Abstract
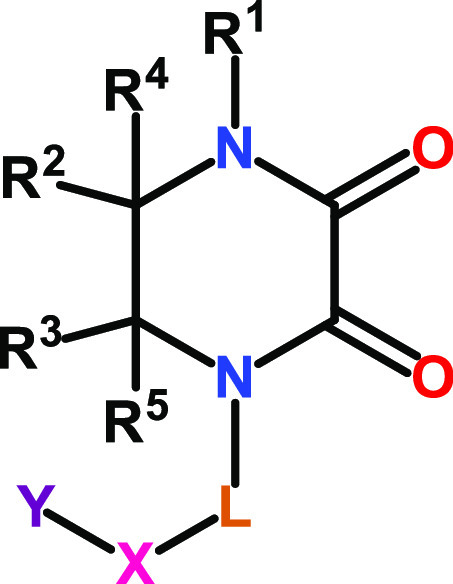
The invention in this patent application relates to piperazine-2,3-dione derivatives represented generally by formula 1. These compounds show activities as selective interleukin 4 induced protein 1 (IL4I1) inhibitors and may potentially be useful in preventing and treating IL4Il-related diseases, such as endometrial, ovarian, and triple negative breast cancers.
Important Compound Classes
Title
IL4I1 Inhibitors and Methods of Use
URL
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2022227015&_cid=P10-LAGZH1-09942-1
Patent Publication Number
WO 2022/227015 A1
Publication Date
November 3, 2022
Priority Application
PCT/CN 2021/091534
Priority Date
April 30, 2021
Inventors
Giambasu, G. M.; Haidle, A. M.; Hopkins, B. A.; Jewell, J. P.; Larsen, M. A.; Lesburg, C. A.; Liu, P.; Pu, Q.; Sanyal, S.; Siliphaivanh, P.; Tudor, M.; White, C. M.; Yan, X.; Zhao, L.; Zheng, X.-M.
Assignee Company
Merck Sharp & Dohme Corp., 770 Sumneytown Pike, West Point, Pennsylvania 19486, USA; MSD R&D Co., Ltd. [CN/CN], SF, Building A, Zongbuyuan Erqi, No. 1582 Gumei Road, Xuhui District, Shanghai 200233, China
Disease Area
Cancer, including endometrial, ovarian, and triple negative breast cancer
Biological Target
Interleukin 4 induced protein 1 (IL4I1)
Summary
The glycosylated protein interleukin 4
induced protein 1 (IL4I1) is a member of the l-amino
acid oxidase (LAAO) family of flavin adenine dinucleotide (FAD)-bound
enzymes. It is expressed in myeloid cells as well as T and B lymphocytes.
IL4I1 performs primarily the oxidative deamination of phenylalanine
(Phe) in the presence of H2O and O2 to produce
ammonium 3-phenylpyruvate and H2O2 according
to the following equation: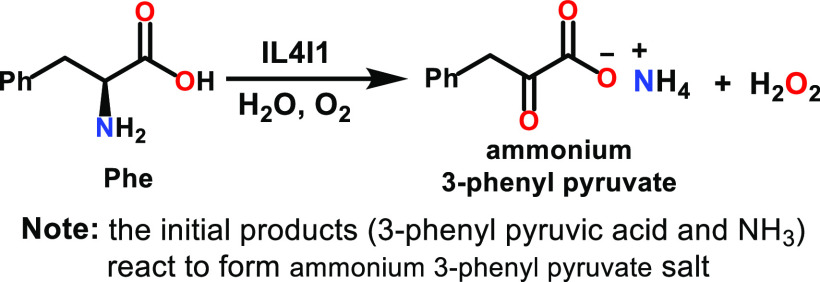 This activity has given this enzyme the name l-phenylalanine
oxidase. However, it also shows activity toward catalyzing the oxidative
deamination of other l-α-amino acids containing aromatic
residues, including tyrosine (Tyr) and tryptophan (Trp), to produce
3-(4-hydroxyphenyl)pyruvate and indole-3-pyruvate, respectively.
In addition, IL4I1 shows weaker activity toward the oxidative deamination
of l-arginine.
This activity has given this enzyme the name l-phenylalanine
oxidase. However, it also shows activity toward catalyzing the oxidative
deamination of other l-α-amino acids containing aromatic
residues, including tyrosine (Tyr) and tryptophan (Trp), to produce
3-(4-hydroxyphenyl)pyruvate and indole-3-pyruvate, respectively.
In addition, IL4I1 shows weaker activity toward the oxidative deamination
of l-arginine.
IL4I1 is highly produced in cells of myeloid origin (monocytes/macrophages and dendritic cells) of the human immune system. Its production is particularly elevated as a result of stimulation by inflammatory and T helper type 1 (Th1) stimuli. Accordingly, IL4I1 is strongly produced by dendritic cell and macrophage populations from chronic Th1 granulomas of sarcoidosis and tuberculosis, but not Th2 granulomas (schistosomiasis). It is also strongly produced by tumor-infiltrating macrophages from various histological types of tumors.
