Abstract
Molecular targeted therapy plays an increasingly important role in the treatment of metastatic pheochromocytomas and paragangliomas (PPGLs), which are rare tumors but remain difficult to treat. This mini-review provides an overview of established molecular targeted therapies in present use, and perspectives on those currently under development and evaluation in clinical trials. Recently published research articles, guidelines, and expert views on molecular targeted therapies in PPGLs are systematically reviewed and summarized. Some tyrosine kinase inhibitors (sunitinib, cabozantinib) are already in clinical use with some promising results, but without formal approval for the treatment of PPGLs. Sunitinib is the only therapeutic option which has been investigated in a randomized placebo-controlled clinical trial. It is clinically used as a first-, second-, or third-line therapeutic option for the treatment of progressive metastatic PPGLs. Some other promising molecular targeted therapies (hypoxia-inducible factor 2 alpha [HIF2α] inhibitors, tumor vaccination together with checkpoint inhibitors, antiangiogenic therapies, kinase signaling inhibitors) are under evaluation in clinical trials. The HIF2α inhibitor belzutifan may prove to be particularly interesting for cluster 1B-/VHL/EPAS1-related PPGLs, whereas antiangiogenic therapies seem to be primarily effective in cluster 1A-/SDHx-related PPGLs. Some combination therapies currently being evaluated in clinical trials, such as temozolomide/olaparib, temozolomide/talazoparib, or cabozantinib/atezolizumab, will provide data for novel therapy for metastatic PPGLs. It is likely that advances in such molecular targeted therapies will play an essential role in the future treatment of these tumors, with more personalized therapy options paving the way towards improved therapeutic outcomes.
Keywords: molecular targeted therapy, metastatic, pheochromocytoma, paraganglioma
Pheochromocytomas and paragangliomas are a group of neuroendocrine neoplasms that originate from the adrenal medulla (pheochromocytomas) or the sympathetic or parasympathetic extra-adrenal paraganglia (paragangliomas). These tumors, collectively referred to as PPGLs, show the highest rate of heritability or genetically known causes among all endocrine tumors.
In recent years, an increasing number of variants in genes involved in PPGL tumor pathogenesis have been discovered, as previously reviewed (1). Germline mutations are known to be present in up to 30% to 35% of PPGL patients, whereas somatic mutations in similar genes can be found in up to one-half of patients (2-8). Thus, around 70% to 80% of all patients show germline or somatic mutations in known PPGL disease-causing genes, and genetic testing is recommended for every patient because this may guide their management and improve their clinical outcome (9-12).
PPGLs can be assigned to 1 of 3 main molecular clusters depending on their genetic signature: pseudohypoxia-related cluster 1 (1A or 1B), kinase signaling-related cluster 2, or Wnt signaling-related cluster 3 (Fig. 1). These clusters are associated with distinct biochemical profiles, imaging-related functionalities, clinical presentations, and prognostic differences. Genetic profiling of PPGLs therefore allows for personalized diagnostics and follow-up of these tumors. Although cluster-specific biochemical phenotyping, imaging, and follow-up have already entered routine clinical practice (12), therapy has largely remained nonspecific and unrelated to mutation status.
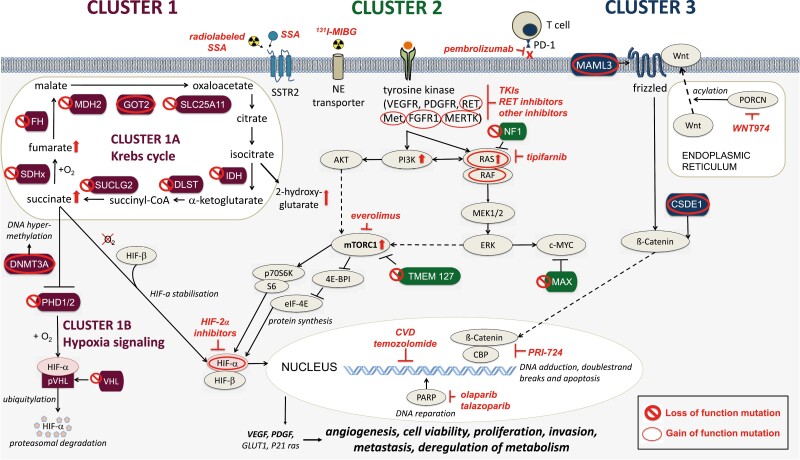
Figure 1.
The 3 main molecular clusters of PPGLs and their associated gain (○) or loss (○) of function mutations. Cluster 1 mutations (crimson) include mutations in cluster 1A/Krebs cycle-related genes (SDHx, FH, MDH2, GOT2, SLC25A11, IDH, DLST, SUCLG2) and cluster 1B/hypoxia signaling-related genes (PHD1/2, VHL, HIF2A/EPAS1). These mutations lead to an accumulation of oncometabolites, increased DNS hypermethylation, decreased HIF-α degradation and HIF-α stabilization. Cluster 2 mutations (green) disrupt the kinase signaling pathway and lead to their overactivation (RET, MET, FGFR1, MERTK, NGFR, NF1, HRAS, BRAF, TMEM127, MAX). Cluster 3 mutations (blue) affect the Wnt signaling pathway (MAML3, CSDE1). All mutations may lead to increased angiogenesis, cell proliferation, invasion, metastasis, and deregulation of metabolism. Potential therapies are shown in red. ⇧ protein activation or upregulation; ⊥ protein inhibition.
In terms of treatment, options are overall still limited for PPGL patients with metastatic disease, and there are no treatment options that may offer a complete cure to this disease. The only officially approved therapy currently available is high specific activity (HSA) [131I]-MIBG therapy that is approved only in the United States (13). Around 10% to 15% of all patients with pheochromocytomas, plus a significantly higher proportion of patients with paragangliomas (35%-40%), develop metastases (14-21). Although cluster 1 tumors, particularly SDHB- and SDHA-mutant PPGLs, show a high metastatic risk of up to 75% (2, 20, 22-24), of the 3 clusters, cluster 2 tumors are associated with the lowest metastatic risk of 3% to 10% (2, 24, 25). Cluster 3 tumors are relatively rare but show aggressive behavior and a high metastatic risk (2, 26). Overall, 5- and 10-year mortality rates for metastatic patients have been reported to be 37% and 29%, respectively (27), with SDHB mutations in particular associated with decreased survival in metastatic PPGL patients (28).
Therefore, with only few established therapeutic options available for metastatic PPGLs, novel therapeutic approaches are urgently needed (12, 29, 30). In recent years, personalized and genetically guided therapy has become increasingly investigated, with some molecular targeted therapies already playing a role in the therapy of metastatic PPGLs. Molecular targeted therapy is defined as a treatment that targets specific molecules that play key roles in cancer growth and survival, leading to an inhibition of tumor cell growth and progression, or a promotion of tumor cell death (31, 32).
This mini-review focuses on molecular targeted therapies for metastatic PPGLs, providing an overview of existing therapeutic options and their efficacy, and highlights the current development of novel personalized molecular targeted therapies. Recently published research articles, guidelines, and expert views on molecular targeted therapies in PPGLs were systematically reviewed, and are summarized in this mini-review.
Management of Metastatic PPGLs
The diagnosis of metastatic PPGL patients is based, similarly to nonmetastatic PPGL patients, either on their clinical presentation with typical signs and symptoms, on the presence of an adrenal incidentaloma, or following surveillance because of a personal or family history (11). However, compared with nonmetastatic PPGL patients, metastatic disease may more often lead to a clinical presentation with severe hypertension or fluctuation in blood pressure because of a higher tumor burden (11, 33). To confirm or rule out a PPGL, subsequent biochemical testing and imaging is indicated (34).
