Abstract
Proton pump inhibitors (PPIs) are widely prescribed and are indicated for the treatment of several GI disorders. Allergists may prescribe PPIs due to the co-incidence of gastroesophageal reflux disease (GERD) with asthma or rhinitis, or when GERD presents as chronic cough. Further, long-term high-dose PPI therapy is a recommended option for management of eosinophilic esophagitis, resulting in histologic remission in approximately 40% of patients. Here, we discuss current recommendations for PPI use, de-escalation, and their side effect profile. We review evidence supporting the epidemiologic link between use of acid-suppressant medication and subsequent development of allergic disorders.
Keywords: proton pump inhibitor, asthma, eosinophilic esophagitis, hypersensitivity, adverse events
Introduction
Acid-suppressant medications are a class of medication that inhibit gastric acid secretion and include histamine-2 receptor antagonists (H2RAs) and proton-pump inhibitors (PPIs). Approximately 15 million U.S. adults, or 7.8% of the American population are estimated to use PPIs.1 They are standard treatment for several conditions including gastroesophageal reflux disease, peptic ulcer disease, Helicobacter pylori infection (in combination with antibiotics), upper gastrointestinal (GI) bleeding prophylaxis, and hypersecretory conditions like Zollinger-Ellison Syndrome.2 Proton pump inhibitors are frequently prescribed, available over the counter, and commonly continued for longer than may be clinically indicated. Use in pregnancy, infancy and early childhood is common despite guidance to avoid unnecessary use of PPIs and little data to support PPI efficacy for many indications.3,4 Over time, there has been increasing focus on potential side effects attributable to PPI use.5–7 Care should be taken to limit PPI use to indications where benefit is expected, and to consider de-escalation of therapy when feasible.8 In this review, we discuss data supporting use of PPIs in allergic conditions, risks of chronic PPI use, and potential impact of PPIs on development of allergic disorders.9
Role of proton pump inhibitor treatment in allergic disorders
Gastroesophageal reflux disease frequently occurs together with rhinitis, asthma, and eosinophilic esophagitis (EoE), and can exacerbate these conditions. The symptoms of GERD, in particular cough,10 can mimic uncontrolled asthma. Approaches to address this concern have been reviewed in depth elsewhere.11 There has been significant interest in the potential role of PPI as a primary or adjunctive treatment for atopic conditions, in particular asthma and eosinophilic esophagitis. We will review the data for these below.
Asthma
GERD frequently coexists with asthma, however the results of trials regarding the role of acid suppression in the management of asthma have been inconsistent. While several trials have demonstrated improved patient-reported asthma symptoms following acid suppression therapy for GERD,12–17 there is less evidence to support objective improvement in asthma control with reflux therapy.18–20 A recent Cochrane-review concluded there was moderate-certainty evidence for improved forced expiratory volume in one-second (FEV1, mean difference 0.1 L 95% CI 0.05 to 0.15, 1333 participants, 7 studies) and decreased use of rescue medications (−0.71 puffs per day, 95% CI −1.20 to −0.22; 239 participants, 2 studies) following GERD therapy.19 However, the authors identified no studies focused on their targeted primary outcomes including hospital admissions, emergency room visits, or unscheduled doctor visits. Therefore, the impact of PPI treatment on asthma exacerbation risk and hospital utilization remains unclear.
Similarly, a recent meta-analysis reported no discernable benefit with PPI treatment for GERD on morning peak-expiratory flow across fourteen randomized clinical trials including a total of 2,182 participants.20 A separate study reported limited clinical benefit in patients who have symptomatic GERD and nighttime asthma symptoms,15 and these patients may represent the group most likely to see improvement in asthma symptoms with PPI treatment.
Current asthma guidelines do not recommend empiric treatment of GERD for patients with asthma.21,22 Rather, current recommendations support treatment of reflux guided by patient symptoms.21
One use of PPI relevant to asthma care is for stress ulcer prophylaxis in the setting of systemic corticosteroid use or critical illness. Patients in the ICU can have multiple additive risks for GI bleeding including lack of enteral intake, poor GI perfusion, coagulopathy, and anticoagulant use. An open-label crossover study of 26,828 adult patients requiring mechanical ventilation in the intensive care unit showed no significant difference in the rates of in-hospital mortality, GI bleeding, or C.difficile infection in patients receiving a PPI or H2RB.23 In the setting of critically ill patients, guidelines recommend PPI over histamine-2 receptor blocker (H2RB) and assessment of individual patient bleeding risk.24
Recently, increasing data suggest that the risk for stress ulcers in asthma patients who are receiving corticosteroids may relate to patient-specific risk factors including comorbidities and concurrent medication use. A recent retrospective study of 30,177 pediatric patients admitted to the ICU for critical asthma found that whereas medical prophylaxis to prevent stress ulcers has been increasingly prescribed over time, there were no episodes of GI bleeding noted over a 10-year period.25 Compared with children, adult patients may have multiple risks for GI bleeding including use of nonsteroidal anti-inflammatory drugs and anticoagulants. Studies have indicated that GI prophylaxis with PPI may be underutilized in older adults at sustained risk of GI bleeding owing to therapy with these classes of medications.26–28
Eosinophilic Esophagitis
Uniquely among atopic disorders, PPIs are recommended as a treatment for eosinophilic esophagitis. Importantly, a trial of PPI therapy is no longer included as a necessary step for diagnosis in most recent EoE guidelines.29,30 EoE can be confirmed in a patient with symptoms of esophageal dysfunction who has at least 15 eosinophils per high powered field (~60 eosinophils per mm2) on esophageal biopsy and no other identifiable causes of esophageal eosinophilia on medical evaluation. When used for treatment of EoE, the current twice-daily dosing recommendations are not explicitly addressed by the US Food and Drug Association (FDA) in PPI packaging inserts (Table 1).
