Full Sample n = 29 |
| General Memory Index | M (SD) | 91.1 (15.5) | 88.6 (13.8) | 89.5 (23.7) | 90.1 (17.9) |
| Range | 55–117 | 66–101 | 55–123 | 55–123 | |
| Verbal Memory Index | M (SD) | 98.5 (15.5) | 96.8 (7.7) | 95.4 (23.8) | 97.1 (17.4) |
| Range | 55–114 | 85–105 | 55–132 | 55–132 | |
| Visual Memory Index | M (SD) | 88.9 (15.0) | 89.8 (14.8) | 89.2 (21.9) | 89.1 (17.1) |
| Range | 55–106 | 67–106 | 55–121 | 55–121 | |
| Attention/Concentration Index | M (SD) | 90.5 (17.3) | 88.0 (17.4) | 87.9 (19.3) | 89.2 (17.4) |
| Range | 55–117 | 67–106 | 55–117 | 55–117 | |
The single child with a mutation in BRAF, who met clinical criteria and was administered testing (but was excluded from the group analyses in this study), had overall lower memory ability relative to the mean of the NS group (GMI = 62). However, this individual’s scores fell well within the range of the NS group as a whole. Furthermore, this individual was not significantly impaired on verbal memory subtests (Verbal Memory Index = 80), which suggests relatively intact functioning in some areas of learning and memory.
Comparison of Memory Abilities across Domains
Pairwise comparisons using Bonferroni adjusted alpha levels of .017 were conducted to examine performance across the three subdomains in our cohort of children with NS. Performance on the verbal memory tasks was significantly better than performance on the visual memory tasks, t(28) = 3.26, p = .003, and the WM tasks, t(28) = 3.27, p =.003. The pairwise comparison of the visual memory and WM domains was non-significant (p = .99). With regard to the percentage of individuals showing at least moderate deficits (standard score below 80; < 10th percentile) in each domain, 10% of participants had deficits of verbal memory, 34% of visual memory and 34% of WM. One-sample t-tests indicated that children with NS scored significantly lower than the normative sample on the core tests of visual memory, t(28) = −3.43, p = .002, and WM, t(28) = −3.35, p = .002, but not on verbal memory tests (p = .384).
Verbal Memory: Delayed Recall vs. Delayed Recognition
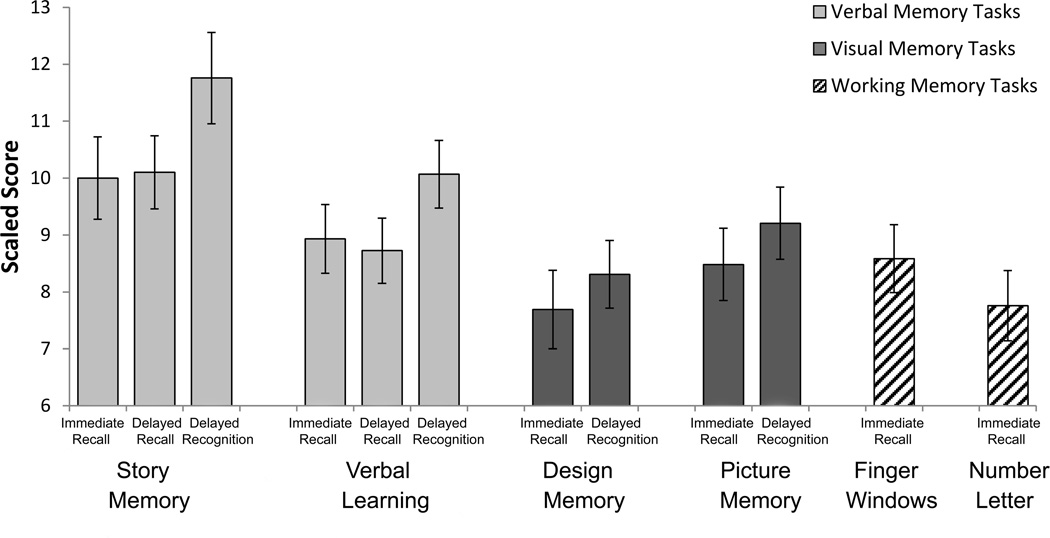
Performance of participants with NS on the each of the WRAML2 subtests is depicted in Figure 1. In order to test the hypothesis that free recall tasks are more difficult than familiarity-based recognition tasks for individuals with NS, performance on delayed recall and delayed recognition tasks for the two verbal subtests (Story Memory and Verbal Learning) was compared. Since the WRAML2 does not have a combined index for the delayed recall tests, scaled scores for the Story Memory and Verbal Learning subtests were averaged to create a Verbal Delayed Recall composite; scaled scores for the recognition tasks associated with these two subtests were similarly combined to create a Verbal Delayed Recognition composite. A planned repeated measures comparison indicated that performance measured by the Verbal Delayed Recall composite (M = 9.41, SD = 2.96) was significantly poorer than performance on the Verbal Delayed Recognition composite (M = 10.91, SD = 3.46), F(1,28) = 21.75, p < .001, partial η2 = .437.
