Abstract
Background
Certain classes of multiple sclerosis (MS) disease modifying therapies (DMTs) have been associated with an increased risk of severe COVID-19, resulting in prescribers considering changes in their practice habits during the COVID-19 pandemic. This study assessed for differences in prescribing patterns of DMTs along with the reason(s) for modification of therapy over time.
Methods
A retrospective review of medical records at Johns Hopkins Health System was performed. The timeframe of the study, April 2019 to December 2021, was divided into three subcategories: pre-pandemic (April 2019–March 2020), pre-vaccine availability (April 2020–March 2021), and post-vaccine availability (April 2021–December 2021). Patients were identified through dispense reports from the pharmacy dispensing system, and prescribing report from the health-system electronic health record (EHR). The health-system EHR was also utilized to conduct chart reviews for a subset of patients that had a modification in their therapy during the specified timeframes. The study included adult patients that were prescribed at least one DMT through the Johns Hopkins Pharmacy Services during the study timeframe and those who stayed on their DMT for at least 2 months without tolerability issues. Descriptive statistics were used to compare the prescribing practices during the timeframes with the percentage of prescribing for each type of treatment and to assess the percentage of patients that switched therapies in the different time periods.
Results
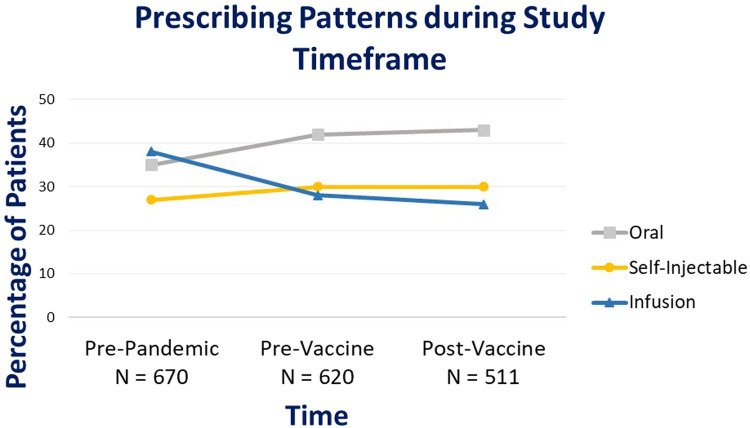
Based on prescribing report data, 670 patients were prescribed a DMT during the pre-pandemic period with infusion therapies being the most prescribed therapies during this timeframe (38%), followed by oral therapies at 35%. In comparison, a total of 620 patients were prescribed a DMT during pre-vaccine pandemic and the percentage of prescriptions of infusion therapies decreased to 28% (-10%) during this timeframe, whereas oral prescriptions increased to 42% (+7%). These trends continued during the post-vaccine timeframe where infusion therapies decreased to 26% (-12%) and oral therapies increased to 43% (+8%) in reference to the pre-pandemic period. Prescribing patterns of self-injectable therapies remained stable throughout the 3 timeframes. A dispensing report cohort of 500 patients were randomly selected for chart reviews to assess therapy modifications due to COVID-19. The percentage of therapy change due to COVID-19 increased to 45.2% during pre-vaccine period and remained at 38.4% during post-vaccine period when compared to the pre-pandemic reference period. The majority of changes due to COVID-19 were delays in infusion therapies (96% during pre-vaccine, and 94% during post-vaccine), not medication changes.
Conclusion
Prescribing patterns and therapy modifications of DMTs for MS patients were impacted by COVID-19, with the greatest changes observed for the infusion therapies, including reduction in percentage of infusion prescriptions and delays in infusion therapies. Prescribing patterns of lower efficacy self-injectable therapies (interferon-beta and glatiramer acetate) remained stable. The outcomes of this study provide background for future outcomes-focused research studies in MS.