Table 1.
|
Multiple studies have evaluated the efficacy of PPIs in EoE, however there have been no randomized controlled studies comparing the efficacy of PPI monotherapy to other EoE therapies like swallowed corticosteroids or dietary exclusion. In prior meta-analyses, the overall percentage of adults who achieved histologic remission on PPI therapy was approximately 50%.31–33 The American Gastroenterological Association and the Joint Task Force on Allergy-Immunology Practice Parameters recently published a joint guideline on EoE treatment reviewing 23 clinical studies of PPI treatment in EoE patients using Grading of Recommendations, Assessment, Development, and Evaluations methodology.34 That review found an unweighted histologic response of 42% to PPI, with a high level of observed intertrial variability owing to inconsistent patient selection criteria, dosing, and therapy duration across PPI trials.34 Additional concerns the contributed to an assessment of very low quality metric of evidence in the Grading of Recommendations, Assessment, Development, and Evaluations study were that a significant portion of PPI trials were retrospective, single-arm studies without comparison to other types of EoE therapy. The risk ratio for PPI treatment compared to placebo (RR 0.66, 95% CI, 0.61–0.72) was consistent with the likely benefit of PPI for a subset of patients. Proton pump inhibitors are relatively inexpensive, have a long-standing safety profile, and are they are easily administered. Proton pump inhibitor treatment should be discussed with patients along with risks and benefits of other forms of EoE therapy when choosing initial therapy or reevaluating therapy options owing to persistent disease activity.29,34 There are no validated biomarkers to predict the subgroup of EoE patients that will maximally benefit from PPI or other EoE therapy, therefore a shared decision making approach regarding the choice of EoE therapy options with the individual patient is recommended. An additional consideration is that GERD and EoE can co-exist, and some EoE patients derive additional symptomatic relief with use of PPI while on another form of EoE therapy.
Current consensus guidelines recognize EoE as an indication for long-term PPI treatment.8 When PPI monotherapy is used for EoE, empiric trials of PPI deprescribing are not indicated and PPI should be continued unless there is shared decision making to switch to another form of EoE therapy. There have been few studies of the long-term efficacy of PPI use in EoE patients. A recent retrospective analysis of 138 adult EoE patients observed for a mean follow up time of 3.6 ± 2.9 years demonstrated that 60% of patients with long-term follow up maintained histologic remission on high-dose PPI therapy.35 Patient demographics, symptoms, atopic status, endoscopic findings and PPI doses were similar between the groups but there was a trend for pre-PPI dilation being more common in patients who did not respond to PPI therapy (60% vs 33%, P=0.06). A separate retrospective analysis of a cohort of 75 adult EoE patients indicated a higher long-term response rate of 87% after 1–2 years’ time.36 Recurrence of esophageal eosinophilia while on PPI were more likely to occur in those with rhinoconjunctivitis as compared to those without it (40% vs. 13%, respectively, P = 0.007) and with a cytochrome P450 family 2 subfamily C member 19 (CYP2C19) rapid metabolizer genotype as compared to those without (36% vs. 6%, respectively, P = 0.01).36 This CYP2C19 rapid metabolizer genotype the has been associated with lower serum PPI levels and in a prospective clinical trial of high-dose PPI therapy in 92 pediatric patients. Binary logistic modeling in a study of 92 pediatric patients demonstrated that the CYP2C19 rapid metabolizer genotype was associated with a PPI-nonresponsive EoE phenotype (odds ratio (OR) [95% confidence interval (CI)] = 7.71 [1.21, 49.11], P = 0.031).37 These findings raise the question of if patient genotype could predict the therapeutic efficacy of high-dose PPI in EoE. The association of CYP2C19 polymorphisms with PPI response in EoE patients requires further prospective validation to determine if this strategy could predict patients’ response to PPI therapy across a diverse population.
Potential Adverse Effects
The short-term effects of PPIs have been extensively studied in randomized controlled studies for GERD, and PPIs have proven quite safe for short-term use. Side effects are reversible with discontinuation of therapy and include headache (<5%) and diarrhea (<5%).6,19,38 With the increasing prevalence of long-term PPI use, there has been growing scrutiny of potential long-term adverse events with these medications. Most studies were performed as case-control or cohort studies with risk for bias and potential effects from residual confounding,7 and have had low effect sizes which call into question their clinical significance. This evidence was rated low to very low quality by expert panels.5
Data from multiple prospective studies has indicated little independent risk of bone mineral density loss or fracture from isolated PPI use.39–42 Additional prospective data on the long-term safety of PPI has come from a randomized controlled trial of 17,598 participants with stable cardiovascular disease randomized to either pantoprazole 40 mg daily or placebo and one of four anticoagulant regimens over three years.43 The data from this study were examined for a variety of long-term side effects seen in prior population-based studies. No significant differences were seen in pneumonia, cardiovascular disease, fractures, gastric atrophy, chronic kidney disease, diabetes, chronic obstructive lung disease, dementia, cancer, hospitalizations, and all-cause mortality. Current guidelines do not recommend routine screening labs for renal function, bone health, or nutritional status in patients receiving long-term PPI.5
Of the outcomes of interest, the only finding was a significant increase in enteric infections in pantoprazole compared to placebo groups (1.4% versus 1.0% in the placebo group; odds ratio, 1.33, 95%CI: 1.01 to 1.75, P=0.04). These included Salmonella, E.coli, Shigella, and Campylobacter infections, and were observed at a lower rate than had been reported in prior review of observational studies.44 There was a nonsignificant increase in Clostridium difficile infection in thirteen individuals in the pantoprazole treated groups.43 Gastric acid is bactericidal and plays an important role in host defense against enteric pathogens. Acid suppression has been confirmed to increase susceptibility to enteric pathogens in murine models, which confirms this suspected mechanism.45,46
Idiosyncratic adverse events:
There have been case reports of idiosyncratic adverse events with PPI use. By definition, these events are unpredictable and nonspecific. For PPI, these events are rare and include acute interstitial nephritis47,48 and subacute cutaneous lupus.49,50
Adverse events associated with polypharmacy
Many patients taking PPI are receiving other medications This is thought to be a contributing factor in certain types of adverse events. There have been reports of rhabdomyolysis in patients concurrently using PPI and β-hydroxy-β-methylglutaryl-CoA reductase inhibitors (statins).51,52 However, these events are rare and rhabdomyolysis is a known complication with statin therapy. Further study is needed to understand whether PPI increases the risk for rhabdomyolysis with statin therapy. Hypomagnesemia has been reported with long-term PPI use. In the absence of other medications, PPI use is associated with low urinary magnesium, which implies that there is reduced uptake of magnesium from the GI tract.53–56 Loop and thiazide diuretics promote urinary magnesium excretion, and the combination of PPI with these medications can increase the risk of hypomagnesemia. Prolonged QT interval and torsades de pointes have been associated with severe hypomagnesemia attributed to PPI use.57,58 Torsades de pointes has also been reported in patients taking both PPI and drugs that prolong the QT-interval.59–61 While reports of these reactions are rare, thorough medication review and discontinuing unnecessary medications can reduce the potential risk for harm.