Figure 1.
Performance of individuals with NS on WRAML2 subtests (normative sample: M = 10, SD = 2).
In order to determine whether this pattern of better delayed recognition compared with recall performance was consistent across the genotypes, we conducted this pairwise comparison individually for each gene group. Verbal Delayed Recall was poorer than Verbal Delayed Recognition for all groups, including those with PTPN11 mutations, t(13) = −2.32, p = .038, SOS1 mutations, t(4) = −10.59, p < .001, and unknown mutations, t(9) = −2.44, p = .037.
Memory for Meaningful vs. Arbitrary Information
It was observed that within each domain, tasks in which the information was presented in a meaningful context appeared to be less difficult for individuals with NS than tasks with more arbitrary or out-of-context information. Hence in the verbal domain, scores tended to be higher on the Story Memory Task, which presents information within a coherent story, than the Verbal Learning task, which presents information as a list of unrelated words. Similarly, scores were higher for the Picture Memory task, which involves everyday scenes, compared with the Design Memory task, which involves unrelated geometric forms.
In this vein, we conducted an exploratory analysis to examine the possibility that information presented in a richer context is more easily retained by individuals with NS than information presented in more impoverished context, relative to the normative sample. Pairwise comparisons were conducted to examine performance on the meaningful vs. arbitrary tasks, both for the verbal domain and the visual domain. Scaled scores for the delayed recognition tasks for each subtest were compared, due to the fact that the response demands were very similar for each of the four subtests in the recognition trial (i.e., they required the participant to simply respond in a multiple choice or yes/no format regarding whether the items was part of the original subtest content). Bonferroni adjusted alpha levels of .025 were used to account for multiple comparisons. For the verbal recognition tasks, performance on the meaningful Story Memory task (M = 11.76, SD = 4.32), was significantly better than performance on the arbitrary Verbal Learning task (M = 10.07, SD = 3.21), t(28) = 2.86, p = .008. For the visual tasks, the difference between performance on the more contextual Picture Memory task (M = 9.21, SD = 3.42) and the more arbitrary Design Memory task (M = 8.31, SD = 3.20) failed to reach significance, t(28) = 1.70, p = .101.
DISCUSSION
The current study aimed to provide a detailed characterization of learning and memory abilities in children and adolescents with NS. Based on a previous study by Alfieri and colleagues [2011b], which reported that children with NS had more frequent deficits in verbal free recall compared with visual and spatial recognition memory tasks, it was hypothesized that verbal memory might be a weaker aspect of memory among individuals with NS than other domains such as visual memory. This hypothesis was not borne out by our results, which indicated that immediate memory for verbal information was actually better than for visual information, and that verbal memory was also a strength relative to working memory. This pattern of strengths and weaknesses was observed in all genotype groups (Table II). Indeed, verbal memory was the only domain in which immediate recall performance of our NS cohort did not significantly differ from the normative population.