Keywords: Multiple sclerosis, Disease modifying therapies, COVID-19
1. Introduction
1.1. Background
Multiple Sclerosis (MS) is the most common immune-mediated demyelinating disease of the central nervous system (CNS) in young adults. (Weiner, 2004) The clinical presentation is heterogeneous and histopathologically there is an admixture of inflammation, demyelination, axonal injury, and neurodegeneration. The adaptive and innate immune systems, which are composed of immune cells (e.g., T cells, B cells, myeloid cells, and other immune cell populations) contribute to lesions within the CNS and disrupt the neuronal pathways and the blood-brain-barrier. (Weiner, 2004; Compston and Coles, 2008) MS disease-modifying therapies (DMTs) target different aspects of the altered immune system in order to help prevent new inflammatory activity and ensuing disability. The current era of DMTs has proven to be effective in preventing new MS disease activity; however, many of these therapies increase the risk of infections, infection-related hospitalizations, and mortality. (Wijnands et al., 2017)
Coronavirus disease 2019 (COVID-19) is a severe acute respiratory syndrome caused by a novel coronavirus (SARS-CoV-2), which was first detected in Wuhan, China, in December 2019 and then spread to the entire world, leading to the declaration of this outbreak as a pandemic by World Health Organization (WHO) in March 2020. COVID-19 has resulted in a great deal of uncertainty for the MS patient population and their clinicians as immunosuppression is reported to be a risk factor for COVID-19 by the US Centers for Disease Control and Prevention (CDC). (Wijnands et al., 2017)
Several studies have revealed that the major risk factors of severe COVID-19 in patients with MS are the same as in the general population, including age, medical comorbidities, black race, etc. (Salter et al., 1; Sormani, 2020; Barzegar et al., 2021; Preziosa et al., 2020) However, there continues to be concern surrounding the safety of DMTs in the ongoing COVID-19 pandemic. Several recent studies have identified therapies targeting CD20 – ocrelizumab and rituximab – may be linked to an increased chance of severe COVID-19 compared to other DMTs. (Salter et al., 1; Hartung et al., 2021; Reder et al., 2021 Mar)
Several surveys have been conducted to understand the prescribers’ perception and changes in clinical practice of MS due to COVID-19. Mateen and colleagues conducted a survey study with 250 US- and Canada-based neurologists that showed that 94% of the respondents believed their MS patients were at a higher risk of acquiring COVID-19, in comparison to the general population. Most prescribers agreed to avoid prescribing high efficacy agents during COVID-19: alemtuzumab (61%), cladribine (47%), ocrelizumab (38%), rituximab (35%), and natalizumab (34%). Lower efficacy oral and self-injectable DMTs such as glatiramer acetate, interferons, and dimethyl fumarate were considered safe DMTs by many respondents. (Mateen et al., 2020)
In our study, we explored how COVID-19 has altered prescribing patterns of MS DMTs (in general) at a large academic institution along with evaluate the specific changes in DMTs, and assess the reason for therapy modification during pre-pandemic, pre-vaccine pandemic, and post-vaccine pandemic timeframes.
2. Materials and methods
2.1. Study design
This observational study involved a retrospective review of medical records at Johns Hopkins Health System (JHHS) between April 2019 – December 2021. The study period was divided into 3 different timeframes: pre-pandemic (April 2019 – March 2020), pre-vaccine availability (April 2020 – March 2021), and post-vaccine availability (April 2021 – December 2021). The study was reviewed by the Johns Hopkins Medicine Institutional Review Board and deemed a quality assurance/ quality improvement project, and exempt from review.
2.2. Study participants
The inclusion criteria for the study included adult patients (18 years or older) with relapsing and progressive MS that were prescribed at least one DMT at Johns Hopkins Pharmacy Services (JHPS), including Johns Hopkins Outpatient Pharmacies (JHOP) and Johns Hopkins infusion services (JHIS-Green Spring Station and Howard County), during the study timeframe. Patients were required to have been on their DMT for at least 2 months without tolerability issues.
Patients who had prescriptions sent to JHPS from non-Johns Hopkins providers were excluded from this study as well as DMTs prescribed for a non-MS diagnosis. Additionally, patients that had a DMT modification due to pregnancy/ breastfeeding or those who were lost to follow up 2 months after initial DMT were excluded from the study.
2.3. Data collection
Two sets of reports were utilized for data collection: prescribing reports and dispensing reports.