Drug allergy
Awareness of potential drug hypersensitivity reactions are of particular importance to the practicing allergist. Although PPIs are generally well-tolerated, there have been cases of both immediate- and cell-mediated hypersensitivity to oral and IV forms of PPI, including omeprazole, esomeprazole, rabeprazole, lansoprazole, and pantoprazole. There have been rare reports of severe cutaneous reactions to PPI.62,63 A recent review of case reports and small case series describing hypersensitivity reactions to PPI found that most were immediate reactions (309 out of 443, 69%), with anaphylaxis described in 53.6% of patients and angioedema or urticaria in 44.1% of patients.64 PPIs triggered 0.8% of anaphylaxis cases in the Uppsala Monitoring Centre database of suspected adverse drug reactions.65 In a study of 2119 patients in the French pharmacovigilance database, PPI elicited and urticaria or angioedema in 6 patients and anaphylaxis in 14 patients.66
The validity of skin prick testing for evaluation of immediate hypersensitivity to PPIs has been evaluated and varying non-irritating concentrations for skin prick and intradermal testing have been reported.67–71 Recommendations for nonirritating testing concentrations have been reviewed in detail elsewhere, including current recommendations to maximize sensitivity and specificity.72 Intradermal testing should only be performed using injectable intravenous preparations of PPIs, as crushed tablets and oral solutions can be irritating.72 Recent prospective study of skin testing for PPI in 65 patients with history suggestive of immediate hypersensitivity to PPI and 30 controls was performed using standardized skin prick and intradermal concentrations for omeprazole (SPT: 20mg/mL, 4mg/mL, 0.4 mg/mL; IDT: 0.004 mg/ml, 0.04 mg/ml, 0.4 mg/ml), lansoprazole (SPT: 30 mg/ml), pantoprazole (SPT: 40 mg/ml, 4 mg/ml, 0.4 mg/ml; IDT: 0.004 mg/ml, 0.04 mg/ml, 0.4 mg/ml), rabeprazole (SPT: 20 mg/ml), and esomeprazole (SPT: 20 mg/ml, 8 mg/ml, 0.8 mg/ml; IDT: 0.008 mg/ml, 0.08 mg/ml, 0.8 mg/ml).68 Oral provocation tests with the suspected culprit PPI (n=12) and other PPIs (n=61) were performed. Calculated sensitivity was 58.8%, specificity 100%, negative predictive value 70.8 %, and positive predictive value 100 %.68 The observed sensitivity and negative predictive value was slightly lower than what had been observed with a prior study of 53 patients in which 41 patients completed challenge.67 Those authors performed SPT using the undiluted commercial preparation (40 mg/mL) for omeprazole, esomeprazole, and pantoprazole, and rabeprazole (40 mg/mL) and lansoprazole (30 mg/mL) were prepared by dissolving the powder in saline. IDT were performed with the injectable preparations of omeprazole, esomeprazole, and pantoprazole at 0.4 and 4 mg/mL. In this study, only 1 of 9 patients with a prior grade III hypersensitivity reaction consented to oral challenge, therefore patients participating in oral challenge had more mild reactions. The sensitivity of skin testing was 61.3%, specificity was 100%, negative predictive value was 91.9%, and positive predictive values was 100%.67
Cross reactivity patterns between PPIs have been described, and likely relate to the structures of the drugs.72 Patients with hypersensitivity to pantoprazole have been shown to have positive SPT or reactions to omeprazole or esopmeprazole.67,70,73
Of note, enteric coatings on delayed-release formulations of PPI can contain gelatin.74 Full ingredient lists and other prescribing information of FDA-approved medications can be checked at dailymed.nlm.nih.gov. There have been reports of anaphylaxis induced by gelatin in enteric coatings in gelatin-sensitized individuals,75 and it may be necessary to perform skin testing or challenge to both gelatin and the drug to differentiate the cause of anaphylaxis.65
Risk of allergic disease following PPI use
The development of atopic disease is complex and is thought to result from a combination of genetic predisposition and prenatal and early life environmental exposures.76 Exposure to acid suppressant medications, both prenatally and in childhood, have been linked to the development of multiple atopic diseases including asthma, food allergy and eosinophilic esophagitis (Table 2).77
Table 2.
Proton pump exposure and risk for allergic disease
| Author, Year | Study Type | Exposure | Allergic Outcome and Finding |
|---|---|---|---|
| Devine, et al., 201780 | Meta-analysis | Prenatal PPI | Asthma: HR 1.30 (95%CI:1.07–1.56) |
| Lai et al., 201879 | Meta-analysis | Prenatal PPI | Asthma: RR 1.34 (95%CI 1.18–1.52) |
| Mitre et al., 201881 | Cohort study | PPI during infancy (age <6 months) | Asthma aHR 1.41 (95%CI: 1.31–1.52) Food Allergy aHR 2.59 (95%CI: 2.25–3.00) Allergic Rhinitis aHR 1.44 (95%CI: 1.36–1.52) |
| Wang et al., 202182 | Cohort study | PPI during childhood (age < 18 years) | Asthma: HR 1.57 (95%CI: 1.49–1.64) |
| DeMuth, et al., 201383 | Cross-sectional | PPI and other ASM during childhood | Food Allergy aPR 1.70 (95%CI 1.10–2.50) |
| Jensen et al., 201884 | Case Control | PPI exposure during infancy | EoE aOR 6.05 (95%CI: 2.55–14.0) |
ASM: acid suppressant medications, CI: confidence interval; EOE: eosinophilic esophagitis, aHR, adjusted hazard ratio, HR: hazard ratio, aOR: adjusted odds ratio, PPI: proton pump inhibitor, aPR: adjusted prevalence ratio
The most well-studied association described to date is between exposure to ASMs and PPI and subsequent development of asthma. In 2009, the first association between prenatal exposure to PPIs and H2RAs increased risk of asthma in childhood was described.78 Subsequently, two meta-analyses confirmed these initial findings and reported that prenatal PPIs were associated with an overall increased risk of asthma in childhood (RR = 1.34; 95% CI 1.18–1.52; I2 = 46% and HR 1.30; 95% CI, 1.07–1.56; I2 = 45.2%).79,80
A large cohort study found children prescribed PPIs during infancy (first 6 months of life) had an increased risk for multiple atopic conditions including asthma (aHR for PPI 1.41,95%CI:1.31–1.52), food allergy (aHR for PPI 2.59,95%CI: 2.25–3.00) and allergic rhinitis (aHR for PPI 1.44,95%CI: 1.36–1.52.).81 More recently the relationship between childhood exposure to PPI and development of asthma was confirmed among a large cohort of children in Sweden.82 This study found that PPI exposure during childhood was associated with increased risk of incident asthma (HR of 1.57 [95% CI, 1.49–1.64]). The highest risk of asthma was among those exposed as infants and toddlers (HR of 1.83 [95% CI, 1.65–2.03]).