The management of metastatic PPGLs is highly dependent on their biochemical phenotype, ideally determined by measurement of plasma-free metanephrines using liquid chromatography-tandem mass spectrometry (12). Cluster 1 PPGLs predominantly present with a noradrenergic phenotype, defined by an increase of normetanephrine either without an increase in metanephrines or with an increase of metanephrine less than 5% of the increase in both metabolites (35). Less commonly, 3-methoxytyramine may also be increased—defining a dopaminergic phenotype (36). Cluster 2 PPGLs are predominantly adrenergic, defined by an increase in plasma metanephrine more than 5% of the increase of all metabolites (35, 36). The precise biochemical phenotype of cluster 3 PPGLs is still unknown (12).
The imaging modalities chosen for screening are dependent on many factors including primary tumor location (adrenal vs extra-adrenal), mutation and patient age. Computed tomography (CT) imaging is preferred for the screening of adrenal tumors and shows higher sensitivity than magnetic resonance imaging (MRI) scans in the detection of lung metastases (12). MRI is now the preferred imaging modality for the screening of extra-adrenal tumors and for the detection of liver metastases. MRI is also preferably used in children and for long-term follow-up of all patients (37). If functional imaging is indicated, the use of the 68Gallium-labeled somatostatin analogue positron emission tomography-CT ([68Ga]-DOTA-SSA PET/CT) is recommended for cluster 1A-related PPGL patients, whereas [18F]-fluorodihydroxyphenylalanine positron emission tomography-CT ([18F]-FDOPA PET/CT) is recommended as first-line functional imaging for cluster 1B- and cluster 2-related PPGL patients (12, 38).
Following the initial diagnosis of a PPGL, genetic counseling and testing should be recommended for every patient (9, 12). Certain mutations (eg, SDHB, ARTX) as well as a tumor size >5 cm, multifocality, previously detected metastases, or a noradrenergic/dopaminergic biochemical phenotype, are all characteristics associated with a higher risk of the development of future metastases (28, 39-41).
Individualized therapy decisions, particularly for metastatic patients, should be made in a multidisciplinary tumor board, preferably in a specialized center (9, 12). In general, surgery is the only curative therapy available, and is indicated as first-line therapy for locoregional disease or maybe oligometastatic disease in selected cases, but may also be used to provide symptomatic relief (eg, by lowering catecholamine levels) in the case of catecholamine-related signs and symptoms, or to reduce tumor mass effects for patients with widespread metastases (12, 42). Furthermore, some studies have suggested resection of the primary tumor and of the metastases to be beneficial for metastatic PPGL patients (39, 43-46); however, more conclusive evidence is still needed.
In functional PPGLs, alpha-adrenoreceptor blockade is usually indicated for 7 to 14 days before any treatment intervention, surgical or otherwise, and should be continued for at least 3 days after ablative or systemic therapies (9, 11, 42). Moreover, alpha-adrenoreceptor blockade should be considered in each patient with metastatic disease with catecholamine-related signs and symptoms.
Because there are no officially approved systemic therapies available for metastatic PPGLs, apart from HSA [131I]-MIBG therapy in the United States, therapy is largely based on past practice and experience.
Therapy of Metastatic PPGLs With a Special Focus on Molecular Targeted Therapies
The treatment algorithm for metastatic PPGL patients should be personalized, based on the rate of progression, overall tumor burden, location of metastases, and the general condition of each patient including assessment of co-morbidities. A flow chart of the practical therapy standards is shown in Fig. 2. The original data and studies supporting the practical therapy standards are summarized and reviewed in Nölting et al (12).
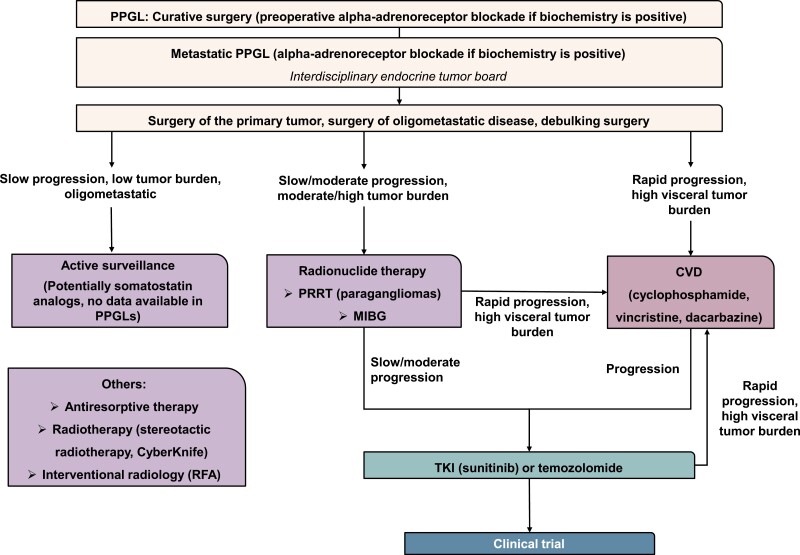
Figure 2.
Simplified flow chart of the practical therapy standards in metastatic PPGLs (12). Each metastatic PPGL patient should be discussed in an interdisciplinary endocrine tumor board. Surgery of the primary tumor, of oligometastatic disease or debulking surgery, should always be considered. In the case of slow progression, low tumor burden and oligometastatic disease, active surveillance may be considered. In patients with slow-to-moderate progression, moderate-to-high tumor burden and positivity on SSTR2 or MIBG imaging, radionuclide therapy using either PRRT or MIBG (depending on avidity on molecular imaging) may be applied. For slowly/moderately progressing tumors that are not eligible for PRRT or MIBG, TKIs or temozolomide may be considered as first-line therapies. In the case of rapid progression or high visceral tumor burden, CVD chemotherapy may be applied. In the case of slow-to-moderate progression following radionuclide therapy, TKIs or temozolomide may be considered. In the case of rapid progression and high visceral tumor burden following other systemic therapies, CVD should be considered. Following progression to CVD, TKIs, or temozolomide may be considered. In case of further progression, inclusion in clinical trials may be considered. MIBG, meta-[131I] iodobenzylguanidine; PRRT, somatostatin receptor-based radionuclide therapy; RFA, radiofrequency ablation; TKI, tyrosine kinase inhibitor.
Various modalities can be used to affect symptomatic control, including those from mass effects and catecholamine-related signs and symptoms, in appropriate circumstances; these would include palliative resection of the primary or metastases, alpha-adrenoreceptor blockade, interventional radiology, or radiotherapy (12).
Although molecular targeted therapy is being increasingly studied in patients with metastatic PPGLs, systemic therapy is still largely based on conventional chemotherapy or based on some specific characteristics as with targeted radionuclide therapy. Moreover, such practical therapy standards are mostly based on retrospective data, with few prospective trials and only 1 completed randomized placebo-controlled clinical trial (FIRST-MAPPP) (47).
Molecular targeted therapies include therapeutic approaches such as antiangiogenic agents and hypoxia-inducible factor 2 alpha (HIF2α) inhibitors, especially for cluster 1 tumors, inhibitors of kinase signaling pathways (PI3K/AKT/mTOR, Ras/Raf/MEK/ERK), especially for cluster 2 tumors, and potentially Wnt signaling inhibitors for cluster 3 tumors. All ongoing clinical trials investigating molecular targeted therapy in PPGL patients are listed in Table 1.
Table 1.