The purpose of prescribing reports was to assess the total number of prescriptions that were prescribed by the JH neurology providers and sent to JHPS during the pre-pandemic, pre-vaccine, and post-vaccine time periods. Prescribing reports were utilized to understand the overall prescribing patterns of DMTs that were sent to JHPS but did not confirm that the medications sent to JHPS were eventually dispensed from the pharmacy. Prescribing data were collected through 3 different prescribing reports utilizing Epic Hyperspace, pharmacy dispensing system (EnterpriseRx), and infusion dispensing system (HomeCare 360).
The purpose of dispensing reports was to identify DMT prescriptions that were sent to JHPS and also dispensed from the pharmacy dispensing systems. These reports allowed the authors to conduct detailed chart reviews for a convenient sample of 500 patients to obtain information such as type and reason for therapy modifications. Dispensing data were collected from EnterpriseRx and HomeCare 360 and combined into a single dispense report (See appendix 1 and 2). During the chart review process, reasons for therapy modification were divided into 3 categories: 1) clinical reason for therapy modification including clinical worsening (relapses, progression, new MRI lesions) or side effects/ intolerance; 2) therapy modification due to COVID-19 including COVID-19 pandemic and vaccine related concerns; 3) unknown/ other reasons for therapy modifications due to unclear or insurance issues. Additionally, the study assessed the types of therapy modifications that occurred, which were divided into 6 categories: 1) self-administered medication to infusion; 2) self-administered to self-administered; 3) infusion to self-administered; 4) infusion to infusion; 5) delay in therapy; 6) unknown/ other. Self-administered medications included oral and self-injectable DMTs and infusion therapies included only those administered within the JHIS.
2.4. Statistical analysis
Microsoft Excel (Redmond, Washington) was utilized for descriptive statistics to assess the changes in prescribing patterns and therapy modifications due to COVID-19.
3. Results
3.1. Changes in prescribing patterns
A total of 670 patients were prescribed a DMT during the pre-pandemic period. The infusion DMTs (ocrelizumab, rituximab, and natalizumab) were the highest prescribed medications (n = 256, 38%) compared to oral (35%) or self-injectable DMTs (27%). In comparison, 620 patients were prescribed a DMT during the pre-vaccine period and the percentage of infusion medications decreased to 28% (−10% compared to the pre-pandemic period). In the post-vaccine period, a total of 511 patients were prescribed a DMT and 26% of these prescriptions were infusion DMTs; a 12% decline compared to the pre-pandemic period. For self-administered DMTs including oral and self-injectable therapies, an overall increase in the number of prescriptions was observed from pre-pandemic to pre-vaccine and post-vaccine pandemic time periods (Fig. 1 ).
Fig. 1.
Prescribing patterns of DMTs during study timeframe.
3.2. Therapy modifications observed during chart reviews
As previously mentioned, a convenience sample of 500 patients was pulled from the dispensing reports to analyze the types of therapy modifications. A total of 398 patients met the inclusion criteria and the median age of these patients was 46 years with the majority being white females, with relapsing-remitting MS. Sixty-one percent of the patients were on infusion therapies as their baseline DMT (Table 1 ).
Table 1.
Patient Demographics and Clinical Characteristics.