An alternative hypothesis to explain the results of Alfieri et al. [2011] is that individuals with NS may be better at recognition tasks relative to free recall tasks. Our results support this position. Within the WRAML2 verbal tasks, delayed recognition memory was significantly better than delayed recall. Furthermore, this result was consistent across all genotype groups.
Results from our memory assessment can be brought to bear on hypotheses regarding the neural mechanisms that may be affected by altered Ras signaling. One line of research suggests that structural brain anomalies resulting from aberrant RAS-MAPK signaling could be responsible for neuropsychological deficits observed in the RASopathies. Experimental animal data has shown that altered signaling within this pathway can result in developmental changes in cortical volume, thickness, patterning and myelination [see Samuels et al., 2009 for a review], as well as inhibited growth of astrocytes and other central nervous system anomalies [Gauthier et al., 2007]. Nevertheless, although frank neuroanatomical abnormalities are frequent in cardiofaciocutaneous and Costello syndromes [Gripp et al., 2010; Yoon et al., 2007], abnormal brain findings appear to be rare among patients with NS [e.g., Holder-Espinasse and Winter, 2003]. Therefore, the potential for obvious alteration of brain structure to explain learning disabilities in NS may be limited.
Recently, insights regarding the role of the RAS-MAPK signaling pathway in memory formation and consolidation have led to a major paradigm shift in how neurocognitive impairments in these disorders are conceptualized [Sweatt, 2004]. A growing body of research confirms the essential role of molecules in this pathway for synaptic plasticity in the mammalian brain, including long-term potentiation (LTP) [Davis and Laroche, 2006]. The process of LTP is thought to be a key mechanism for formation and storage of long-term memories in brain structures including the hippocampus, amygdala and regions of neocortex [Bliss and Collingridge, 1993; Lynch, 2004].
In a series of groundbreaking studies, Silva and colleagues demonstrated that hyperactivation of Ras in a genetically engineered, heterozygous mouse model of NF1 (Nf1+/−) resulted in disruption of LTP at Schaffer collateral/CA1 synapses in the hippocampus [Costa et al., 2002], suggesting a plausible mechanistic explanation for the mild to moderate learning disabilities typically seen in humans with NF1 [Shilyansky et al., 2010b]. Importantly, the Nf1+/− mice showed behavioral deficits in hippocampal-dependent spatial learning tasks. In contrast, deficits in other types of learning such as associative learning (e.g., fear conditioning) were not observed [Costa et al., 2001; Silva et al., 1997]. Furthermore, following genetic and pharmacological manipulations that reduced Ras signaling (including use of statin drugs), the learning deficits in the Nf1+/− mice were rescued and neurophysiological function normalized [Costa et al., 2002; Cui et al., 2008; Li et al., 2005]. These findings have precipitated initial clinical trials of statin-based drugs as prospective therapies to improve cognition and behavior in individuals with NF1 [Chabernaud et al., 2012; Krab et al., 2008].
In light of these exciting advances, a closer examination of memory abilities in individuals with NS and other RASopathies is of keen interest, both to better understand the nature of cognitive and learning deficits that result from abnormal RAS-MAPK signaling as well as to establish potential targets for therapeutic interventions. Results from the current study demonstrate that children with NS show relatively intact verbal recognition processes relative to verbal recall. These findings are consistent with hypothesis that neurobiological processes of LTP in the hippocampus could be adversely affected in some individuals with NS, but that neighboring cortical regions that support familiarity-based judgments function relatively well.
An alternative explanation for our findings is that executive functions necessary for organized retrieval of information from memory could be affected in NS. Indeed, the prefrontal cortex, a region important for executive processes, is known to be involved in strategic free recall of items from memory [Dickerson et al., 2007; Long et al., 2010]. Our results from the two WM tasks comprising the “Attention/Concentration Index” of the WRAML2 indicate that a significant proportion of our NS cohort (34%) showed impairments on tasks which require top-down control. Thus, poor prefrontal executive functions could be an important contributor to the greater difficulty with free recall tasks seen in many individuals.
