Abstract
Background
Hospital-acquired pneumonia accounts for 25% of all health care–associated infections and is classified as either ventilator-associated or non–ventilator-associated pneumonia. Hospital-acquired pneumonia most frequently results from aspiration of oropharyngeal secretions into the lungs. Although preventive measures for ventilator-associated pneumonia are well established, few preventive measures exist for the nonventilator type.
Objective
To (1) explore oral microbes associated with ventilator-associated and non–ventilator-associated pneumonia in acutely ill, adult hospitalized patients, and (2) provide evidence-based recommendations for measures to prevent pneumonia in hospitalized patients.
Methods
A literature search was conducted using CINAHL, Academic Search Premier, Medline, and the Cochrane Library.
Results
Ten studies were found that identified common oral microbes in ventilator-associated and non–ventilator-associated pneumonia, including Acinetobacter baumannii, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus, S aureus, and Streptococcus pneumoniae. Collectively, oral colonization with E coli, P aeruginosa, methicillin-resistant S aureus, and S aureus increased the risk of nonventilator pneumonia. Findings also suggested microaspiration of colonized oral microbes into the lungs. Non–ventilator-associated pneumonia had similar colonization rates of gram-positive and gram-negative bacteria, whereas ventilator-associated pneumonia had greater colonization with gram-negative bacteria. The literature did not indicate a standard of oral care effective in all patient populations.
Discussion
Oral care is an effective intervention to prevent hospital-acquired pneumonia by reducing pathogenic oral microbial colonization. The impact of different methods and timing of oral care on oral microbes should be further explored, particularly in patients not receiving mechanical ventilation.
Conclusions
Findings reaffirm the importance of consistent oral care in hospitalized patients. In addition, practices should be different in patients receiving mechanical ventilation versus patients not receiving ventilation. Results may also provide knowledge to inform future preventive measures for pneumonia, particularly for nonventilator pneumonia.
Hospital-acquired pneumonia (HAP) is a common problem in health care, accounting for 25% of all health care–associated infections.1 Hospital-acquired pneumonia develops after 48 hours of hospital admission and is typically categorized as either ventilator-associated pneumonia (VAP) or non–ventilator-associated hospital-acquired pneumonia (NV-HAP).2,3 Ventilator-associated pneumonia occurs in critically ill, intubated patients and has been an important research focus owing to its high mortality rate, negative clinical outcomes, and high costs per case.4,5 Unlike VAP, NV-HAP can affect any hospitalized patient, not just those who are critically ill. Interest in NV-HAP has increased owing to its high rate of occurrence, high mortality rate, and increased costs.6 Currently, NV-HAP occurs in 1.2 to 8.9 patients per 1000 patient days, although rates are likely underestimated because hospitals are not required to report cases of NV-HAP, as they are for VAP.6 The costs of NV-HAP vary from $28 000 to $40 000 per case, and mortality rates among adults range from 13% to 30%.6
Etiology of HAP
Many different types of microbes colonize the mouth and upper respiratory tract in all individuals, including hospitalized patients.7 Hospitalization itself changes the microbial colonization of the mouth and worsens oral health in adult patients.5,8,9 Hospital-acquired pneumonia results from aspiration of oropharyngeal secretions into the lungs,9,10 highlighting the importance of adequate oral health. Owing to the causal relationship between the oral microbial environment and the occurrence of HAP, it is useful to compare microbial colonization in the mouth versus the lungs.
Dental plaque, which is found in both natural teeth and dentures, is a biofilm of microbes that is frequently a source of pneumonia development.8,11 Additional sources of microbial colonization associated with HAP include medical devices situated in the gastrointestinal or pulmonary systems (such as feeding tubes, gastric tubes, and endotracheal tubes), transfer of microbes between staff members (lack of adequate hand hygiene), host or treatment colonization risk factors (eg, antibiotics, surgery, underlying disease severity, invasive devices), and the environment.10
Bacteria are the main cause of HAP.10 Viral and fungal causes of HAP are much less common and are typically seen in patients who are immunocompromised.12 Most bacterial cases of HAP are caused by gram-negative bacteria, with only 20% to 30% of cases being caused by gram-positive bacteria.13 Hospital-acquired pneumonia is also classified as either early onset or late onset. Early-onset HAP occurs within the first 4 days of hospitalization and is generally caused less frequently by drug-resistant bacteria compared with late-onset HAP.13
Multidrug-resistant (MDR) bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA), are found more frequently in HAP compared with community-acquired pneumonia, and MDR infections are increasing in both NV-HAP and VAP cases.14 Immune suppression, antibiotic use and resistance, and hospitalization within the last 3 months are risk factors for experiencing an MDR infection.14 In intubated patients, greater time receiving mechanical ventilation increases the likelihood of experiencing an MDR infection.15 Early-onset HAP cases are generally associated with more positive clinical outcomes compared with late-onset HAP (owing to the virulence of the microbes found in the latter). In addition, late-onset HAP is often polymicrobial, making it more difficult for clinicians to identify and manage.13
Clinical Management of HAP
Diagnosis and management of HAP rely on understanding causative mechanisms and individualizing treatment on the basis of the causative microbes.15–16 Evidence-based guidelines for HAP management suggest that patients with NV-HAP be managed in a similar manner to those with VAP by identifying risk for pneumonia infection with specific microbes (such as MDR pathogens).10 Patients with NV-HAP should be treated in accordance with specific microbes identified from noninvasive samples.16 Cultures are obtained from different specimen types including from the lungs and oropharyngeal or nasotracheal secretions.10,13 Lung specimens are obtained using bronchoalveolar lavage (BAL) or protected BAL fluid. Bronchoalveolar lavage sampling is performed during bronchoscopy by instillation of sterile normal saline into a section of the lung and suctioning to collect the fluid for analysis.17 Protected BAL uses a sterile protected brush to obtain the specimen from the lung.18 Oropharyngeal secretions may be collected using a mouth swab and/or a sputum sample. Analyses of microbial colonization in dental plaque are also used in clinical research19 but are not commonly performed in the clinical setting.
