Abstract
Background
In 2018, the Centers for Medicaid and Medicare Services (CMS) issued a protocol for the treatment of sepsis. This bundle protocol, titled SEP-1 is a multicomponent 3 h and 6 h resuscitation treatment for patients with the diagnosis of either severe sepsis or septic shock. The SEP-1 bundle includes antibiotic administration, fluid bolus, blood cultures, lactate measurement, vasopressors for fluid-refractory hypotension, and a reevaluation of volume status. We performed a retrospective analysis of patients diagnosed with either severe sepsis or septic shock comparing mortality outcomes based on compliance with the updated SEP-1 bundle at a rural community hospital.
Methods
Mortality outcome and readmission data were extracted from an electronic medical records database from January 1, 2019, to June 30, 2020. International Classification of Diseases (ICD)-10 codes were used to identify patients with either severe sepsis or septic shock. Once identified, patients were separated into four populations: patients with severe sepsis who met SEP-1, patients with severe sepsis who failed SEP-1, patients with septic shock who met SEP-1, and patients with septic shock who failed SEP-1. A patient who met bundle criteria (SEP-1 criteria) received each component of the bundle in the time allotted. Using chi-squared test of homogeneity, mortality outcomes for population proportions were investigated. Two sample proportion summary hypothesis test and 95% confidence intervals (CI) determined significance in mortality outcomes.
Results
Out of our 1122 patient population, 437 patients qualified to be measured by CMS criteria. Of the 437 patients, 195 met the treatment bundle and 242 failed the treatment bundle. Upon comparing the two groups, we found the probable difference in mortality rate between the met(14.87%) and failed bundle(27.69%) groups to be significant(95% CI: 5.28–20.34, P=0.0013). However, the driving force of this result lies in the subgroup of patients with severe sepsis with septic shock, which show a higher mortality rate compared to the subgroup with just severe sepsis. The difference was within the range of 3.31% to 29.71%.
Conclusion
This study shows that with septic shock obtained a benefit, decreased mortality, when the SEP-1 bundle was met. However, meeting the SEP-1 bundle had no benefit for patients who had the diagnosis of severe sepsis alone. The significant difference in mortality, found between the met and failed bundle groups, is primarily due to the number of patients with septic shock, and whether or not those patients with septic shock met or failed the bundle.
Keywords: SEP-1, Sepsis, Early goal-directed therapy, Centers for Medicaid and Medicare Services, Quality improvement, Reimbursement
Introduction
Sepsis treatment continues to be an ever-evolving topic. With controversial recommendations that occurred in 2004 [1] to updated terminology in 2016,[2] sepsis continues to be a difficult pathology to treat. It continues to have focused attention because of the complex physiology,[3] high rate of mortality, and high healthcare burden.[4]
Rivers et al,[5] in 2001, published a study on early goal-directed therapy (EGDT) and the management of sepsis. While this study is a landmark paper and was considered to be the momentum needed to decrease sepsis mortality, very few studies have since been able to achieve the same results.[6], [7], [8] Despite this, Rivers’ paper is still considered a cornerstone for recommendations and guidelines. In 2002, the Surviving Sepsis Campaign was initiated to increase awareness, improve outcomes, and develop recommendations that physicians could use while treating sepsis.[9] While these recommendations never intended to replace the bedside clinician's decision-making capabilities regarding a “patient's unique set of clinical variables”,[1] they appear to have become a backbone for the Centers for Medicaid and Medicare Services (CMS's) SEP-1 guideline.