An interesting result of the present study is that girls with NS outperformed boys on the learning and memory tests. This finding differs from other studies of individuals with NS, which have generally found no gender differences on measures of intellectual functioning [Pierpont et al., 2009] or memory [Alfieri et al., 2011b]. This finding could potentially reflect a higher rate of learning disabilities or Attention Deficit Hyperactivity Disorder (ADHD) among males compared with females in the general population [Liederman et al., 2005], and/or among children with NS. An examination of previous psychiatric diagnoses (collected via a demographic form completed by parents of participants) indicated that the rate of ADHD among the males in NS in our sample (50%) was much higher than the rate among the females (8%). Thus, the executive dysfunction commonly seen in ADHD could have plausibly led to the poorer performance on WRAML2 tests among the boys.
An additional recent discovery relevant to the current study is that some of the variation in intellectual and adaptive skills of individuals with NS and other RASopathies can be attributed to the specific gene and type of mutation that occurs [Cesarini et al., 2009; Pierpont et al., 2010b; Pierpont et al., 2009]. Despite low statistical power to detect genotype effects, some descriptive findings from the current study are of interest. For example, it was observed that the pattern of strong performance on verbal tasks relative to the visual and WM tasks was observed across all genotype groups (see Table II). The groups also did not significantly differ with regard to overall memory ability. In addition, individuals with p.N308D and p.N308S mutations in PTPN11 appear to have intact memory, consistent with previous literature indicating a low rate of cognitive deficits in this group [Tartaglia et al., 2002]. Finally, we observed that a child with a BRAF mutation (p.Q257R) scored within the range seen in the NS group on memory testing, despite having a lower GMI score than the overall NS group mean. This suggests that the spectrum of memory abilities in children with RASopathies may be on a continuum, with overlapping distributions based on gene classification.
Finally, a unique aspect of the present study concerns the examination of the possibility that information presented in a contextual, or meaningful, format may be better retained by participants with NS than information presented in a rote fashion. At least for the verbal domain, our exploratory analyses indicate children with NS had greater difficulty learning arbitrary or list-like information. This suggests that presenting information in a contextual rather than rote format may be especially supportive of memory in individuals with NS relative to the normative population.
Results of the current study must be interpreted in light of several limitations. First, the overall sample size of the study is small, reflecting the overall low prevalence of NS. Second, it was not possible to obtain confirmation of a specific mutation for all participants, due to the fact that some causative genes remain to be discovered, as well as the clinical reality that molecular testing is an ongoing process for many patients. Nevertheless, the rate of unknown mutations in our cohort (34%) is consistent with the percentage of NS cases with unknown mutations in recent genetics studies using clinically referred cohorts [Tartaglia et al., 2010]. Additionally, use of the WRAML2 to measure memory ability only enabled the investigation of delayed free recall vs. delayed recognition processes within the verbal domain. Future studies using other instruments may assist in determining whether recognition is also stronger within the visual domain.
CONCLUSIONS
Recent progress in understanding the effects of altered RAS-MAPK signaling on mammalian neurobiology and neurophysiology have led to an increased research focus on human genetic syndromes associated with mutations in this pathway. In particular, there is a growing literature indicating that RAS-MAPK mutations can disrupt memory formation and consolidation. The results of the current study support the position that recall processes, which depend on the hippocampus and prefrontal cortex, are more difficult for individuals with NS than recognition memory processes which rely on more distributed temporal cortical regions. Additionally, verbal recall and recognition were not impaired relative to the normative population, whereas visual memory and WM were an area of weakness for many of the children with NS.
