A growing and compelling body of evidence demonstrates that children born into poverty, whether in high-, middle-, or low-income countries,1 are at heightened risk for compromised health and developmental outcomes throughout the life course.2–6 It is estimated that 80.8 million children ages 3 and 4 years in low- and middle-income countries experienced low cognitive and/or socioemotional development in 2010 based on Early Childhood Development Index scores, with the largest number of affected children in sub-Saharan Africa, South Asia, and the East Asia and Pacific region.6 Recent evidence has also underscored the importance of interventions to foster healthy neurodevelopment from preconception through adolescence in light of findings that the early years of life are a sensitive period for countering adverse exposures that threaten the integrity of neural, neuroendocrine, and immune systems.7
Consequently, there is an increasing recognition by the global health community of the need to expand initiatives to address not only the ongoing need for reduced child mortality, but also to decrease child morbidity and adverse exposures toward improving health and developmental outcomes. The bridging of the child survival and child development fields has recently been prioritized by the World Health Organization (WHO) and other agencies as being essential for optimizing global health, equity, and sustainable development.8 In fact, a recent review found significant overlap between public health strategies for improving child survival and child development interventions, the latter of which typically include enriching learning components.9
The newly developed United Nations Sustainable Development Goals (SDGs) for 2015 to 2030 additionally reinforce that human development is essential for sustainable global development.10 In April 2016, an alliance was established by the World Bank and the United Nations Children’s Fund (UNICEF) to make gains on these SDGs by supporting country-led efforts to invest in nutrition, early stimulation, learning, and protection for every child.11 The WHO has also recommended several essential interventions worldwide for child survival, growth, and development.7,12 However, research is needed on the extent to which these interventions can be applied and/or adapted to diverse populations as well as implementation science on the optimal approaches for scaling up these interventions, particularly in low-resource settings (LRS).
Furthermore, for children living in poverty, there are a host of adverse exposures that dramatically increase the risk for altering the course of neurodevelopment. Paramount among these is the intersection between nutrition and inflammation (ie, acute and chronic), a hallmark of both infectious and noncommunicable diseases. Neurodevelopmental outcomes are of particular interest to the global community given their implications for long-term individual and population health and prosperity, as the current Zika virus outbreak demonstrates. Although some evidence exists linking pediatric neurodevelopment, nutrition, and inflammation, much remains to be learned about underlying mechanisms, biomarkers, assessment, interventions, and risk and protective factors, among other considerations. At the forefront, it is essential to develop tools for the assessment of neurodevelopment and the nutritional, inflammatory, and other influencing factors that impact it, that are readily deployable in the field, particularly in culturally diverse LRS.
To address these knowledge gaps and identify the next set of research priorities, a global health meeting was held by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) on February 11th and 12th, 2015 on the Bethesda, Maryland campus of the United States National Institutes of Health (NIH). This meeting, entitled “Research Gaps at the Intersection of Child Neurodevelopment, Nutrition, and Inflammation in Low-Resource Settings,” included 80 academic researchers and representatives from multiple organizations, including: several offices and institutes from the United States Department of Health and Human Services (HHS), including the NIH, HHS Office of Global Affairs, and United States Centers for Disease Control and Prevention (CDC); the United States Agency for International Development (USAID); National Academy of Sciences (NAS); the WHO; World Bank; the Bill and Melinda Gates Foundation (BMGF); Grand Challenges Canada; and the Sackler Institute for Nutrition Science at the New York Academy of Sciences.
The primary objective of this NICHD Global Health Meeting was to identify research priorities that are key to understanding the interrelationships and impact of nutritional status and inflammation due to specific infections or noncommunicable diseases on child neurodevelopment and brain function from fetal life through adolescence in LRS. Multidisciplinary panels, with experts in child neurodevelopment, nutrition, and infectious disease/inflammation, identified research gaps and assessment needs during 4 developmental periods: (1) pregnancy/fetal life, (2) infancy (birth to 2 years of age), (3) early to middle childhood (age 3 to 12 years), and (4) adolescence (age 13 to 18 years).
After these panel presentations, multidisciplinary working groups, 4 focused on targeted developmental periods and a fifth on assessment needs, were charged with responding to the 5 thematic areas in Table 1 and considering (1) existing knowledge, (2) evidence gaps, and (3) research priorities, as well as developing outlines for the articles contained in this supplement. The working groups were also encouraged to break down existing silos between disciplines in child neurodevelopment, nutrition, and inflammation (ie, due to infectious and noninfectious causes) to identify an integrated, multidisciplinary approach for conducting research, implementing programs, and developing policies.
TABLE 1.