SEP-1 is a severe sepsis and septic shock treatment guideline developed by CMS with a goal of promoting quality and cost-effective care nationally.[10] This guideline was established by CMS as a quality measure with compliance being tied to hospital reimbursement. This CMS quality measure requires hospitals to report their compliance with a multicomponent 3 h and 6 h treatment and resuscitation bundle for patients with severe sepsis or septic shock. This bundle includes antibiotic administration, fluid bolus, blood culture, lactate measurement, the use of vasopressors for fluid-refractory hypotension, and reevaluation of volume status.[11] The first SEP-1 performance measure was initiated in 2015 with data showing variability in results.[12], [13], [14], [15] The difficulty in studying sepsis may be in part due to the ever-changing guidelines and definitions. Since 1991 when sepsis began receiving worldwide attention, there have been four Surviving Sepsis Campaign (SSC) recommendations[1], [16], [17], [18] and three International Consensus Definitions (Sepsis-1[19], Sepsis-2,[20] and Sepsis 3[2]). To add to the confusion, CMS is still using the Sepsis-2 definition that includes the diagnoses sepsis, severe sepsis, and septic shock. Despite treatment complexity, the treatment of sepsis has been improving since 1991, and new treatments and molecular markers are continually being considered for clinical management.[21]
In 2018, CMS unveiled its newest version of sepsis guidelines, still titled SEP-1, and is the same “bundle” type of treatment. While very similar to the 2015 version of SEP-1, it contains updates provided in Table 1. These guidelines are the standard for clinical performance measurement and tool for clinicians’ guided behaviors in the management of patients with sepsis and septic shock. Researchers wanted to instigate mortality rates in patients by looking at both SEP-1 guidelines and International Classification of Diseases (ICD)-10 code patient diagnosis. ICD-10 code definitions for the diagnosis of sepsis and septic shock may not be recognized in consensus but are still used as the documented diagnosis on patient charts. To our knowledge, no studies have investigated outcomes related to the implementation of the most recent version of SEP-1 guidelines. Furthermore, previous studies have failed to break down the mortality rates within the met vs. failed bundle group by looking at patient subgroups with either severe sepsis or septic shock, despite reporting differences in mortality.[22,23] The purpose of this study is to elucidate those subgroups as variables and determine whether they are associated with the efficacy of the SEP-1 bundle and in improving patient outcomes. Specifically, we wanted to compare the outcomes among the four population sub-groups: met severe sepsis, failed severe sepsis, met septic shock, and failed septic shock.
Table 1.
SEP-1 guidelines for sepsis treatment.
| Diagnosis | Within 3 h | Within 6 h |
| Severe Sepsis (ICD-10 R65.2) | Lactate Blood culture before antibiotics Antibiotics administration 30 mL/kg IVF (if hypotensive) |
Repeat lactate Perform a volume status or perfusion exam |
| Septic Shock (ICD-10 R65.21) | Lactate Blood culture before antibiotics Antibiotics administration 30 mL/kg IVF |
Repeat lactate Vasopressors (if systolic blood pressure <90 after 30 mL/kg IVF) Perform a volume status or perfusion exam |
ICD: International Classification of Diseases; IVF: Intravenous fluids; SEP-1: The severe sepsis and septic shock management bundle.
Methods
Data source
Clinical data were obtained from a rural community teaching hospital with 339 beds that resides in Southwest Missouri. Outcome and readmission data were extracted from the electronic medical records database from January 1, 2019, to June 30, 2020. The initial data set consisted of 1122 patients whose medical records contained at least one of the ICD-10 codes listed in Table 2. The patient population served by this hospital is primarily Caucasian and contains the highest rate of uninsured patients in Missouri.[24]
Table 2.
ICD-10 codes of the initial patient population.
| Sepsis ICD-10 codes | Diagnosis |
| A400 A401 A403 A408 A409 A4101 A4102 A411 A412 A413 A414 A4150 A4151 A4152 A4153 A4159 A4181 A4189 A419 R6520 R6521 |
Sepsis due to Streptococcus, group A Sepsis due to Streptococcus, group B Sepsis due to Streptococcus pneumoniae Other Streptococcal sepsis Streptococcal sepsis, unspecified Sepsis due to methicillin-susceptible Staphylococcus aureus Sepsis due to methicillin-resistant Staphylococcus aureus Sepsis due to other specified Staphylococcus Sepsis due to unspecified Staphylococcus Sepsis due to Haemophilus influenzae Sepsis due to anaerobes Gram-negative sepsis, unspecified Sepsis due to Escherichia coli (E. coli) Sepsis due to Pseudomonas Sepsis due to Serratia Other Gram-negative sepsis Sepsis due to Enterococcus Other specified sepsis Sepsis, unspecified organism Severe sepsis without septic shock Severe sepsis with septic shock |
ICD: International Classification of Diseases.