Ongoing clinical trials investigating molecular targeted therapy in PPGLs, listed in order of their mention in the text
| Ongoing clinical trials | Intervention/treatment | Study design | Phase | Locationa | Status |
|---|---|---|---|---|---|
| NCT04394858 | Olaparib (PARP inhibitor) plus temozolomide (chemotherapeutic) | Prospective | 2 | US | Recruiting |
| NCT05142241 (RARE 2) | Talazoparib (PARP inhibitor) plus temozolomide (chemotherapeutic) | Prospective | 2 | US | Recruiting |
| NCT00107289 | [131I]-MIBG | Prospective | 2 | US | Recruiting |
| NCT01850888 | [131I]-MIBG in palliative patients | Prospective | NA | US | Recruiting |
| NCT04770831 | [131I]-MIBG | Prospective | 2 | US | Recruiting |
| NCT00874614 | HSA [131I]-MIBG | Prospective | 2 | US | Unknown |
| NCT03206060 | [177Lu] DOTATATE (PRRT) | Prospective | 2 | US | Recruiting |
| NCT04276597 | [177Lu] DOTATOC (PRRT) | Prospective | 2 | US | Recruiting |
| NCT04711135 | [177Lu] DOTATATE (PRRT) in adolescents | Prospective | 2 | US, Europe, UK | Recruiting |
| NCT04029428 | [177Lu] DOTATATE vs [90Y] DOTATATE vs mix of 50% each (PRRT) | Prospective | 2 | Poland | Unknown |
|
NCT00843037 (SNIPP) |
Sunitinib (TKI) | Prospective | 2 | Canada, Netherlands | Active, not recruiting |
| NCT02302833 | Cabozantinib s-malate (TKI) | Prospective | 2 | US | Recruiting |
| NCT01371201 (FIRST-MAPPP) | Sunitinib (TKI) | Randomized, double-blind, placebo-controlled | 2 | Europe | Closed (data arriving soon) |
| NCT03946527 (LAMPARA) | Lanreotide (SSTR analog) | Prospective | 2 | US | Recruiting |
| NCT03839498 | Axitinib (TKI) | Prospective | 2 | US | Recruiting |
| NCT03008369 | Lenvatinib (TKI) | Prospective | 2 | US | Active, not recruiting |
| NCT04860700 | Anlotinib (TKI) | Prospective | 2 | China | Recruiting |
| NCT05133349 | Anlotinib (TKI) | Prospective | 2 | China | Recruiting |
| NCT02721732 | Pembrolizumab (Immunotherapeutic) | Prospective | 2 | US | Active, not recruiting |
| NCT04400474 (CABATEN) | Cabozantinib (TKI) plus atezolizumab (immunotherapeutic) | Prospective | 2 | Spain | Recruiting |
| NCT04924075 (MK-6482-015) | Belzutifan (HIF2α inhibitor) | Prospective | 2 | US, Canada, Europe, UK, Russia, Turkey | Recruiting |
| NCT04895748 | DFF332 (HIF2α inhibitor) plus everolimus (mTORC1 inhibitor) or DFF332 plus spartalizumab (immunotherapeutic) plus taminadenant (A2A receptor antagonist) | Prospective | 1 | US, Europe, Japan, Singapore | Recruiting |
| NCT04284774 (MATCH) | Tipifarnib (farnesyltransferase inhibitor) | Prospective | 2 | US | Recruiting |
| NCT04187404 (Spencer) | EO2401 (therapeutic vaccine) plus nivolumab (immunotherapeutic) | Prospective | 1/2 | US and Europe | Recruiting |
| NCT03034200 | ONC201 (small molecule DRD2 antagonist) | Prospective | 2 | US | Active, not recruiting |
Abbreviations: HSA, high specific activity; HIF2α, hypoxia-inducible factor 2 alpha; MIBG, meta-iodobenzylguanidine; NA, not applicable; PARP, poly (ADP-ribose) polymerase; PRRT, peptide receptor radionuclide therapy; TKI, tyrosine kinase inhibitor.
a Trial locations at the timepoint of the writing of this paper.
Chemotherapy
Cytotoxic chemotherapy using cyclophosphamide/vincristine/dacarbazine (CVD, Averbuch scheme) or temozolomide are conventional therapeutic options for metastatic PPGL patients. These therapies are only briefly mentioned here to give an overview of the practical therapy standards but are not considered targeted therapy. For metastatic PPGLs with rapid progression and a high visceral tumor burden, CVD chemotherapy may be the treatment of choice (12, 42). The largest meta-analysis on CVD therapy reported a partial response concerning tumor volume in 37% of patients (4 studies), and a partial response concerning catecholamine excess in 40% of patients (2 studies) (48). However, complete responses regarding tumor volume and catecholamine excess were only seen in 4% and 14%, respectively.
Although temozolomide has also shown promising efficacy in metastatic, particularly SDHB-mutant, PPGLs in retrospective studies (49, 50), prospective data are still lacking. At present, probably the main place of temozolomide is in patients showing slow-to-moderate progression and who are not eligible for peptide (somatostatin) receptor (SSTR)-based radionuclide therapy (PRRT) or MIBG therapy, or who show slow-to-moderate progression after such treatment (12, 49, 50).
Combination therapy: temozolomide plus poly (ADP-ribose) inhibitor (targeted therapy)
A preclinical study showed that combining temozolomide with a poly (ADP-ribose) polymerase (PARP) inhibitor may be a novel therapeutic approach in SDHB-mutant PPGLs (51), and a prospective randomized clinical phase 2 study investigating temozolomide vs temozolomide plus the PARP inhibitor olaparib in metastatic PPGL is currently recruiting (NCT04394858). Another phase 2 trial investigating temozolomide in combination with the PARP inhibitor talazoparib in advanced cancers, including PPGLs, is also now recruiting (RARE 2, NCT05142241).
Targeted Radionuclide Therapy
In patients with slow-to-moderate progression and moderate-to-high tumor burden, targeted radionuclide therapy using peptide PRRT or meta-[131I] iodobenzylguanidine ([131I]-MIBG) may currently be used as first-line therapeutic options (11-13). However, such PPRT is only indicated if the tumor is positive on [68Ga]-DOTA-SSA imaging (12, 52), whereas HSA or conventional [131I]-MIBG therapy may be applied in patients with tumors that show uptake on [123I]-MIBG imaging (13, 42).
HSA [131I]-MIBG therapy has been US Food and Drug Administration (FDA)-approved based on a phase 2 study with good results (n = 64, partial response or stable disease in 92%, median overall survival 36.7 months) (13). However, studies have shown that metastatic cluster 1-, particularly SDHB-related, PPGLs may be less frequently positive on [123I]-MIBG imaging (53). Therefore, other radionuclide therapies, such as PRRT, may be particularly interesting for cluster 1-related PPGLs, which often show strong SSTR2 expression and positivity on [68Ga]-DOTA-SSA imaging (38, 54, 55). A prospective study has also shown particularly long overall survival (82 months) in metastatic paraganglioma patients (n = 28) following [90Y] DOTATOC therapy, further suggesting a high therapeutic potential of PRRT in metastatic paragangliomas (56).
Other types of PRRT are now also being evaluated. PRRT using alpha-particle emitting radionuclides such as 225Ac-DOTATATE has shown promising results in metastatic gastro-enteropancreatic neuroendocrine tumor (NET) patients who are refractory to or have reached the maximum therapy cycles of 177Lu-DOTATATE therapy and may also prove to be valuable for metastatic PPGL patients (57). PRRT using SSTR antagonists, which may have higher tumor-binding affinity than SSTR agonists (58), has been shown to be clinically feasible and effective (59). However, there are still no completed or active clinical trials investigating these types of PRRT in patients with metastatic PPGL.
Several clinical trials further investigating [131I]-MIBG therapy and PRRT in metastatic PPGL patients (adult or adolescent) are now recruiting (Table 1).
For slowly/moderately progressing tumors that are not eligible for PRRT or MIBG, tyrosine kinase inhibitors (TKIs) or temozolomide may be considered as first-line therapeutic options (12).
Tyrosine kinase inhibitors
In the case of progression to CVD or radionuclide therapy, TKIs may be used (12). Targeting angiogenesis, which is a hallmark of metastatic PPGL development (60), by using TKIs is an important therapeutic strategy since both cluster 1, particularly SDHB, and cluster 2 mutations may predispose to angiogenesis (61, 62).