Purpose of Integrative Review
Oral microbes play an important role in the occurrence of HAP.8 To our knowledge, no other published articles have explored the commonalities and differences among oral microbes found in NV-HAP and VAP. Identifying certain patterns of microbial colonization may also provide a foundation for development of a preventive regimen for NV-HAP. The purpose of this integrative review was to (1) explore common oral microbial species associated with NV-HAP and VAP in acutely ill, hospitalized adults and (2) provide evidence-based recommendations for prevention of HAP.
Methods
The databases used for this integrative review were CINAHL, Academic Search Premier, MEDLINE, the Cochrane Central Register of Controlled Trials, and the Cochrane Database of Systematic Reviews. The search strategy used was pneumonia* AND hospital acquired* OR nosocomial infection* OR cross infection* AND oral microbe* OR oral bacteria* OR oral colonization*.
Articles were included if they were peer-reviewed research articles, were published in the English language, focused on adult hospitalized patients with either NV-HAP or VAP, and made mention of oral microbial colonization in relation to NV-HAP or VAP. Articles were excluded if they did not focus on the population described, did not include discussion of mechanical ventilation status, made no mention of oral microbe colonization in relation to HAP, or were literature reviews or evidence-based practice guidelines.
The results of the search process are shown in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram in the Figure. The initial search yielded 388 articles (305 articles after duplicates were removed and 2 additional articles were identified through searches of references in relevant articles), of which 295 were excluded. Thus, 10 articles were included in this review. We completed a critical appraisal of each article using the Joanna Briggs appraisal tools specific to study design.20 The levels of evidence found for the 10 articles were as follows: level I (experimental design), 1 article; level II (quasi-experimental design), 2 articles; level III (nonexperimental study design), 6 articles; and level V (case study), 1 article.21
Figure.
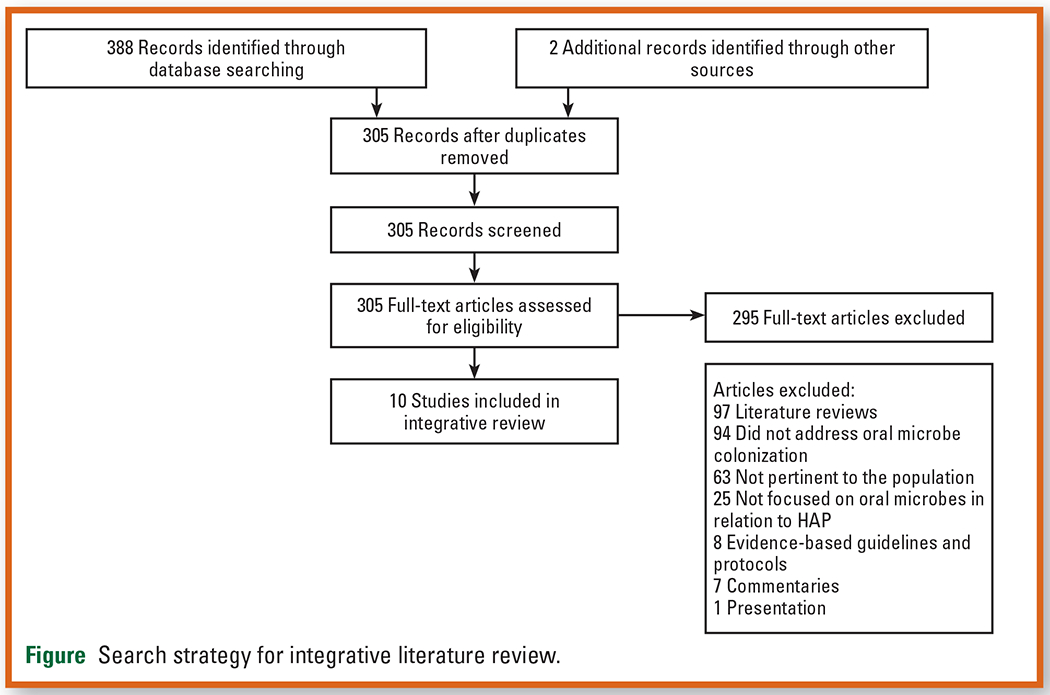
Search strategy for integrative literature review.
Results
The 10 articles included in this review are listed in the Table.8,11,19,22-28 Collectively, the studies provide an overview of commonalities and differences among oral microbes in the different types of pneumonia.