While these studies included large numbers of patients, the effect sizes seen were relatively small. Further, the studies were retrospective and may fail to account for all potential biases due to confounding. One particular concern for exposure during childhood is protopathic bias; where symptoms of cough may be attributed to reflux and treated with PPI but in time manifest more completely as asthma.
Less is known about the association between PPI exposure and the specific risk of IgE-mediated food allergy, however several studies have found an increased risk of food allergy. A small cross-sectional study found an increased risk of food allergy following childhood exposure to PPI and all other ASM (adjusted prevalence ratio 1.70, 95%CI 1.10–2.50).83 Another smaller case control study found a large increased risk of subsequent development in eosinophilic esophagitis (EoE) in those exposed to ASM during infancy (aOR 6.05 95%CI: 2.55–14.0).84
Several mechanisms have been suggested as the potential causal link between PPI use and development of atopy. These possible mechanisms include allergic sensitization, type 2 (T2) cytokine skewing and alterations in the microbiome. Mouse models suggest that ASM exposure increases formation of IgE to dietary antigens, type 2 cytokines, and clinical food allergy.85 In a subsequent study of adults treated with 3 months of either PPI or H2RA for dyspepsia or chronic gastritis, food allergen sensitization was examined before and after ASM use.86 Of the patients with pre-existing food specific IgE, 10% were noted to have a rise in serum food-specific IgE levels. However, 15% of the subjects developed de novo allergen sensitization, suggesting that ASM use could contribute to food-allergen sensitization in patients who may be at risk of food allergy.
Currently, the most compelling potential mechanism is the alteration of the microbiome leading to dysbiosis (Figure 1). It is increasingly recognized that the gut and airway microbiome play a key role in the pathobiology of allergic disease and dysbiosis increases risk of allergic disorders87,88 PPIs alter the gut and possibly even the airway microbiome leading to dysbiosis including a decrease in microbial diversity, lower abundance of gut commensal, and an increase in abundance of oral and upper gastrointestinal commensals.89,90 These deleterious alterations may have lasting impacts when exposure occurs during the critical window for immune development (prenatal and early life).
Figure 1:
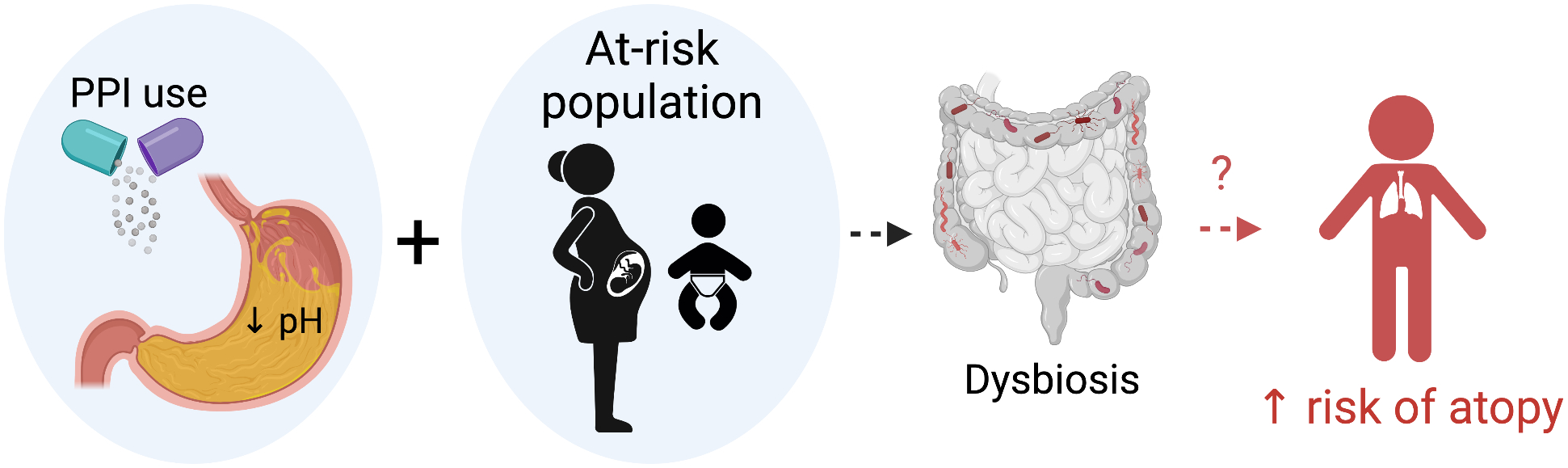
Schematic of hypothesis of mechanistic link between early life PPI exposure and risk of subsequent atopy. In susceptible individuals, prenatal or early life PPI exposure reduces gastric acid. While this mechanism is not fully understood, microbial dysbiosis and later atopic disease may occur in a subset of patients. Figure created with biorender.com.
There is a growing body of evidence suggesting that there is a true association between exposure to ASMs and the risk for developing allergic disease, but we must consider the possibility that the results are affected by unmeasured confounding or by cofounding by indication. For example, failure to thrive or vomiting in young infants may be attributed to reflux and treated with PPI, but ultimately are early signs of other underlying conditions such as EoE or milk protein intolerance. Further replication of results in multiple populations and using detailed prospective data will help to answer this important clinical question. For now, it is wise to carefully consider the risks and benefits of acid suppressant medication therapy during pregnancy, infancy and childhood and to restrict use of these medications to indications for which they are clinically indicated.
Conclusions:
Although PPI have a demonstrated role for the treatment of both short- and long-term conditions, they are frequently over-prescribed and are available without a prescription in many countries. Within the context of routine allergy care, PPIs are used to treat patients with comorbid GERD and in management of EoE.31,34 EoE is considered an indication for continuous long-term PPI therapy.8
PPI have been extensively studied in short-term randomized controlled studies and have a well-described and acceptable short term safety profile. Care should be exercised when beginning PPI therapy in individuals on multiple medications due to the risk for adverse drug-drug interactions. The potential long-term adverse effects of PPIs have been scrutinized, with many initial associations identified. However, the current body of evidence does not entirely support a strong association between PPIs use and many potential long term adverse effects. Prospective studies of PPI have demonstrated an increased risk for enteric infection, including C.difficile infections.43. Data regarding long-term adverse effects is particularly lacking among pediatric and young-adult populations, which has relevance to long-term use in EoE.