| Patient Demographics | Total Patients (n = 398) |
|---|---|
| Median age – years (range) | 46 (18 – 81) |
| Female sex – no. of patients (%) | 299 (75) |
Race/ Ethnicity – no. of patients (%)
|
263 (66) 106 (27) 29 (7) |
| Patients with ≥ 1 comorbidities – no. of patients (%) | 142 (36) |
MS Subtype – no. of patients (%)
|
357 (90) 20 (5) 19 (5) |
Ambulation Status – no. of patients (%)
|
330 (83) 60 (15) 8 (2) |
Baseline DMTs
|
244 (61) 154 (39) |
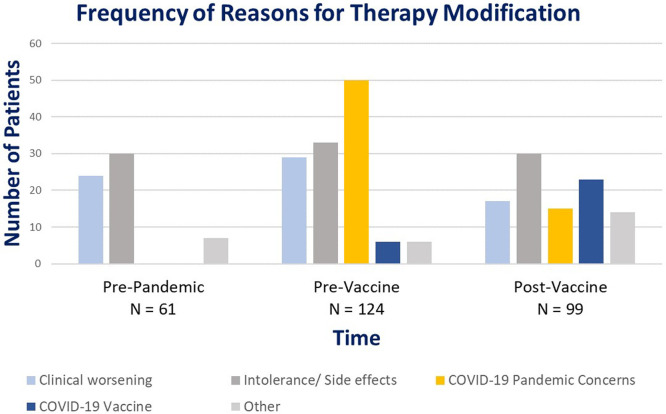
Out of the 398 patients included in chart reviews, 57% (225/398) had a therapy modification due to one of the following reasons: clinical worsening or side effects, COVID-19 related changes, or unknown/ other (Fig. 2 ). During the pre-pandemic period, none of the therapy modifications were COVID-19 related; therefore, this group served as the reference group. In comparison, out of the total 124 patients that had a change in their therapy during the pre-vaccine pandemic, 45% (56/124) had a therapy modification related to COVID-19. The majority of COVID-19 related therapy modifications during the pre-vaccine pandemic were due to COVID-19 pandemic related concerns (n = 50; 40%), followed by a small group of 6 patients (4%) with a change in therapy related to the COVID-19 vaccine. During the post-vaccine pandemic, a total of 99 patients had a therapy modification of which 38 patients (38%) had a therapy modification related to COVID-19. The majority of the COVID-19 related therapy modifications during the post-vaccine pandemic were due to the COVID-19 vaccine (n = 23; 23%) and there was a decrease in the number of patients with a therapy modification due to pandemic-related concerns (n = 15; 15%) during this period (Table 2 ).
Fig. 2.
Categorization of Reasons for Therapy Modification by Time Period.
Table 2.
COVID-19 Related Therapy Modifications.
| Therapy Modifications* | No. Of patients (%) |
|---|---|
Pre-Pandemic (n = 61)
|
0 (0) 0 (0) 0 (0) |
Pre-Vaccine Pandemic (n*=124)
|
56 (45) 50 (89) 6 (11) |
Post-Vaccine Pandemic (n*=99)
|
38 (38) 15 (39) 23 (61) |
N=total number of therapy modifications in each time period.
The convenience sample of 500 patients from dispensing reports was also utilized to assess the types of therapy modification due to COVID-19. As expected, the pre-pandemic group, which served as the reference time, did not have any therapy modifications due to COVID-19. During the pre-vaccine pandemic time period, a total of 56 patients had a therapy modification due to COVID-19, the majority of which were due to a delay in their therapy (96%). Delays in therapy were seen with infusion medications where patients that were scheduled for their next dose had to delay their dose by several months due to COVID-19 related concerns such as patient/ prescriber hesitancy to come to infusion centers, fear of exposure to COVID-19, change in infusion dosing frequency from standard to extended dose intervals, and change in dosing schedule due to COVID-19 vaccine. Similarly, 36 (95%) out of the 38 patients during the post-vaccine pandemic had a delay in therapy, followed by 2 patients (5%) who switched from an infusion DMT to a self-administered medication due to COVID-19 (Table 3 ). The delay in infusion therapy was most often seen with ocrelizumab and rituximab.
Table 3.
Types of Therapy Modification due to COVID-19.
| No. Of COVID-19 Related Therapy Modifications | Types of Therapy Modifications due to COVID-19 – No. Of pts (%) |
| Pre-Pandemic (n = 0) | None |
| Pre-Vaccine (n = 56) |
|
| Post-Vaccine (n = 38) |
|
4. Discussion
Based on this retrospective study assessing the changes in DMTs due to COVID-19 within one health system, it was observed that the pandemic mostly impacted the prescribing patterns of higher efficacy, anti-CD20 B cell depleting infusion therapies. There was a decrease in the number of infusion medications prescribed from the pre- to post-vaccine time period. In contrast, lower risk self-administered DMTs, including oral and self-injectable medications, saw a consistent increase in the number of prescriptions throughout the study timeframe. These results align with the results of a study conducted in England by Williams and colleagues, which also reported a reduction in prescribing of high-efficacy monoclonal antibody therapies during the pandemic whereas the self-administered medications showed a consistent upward trend. (Williams et al., 2022) In our study, patients who were already on a DMT, therapy modifications were primarily delays in treatment administration and not true medication changes.
