Abstract
Gram-negative sepsis is a severe clinical syndrome associated with significant morbidity and mortality. Lipopolysaccharide (LPS), expressed on Gram-negative bacteria, is a potent pro-inflammatory toxin that induces inflammation and coagulation via two separate receptor systems. One is Toll-like receptor 4 (TLR4), expressed on cell surfaces and in endosomes, and the other is the cytosolic receptor caspase-11 (caspases-4 and -5 in humans). Extracellular LPS binds to high mobility group box 1 (HMGB1) protein, a cytokine-like molecule. The HMGB1–LPS complex is transported via receptor for advanced glycated end products (RAGE)-endocytosis to the endolysosomal system to reach the cytosolic LPS receptor caspase-11 to induce HMGB1 release, inflammation, and coagulation that may cause multi-organ failure. The insight that LPS needs HMGB1 assistance to generate severe inflammation has led to successful therapeutic results in preclinical Gram-negative sepsis studies targeting HMGB1. However, to date, no clinical studies have been performed based on this strategy. HMGB1 is also actively released by peripheral sensory nerves and this mechanism is fundamental for the initiation and propagation of inflammation during tissue injury. Homeostasis is achieved when other neurons actively restrict the inflammatory response via monitoring by the central nervous system and the vagus nerve through the cholinergic anti-inflammatory pathway. The neuronal control in Gram-negative sepsis needs further studies since a deeper understanding of the interplay between HMGB1 and acetylcholine may have beneficial therapeutic implications. Herein, we review the synergistic overlapping mechanisms of LPS and HMGB1 and discuss future treatment opportunities in Gram-negative sepsis.
Keywords: Sepsis, Lipopolysaccharide (LPS), High mobility group box 1 (HMGB1), Toll-like receptor 4 (TLR4), Receptor for advanced glycated end products (RAGE), Caspase-11
Introduction
Gram-negative sepsis is a life-threatening systemic response to Gram-negative bacterial infections activating dysregulated inflammation that mediates organ dysfunction. The most frequently affected organs in Gram-negative sepsis are respiratory, renal, cardiovascular, gastrointestinal, hepatic, neurological, and coagulation system.[1], [2], [3] Timely administration of antibiotics, fluids, and oxygen has led to improved survival, but there is a great demand to develop additional means to advance the clinical outcome in sepsis.
Endotoxin (lipopolysaccharide [LPS]), abundantly expressed on the cell surface of Gram-negative bacteria, is one of the most powerful pro-inflammatory molecules known in nature and is central in the pathogenesis of Gram-negative sepsis.[4] There are two different LPS-receptor systems that are pivotal in the pathogenesis of severe Gram-negative infections. One LPS-sensing pathway utilizes the Toll-like receptor 4 (TLR4) and myeloid differentiation factor 2 (TlR4/MD-2) receptor system located on the cell surface and in endosomes in many cell types [Figure 1].[5]
Figure 1.

LPS binds to MD-2 and activates the TLR4/MD-2 receptor complex via two separate intracellular signal pathways that mediate HMGB1 release. Pro-inflammatory cytokines are formed when the adaptor molecule TIRAP gets associated with TLR4 and the MyD88 that activates the NF-κB signaling pathway. Interferon-β is produced when TRAM associates with TRIF. HMGB1: High mobility group box 1; LPS: Lipopolysaccharide; MD-2: myeloid differentiation factor 2; MyD88: Myeloid differentiation factor 88; NF-κB: Nuclear factor-kappa B; TIRAP: Toll/interleukin-1 receptor(TIR) domain-containing adapter protein; TLR4: Toll-like receptor 4; TRAM: TRIF-related adaptor molecule; TRIF: TIR-domain-containing adapter-inducing interferon-β.
The second LPS-sensing system is located intracellularly and depends on the cytosolic LPS-receptors caspase-11 in mice and caspases-4 and −5 in humans [Figure 2].[6], [7] The triggering of these caspases results in inflammasome activation, gasdermin D cleavage, pyroptosis, and coagulopathy.[8] This biology occurs predominantly in myeloid and endothelial cells, and in a few additional cell types. Extracellular LPS needs CD14 and LPS-binding protein to successfully interact with MD-2 in the TLR4 complex and requires intracellular support by high mobility group box 1 (HMGB1) protein to reach its cytosolic receptor caspase-11.[9] LPS activation of TLR4 and caspase-11 both generate extracellular HMGB1 release.[9], [12]
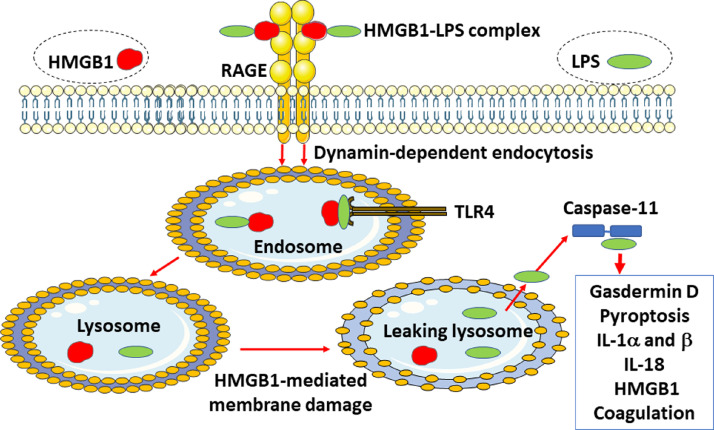
Figure 2.
LPS needs HMGB1 to trigger fulminant inflammation. The initial event in LPS toxicity is due to extracellular LPS activation of cell surface TLR4, which generates the release of many pro-inflammatory molecules including HMGB1. HMGB1 has two LPS-binding sites and thus forms an extracellular HMGB1–LPS complex that gets endocytosed via RAGE to finally reach the cytosol culminating in activation of the intracellular LPS sensor caspase-11 (caspases-4 and −5 in humans) that instigates inflammation and coagulation. HMGB1: High mobility group box 1; IL: interleukin; LPS: Lipopolysaccharide; RAGE: Receptor for advanced glycated end products; TLR4: Toll-like receptor 4.
TLR4−/− mice are not protected when lethal LPS doses are administered concomitantly with agents promoting endogenous type 1 interferon release, while caspase-11−/− mice are resistant to lethal endotoxemic shock.[6], [7] Type 1 interferons most efficiently induce HMGB1 release[13], [14] and the released HMGB1 can bind LPS and forms heterocomplexes that are endocytosed via receptor for advanced glycated end products (RAGE) to finally trigger caspase-11.[9] Taken together, these results suggest that intracellular LPS sensors might be more important for the development of severe Gram-negative sepsis and septic shock than recognition of LPS by TLR4. The intricate interplay between LPS and HMGB1 in the pathogenesis of Gram-negative sepsis offers a broadened understanding of key mechanisms and provides clues to novel therapeutic options that will be discussed in this review.
Extracellular HMGB1 Biology
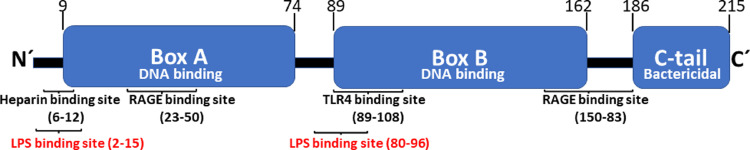
HMGB1 is a nuclear protein present in all animal cell types and codes for 215 amino acid residues arranged in two positively charged DNA-binding domains and a negatively charged C-terminal region [Figure 3].[15] HMGB1 serves vital intranuclear functions needed for transcription, nucleosome formation, DNA repair, and appropriate DNA conformation. Global HMGB1 gene deficiency is lethal. HMGB1 is exceptionally preserved during evolution and is 99% identical in mammals. Two nuclear localization sites (NLSs) secure a dominant nuclear HMGB1 localization during cellular homeostasis.
Figure 3.
HMGB1 structure and location of binding sites for the HMGB1-receptors TLR4 and RAGE, heparin, and LPS. HMGB1: High mobility group box 1; LPS: Lipopolysaccharide; RAGE: Receptor for advanced glycated end products; TLR4: Toll-like receptor 4.
Active extracellular HMGB1 release, induced by LPS and by additional stimuli, requires posttranslational modifications of the nuclear HMGB1 to enable a cytoplasmic translocation necessary for further extracellular release [Figure 4]. These modifications include hyperacetylation of critical lysines in the NLSs preventing the continuous bidirectional shuttle of HMGB1 between the cytoplasm and the nucleus present in all cells and leads to cytoplasmic HMGB1 accumulation.[16] Nuclear hyperacetylation is accomplished via increased histone-acetylase activity (HAT) as well as decreased histone-deacetylase (HDAC) activity.[16], [17], [18] Metformin, resveratrol, and curcumin enhance sirtuin 1 (SIRT1) deacetylase activity and thus decrease extracellular HMGB1 release and HMGB1-dependent inflammation.[19], [20], [26] SIRT1 activity declines during aging and may contribute to hyperinflammation in elderly patients.[27], [28] SIRT1-specific agonists are of considerable interest for future studies in Gram-negative sepsis.[19], [26]
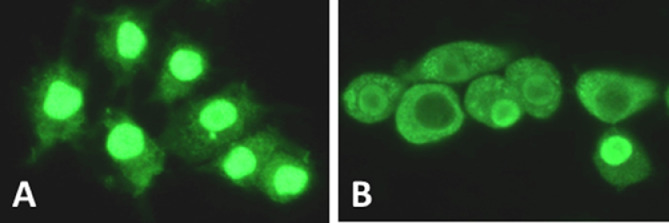
Figure 4.
Intracellular HMGB1 localization in macrophages before (A) and after (B) 24 h-LPS activation. HMGB1, visualized with green immunofluorescent staining is predominately localized in the nucleus in resting human monocytes, while actively translocated from the nucleus to the cytoplasm after LPS activation. HMGB1: High mobility group box 1; LPS: Lipopolysaccharide.