Epidemiologic data suggests that PPI exposure during pregnancy and childhood is associated with risk of childhood allergic disease including asthma, food allergy and EoE. The most compelling potential causal mechanism is alteration of the microbiome. However, the possibility of uncontrolled cofounding remains, and additional prospective studies would be beneficial to clarify the magnitude of this effect.
Concurrent with national guidelines, allergists should use PPIs only when clinically indicated and therefore most likely to have clinical benefit.5 Risks and benefits can be discussed with patients. Choosing the lowest effective dose for the shortest required duration of therapy is an effective option to minimize the risk of harm. For patients on chronic PPI, a trial of PPI de-escalation use should be considered in patients if there is not an indication for continuous long-term PPI therapy.8
Conflict of Interest:
Dr. Robinson is employed by both Lahey Clinic and Sanofi. Dr. Ruffner receives research funding related to EoE from NIH K08 AI148456, AAAAI Foundation Faculty Development Award, and The Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR). CEGIR is part of the Rare Diseases Clinical Research Network (RDCRN), and is funded under grant number U54AI117804 as a collaboration between NCATS, the National Institute of Allergy and Infectious Diseases (NIAID), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Funding:
No funding was received for this work.
Abbreviations:
- aHR
adjusted hazard ratio
- ASM
acid suppressant medications
- aOR
adjusted odds ratio
- CI
confidence interval
- CYP2C19
Cytochrome P450 family 2 subfamily C member 19
- EoE
eosinophilic esophagitis
- FEV1
Forced expiratory volume in the first second
- FDA
Food & Drug Association
- GERD
gastroesophageal reflux disease
- GRADE
Grading of Recommendations, Assessment, Development and Evaluations
- H2RA
histamine-2 receptor antagonist
- HR
hazard ratio
- HMG-CoA
β-Hydroxy β-methylglutaryl-CoA
- IgE
immunoglobulin E
- PPI
proton pump inhibitor
- T2
type 2
- RR
relative risk
References
- 1.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in Prescription Drug Use Among Adults in the United States From 1999–2012. JAMA. 2015;314:1818–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aihara T, Nakamura E, Amagase K, Tomita K, Fujishita T, Furutani K, et al. Pharmacological control of gastric acid secretion for the treatment of acid-related peptic disease: past, present, and future. Pharmacol Ther. 2003;98:109–27. [DOI] [PubMed] [Google Scholar]
- 3.Quinonez RA, Garber MD, Schroeder AR, Alverson BK, Nickel W, Goldstein J, et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8:479–85. [DOI] [PubMed] [Google Scholar]
- 4.Body C, Christie JA. Gastrointestinal Diseases in Pregnancy: Nausea, Vomiting, Hyperemesis Gravidarum, Gastroesophageal Reflux Disease, Constipation, and Diarrhea. Gastroenterol Clin North Am. 2016;45:267–83. [DOI] [PubMed] [Google Scholar]
- 5.Freedberg DE, Kim LS, Yang YX. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology. 2017;152:706–15. [DOI] [PubMed] [Google Scholar]
- 6.Katzka DA, Kahrilas PJ. Advances in the diagnosis and management of gastroesophageal reflux disease. BMJ. 2020;371:m3786. [DOI] [PubMed] [Google Scholar]
- 7.Vaezi MF, Yang YX, Howden CW. Complications of Proton Pump Inhibitor Therapy. Gastroenterology. 2017;153:35–48. [DOI] [PubMed] [Google Scholar]
- 8.Targownik LE, Fisher DA, Saini SD. AGA Clinical Practice Update on De-Prescribing of Proton Pump Inhibitors: Expert Review. Gastroenterology. 2022;162:1334–42. [DOI] [PubMed] [Google Scholar]
- 9.Arai N, Nakamizo T, Ihara H, Koide T, Nakamura A, Tabuse M, et al. Histamine H2-Blocker and Proton Pump Inhibitor Use and the Risk of Pneumonia in Acute Stroke: A Retrospective Analysis on Susceptible Patients. PLoS ONE. 2017;12:e0169300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irwin RS. Chronic Cough Due to Gastroesophageal Reflux Disease: ACCP Evidence-Based Clinical Practice Guidelines. Chest. 2006;129:80S–94S. [DOI] [PubMed] [Google Scholar]
- 11.Gibson P, Wang G, McGarvey L, Vertigan AE, Altman KW, Birring SS, et al. Treatment of Unexplained Chronic Cough: CHEST Guideline and Expert Panel Report. Chest. 2016;149:27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irwin RS, Curley FJ, French CL. Difficult-to-control asthma. Contributing factors and outcome of a systematic management protocol. Chest. 1993;103:1662–9. [DOI] [PubMed] [Google Scholar]
- 13.Meier JH, McNally PR, Punja M, Freeman SR, Sudduth RH, Stocker N, et al. Does omeprazole (Prilosec) improve respiratory function in asthmatics with gastroesophageal reflux? A double-blind, placebo-controlled crossover study. Dig Dis Sci. 1994;39:2127–33. [DOI] [PubMed] [Google Scholar]
- 14.Harding SM, Richter JE, Guzzo MR, Schan CA, Alexander RW, Bradley LA. Asthma and gastroesophageal reflux: acid suppressive therapy improves asthma outcome. Am J Med. 1996;100:395–405. [DOI] [PubMed] [Google Scholar]
- 15.Kiljander TO, Harding SM, Field SK, Stein MR, Nelson HS, Ekelund J, et al. Effects of esomeprazole 40 mg twice daily on asthma: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2006;173:1091–7. [DOI] [PubMed] [Google Scholar]
- 16.Kiljander TO. The role of proton pump inhibitors in the management of gastroesophageal reflux disease-related asthma and chronic cough. Am J Med. 2003;115 Suppl 3A:65S–71S. [DOI] [PubMed] [Google Scholar]
- 17.Kiljander TO, Salomaa ER, Hietanen EK, Terho EO. Gastroesophageal reflux in asthmatics: A double-blind, placebo-controlled crossover study with omeprazole. Chest. 1999;116:1257–64. [DOI] [PubMed] [Google Scholar]