Several COVID-19 related factors could have contributed to the prescribing changes and therapy modifications of infusion therapies in this study. It was observed that 2–3 months of delays in infusion therapies during the initial months of the pandemic occurred due to uncertainty regarding the impact of DMTs, particularly the anti-CD20 agents, on the severity of COVID-19. During the post-vaccine pandemic period, the majority of the delays in therapies were due to COVID-19 vaccines as patients and prescribers worked the DMT doses around COVID-19 vaccine schedules. This delay in therapy was seen due to concerns about certain DMTs such as anti-CD20 medications having an impact on vaccine response. Patients with fewer risk factors for MS disease activity such as older age, fewer comorbidities, and longitudinal stability on a DMT (clinically and radiologically) were switched from infusion therapies standard dosing to extended interval dosing due to uncertainty and limited knowledge of risk versus benefits of these medications during the pandemic.
A factor independent of the COVID-19 pandemic that may have also influenced the prescribing patterns of DMTs was the approval of ofatumumab, a self-injectable anti-CD20 DMT, which received FDA approval during the study timeframe. Some clinicians feel that this medication has comparable efficacy as ocrelizumab, but it is unknown whether there is a similar COVID-19 risk as other anti-CD20 DMTs. It does offer easy dose administration and eliminates the need to go to an infusion center, unlike infusion therapies. (Ofatumumab 2020; Cross et al., 2022) This study revealed an increase in the number of ofatumumab prescriptions from pre-vaccine pandemic to post-vaccine pandemic, making it a confounding factor that could have contributed to the reduction of infusion prescriptions observed during the ongoing pandemic.
Several limitations were identified in the current study. First, this was a retrospective study from a single health system. Second, the study included only patients getting their DMTs through JHPS which only accounts for approximately 15% of the prescriptions written by JH MS neurology providers. However, detailed pharmacy and DMT related information could be gathered with such approach. Lastly, the study does not capture ongoing post-COVID-19 vaccine-related changes in DMTs as more patients continue to get vaccinated.
5. Conclusion
The COVID-19 pandemic has resulted in uncertainty regarding the use of certain DMTs, especially the B cell depleting therapies due to their linkage to severe COVID-19 outcomes. Our study revealed that COVID-19 has impacted the overall prescribing rates of DMTs at JHHS as seen by the reduction in the prescriptions of infusion therapies during the pandemic. Additionally, the pandemic resulted in delays in therapies, all of which occurred with infusion medications, but not true medication changes. Further research is required to explore outcome-focused studies to assess the impact of delays in therapies or patients transitioning to extended-interval dosing of infusion medications on MS disease activity and/or disability. Lastly, ongoing studies will hopefully identify the full impact of DMTs on COVID-19 vaccine response.
CRediT authorship contribution statement
Reemal Zaheer: Methodology, Data curation, Formal analysis, Writing – original draft. Roma Amin: Writing – original draft, Data curation, Formal analysis, Writing – review & editing. LaTasha Riddick: Writing – original draft, Data curation, Formal analysis, Writing – review & editing. Shuvro Roy: Writing – original draft, Data curation, Formal analysis, Writing – review & editing. Sujin Wolff: Writing – original draft, Data curation, Formal analysis, Writing – review & editing. Amy Nathanson: Writing – original draft, Data curation, Formal analysis, Writing – review & editing. Scott Newsome: Writing – original draft, Data curation, Formal analysis, Writing – review & editing.
Declaration of Competing Interest
Dr. Newsome has received consultant fees for scientific advisory boards from Biogen, Genentech, Bristol Myers Squibb, EMD Serono, Greenwich Biosciences, Novartis, and Horizon Therapeutics, is an advisor for Autobahn, is the study lead PI for a Roche clinical trial, was a clinical adjudication committee member for a medDay Pharmaceuticals clinical trial, and has received research funding (paid directly to institution) from Biogen, Roche, Genentech, The Stiff Person Syndrome Research Foundation, National Multiple Sclerosis Society, Department of Defense, and Patient Centered Outcomes Institute.