Five Thematic Areas Addressed by NICHD Global Health Meeting Working Groups
| I. What is the existing knowledge and what are the evidence gaps and research priorities related to the impact of: |
| a. nutritional status on nerodevelopment and brain function? |
| b. inflammation on neurodevelopment and brain function? |
| c. interactions between inflammation and nutritional status on neurodevelopment and brain function? |
| d. protective factors (including environmental and behavioral), plasticity, and resilience on neurodevelopmental outcomes? |
| II. What underlying mechanisms help explain the complex interactions among neurodevelopment, inflammation, and nutritional status? |
| III. What types of measures, metrics, biomarkers, and tools are needed to evaluate the relationships of neurodevelopment/brain function, nutritional status, and inflammation? |
| IV. What is the potential impact of therapeutics that target inflammation (ie, the effects of nutrients and drugs considered individually as well as their interactions) on neurodevelopment/brain function during childhood? |
| V. Identify important considerations throughout the continuum from basic research and mechanisms to translational research and clinical care (including environmental, cultural, family, community, etc). |
During the second meeting day, a policy roundtable was held that focused on translational research, program implementation, and policy implications targeting child neurodevelopment, nutrition, and infectious diseases/inflammation. Roundtable participants included representatives from several domestic (HHS Office of Global Affairs, NIH, CDC, USAID, NAS) and international (WHO, World Bank, BMGF, Grand Challenges Canada, Sackler Institute) agencies that were supporting research, programs, or policies on key global health topics. There was general consensus among roundtable participants that it is important to maintain an ongoing dialogue between researchers, program implementers, and policy makers to ensure that research priority-setting is informed by current global health challenges in the field and, in turn, for research evidence to inform program and policy development. Furthermore, in light of the complex global health landscape and extensive needs in LRS, the value of public–private partnerships was underscored.
During the policy roundtable discussion, ideas for linking research evidence to program and policy development included: promoting research capacity building in LRS; pooling data for meta-analyses; conducting implementation science; facilitating open access; sharing research protocols, data, and plans; linking the results of cohort studies to well-framed policy statements; studying the effectiveness of integrated delivery of interventions; establishing professional standards of care; including economic analyses when framing research plans and findings; and developing guidelines for governments for selecting and scaling up evidence-based interventions.
This NICHD Global Health Meeting also aimed to build on research findings and related work of NIH-led interagency research platforms, which are referred to in several articles within this supplement. These include: the NICHD Biomarkers of Nutrition for Development (BOND) study13; the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) study14; the Inflammation and Nutritional Science for Programs/Policies and Interpretation of Research Evidence (INSPIRE) project15; the NICHD Global Network for Women’s and Children’s Research16; the Interactions of Malnutrition and Enteric Infections: Consequences for Child Health and Development (MAL-ED) study17; and the Fogarty International Center (FIC) Global Brain Disorders Program,18 among others. Working definitions for key science areas addressed in this journal supplement were extracted from and/or at least in part developed based on the work of these ongoing NIH-led research collaborations. A more detailed summary of definitions related to this journal supplement can be found in Table 1 of the article “Assessment of Neurodevelopment, Nutrition, and Inflammation From Fetal Life to Adolescence in Low-Resource Settings.”
For the purposes of this journal supplement, neurodevelopment is defined as the dynamic interrelationship between environment, genes, and the brain whereby the brain develops across time to establish sensory, motor, cognitive, socioemotional, cultural, and behavioral adaptive functions. This definition has been modified for this effort from an earlier version recently published in Nature.19 Nutrition is defined as the science of food, the nutrients and other substances therein, their action, interaction, and balance in relation to health and disease, and the processes of ingestion, absorption, use, and excretion.20 Furthermore, malnutrition has 3 principal constituents: undernutrition (ie, poor growth, including underweight, stunting, and wasting), overnutrition (ie, overweight/obesity), and deficiencies in micronutrients.20,21
Finally, both infection and inflammation are addressed within the articles of this journal supplement. The term “infection” describes the interaction between the action of microbial invasion and the reaction of the body’s inflammatory defensive response; the 2 components are considered together when discussing an infection, and the word infection is used to imply a microbial invasive cause for the observed inflammatory reaction.22 In contrast, inflammation is the stereotypical physiologic response to infections, tissue injury, psychological stress, and other insults.23,24
It is also important to distinguish between acute and chronic inflammation. Inflammation, as characterized by the acute phase response, is an innate body defense that triggers a sequence of physiologic changes in response to a myriad of stressors, including microbial invasion, tissue injury, immunologic reactions, and inflammatory processes.15 Inflammation is generally protective to the host because it removes injurious stimuli and promotes the healing of damaged tissue. However, chronic inflammation may result when there is overproduction of inflammatory mediators that may amplify the acute phase response on an ongoing basis and become detrimental to the host.23 For the purposes of this journal supplement, the term “inflammation” will include both infectious and noninfectious as well as acute and chronic causes of inflammation, unless otherwise specified.