- 18.American Lung Association Asthma Clinical Research Centers, Mastronarde JG, Anthonisen NR, Castro M, Holbrook JT, Leone FT, et al. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopsaftis Z, Yap HS, Tin KS, Hnin K, Carson-Chahhoud KV. Pharmacological and surgical interventions for the treatment of gastro-oesophageal reflux in adults and children with asthma. Cochrane Database Syst Rev. 2021;5:CD001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng Z, Luo Y, Li J, Gao J. Randomised trials of proton pump inhibitors for gastro-oesophageal reflux disease in patients with asthma: an updated systematic review and meta-analysis. BMJ Open. 2021;11:e043860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, et al. Global Initiative for Asthma Strategy 2021: executive summary and rationale for key changes. Eur Respir J. 2022;59:2102730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146:1217–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The PEPTIC Investigators for the Australian and New Zealand Intensive Care Society Clinical Trials Group AHSCCSCN and the Irish Critical Care Trials Group. Effect of Stress Ulcer Prophylaxis With Proton Pump Inhibitors vs Histamine-2 Receptor Blockers on In-Hospital Mortality Among ICU Patients Receiving Invasive Mechanical Ventilation: The PEPTIC Randomized Clinical Trial. JAMA. 2020;323:616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye Z, Blaser AR, Lytvyn L, Wang Y, Guyatt GH, Mikita JS, et al. Gastrointestinal bleeding prophylaxis for critically ill patients: a clinical practice guideline. BMJ. 2020;368:l6722. [DOI] [PubMed] [Google Scholar]
- 25.Roberts AR, Roddy M, Wilsey MJ, McKinley SD, Sanchez-Teppa B, Sochet AA. Stress Ulcer Prophylaxis for Critical Asthma. Pediatrics. 2022;149:e2021054527. [DOI] [PubMed] [Google Scholar]
- 26.Kurlander JE, Gu X, Scheiman JM, Haymart B, Kline-Rogers E, Saini SD, et al. Missed opportunities to prevent upper GI hemorrhage: The experience of the Michigan Anticoagulation Quality Improvement Initiative. Vasc Med Lond Engl. 2019;24:153–5. [DOI] [PubMed] [Google Scholar]
- 27.Kurlander JE, Rubenstein JH, Richardson CR, Krein SL, De Vries R, Zikmund-Fisher BJ, et al. Physicians’ Perceptions of Proton Pump Inhibitor Risks and Recommendations to Discontinue: A National Survey. Am J Gastroenterol. 2020;115:689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ray WA, Chung CP, Murray KT, Smalley WE, Daugherty JR, Dupont WD, et al. Association of Proton Pump Inhibitors With Reduced Risk of Warfarin-Related Serious Upper Gastrointestinal Bleeding. Gastroenterology. 2016;151:1105–1112.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dellon ES, Liacouras CA, Molina-Infante J, Furuta GT, Spergel JM, Zevit N, et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology. 2018;155:1022–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucendo AJ, Molina-Infante J, Arias Á, von Arnim U, Bredenoord AJ, Bussmann C, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur Gastroenterol J. 2017;5:335–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucendo AJ, Arias Á, Molina-Infante J. Efficacy of Proton Pump Inhibitor Drugs for Inducing Clinical and Histologic Remission in Patients With Symptomatic Esophageal Eosinophilia: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2016;14:13–22.e1. [DOI] [PubMed] [Google Scholar]
- 32.Rank MA, Sharaf RN, Furuta GT, Aceves SS, Greenhawt M, Spergel JM, et al. Technical Review on the Management of Eosinophilic Esophagitis: A Report From the AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters. Gastroenterology. 2020;158:1789–1810.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franciosi JP, Mougey EB, Dellon ES, Gutierrez-Junquera C, Fernandez-Fernandez S, Venkatesh RD, et al. Proton Pump Inhibitor Therapy for Eosinophilic Esophagitis: History, Mechanisms, Efficacy, and Future Directions. J Asthma Allergy. 2022;15:281–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirano I, Chan ES, Rank MA, Sharaf RN, Stollman NH, Stukus DR, et al. AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters Clinical Guidelines for the Management of Eosinophilic Esophagitis. Gastroenterology. 2020;158:1776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thakkar KP, Fowler M, Keene S, Iuga A, Dellon ES. Long-term efficacy of proton pump inhibitors as a treatment modality for eosinophilic esophagitis. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2022;S1590–8658(22)00209–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molina-Infante J, Rodriguez-Sanchez J, Martinek J, van Rhijn BD, Krajciova J, Rivas MD, et al. Long-Term Loss of Response in Proton Pump Inhibitor-Responsive Esophageal Eosinophilia Is Uncommon and Influenced by CYP2C19 Genotype and Rhinoconjunctivitis. Am J Gastroenterol. 2015;110:1567–75. [DOI] [PubMed] [Google Scholar]
- 37.Mougey EB, Williams A, Coyne AJK, Gutiérrez-Junquera C, Fernández-Fernández S, Cilleruelo ML, et al. CYP2C19 and STAT6 Variants Influence the Outcome of Proton Pump Inhibitor Therapy in Pediatric Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr. 2019;1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sigterman KE, van Pinxteren B, Bonis PA, Lau J, Numans ME. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. 2013;CD002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gray SL, LaCroix AZ, Larson J, Robbins J, Cauley JA, Manson JE, et al. Proton Pump Inhibitor Use, Hip Fracture and Change in Bone Density In Postmenopausal Women Results from the Women’s Health Initiative. Arch Intern Med. 2010;170:765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Targownik LE, Leslie WD, Davison KS, Goltzman D, Jamal SA, Kreiger N, et al. The Relationship Between Proton Pump Inhibitor Use and Longitudinal Change in Bone Mineral Density: A Population-Based From the Canadian Multicentre Osteoporosis Study (CaMos). Am J Gastroenterol. 2012;107:1361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Targownik LE, Lix LM, Leung S, Leslie WD. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology. 2010;138:896–904. [DOI] [PubMed] [Google Scholar]