The present study suggests several potential ways to manage or circumvent memory problems in individuals with NS. First, our results suggest individuals with NS may have greater knowledge than they are able to provide on free-form testing. Thus, when asked to recall knowledge of words, details, or themes that have been learned, they may better be able to reflect their knowledge when provided with choices. Second, for many individuals with NS it may be more difficult to learn visually than verbally; therefore, verbal or multimodal methods of presenting new information may be most effective. Third, information presented in a meaningful context may be better retained than arbitrary or rote information. Finally, children with NS may benefit from supports to aid possible difficulties in WM and executive functioning. Additional research is needed to explore abilities in this domain more extensively.
ACKNOWLEDGMENTS
We are extremely indebted to all of the families that participated in this research. The study was supported by a Boston Children’s Hospital Clinical Research Program Grant awarded to the authors, with support from The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award #UL1 RR 05758 and financial contributions from Harvard University and its affiliated academic health centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health.
REFERENCES
- Alfieri P, Cesarini L, De Rose P, Ricci D, Selicorni A, Menghini D, Guzzetta A, Baranello G, Tinelli F, Mallardi M, Zampino G, Vicari S, Atkinson J, Mercuri E. Visual processing in Noonan syndrome: dorsal and ventral stream sensitivity. Am J Med Genet A. 2011a;155A:2459–2464. doi: 10.1002/ajmg.a.34229. [DOI] [PubMed] [Google Scholar]
- Alfieri P, Cesarini L, Mallardi M, Piccini G, Caciolo C, Leoni C, Mirante N, Pantaleoni F, Digilio MC, Gambardella ML, Tartaglia M, Vicari S, Mercuri E, Zampino G. Long term memory profile of disorders associated with dysregulation of the RAS-MAPK signaling cascade. Behav Genet. 2011b;41:423–429. doi: 10.1007/s10519-011-9446-5. [DOI] [PubMed] [Google Scholar]
- Allanson JE. Noonan syndrome. Am J Med Genet C. 2007;145C:274–279. doi: 10.1002/ajmg.c.30138. [DOI] [PubMed] [Google Scholar]
- Ariza M, Pueyo R, Junque C, Mataro M, Poca MA, Mena MP, Sahuquillo J. Differences in visual vs. verbal memory impairments as a result of focal temporal lobe damage in patients with traumatic brain injury. Brain Inj. 2006;20:1053–1059. doi: 10.1080/02699050600909862. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working Memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Cesarini L, Alfieri P, Pantaleoni F, Vasta I, Cerutti M, Petrangeli V, Mariotti P, Leoni C, Ricci D, Vicari S, Selicorni A, Tartaglia M, Mercuri E, Zampino G. Cognitive profile of disorders associated with dysregulation of the RAS/MAPK signaling cascade. Am J Med Genet A. 2009;149A:140–146. doi: 10.1002/ajmg.a.32488. [DOI] [PubMed] [Google Scholar]
- Chabernaud C, Mennes M, Kardel PG, Gaillard WD, Kalbfleisch ML, Vanmeter JW, Packer RJ, Milham MP, Castellanos FX, Acosta MT. Lovastatin regulates brain spontaneous low-frequency brain activity in Neurofibromatosis type 1. Neurosci Lett. 2012;515:28–33. doi: 10.1016/j.neulet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RM, Federov NB, Kogan JH, Murphy GG, Stern J, Ohno M, Kucherlapati R, Jacks T, Silva AJ. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature. 2002;415:526–530. doi: 10.1038/nature711. [DOI] [PubMed] [Google Scholar]
- Costa RM, Yang T, Huynh DP, Pulst SM, Viskochil DH, Silva AJ, Brannan CI. Learning deficits, but normal development and tumor predisposition, in mice lacking exon 23a of Nf1. Nat Genet. 2001;27:399–405. doi: 10.1038/86898. [DOI] [PubMed] [Google Scholar]