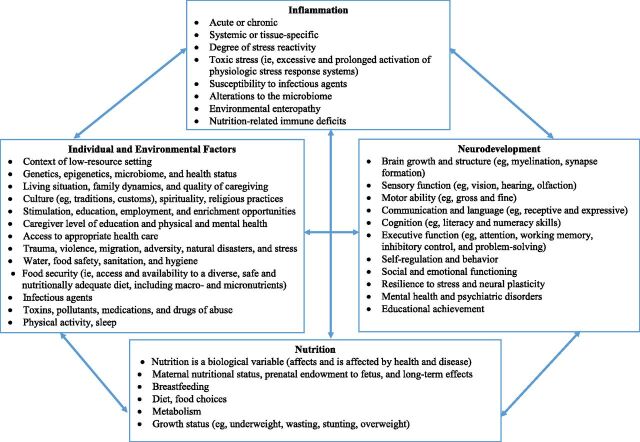
Figure 1 conceptualizes examples of individual and environmental factors on both nutrition and inflammation that, separately and collectively, affect child neurodevelopment, the focus of this overview. Examples of important variables include caregiver factors, such as education, physical and mental health, and the environment in which children grow up, including the effects of poverty, violence, toxic stress, pathogen and toxin exposure, and the level of nurturance, enrichment, and education provided to the child. It is important to note that factors affecting neurodevelopment are often bidirectional and include both direct and indirect effects, highlighting opportunities to evaluate interventions. A detailed review of all factors affecting child neurodevelopment is beyond the scope of this supplement, which will specifically focus on the interrelationships among nutrition, inflammation, and neurodevelopment, as described in more detail in subsequent supplement articles.
FIGURE 1.
Relationships among individual and environmental factors, inflammation, nutrition, and neurodevelopment.
Emerging Research Areas
This section summarizes emerging research areas at the intersection of child neurodevelopment, nutrition, and inflammation in LRS, first discussed at the aforementioned February 2015 NICHD Global Health Meeting and explored in more depth within the articles contained in this journal supplement. They are listed below in no particular order of priority.
Need for an integrated approach. Determine how nutritional and inflammatory status, independently and in relationship with each other, impact brain and neurodevelopment from preconception (including maternal and paternal factors) through gestation, infancy, childhood, adolescence, and beyond and what interventions can favorably alter these processes. Children living in impoverished settings are at risk for exposure to early and compounding adversities that dramatically increase the potential for altered neurodevelopment.2–6 Nutrition and inflammation play essential roles throughout early brain development, and, in the face of adversities, children may experience lasting deficits to their growth and development. For example, micronutrient deficiency, infection, and inflammation interact in complex ways. Micronutrient deficiency may predispose children to or protect them from infection or inflammation, but, conversely, inflammation and infection may lead to micronutrient deficiency. Over time, as a child is distanced from a specific insult or experiences ongoing adversities, environmental and contextual factors become even more important. Integrated interventions that combine nutrients with opportunities for responsive caregiving and early learning have been recommended,25 and evidence suggests that trials that include both early education and nutrition are more likely to result in cognitive benefits than single intervention trials,26 but relatively few integrated trials or programs have been evaluated systematically.27
New tools for neurodevelopmental assessment. Develop tools for the assessment of neurodevelopment (ie, birth to adolescence) that are field-friendly in LRS for diverse populations. There is a lack of standardized, validated neurodevelopmental assessment tools for children from birth through adolescence that are field-friendly in LRS for diverse populations. Currently available tools are relatively coarse behavioral measures (eg, developmental exams) that lack sensitivity to underlying neural mechanisms, cannot be used early in life given limited behavioral repertoires, and are largely developed in western, high-resource countries. Cutting-edge tools are needed, including brain imaging, which can provide a more direct assessment of the physical development of the brain and can be combined with behavioral measures for a more comprehensive assessment. Generally, neurocognitive assessment tools have included less emphasis on executive functions, such as socioemotional regulation, impulse control, and the ability to sustain attention,28 although these areas of neurodevelopment may be particularly affected by nutritional deficiencies or inflammatory responses.
Standardized norms for neurodevelopment. Establish whether there is a standardized “norm” by sex for neurodevelopment that is applicable across diverse populations. If it were determined that neurodevelopment unfolds in a universally consistent manner in girls and boys across cultural, socioeconomic, and geographic contexts, this would provide support for neurodevelopmental assessment measures developed in high-resource countries, in a valid and consistent manner, being reasonably adapted for use in LRS. However, if the ecological and cultural context or differences in biological factors overshadow the more universal dimensions of child neurodevelopment, then more time and effort would be required to develop local, context-specific neurodevelopmental measures. Toward developing standardized norms for neurodevelopment, a large-scale longitudinal study that tracks the neurodevelopment of “healthy” children in multiple countries is recommended.
Critical and sensitive periods. Build on recent advances in neurobiology related to critical and sensitive periods to determine whether they can be rescued later in life. Adverse environmental exposures are most deleterious to the developing brain when they occur during a sensitive or critical period of development.29,30 A sensitive period is a time in development during which the brain is particularly responsive to stimuli or insults followed by an extended period of ongoing responsiveness, but to a lesser degree (eg, language development); by contrast, a critical period refers to a time in development when the presence or absence of an experience results in irreversible change (eg, binocular vision). Although the young brain is resilient and demonstrates potential for recovery, early-life insults, particularly during sensitive or critical periods of development, can result in long-term deficits and adverse outcomes later in life.31 However, studies of resilience and recent advances in the neurobiology of sensitive periods may lead to new discoveries that enable the rescue of sensitive periods through targeted interventions later in life.32
Mild but diffuse deficits in neurodevelopment. Explore the functional significance of mild but diffuse deficits in neurodevelopment. Although reliable estimates are not readily available, the global burden of mild but diffuse neurodevelopmental deficits is likely to be substantial. Additional research is needed, including long-term follow-up studies, to better understand etiologies and prognoses of mild deficits in neurodevelopment toward developing effective preventive and therapeutic interventions (eg, behavioral and family programs, trauma care, educational initiatives).