Systemic HMGB1 accumulation during endotoxemia occurs much later than most other inflammatory mediators induced after LPS activation. Serum HMGB1 is minimally detectable by 8 h post LPS exposure and increased to a prolonged plateau level from 16 h to 32 h after LPS administration.[10] Cytoplasmic HMGB1 is actively released extracellularly via several separate mechanisms and the extracellular HMGB1 then acts via a few different receptors, one of which is the RAGE.[29], [30] Myeloid and endothelial cells use RAGE to bind and endocytose extracellular LPS–HMGB1 complexes generated via two LPS-specific binding sites in the HMGB1 molecule [Figures 2 and 3].[31], [32] The LPS–HMGB1 complexes finally enter the lysosomes where HMGB1 in the acidic environment disrupts the lysosomal membrane and allows LPS to leak out in the cytoplasm to bind to caspase-11.[9] This interaction generates gasdermin D cleavage and the truncated gasdermin D then forms oligomerized molecules producing nanopores in the plasma membrane culminating in pyroptotic cell death. This activity in live and dying cells mediates the release of interleukin(IL)−1α, IL-1β, IL-18, and HMGB1.[11], [33], [34] Pyroptosis has been implicated as one major pathway for HMGB1 release during Gram-negative sepsis when caspase-1/caspase-11 double-deficient mice express markedly reduced systemic HMGB1 levels.[9] One additional route for active HMGB1 release from myeloid cells occurs via exocytosis of secretory lysosomes, a pathway also used for IL-1β secretion, although HMGB1 and IL-1β are stored in separate vesicles.[35]
However, the major cellular source for circulating HMGB1 in Gram-negative sepsis is neither the macrophage nor the endothelial cell but the hepatocyte.[12] Deletion of Hmgb1 in hepatocytes significantly improved survival in lethal endotoxemia and prevented the accumulation of HMGB1 in the circulation, while myeloid-specific deletion of Hmgb1 did not reduce the systemic HMGB1 levels and did not improve survival.[9] Thus, hepatocytes and inflammatory cells act on separate receptor pathways to release HMGB1[12] because hepatocytes utilize cell-surface expressed TLR4 to recognize and endocytose extracellular LPS for delivery to caspase-11 followed by cleavage and activation of gasdermin D. These events do not lead to pyroptosis in hepatocytes but result in calcium-dependent phosphorylation of nuclear HMGB1 generating cytoplasmic HMGB1 accumulation and enabling extracellular release that occurs via exosomes. Thus, LPS-dependent HMGB1 release in hepatocytes involves a coordinated interaction between the two canonical LPS sensing receptor systems, such as TLR4 and caspase-11.
Additionally, HMGB1 can be released expressed in microparticles derived from activated platelets.[36], [37] Vascular injury induces the massive extracellular release of HMGB1 from platelets displaying an important role in the pathogenesis of thrombosis formation and neutrophil activation.[38], [40] Furthermore, one recent study intriguingly reports that lactate may promote macrophage HMGB1 acetylation, and exosomal release in polymicrobial sepsis.[41] Macrophages can internalize extracellular lactate, but additional studies are needed to elucidate how lactate stimulates HMGB1 release.
HMGB1 receptor usage and subsequent biological activities depend on the redox state of the three HMGB1 cysteines (Cys23, Cys45, and Cys106). These HMGB1 cysteines reside in a fully reduced state in a quiescent cell[15] However, the redox state of HMGB1 is dynamic and may change depending on the redox balance in the intracellular and extracellular environment, which alters the immune function of the molecule. Necrosis will generate the release of fully reduced HMGB1, which exerts chemotactic activity by forming a heterocomplex with the chemokine CXCL12 (SDF-1) that binds to the CXCL12-reciprocal receptor CXCR4 and initiates chemotaxis in a synergistic way compared with CXCL12 alone.[42], [43] Mild HMGB1 oxidation generates a disulfide bond between Cys23 and Cys45 while keeping Cys106 in the reduced form. This modification converts extracellular HMGB1 to a potent pro-inflammatory cytokine capable of high-affinity binding to MD-2 in the TLR4 receptor complex.[44], [45] HMGB1 and LPS thus both bind MD-2 and signal via TLR4. The fully reduced form of HMGB1, with or without CXCL12, cannot activate the TLR4 signaling pathway and disulfide HMGB1 cannot activate the CXCR4 pathway. Further oxidation of HMGB1 will transform the molecule irreversibly by generating sulfonyl groups on any or all three cysteine residues creating an HMGB1 isoform without pro-inflammatory activities. Recent findings in tumor biology indicate that sulfonyl HMGB1 is an anti-inflammatory molecule signaling via RAGE to recruit immunosuppressive cells including regulatory T cells, M2 macrophages, and myeloid-derived suppressor cells.[46] Sulfonyl HMGB1 also downregulates functions of antigen-presenting cells including dendritic cells and plasmacytoid dendritic cells. It is presently unknown whether sulfonyl HMGB1 contributes to phases of immunosuppression occurring during sepsis and in sepsis survivors. If the reticuloendothelial system during sepsis is incapable of degrading apoptotic cells, then large quantities of sulfonyl HMGB1 may be released via secondary necrosis of apoptotic bodies. One unique feature of HMGB1 is that pathologically increased systemic levels prevail for weeks or even months after the acute phase of sepsis for reasons that are presently unknown. Moreover, the principle cellular sources of HMGB1 have not yet been identified[47], [48] and the redox form of these long-lasting increased plasma HMGB1 levels is also unknown.
Although there are at least 14 receptor systems implicated as HMGB1 receptors, there are only two, TLR4 and RAGE, that play a dominant role in HMGB1-mediated inflammatory mechanisms. The other receptors are likely important for binding complexes of HMGB1 bound to other molecules. As noted above, HMGB1 binds LPS[49] but also other immune-activating molecules such as DNA, RNA, histones, nucleosomes, IL-1α, IL-1β, and additional molecules, which are high-affinity ligands of other HMGB1 receptors[50,[52], [53], [54], [55], [56], [57], [58], [59], [60]]. A common denominator for many of the HMGB1-partner molecules is that they are problematic targets for endogenous antibody formation. The HMGB1 heterocomplexes are endocytosed via RAGE into endosomes, where certain HMGB1 partner molecules will stimulate various TLR receptors. The complexes then get translocated to the lysosomal compartment where the complexes dissociate in the acid environment and HMGB1 acts as a detergent and disrupts the lysosomal membrane allowing transported damage-associated molecular pattern molecules (DAMPs) and pathogen-associated molecular pattern molecules (PAMPs) access to pro-inflammatory cytosolic sensors including inflammasomes, c-GAS, and RIG-I.
HMGB1 in the Development of Sepsis-induced Organ Failures
A substantial number of preclinical studies of Gram-negative sepsis and endotoxemia document how HMGB1 and LPS cause tissue damage responsible for multi-organ failure via different mechanisms (reviewed in Ref. [61]).
Acute lung injury
A common denominator for many features of HMGB1-caused acute pathology is that HMGB1 acts as a mediator of epithelial and endothelial barrier failure. HMGB1 activates the Rho-kinase 1 pathway via RAGE as a mechanism that creates the pulmonary endothelial barrier disruption causing hyperpermeability.[62] RAGE is constitutively highly expressed in the lungs only, while other tissues show little to no RAGE cell surface expression during basic conditions in contrast to after inflammatory stimulation.[29,63,64] Patients with acute respiratory distress syndrome have been reported to express elevated HMGB1 concentrations in the epithelial lining fluid, obtained by bronchoscopic microsampling, and at 1000-fold higher than in the plasma.[65] Sepsis-induced acute lung injury generates disturbed gas exchange, severe inflammation, increased permeability to proteins, and pulmonary dysfunction due to alveoli and pulmonary endothelial cell injury.[66] Intratracheal administration of HMGB1 causes neutrophil accumulation, interstitial edema, and increased production of pro-inflammatory cytokines in the lungs.[67] Anti-HMGB1 antibodies attenuate endotoxin-induced acute respiratory distress syndrome, but not the early release of tumor necrosis factor (TNF) -α and IL-1β, signifying that HMGB1 is a late mediator of endotoxin-induced acute lung injury.[65] HMGB1 also aggravates sepsis-induced respiratory dysfunction by inhibiting the neutrophil ability to clear bacteria due to HMGB1-provoked decreased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity.[68,69] Treatment with anti-HMGB1 antibodies significantly diminished Gram-negative sepsis-induced dysfunction of neutrophil NADPH oxidase activity.[68] HMGB1 plays a critical role in endothelial cell barrier disruption, the hallmark of acute lung injury, by rearranging the actin cytoskeleton into a contractile phenotype.[70] Other injurious sequelae induced by excessive HMGB1 involve extensive pulmonary macrophage pyroptosis causing inflammation and coagulation.[71]
Acute kidney injury
Endotoxemia in Gram-negative sepsis causes acute kidney injury.[72] It has been demonstrated that LPS damages podocytes, which constitute the glomerular filtration barrier.[73,74] Knockdown of HMGB1 attenuates the endotoxin-induced injury in cultured podocytes.[73] Furthermore, tubular epithelial cells express mitochondrial dysfunction after HMGB1 exposure.[73] Another study reports that HMGB1 accumulates in renal tissue during Gram-negative sepsis and enters the urine, and the interaction between HMGB1 and TLR4 turns tubular epithelial cells into inflammatory promoters.[75] Further support for the perception that HMGB1 plays an important role in the development of acute kidney injury is provided by results establishing that SIRT1-mediated HMGB1 deacetylation that subsequently inhibited HMGB1 release also ameliorated sepsis-associated acute kidney injury.[76]
Acute cardiovascular injury
Endotoxemia causes reductions in left ventricle function, cardiac output, and blood pressure, and triggers elevated heart and respiratory rate. LPS-induced pathological HMGB1 levels harm the systemic circulation by causing myocardial dysfunction[77,78] and by disrupting vascular endothelial barriers.[79,80] LPS induces increased myocardial HMGB1 expression and secretion in vivo via TLR4-dependent signaling and the released HMGB1 mediates negative inotropic effects.[77,78] The LPS-induced depression of myocardial contractility in endotoxemic mice can be prevented by the administration of several different HMGB1 antagonists.[78] HMGB1 causes microvascular leakage via impairment of tight junctions, rearrangement of actin filament cytoskeleton, and release of large quantities of cytokines and chemokines, which among other things promote cell surface adhesion molecules like intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) on the vascular cells.[80,81] Treatment with HMGB1-specific antagonists reverses the damage in the endothelial barrier in several experimental Gram-negative sepsis studies.[79,[82], [83],84]
Acute gastrointestinal injury
Endotoxemia causes impaired intestinal barrier function with increased mucosal permeability to hydrophilic macromolecules[85] and increased translocation of viable bacteria that will aggravate a Gram-negative sepsis.[86] The combined LPS/HMGB1-induced mucosal leakage results from decreased expression of zonula occludens-1 necessary for intercellular tight junctions.[87], [88], [89] In vitro studies indicate that RAGE signaling and inducible nitric oxide synthase (iNOS) induction are important pathways in this pathology.[87] Bile HMGB1 contributes to the gut barrier dysfunction in experimental endotoxemia, while HMGB1 removal from the bile of endotoxemic rats partially reverses the gut hyperpermeability.[90] Enterocytes exposed to various endogenous and exogenous pro-inflammatory stimuli actively secrete huge amounts of HMGB1 associated with exosomes.[89] The gastrointestinal dysfunction is mechanistically closely connected to the development of acute lung injury.[88,[91], [92],93]
Acute hepatic injury
The liver is another organ that commonly undergoes functional failure during severe Gram-negative sepsis and septic shock. Sepsis-induced hepatic ischemia can result in acute liver failure with the presentation of abnormal levels of liver enzymes, coagulopathy, and systemic edema due to the disturbance of the synthetic function of the liver. Several studies demonstrated that extracellular histones and HMGB1 are associated with hepatic inflammation and activate the TLR-mediated signaling pathway of Kupffer cells to generate a cytokine storm.[94,95] Several preclinical studies indicate that HMGB1 antagonists may ameliorate LPS-induced acute liver failure.[12,[96], [97],98]
Acute neurological injury
Increased systemic HMGB1 levels generate blood–brain barrier disruption that will allow LPS, HMGB1, and other pro-inflammatory molecules to cause inflammation in the central nervous system. The neuroinflammation which is further aggravated by local HMGB1 release from activated neurons, microglia cells, and astrocytes[99,100] causes multiple central nervous dysfunctions including cognitive decline. This sepsis-associated encephalopathy was recently studied in a mouse model of Gram-negative polymicrobial sepsis.[101] The systemic infection caused increased extracellular amounts in the central nervous system of disulfide HMGB1 that via TLR4–MD-2 receptors enhanced NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome activity, thereby exacerbating neuroinflammation and cognitive impairment. Another study, also performed in a mouse model of Gram-negative polymicrobial sepsis, demonstrated that HMGB1 mediates cognitive impairment in sepsis survivors and that treatment with anti-HMGB1 antibodies may prevent or reverse the cognitive decline.[48]
A similar cognitive decline, including memory dysfunction, is a common complication after surgical trauma particularly in patients over 65 years of age and in aged animals after surgery.[[102], [103],105] Experimental postoperative neuroinflammation and cognitive decline can be prevented by HMGB1-specific antagonists.[104,105] Overall, these data demonstrate a pivotal role for HMGB1 in provoking neuroinflammation during severe aseptic as well infectious systemic inflammation.