- 42.Research C for DE and. FDA Drug Safety Communication: Possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors [Internet]. FDA. FDA; 2019. [cited 2022 Aug 9]. Available from: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-possible-increased-risk-fractures-hip-wrist-and-spine-use-proton-pump [Google Scholar]
- 43.Moayyedi P, Eikelboom JW, Bosch J, Connolly SJ, Dyal L, Shestakovska O, et al. Safety of Proton Pump Inhibitors Based on a Large, Multi-Year, Randomized Trial of Patients Receiving Rivaroxaban or Aspirin. Gastroenterology. 2019;157:682–691.e2. [DOI] [PubMed] [Google Scholar]
- 44.Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol. 2007;102:2047–56. [DOI] [PubMed] [Google Scholar]
- 45.Tennant SM, Hartland EL, Phumoonna T, Lyras D, Rood JI, Robins-Browne RM, et al. Influence of gastric acid on susceptibility to infection with ingested bacterial pathogens. Infect Immun. 2008;76:639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yasutomi E, Hoshi N, Adachi S, Otsuka T, Kong L, Ku Y, et al. Proton Pump Inhibitors Increase the Susceptibility of Mice to Oral Infection with Enteropathogenic Bacteria. Dig Dis Sci. 2018;63:881–9. [DOI] [PubMed] [Google Scholar]
- 47.Muriithi AK, Leung N, Valeri AM, Cornell LD, Sethi S, Fidler ME, et al. Biopsy-proven acute interstitial nephritis, 1993–2011: a case series. Am J Kidney Dis Off J Natl Kidney Found. 2014;64:558–66. [DOI] [PubMed] [Google Scholar]
- 48.Al-Aly Z, Maddukuri G, Xie Y. Proton Pump Inhibitors and the Kidney: Implications of Current Evidence for Clinical Practice and When and How to Deprescribe. Am J Kidney Dis. 2020;75:497–507. [DOI] [PubMed] [Google Scholar]
- 49.Kaur S, Singla P, Kaur S, Kansal A, Bansal A, Singh A. Proton Pump Inhibitor Induced Subacute Cutaneous Lupus Erythematosus: A Case Series of 7 Patients and Brief Review of Literature. Indian Dermatol Online J. 2022;13:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poh YJ, Alrashid A, Sangle SR, Higgins E, Benton E, McGibbon D, et al. Proton pump inhibitor induced subacute cutaneous lupus erythematosus: Clinical characteristics and outcomes. Lupus. 2022;9612033221104236. [DOI] [PubMed] [Google Scholar]
- 51.Duncan SJ, Howden CW. Proton Pump Inhibitors and Risk of Rhabdomyolysis. Drug Saf. 2017;40:61–4. [DOI] [PubMed] [Google Scholar]
- 52.Capogrosso Sansone A, Convertino I, Galiulo MT, Salvadori S, Pieroni S, Knezevic T, et al. Muscular Adverse Drug Reactions Associated with Proton Pump Inhibitors: A Disproportionality Analysis Using the Italian National Network of Pharmacovigilance Database. Drug Saf. 2017;40:895–909. [DOI] [PubMed] [Google Scholar]
- 53.Broeren MAC, Geerdink EAM, Vader HL, van den Wall Bake AWL. Hypomagnesemia Induced by Several Proton-Pump Inhibitors. Ann Intern Med. 2009;151:755–6. [DOI] [PubMed] [Google Scholar]
- 54.Hoorn EJ, van der Hoek J, de Man RA, Kuipers EJ, Bolwerk C, Zietse R. A Case Series of Proton Pump Inhibitor–Induced Hypomagnesemia. Am J Kidney Dis. 2010;56:112–6. [DOI] [PubMed] [Google Scholar]
- 55.Koulouridis I, Alfayez M, Tighiouart H, Madias NE, Kent DM, Paulus JK, et al. Out-of-Hospital Use of Proton Pump Inhibitors and Hypomagnesemia at Hospital Admission: A Nested Case-Control Study. Am J Kidney Dis. 2013;62:730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tamura T, Sakaeda T, Kadoyama K, Okuno Y. Omeprazole- and esomeprazole-associated hypomagnesaemia: data mining of the public version of the FDA Adverse Event Reporting System. Int J Med Sci. 2012;9:322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lazzerini PE, Bertolozzi I, Finizola F, Acampa M, Natale M, Vanni F, et al. Proton Pump Inhibitors and Serum Magnesium Levels in Patients With Torsades de Pointes. Front Pharmacol. 2018;9:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chrysant SG. Proton pump inhibitor-induced hypomagnesemia complicated with serious cardiac arrhythmias. Expert Rev Cardiovasc Ther. 2019;17:345–51. [DOI] [PubMed] [Google Scholar]
- 59.Rossi M, Marzi F, Natale M, Porceddu A, Tuccori M, Lazzerini PE, et al. Drug-Associated QTc Prolongation in Geriatric Hospitalized Patients: A Cross-Sectional Study in Internal Medicine. Drugs - Real World Outcomes. 2021;8:325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Asajima H, Saito N, Ohmura Y, Ohmura K. Lansoprazole precipitated QT prolongation and torsade de pointes associated with disopyramide. Eur J Clin Pharmacol. 2012;68:331–3. [DOI] [PubMed] [Google Scholar]
- 61.Bibawy JN, Parikh V, Wahba J, Barsoum EA, Lafferty J, Kowalski M, et al. Pantoprazole (proton pump inhibitor) contributing to Torsades de Pointes storm. Circ Arrhythm Electrophysiol. 2013;6:e17–19. [DOI] [PubMed] [Google Scholar]
- 62.Salloum A, Nasr D, Maalouf D. Dermatologic adverse reactions to proton-pump inhibitors: A synthetized review. J Cosmet Dermatol. 2021;20:1073–9. [DOI] [PubMed] [Google Scholar]
- 63.Lin CY, Wang CW, Hui CYR, Chang YC, Yang CH, Cheng CY, et al. Delayed-type hypersensitivity reactions induced by proton pump inhibitors: A clinical and in vitro T-cell reactivity study. Allergy. 2018;73:221–9. [DOI] [PubMed] [Google Scholar]
- 64.Kepil Özdemir S, Bavbek S. Hypersensitivity reactions to proton-pump inhibitors: Clinical presentation, diagnosis, and management. Allergy Asthma Proc. 2020;41:e37–44. [DOI] [PubMed] [Google Scholar]
- 65.Natsch S, Vinks MH, Voogt AK, Mees EB, Meyboom RH. Anaphylactic reactions to proton-pump inhibitors. Ann Pharmacother. 2000;34:474–6. [DOI] [PubMed] [Google Scholar]
- 66.Tourillon C, Mahe J, Baron A, Lambert A, Yélehé-Okouma M, Veyrac G, et al. Immediate-Type Hypersensitivity Cross-Reactions to Proton Pump Inhibitors: A Descriptive Study of Data from the French National Pharmacovigilance Database. Int Arch Allergy Immunol. 2019;178:159–66. [DOI] [PubMed] [Google Scholar]