More precise biomarkers for nutrition and inflammation. Identify field-friendly, standardized, and cost-effective biomarkers for nutritional, inflammatory, and other influencing factors linked to specific health outcomes across the life span. There is a need for the development of low-cost, point-of-care, accurate diagnostics for nutrition, infection, and inflammation in LRS. A biomarker is a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.33 Although biomarkers may have biological use, their measures should ideally also be of use in clinical or programmatic settings. There is a need for field-friendly, age-standardized norms for nutritional and inflammatory biomarkers. Ideally, nutrient-driven alterations in brain function that are measureable (ie, signature effects that may vary by nutrient and age of the subject) should be determined. Better markers of inflammation, tissue injury, and host response, including noninvasive surrogate markers of central nervous system (CNS) infection, inflammation, and injury are needed.
Impact of malnutrition (ie, undernutrition, overnutrition, and micronutrient deficiency) on neurodevelopment. Characterize the magnitude and duration of the effects of nutritional status on neurodevelopmental outcomes. The vulnerability of a brain region to a nutrient deficit will depend on the timing of the event, based on the region’s requirement for the nutrient at that time.31 This basic principle exists from conception through the end of brain development, but is particularly accentuated during periods of rapid brain growth and differentiation. Poor nutrition and growth (ie, intrauterine growth restriction, small for gestational age, and stunting) are associated with impaired neurodevelopmental outcomes.3,34–37 Moreover, most of the research linking nutrition with inflammation and neurodevelopment focuses on undernutrition and overnutrition, including maternal and childhood obesity, that have also become a global concern. Obesity begins early in life, increases during childhood and adolescence, and has been associated with impaired cognition.38 Possible mechanisms include inflammation, oxidative stress, decreased motor performance associated with a degraded musculoskeletal system, and alterations in brain structure, leptin/insulin regulation, cerebrovascular function, and/or blood-brain barrier function.
Infections, inflammation, and neurodevelopment. Elucidate the role of and mechanisms through which infectious diseases and inflammation impact neurodevelopment. A recent review of infections that affect the nervous system noted that, for most pathogens, reliable estimates of the incidence of infection and infection-related neurodevelopmental impairment are not currently available.39 The burden of neurodevelopmental impairment from specific infectious agents and/or the accompanying inflammatory response, and how exposure at a given time point impacts later neurodevelopment, need to be explored. For infectious diseases and a variety of other early nutritional, inflammatory, or toxic environmental insults, duration and severity may be as important as timing. Infection may lead to neurodevelopmental impairment through direct CNS injury by the infectious pathogen or through pathways that may involve inflammation as 1 component, but may not be traditionally defined as inflammation.40
Noninfectious inflammation and neurodevelopment. Describe the role of and mechanisms through which noninfectious inflammation (eg, chronic diseases, exposure to environmental toxins, and psychological stress) impacts neurodevelopment. The evidence base for the direct impact of noninfectious inflammation on neurodevelopment is even more limited than for infectious causes of inflammation. Nonetheless, growing evidence indicates that a variety of early-life stresses can contribute to enduring abnormalities in brain organization and structure, as well as in endocrine regulatory processes, among other consequences.41–44 These life stressors contribute to a physiologic response referred to as toxic stress by the National Scientific Council on the Developing Child, defined as the excessive or prolonged activation of the physiologic stress response systems in the absence of the buffering protection afforded by stable, responsive relationships, and the result of cumulative adverse childhood experiences. Animal and human studies have found associations between early-life adversity and toxic stress to changes in brain architecture and gene expression, potentially resulting in long-term and even intergenerational physical and mental health consequences. Importantly, toxic stress is most deleterious to the developing brain when it occurs during a sensitive or critical period of development and may have lifelong effects. Additionally, longitudinal studies have shown that early malnutrition has effects on inflammatory status in adulthood.
Environmental enteropathy. Define the pathways by which environmental enteropathy leads to neurodevelopmental impairments and interacts with the microbiome. Children in LRS are at higher risk for environmental enteropathy caused by fecal–oral contamination, which results in the blunting of intestinal villi and inflammation.45 This can lead to chronic intestinal problems with absorbing nutrients, which may result in malnutrition, growth stunting, and other developmental deficits in children.46 Environmental enteropathy, involving intestinal inflammation without overt diarrhea, also seems to affect the risk of both malnutrition and impaired child neurodevelopment.45 In fact, environmental enteropathy may contribute to the failure of nutritional interventions and oral vaccines in LRS. Furthermore, environmental enteropathy and alterations in the microbiome interact to increase microbial translocation and cause inflammation in the CNS in animals47–49 and humans,49,50 as evidenced by findings that markers in gut inflammation are associated with schizophrenia and other psychiatric disorders.51–53
Microbiome. Identify microbiome profiles associated with improved micronutrient bioavailability, inflammatory status, functional neurodevelopmental outcomes, and psychiatric disorders. We need improved understanding of the role of the microbiome along the life span, including prenatal life, on child neurodevelopment. Research has shown that inflammation and malnutrition can alter the microbiome, and, conversely, that the microbiome can affect both nutrition and systemic inflammation.54 Changes in the microbiome have been hypothesized to potentially influence child neurodevelopment and behavior through the “microbiome-gut–brain axis.”55 Alterations in the microbiome are very likely to occur in environmental enteropathy and indeed might be part of the pathogenesis. Future research studies should assess how both conditions may affect child neurodevelopment, how each condition affects the other, and how inflammation, malnutrition, and micronutrient deficiency contribute to and interact with the microbiome and environmental enteropathy.