Study design
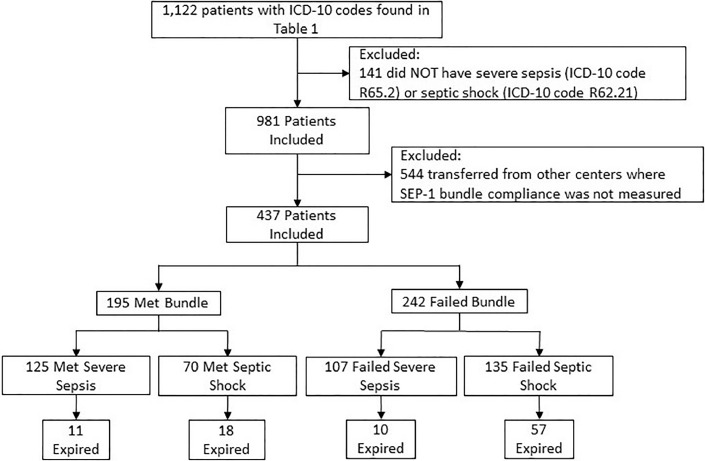
The study was approved by the Institutional Review Board of Freeman Health System under the protocol title: The Surviving Sepsis Campaign and Its Effect on Patient Populations with Sepsis and Pre-existing Comorbidities. Due to its retrospective nature, informed consent was not required. This was a retrospective study analyzing the outcomes of community or hospital-onset of severe sepsis (ICD-10 code R65.2) or septic shock (ICD-10 code R62.21) following the new implementation of 2018 SEP-1. These two ICD-10 diagnoses codes were chosen since SEP-1 does not contain a protocol for the treatment of sepsis but is targeted toward severe sepsis and septic shock. These inclusion criteria reduced our population of 1122 to 981. In addition, 544 patients were excluded from the study because they were transferred from other acute care centers and therefore SEP-1 bundle compliance was not measured, per CMS guidelines. The total number of patients included in this study is 437 [Figure 1].
Figure 1.
Sepsis treatment subgroup categorization with the number of patients per group. ICD: International Classification of Diseases; SEP-1: The severe sepsis and septic shock management bundle.
All patients 18 years or older, who were not transferred from another acute care facility, qualified to be measured by CMS SEP-1 2018 version were included in the study. All patients had to have an ICD-10 code of R65.2 or R65.21, which are diagnoses of severe sepsis and septic shock, respectively.
Patients were categorized based on the physician's compliance with SEP-1 during the patient's treatment in the hospital. The SEP-1 protocol is considered an “all-or-none” CMS measure; therefore, if a physician completed the SEP-1 protocol, the patient was classified as met. If a physician did not complete one or more of the components in the time allotted, the patient was classified as failed.
Patients with ICD R65.2 (severe sepsis) failed SEP-1 if the physician did not complete one or more of the tasks noted in Table 1 within the allotted time.
Patients with ICD R65.21 failed SEP-1 (septic shock) if the physician did not complete one or more of the tasks noted in Table 1 within the referenced time.
Statistical analysis
We first determined the population proportions using chi-squared test of homogeneity. These population proportions were calculated for the outcome variable mortality. For outcomes, it was assumed the samples were representative of their respective populations. Furthermore, the following rules were applied: 10% condition in which the samples are clearly <10% of the population and a dependent variable (lived, expired) had to have 10 or more patients. These outcomes were further analyzed using the statistical test “95% confidence intervals” (CIs). Non-overlapping intervals were indicators of where differences occurred, and these differences were further investigated with two sample proportion summary hypothesis tests. The primary outcome was in-hospital mortality. Confounding variables included age, sex, and medical specialty that diagnosed sepsis.
Results
Differences in study population
Data on 437 patients with a diagnosis of either severe sepsis or septic shock was collected. These patients were then separated based on the completion of the SEP-1 treatment bundle: met or failed. A total of 195 patients completed or met the bundle, while 242 patients had a documented failed bundle, as shown in Figure 1. The patients in these two groups were further broken down by severity of illness. In the failed group, 107 patients (44.2%) had severe sepsis and 135 patients (55.8%) had septic shock. In the met group, 125 patients (64.1%) had severe sepsis compared to 70 patients (35.9%) with septic shock. This shows a minor discrepancy in the severity of illness between the two data groups of met vs. failed; the average age was similar between groups.