Table.
Details and findings of studies included in the review
| Source | Design/setting | Pneumonia type | Colonization site | Common microbes | Level of evidencea |
|---|---|---|---|---|---|
| Bonten et al,22 1996 | Experimental (subanalysis of RCT) in an ICU | VAP | Oropharynx | Enteric gram-negative bacteria and Pseudo-monadaceae | I |
| Chen et al,23 2016 | Nonexperimental (prospective observational) in an emergency ICU | NV-HAP and VAP | Sputum (NV-HAP) and lungs (VAP) | A baumannii and MRSA | III |
| El Attar et al,19 2010 | Nonexperimental (case-control design) in a respiratory ICU | NV-HAP | Oropharynx, dental plaque, and lungs | S aureus | III |
| El-Solh et al,11 2004 | Nonexperimental (prospective cohort study) in a critical care unit | VAP | Oropharynx, dental plaque, and lungs | S aureus | III |
| Ewan et al,8 2015 | Nonexperimental (prospective cohort study) in orthopedic units | NV-HAP | Dental plaque | S aureus, MRSA, P aeruginosa, and E coli associated with increased risk of HAP (P=.002) | III |
| Gaber et al,24 2020 | Nonexperimental (prospective observational) in a university hospital | NV-HAP | Sputum, pleural fluid, and lungs | P aeruginosa and A baumannii | III |
| Garrouste-Orgeas et al,25 1997 | Nonexperimental (prospective observational) in a medical-surgical ICU | VAP | Oropharynx | S aureus, A baumannii, and P aeruginosa | III |
| Mori et al,26 2006 | Quasi-experimental trial with historical controls in a medical-surgical ICU | VAP | Oropharynx | P aeruginosa, MRSA, and Candida | II |
| Nicolosi et al,27 2014 | Quasi-experimental in patients undergoing cardiac surgery | VAP | Dental plaque | K pneumoniae, S aureus, and P aeruginosa | II |
| Ohkoshi et al,28 2018 | Nonexperimental (case study) in an ICU | VAP | Sputum | S pneumoniae and H influenzae | V |
Abbreviations: A baumannii; Acinetobacter baumannii; E coli, Escherichia coli; H influenzae, Haemophilus influenzae; ICU, intensive care unit; K pneumoniae, Klebsiella pneumoniae; MRSA, methicillin-resistant Staphylococcus aureus; NV-HAP, non-ventilator-associated hospital-acquired pneumonia; P aeruginosa, Pseudomonas aeruginosa; RCT, randomized controlled trial; S aureus, Staphylococcus aureus; S pneumoniae, Streptococcus pneumoniae; VAP, ventilator-associated pneumonia.
Adapted from Dearholt and Dang.21 Level I, experimental studies; level II, quasi-experimental studies; level III, nonexperimental studies; level II, quasiexperimental studies; and level V, case reports.
Microbes in NV-HAP
Patients with NV-HAP had similar colonization rates of gram-positive bacteria and gram-negative bacteria. The most common oral microbes in NV-HAP were Acinetobacter baumannii, Pseudomonas aeruginosa, and S aureus.19,24 Patients who had a combination of oropharyngeal colonization with Escherichia coli, P aeruginosa, MRSA, and S aureus were more than 9 times as likely to develop NV-HAP (odds ratio, 9.48; 95% CI, 2.28-38.78; P = .002).8 The presence of E coli and S aureus independently increased the risk of NV-HAP occurrence.8 In contrast, some oral microbes, including Haemophilus influenzae and Streptococcus pneumoniae, were actually protective against NV-HAP.8 Findings for S pneumoniae were conflicting, as this bacterium was causative in 17% of NV-HAP cases in patients with moderate to severe chronic periodontitis.19
Microbes identified in dental plaque were also associated with the occurrence of NV-HAP.8,19 In most patients with NV-HAP, dental plaque was colonized with 1 or more microbes, with S aureus being the most common.19 Other bacteria identified in dental plaque included Bacteroides species, coagulase-negative staphylococci, and S pneumoniae.19
Specimens obtained by BAL from patients with NV-HAP contained similar microbes to those found in the oropharynx and dental plaque,19 suggesting microaspiration of oropharyngeal secretions into the lungs. The most common microbe found in the lungs was S aureus.19
Different oral care regimens did not significantly change oral bacteria in patients with NV-HAP, aside from greater colonization with Stenotrophomonas maltophilia in patients who had oral care with 0.2% chlorhexidine gluconate (CHG) compared with patients who had oral care with 0.08% metronidazole.23 Metronidazole is an antibacterial agent, whereas CHG is an antiseptic agent,29 which could account for differences in oral microbial findings. Oral colonization with S maltophilia could also have been due to water contamination.