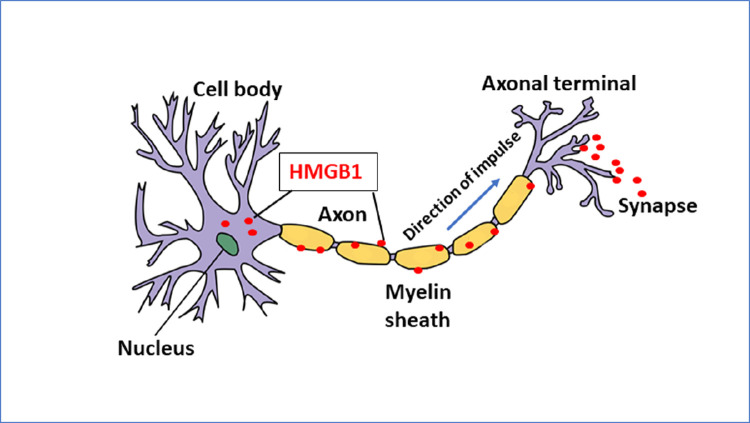
Recent discoveries demonstrate that sensory neurons also actively release HMGB1 and upregulate inflammation in the periphery. Activation of these nociceptive nerves turns on localized inflammation by actively releasing HMGB1 in a retrograde manner within the innervated area.[106,107].
This peripheral neuronal pro-inflammatory system may conceivably form a major threat for exacerbating Gram-negative sepsis. Neuronally-derived HMGB1 is essential for the development of neuroinflammation, since Hmgb1 gene deletion limited to neurons or neutralization via HMGB1-neutralizing antibodies potently ameliorate injury in inflammatory models of experimental polyarthritis and nerve damage.[107] [Figure 5] Thus, nociceptor HMGB1 is an essential mediator of the neuroinflammatory response to different forms of tissue injury but the importance of these predictions has not yet been studied in sepsis. There is now a distinct opportunity to study whether targeting neuronal HMGB1 will benefit patients with Gram-negative sepsis.
Figure 5.
Stimulated sensory neurons actively secrete HMGB1 in an antidromic fashion by molecular mechanisms that remain elusive[107] HMGB1: High mobility group box 1.
Coagulopathy
Coagulopathy is a common phenomenon in Gram-negative sepsis ranging from subclinical activation of blood coagulation with a risk of venous thromboembolism to life-threatening acute disseminated intravascular coagulation (DIC).[108] DIC causes extensive microvascular thrombosis and consumption of platelets and coagulation proteins, eventually provoking bleeding. Coagulation is part of an innate immune system in the effort to trap microbes in the microvasculature but with the risk that DIC may develop when a local bacterial infection escalates to sepsis. The initial step in this coagulation process is that LPS gets HMGB1-assistance to be imported to the cytosol to reach caspase-11, which then enzymatically cleaves gasdermin D into peptides that form nanopores in the cytoplasmic membrane.[9,109,110] In the context of coagulation these pores in macrophages enable calcium influx rather than pyroptosis. The calcium flux activates a phospholipid scramblase to externalize phosphatidylserin.[109] These cell surface molecules subsequently increase tissue factor activity and enhance the assembly of cofactor–protease complexes on vascular endothelial cells starting the extrinsic coagulation cascade.[110] Extracellular HMGB1 will further increase the expression of tissue factor in endothelial cells via TLR4 signaling and activation of the transcription factors nuclear factor kappa B (NF-κB) and early growth response-1(Egr-1).[111]
There is an additional notable mechanism by which LPS and HMGB1 may cause coagulopathy in Gram-negative sepsis, because HMGB1 is stored in granules in platelets and is translocated to the cell surface when platelets are activated.[112] TLR4-mediated LPS activation of platelets leads to upregulation of surface HMGB1 expression and platelet aggregation.[113] Several reports established that platelet-derived disulfide HMGB1 is a critical mediator of thrombosis formation.[38,39] In contrast, mice with ablated platelet HMGB1 are partially protected from thrombus generation, platelet aggregation, inflammation, and organ damage during experimental shock.[38] Taken together, these observations suggest that platelet HMGB1 may contribute to the development of microvascular thrombosis in Gram-negative sepsis.
Post-sepsis cognitive dysfunction
Long-lasting cognitive impairment after Gram-negative sepsis remains a major challenge for many surviving patients.[114] Serum HMGB1 levels peaked 3–4 weeks post-injury and only returned to baseline levels by week 12 in a murine cecal ligation and puncture (CLP)-sepsis model.[48] The animals concurrently acquired long-term impairments in learning and memory, and anatomic changes in the hippocampus associated with a loss of synaptic plasticity. Systemic administration of neutralizing anti-HMGB1 monoclonal antibodies given during the second week after onset of peritonitis improved learning and memory impairments and brain pathology.[48] Injections of disulfide HMGB1 to naïve mice recapitulated these cognitive impairments. Furthermore, endotoxemia combined with increased systemic HMGB1 levels generated hippocampal inflammation and cognitive impairment via microglia cell activation in mice with chronic colitis.[115] There is a more than five-fold variation in the density of microglia cells between different regions in the brain, and the hippocampus is the most densely populated region.[116] RAGE mediated sepsis-triggered brain amyloid-β peptide accumulation and tau phosphorylation combined with cognitive impairment[117] and these harmful events were significantly prevented by injections of anti-RAGE antibodies in the hippocampus region. Clinical observation of significant interest is that many intensive care unit survivors, including patients with Gram-negative sepsis, express increased plasma HMGB1 levels associated with cognitive impairment still 6 months after the acute stage.[47] The cellular source of the prevailing HMGB1 levels is presently unknown. Taken together, these preclinical and clinical results suggest that it might be possible to prevent or reverse cognitive impairments in sepsis-surviving patients by administration of agents reducing pathogenic amounts of extracellular HMGB1.
Experimental models of sepsis-induced persistent neuroinflammation may provide additional clues to the origin of the major clinical problem of post-sepsis cognitive dysfunction. Mice surviving CLP-induced sepsis express neuroinflammation and a significantly reduced number of cholinergic neurons in the basal forebrain area.[118] This is of distinct interest since basal forebrain cholinergic neurons are implicated in the regulation of cognitive functions, including attention, learning, memory, and motivation.[119] Furthermore, the enzymatic degradation of acetylcholine was reported to be increased in the hippocampal area.[118] Cholinergic dysfunction may thus be an important underlying event contributing to the cognitive deficits in sepsis survivors. Accordingly, enhancing brain cholinergic signaling might provide an option to treat cognitive deterioration in sepsis survivors.
Tentative Novel Therapeutic Options in Ggram-negative Sepsis
Administration of low-dose heparin or non-anticoagulant heparin
Realizing that LPS needs HMGB1 to mediate its full toxicity in Gram-negative sepsis suggests new therapeutic strategies centered on inhibition of intracellular import of LPS to interact with caspase-11 (in humans caspases-4 and −5).[120] Heparin is a mammalian polysaccharide that binds to specific sites on HMGB1 and prevents LPS from binding to HMGB1 [Figure 3][121] and HMGB1 from binding to RAGE,[51,121] which restricts caspase-11-dependent inflammation and coagulation in endotoxic mice.[120] Furthermore, non-anticoagulant heparin can also bind HMGB1 as well as extracellular histones and prevent histone-mediated cytotoxicity, which is another significant pathogenic pathway in severe sepsis.[120,122] The doses of heparin required to inhibit LPS–HMGB1 toxicity are much lower than those needed for the anticoagulant effects [120] and modified heparin lacking anticoagulant activity prevents LPS from binding to HMGB1 and prevents RAGE-mediated endocytosis of HMGB1. These observations are important to optimize the design of possible future clinical sepsis trials to minimize the risk of bleeding complications with reduced doses of heparin or with non-anticoagulant heparin. The same study found that heparin treatment that controlled experimental Gram-negative sepsis failed to protect mice with sepsis caused by Staphylococcus aureus, supporting that the pathogenic mechanisms operating in Gram-positive vs. Gram-negative sepsis are separate. We have previously reported that LPS and Staphylococcal superantigens induce separate cytokine patterns.[123,124] LPS predominantly induces pro-inflammatory monokines, while Staphylococcal and Streptococcal superantigens mainly generate pro-inflammatory cytokines made by lymphocytes and natural killer (NK) cells. Future clinical studies of heparin treatment in sepsis should thus preferably include a stratification of patients based on the type of invading pathogens. There are several published therapeutic heparin studies in sepsis patients but the results are conflicting, possibly because no such stratification was performed.