Sunitinib is a clinically available TKI that has been investigated in prospective phase 2 trials in PPGL patients: 1 prospective phase 2 trial (SNIPP trial, NCT00843037) showed a partial response of 13% (n = 25, disease control rate [DCR] over 12 weeks, 83% median progression-free survival [PFS] 13.4 months), and all SDHx-mutant patients showed partial responses or stable disease (63). The first randomized placebo-controlled phase 2 study in patients with metastatic PPGL (FIRST-MAPPP, NCT01371201) investigated sunitinib vs placebo, and demonstrated promising preliminary results (PFS at 12 months: sunitinib group 35.9% vs placebo 18.9%; median PFS sunitinib 8.9 months vs placebo 3.6 months) (abstract) (47). A retrospective clinical trial described a partial response to sunitinib in 21% of patients, with 62.5% of cases with stable disease or a partial response in SDHB mutation carriers (64).
The TKI cabozantinib is also in clinical use and is being investigated in a clinical phase 2 trial in metastatic PPGL (NCT02302833) with promising preliminary results (partial response 37%, stable disease 55%, DCR 92%, PFS 16 months; responders included SDHB-mutant patients [preliminary data published in a review]) (62). Consistent with these data, our preclinical study on human PPGL primary cultures showed significantly stronger efficacy of cabozantinib in cluster 1 tumors, particularly SDHB-related tumors, compared with cluster 2 tumors (65).
Although the prospective and retrospective studies, as well as our preclinical study on human PPGL primary cultures, indicated particular efficacy of sunitinib and cabozantinib in cluster 1 SDHx-, particularly SDHB-related tumors (63-66), it still remains to be seen from the FIRST-MAPPP trial whether patients with these mutations are the best candidates for sunitinib (final detailed data are awaited). Moreover, it has to be kept in mind that patients with cluster 1-related PPGLs are often younger and have more aggressive tumors, compared with patients with cluster 2-related tumors. This may add to the better efficacy and tolerability of some drugs in patients with cluster 1-related tumors.
Other TKIs, including axitinib, pazopanib, lenvatinib, and anlotinib, have not been extensively clinically used in PPGLs as yet, but have shown moderate efficacy in small phase 2 trials (axitinib, n = 9, partial response in 3/9 patients [abstract]; pazopanib, n = 6, partial response in 1/6 patients, study halted from poor recruitment) (67, 68). Another phase 2 trial on axitinib is now recruiting (NCT03839498). A small retrospective study on the TKI lenvatinib showed promising results (n = 11, 5/11 SDHB-mutant, n = 8 with measurable disease, PFS at 12 months 61.4%, median PFS 14.7 months, partial response 5/8, stable disease 3/8), but a worsening of hypertension in the majority of patients (9/11) (69). Lenvatinib is currently being studied in another small phase 2 trial in metastatic PPGLs (NCT03008369). Two phase 2 trials studying TKI anlotinib in advanced PPGLs are now recruiting (NCT04860700, NCT05133349).
Immunotherapy
Pembrolizumab, a monoclonal antibody targeting PD-1, showed modest efficacy in 2 clinical phase 2 studies (n = 11, objective response rate [ORR] 9%, DCR 73%, median PFS 5.7 months and n = 9, ORR 0%, DCR 75% over 4 months, PFS at 27 weeks 43%, respectively) (NCT02721732) (70, 71).
Combination therapy: immunotherapy plus TKI
Because antiangiogenic therapy, through targeting vascular endothelial growth factor, promotes immune cell mobilization and enhances the efficacy of immunotherapy (62), the evaluation of TKIs in combination with immunotherapeutics may be of particular interest for metastatic PPGL patients. TKI plus immunotherapeutic combination therapies have already been approved for the therapy of advanced renal cell carcinoma (lenvatinib/pembrolizumab and cabozantinib/nivolumab) (72, 73), but there are only limited data available in PPGLs. One case study showed that cabozantinib plus nivolumab resulted in a major response in a metastatic PPGL patient until the end of the observation period (22 months after combination therapy initiation) (74). Furthermore, a multicohort phase 2 study of cabozantinib plus the immunotherapeutic atezolizumab in advanced endocrine tumors, including PPGLs, is currently recruiting, and may provide important clinical data (CABATEN, NCT04400474).
HIF2α Inhibitors
The HIF2α inhibitor belzutifan has received FDA approval for therapy of cancers associated with von Hippel-Lindau (VHL) disease (75), based on promising results from a phase 2 study on VHL-associated renal cell carcinoma (RCC) (n = 61, ORR 49%, partial response in 49% of patients, PFS at 24 months 96%) (MK-6482-004, NCT03401788) (76). Although PPGL patients have not been included in the studies so far, another phase 2 trial on belzutifan in advanced PPGLs and NETs is now recruiting (MK-6482-015, NCT04924075). Although some preclinical in vitro studies have shown a lack of efficacy of HIF2α inhibitors in PPGL cells, this was possibly because of the limitations of in vitro experiments (24, 65).
Other HIF2α inhibitors currently under investigation include PT2385, evaluated in a phase 2 study in VHL-associated clear cell (cc)RCC patients (NCT03108066), and DFF332 (in combination with either the mTORC1 inhibitor everolimus or the immunotherapeutic spartalizumab, plus the adenosine A2A receptor antagonist taminadenant), investigated in a phase 1 trial in tumor patients with HIF-stabilizing mutations, including PPGLs (NCT04895748).
Although there are currently no clinical data available concerning the efficacy of HIF2α inhibitors in PPGLs, these drugs theoretically offer important treatment potential for metastatic, particularly cluster 1-associated tumors (24, 77, 78), and the MK-6482-015 trial is likely to provide highly relevant data for metastatic PPGL patients.
Combination therapy: HIF2α inhibitor plus TKI
A potentially interesting combination therapy—belzutifan plus the TKI cabozantinib—is currently being investigated in a phase 2 trial in patients with advanced ccRCC (MK-6482-003, NCT03634540), with promising preliminary results (n = 41, ORR 22%, DCR 92.7% over 6 months, median PFS 16.8 months, PFS at 6 months 78.3% [in abstract]) (79).
Kinase Signaling Inhibitors
The kinase signaling pathways PI3K/AKT/mTOR or Ras/Raf/MEK/ERK are often overactivated in cluster 2-related PPGLs, and may be targeted by kinase signaling inhibitors (3, 12). TKIs have been discussed previously and may be used in both cluster 1- and cluster 2-related tumors.
The mTORC1 inhibitor everolimus is approved for the therapy of progressive NETs but has shown only slight to moderate efficacy in PPGLs in a small prospective and another small retrospective study (n = 4, DCR 25% and n = 7, DCR 71%, median PFS 3.8 months, respectively) (80, 81).
The selective RET inhibitor selpercatinib is approved for treatment of RET-mutant lung and thyroid cancers on the basis of a phase 1/2 clinical study in RET-mutant solid tumors, and medullary thyroid carcinomas (LIBRETTO-001, NCT03157128) (82). Although selpercatinib has also shown strong efficacy in a case report of a RET fusion-positive metastatic PPGL patient (83), our preclinical studies found only moderate efficacy of selpercatinib in RET-mutant PPGL primary cultures (65), although this was based on a small sample size. Moreover, it is worth mentioning that the RET-mutant tumors in the primary culture study were all nonmetastatic tumors and, in general, cluster 2-related PPGLs show a very low metastatic risk (3%-10%) (2, 24, 25).
Tipifarnib, a farnesyl-transferase inhibitor that disrupts HRAS function, particularly in HRAS-mutant cancers, has received FDA “breakthrough therapy” designation for the treatment of recurrent or metastatic HRAS-mutant head-and-neck squamous cell carcinoma, based on the results of a phase 2 study (84). A phase 2 pediatric trial studying tipifarnib in patients with HRAS-mutant pheochromocytomas, among others, is now recruiting (MATCH, NCT04284774), and should provide important data for PPGL therapy.
Combination therapy: mTOR inhibitor plus TKI
Because everolimus usually leads to the development of resistance in patients with NETs after less than 1 year, through compensatory activation of other kinase signaling pathways (85, 86), the combination of mTOR inhibitors with TKIs may be a promising therapeutic option for NETs and also PPGLs, as shown by our preclinical studies in patient-derived PPGL primary cultures (a synergistic effect of everolimus plus cabozantinib was observed and an additive effect of everolimus plus sunitinib) (65, 87). Moreover, combination therapy of a TKI (lenvatinib) plus an mTOR inhibitor (everolimus) has been approved for other cancers (88), showing good efficacy and tolerability (89).