Differences in mortality outcomes
We found a significant difference while comparing the mortality rate between patients who met the SEP-1 and failed the SEP-1 bundle (P=0.0013). This difference is better understood while dividing met and failed populations into the four subgroups, as shown in Figure 1. Adherence to the SEP-1 bundle showed no benefit in survival for patients diagnosed with severe sepsis (P=0.8852). In fact, the averages and 95% CI for the mortality rate of severe sepsis patients in the met and failed populations are very similar: met severe sepsis average mortality of 8.80% (95% CI: 3.83–13.77%) and failed severe sepsis average of 9.35% (95% CI: 3.83–14.86%). However, adherence to the SEP-1 bundle does improve mortality in patients diagnosed with septic shock. Using a 95% CI, the proportionate difference found was 3.31–29.71% (P = 0.0200). Furthermore, the met septic shock subgroup's average mortality rate was 25.71% (95% CI: 15.47–35.95%) and the failed septic shock subgroup's average mortality rate was 42.22% (95% CI: 33.89–50.55%). Of note, the subgroup with the lowest average mortality rate, 8.80%, was the severe sepsis patients who met the SEP-1 bundle. The subgroups with the highest average mortality rate, 42.22%, were found among the septic shock patients who failed to meet the SEP-1 bundle [Table 3].
Table 3.
Population subgroups and their respective proportion of mortality.
| Populations | n | Deaths | Mortality (%) | 95% CI for mortality (%) | Mortality difference (%) | 95% CI for mortality difference (%) | P-value |
| Includes both severe sepsis and septic shock | 12.81 | 5.28–20.34 | 0.0013 | ||||
| Failed bundle | 242 | 67 | 27.69 | 22.05–33.32 | |||
| Met SEP-1 bundle | 195 | 29 | 14.87 | 9.88–19.87 | |||
| Includes only severe sepsis | 0.54 | NA | 0.8852 | ||||
| Failed bundle (sepsis only) | 107 | 10 | 9.35 | 3.83–14.86 | |||
| Met SEP-1 bundle (sepsis only) | 125 | 11 | 8.80 | 3.83–13.76 | |||
| Includes only septic shock | 16.51 | 3.31–29.71 | 0.0200 | ||||
| Failed bundle (shock only) | 135 | 57 | 42.22 | 33.89–50.55 | |||
| Met SEP-1 bundle (shock only) | 70 | 18 | 25.71 | 15.47–35.95 | |||
CI: Confidence interval; ICD: International Classification of Diseases; NA: Not applicable; SEP-1: The severe sepsis and septic shock management bundle.
Discussion
The data suggest when physicians follow the SEP-1 guidelines there are lower rates of mortality and therefore, a greater probability of survival in patients with severe sepsis and septic shock in whom the SEP-1 bundle was met. However, when the met and failed bundles are broken into subgroups based on their respective diagnoses of severe sepsis and septic shock, the significance of adherence to the SEP-1 guidelines becomes clear. For patients with severe sepsis, adherence to the SEP-1 bundle showed no statistical difference in outcome when compared to patients with severe sepsis who did not meet, or failed, the SEP-1 bundle. In fact, the benefit of adherence to the SEP-1 bundle to the group as a whole (patients with severe sepsis and patients with septic shock) appears to be driven by the improvement in mortality seen in the septic shock patients who met, or received, the SEP-1 bundle. Because no other studies separated the subgroups and compared the diagnoses of severe sepsis and septic shock, it is difficult to relate these findings to other study results. For this patient population, these results show that adherence to the SEP-1 bundle improves mortality only in patients with a diagnosis of septic shock. Adherence to the SEP-1 bundle or lack thereof, has little to no effect on mortality in patients with a diagnosis of severe sepsis alone.
Furthermore, the SEP-1 bundle has no exclusion criteria based on physician recommendations, unlike ARISE, PROCESS, and PROMISE trials. These trials allowed the physician to deem aggressive fluid resuscitation unsuitable which contrasts with the current guidelines. SEP-1 is an “all-or-none” quality measure that requires all components of the bundle to be administered within a certain timeframe regardless of the physician's clinical opinion. This could possibly explain why the fluid bolus is the most common reason for failure in our study. As with the three clinical trials, a physician is unlikely to administer aggressive intravenous fluid treatment if it has the potential to cause more harm, specifically in patient populations that are at risk of hypervolemia.
The complexity of sepsis and the variability in studies suggest the need for further evolution in the management of sepsis. Consulting with an infectious disease boarded physician when a patient has been identified as having sepsis, in combination with the 3-h bundle treatment given within 12 h, showed decreased mortality rates.[25] This infectious disease consult approach could be implemented into future guidelines.
In addition, this study also reveals insight into the effect of SEP-1 implementation on the various subclassifications of sepsis (severe sepsis and septic shock). With a higher rate of mortality shown in septic shock, this study highlights the useful role that lactate may serve while studying severe sepsis vs. septic shock. While this marker proves to be controversial due to its reliability to detect hypoperfusion,[26] specifically in patients with one or more comorbidities, it is the only lab marker that can differentiate and diagnose septic shock per CMS guidelines. With an earlier detection of septic shock, the SEP-1 protocol could be implemented and provide better outcomes for patients.