Microbes in VAP
Intubated patients with VAP had greater colonization with gram-negative bacteria than with gram-positive bacteria. The most common oral microbes found in VAP cases were A baumannii, Klebsiella pneumoniae, P aeruginosa, MRSA, and S aureus.11,26,27 In a study in which 29% (14 of 49) of patients experienced VAP, S aureus was the most common microbe found in all specimen types.11 One case study that examined the microbiological sputum profile of a patient with VAP found high degrees of colonization with H influenzae and S pneumoniae.28
In another study, gram-negative bacteria were primarily responsible for all 30 documented cases of VAP.25 The most common gram-negative bacteria colonized in the oropharynx included A baumannii and P aeruginosa.25 A similar study indicated that 18% (26 of 141) of patients with VAP had enteric gram-negative bacteria and Pseudomonadaceae in the oropharynx.22
Causative agents differed in early- versus late-onset VAP. In a small sample of 16 intubated patients, early-onset VAP was caused primarily by P aeruginosa.26 Patients with late-onset VAP still had frequent colonization with P aeruginosa; however, they had a higher incidence of infections with more resistant microbes, including MRSA.
In patients who received oral care and experienced VAP, K pneumoniae, P aeruginosa, MRSA, and S aureus were frequently identified.26,27 In patients who did not receive oral care and experienced VAP, P aeruginosa was predominant.26,27 Several other gram-negative and gram-positive bacteria were identified in patients who did not receive oral care.26,27 In addition, certain types of oral care influenced the type of bacteria found in patients with VAP.23 Intubated patients who received oral care with 0.2% CHG had significantly greater colonization with gram-negative bacteria in the lungs compared with patients who received oral care with 0.08% metronidazole (P = .02).23
Discussion
Implications of Microbial Findings
The studies in this review explored microbes found in the oropharynx, dental plaque, and lungs of patients with NV-HAP and VAP. Oral microbial findings were similar between pneumonia types, including A baumannii, E coli, K pneumoniae, P aeruginosa, MRSA, S aureus, and S pneumoniae. Gram-positive bacteria, such as S aureus and S pneumoniae, are common in the community setting and frequently found on the human body.13 For instance, in healthy individuals, MRSA and S aureus are sometimes found in the nares and S aureus on the skin.30,31 Hospital-acquired pneumonia infections caused by gram-positive bacteria (such as MRSA) are concerning owing to emerging resistant strands and high costs of treatment.32 Patients at risk for development of Staphylococcus HAP infections include those with chronic conditions (such as diabetes) and immunocompromised patients who undergo invasive procedures.30
Cases of VAP are caused primarily by gram-negative bacteria, as reaffirmed in our review.33 This finding may be due to the frequent colonization of the oropharynx and gut by gram-negative bacteria, followed by common mechanisms such as gastric reflux into the oropharynx, and through transmission by health care workers. Both of these situations could lead to VAP.34
Gram-negative bacteria are associated with severe health consequences, including pneumonia, septicemia, meningitis, and surgical site or wound infections.30 Many gram-negative bacteria are becoming resistant to antibiotics, which is a growing concern in the health care setting owing to the serious infections that may result and limited antibiotic treatments available.30,35
A particularly concerning gram-negative bacterium found in both pneumonia types is P aeruginosa, which is often waterborne.36 Common environmental reservoirs of P aeruginosa include sinks, sink faucets, respiratory therapy equipment, and portable water, among others.37 Pseudomonas aeruginosa is of great concern in hospitals owing to its increasing presence in cases of VAP and antimicrobial resistance, making it difficult to treat.38
Another disconcerting bacterium found in the mouth in cases of both NV-HAP and VAP was E coli. Although E coli normally resides in the gut of healthy individuals, oropharyngeal colonization with E coli is rare in the community setting.39 Oropharyngeal colonization with E coli is concerning because of its ability to cause HAP and associated negative health outcomes, including longer intensive care unit and hospital stays, high mortality and costs, and increased antibiotic use.39 In addition, antibiotic-resistant strains of E coli have been emerging, which are associated with worse clinical outcomes.39 Oropharyngeal colonization with E coli occurs more often in critically ill hospitalized patients, most likely owing to a multifactorial process.39 Factors that may increase oropharyngeal colonization with E coli include increased supine positioning, gastric reflux, gut-lung translocation, altered gastric pH from proton pump inhibitors, altered local immunity, and/or contamination from health care workers (resulting from poor hand hygiene).39
Few MDR pathogens were noted among both types of HAP. Our review found similar oral bacteria in early-and late-onset VAP, with P aeruginosa being the most common. However, late-onset VAP cases had greater colonization with resistant bacteria (mainly MRSA).26 Supporting literature shows that infecting microbes are more likely to respond to antibiotics in early-onset than in late-onset VAP, which is frequently caused by resistant bacteria.40 Multidrug-resistant pathogens were found in nearly all VAP cases regardless of when the pneumonia developed, suggesting that the microbial cause of early VAP may be shifting.41
Clinical Practice Recommendations
Our review found that a variety of potentially pathogenic microbes are associated with the development of HAP. Oral care is an effective preventive measure against pneumonia; however, review of the literature did not isolate a standard of oral care effective in all patient populations. Hospitalized patients may need different oral care regimens depending on their level of acuity and individualized risk factors for HAP. The oral care recommendations below are not inclusive but are evidence-based oral care practices.
Patients in Acute Care Settings Not Receiving Mechanical Ventilation.
Toothbrushing and cleansing of gums and dentures may be effective methods of reducing plaque and microbe accumulation in the mouth, but further research is required to identify best practices that improve outcomes.1,9,42 Recommendations regarding routine use of CHG in patients who are not receiving mechanical ventilation are conflicting and need further study.43,44
Patients Receiving Mechanical Ventilation.