Epigenetic mechanisms and intergenerational effects. Understand epigenetic mechanisms and potential intergenerational effects whereby adverse life experiences are biologically embedded to tailor interventions to specific neural and behavioral systems. Areas in which there is interesting preliminary data, but for which additional studies are needed, include the role of genetic factors in terms of susceptibility to infections as well as the role of infections, nutrition, stress, and other factors in modulating gene expression through epigenetic changes in DNA, including the role of DNA methylation53 and modifications of DNA binding proteins, such as histones.56,57 The Barbados Nutrition Study is elucidating some intergenerational effects of infant malnutrition due to DNA methylation.56 Epigenetic changes have been found in the first and second generation of study participants and the imprinted genes are seen in both generations. The gut microbiome in pregnancy also presents an epigenetic pattern that affects inflammatory trajectories. Malnutrition, the microbiome, and inflammation can all lead to epigenetic changes that can be transmitted to subsequent generations.
Sleep, immune function, and neurodevelopment. Determine how sleep deprivation or altered sleep schedules (circadian rhythm) affect immune function, inflammation, and neurodevelopmental outcomes. The importance of the relationship between healthy sleep duration and quality and neurodevelopment is increasingly being recognized across the life span.58,59 Sleep represents an important modifiable risk factor that can impact neurodevelopment and overall health or chronic disease progression, including gene expression.60 Populations in LRS may be disproportionately affected by poor sleep due to various environmental and cultural factors, such as noise, hunger, insect or rodent infestation, or psychological stress. Poor sleep parameters may also exert a role on neurodevelopment through alterations in nutrient use, immune function, and inflammation, particularly during sensitive or critical periods of brain development. A better understanding of these mechanisms and interactions is necessary to develop and target evidence-based interventions.
Resiliency and protective factors. Identify exposures, influences, and interventions that contribute to resiliency. The chronic exposure of millions of children to early childhood malnutrition, infections, and environmental insults creates an even more urgent need for studies of resilience and protective factors in these settings. This research needs to include longitudinal studies and data collection at the individual, family, and societal levels. The sex of the child and ethnic or cultural identity also need to be considered because they may modify the benefits attributable to any protective factor. In addition, modern genetic, epigenetic, and neuroimaging techniques may help us to better identify unique variability and characteristics of individuals displaying greater resilience and change the concepts of “critical and sensitive periods” and have implications for interventions. Furthermore, because nearly one-third of all women globally undergo pregnancy during their adolescent years, interventions during this period of life not only impact the adolescents themselves, but also have substantial effects on the well-being of the next generation.
Summary and Next Steps
The subsequent articles in this supplement will additionally explore and develop the concepts and themes introduced in this executive summary from preconception through adolescence. An introductory article describes the current global child health context with an emphasis on neurodevelopment and the impact and interrelationships of nutrition and inflammation. A second article considers available assessment methodologies and tools in these 3 scientific areas. Four subsequent articles address specific developmental considerations for these topics during fetal life, infancy, childhood, and adolescence. Within each article, the existing knowledge, evidence gaps, and research priorities are explored.
Acknowledgments
The NICHD Global Health Meeting held on February 11th and 12th, 2015 was organized by the Office of Global Health and sponsored by the Office of the Director and several branches at NICHD, which included both financial support and valuable scientific input. The Office of Dietary Supplements in the NIH Office of the Director also made a generous financial contribution and had a representative at this NICHD Meeting.