The combination of sunitinib plus the mTOR inhibitor rapamycin is also clinically well tolerated (90) and showed efficacy in at least 1 SDHB-mutant metastatic PPGL patient described in the literature (64). Stable disease was observed until the end of the observation period (3 years after initiating sunitinib, 18 months after addition of rapamycin), suggesting that molecular targeted combination therapies may prolong PFS at effective and clinically well-tolerated low doses. However, further clinical studies are warranted in metastatic PPGLs.
Combination therapy: tipifarnib plus TKI
A phase 1 trial of tipifarnib plus the TKI sorafenib in thyroid cancer showed good tolerability and promising results through inhibition of Ras/Raf/MAPK kinase/ERK and RET kinase pathways (n = 35, 8 BRAF-mutant, 8 RET-mutant, median PFS 18 months, overall survival at 24 months 80%) (91). These results also suggest a particular efficacy of combination therapy using inhibitors of the kinase signaling pathways, and this may potentially be transferable to PPGL patients. Furthermore, our preclinical studies in PPGL primary cultures have also shown notable efficacy of molecular targeted combination therapy, especially in cluster 2-, but also in cluster 1-related, PPGL primary cultures, through multiple targeting of kinase signaling pathways (65, 87).
Wnt Signaling Inhibitors
Because cluster 3-related PPGLs are relatively rare, there are no established specific therapies available for these tumors at the current time. However, targeting Wnt signaling is another therapeutic approach that should be further explored because these PPGLs harbor an aggressive phenotype with high metastatic potential (3, 26). Potential therapies include the Porcupine O-Acyltransferase inhibitor WNT974 and ß-catenin inhibitor PRI-724, which have shown good efficacy in a preclinical study in neuroendocrine tumor cell lines (92).
Bone-targeted Agents
Because metastatic PPGLs commonly spread to the skeletal system, the treatment of bone metastases, particularly if symptomatic and progressive, is also an important part of PPGL therapy. The use of bone-targeted agents such as the monoclonal antibody denosumab or the bisphosphonate zoledronic acid, may be considered as standard practice (42) because they are effective in reducing the risk of pathologic fractures and the need for radiation compared with placebo, as shown in a network meta-analysis (93). Moreover, zoledronic acid may also reduce neoplastic progression (both breast cancers and nonbreast cancers), as shown in osteopenic postmenopausal women (hazard ratio 0.67) (94), through inhibition of cancer cell proliferation and viability (65, 95). Our own PPGL primary culture studies have also revealed an antitumor effect of zoledronic acid in PPGLs (65). Other therapeutic options in the case of metastases, in the skeleton or other locations, include conventional external beam radiation therapy, stereotactic radiosurgery, and interventional radiology (radiofrequency ablation, cryoablation) (12, 96, 97).
Biotherapy: Somatostatin Analogs
The use of SSTR analogs may be considered in patients with strong SSTR2 expression (often cluster 1 SDHx-related PPGL) (12, 42). The rationale comes from patients with metastatic NETs where both lanreotide and octreotide prolonged PFS (median PFS lanreotide not reached vs placebo 18 months, estimated PFS lanreotide at 24 months 65.1% vs placebo 33.0%; median PFS octreotide LAR 14.3 months vs placebo 6 months) (98, 99). For PPGL patients, data are still lacking: only a few case reports have been published so far (100-103). However, a phase 2 trial on lanreotide in metastatic PPGL patients is now recruiting (LAMPARA, NCT03946527). One could consider the use of such analogs in patients with slow progression before the use of other systemic therapies, given its paucity of adverse effects.
Outlook and Conclusions
Although cluster specific pathogenesis, biochemical phenotyping, diagnostics, and follow-up are already widely used for PPGLs, much therapy still remains largely nonspecific (12).
Two anecdotal reports highlight the importance of mutational analysis in determining the optimal therapeutic strategy for individual PPGL patients. A metastatic PPGL patient with a novel germline ALK mutation received individualized molecular targeted therapy with the ALK inhibitor brigatinib, leading to disease remission and a sustained partial response until the end of the observation period (10 months after therapy initiation) (104). Another metastatic nonhereditary PPGL patient with a novel somatic RET-SEPTIN9 fusion was accordingly treated with the selective RET inhibitor selpercatinib, resulting in a partial response after 12 weeks of treatment and an ongoing treatment response until week 23 (83). Such individualized (particularly molecular targeted) therapy may therefore follow genetic testing and the molecular classification of metastatic PPGLs, but both germline and somatic mutation testing will need to be widely implemented in the management of PPGLs for this to be practicable.
Ongoing trials investigating molecular targeted therapies, as well as other therapeutic strategies (eg, novel therapeutic tumor vaccines together with check-point inhibitors; Spencer, NCT04187404) and small molecules, such as the DRD2 antagonist ONC201 (NCT03034200), will also provide important novel data regarding the therapy of metastatic PPGLs.
In conclusion, this mini-review has provided an overview of the current development and use of novel and promising molecular targeted therapies in metastatic PPGL patients. Molecular targeted therapeutics are now being increasingly clinically applied and are often effective and well tolerated. Combined molecular targeted therapies are also being studied with promising results, with a need for awareness of adverse events. With therapeutic strategies constantly being optimized and novel treatment strategies being developed and tested, the outlook for these rare tumors seems promising.
Abbreviations
- ccRCC
clear cell renal cell carcinoma
- CT
computed tomography
- CVD
cyclophosphamide/vincristine/dacarbazine
- DCR
disease control rate
- FDA
US Food and Drug Administration
- [18F]-FDOPA PET/CT
[18F]-fluorodihydroxyphenylalanine positron emission tomography-CT
- HIF2α
hypoxia-inducible factor 2 alpha
- HSA
high specific activity
- MRI
magnetic resonance imaging
- NET
neuroendocrine tumor
- ORR
objective response rate
- PARP
poly (ADP-ribose) polymerase
- PFS
progression-free survival
- PPGL
pheochromocytoma and paraganglioma
- PRRT
peptide (somatostatin) receptor (SSTR)-based radionuclide therapy
- RCC
renal cell carcinoma
- SSTR
somatostatin receptor
- TKI
tyrosine kinase inhibitor
- VHL
von Hippel-Lindau
- [68Ga]-DOTA-SSA PET/CT
68Gallium-labeled somatostatin analogue positron emission tomography-computed tomography
Contributor Information
Katharina Wang, Department of Internal Medicine IV, University Hospital, LMU Klinikum, Ludwig Maximilian University of Munich, 80336 Munich, Germany.
Joakim Crona, Department of Medical Sciences, Uppsala University, 75185 Uppsala, Sweden.
Felix Beuschlein, Department of Internal Medicine IV, University Hospital, LMU Klinikum, Ludwig Maximilian University of Munich, 80336 Munich, Germany; Department of Endocrinology, Diabetology and Clinical Nutrition, University Hospital Zurich (USZ) and University of Zurich (UZH), 8091 Zurich, Switzerland.
Ashley B Grossman, Green Templeton College, University of Oxford, Oxford OX2 6HG, United Kingdom; NET Unit, ENETS Centre of Excellence, Royal Free Hospital, London NW3 2QG, United Kingdom.
Karel Pacak, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland 20892-1109, USA.
Svenja Nölting, Department of Internal Medicine IV, University Hospital, LMU Klinikum, Ludwig Maximilian University of Munich, 80336 Munich, Germany; Department of Endocrinology, Diabetology and Clinical Nutrition, University Hospital Zurich (USZ) and University of Zurich (UZH), 8091 Zurich, Switzerland.
Funding
This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft [DFG]) within the CRC/Transregio 205/2, Project number: 314061271 – TRR 205 ‘The Adrenal: Central Relay in Health and Disease’ (to S.N. and F.B.) and the Immuno-TargET project under the umbrella of University Medicine Zurich (to S.N. and F.B.).
