Abstract
Background
Pain is commonly experienced by patients with inflammatory bowel disease (IBD). Unfortunately, pain management is a challenge in IBD care, as currently available analgesics are associated with adverse events. Our understanding of the impact of opioid use on healthcare utilization among IBD patients remains limited.
Methods
A systematic search was completed using PubMed, Embase, the Cochrane Library, and Scopus through May of 2020. The exposure of interest was any opioid medication prescribed by a healthcare provider. Outcomes included readmissions rate, hospitalization, hospital length of stay, healthcare costs, emergency department visits, outpatient visits, IBD-related surgeries, and IBD-related medication utilization. Meta-analysis was conducted on study outcomes reported in at least 4 studies using random-effects models to estimate pooled relative risk (RR) and 95% confidence interval (CI).
Results
We identified 1969 articles, of which 30 met inclusion criteria. Meta-analysis showed an association between opioid use and longer length of stay (mean difference, 2.25 days; 95% CI, 1.29-3.22), higher likelihood of prior IBD-related surgery (RR, 1.72; 95% CI, 1.32-2.25), and higher rates of biologic use (RR, 1.38; 95% CI, 1.13-1.68) but no difference in 30-day readmissions (RR, 1.17; 95% CI, 0.86-1.61), immunomodulator use (RR, 1.13; 95% CI, 0.89-1.44), or corticosteroid use (RR, 1.36; 95% CI, 0.88-2.10) in patients with IBD. On systematic review, opioid use was associated with increased hospitalizations, healthcare costs, emergency department visits, outpatient visits, and polypharmacy.
Discussion
Opioids use among patients with IBD is associated with increased healthcare utilization. Nonopioid alternatives are needed to reduce burden on the healthcare system and improve patient outcomes.
Keywords: Inflammatory Bowel Disease, Ulcerative Colitis, Crohn’s Disease, Opioids, Healthcare utilization
Introduction
Approximately 1.4 million Americans suffer from inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC).1 While the clinical presentation of IBD varies by disease extent and location, abdominal pain is common symptom in both patients with CD and UC.2 However, pain management presents a challenge in IBD, as available analgesics have significant potential for adverse events in this population. For example, analysis of a prospective cohort of over 6000 patients with CD in the TREAT (Therapy, Resource, Evaluation, and Assessment Tool) registry demonstrated increased mortality in patients taking chronic opioids.3 A similar mortality risk has also been identified in both patients with UC and CD who are prescribed high doses of opioids.4,5 Opioid use in patients with IBD has also been linked to increased risk of serious infection and opioid use disorder.3,6,7 Despite the negative safety profile, it is estimated that 21% of outpatients and 62% of inpatients with IBD are prescribed opioids at some point in their disease course.8
The cost of IBD care is primarily driven by hospitalizations, emergency department (ED) visits, surgeries, and pharmaceuticals.9 As the cost of managing IBD continues to rise, it is vital that we identify potential risk factors for high expenditure in the IBD population.10 Recent literature has established opioid use, along with psychiatric disorders, anemia, biologic use, corticosteroid use, and disease severity, as a risk factor for increased healthcare spending in patients with IBD.11–13 While opioid use may predict increased spending, it remains unclear which aspects of healthcare utilization are driving costs in patients with IBD who use opioids. Several studies have attempted to draw connections between opioid use and readmission rates, hospitalizations, length of stay (LOS), and IBD-related surgeries; however, results have been mixed. Furthermore, there is significantly variability in patient characteristics and in the definition of opioid use. Given the degree of variability between studies, a pooled analysis is needed to better understand this relationship between healthcare utilization and opioid use in patients with IBD. Therefore, we performed a systematic review and meta-analysis to determine if opioid use is associated with increased healthcare utilization among patients with IBD.
Methods
Study Eligibility
The aim of this study was to determine if prescription opioid use is a risk factor for high healthcare utilization in the IBD population. Studies that reported healthcare utilization outcomes in patients with IBD using opioids were eligible for inclusion. For the purposes of this review, any study documenting an opioid prescribed by a medical provider during the study period was included. This included opioids prescribed in the outpatient setting and during a single inpatient admission. Studies examining opioid use disorder were excluded from the main analysis; however, they were incorporated into the sensitivity analysis. The decision to exclude studies using opioid use disorder as a surrogate for opioid use was due to the fact that opioid use disorder does not necessarily represent active opioid or prescription opioid use. Randomized controlled trials, prospective and retrospective cohort studies, longitudinal, and case-control studies were eligible for inclusion. Case series and case reports were excluded due to low-quality methodology, as were conference abstracts due to incomplete data reporting.