Ventilator-associated pneumonia prevention bundles often include oral care with CHG.42,45 Chlorhexidine reduces the risk for VAP from 26% to 18%, but there is no evidence that it reduces mortality, duration of mechanical ventilation, or intensive care unit length of stay.45 Concentrations of CHG vary and influence outcomes. A meta-analysis found that oral care with 2% CHG reduced the incidence of VAP (relative risk, 0.53; 95% CI, 0.31-0.91), but lower concentrations had no effect.46 Findings have been mixed regarding whether higher concentrations of oral CHG may have adverse effects on the oral mucosa, such as lesions, ulcerations, and bleeding.47,48 An increased risk of oral mucosal lesions was associated with mechanical ventilation, receiving 2% CHG for long periods of time, and severe illness.47 A recent multisite study of 14 333 patients undergoing ventilation indicated that CHG was associated with increased odds of death and sepsis and had no effect on VAP.49
Hand Hygiene.
Consistent hand hygiene is also important for patients and staff members to prevent oropharyngeal colonization with pathogens like E coli, which was commonly found in HAP cases. This organism is not normally found in the mouth but can be spread via the fecal-to-oral route through inadequate hand hygiene.
Research Recommendations
Future research should further explore oral microbes found in the hospitalized population not receiving mechanical ventilation, as the evidence on this topic is insufficient. Most articles included in our review focused on VAP, and many articles related to NV-HAP were outdated. Research should explore how oral microbes change over the course of hospitalization and with different treatment regimens. Understanding these changes will help clinicians individualize patient care, which will improve clinical outcomes. Oral bacteria may differ across patients, making it important to explore and better understand contributing factors. Other factors such as diet (eg, vegetarian) can also influence the mix of oral microbes in an individual patient.50
Second, future NV-HAP research should focus on the impact of different types of oral care on oral microbes. Our review found that specific oral microbes were associated with NV-HAP, including E coli, P aeruginosa, MRSA, and S aureus. We also found that certain types of oral microbes, such as H influenzae and S pneumoniae, may actually be protective against NV-HAP,8 helping to maintain an equilibrium of the oral microbiome for both oral and systemic health.51 Different oral care methods and/or products may have varying effects on oral microbial colonization. For instance, investigators in a randomized clinical trial found that 1% CHG oral care with a toothbrush reduced oral colonization with S aureus (one of the most common causes of HAP) by 42% during a 6-month period.52 The frequency of oral care with CHG was not specified, although the concentration of CHG is a lower one than that used for VAP prevention in critically ill patients. The impact of different oral care regimens on the type of oral microbe development in different patient populations should be further explored. Different concentrations of CHG should be explored to determine which is most safe and effective.
Finally, aside from oral care, few prevention interventions have been systematically explored to prevent NV-HAP.14 Future studies are needed to develop a comprehensive interdisciplinary approach to preventing NV-HAP.
Limitations
A limitation of this integrative review is the lack of studies that examined oral microbes associated with particular types of pneumonia, especially NV-HAP. Few studies focused solely on oral microbes in pneumonia, and they mainly provided descriptive statistics. Other studies not included in this review explore microbes found in the lungs of intubated patients and patients with VAP. However, the focus of this review was oral microbes, so these articles were not included. In addition, several studies had small sample sizes, limiting the generalizability of the findings. one study was specific to chronic periodonititis, limiting the generalizability of its findings to other NV-HAP cases. Finally, most articles included in the review were published more than 5 years ago. The prevalence of specific microbes may have changed over time; thus, the findings may not be applicable to the current clinical setting. Recent research has been published on oral care for intubated patients; however, this research was not included because this topic was not the focus of this review.
Conclusion
Our review found common oral microbes among cases of NV-HAP and VAP. The former had similar rates of oral colonization with gram-positive and gram-negative bacteria, whereas the latter had greater colonization with gram-negative than with gram-positive bacteria. The findings provide a foundation for understanding oral microbes associated with pneumonia, particularly in patients not undergoing mechanical ventilation, which may inform future preventive measures and research trajectories. Microaspiration of oropharyngeal secretions, including oral microbes, was noted, reaffirming the importance of consistent and individualized oral care in all hospitalized patients. It is important for nurses to recognize that current evidence supports different oral care practices for patients receiving versus not receiving mechanical ventilation. Adherence to isolation protocols and proper hand hygiene are also essential in reducing the spread of pathogens.
Hospital-acquired pneumonia (HAP) most frequently results from aspiration of oropharyngeal secretions into the lungs. In this article, the authors explore oral microbes associated with ventilator-associated pneumonia (VAP) and non–ventilator-associated pneumonia (NV-HAP) in acutely ill patients, and provide evidence-based recommendations for measures to prevent pneumonia in hospitalized patients.
Bacteria are the main cause of HAP. Multidrug-resistant (MDR) bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA), are found more frequently in HAP compared with community-acquired pneumonia.
Hospital-acquired pneumonia infections caused by gram-positive bacteria (such as MRSA) are concerning owing to emerging resistant strands and high costs of treatment.