The oxidative deamination activity of IL4I1 causes the depletion of essential amino acids while liberating several toxic metabolites that may block the actions of anti-tumor effector T cells. Researchers have observed the expression of IL4I1 in the tumor-associated macrophage (TAM) population of many types of tumors, and it was also detected in tumor cells of several B lymphoma subtypes. The presence of IL4I1-producing cells in the tumor cell microenvironment inhibits the anti-tumor immune response either directly by limiting the proliferation and functionality of cytotoxic T cells and Th1 cells or indirectly by facilitating the accumulation of Treg cells. Analyses of biopsy samples of human tumor and normal tissues have identified increased expression of both IL4I1 mRNA and protein in tumor-infiltrating myeloid cells.
The Cancer Genome Atlas (TCGA) indicates that, among solid tumors, endometrial carcinoma contains the highest levels of IL4I1 mRNA expression, followed by serious ovarian and triple negative breast cancers. Additionally, elevated levels of phenylpyruvic acid (the product of oxidative deamination of phenylalanine by IL4I1) were detected in endometrial and ovarian tumor samples compared to matched adjacent tissues obtained from the same patients. Furthermore, the accumulation of detectable levels of phenylpyruvic acid in tumor samples is dependent on the presence of IL4I1 itself.
The above data point to the inhibition of IL4I1 as a potentially useful biological target for the treatment of cancer indications where IL4I1 is most expressed and/or active, including endometrial, ovarian, and triple negative breast cancers.
Therefore, there is a need for specific inhibitors of IL4I1. Currently, however, there are no available specific inhibitors of IL4I1, and while researchers have identified some molecules capable of inhibiting related LAAOs found in snake venom, they were generally non-selective, with little activity against IL4I1.