During the second day of the NICHD Global Health Meeting and 2 subsequent conference calls, 5 working groups (ie, Assessment, Pregnancy/Fetal Development, Infancy, Early/Middle Childhood, and Adolescence) convened to brainstorm existing research evidence and knowledge gaps related to the intersection of child neurodevelopment, nutrition, and inflammation in low resource settings. These working groups were chaired by the panelists at the NICHD Global Health Meeting, who subsequently served as the lead and co-authors of the manuscripts in this supplement. We would like to acknowledge the valuable contribution of these working group members, who are listed in alphabetical order: Jere Behrman (University of Pennsylvania), Sharon Bergquist (BMGF), Robert Black (Johns Hopkins Bloomberg School of Public Health), Kimber Bogard (National Academy of Medicine), Pim Brouwers (NIMH/NIH), Germaine Buck Louis (NICHD/NIH), Rebecca Clark (NICHD/NIH), Cindy Davis (ODS/NIH), Tarun Dua (WHO), Linda Duffy (NCCIH/NIH), Eleanore Edson (BMGF), Henry Falk (CDC), Sarah Glavin (NICHD/NIH), Devasena Gnanashanmugam (NIAID/NIH), Lynne Haverkos (NICHD/NIH), Yiwu He (BMGF), Van Hubbard (NIDDK/NIH), Terrie Inder (Brigham and Women’s Hospital), Elizabeth Jordan-Bell (USAID), Alice Kau (NICHD/NIH), Patrick Kelley (National Academies of Sciences, Engineering, and Medicine), Marion Koso-Thomas (NICHD/NIH), Danuta Krotoski (NICHD/NIH), Ty Lawson (OD/NIH), Anne ML Lee (Brigham and Women’s Hospital, Harvard Medical School), Sonia Lee (NICHD/NIH), Daniel Marks (BMGF), Mireille Seneclauze Mclean (Sackler Institute for Nutrition Science at the New York Academy of Sciences), Dominique McMahon (Grand Challenges Canada), Kathleen Michels (FIC/NIH), Jeffrey Murray (BMGF), Melissa Parisi (NICHD/NIH), Cristina Rabadan-Diehl (HHS), Tonse Raju (NICHD/NIH), Zeba Rasmussen (FIC/NIH), Dianne Rausch (NIMH/NIH), Rebecca Scharf (University of Virginia Children’s Hospital), Meera Shekar (World Bank), George Siberry (NICHD/NIH), Caroline Signore (NICHD/NIH), Donald Silberberg (University of Pennsylvania Medical Center), Rachel Sturke (FIC/NIH), David Weinberg (NICHD/NIH), Linda Wright (NICHD/NIH), Edwina Yeung (NICHD/NIH).
We thank Ty Lawson and Rosalina Bray at NICHD/NIH for their assistance with meeting preparations for the February 2015 NICHD Global Health Meeting; Annie E. Berens at the Harvard Medical School for her development of the "Early Adversity Causal Model" included in the article, "Neurodevelopment, Nutrition, and Inflammation: The Evolving Global Child Health Landscape"; and Arielle G. Rabinowitz at the Yale School of Medicine and Rebecca S. Hock at the Massachusetts General Hospital and Harvard Medical School for their assistance with manuscript preparation.
Glossary
- BMGF
Bill and Melinda Gates Foundation
- CDC
United States Centers for Disease Control and Prevention
- CNS
central nervous system
- FIC
John E. Fogarty International Center
- HHS
United States Department of Health and Human Services
- LRS
low-resource setting
- NAS
National Academy of Sciences
- NICHD
Eunice Kennedy Shriver National Institute of Child Health and Human Development
- NIH
United States National Institutes of Health
- SDGs
United Nations Sustainable Development Goals
- UNICEF
United Nations Children's Fund
- USAID
United States Agency for International Development
- WHO
World Health Organization
Footnotes
Dr Kutlesic conceptualized the article, coordinated the author team, compiled and drafted the initial manuscript, and drafted the final manuscript; Dr Brewinski Isaacs conceptualized the article, helped coordinate the author team, helped compile and draft the initial manuscript, and revised the final manuscript; Drs Freund, Hazra, and Raiten conceptualized the article and revised the initial and final manuscripts; and all authors approved the final manuscript as submitted and are accountable for all aspects of the work.
FUNDING: This supplement was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) at the United States National Institutes of Health (NIH).
Contributor Information
Vesna Kutlesic, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland.
Margaret Brewinski Isaacs, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland.
Lisa S. Freund, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland.
Rohan Hazra, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland.
Daniel J. Raiten, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland.
References
- 1.World Bank . How does the World Bank classify countries? Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/378834-how-does-the-world-bank-classify-countries. Accessed June 9, 2016
- 2.Evans GW. The environment of childhood poverty. Am Psychol. 2004;59(2):77–92 [DOI] [PubMed] [Google Scholar]
- 3.Walker SP, Wachs TD, Grantham-McGregor S, et al. Inequality in early childhood: risk and protective factors for early child development. Lancet. 2011;378(9799):1325–1338 [DOI] [PubMed] [Google Scholar]
- 4.Johnson SB, Riis JL, Noble KG. Poverty and the developing brain. Pediatrics. 2016;137(4):e20153075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gitterman BA, Flanagan PJ, Cotton WH, et al. ; Council on Community Pediatrics . Poverty and child health in the United States. Pediatrics. 2016;137(4):e20160339. [DOI] [PubMed] [Google Scholar]