Preventing HMGB1-mediated lysosomal rupture
A recent phenotypic screening study of several hundred compounds for their capacity to inhibit the LPS–HMGB1–caspase-11 pathway resulted in the identification of one small molecule with a porphyrin structure (FeTPPS) that inhibited caspase-11 activation and subsequent inflammation and coagulation.[125] Most critically, FeTPPS treatment prevented lethality in experimental Gram-negative sepsis. The mode of action by this molecule ensued via disruption of LPS–HMGB1 binding and reduced HMGB1 capacity to permeabilize lysosomal membranes. Both mechanisms thus diminished the cytosolic delivery of LPS in macrophages.
Inhibiting HMGB1 release via histone deacetylase agonists
Acetylation of multiple lysine residues positioned in the two NLSs in HMGB1 is mandatory for the nuclear HMGB1 export necessary for further active extracellular HMGB1 release.[16] HMGB1 acetylation is regulated by acetylation–deacetylation reactions catalyzed by histone acetyltransferases and HDACs including SIRT1. LPS shifts the balance toward HMGB1 hyperacetylation by triggering the degradation of HDAC4, while SIRT1 is stable under conditions of inflammation.[126] Molecules that specifically booster SIRT1 activity offer tools to promote nuclear HMGB1 sequestration and thus reduce HMGB1 release in Gram-negative sepsis. Such agents have been successfully administered in several different models of LPS-mediated severe inflammatory conditions.[19,[21], [22], [23], [24], [25],26,76,[127], [128], [129], [130], [131], [132], [133]] However, there are yet no published clinical studies using specific SIRT1 agonists.
Preserving mitochondrial integrity using ethyl pyruvate administration
Ethyl pyruvate is a derivative of pyruvic acid, which is an important endogenous metabolite that can scavenge reactive oxygen species (ROS).[134] Treatment with ethyl pyruvate confers significant protection against experimental Gram-negative sepsis- and endotoxemia-induced lethality.[135,136] Mechanistically, ethyl pyruvate significantly attenuates mitochondrial damage and cytoplasmic translocation of mitochondrial DNA during inflammation.[137] Mitochondrial DNA is a potent activator of NLRP3 inflammasomes which contribute to the release of HMGB1,[11,[138], [139], [140] 141] which is dose-dependently inhibited by ethyl pyruvate.[136,137] ROS-induced mitochondrial dysfunction is the major cellular cause of insufficient energy production mediating failed cellular homeostasis in sepsis. It is thus highly significant that ethyl pyruvate preserves mitochondrial integrity and merits further studies in Gram-negative sepsis.
Antibody-mediated neutralization of HMGB1
Autoantibodies against HMGB1 can be produced during sepsis and are associated with a favorable outcome in patients undergoing septic shock.[142,143] Furthermore, there are many successful preclinical studies in Gram-negative sepsis using either polyclonal or monoclonal anti-HMGB1 antibodies (mAb) (reviewed in Ref. [144]). However, the challenge for antibody-based therapy in HMGB1-mediated pathology is the risk of an obstructive steric hindrance for antibody-recognition of HMGB1 caused by complex-bound DAMP and PAMP partner molecules. From one aspect HMGB1 offers an attractive, unique temporal therapeutic window for successful antagonistic antibody therapy by being released late during the onset of Gram-negative sepsis. It takes 16–32 h after LPS exposure for maximal HMGB1 release to occur and treatment with anti-HMGB1 mAb can be successfully administered up to 24 h after onset of CLP-sepsis.[145] Treatment with antagonists targeting pro-inflammatory cytokines does not mediate beneficial effects when administered at this late time point in CLP-sepsis. However, anti-HMGB1 antibody therapy initiated 36 h after onset of CLP does not confer lethality protection despite that increased systemic HMGB1 levels prevail for many weeks during and after Gram-negative sepsis. The reasons why therapy based on HMGB1-specific antibodies failed when initiated 36 h after onset of CLP are not fully comprehended. One interpretation might be that during the sepsis course, due to accelerating cell death, there will be an accumulation of extracellular PAMPs and DAMPs including DNA, RNA, histones, nucleosomes, and additional molecules that all avidly bind to extracellular HMGB1 and thus may block the antibody recognition. If this analysis of the ongoing pathology is correct, that implies that the timing of initiation of HMGB1-targeted antibody-based therapy is very critical. Early recognition and administration of antibodies that recognize HMGB1 epitopes not occupied by LPS or other HMGB1-partner molecules might offer a reasonable chance to bring beneficial therapeutic effects to patients with Gram-negative sepsis, in analogy to results recorded in preclinical sepsis studies.
Activating the endogenous cholinergic anti-inflammatory pathway
Inflammation, including Gram-negative sepsis, is regulated by the nervous system, but this insight has so far not led to any clinical therapy for sepsis. Neuronally released HMGB1 will accelerate inflammation, while acetylcholine, released via the cholinergic anti-inflammatory pathway, will in contrast downregulate excessive inflammation.[146,147] The cholinergic mechanism inhibits pro-inflammatory cytokine release via signals that require the vagus nerve and α7 nicotinic acetylcholine receptors (α7nAChR).[148] α7nAChR-mediated signaling also inhibits HMGB1 release,[[149], [150], [151], [152], [153], [154],156] RAGE-mediated endocytosis of HMGB1 complexes [50], and TLR4/myeloid differentiation factor 88 (MyD88)/NF-κB signaling.[155] SIRT1 function has been reported to be enhanced by α7nAChR-specific agonist stimulation and inhibited by α7nAChR-specific antagonist exposure supporting a role for α7nAChR signaling to mediate increased SIRT1 activity that inhibits HMGB1 release.[156]
Surgical implantation of vagus nerve pacemakers programmed to activate the cholinergic anti-inflammatory system has demonstrated beneficial results in patients with rheumatoid arthritis and inflammatory bowel disease.[157,158] Vagus nerve stimulation or administration of α7nAChR agonists have also conferred beneficial therapeutic effects in experimental Gram-negative sepsis and in endotoxemia (reviewed in Refs. [159,160]). To date, clinical trial results of these approaches in sepsis have not been reported. A need for surgical device implantation can be circumvented by transcutaneous auricular vagus nerve stimulation using an external pulse generator.[161,162] This approach has been successfully utilized in murine polymicrobial sepsis resulting in improved survival and reduced systemic HMGB1 levels.[163] Further studies are warranted to translate the beneficial effects via the cholinergic anti-inflammatory pathway into clinical use for patients with Gram-negative sepsis.
Conclusions
As outlined in this review, there is a close collaboration between LPS and HMGB1 in the pathogenesis of Gram-negative sepsis. Clinical studies aimed to neutralize LPS have failed to improve the outcome in sepsis and no clinical studies to specifically neutralize or eliminate HMGB1 have been performed. One reason is that HMGB1 is a very challenging molecule to target in vivo with HMGB1-binding antagonists because HMGB1 may change its structure during dynamic biological processes. Extracellular HMGB1 forms complexes with many other molecules that gradually accumulate extracellularly during tissue injury, and these complexes may alter receptor occupancies. The occurrence of various post-translational modifications of HMGB1 is another characteristic feature of HMGB1 biology generating complications for HMGB1 binders to recognize the molecule. An alternative strategy that has not yet been tested clinically would be to inhibit active HMGB1 release. Active HMGB1 release in response to a stimulus requires distinct molecular post-translational modification activities to translocate the nuclear HMGB1 to the cytosol, which is a mandatory initial step for further extracellular release. Different strategies aimed to inhibit specific post-translational modifications of HMGB1 needed for nuclear HMGB1 export may conceivably provide opportunities to pacify HMGB1 in Gram-negative sepsis to the benefit of patients.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We gratefully acknowledge the critical reading of the manuscript by Professor Kevin Tracey, New York.
Managing Editor: Jingling Bao
Footnotes
Given their role as Editorial Board Members, Prof. Huan Yang and Prof. Ulf Andersson had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Prof. Ben Lu took the responsibility for peer-review progress. Prof. Dechang Chen who is the co-editor-in-chief made the final decision.