Immune suppression, antibiotic use and resistance, and hospitalization within the last 3 months are risk factors for experiencing an MDR infection. In intubated patients, greater time receiving mechanical ventilation increases the likelihood of experiencing an MDR infection.
Oral care is an effective preventive measure against pneumonia; however, review of the literature did not isolate a standard of oral care effective in all patient populations. Hospitalized patients may need different oral care regimens depending on their level of acuity and individualized risk factors for HAP.
Patients in Acute Care Settings Not Receiving Mechanical Ventilation. Toothbrushing and cleansing of gums and dentures may be effective methods of reducing plaque and microbe accumulation in the mouth, but further research is required to identify best practices that improve outcomes.
Patients Receiving Mechanical Ventilation. Ventilator-associated pneumonia prevention bundles often include oral care with CHG. Chlorhexidine reduces the risk for VAP from 26% to 18%, but there is no evidence that it reduces mortality, duration of mechanical ventilation, or intensive care unit length of stay. Concentrations of CHG vary and influence outcomes. Findings have been mixed regarding whether higher concentrations of oral CHG may have adverse effects on the oral mucosa, such as lesions, ulcerations, and bleeding.
Hand Hygiene. Consistent hand hygiene is also important for patients and staff members to prevent oropharyngeal colonization with pathogens like Escherichia coli, which was commonly found in HAP cases.
It is important for nurses to recognize that current evidence supports different oral care practices for patients receiving versus not receiving mechanical ventilation. Adherence to isolation protocols and proper hand hygiene are also essential in reducing the spread of pathogens.
Financial Disclosures
This research was supported by a grant from the National Institute of Nursing Research, National Institutes of Health (1F31NR019518-01A1).
Footnotes
To purchase electronic or print reprints, contact the American Association of Critical-Care Nurses, 27071 Aliso Creek Rd, Aliso Viejo, CA 92656. Phone, (800) 899-1712 or (949) 362-2050 (ext 532); fax, (949) 362-2049; reprints@aacn.org.
Contributor Information
Kimberly Paige Rathbun, PhD student, predoctoral fellow, and graduate student research assistant at the University of Central Florida College of Nursing, Orlando.
Annette M. Bourgault, associate professor at the University of Central Florida College of Nursing.
Mary Lou Sole, dean, professor, and Orlando Health Endowed Chair in Nursing at the University of Central Florida College of Nursing.
References
- 1.Munro S, Baker D. Reducing missed oral care opportunities to prevent non-ventilator associated hospital acquired pneumonia at the Department of Veterans Affairs. Appl Nurs Res. 2018;44:48–53. [DOI] [PubMed] [Google Scholar]
- 2.National Healthcare Safety Network, Centers for Disease Control and Prevention. Identifying healthcare-associated infections (HAI) for NHSN surveillance. January 2022. Accessed November 12, 2021. https://www.cdc.gov/nhsn/PDFs/pscManual/2PSC_IdentifyingHAIs_NHSNcurrent.pdf
- 3.National Healthcare Safety Network, Centers for Disease Control and Prevention. Pneumonia (ventilator-associated [VAP] and non-ventilator-associated pneumonia [PNEU]) event. January 2022. Accessed November 12, 2021. https://www.cdc.gov/nhsn/pdfs/pscmanual/6pscvapcurrent.pdf
- 4.Papazian L, Klompas M, Luyt CE. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. 2020;46(5):888–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munro CL, Grap MJ, Elswick RK Jr, McKinney J, Sessler CN, Hummel RS III. Oral health status and development of ventilator-associated pneumonia: a descriptive study. Am J Crit Care. 2006;15(5):453–460. [PubMed] [Google Scholar]
- 6.Giuliano KK, Baker D, Quinn B. The epidemiology of nonventilator hospital-acquired pneumonia in the United States. Am J Infect Control. 2018;46(3):322–327. [DOI] [PubMed] [Google Scholar]
- 7.Kumpitsch C, Koskinen K, Schöpf V, Moissl-Eichinger C. The microbiome of the upper respiratory tract in health and disease. BMC Biol. 2019;17(1):87. doi: 10.1186/s12915-019-0703-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ewan VC, Sails AD, Walls AWG, Rushton S, Newton JL. Dental and microbiological risk factors for hospital-acquired pneumonia in non-ventilated older patients. PLoS One. 2015;10(4):e0123622. doi: 10.1371/journal.pone.0123622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinn B, Baker DL, Cohen S, Stewart JL, Lima CA, Parise C. Basic nursing care to prevent nonventilator hospital-acquired pneumonia. J Nurs Scholarsh. 2014;46(1):11–19. doi: 10.1111/jnu.12050 [DOI] [PubMed] [Google Scholar]