- 6.McCoy DC, Peet ED, Ezzati M, et al. Early childhood developmental status in low- and middle-income countries: national, regional, and global prevalence estimates using predictive modeling. PLoS Med. 2016;13(6):e1002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wachs TD, Georgieff M, Cusick S, McEwen BS. Issues in the timing of integrated early interventions: contributions from nutrition, neuroscience, and psychological research. Ann N Y Acad Sci. 2014;1308:89–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan M. Linking child survival and child development for health, equity, and sustainable development. Lancet. 2013;381(9877):1514–1515 [DOI] [PubMed] [Google Scholar]
- 9.Shonkoff JP, Richter L, van der Gaag J, Bhutta ZA. An integrated scientific framework for child survival and early childhood development. Pediatrics. 2012;129(2). Available at: www.pediatrics.org/cgi/content/full/129/2/e460 [DOI] [PubMed] [Google Scholar]
- 10.United Nations . Transforming our world: the 2030 agenda for sustainable development. Available at: https://docs.google.com/gview?url=http://sustainabledevelopment.un.org/content/documents/21252030%20Agenda%20for%20Sustainable%20Development%20web.pdf&embedded=true. Accessed June 13, 2016
- 11.World Bank . World Bank and UNICEF alliance to advance early childhood development. Available at: http://sd.iisd.org/news/world-bank-unicef-establish-alliance-to-advance-early-childhood-development/. Accessed June 13, 2016
- 12.Partnership for Maternal, Newborn, and Child Health . Essential interventions, commodities and guidelines: a global review of the key interventions related to reproductive, maternal, newborn, and child health (RMNCH). Available at: www.who.int/pmnch/topics/part_publications/essential_interventions_18_01_2012.pdf. Accessed June 13, 2016
- 13.Raiten DJ, Namasté S, Brabin B, et al. Executive summary--biomarkers of nutrition for development: building a consensus. Am J Clin Nutr. 2011;94(2):633S–650S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suchdev PS, Namaste S, Aaron G, Raiten D, Brown KH, Flores-Ayala R; BRINDA Working Group . Overview of the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) Project. Adv Nutr. 2016;7(2):349–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raiten DJ, Sakr Ashour FA, Ross AC, et al. ; INSPIRE Consultative Group . Inflammation and Nutritional Science for Programs/Policies and Interpretation of Research Evidence (INSPIRE). J Nutr. 2015;145(5):1039S–1108S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eunice Kennedy Shriver National Institute of Child Health and Human Development . Global Network for Women’s and Children’s Health Research. Available at: www.nichd.nih.gov/research/supported/Pages/globalnetwork.aspx. Accessed June 7, 2016
- 17.MAL-ED Network Investigators . The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. Clin Infect Dis. 2014;59(4 suppl 4):S193–S206 [DOI] [PubMed] [Google Scholar]
- 18.Fogarty International Center, National Institutes of Health . Global brain disorders research. Available at: www.fic.nih.gov/Programs/Pages/Brain-Disorders.aspx. Accessed June 7, 2016
- 19.Boivin MJ, Kakooza AM, Warf BC, Davidson LL, Grigorenko EL. Reducing neurodevelopmental disorders and disability through research and interventions. Nature. 2015;527(7578):S155–S160 [DOI] [PubMed] [Google Scholar]
- 20.Lagua RT, Claudio VS. Nutrition and Diet Therapy Reference Dictionary. New York, NY: Chapman & Hall; 1995 [Google Scholar]
- 21.International Food Policy Research Institute . Global nutrition report 2014: actions and accountability to accelerate the world’s progress on nutrition. Available at: http://ebrary.ifpri.org/utils/getfile/collection/p15738coll2/id/128484/filename/128695.pdf. Accessed June 7, 2016 [DOI] [PMC free article] [PubMed]
- 22.Abbas AB, Lichtman AH. Innate Immunity. In: Abbas AB, Lichtman AH, eds. Basic Immunology: Functions and Disorders of the Immune System. Philadelphia, PA: Saunders/Elsevier; 2009 [Google Scholar]
- 23.Calder PC, Albers R, Antoine JM, et al. Inflammatory disease processes and interactions with nutrition. Br J Nutr. 2009;101(suppl 1):S1–S45 [DOI] [PubMed] [Google Scholar]
- 24.Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun. 2002;16(6):622–653 [DOI] [PubMed] [Google Scholar]
- 25.Black MM, Dewey KG. Promoting equity through integrated early child development and nutrition interventions. Ann N Y Acad Sci. 2014;1308:1–10 [DOI] [PubMed] [Google Scholar]
- 26.Nores M, Barnett WS. Benefits of early childhood interventions across the world: (under) investing in the very young. Econ Educ Rev. 2010;29(2):271–282 [Google Scholar]
- 27.Grantham-McGregor SM, Fernald LC, Kagawa RM, Walker S. Effects of integrated child development and nutrition interventions on child development and nutritional status. Ann N Y Acad Sci. 2014;1308:11–32 [DOI] [PubMed] [Google Scholar]
- 28.Gladstone M, Mallewa M, Alusine Jalloh A, et al. Assessment of neurodisability and malnutrition in children in Africa. Semin Pediatr Neurol. 2014;21(1):50–57 [DOI] [PubMed] [Google Scholar]
- 29.Bruer JT. A critical and sensitive period primer. In: Bailey DB, Bruer JT, Symons FJ, Lichtman JW, eds. Critical Thinking About Critical Periods. Baltimore, MD: Paul H. Brookes Publishing; 2001:3–26 [Google Scholar]
- 30.Fox SE, Levitt P, Nelson CA III. How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 2010;81(1):28–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cusick SE, Georgieff MK. The role of nutrition in brain development: the golden opportunity of the “first 1000 days.” J Pediatr. 2016;175:16–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hensch TK, Bilimoria PM. Re-opening windows: manipulating critical periods for brain development. Cerebrum. July–August 2012:11. [PMC free article] [PubMed] [Google Scholar]
- 33.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95 [DOI] [PubMed] [Google Scholar]