Disclosures
The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Jhawar S, Arakawa Y, Kumar S, et al. New insights on the genetics of pheochromocytoma and paraganglioma and its clinical implications. Cancers 2022;14(3). doi 10.3390/cancers14030594 [published Online First: Epub Date]| [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crona J, Lamarca A, Ghosal S, Welin S, Skogseid B, Pacak K. Genotype-phenotype correlations in pheochromocytoma and paraganglioma: a systematic review and individual patient meta-analysis. Endocr Relat Cancer. 2019;26(5):539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fishbein L, Leshchiner I, Walter V, et al. Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell 2017;31(2):181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luchetti A, Walsh D, Rodger F, et al. Profiling of somatic mutations in phaeochromocytoma and paraganglioma by targeted next generation sequencing analysis. Int J Endocrinol 2015;2015:138573. doi: 10.1155/2015/138573 [published Online First: Epub Date]| [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gieldon L, William D, Hackmann K, et al. Optimizing genetic workup in pheochromocytoma and paraganglioma by integrating diagnostic and research approaches. Cancers 2019;11(6). doi 10.3390/cancers11060809 [published Online First: Epub Date]| [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang J, Zhang J, Pang Y, et al. Sino-European differences in the genetic landscape and clinical presentation of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2020;105(10):3295–3307. [DOI] [PubMed] [Google Scholar]
- 7. Jochmanova I, Pacak K. Genomic landscape of pheochromocytoma and paraganglioma. Trends Cancer 2018;4(1):6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burnichon N, Vescovo L, Amar L, et al. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum Mol Genet. 2011;20(20):3974–3985. [DOI] [PubMed] [Google Scholar]
- 9. Lenders JW, Duh QY, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915–1942. [DOI] [PubMed] [Google Scholar]
- 10. Buffet A, Ben Aim L, Leboulleux S, et al. Positive impact of genetic test on the management and outcome of patients with paraganglioma and/or pheochromocytoma. J Clin Endocrinol Metab. 2019;104(4):1109–1118. [DOI] [PubMed] [Google Scholar]
- 11. Lenders JWM, Kerstens MN, Amar L, et al. Genetics, diagnosis, management and future directions of research of phaeochromocytoma and paraganglioma: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens. 2020;38(8):1443–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nölting S, Bechmann N, Taieb D, et al. Personalized management of pheochromocytoma and paraganglioma. Endocr Rev. 2022;43(2):199–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pryma DA, Chin BB, Noto RB, et al. Efficacy and safety of high-specific-activity (131)I-MIBG therapy in patients with advanced pheochromocytoma or paraganglioma. J Nucl Med. 2019;60(5):623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eisenhofer G, Lenders JW, Siegert G, et al. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 2012;48(11):1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel D, Phay JE, Yen TWF, et al. Update on pheochromocytoma and paraganglioma from the SSO Endocrine/Head and Neck Disease-Site Work Group. Part 1 of 2: advances in pathogenesis and diagnosis of pheochromocytoma and paraganglioma. Ann Surg Oncol. 2020;27(5):1329–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Remine WH, Chong GC, Van Heerden JA, Sheps SG, Harrison EG Jr. Current management of pheochromocytoma. Ann Surg. 1974;179(5):740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Proye CA, Vix M, Jansson S, Tisell LE, Dralle H, Hiller W. “The” pheochromocytoma: a benign, intra-adrenal, hypertensive, sporadic unilateral tumor. Does it exist? World J Surg. 1994;18(4):467–472. [DOI] [PubMed] [Google Scholar]
- 18. Goldstein RE, O’NeillJA, Jr., HolcombGW, 3rd, et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229(6):755–764; discussion 64-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mannelli M, Ianni L, Cilotti A, Conti A. Pheochromocytoma in Italy: a multicentric retrospective study. Eur J Endocrinol. 1999;141(6):619–624. [DOI] [PubMed] [Google Scholar]
- 20. Amar L, Servais A, Gimenez-Roqueplo AP, Zinzindohoue F, Chatellier G, Plouin PF. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J Clin Endocrinol Metab. 2005;90(4):2110–2116. [DOI] [PubMed] [Google Scholar]
- 21. Edstrom Elder E, Hjelm Skog AL, Hoog A, Hamberger B. The management of benign and malignant pheochromocytoma and abdominal paraganglioma. Eur J Surg Oncol. 2003;29(3):278–283. [DOI] [PubMed] [Google Scholar]
- 22. Brouwers FM, Eisenhofer G, Tao JJ, et al. High frequency of SDHB germline mutations in patients with malignant catecholamine-producing paragangliomas: implications for genetic testing. J Clin Endocrinol Metab. 2006;91(11):4505–4509. [DOI] [PubMed] [Google Scholar]
- 23. Schovanek J, Martucci V, Wesley R, et al. The size of the primary tumor and age at initial diagnosis are independent predictors of the metastatic behavior and survival of patients with SDHB-related pheochromocytoma and paraganglioma: a retrospective cohort study. BMC Cancer 2014;14:523. doi: 10.1186/1471-2407-14-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bechmann N, Moskopp ML, Ullrich M, et al. HIF2alpha supports pro-metastatic behavior in pheochromocytomas/paragangliomas. Endocr Relat Cancer. 2020;27(11):625–640. [DOI] [PubMed] [Google Scholar]
- 25. Kumar S, Lila AR, Memon SS, et al. Metastatic cluster 2-related pheochromocytoma/paraganglioma: a single-center experience and systematic review. Endocr Connect 2021;10(11):1463–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alzofon N, Koc K, Panwell K, et al. Mastermind like transcriptional coactivator 3 (MAML3) drives neuroendocrine tumor progression. Mol Cancer Res. 2021;19(9):1476-1485 doi: 10.1158/1541-7786.MCR-20-0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hamidi O, YoungWF, Jr., Gruber L, et al. Outcomes of patients with metastatic phaeochromocytoma and paraganglioma: a systematic review and meta-analysis. Clin Endocrinol 2017;87(5):440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Turkova H, Prodanov T, Maly M, et al. Characteristics and outcomes of metastatic sdhb and sporadic pheochromocytoma/paraganglioma: an National Institutes of Health Study. Endocr Pract. 2016;22(3):302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nölting S, Ullrich M, Pietzsch J, et al. Current management of pheochromocytoma/paraganglioma: a guide for the practicing clinician in the era of precision medicine. Cancers 2019;11(10). Doi: 10.3390/cancers11101505 [published Online First: Epub Date]| [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nölting S, Grossman A, Pacak K. Metastatic phaeochromocytoma: spinning towards more promising treatment options. Exp Clin Endocrinol Diabetes. 2018;127(2-03):117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: treating cancer with specificity. Eur J Pharmacol. 2018;834:188–196. doi: 10.1016/j.ejphar.2018.07.034. [DOI] [PubMed] [Google Scholar]
- 32. Rosland GV, Engelsen AS. Novel points of attack for targeted cancer therapy. Basic Clin Pharmacol Toxicol. 2015;116(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet 2005;366(9486):665–675. [DOI] [PubMed] [Google Scholar]
- 34. Pacak K, Linehan WM, Eisenhofer G, Walther MM, Goldstein DS. Recent advances in genetics, diagnosis, localization, and treatment of pheochromocytoma. Ann Intern Med. 2001;134(4):315–329. [DOI] [PubMed] [Google Scholar]
- 35. Eisenhofer G, Klink B, Richter S, Lenders JW, Robledo M. Metabologenomics of phaeochromocytoma and paraganglioma: an integrated approach for personalised biochemical and genetic testing. Clin Biochem Rev 2017;38(2):69–100. [PMC free article] [PubMed] [Google Scholar]