Search Strategy
A systematic search of opioid use in IBD was performed by an experienced health sciences librarian (C.S.) using MEDLINE (PubMed), EMBASE, the Cochrane Library, and Scopus. No filters were used in the initial search to reduce the risk of bias. The initial search and included studies in any language, publication year, or journal. Both published and nonpublished (conference abstracts, oral presentations) were also included in the initial search. Findings were reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement, elaboration, and explanation and the Statement for Reporting Literature Searches for Systematic Reviews.14–16 All searches were completed by May 2020.
Outcomes
Healthcare utilization is defined as the “quantification or description of the individual use of health services for the purposes of disease prevention or cure,” and it is typically measured by the number of services in a given period of time divided by the population or as a total aggregate number.17 For the purposes of this study, we focused our primary outcomes on 30-day readmission rates, hospitalizations, LOS, total healthcare costs, ED visits, outpatient visits, polypharmacy, and use of private or public insurance. Secondary outcomes included IBD-related surgeries and medications as potential markers for disease severity. Surgeries included both a prior history of IBD-related surgery and surgeries that occurred during the study period. Medications included biologics (infliximab, adalimumab, certolizumab pegol, golimumab, vedolizumab, and ustekinumab), immunomodulators (azathioprine, 6-mercaptapurine, and methotrexate), steroids (prednisone, methylprednisolone, or budesonide), and small molecules (tofacitinib). Meta-analysis was conducted on outcomes that were reported in at least 4 studies. Studies were included in the systematic review if they were not eligible for inclusion in the meta-analysis but otherwise met the study inclusion criteria.
Study Selection and Data Extraction
Titles and abstracts were independently reviewed by J.L.S. and J.J. for study eligibility and data extraction. If study selection were discordant, the full text was reviewed, and consensus was reached via discussion between the 2 reviewers (J.L.S. and J.J.). A third reviewer, J.A.B., was available for adjudication if no consensus on inclusion could be reached.
Data extraction was independently performed by J.L.S., J.J., and L.G.-H. Study demographics, including author, year of publication, country of publication, study design, sample size, age range, IBD subtype, and definition of opioid use were extracted independently by JLS and L.G.-H. For discordant data, a consensus was reached by discussion between the 2 reviewers (J.L.S. and L.G.-H.). The remaining data were extracted by J.L.S. and J.J. using a customizable data extraction tool created in DistillerSR Literature Review Software version 2.34.1 (Evidence Partners, Ottawa, ON, Canada). For studies eligible for meta-analysis, the data extraction tool contained 2 × 2 tables used to collect raw data calculated from odds ratios (ORs) and relative risks (RRs). The data collection tool was also used to collect descriptions of the statistical findings for each of the systematic review outcomes (hospitalizations, ED visits, costs, insurance type, and polypharmacy). Discrepant data were re-evaluated by J.L.S. and J.J. and resolved via discussion between reviewers. Both E.M.B. and J.A.B. were available for adjudication if the discrepancy could not be resolved to consensus. We were unable to extract data from Anderson et al19 and Tinsley et al.29 The corresponding authors were contacted but the critical data were either unavailable or no response was received.
Risk of Bias Assessment and Sensitivity Analysis
A risk of bias assessment was independently performed by J.L.S. and E.M.B. The Newcastle-Ottawa Scale (NOS) was used to assess the quality of all cohort and case-control studies included in the analysis (Table 1).42 An NOS score of 7 or higher was used to identify studies with a low risk of bias.43 As proposed by Egger et al44 a visual inspection of funnel plots for asymmetry was used to assess publication bias. Floor and ceiling effects were identified in funnel plots when relevant (Supplemental Figure 1).
Table 1.
Study characteristics.