- 34.Pollitt E, Gorman KS. Nutritional deficiencies as developmental risk factors. In: Nelson CA III, ed. Threats to Optimal Development: Integrating Biological, Psychological, and Social Risk Factors. Hillsdale, NJ: Lawrence Erlbaum Associates, Publishers; 1994:121–144 [Google Scholar]
- 35.Levine TA, Grunau RE, McAuliffe FM, Pinnamaneni R, Foran A, Alderdice FA. Early childhood neurodevelopment after intrauterine growth restriction: a systematic review. Pediatrics. 2015;135(1):126–141 [DOI] [PubMed] [Google Scholar]
- 36.Nishimura T, Takei N, Tsuchiya KJ, Asano R, Mori N. Identification of neurodevelopmental trajectories in infancy and of risk factors affecting deviant development: a longitudinal birth cohort study. Int J Epidemiol. 2016;45(2):543–553 [DOI] [PubMed] [Google Scholar]
- 37.Prendergast AJ, Humphrey JH. The stunting syndrome in developing countries. Paediatr Int Child Health. 2014;34(4):250–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang C, Chan JS, Ren L, Yan JH. Obesity reduces cognitive and motor functions across the lifespan. Neural Plast. 2016;2016:2473081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.John CC, Carabin H, Montano SM, Bangirana P, Zunt JR, Peterson PK. Global research priorities for infections that affect the nervous system. Nature. 2015;527(7578):S178–S186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Idro R, Marsh K, John CC, Newton CR. Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr Res. 2010;68(4):267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shonkoff JP, Garner AS; Committee on Psychosocial Aspects of Child and Family Health; Committee on Early Childhood, Adoption, and Dependent Care; Section on Developmental and Behavioral Pediatrics . The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1). Available at: www.pediatrics.org/cgi/content/full/129/1/e232 [DOI] [PubMed] [Google Scholar]
- 42.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259 [DOI] [PubMed] [Google Scholar]
- 43.Center on the Developing Child at Harvard University . From best practices to breakthrough impacts: a science-based approach to building a more promising future for young children and families. Available at: http://developingchild.harvard.edu/resources/from-best-practices-to-breakthrough-impacts/. Accessed August 7, 2016
- 44.Cowan CSM, Callaghan BL, Kan JM, Richardson R. The lasting impact of early-life adversity on individuals and their descendants: potential mechanisms and hope for intervention. Genes Brain Behav. 2016;15(1):155–168 [DOI] [PubMed] [Google Scholar]
- 45.Gilmartin AA, Petri WA Jr. Exploring the role of environmental enteropathy in malnutrition, infant development and oral vaccine response. Philos Trans R Soc Lond B Biol Sci. 2015;370(1671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keusch GT, Rosenberg IH, Denno DM, et al. Implications of acquired environmental enteric dysfunction for growth and stunting in infants and children living in low- and middle-income countries. Food Nutr Bull. 2013;34(3):357–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood-brain barrier permeability in mice [published correction appears in Sci Transl Med. 2014;6(266):266er7]. Sci Transl Med. 2014;6(263):263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481 [DOI] [PubMed] [Google Scholar]
- 49.Blanton LV, Charbonneau MR, Salih T, et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. 2016;351(6275):aad3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keusch GT, Denno DM, Black RE, et al. Environmental enteric dysfunction: pathogenesis, diagnosis, and clinical consequences. Clin Infect Dis. 2014;59(suppl 4):S207–S212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nemani K, Hosseini Ghomi R, McCormick B, Fan X. Schizophrenia and the gut-brain axis. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:155–160 [DOI] [PubMed] [Google Scholar]
- 52.Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry. 2015;2(3):258–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sabunciyan S, Maher B, Bahn S, Dickerson F, Yolken RH. Association of DNA methylation with acute mania and inflammatory markers. PLoS One. 2015;10(7):e0132001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahmed T, Auble D, Berkley JA, et al. An evolving perspective about the origins of childhood undernutrition and nutritional interventions that includes the gut microbiome. Ann N Y Acad Sci. 2014;1332:22–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keunen K, van Elburg RM, van Bel F, Benders MJ. Impact of nutrition on brain development and its neuroprotective implications following preterm birth. Pediatr Res. 2015;77(1-2):148–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galler J, Rabinowitz DG. The intergenerational effects of early adversity. Prog Mol Biol Transl Sci. 2014;128:177–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bagot RC, Labonté B, Peña CJ, Nestler EJ. Epigenetic signaling in psychiatric disorders: stress and depression. Dialogues Clin Neurosci. 2014;16(3):281–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Colten HR, Altevogt BM. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington, DC: National Academies Press; 2006 [PubMed] [Google Scholar]
- 59.Galland BC, Taylor BJ, Elder DE, Herbison P. Normal sleep patterns in infants and children: a systematic review of observational studies. Sleep Med Rev. 2012;16(3):213–222 [DOI] [PubMed] [Google Scholar]
- 60.Luyster FS, Strollo PJ Jr, Zee PC, Walsh JK; Boards of Directors of the American Academy of Sleep Medicine and the Sleep Research Society . Sleep: a health imperative. Sleep. 2012;35(6):727–734 [DOI] [PMC free article] [PubMed] [Google Scholar]