Limitations
The primary limitation of our study was the sample size. While the total patient population was similar in size to previous studies,[12], [13], [14], [15],[27], [28], [29], [30] those studies did not separate the patient population into four subgroups. In doing so, the power of this study was decreased. In addition, the use of ICD-10 codes authors acknowledge should be used for diagnosis and not for monitoring the progression of critical illnesses, such as that needed for septic patients. Records obtained for our study were from a single visit, not a patient's entire electronic health record (EHR); they did not allow us to define the personal history of a patient or a baseline of severity. Due to COVID-19 protocols, access to patient chart review at Freeman Health System was limited. Thus, we were unable to investigate our sample group's Sequential organ failure assessment(SOFA) or Acute physiology and chronic health evaluation (APACHE) scores; both of which could have varied widely within our sample groups. Furthermore, the samples were not randomly selected from the population. Consequently, it is unclear whether the samples are representative of their respective populations as a whole. Another limitation of the study was patient diversity. Due to location, the patient population was primarily Caucasian, therefore, possibly limiting the understanding of SEP-1′s treatment effect on patients of different ethnicities. Lastly, a limitation that is congruent with other observation studies is the possibility of residual confounding variables within this severely ill population. The sample group selected for contained only severely ill individuals diagnosed with sepsis in the hospital, not just patients who were admitted through the Emergency Department. While age and numbers were similar across the four groups, confounding variables such as secondary comorbidities, the initial presentation of sepsis, or the reason for failed bundle compliance could not be considered. Thus, the focus of this study was on mortality, a categorical variable. A quantitative analysis such as multivariable regression could not be performed due to an insufficient sample size. To address this, future studies should consider using multicenter analysis, larger hospitals, or combined health care systems that would allow a greater number of patients to be analyzed. In addition, access onsite to such facilities would allow additional data points to be collected via patient chart review.
Conclusions
Our study suggests that patients with a diagnosis of septic shock benefit from adherence to the SEP-1 guidelines while adherence to the SEP-1 guidelines offers little or no benefit to patients with a diagnosis of severe sepsis alone. A one-size-fits-all approach to sepsis management may not be beneficial to certain patient subsets within the diagnosis of sepsis. Based on the results of this study, further research assessing the impact of adherence to the SEP-1 guidelines on various subpopulations of septic patients is needed.
Funding
This research did not receive grant funding from any agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
All authors have declared no financial support was received from any organization for the submitted work. All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgments
A special thank you to Nova Beyersdorfer, for her support and assistance on this project.
Managing Editor: Jingling Bao
References
- 1.Dellinger R.P., Carlet J.M., Masur H., Gerlach H., Calandra T., Cohen J., et al. Surviving sepsis campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 2.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotchkiss R.S., Karl I.E. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 4.Angus D.C., Linde-Zwirble W.T., Lidicker J., Clermont G., Carcillo J., Pinsky M.R. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Rivers E., Nguyen B., Havstad S., Ressler J., Muzzin A., Knoblich B., et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 6.Yealy D.M., Kellum J.A., Huang D.T., Barnato A.E., Weissfeld L.A., et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. ProCESS Investigators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ARISE Investigators, ANZICS Clinical Trials Group. Peake S.L., Delaney A., Bailey M., Bellomo R., et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 8.Mouncey P.R., Osborn T.M., Power G.S., Harrison D.A., Sadique M.Z., Grieve R.D., et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 9.SCCM History. 2022. Society of critical care medicine (SCCM). Available from: https://www.sccm.org/SurvivingSepsisCampaign/About-SSC/History/BarcelonaDeclaration [Last accessed on 2022 March 18].
- 10.Wang J., Strich J.R., Applefeld W.N., Sun J., Cui X., Natanson C., et al. Driving blind: instituting SEP-1 without high quality outcomes data. J Thorac Dis. 2020;12 doi: 10.21037/jtd.2019.12.100. Suppl 1):S22–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.QualityNet Home (cms.gov), 2022. Available from: https://qualitynet.cms.gov/inpatient/specifications-manuals/sepsis-resources [Last accessed on 2022 March 18].