- 10.American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. [DOI] [PubMed] [Google Scholar]
- 11.El-Solh AA, Pietrantoni C, Bhat A, et al. Colonization of dental plaques: a reservoir of respiratory pathogens for hospital-acquired pneumonia in institutionalized elders. Chest. 2004;126(5):1575–1582. [DOI] [PubMed] [Google Scholar]
- 12.Kelliher K, Kirton OC. Infections in critically ill patients. In: Hupp JR, Ferneini EM, eds. Head, Neck, and Orofacial Infections: An Interdisciplinary Approach. Elsevier; 2016:383–394. [Google Scholar]
- 13.Cilloniz C, Martin-Loeches I, Garcia-Vidal C, San Jose A, Torres A. Microbial etiology of pneumonia: epidemiology, diagnosis and resistance patterns. Int J Mol Sci. 2016;17(12):2120. doi: 10.3390/ijms17122120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pássaro L, Harbarth S, Landelle C. Prevention of hospital-acquired pneumonia in non-ventilated adult patients: a narrative review. Antimicrob Resist Infect Control. 2016;5:43. doi: 10.1186/s13756-016-0150-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalanuria AA, Zai W, Mirski M. Ventilator-associated pneumonia in the ICU. Crit Care. 2014;18(2):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalil AC, Metersky ML, Klompas M, et al. Executive summary: Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):575–582. doi: 10.1093/cid/ciw504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel PH, Antoine M, Ullah S. Bronchoalveolar lavage. StatPearls. 2020. Accessed November 4, 2021. https://www.ncbi.nlm.nih.gov/books/NBK430762 [PubMed] [Google Scholar]
- 18.Grønseth R, Drengenes C, Wilker HG, et al. Protected sampling is preferable in bronchoscopic studies of the airway microbiome. ERJ Open Res. 2017;3(3):00019–2017. doi: 10.1183/23120541.00019-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Attar MM, Zaghloup MZ, Elmenoufr HS. Role of periodontitis in hospital-acquired pneumonia. East Mediterr Health J. 2010;16(5):563–569. [PubMed] [Google Scholar]
- 20.Joanna Briggs Institute. Critical appraisal tools. Accessed October 8, 2021. https://jbi.global/critical-appraisal-tools
- 21.Dearholt SL, Dang D. Johns Hopkins Nursing Evidence-Based Practice: Model and Guidelines. 2nd ed. Sigma Theta Tau International; 2012. [DOI] [PubMed] [Google Scholar]
- 22.Bonten MJ, Bergmans DC, Ambergen AW, et al. Risk factors for pneumonia, and colonization of respiratory tract and stomach in mechanically ventilated ICU patients. Am J Respir Crit Care Med. 1996;154(5):1339–1346. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Mao EQ, Yang YJ, et al. Prospective observational study to compare oral topical metronidazole versus 0.2% chlorhexidine gluconate to prevent nosocomial pneumonia. Am J Infect Control. 2016;44(10):1116–1122. [DOI] [PubMed] [Google Scholar]
- 24.Gaber SN, Hemeda EEM, Elsayeh HAS, Wahed WYA, Khalil MAF, Ibrahim EG. Propolis extract: a possible antiseptic oral care against multidrug-resistant non-fermenting bacteria isolated from non-ventilator hospital-acquired pneumonia. J Pure Appl Microbiol. 2020;14(1):123–131. [Google Scholar]
- 25.Garrouste-Orgeas M, Chevret S, Arlet G, et al. Oropharyngeal or gastric colonization and nosocomial pneumonia in adult intensive care unit patients: a prospective study based on genomic DNA analysis. Am J Respir Crit Care Med. 1997;156(5):1647–1655. doi: 10.H64/ajrccm.156.5.96-04076 [DOI] [PubMed] [Google Scholar]
- 26.Mori H, Hirasawa H, Oda S, Shiga H, Matsuda K, Nakamura M. Oral care reduces incidence of ventilator-associated pneumonia in ICU populations. Intensive Care Med. 2006;32(2):230–236. doi: 10.1007/s00134-005-0014-4 [DOI] [PubMed] [Google Scholar]
- 27.Nicolosi LN, del Carmen Rubio M, Martinez CD, González NN, Cruz ME. Effect of oral hygiene and 0.12% chlorhexidine gluconate oral rinse in preventing ventilator-associated pneumonia after cardiovascular surgery. Respir Care. 2014;59(4):504–509. doi: 10.4187/respcare.02666 [DOI] [PubMed] [Google Scholar]
- 28.Ohkoshi Y, Sato T, Wada T, et al. Whole genome analysis of a multidrug-resistant Streptococcus pneumoniae isolate from a patient with invasive pneumococcal infection developing disseminated intravascular coagulation. J Infect Chemother. 2018;24(8):674–681. doi: 10.1016/j.jiac.2018.01.012 [DOI] [PubMed] [Google Scholar]
- 29.Pradeep AR, Kumari M, Priyanka N, Naik SB. Efficacy of chlorhexidine, metronidazole and combination gel in the treatment of gingivitis—a randomized clinical trial. J Int Acad Periodontol. 2012;14(4):91–96. [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Healthcare-associated infections. 2016. Accessed November 10, 2021. https://www.cdc.gov/hai/index.html
- 31.Centers for Disease Control and Prevention. Healthcare settings. 2019. Accessed November 15, 2021. https://www.cdc.gov/mrsa/healthcare/index.html