- 36. Eisenhofer G, Deutschbein T, Constantinescu G, et al. Plasma metanephrines and prospective prediction of tumor location, size and mutation type in patients with pheochromocytoma and paraganglioma. Clin Chem Lab Med. 2020;59(2):353–363. [DOI] [PubMed] [Google Scholar]
- 37. Amar L, Pacak K, Steichen O, et al. International consensus on initial screening and follow-up of asymptomatic SDHx mutation carriers. Nat Rev Endocrinol. 2021;17(7):435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Taieb D, Hicks RJ, Hindie E, et al. European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2019;46(10):2112–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hamidi O, YoungWF, Jr., Iniguez-Ariza NM, et al. Malignant pheochromocytoma and paraganglioma: 272 patients over 55 years. J Clin Endocrinol Metab 2017;102(9):3296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hescot S, Curras-Freixes M, Deutschbein T, et al. Prognosis of Malignant Pheochromocytoma and Paraganglioma (MAPP-Prono Study): a European Network for the study of adrenal tumors retrospective study. J Clin Endocrinol Metab. 2019;104(6):2367–2374. [DOI] [PubMed] [Google Scholar]
- 41. Mei L, Khurana A, Al-Juhaishi T, et al. Prognostic factors of malignant pheochromocytoma and paraganglioma: a combined SEER and TCGA databases review. Horm Metab Res. 2019;51(7):451–457. [DOI] [PubMed] [Google Scholar]
- 42. Fishbein L, Del Rivero J, Else T, et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for surveillance and management of metastatic and/or unresectable pheochromocytoma and paraganglioma. Pancreas 2021;50(4):469–493. [DOI] [PubMed] [Google Scholar]
- 43. Roman-Gonzalez A, Zhou S, Ayala-Ramirez M, et al. Impact of surgical resection of the primary tumor on overall survival in patients with metastatic pheochromocytoma or sympathetic paraganglioma. Ann Surg. 2018;268(1):172–178. [DOI] [PubMed] [Google Scholar]
- 44. Strajina V, Dy BM, Farley DR, et al. Surgical treatment of malignant pheochromocytoma and paraganglioma: retrospective case series. Ann Surg Oncol. 2017;24(6):1546–1550. [DOI] [PubMed] [Google Scholar]
- 45. Wei S, Wu D, Yue J. Surgical resection of multiple liver metastasis of functional malignant pheochromocytoma: a case report and literature review. J Cancer Res Ther. 2013;9(Suppl):S183–S185. [DOI] [PubMed] [Google Scholar]
- 46. Arnas-Leon C, Sanchez V, Santana Suarez AD, Quintana Arroyo S, Acosta C, Martinez Martin FJ. Complete remission in metastatic pheochromocytoma treated with extensive surgery. Cureus 2016;8(1):e447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baudin E, Goichot B, Berruti A, et al. First International Randomized Study in Malignant Progressive Pheochromocytoma and Paragangliomas (FIRSTMAPPP): an academic double-blind trial investigating sunitinib. Ann Oncol. 2021;32:S621–S25. Doi: 10.1016/annonc/annonc700 [DOI] [Google Scholar]
- 48. Niemeijer ND, Alblas G, van Hulsteijn LT, Dekkers OM, Corssmit EP. Chemotherapy with cyclophosphamide, vincristine and dacarbazine for malignant paraganglioma and pheochromocytoma: systematic review and meta-analysis. Clin Endocrinol 2014;81(5):642–651. [DOI] [PubMed] [Google Scholar]
- 49. Hadoux J, Favier J, Scoazec JY, et al. SDHB mutations are associated with response to temozolomide in patients with metastatic pheochromocytoma or paraganglioma. Int J Cancer. 2014;135(11):2711–2720. [DOI] [PubMed] [Google Scholar]
- 50. Kulke MH, Stuart K, Enzinger PC, et al. Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J Clin Oncol. 2006;24(3):401–406. [DOI] [PubMed] [Google Scholar]
- 51. Pang Y, Lu Y, Caisova V, et al. Targeting NAD(+)/PARP DNA repair pathway as a novel therapeutic approach to SDHB-mutated cluster i pheochromocytoma and paraganglioma. Clin Cancer Res. 2018;24(14):3423–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kong G, Grozinsky-Glasberg S, Hofman MS, et al. Efficacy of peptide receptor radionuclide therapy for functional metastatic paraganglioma and pheochromocytoma. J Clin Endocrinol Metab. 2017;102(9):3278–3287. [DOI] [PubMed] [Google Scholar]
- 53. Fonte JS, Robles JF, Chen CC, et al. False-negative (1)(2)(3)I-MIBG SPECT is most commonly found in SDHB-related pheochromocytoma or paraganglioma with high frequency to develop metastatic disease. Endocr Relat Cancer. 2012;19(1):83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ziegler CG, Brown JW, Schally AV, et al. Expression of neuropeptide hormone receptors in human adrenal tumors and cell lines: antiproliferative effects of peptide analogues. Proc Natl Acad Sci USA. 2009;106(37):15879–15884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Van Essen M, Krenning EP, De Jong M, Valkema R, Kwekkeboom DJ. Peptide Receptor Radionuclide Therapy with radiolabelled somatostatin analogues in patients with somatostatin receptor positive tumours. Acta Oncol. 2007;46(6):723–734. [DOI] [PubMed] [Google Scholar]
- 56. Imhof A, Brunner P, Marincek N, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29(17):2416–2423. [DOI] [PubMed] [Google Scholar]
- 57. Ballal S, Yadav MP, Bal C, Sahoo RK, Tripathi M. Broadening horizons with (225)Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to (177)Lu-DOTATATE PRRT: first clinical experience on the efficacy and safety. Eur J Nucl Med Mol Imaging. 2020;47(4):934–946. [DOI] [PubMed] [Google Scholar]
- 58. Fani M, Braun F, Waser B, et al. Unexpected sensitivity of sst2 antagonists to N-terminal radiometal modifications. J Nucl Med. 2012;53(9):1481–1489. [DOI] [PubMed] [Google Scholar]
- 59. Wild D, Fani M, Fischer R, et al. Comparison of somatostatin receptor agonist and antagonist for peptide receptor radionuclide therapy: a pilot study. J Nucl Med. 2014;55(8):1248–1252. [DOI] [PubMed] [Google Scholar]
- 60. Jimenez C. Treatment for patients with malignant pheochromocytomas and paragangliomas: a perspective from the hallmarks of cancer. Front Endocrinol 2018;9:277. doi: 10.3389/fendo.2018.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Favier J, Igaz P, Burnichon N, et al. Rationale for anti-angiogenic therapy in pheochromocytoma and paraganglioma. Endocr Pathol. 2012;23(1):34–42. [DOI] [PubMed] [Google Scholar]
- 62. Jimenez C, Fazeli S, Roman-Gonzalez A. Antiangiogenic therapies for pheochromocytoma and paraganglioma. Endocr Relat Cancer. 2020;27(7):R239–R254. [DOI] [PubMed] [Google Scholar]
- 63. O’Kane GM, Ezzat S, Joshua AM, et al. A phase 2 trial of sunitinib in patients with progressive paraganglioma or pheochromocytoma: the SNIPP trial. Br J Cancer. 2019;120(12):1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ayala-Ramirez M, Chougnet CN, Habra MA, et al. Treatment with sunitinib for patients with progressive metastatic pheochromocytomas and sympathetic paragangliomas. J Clin Endocrinol Metab. 2012;97(11):4040–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang K, Schutze I, Gulde S, et al. Personalized drug testing in human pheochromocytoma/paraganglioma primary cultures. Endocr Relat Cancer. 2022;29(6):285-306. doi: 10.1530/ERC-21-0355. [DOI] [PubMed] [Google Scholar]
- 66. Jimenez C, Busaidy N, Habra M, Waguespack S, Jessop A. A phase 2 study to evaluate the effects of cabozantinib in patients with unresectable metastatic pheochromocytomas and paragangliomas. In: International Symposium on Pheochromocytoma and Paraganglioma Sydney, Australia, 2017. [Google Scholar]
- 67. Burotto Pichun ME, Edgerly M, Velarde M, et al. Phase II clinical trial of axitinib in metastatic pheochromocytomas and paragangliomas (P/PG): preliminary results. J Clin Oncol. 2015;33(7_suppl):457–457. [Google Scholar]