- 12.Ramsdell T.H., Smith A.N., Kerkhove E. Compliance with updated sepsis bundles to meet new sepsis core measure in a tertiary care hospital. Hosp Pharm. 2017;52:177–186. doi: 10.1310/hpj5203-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esposito A., Silverman M.E., Diaz F., Fiesseler F., Magnes G., Salo D. Sepsis core measures – are they worth the cost. J Emerg Med. 2018;55:751–757. doi: 10.1016/j.jemermed.2018.07.033. [DOI] [PubMed] [Google Scholar]
- 14.Rhee C., Filbin M.R., Massaro A.F., Bulger A.L., McEachern D., Tobin K.A., et al. Compliance with the national SEP-1 quality measure and association with sepsis outcomes: a multicenter retrospective cohort study. Crit Care Med. 2018;46(10):1585–1591. doi: 10.1097/CCM.0000000000003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitfield P.L., Ratliff P.D., Lockhart L.L., Andrews D., Komyathy K.L., Sloan M.A., et al. Implementation of an adult code sepsis protocol and its impact on SEP-1 core measure perfect score attainment in the ED. Am J Emerg Med. 2020;38:879–882. doi: 10.1016/j.ajem.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Rhodes A., Evans L.E., Alhazzani W., Levy M.M., Antonelli M., Ferrer R., et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 17.Dellinger R.P., Levy M.M., Rhodes A., Annane D., Gerlach H., Opal S.M., et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 18.Dellinger R.P., Levy M.M., Carlet J.M., Bion J., Parker M.M., Jaeschke R., et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 19.Bone R.C., Balk R.A., Cerra F.B., Dellinger R.P., Fein A.M., Knaus W.A., et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee. American college of chest physicians/society of critical care medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 20.Levy M.M., Fink M.P., Marshall J.C., Abraham E., Angus D., Cook D., et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Intensive Care Med. 2003;29:530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 21.Blangy-Letheule A., Persello A., Rozec B., Waard M., Lauzier B. New approaches to identify sepsis biomarkers: the importance of model and sample source for mass spectrometry. Oxid Med Cell Longev. 2020;2020 doi: 10.1155/2020/6681073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shankar-Hari M., Phillips G.S., Levy M.L., Seymour C.W., Liu V.X., Deutschman C.S., et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:775–787. doi: 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaukonen K.M., Bailey M., Suzuki S., Pilcher D., Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311:1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 24.Missouri Health Assessment, Missouri Health Assessment Health & Senior Services (mo.gov), Source: missouri department of health and senior services and centers for disease control and prevention behavioral risk factor surveillance system (BRFSS), 2022. Available from: https://www.mo.gov/. [Last accessed on 2022 March 18].
- 25.Madaline T., Wadskier Montagne F., Eisenberg R., Mowrey W., Kaur J., Malik M., et al. Early infectious disease consultation is associated with lower mortality in patients with severe sepsis or septic shock who complete the 3-hour sepsis treatment bundle. Open Forum Infect Dis. 2019;6:ofz408. doi: 10.1093/ofid/ofz408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marik P.E. SEP-1: the lactate myth and other fairytales. Crit Care Med. 2018;46:1689–1690. doi: 10.1097/CCM.0000000000003313. [DOI] [PubMed] [Google Scholar]
- 27.Venkatesh A.K., Slesinger T., Whittle J., Osborn T., Aaronson E., Rothenberg C., et al. Preliminary performance on the new CMS sepsis-1 national quality measure: early insights from the emergency quality network (E-QUAL) Ann Emerg Med. 2018;71 doi: 10.1016/j.annemergmed.2017.06.032. 10.e–5.e. [DOI] [PubMed] [Google Scholar]
- 28.Liao J., Aaronson E., Kim J., Liu X., Snydeman C., Goldfarb I., et al. Association of hospital characteristics with early SEP-1 performance. Am J Med Qual. 2020;35:110–116. doi: 10.1177/1062860619857028. [DOI] [PubMed] [Google Scholar]
- 29.Greenwood-Ericksen M.B., Rothenberg C., Mohr N., Andrea S.D., Slesinger T., Osborn T., et al. Urban and rural emergency department performance on national quality metrics for sepsis care in the United States. J Rural Health. 2019;35:490–497. doi: 10.1111/jrh.12339. [DOI] [PubMed] [Google Scholar]
- 30.Barbash I.J., Kahn J.M. Sepsis quality in safety-net hospitals: an analysis of Medicare's SEP-1 performance measure. J Crit Care. 2019;54:88–93. doi: 10.1016/j.jcrc.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]