References
- 1.Minasyan H. Sepsis and septic shock: pathogenesis and treatment perspectives. J Crit Care. 2017;40:229–242. doi: 10.1016/j.jcrc.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Deutschman C.S., Tracey K.J. Sepsis: current dogma and new perspectives. Immunity. 2014;40(4):463–475. doi: 10.1016/j.immuni.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Singer M. The new sepsis consensus definitions (Sepsis-3): the good, the not-so-bad, and the actually-quite-pretty. Intensive Care Med. 2016;42(12):2027–2029. doi: 10.1007/s00134-016-4600-4. [DOI] [PubMed] [Google Scholar]
- 4.Raetz C.R., Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park B.S., Lee J.O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med. 2013;45(12):e66. doi: 10.1038/emm.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagar J.A., Powell D.A., Aachoui Y., Ernst R.K., Miao E.A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341(6151):1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kayagaki N., Wong M.T., Stowe I.B., Ramani S.R., Gonzalez L.C., Akashi-Takamura S., et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341(6151):1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 8.Rathinam V., Zhao Y., Shao F. Innate immunity to intracellular LPS. Nat Immunol. 2019;20(5):527–533. doi: 10.1038/s41590-019-0368-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng M., Tang Y., Li W., Wang X., Zhang R., Zhang X., et al. The endotoxin delivery protein HMGB1 mediates caspase-11-dependent lethality in sepsis. Immunity. 2018;49(4):740–753. doi: 10.1016/j.immuni.2018.08.016. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H., Bloom O., Zhang M., Vishnubhakat J.M., Ombrellino M., Che J., et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 11.Lamkanfi M., Sarkar A., Vande Walle L., Vitari A.C., Amer A.O., Wewers M.D., et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J Immunol. 2010;185(7):4385–4392. doi: 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W., Deng M., Loughran P.A., Yang M., Lin M., Yang C., et al. LPS induces active HMGB1 release from hepatocytes into exosomes through the coordinated activities of TLR4 and caspase-11/GSDMD signaling. Front Immunol. 2020;11:229. doi: 10.3389/fimmu.2020.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu B., Antoine D.J., Kwan K., Lundbäck P., Wähämaa H., Schierbeck H., et al. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci U S A. 2014;111(8):3068–3073. doi: 10.1073/pnas.1316925111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin K., Gribbin E., Wang H. Interferon-gamma inhibition attenuates lethality after cecal ligation and puncture in rats: implication of high mobility group box-1. Shock. 2005;24(4):396–401. doi: 10.1097/01.shk.0000175556.03300.c6. [DOI] [PubMed] [Google Scholar]
- 15.Kang R., Chen R., Zhang Q., Hou W., Wu S., Cao L., et al. HMGB1 in health and disease. Mol Aspects Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonaldi T., Talamo F., Scaffidi P., Ferrera D., Porto A., Bachi A., et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22(20):5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evankovich J., Cho S.W., Zhang R., Cardinal J., Dhupar R., Zhang L., et al. High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histone deacetylase activity. J Biol Chem. 2010;285(51):39888–39897. doi: 10.1074/jbc.M110.128348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou J.Y., Crews F.T. Release of neuronal HMGB1 by ethanol through decreased HDAC activity activates brain neuroimmune signaling. PLoS ONE. 2014;9(2):e87915. doi: 10.1371/journal.pone.0087915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang J.S., Choi H.S., Ham S.A., Yoo T., Lee W.J., Paek K.S., et al. Deacetylation-mediated interaction of SIRT1-HMGB1 improves survival in a mouse model of endotoxemia. Sci Rep. 2015;5:15971. doi: 10.1038/srep15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao P.C., Xu S.N., Huang Z.S., Jiang G.W., Deng P.C., Zhang Y.M. Hyperbaric oxygen via mediating SIRT1-induced deacetylation of HMGB1 improved cReperfusion inj/reperfusion injury. Eur J Neurosci. 2021;54(9):7318–7331. doi: 10.1111/ejn.15458. [DOI] [PubMed] [Google Scholar]
- 21.Karkischenko V.N., Skvortsova V.I., Gasanov M.T., Fokin Y.V., Nesterov M.S., Petrova N.V., et al. Inhaled [D-Ala(2)]-dynorphin 1-6 prevents hyperacetylation and release of high mobility group box 1 in a mouse model of acute lung injury. J Immunol Res. 2021;2021 doi: 10.1155/2021/4414544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le K., Chibaatar Daliv E., Wu S., Qian F., Ali A.I., Yu D., et al. SIRT1-regulated HMGB1 release is partially involved in TLR4 signal transduction: a possible anti-neuroinflammatory mechanism of resveratrol in neonatal hypoxic-ischemic brain injury. Int Immunopharmacol. 2019;75 doi: 10.1016/j.intimp.2019.105779. [DOI] [PubMed] [Google Scholar]
- 23.Rabadi M.M., Xavier S., Vasko R., Kaur K., Goligorksy M.S., Ratliff B.B. High-mobility group box 1 is a novel deacetylation target of Sirtuin1. Kidney Int. 2015;87(1):95–108. doi: 10.1038/ki.2014.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu S., Zeng Z., Zhao M., Huang Q., Gao Y., Dai X., et al. Evidence for SIRT1 mediated HMGB1 release from kidney cells in the early stages of hemorrhagic shock. Front Physiol. 2019;10:854. doi: 10.3389/fphys.2019.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng X., Chen W., Ni X., Little P.J., Xu S., Tang L., et al. Metformin, macrophage dysfunction and atherosclerosis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.682853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu S., Zhou X., Xiang H., Wang S., Cui Z., Zhou J. Resveratrol reduced liver damage after liver resection in a rat model by upregulating sirtuin 1 (SIRT1) and inhibiting the acetylation of high mobility group box 1 (HMGB1) Med Sci Monit. 2019;25:3212–3220. doi: 10.12659/MSM.913937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haigis M.C., Guarente L.P. Mammalian sirtuins – Emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20(21):2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 28.Imai S., Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24(8):464–471. doi: 10.1016/j.tcb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bierhaus A., Humpert P.M., Morcos M., Wendt T., Chavakis T., Arnold B., et al. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berl) 2005;83(11):876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 30.Kierdorf K., Fritz G. RAGE regulation and signaling in inflammation and beyond. J Leukoc Biol. 2013;94(1):55–68. doi: 10.1189/jlb.1012519. [DOI] [PubMed] [Google Scholar]
- 31.Merenmies J., Pihlaskari R., Laitinen J., Wartiovaara J., Rauvala H. 30-kDa heparin-binding protein of brain (amphoterin) involved in neurite outgrowth. Amino acid sequence and localization in the filopodia of the advancing plasma membrane. J Biol Chem. 1991;266(25):16722–16729. [PubMed] [Google Scholar]
- 32.LeBlanc P.M., Doggett T.A., Choi J., Hancock M.A., Durocher Y., Frank F., et al. An immunogenic peptide in the A-box of HMGB1 protein reverses apoptosis-induced tolerance through RAGE receptor. J Biol Chem. 2014;289(11):7777–7786. doi: 10.1074/jbc.M113.541474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu B., Wang H., Andersson U., Tracey K.J. Regulation of HMGB1 release by inflammasomes. Protein Cell. 2013;4(3):163–167. doi: 10.1007/s13238-012-2118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan S.W., Zhao Y., Li P., Ning Y.L., Huang Z.Z., Yang N., et al. HMGB1 mediates cognitive impairment caused by the NLRP3 inflammasome in the late stage of traumatic brain injury. J Neuroinflammation. 2021;18(1):241. doi: 10.1186/s12974-021-02274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardella S., Andrei C., Ferrera D., Lotti L.V., Torrisi M.R., Bianchi M.E., et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3(10):995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mobarrez F., Vikerfors A., Gustafsson J.T., Gunnarsson I., Zickert A., Larsson A., et al. Microparticles in the blood of patients with systemic lupus erythematosus (SLE): phenotypic characterization and clinical associations. Sci Rep. 2016;6:36025. doi: 10.1038/srep36025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pisetsky D.S., Gauley J., Ullal A.J. HMGB1 and microparticles as mediators of the immune response to cell death. Antioxid Redox Signal. 2011;15(8):2209–2219. doi: 10.1089/ars.2010.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogel S., Bodenstein R., Chen Q., Feil S., Feil R., Rheinlaender J., et al. Platelet-derived HMGB1 is a critical mediator of thrombosis. J Clin Invest. 2015;125(12):4638–4654. doi: 10.1172/JCI81660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stark K., Philippi V., Stockhausen S., Busse J., Antonelli A., Miller M., et al. Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice. Blood. 2016;128(20):2435–2449. doi: 10.1182/blood-2016-04-710632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maugeri N., Campana L., Gavina M., Covino C., De Metrio M., Panciroli C., et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J Thromb Haemost. 2014;12(12):2074–2088. doi: 10.1111/jth.12710. [DOI] [PubMed] [Google Scholar]
- 41.Yang K., Fan M., Wang X., Xu J., Wang Y., Tu F., et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. 2022;29(1):133–146. doi: 10.1038/s41418-021-00841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venereau E., Casalgrandi M., Schiraldi M., Antoine D.J., Cattaneo A., De Marchis F., et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209(9):1519–1528. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiraldi M., Raucci A., Muñoz L.M., Livoti E., Celona B., Venereau E., et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209(3):551–563. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang H., Hreggvidsdottir H.S., Palmblad K., Wang H., Ochani M., Li J., et al. A critical cysteine is required for HMGB1 binding to toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010;107(26):11942–11947. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang H., Wang H., Ju Z., Ragab A.A., Lundbäck P., Long W., et al. MD-2 is required for disulfide HMGB1-dependent TLR4 signaling. J Exp Med. 2015;212(1):5–14. doi: 10.1084/jem.20141318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hubert P., Roncarati P., Demoulin S., Pilard C., Ancion M., Reynders C., et al. Extracellular HMGB1 blockade inhibits tumor growth through profoundly remodeling immune microenvironment and enhances checkpoint inhibitor-based immunotherapy. J Immunother Cancer. 2021;9(3) doi: 10.1136/jitc-2020-001966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brück E., Lasselin J., Andersson U., Sackey P.V., Olofsson P.S. Prolonged elevation of plasma HMGB1 is associated with cognitive impairment in intensive care unit survivors. Intensive Care Med. 2020;46(4):811–812. doi: 10.1007/s00134-020-05941-7. [DOI] [PubMed] [Google Scholar]