The compounds of formula 1 described in this patent application have displayed specific activities against IL4I1 and may potentially provide useful treatment of cancer indications where IL4I1 is most expressed and/or active, including endometrial, ovarian, and triple negative breast cancers.
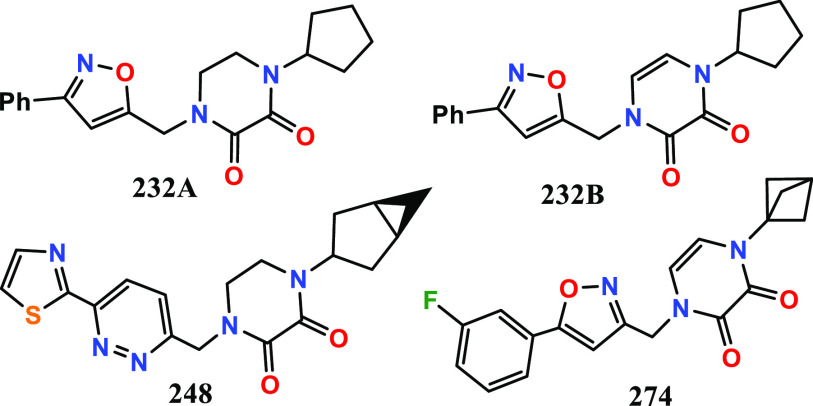
Key Structures
The inventors reported the structures
and methods of synthesis of 278 examples of formula 1, including the
following representative examples: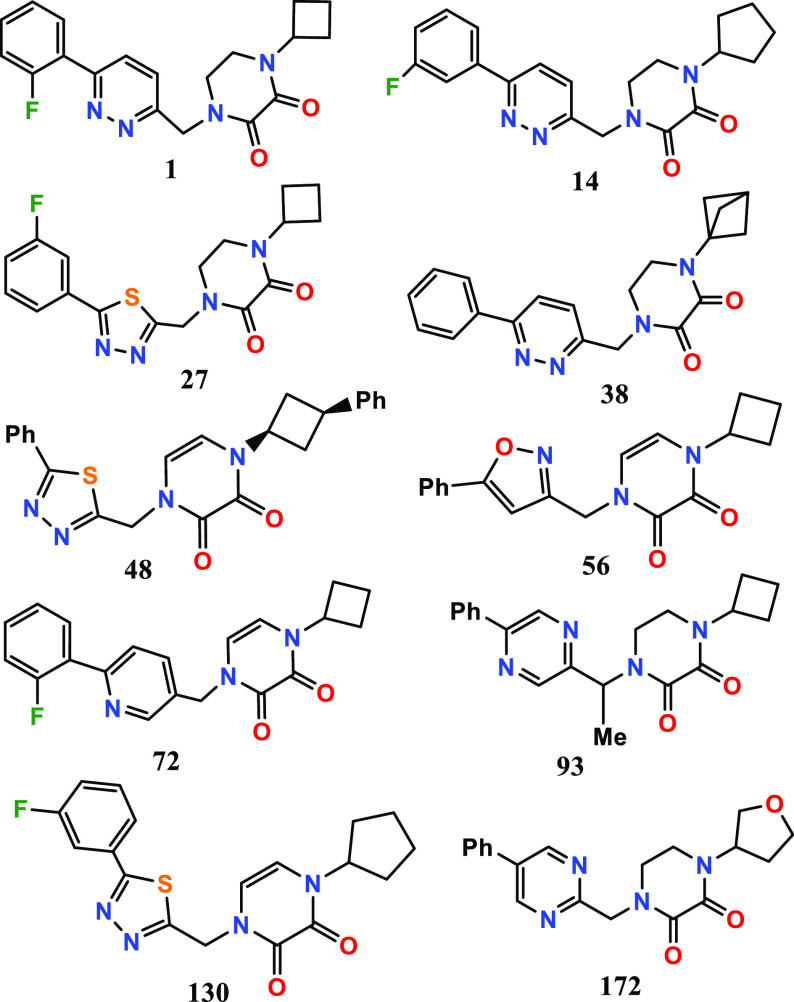
Biological Assay
IL4I1 Enzymatic Assay
Biological Data
The inhibitory effect of the compounds
of formula 1 (EC50) on IL4I1 was assessed by measuring
the effectiveness of these compounds in inhibiting the production
of H2O2. The data obtained from testing the
above representative examples by this assay are outlined in the following
table: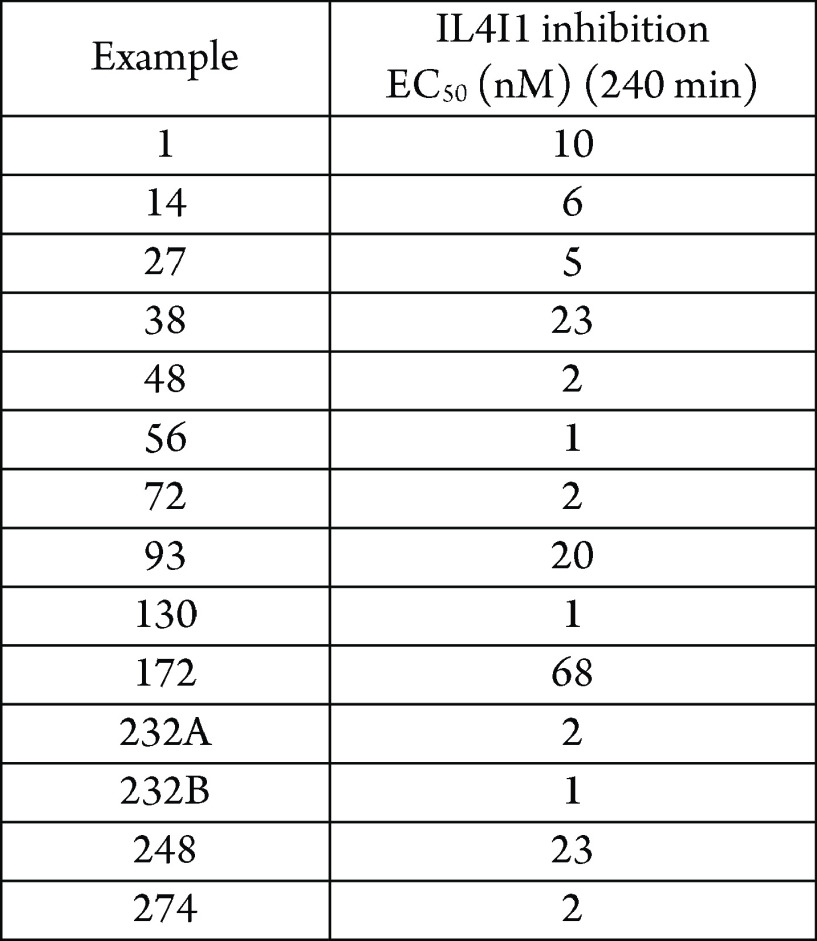
Recent Review Articles
The author declares no competing financial interest.
References
- Prevost-Blondel A.; Richard Y. Interleukin 4-Induced Gene 1 as an Emerging Regulator of B-Cell Biology and its Role in Cutaneous Melanoma. Crit. Rev. Immunol. 2019, 39 (1), 39–57. 10.1615/CritRevImmunol.2019030020. [DOI] [PubMed] [Google Scholar]
- Molinier-Frenkel V.; Prevost-Blondel A.; Castellano F. The IL4I1 Enzyme: A New Player in the Immunosuppressive Tumor Microenvironment. Cells 2019, 8 (7), 757. 10.3390/cells8070757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasoudris F.; Cousin C.; Prevost-Blondel A.; Martin-Garcia N.; Abd-Alsamad I.; Ortonne N.; Farcet J.-P.; Castellano F.; Molinier-Frenkel V. IL4I1: an inhibitor of the CD8+ antitumor T-cell response in vivo. Eur. J. Immunol. 2011, 41 (6), 1629–1638. 10.1002/eji.201041119. [DOI] [PMC free article] [PubMed] [Google Scholar]