- 68. Jasim S, Suman VJ, Jimenez C, et al. Phase II trial of pazopanib in advanced/progressive malignant pheochromocytoma and paraganglioma. Endocrine 2017;57(2):220–225. [DOI] [PubMed] [Google Scholar]
- 69. Hassan Nelson L, Fuentes-Bayne H, Yin J, et al. Lenvatinib as a therapeutic option in unresectable metastatic pheochromocytoma and paragangliomas. J Endocr Soc. 2022;6(5):bvac044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jimenez C, Subbiah V, Stephen B, et al. Phase II clinical trial of pembrolizumab in patients with progressive metastatic pheochromocytomas and paragangliomas. Cancers 2020;12(8). doi 10.3390/cancers12082307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Naing A, Meric-Bernstam F, Stephen B, et al. Phase 2 study of pembrolizumab in patients with advanced rare cancers. J ImmunoTher Cancer. 2020;8(1). doi 10.1136/jitc-2019-000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–1300. [DOI] [PubMed] [Google Scholar]
- 73. Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Economides MP, Shah AY, Jimenez C, Habra MA, Desai M, Campbell MT. A durable response with the combination of nivolumab and cabozantinib in a patient with metastatic paraganglioma: a case report and review of the current literature. Front Endocrinol 2020;11:594264. doi: 10.3389/fendo.2020.594264 [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Deeks ED. Belzutifan: first approval. Drugs. 2021;81(16):1921–1927. [DOI] [PubMed] [Google Scholar]
- 76. Jonasch E, Donskov F, Iliopoulos O, et al. Belzutifan for renal cell carcinoma in von Hippel-Lindau disease. N Engl J Med. 2021;385(22):2036–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Toledo RA. New HIF2alpha inhibitors: potential implications as therapeutics for advanced pheochromocytomas and paragangliomas. Endocr Relat Cancer 2017;24(9):C9–C19. [DOI] [PubMed] [Google Scholar]
- 78. Peng S, Zhang J, Tan X, et al. The VHL/HIF axis in the development and treatment of pheochromocytoma/paraganglioma. Front Endocrinol 2020;11:586857. doi: 10.3389/fendo.2020.586857 [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Choueiri TK, Bauer TM, McDermott DF, et al. Phase 2 study of the oral hypoxia-inducible factor 2α (HIF-2α) inhibitor MK-6482 in combination with cabozantinib in patients with advanced clear cell renal cell carcinoma (ccRCC). J Clin Oncol. 2021;39(6_suppl):272–272. [Google Scholar]
- 80. Druce MR, Kaltsas GA, Fraenkel M, Gross DJ, Grossman AB. Novel and evolving therapies in the treatment of malignant phaeochromocytoma: experience with the mTOR inhibitor everolimus (RAD001). Horm Metab Res. 2009;41(9):697–702. [DOI] [PubMed] [Google Scholar]
- 81. Oh DY, Kim TW, Park YS, et al. Phase 2 study of everolimus monotherapy in patients with nonfunctioning neuroendocrine tumors or pheochromocytomas/paragangliomas. Cancer 2012;118(24):6162–6170. [DOI] [PubMed] [Google Scholar]
- 82. Wirth LJ, Sherman E, Robinson B, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. 2020;383(9):825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mweempwa A, Xu H, Vissers JHA, et al. Novel RET fusion RET-SEPTIN9 predicts response to selective RET inhibition with selpercatinib in malignant pheochromocytoma. JCO Precis Oncol 2021;5:1160–1165. doi: 10.1200/PO.21.00127 [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 84. Ho AL, Brana I, Haddad R, et al. Tipifarnib in head and neck squamous cell carcinoma with HRAS mutations. J Clin Oncol. 2021;39(17):1856–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 2016;387(10022):968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fankhauser M, Bechmann N, Lauseker M, et al. Synergistic highly potent targeted drug combinations in different pheochromocytoma models including human tumor cultures. Endocrinology 2019;160(11):2600–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16(15):1473–1482. [DOI] [PubMed] [Google Scholar]
- 89. Wiele AJ, Bathala TK, Hahn AW, et al. Lenvatinib with or without everolimus in patients with metastatic renal cell carcinoma after immune checkpoint inhibitors and vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapies. Oncologist 2021;26(6):476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Waqar SN, Gopalan PK, Williams K, Devarakonda S, Govindan R. A phase I trial of sunitinib and rapamycin in patients with advanced non-small cell lung cancer. Chemotherapy 2013;59(1):8–13. [DOI] [PubMed] [Google Scholar]
- 91. Hong DS, Cabanillas ME, Wheler J, et al. Inhibition of the Ras/Raf/MEK/ERK and RET kinase pathways with the combination of the multikinase inhibitor sorafenib and the farnesyltransferase inhibitor tipifarnib in medullary and differentiated thyroid malignancies. J Clin Endocrinol Metab. 2011;96(4):997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Jin XF, Spoettl G, Maurer J, Nolting S, Auernhammer CJ. Inhibition of Wnt/beta-catenin signaling in neuroendocrine tumors in vitro: antitumoral effects. Cancers 2020;12(2). doi 10.3390/cancers12020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang Z, Qiao D, Lu Y, et al. Systematic literature review and network meta-analysis comparing bone-targeted agents for the prevention of skeletal-related events in cancer patients with bone metastasis. Oncologist 2015;20(4):440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Reid IR, Horne AM, Mihov B, et al. Effects of zoledronate on cancer, cardiac events, and mortality in osteopenic older women. J Bone Miner Res. 2020;35(1):20–27. [DOI] [PubMed] [Google Scholar]
- 95. Wang L, Fang D, Xu J, Luo R. Various pathways of zoledronic acid against osteoclasts and bone cancer metastasis: a brief review. BMC Cancer 2020;20(1):1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kohlenberg J, Welch B, Hamidi O, et al. Efficacy and safety of ablative therapy in the treatment of patients with metastatic pheochromocytoma and paraganglioma. Cancers 2019;11(2). doi 10.3390/cancers11020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Breen W, Bancos I, Young WF Jr, et al. External beam radiation therapy for advanced/unresectable malignant paraganglioma and pheochromocytoma. Adv Radiat Oncol 2018;3(1):25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Caplin ME, Pavel M, Cwikla JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–233. [DOI] [PubMed] [Google Scholar]
- 99. Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656–4663. [DOI] [PubMed] [Google Scholar]
- 100. Tonyukuk V, Emral R, Temizkan S, Sertcelik A, Erden I, Corapcioglu D. Case report: patient with multiple paragangliomas treated with long acting somatostatin analogue. Endocr J. 2003;50(5):507–513. [DOI] [PubMed] [Google Scholar]
- 101. van Hulsteijn LT, van Duinen N, Verbist BM, et al. Effects of octreotide therapy in progressive head and neck paragangliomas: case series. Head Neck 2013;35(12):E391–E396. [DOI] [PubMed] [Google Scholar]
- 102. Tena I, Gupta G, Tajahuerce M, et al. Successful second-line metronomic temozolomide in metastatic paraganglioma: case reports and review of the literature. Clin Med Insights Oncol 2018;12. doi: 10.1177/1179554918763367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jha A, Patel M, Baker E, et al. Role of (68)Ga-DOTATATE PET/CT in a case of SDHB-related pterygopalatine fossa paraganglioma successfully controlled with octreotide. Nucl Med Mol Imaging 2020;54(1):48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Heregger R, Huemer F, Hutarew G, et al. Sustained response to brigatinib in a patient with refractory metastatic pheochromocytoma harboring R1192P anaplastic lymphoma kinase mutation: a case report from the Austrian Group Medical Tumor Therapy next-generation sequencing registry and discussion of the literature. ESMO Open 2021;6(4):100233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.