- 67.Bonadonna P, Lombardo C, Bortolami O, Bircher A, Scherer K, Barbaud A, et al. Hypersensitivity to proton pump inhibitors: Diagnostic accuracy of skin tests compared to oral provocation test. J Allergy Clin Immunol. 2012;130:547–9. [DOI] [PubMed] [Google Scholar]
- 68.Kepil Özdemir S, Yılmaz I, Aydin Ö, Büyüköztürk S, Gelincik A, Demirtürk M, et al. Immediate-type hypersensitivity reactions to proton pump inhibitors: usefulness of skin tests in the diagnosis and assessment of cross-reactivity. Allergy. 2013;68:1008–14. [DOI] [PubMed] [Google Scholar]
- 69.Sobrevia Elfau MT, Garcés Sotillos M, Ferrer Clavería L, Segura Arazuri N, Monzón Ballarin S, Colás Sanz C. Study of cross-reactivity between proton pump inhibitors. J Investig Allergol Clin Immunol. 2010;20:157–61. [PubMed] [Google Scholar]
- 70.Garrido Fernández S, Cumplido JA, Rábano A, Martínez D, Blanco C, Carrillo T. Allergy to proton pump inhibitors: diagnosis and assessment of cross-reactivity. J Investig Allergol Clin Immunol. 2008;18:140–1. [PubMed] [Google Scholar]
- 71.Porcel S, Rodríguez A, Jiménez S, Alvarado M, Hernández J. Allergy to lansoprazole: study of cross-reactivity among proton-pump inhibitors. Allergy. 2005;60:1087–8. [DOI] [PubMed] [Google Scholar]
- 72.Otani IM, Banerji A. Immediate and Delayed Hypersensitivity Reactions to Proton Pump Inhibitors: Evaluation and Management. Curr Allergy Asthma Rep. 2016;16:17. [DOI] [PubMed] [Google Scholar]
- 73.Pérez Pimiento AJ, Prieto Lastra L, Rodríguez Cabreros MI, González Sánchez LA, Mosquera MR, Cubero AG. Hypersensitivity to lansoprazole and rabeprazole with tolerance to other proton pump inhibitors. J Allergy Clin Immunol. 2006;117:707–8. [DOI] [PubMed] [Google Scholar]
- 74.Reker D, Blum SM, Steiger C, Anger KE, Sommer JM, Fanikos J, et al. ‘Inactive’ ingredients in oral medications. Sci Transl Med. 2019;11:eaau6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Land MH, Piehl MD, Burks AW. Near fatal anaphylaxis from orally administered gelatin capsule. J Allergy Clin Immunol Pract. 2013;1:99–100. [DOI] [PubMed] [Google Scholar]
- 76.Reynolds LA, Finlay BB. Early life factors that affect allergy development. Nat Rev Immunol. 2017;17:518–28. [DOI] [PubMed] [Google Scholar]
- 77.Robinson LB, Camargo CA. Acid suppressant medications and the risk of allergic diseases. Expert Rev Clin Immunol. 2018;14:771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dehlink E, Yen E, Leichtner AM, Hait EJ, Fiebiger E. First evidence of a possible association between gastric acid suppression during pregnancy and childhood asthma: a population-based register study. Clin Exp Allergy J Br Soc Allergy Clin Immunol. 2009;39:246–53. [DOI] [PubMed] [Google Scholar]
- 79.Lai T, Wu M, Liu J, Luo M, He L, Wang X, et al. Acid-Suppressive Drug Use During Pregnancy and the Risk of Childhood Asthma: A Meta-analysis. Pediatrics. 2018;141:e20170889. [DOI] [PubMed] [Google Scholar]
- 80.Devine RE, McCleary N, Sheikh A, Nwaru BI. Acid-suppressive medications during pregnancy and risk of asthma and allergy in children: A systematic review and meta-analysis. J Allergy Clin Immunol. 2017;139:1985–1988.e12. [DOI] [PubMed] [Google Scholar]
- 81.Mitre E, Susi A, Kropp LE, Schwartz DJ, Gorman GH, Nylund CM. Association Between Use of Acid-Suppressive Medications and Antibiotics During Infancy and Allergic Diseases in Early Childhood. JAMA Pediatr. 2018;172:e180315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang YH, Wintzell V, Ludvigsson JF, Svanström H, Pasternak B. Association Between Proton Pump Inhibitor Use and Risk of Asthma in Children. JAMA Pediatr. 2021;175:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DeMuth K, Stecenko A, Sullivan K, Fitzpatrick A. Relationship between treatment with antacid medication and the prevalence of food allergy in children. Allergy Asthma Proc. 2013;34:227–32. [DOI] [PubMed] [Google Scholar]
- 84.Jensen ET, Kuhl JT, Martin LJ, Rothenberg ME, Dellon ES. Prenatal, intrapartum, and postnatal factors are associated with pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2018;141:214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Untersmayr E, Schöll I, Swoboda I, Beil WJ, Förster-Waldl E, Walter F, et al. Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in BALB/c mice. J Allergy Clin Immunol. 2003;112:616–23. [DOI] [PubMed] [Google Scholar]
- 86.Untersmayr E, Bakos N, Schöll I, Kundi M, Roth-Walter F, Szalai K, et al. Anti-ulcer drugs promote IgE formation toward dietary antigens in adult patients. FASEB J Off Publ Fed Am Soc Exp Biol. 2005;19:656–8. [DOI] [PubMed] [Google Scholar]
- 87.Koidl L, Untersmayr E. The clinical implications of the microbiome in the development of allergy diseases. Expert Rev Clin Immunol. 2021;17:115–26. [DOI] [PubMed] [Google Scholar]
- 88.Huang YJ, Marsland BJ, Bunyavanich S, O’Mahony L, Leung DYM, Muraro A, et al. The microbiome in allergic disease: Current understanding and future opportunities-2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J Allergy Clin Immunol. 2017;139:1099–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65:749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rosen R, Hu L, Amirault J, Khatwa U, Ward DV, Onderdonk A. 16S community profiling identifies proton pump inhibitor related differences in gastric, lung, and oropharyngeal microflora. J Pediatr. 2015;166:917–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Proton Pump Inhibitors: U.S. Food and Drug Administration-Approved Indications and Dosages for Use in Adults [Internet]. [cited 2022 Aug 17]. Available from: https://www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/Medicaid-Integrity-Education/Pharmacy-Education-Materials/Downloads/ppi-adult-dosingchart11-14.pdf
- 92.Proton Pump Inhibitors: Use in Adults [Internet]. Proton Pump Inhibitors: Use in Adults. [cited 2022 Aug 17]. Available from: https://www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/Medicaid-Integrity-Education/Pharmacy-Education-Materials/Downloads/ppi-adult-factsheet11-14.pdf