- 48.Chavan S.S., Huerta P.T., Robbiati S., Valdes-Ferrer S.I., Ochani M., Dancho M., et al. HMGB1 mediates cognitive impairment in sepsis survivors. Mol Med. 2012;18(1):930–937. doi: 10.2119/molmed.2012.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Youn J.H., Kwak M.S., Wu J., Kim E.S., Ji Y., Min H.J., et al. Identification of lipopolysaccharide-binding peptide regions within HMGB1 and their effects on subclinical endotoxemia in a mouse model. Eur J Immunol. 2011;41(9):2753–2762. doi: 10.1002/eji.201141391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang H., Liu H., Zeng Q., Imperato G.H., Addorisio M.E., Li J., et al. Inhibition of HMGB1/RAGE-mediated endocytosis by HMGB1 antagonist box A, anti-HMGB1 antibodies, and cholinergic agonists suppresses inflammation. Mol Med. 2019;25(1):13. doi: 10.1186/s10020-019-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ling Y., Yang Z.Y., Yin T., Li L., Yuan W.W., Wu H.S., et al. Heparin changes the conformation of high-mobility group protein 1 and decreases its affinity toward receptor for advanced glycation endproducts in vitro. Int Immunopharmacol. 2011;11(2):187–193. doi: 10.1016/j.intimp.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 52.Porat A., Giat E., Kowal C., He M., Son M., Latz E., et al. DNA-mediated interferon signature induction by SLE serum occurs in monocytes through two pathways: a mechanism to inhibit both pathways. Front Immunol. 2018;9:2824. doi: 10.3389/fimmu.2018.02824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin H.J., Jiang Z.P., Lo H.R., Feng C.L., Chen C.J., Yang C.Y., et al. Coalescence of RAGE in lipid rafts in response to cytolethal distending toxin-induced inflammation. Front Immunol. 2019;10:109. doi: 10.3389/fimmu.2019.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jia C., Zhang J., Chen H., Zhuge Y., Chen H., Qian F., et al. Endothelial cell pyroptosis plays an important role in Kawasaki disease via HMGB1/RAGE/cathespin B signaling pathway and NLRP3 inflammasome activation. Cell Death Dis. 2019;10(10):778. doi: 10.1038/s41419-019-2021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Mingo Pulido Á., Hänggi K., Celias D.P., Gardner A., Li J., Batista-Bittencourt B., et al. The inhibitory receptor TIM-3 limits activation of the cGAS-STING pathway in intra-tumoral dendritic cells by suppressing extracellular DNA uptake. Immunity. 2021;54(6):1154–1167. doi: 10.1016/j.immuni.2021.04.019. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu J., Yue Y., Xiong S. Extracellular HMGB1 augments macrophage inflammation by facilitating the endosomal accumulation of ALD-DNA via TLR2/4-mediated endocytosis. Biochim Biophys Acta Mol Basis Dis. 2021;1867(10) doi: 10.1016/j.bbadis.2021.166184. [DOI] [PubMed] [Google Scholar]
- 57.Liu L., Yang M., Kang R., Dai Y., Yu Y., Gao F., et al. HMGB1-DNA complex-induced autophagy limits AIM2 inflammasome activation through RAGE. Biochem Biophys Res Commun. 2014;450(1):851–856. doi: 10.1016/j.bbrc.2014.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sha Y., Zmijewski J., Xu Z., Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 2008;180(4):2531–2537. doi: 10.4049/jimmunol.180.4.2531. [DOI] [PubMed] [Google Scholar]
- 59.Pisetsky D.S. The complex role of DNA, histones and HMGB1 in the pathogenesis of SLE. Autoimmunity. 2014;47(8):487–493. doi: 10.3109/08916934.2014.921811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raucci A., Di Maggio S., Scavello F., D'Ambrosio A., Bianchi M.E., et al. The Janus face of HMGB1 in heart disease: a necessary update. Cell Mol Life Sci. 2019;76(2):211–229. doi: 10.1007/s00018-018-2930-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deng C., Zhao L., Yang Z., Shang J.J., Wang C.Y., Shen M.Z., et al. Targeting HMGB1 for the treatment of sepsis and sepsis-induced organ injury. Acta Pharmacol Sin. 2021 doi: 10.1038/s41401-021-00676-7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao M.J., Jiang H.R., Sun J.W., Wang Z.A., Hu B., Zhu C.R., et al. Roles of RAGE/ROCK1 pathway in HMGB1-induced early changes in barrier permeability of human pulmonary microvascular endothelial cell. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.697071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brett J., Schmidt A.M., Yan S.D., Zou Y.S., Weidman E., Pinsky D., et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143(6):1699–1712. [PMC free article] [PubMed] [Google Scholar]
- 64.Oczypok E.A., Perkins T.N., Oury T.D. All the “RAGE” in lung disease: the receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses. Paediatr Respir Rev. 2017;23:40–49. doi: 10.1016/j.prrv.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ueno H., Matsuda T., Hashimoto S., Amaya F., Kitamura Y., Tanaka M., et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 2004;170(12):1310–1316. doi: 10.1164/rccm.200402-188OC. [DOI] [PubMed] [Google Scholar]
- 66.Liu H., Yu X., Yu S., Kou J. Molecular mechanisms in lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Int Immunopharmacol. 2015;29(2):937–946. doi: 10.1016/j.intimp.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 67.Abraham E., Arcaroli J., Carmody A., Wang H., Tracey K.J. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165(6):2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 68.Grégoire M., Tadié J.M., Uhel F., Gacouin A., Piau C., Bone N., et al. Frontline science: HMGB1 induces neutrophil dysfunction in experimental sepsis and in patients who survive septic shock. J Leukoc Biol. 2017;101(6):1281–1287. doi: 10.1189/jlb.5HI0316-128RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tadié J.M., Bae H.B., Banerjee S., Zmijewski J.W., Abraham E. Differential activation of RAGE by HMGB1 modulates neutrophil-associated NADPH oxidase activity and bacterial killing. Am J Physiol Cell Physiol. 2012;302(1):C249–C256. doi: 10.1152/ajpcell.00302.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolfson R.K., Chiang E.T., Garcia J.G. HMGB1 induces human lung endothelial cell cytoskeletal rearrangement and barrier disruption. Microvasc Res. 2011;81(2):189–197. doi: 10.1016/j.mvr.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang J., Li R., Peng Z., Hu B., Rao X., Li J. HMGB1 participates in LPS‑induced acute lung injury by activating the AIM2 inflammasome in macrophages and inducing polarization of M1 macrophages via TLR2, TLR4, and RAGE/NF‑κB signaling pathways. Int J Mol Med. 2020;45(1):61–80. doi: 10.3892/ijmm.2019.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bellomo R., Kellum J.A., Ronco C., Wald R., Martensson J., Maiden M., et al. Acute kidney injury in sepsis. Intensive Care Med. 2017;43(6):816–828. doi: 10.1007/s00134-017-4755-7. [DOI] [PubMed] [Google Scholar]
- 73.Gao Z., Lu L., Chen X. Release of HMGB1 in podocytes exacerbates lipopolysaccharide-induced acute kidney injury. Mediators Inflamm. 2021;2021 doi: 10.1155/2021/5220226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang X., Hou X., Chuan L., Wei S., Wang J., Yang X., et al. miR-129-5p alleviates LPS-induced acute kidney injury via targeting HMGB1/TLRs/NF-kappaB pathway. Int Immunopharmacol. 2020;89(Pt A) doi: 10.1016/j.intimp.2020.107016. [DOI] [PubMed] [Google Scholar]
- 75.Zheng S., Pan Y., Wang C., Liu Y., Shi M., Ding G. HMGB1 turns renal tubular epithelial cells into inflammatory promoters by interacting with TLR4 during sepsis. J Interferon Cytokine Res. 2016;36(1):9–19. doi: 10.1089/jir.2015.0067. [DOI] [PubMed] [Google Scholar]
- 76.Wei S., Gao Y., Dai X., Fu W., Cai S., Fang H., et al. SIRT1-mediated HMGB1 deacetylation suppresses sepsis-associated acute kidney injury. Am J Physiol Renal Physiol. 2019;316(1):F20–F31. doi: 10.1152/ajprenal.00119.2018. [DOI] [PubMed] [Google Scholar]
- 77.Hagiwara S., Iwasaka H., Uchino T., Noguchi T. High mobility group box 1 induces a negative inotropic effect on the left ventricle in an isolated rat heart model of septic shock: a pilot study. Circ J. 2008;72(6):1012–1017. doi: 10.1253/circj.72.1012. [DOI] [PubMed] [Google Scholar]
- 78.Xu H., Su Z., Wu J., Yang M., Penninger J.M., Martin C.M., et al. The alarmin cytokine, high mobility group box 1, is produced by viable cardiomyocytes and mediates the lipopolysaccharide-induced myocardial dysfunction via a TLR4/phosphatidylinositol 3-kinase gamma pathway. J Immunol. 2010;184(3):1492–1498. doi: 10.4049/jimmunol.0902660. [DOI] [PubMed] [Google Scholar]
- 79.Zheng Y.J., Xu W.P., Ding G., Gao Y.H., Wang H.R., Pan S.M. Expression of HMGB1 in septic serum induces vascular endothelial hyperpermeability. Mol Med Rep. 2016;13(1):513–521. doi: 10.3892/mmr.2015.4536. [DOI] [PubMed] [Google Scholar]
- 80.Fiuza C., Bustin M., Talwar S., Tropea M., Gerstenberger E., Shelhamer J.H., et al. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101(7):2652–2660. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- 81.Opal S.M., van der Poll T. Endothelial barrier dysfunction in septic shock. J Intern Med. 2015;277(3):277–293. doi: 10.1111/joim.12331. [DOI] [PubMed] [Google Scholar]
- 82.Vincent J.L., Ramesh M.K., Ernest D., LaRosa S.P., Pachl J., Aikawa N., et al. A randomized, double-blind, placebo-controlled, phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Crit Care Med. 2013;41(9):2069–2079. doi: 10.1097/CCM.0b013e31828e9b03. [DOI] [PubMed] [Google Scholar]
- 83.Zhou Y., Li P., Goodwin A.J., Cook J.A., Halushka P.V., Chang E., et al. Exosomes from endothelial progenitor cells improve outcomes of the lipopolysaccharide-induced acute lung injury. Crit Care. 2019;23(1):44. doi: 10.1186/s13054-019-2339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Y., Zhu H., Pan L., Zhang B., Che H. MicroRNA-103a-3p confers protection against lipopolysaccharide-induced sepsis and consequent multiple organ dysfunction syndrome by targeting HMGB1. Infect Genet Evol. 2021;89 doi: 10.1016/j.meegid.2020.104681. [DOI] [PubMed] [Google Scholar]