Appendices
Appendix 1: Data Collection
| Demographic information | Patient age, gender, comorbidities, MS subtype, Race/ ethnicity, ambulation status |
|---|---|
| Comorbidities (Preziosa et al., 2020) |
|
| Ambulation Status Categories | 4 categories:
|
| MS Subtype Categories | 4 categories:
|
| Prescribing Information | Includes prescriber name at baseline and during therapy modification, and dispense dates |
Appendix 2: DMTs included in the study
| DMT Type | Medication Names |
|---|---|
| Oral |
|
| Self-injectable |
|
| Infusion |
|
| Not included in the study |
|
References
- Barzegar M., Mirmosayyeb O., Gajarzadeh M., et al. COVID-19 among patients with multiple sclerosis. Neurol. Neuroimmunol. Neuroinflammation. 2021;8(4):e1001. doi: 10.1212/NXI.0000000000001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A., Coles A. Multiple sclerosis. Lancet. 2008;372:152. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Cross A.H., Delgado S., Habek M., et al. COVID-19 Outcomes and vaccination in people with relapsing multiple sclerosis treated with of atumumab. Neurol. Ther. 2021;11:741–758. doi: 10.1007/s40120-022-00341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung H.P., Meuth S.G., Thompson A.J. Paradigm shifts: early initiation of high-efficacy disease-modifying treatment in multiple sclerosis. Mult. Scler. 2021;27:1473–1476. doi: 10.1177/13524585211033190. [DOI] [PubMed] [Google Scholar]
- Mateen F.J., Rezaei S., Alakel N., et al. Impact of COVID-19 on U.S. and Canadian neurologists’ therapeutic approach to multiple sclerosis: a survey of knowledge, attitudes, and practices. J. Neurol. 2020;267:3467–3475. doi: 10.1007/s00415-020-10045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofatumumab . East Hanover. Novartis Pharmaceuticals Corporation; NJ: 2020. [package insert] [Google Scholar]
- Preziosa P., Rocca M., Filippi M. COVID-19 will change MS care forever – No. Mult. Scler. J. 2020;26(10) doi: 10.1177/1352458520929971. 2020. [DOI] [PubMed] [Google Scholar]
- Reder A.T., et al. COVID-19 in patients with multiple sclerosis: associations with disease-modifying therapies. CNS Drugs. 2021;35(3):317–330. doi: 10.1007/s40263-021-00804-1. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter A., Fox R.J., Newsome S.D., Halper J., Li D.K.B., Kanellis P., Costello K., Bebo B., Rammohan K., Cutter G.R., Cross A.H. Outcomes and risk factors associated with SARS-CoV-2 infection in a north american registry of patients with multiple sclerosis. JAMA Neurol. 2021;78(6):699–708. doi: 10.1001/jamaneurol.2021.0688. Jun 1Erratum in: JAMA Neurol. 2021 Jun 1;78(6):765. PMID: 33739362; PMCID: PMC7980147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M. An italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol. 2020;19(6):481–482. doi: 10.1016/S1474-4422(20)30147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H.L. Multiple sclerosis is an inflammatory T-Cell–mediated autoimmune disease. Arch Neurol. 2004;61(10):1613–1615. doi: 10.1001/archneur.61.10.1613. [DOI] [PubMed] [Google Scholar]
- Wijnands J.M., Kingwell E., Zhu F., et al. Infection-related health care utilization among people with and without multiple sclerosis. Mult. Scler. J. 2017;23:1506–1516. doi: 10.1177/1352458516681198. [DOI] [PubMed] [Google Scholar]
- Williams T., Mishra R., Bharkhada B., et al. Impact of the COVID-19 pandemic on the prescription of disease-modifying therapy for multiple sclerosis in England: a nationwide study. J. Neurol. Neurosurg. Psychiatry. 2022 doi: 10.1136/jnnp-2021-328340. Published Online First10 March. [DOI] [PMC free article] [PubMed] [Google Scholar]