- 32.Woodford N, Livermore DM. Infections caused by Gram-positive bacteria: a review of the global challenge. J Infect. 2009;59(suppl 1):S4–S16. [DOI] [PubMed] [Google Scholar]
- 33.Thakuria B, Singh P, Agrawal S, Asthana V. Profile of infective microorganisms causing ventilator-associated pneumonia: a clinical study from resource limited intensive care unit. J Anaesthesiol Clin Pharmacol. 2013;29(3):361–366. doi: 10.4103/0970-9185.117111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park DR. The microbiology of ventilator-associated pneumonia. Respir Care. 2005;50(6):742–763. [PubMed] [Google Scholar]
- 35.Oliphant CM, Eroschenko K. Antibiotic resistance, part 2: gram-negative pathogens. J Nurse Pract. 2015;11(1):79–86. doi: 10.1016/jnurpra.2014.10.008 [DOI] [Google Scholar]
- 36.Mena KD, Gerba CP. Risk assessment of Pseudomonas aeruginosa in water. Rev Environ Contam Toxicol. 2009;201:71–115. [DOI] [PubMed] [Google Scholar]
- 37.Kerr KG, Snelling AM. Pseudomonas aeruginosa: a formidable and ever-present adversary. J Hosp Infect. 2009;73(4):338–344. [DOI] [PubMed] [Google Scholar]
- 38.Bassetti M, Vena A, Croxatto A, Righi E, Guery B. How to manage Pseudomonas aeruginosa infections. Drugs Context. 2018;7:212527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Lastours V, Malosh RE, Aiello AE, Foxman B. Prevalence of Escherichia coli carriage in the oropharynx of ambulatory children and adults with and without upper respiratory symptoms. Ann Am Thorac Soc. 2015;12(3):461–463. doi: 10.1513/AnnalsATS.201412-586LE [DOI] [PubMed] [Google Scholar]
- 40.Giard M, Lepape A, Allaouchiche B, et al. Early- and late-onset ventilator-associated pneumonia acquired in the intensive care unit: comparison of risk factors. J Crit Care. 2008;23(1):27–33. [DOI] [PubMed] [Google Scholar]
- 41.Khan R, Al-Dorzi HM, Tamim HM, et al. The impact of onset time on the isolated pathogens and outcomes in ventilator associated pneumonia. J Infect Public Health. 2016;9(2):161–171. doi: 10.1016/j.jiph.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 42.Quinn B, Giuliano KK, Baker D. Non-ventilator health care-associated pneumonia (NV-HAP): best practices for prevention of NV-HAP. Am J Infect Control. 2020;48(5):A23–A27. [DOI] [PubMed] [Google Scholar]
- 43.Sharif-Abdullah SS, Chong MC, Surindar-Kaur SS, Kamaruzzaman SB, Ng KH. The effect of chlorhexidine in reducing oral colonisation in geriatric patients: a randomised controlled trial. Singapore Med J. 2016;57(5):262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deschepper M, Waegeman W, Eeckloo K, Vogelaers D, Blot S. Effects of chlorhexidine gluconate oral care on hospital mortality: a hospital-wide, observational cohort study. Intensive Care Med. 2018;44(7):1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao T, Wu X, Zhang Q, Li C, Worthington HV, Hua F. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst Rev. 2020;12(12):CD008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villar CC, Pannuti CM, Nery DM, Morillo CMR, Carmona MJC, Romito GA. Effectiveness of intraoral chlorhexidine protocols in the prevention of ventilator-associated pneumonia: meta-analysis and systematic review. Respir Care. 2016;61(9):1245–1259. doi: 10.4187/respcare.04610 [DOI] [PubMed] [Google Scholar]
- 47.Plantinga NL, Wittekamp BHJ, Leleu K, et al. Oral mucosal adverse events with chlorhexidine 2% mouthwash in ICU. Intensive Care Med. 2016;42(4):620–621. doi: 10.1007/s00134-016-4217-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zand F, Zahed L, Mansouri P, Dehghanrad F, Bahrani M, Ghorbani M. The effects of oral rinse with 0.2% and 2% chlorhexidine on oropharyngeal colonization and ventilator associated pneumonia in adults’ intensive care units. J Crit Care. 2017;40:318–322. doi: 10.1016/j.jcrc.2017.02.029 [DOI] [PubMed] [Google Scholar]
- 49.Parreco J, Soe-Lin H, Byerly S, et al. Multi-center outcomes of chlorhexidine oral decontamination in intensive care units. Surg Infect (Larchmt). 2020;21(8):659–664. doi: 10.1089/sur.2019.172 [DOI] [PubMed] [Google Scholar]
- 50.Lu M, Xuan S, Wang Z. Oral microbiota: a new view of body health. Food Sci Hum Wellness. 2019;8(1):8–15. doi: 10.1016/j.fshw.2018.12.001 [DOI] [Google Scholar]
- 51.Kilian M, Chapple ILC, Hannig M, et al. The oral microbiome—an update for oral healthcare professionals. Br Dent J. 2016;221(10):657–666. [DOI] [PubMed] [Google Scholar]
- 52.Ab Malik N, Razak FA, Yatim SM, et al. Oral health interventions using chlorhexidine—effects on the prevalence of oral opportunistic pathogens in stroke survivors: a randomized clinical trial. J Evid Based Dent Pract. 2018;18(2):99–109. doi: 10.1016/j.jebdp.2017.08.002 [DOI] [PubMed] [Google Scholar]