- Cui Y, Costa RM, Murphy GG, Elgersma Y, Zhu Y, Gutmann DH, Parada LF, Mody I, Silva AJ. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell. 2008;135:549–560. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Laroche S. Mitogen-activated protein kinase/extracellular regulated kinase signalling and memory stabilization: a review. Genes Brain Behav 5 Suppl. 2006;2:61–72. doi: 10.1111/j.1601-183X.2006.00230.x. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Miller SL, Greve DN, Dale AM, Albert MS, Schacter DL, Sperling RA. Prefrontal-hippocampal-fusiform activity during encoding predicts intraindividual differences in free recall ability: an event-related functional-anatomic MRI study. Hippocampus. 2007;17:1060–1070. doi: 10.1002/hipo.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier AS, Furstoss O, Araki T, Chan R, Neel BG, Kaplan DR, Miller FD. Control of CNS cell-fate decisions by SHP-2 and its dysregulation in Noonan syndrome. Neuron. 2007;54:245–262. doi: 10.1016/j.neuron.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, Hopkins E, Doyle D, Dobyns WB. High incidence of progressive postnatal cerebellar enlargement in Costello syndrome: brain overgrowth associated with HRAS mutations as the likely cause of structural brain and spinal cord abnormalities. Am J Med Genet A. 2010;152A:1161–1168. doi: 10.1002/ajmg.a.33391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder-Espinasse M, Winter RM. Type 1 Arnold-Chiari malformation and Noonan syndrome. A new diagnostic feature? Clin Dysmorphol. 2003;12:275. doi: 10.1097/00019605-200310000-00013. [DOI] [PubMed] [Google Scholar]
- Krab LC, de Goede-Bolder A, Aarsen FK, Pluijm SM, Bouman MJ, van der Geest JN, Lequin M, Catsman CE, Arts WF, Kushner SA, Silva AJ, de Zeeuw CI, Moll HA, Elgersma Y. Effect of simvastatin on cognitive functioning in children with neurofibromatosis type 1: a randomized controlled trial. J Am Med Assoc. 2008;300:287–294. doi: 10.1001/jama.300.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DA, Portnoy S, Hill P, Gillberg C, Patton MA, Allanson J, Sarimski K. Psychological profile of children with Noonan syndrome. Dev Med Child Neurol. 2005;47:35–38. doi: 10.1017/s001216220500006x. [DOI] [PubMed] [Google Scholar]
- Lee TM, Yip JT, Jones-Gotman M. Memory deficits after resection from left or right anterior temporal lobe in humans: a meta-analytic review. Epilepsia. 2002;43:283–291. doi: 10.1046/j.1528-1157.2002.09901.x. [DOI] [PubMed] [Google Scholar]
- Li W, Cui Y, Kushner SA, Brown RA, Jentsch JD, Frankland PW, Cannon TD, Silva AJ. The HMG-CoA reductase inhibitor lovastatin reverses the learning and attention deficits in a mouse model of neurofibromatosis type 1. Curr Biol. 2005;15:1961–1967. doi: 10.1016/j.cub.2005.09.043. [DOI] [PubMed] [Google Scholar]
- Liederman J, Kantrowitz L, Flannery K. Male vulnerability to reading disability is not likely to be a myth: a call for new data. J Learn Disabil. 2005;38:109–129. doi: 10.1177/00222194050380020201. [DOI] [PubMed] [Google Scholar]
- Long NM, Oztekin I, Badre D. Separable prefrontal cortex contributions to free recall. J Neurosci. 2010;30:10967–10976. doi: 10.1523/JNEUROSCI.2611-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Neri G, Allanson J, Kavamura MI. No reason yet to change diagnostic criteria for Noonan, Costello and cardio-facio-cutaneous syndromes. J Med Genet. 2008;45:832. doi: 10.1136/jmg.2008.063263. [DOI] [PubMed] [Google Scholar]