- 85.Unno N., Wang H., Menconi M.J., Tytgat S.H., Larkin V., Smith M., et al. Inhibition of inducible nitric oxide synthase ameliorates endotoxin-induced gut mucosal barrier dysfunction in rats. Gastroenterology. 1997;113(4):1246–1257. doi: 10.1053/gast.1997.v113.pm9322519. [DOI] [PubMed] [Google Scholar]
- 86.Deitch E.A., Ma L., Ma W.J., Grisham M.B., Granger D.N., Specian R.D., et al. Inhibition of endotoxin-induced bacterial translocation in mice. J Clin Invest. 1989;84(1):36–42. doi: 10.1172/JCI114164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sappington P.L., Yang R., Yang H., Tracey K.J., Delude R.L., Fink M.P. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002;123(3):790–802. doi: 10.1053/gast.2002.35391. [DOI] [PubMed] [Google Scholar]
- 88.Yang R., Harada T., Mollen K.P., Prince J.M., Levy R.M., Englert J.A., et al. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med. 2006;12(4–6):105–114. doi: 10.2119/2006-00010. Yang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu S., Stolz D.B., Sappington P.L., Macias C.A., Killeen M.E., Tenhunen J.J., et al. HMGB1 is secreted by immunostimulated enterocytes and contributes to cytomix-induced hyperpermeability of Caco-2 monolayers. Am J Physiol Cell Physiol. 2006;290(4):C990–C999. doi: 10.1152/ajpcell.00308.2005. [DOI] [PubMed] [Google Scholar]
- 90.Yang R., Miki K., Oksala N., Nakao A., Lindgren L., Killeen M.E., et al. Bile high-mobility group box 1 contributes to gut barrier dysfunction in experimental endotoxemia. Am J Physiol Regul Integr Comp Physiol. 2009;297(2):R362–R369. doi: 10.1152/ajpregu.00184.2009. [DOI] [PubMed] [Google Scholar]
- 91.Jin S., Ding X., Yang C., Li W., Deng M., Liao H., et al. Mechanical ventilation exacerbates poly (I:C) induced acute lung injury: central role for caspase-11 and gut-lung axis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.693874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim J.Y., Park J.S., Strassheim D., Douglas I., Diaz del Valle F., Asehnoune K., et al. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2005;288(5):L958–L965. doi: 10.1152/ajplung.00359.2004. [DOI] [PubMed] [Google Scholar]
- 93.Sodhi C.P., Jia H., Yamaguchi Y., Lu P., Good M., Egan C., et al. Intestinal epithelial TLR-4 activation is required for the development of acute lung injury after trauma/hemorrhagic shock via the release of HMGB1 from the gut. J Immunol. 2015;194(10):4931–4939. doi: 10.4049/jimmunol.1402490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yamamoto T., Tajima Y. HMGB1 is a promising therapeutic target for acute liver failure. Expert Rev Gastroenterol Hepatol. 2017;11(7):673–682. doi: 10.1080/17474124.2017.1345625. [DOI] [PubMed] [Google Scholar]
- 95.Yang R., Zou X., Tenhunen J., Tønnessen T.I. HMGB1 and extracellular histones significantly contribute to systemic inflammation and multiple organ failure in acute liver failure. Mediators Inflamm. 2017;2017 doi: 10.1155/2017/5928078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Osumi W., Jin D., Imai Y., Tashiro K., Li Z.L., Otsuki Y., et al. Recombinant human soluble thrombomodulin improved lipopolysaccharide/d-galactosamine-induced acute liver failure in mice. J Pharmacol Sci. 2015;129(4):233–239. doi: 10.1016/j.jphs.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 97.Shen C.H., Ma Z.Y., Li J.H., Li R.D., Tao Y.F., Zhang Q.B., et al. Glycyrrhizin improves inflammation and apoptosis via suppressing HMGB1 and PI3K/mTOR pathway in lipopolysaccharide-induced acute liver injury. Eur Rev Med Pharmacol Sci. 2020;24(12):7122–7130. doi: 10.26355/eurrev_202006_21706. [DOI] [PubMed] [Google Scholar]
- 98.Wang G., Jin S., Huang W., Li Y., Wang J., Ling X., et al. LPS-induced macrophage HMGB1-loaded extracellular vesicles trigger hepatocyte pyroptosis by activating the NLRP3 inflammasome. Cell Death Discov. 2021;7(1):337. doi: 10.1038/s41420-021-00729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nishibori M., Wang D., Ousaka D., Wake H. High mobility group box-1 and blood-brain barrier disruption. Cells. 2020;9(12):2650. doi: 10.3390/cells9122650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peek V., Harden L.M., Damm J., Aslani F., Leisengang S., Roth J., et al. LPS primes brain responsiveness to high mobility group box-1 protein. Pharmaceuticals (Basel) 2021;14(6):558. doi: 10.3390/ph14060558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xiong Y., Yang J., Tong H., Zhu C., Pang Y. HMGB1 augments cognitive impairment in sepsis-associated encephalopathy by binding to MD-2 and promoting NLRP3-induced neuroinflammation. Psychogeriatrics. 2021 doi: 10.1111/psyg.12794. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 102.Terrando N., Monaco C., Ma D., Foxwell B.M., Feldmann M., Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107(47):20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.He H.J., Wang Y., Le Y., Duan K.M., Yan X.B., Liao Q., et al. Surgery upregulates high mobility group box-1 and disrupts the blood-brain barrier causing cognitive dysfunction in aged rats. CNS Neurosci Ther. 2012;18(12):994–1002. doi: 10.1111/cns.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vacas S., Degos V., Tracey K.J., Maze M. High-mobility group box 1 protein initiates postoperative cognitive decline by engaging bone marrow-derived macrophages. Anesthesiology. 2014;120(5):1160–1167. doi: 10.1097/ALN.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Terrando N., Yang T., Wang X., Fang J., Cao M., Andersson U., et al. Systemic HMGB1 neutralization prevents postoperative neurocognitive dysfunction in aged rats. Front Immunol. 2016;7:441. doi: 10.3389/fimmu.2016.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang H., Andersson U., Brines M. Neurons are a primary driver of inflammation via release of HMGB1. Cells. 2021;10(10):2791. doi: 10.3390/cells10102791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang H., Zeng Q., Silverman H.A., Gunasekaran M., George S.J., Devarajan A., et al. HMGB1 released from nociceptors mediates inflammation. Proc Natl Acad Sci U S A. 2021;118(33) doi: 10.1073/pnas.2102034118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tang D., Wang H., Billiar T.R., Kroemer G., Kang R. Emerging mechanisms of immunocoagulation in sepsis and septic shock. Trends Immunol. 2021;42(6):508–522. doi: 10.1016/j.it.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang X., Cheng X., Tang Y., Qiu X., Wang Y., Kang H., et al. Bacterial endotoxin activates the coagulation cascade through gasdermin d-dependent phosphatidylserine exposure. Immunity. 2019;51(6):983–996. doi: 10.1016/j.immuni.2019.11.005. e6. [DOI] [PubMed] [Google Scholar]
- 110.Yang X., Cheng X., Tang Y., Qiu X., Wang Z., Fu G., et al. The role of type 1 interferons in coagulation induced by Gram-negative bacteria. Blood. 2020;135(14):1087–1100. doi: 10.1182/blood.2019002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lv B., Wang H., Tang Y., Fan Z., Xiao X., Chen F. High-mobility group box 1 protein induces tissue factor expression in vascular endothelial cells via activation of NF-kappaB and Egr-1. Thromb Haemost. 2009;102(2):352–359. doi: 10.1160/TH08-11-0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rouhiainen A., Imai S., Rauvala H., Parkkinen J. Occurrence of amphoterin (HMG1) as an endogenous protein of human platelets that is exported to the cell surface upon platelet activation. Thromb Haemost. 2000;84(6):1087–1094. [PubMed] [Google Scholar]
- 113.Li R., Hommel C., Nguyen N. Lipopolysaccharide-activated canine platelets upregulate high mobility group box-1 via toll-like receptor 4. Front Vet Sci. 2021;8 doi: 10.3389/fvets.2021.674678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rengel K.F., Hayhurst C.J., Pandharipande P.P., Hughes C.G. Long-term cognitive and functional impairments after critical illness. Anesth Analg. 2019;128(4):772–780. doi: 10.1213/ANE.0000000000004066. [DOI] [PubMed] [Google Scholar]
- 115.Mitchell J., Kim S.J., Howe C., Lee S., Her J.Y., Patel M., et al. Chronic intestinal inflammation suppresses brain activity by inducing neuroinflammation in mice. Am J Pathol. 2022;192(1):72–86. doi: 10.1016/j.ajpath.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lawson L.J., Perry V.H., Dri P., Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39(1):151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- 117.Gasparotto J., Girardi C.S., Somensi N., Ribeiro C.T., Moreira J., Michels M., et al. Receptor for advanced glycation end products mediates sepsis-triggered amyloid-β accumulation, Tau phosphorylation, and cognitive impairment. J Biol Chem. 2018;293(1):226–244. doi: 10.1074/jbc.M117.786756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zaghloul N., Addorisio M.E., Silverman H.A., Patel H.L., Valdés-Ferrer S.I., Ayasolla K.R., et al. Forebrain cholinergic dysfunction and systemic and brain inflammation in murine sepsis survivors. Front Immunol. 2017;8:1673. doi: 10.3389/fimmu.2017.01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ballinger E.C., Ananth M., Talmage D.A., Role L.W. Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron. 2016;91(6):1199–1218. doi: 10.1016/j.neuron.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tang Y., Wang X., Li Z., He Z., Yang X., Cheng X., et al. Heparin prevents caspase-11-dependent septic lethality independent of anticoagulant properties. Immunity. 2021;54(3):454–467. doi: 10.1016/j.immuni.2021.01.007. e6. [DOI] [PubMed] [Google Scholar]
- 121.Li L., Ling Y., Huang M., Yin T., Gou S.M., Zhan N.Y., et al. Heparin inhibits the inflammatory response induced by LPS and HMGB1 by blocking the binding of HMGB1 to the surface of macrophages. Cytokine. 2015;72(1):36–42. doi: 10.1016/j.cyto.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 122.Wildhagen K.C., García de Frutos P., Reutelingsperger C.P., Schrijver R., Aresté C., Ortega-Gómez A., et al. Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood. 2014;123(7):1098–1101. doi: 10.1182/blood-2013-07-514984. [DOI] [PubMed] [Google Scholar]
- 123.Andersson J., Nagy S., Björk L., Abrams J., Holm S., Andersson U. Bacterial toxin-induced cytokine production studied at the single-cell level. Immunol Rev. 1992;127:69–96. doi: 10.1111/j.1600-065x.1992.tb01409.x. [DOI] [PubMed] [Google Scholar]