- Pagani MR, Oishi K, Gelb BD, Zhong Y. The phosphatase SHP2 regulates the spacing effect for long-term memory induction. Cell. 2009;139:186–198. doi: 10.1016/j.cell.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpont EI, Ellis Weismer S, Roberts AE, Tworog-Dube E, Pierpont ME, Mendelsohn NJ, Seidenberg MS. The language phenotype of children and adolescents with Noonan syndrome. J Speech Lang Hear Res. 2010a;53:917–932. doi: 10.1044/1092-4388(2009/09-0046). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpont EI, Pierpont ME, Mendelsohn NJ, Roberts AE, Tworog-Dube E, Rauen KA, Seidenberg MS. Effects of germline mutations in the Ras/MAPK signaling pathway on adaptive behavior: cardiofaciocutaneous syndrome and Noonan syndrome. Am J Med Genet A. 2010b;152A:591–600. doi: 10.1002/ajmg.a.33268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpont EI, Pierpont ME, Mendelsohn NJ, Roberts AE, Tworog-Dube E, Seidenberg MS. Genotype differences in cognitive functioning in Noonan syndrome. Genes Brain Behav. 2009;8:275–282. doi: 10.1111/j.1601-183X.2008.00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels IS, Saitta SC, Landreth GE. MAP'ing CNS development and cognition: an ERKsome process. Neuron. 2009;61:160–167. doi: 10.1016/j.neuron.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheslow D, Adams W. Administration and Technical Manual for the WRAML2. Lutz, FL: Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- Shilyansky C, Karlsgodt KH, Cummings DM, Sidiropoulou K, Hardt M, James AS, Ehninger D, Bearden CE, Poirazi P, Jentsch JD, Cannon TD, Levine MS, Silva AJ. Neurofibromin regulates corticostriatal inhibitory networks during working memory performance. Proc Natl Acad Sci U S A. 2010a;107:13141–13146. doi: 10.1073/pnas.1004829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilyansky C, Lee YS, Silva AJ. Molecular and cellular mechanisms of learning disabilities: a focus on NF1. Annu Rev Neurosci. 2010b;33:221–243. doi: 10.1146/annurev-neuro-060909-153215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Frankland PW, Marowitz Z, Friedman E, Laszlo GS, Cioffi D, Jacks T, Bourtchuladze R. A mouse model for the learning and memory deficits associated with neurofibromatosis type I. Nat Genet. 1997;15:281–284. doi: 10.1038/ng0397-281. [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: Administration, norms, and commentary. Oxford: Oxford University Press; 2006. [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Tartaglia M, Kalidas K, Shaw A, Song X, Musat DL, van der Burgt I, Brunner HG, Bertola DR, Crosby A, Ion A, Kucherlapati RS, Jeffery S, Patton MA, Gelb BD. PTPN11 mutations in Noonan syndrome: molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneity. Am J Hum Genet. 2002;70:1555–1563. doi: 10.1086/340847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia M, Zampino G, Gelb BD. Noonan syndrome: clinical aspects and molecular pathogenesis. Mol syndromol. 2010;1:2–26. doi: 10.1159/000276766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidyman WE, Rauen KA. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr Opin Genet Dev. 2009;19:230–236. doi: 10.1016/j.gde.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Burgt I, Berends E, Lommen E, van Beersum S, Hamel B, Mariman E. Clinical and molecular studies in a large Dutch family with Noonan syndrome. Am J Med Genet. 1994;53:187–191. doi: 10.1002/ajmg.1320530213. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Wingbermuhle E, Egger JI, Verhoeven WM, van der Burgt I, Kessels RP. Affective functioning and social cognition in Noonan syndrome. Psychol Med. 2011;42:419–426. doi: 10.1017/S0033291711001115. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Yoon G, Rosenberg J, Blaser S, Rauen KA. Neurological complications of cardio-facio-cutaneous syndrome. Dev Med Child Neurol. 2007;49:894–899. doi: 10.1111/j.1469-8749.2007.00894.x. [DOI] [PubMed] [Google Scholar]
- Zenker M. Clinical manifestations of mutations in RAS and related intracellular signal transduction factors. Curr Opin Pediatr. 2011;23:443–451. doi: 10.1097/MOP.0b013e32834881dd. [DOI] [PubMed] [Google Scholar]