- 124.Björk L., Andersson J., Ceska M., Andersson U. Endotoxin and Staphylococcus aureus enterotoxin A induce different patterns of cytokines. Cytokine. 1992;4(6):513–519. doi: 10.1016/1043-4666(92)90013-h. [DOI] [PubMed] [Google Scholar]
- 125.Wang X., Li Z., Bai Y., Zhang R., Meng R., Chen F., et al. A small molecule binding HMGB1 inhibits caspase-11-mediated lethality in sepsis. Cell Death Dis. 2021;12(4):402. doi: 10.1038/s41419-021-03652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Walko T.D., 3rd, Di Caro V., Piganelli J., Billiar T.R., Clark R.S., Aneja R.K. Poly(ADP-ribose) polymerase 1-sirtuin 1 functional interplay regulates LPS-mediated high mobility group box 1 secretion. Mol Med. 2015;20(1):612–624. doi: 10.2119/molmed.2014.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chibaatar E., Le K., Abdoulaye I.A., Wu S., Guo Y. Melatonin ameliorates lipopolysaccharide-induced microglial inflammation via triggering SIRT1/HMGB1 signaling axis. J Mol Neurosci. 2021;71(4):691–701. doi: 10.1007/s12031-020-01699-1. [DOI] [PubMed] [Google Scholar]
- 128.Kim J.E., Lee W., Yang S., Cho S.H., Baek M.C., Song G.Y., et al. Suppressive effects of rare ginsenosides, Rk1 and Rg5, on HMGB1-mediated septic responses. Food Chem Toxicol. 2019;124:45–53. doi: 10.1016/j.fct.2018.11.057. [DOI] [PubMed] [Google Scholar]
- 129.Lee W., Ku S.K., Bae J.S. Zingerone reduces HMGB1-mediated septic responses and improves survival in septic mice. Toxicol Appl Pharmacol. 2017;329:202–211. doi: 10.1016/j.taap.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 130.Lee W., Lee H., Lee T., Park E.K., Bae J.S. Inhibitory functions of maslinic acid, a natural triterpene, on HMGB1-mediated septic responses. Phytomedicine. 2020;69 doi: 10.1016/j.phymed.2020.153200. [DOI] [PubMed] [Google Scholar]
- 131.Li Y., Liu T., Li Y., Han D., Hong J., Yang N., et al. Baicalin ameliorates cognitive impairment and protects microglia from LPS-induced neuroinflammation via the SIRT1/HMGB1 pathway. Oxid Med Cell Longev. 2020;2020 doi: 10.1155/2020/4751349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qi Z., Zhang Y., Qi S., Ling L., Gui L., Yan L., et al. Salidroside inhibits HMGB1 acetylation and release through upregulation of SirT1 during inflammation. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/9821543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang S., Lee W., Lee B.S., Lee C., Park E.K., Ku S.K., et al. Aloin reduces HMGB1-mediated septic responses and improves survival in septic mice by activation of the SIRT1 and PI3K/Nrf2/HO-1 signaling axis. Am J Chin Med. 2019;47(3):613–633. doi: 10.1142/S0192415X19500320. [DOI] [PubMed] [Google Scholar]
- 134.Fink M.P. Ethyl pyruvate: a novel treatment for sepsis and shock. Minerva Anestesiol. 2004;70(5):365–371. [PubMed] [Google Scholar]
- 135.Yang R., Zhu S., Tonnessen T.I. Ethyl pyruvate is a novel anti-inflammatory agent to treat multiple inflammatory organ injuries. J Inflamm (Lond) 2016;13:37. doi: 10.1186/s12950-016-0144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ulloa L., Ochani M., Yang H., Tanovic M., Halperin D., Yang R., et al. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A. 2002;99(19):12351–12356. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li S., Liang F., Kwan K., Tang Y., Wang X., Tang Y., et al. Identification of ethyl pyruvate as a NLRP3 inflammasome inhibitor that preserves mitochondrial integrity. Mol Med. 2018;24(1):8. doi: 10.1186/s10020-018-0006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lu B., Nakamura T., Inouye K., Li J., Tang Y., Lundbäck P., et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488(7413):670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nakahira K., Haspel J.A., Rathinam V.A., Lee S.J., Dolinay T., Lam H.C., et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12(3):222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Groß C.J., Mishra R., Schneider K.S., Médard G., Wettmarshausen J., Dittlein D.C., et al. K(+) efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity. 2016;45(4):761–773. doi: 10.1016/j.immuni.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 141.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 142.Barnay-Verdier S., Fattoum L., Borde C., Kaveri S., Gibot S., Maréchal V. Emergence of autoantibodies to HMGB1 is associated with survival in patients with septic shock. Intensive Care Med. 2011;37(6):957–962. doi: 10.1007/s00134-011-2192-6. [DOI] [PubMed] [Google Scholar]
- 143.Barnay-Verdier S., Borde C., Fattoum L., Wootla B., Lacroix-Desmazes S., Kaveri S., et al. Emergence of antibodies endowed with proteolytic activity against high-mobility group box 1 protein (HMGB1) in patients surviving septic shock. Cell Immunol. 2020;347 doi: 10.1016/j.cellimm.2019.104020. [DOI] [PubMed] [Google Scholar]
- 144.Zhu C.S., Wang W., Qiang X., Chen W., Lan X., Li J., et al. Endogenous regulation and pharmacological modulation of sepsis-induced HMGB1 release and action: an updated review. Cells. 2021;10(9) doi: 10.3390/cells10092220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang H., Ochani M., Li J., Qiang X., Tanovic M., Harris H.E., et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101(1):296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Andersson U., Tracey K.J. Reflex principles of immunological homeostasis. Annu Rev Immunol. 2012;30:313–335. doi: 10.1146/annurev-immunol-020711-075015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Andersson U., Tracey K.J. Neural reflexes in inflammation and immunity. J Exp Med. 2012;209(6):1057–1068. doi: 10.1084/jem.20120571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gallowitsch-Puerta M., Tracey K.J. Immunologic role of the cholinergic anti-inflammatory pathway and the nicotinic acetylcholine alpha 7 receptor. Ann N Y Acad Sci. 2005;1062:209–219. doi: 10.1196/annals.1358.024. [DOI] [PubMed] [Google Scholar]
- 149.Li F., Chen Z., Pan Q., Fu S., Lin F., Ren H., et al. The protective effect of PNU-282987, a selective α7 nicotinic acetylcholine receptor agonist, on the hepatic ischemia-reperfusion injury is associated with the inhibition of high-mobility group box 1 protein expression and nuclear factor κB activation in mice. Shock. 2013;39(2):197–203. doi: 10.1097/SHK.0b013e31827aa1f6. [DOI] [PubMed] [Google Scholar]
- 150.Pavlov V.A., Ochani M., Yang L.H., Gallowitsch-Puerta M., Ochani K., Lin X., et al. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med. 2007;35(4):1139–1144. doi: 10.1097/01.CCM.0000259381.56526.96. [DOI] [PubMed] [Google Scholar]
- 151.Wang H., Liao H., Ochani M., Justiniani M., Lin X., Yang L., et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10(11):1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 152.Wang Q., Wang F., Li X., Yang Q., Li X., Xu N., et al. Electroacupuncture pretreatment attenuates cerebral ischemic injury through α7 nicotinic acetylcholine receptor-mediated inhibition of high-mobility group box 1 release in rats. J Neuroinflammation. 2012;9:24. doi: 10.1186/1742-2094-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang Z., Hou L., Yang H., Ge J., Wang S., Tian W., et al. Electroacupuncture pretreatment attenuates acute lung injury through α7 nicotinic acetylcholine receptor-mediated inhibition of HMGB1 release in rats after cardiopulmonary bypass. Shock. 2018;50(3):351–359. doi: 10.1097/SHK.0000000000001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang Z., Liu T., Yin C., Li Y., Gao F., Yu L., et al. Electroacupuncture pretreatment ameliorates anesthesia and surgery-induced cognitive dysfunction via activation of an α7-nAChR signal in aged rats. Neuropsychiatr Dis Treat. 2021;17:2599–2611. doi: 10.2147/NDT.S322047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zi S.F., Li J.H., Liu L., Deng C., Ao X., Chen D.D., et al. Dexmedetomidine-mediated protection against septic liver injury depends on TLR4/MyD88/NF-κB signaling downregulation partly via cholinergic anti-inflammatory mechanisms. Int Immunopharmacol. 2019;76 doi: 10.1016/j.intimp.2019.105898. [DOI] [PubMed] [Google Scholar]
- 156.Li D.J., Huang F., Ni M., Fu H., Zhang L.S., Shen F.M. α7 nicotinic acetylcholine receptor relieves angiotensin II-induced senescence in vascular smooth muscle cells by raising nicotinamide adenine dinucleotide-dependent SIRT1 activity. Arterioscler Thromb Vasc Biol. 2016;36(8):1566–1576. doi: 10.1161/ATVBAHA.116.307157. [DOI] [PubMed] [Google Scholar]
- 157.Koopman F.A., Chavan S.S., Miljko S., Grazio S., Sokolovic S., Schuurman P.R., et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2016;113(29):8284–8289. doi: 10.1073/pnas.1605635113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bonaz B., Sinniger V., Hoffmann D., Clarençon D., Mathieu N., Dantzer C., et al. Chronic vagus nerve stimulation in Crohn's disease: a 6-month follow-up pilot study. Neurogastroenterol Motil. 2016;28(6):948–953. doi: 10.1111/nmo.12792. [DOI] [PubMed] [Google Scholar]
- 159.Pavlov V.A., Chavan S.S., Tracey K.J. Molecular and functional neuroscience in immunity. Annu Rev Immunol. 2018;36:783–812. doi: 10.1146/annurev-immunol-042617-053158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pavlov V.A., Chavan S.S., Tracey K.J. Bioelectronic medicine: from preclinical studies on the inflammatory reflex to new approaches in disease diagnosis and treatment. Cold Spring Harb Perspect Med. 2020;10(3) doi: 10.1101/cshperspect.a034140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Aranow C., Atish-Fregoso Y., Lesser M., Mackay M., Anderson E., Chavan S., et al. Transcutaneous auricular vagus nerve stimulation reduces pain and fatigue in patients with systemic lupus erythematosus: a randomised, double-blind, sham-controlled pilot trial. Ann Rheum Dis. 2021;80(2):203–208. doi: 10.1136/annrheumdis-2020-217872. [DOI] [PubMed] [Google Scholar]
- 162.Hong G.S., Zillekens A., Schneiker B., Pantelis D., de Jonge W.J., Schaefer N., et al. Non-invasive transcutaneous auricular vagus nerve stimulation prevents postoperative ileus and endotoxemia in mice. Neurogastroenterol Motil. 2019;31(3):e13501. doi: 10.1111/nmo.13501. [DOI] [PubMed] [Google Scholar]
- 163.Huston J.M., Gallowitsch-Puerta M., Ochani M., Ochani K., Yuan R., Rosas-Ballina M., et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med. 2007;35(12):2762–2768. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]