| First Author | Year | Country | Study Design | Sample Size | Mean Age (y) | Disease (%CD, %UC, %IC) | Inpatient vs Outpatient Opioid Use | Definition of Opioid Use | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Alley18 | 2019 | United States | Retrospective cohort | 76 171 | 45.3 | 52% CD, 48% UC | Outpatient | Extended opioid use defined as 60-d supply or more of opioids in a single year | 7 |
| Anderson19 | 2018 | United States | Prospective cohort | 447 | 39.4 | 65% CD, 33% UC, 2% IC | Outpatient | 1 or more prescriptions during 2-y study period | 7 |
| Berry20 | 2020 | United States | Retrospective cohort | 57 | 32 | 56% CD, 40% UC, 4% IC | Inpatient | Any opioid use during hospitalization, excluding those for procedural sedation | 7 |
| Buckley21 | 2013 | United States | Cross-sectional | 104 582 | — | 46% CD, 53% UC, 1% IC | Outpatient | Any outpatient opioid dispensed during the study period | NA |
| Burr4 | 2018 | United Kingdom | Retrospective cohort | 8866 | — | 40% CD, 60% UC | Outpatient | Any opioid prescribed by primary care during study period | 7 |
| Cheung22 | 2015 | United States | Cross-sectional | 108 | 50.5 | 100% CD | Outpatient | Any opioid within the month prior to hospital admission | NA |
| Chitnavis23 | 2019 | United States | Retrospective cohort | 497 | 52.9 | 100% UC | Outpatient | 3 consecutive prescriptions for opioids filled or 2 filled in a 6-mo period | 6 |
| Christian24 | 2017 | United States | Retrospective cohort | 498 | 39.4 | 67% CD, 31% UC, 2% ID |
Outpatient | Opioid prescription at discharge | 7 |
| Click13 | 2016 | United States | Prospective cohort | 338 | — | 54% CD, 42% UC, 4% IC |
Outpatient | Any outpatient opioid prescription during the study period | 6 |
| Coates25 | 2020 | United States | Retrospective cohort | 542 | 40.4 | 100% CD | Outpatient | Opioid prescription provided prior to index visit | 6 |
| Cross26 | 2005 | United States | Retrospective cohort | 291 | 40.2 | 100% CD | Outpatient | Any opioid used for purpose of analgesia, excluding 2-mo period following surgery | 5 |
| Dalal27 | 2020 | United States | Retrospective cohort | 862 | 40.4 | 66% CD, 34% UC | Inpatient | IV and non-IV opioids prescribed during hospital admission | 8 |
| Hanson28 | 2009 | United States | Case-control | 200 | 44.9 | 78% CD, 22% UC | Outpatient | Opioid prescription taken for IBD-related pain at initial clinic visit | 7 |
| Hazratjee29 | 2013 | United States | Retrospective cohort | 429 | 41.2 | 72% CD, 38% UC | Both | (1)Any opioids prescribed during admission (2)Opioids prescribed at discharge |
8 |
| Kelso30 | 2017 | United States | Retrospective Cohort | 113 | 48.5 | 48% CD, 52% UC | Inpatient | IV opioid during admission | 7 |
| Li31 | 2016 | United States | Retrospective cohort | 1331 | 41.1 | 100% CD | Both | Any inpatient or outpatient opioid prescription 1 mo prior to surgery | 5 |
| Lian32 | 2010 | United States | Retrospective cohort | 223 | 38.7 | 100% UC | Inpatient | Oral or IV opioids given while inpatient (excluding after colectomy) | 6 |
| Lichtenstein3 | 2012 | United States, Canada | Prospective cohort | 6273 | 42.5 | 100% CD | Both | Any opioid use from enrollment to 6-mo data collection period | 5 |
| Limsrivilai11 | 2017 | United States | Retrospective cohort | 1430 | 40.0 | 60% CD, 40% UC | Both | Any opioid prescription use during the study period | 7 |
| Long33 | 2012 | United States | Retrospective cohort | 117 | 31.9 | 72% CD, 28% UC | Inpatient | Any opioids prescribed during hospital admission, excluding those used for sedation or postoperatively | 6 |
| Mudireddy34 | 2017 | United States | Retrospective cohort | 439 | 38 | 67% CD, 33% UC | Both | (1)Opioids on admission (2)Opioids on discharge |
8 |
| Nugent35 | 2016 | Canada | Case-control | 3694 | 50 | — | Outpatient | At least 1 opioid prescription in the previous year | 8 |
| O’Brien36 | 2020 | United States | Retrospective cohort | 118 | — | 100% CD | Both | Any opioid use 6 mo prior to surgery | 6 |
| Parian37 | 2015 | United States | Retrospective cohort | 190 | 70.2a | 50% CD, 50% UC | Outpatient | Opioids listed on outpatient medication list during clinic visit | 5 |
| Park9 | 2020 | United States | Case-control | 52 782 | 48.3 | 45% CD, 55% UC | Outpatient | Claims data with at least 1 opioid prescription | 7 |
| Pauly38 | 2017 | United States | Retrospective cohort | 47 164 | 41.8 | 100% CD | Outpatient | 90-d supply of opioids in a 6-mo period without any 30-d gaps between prescriptions | 6 |
| Sanford39 | 2014 | Canada | Cross-sectional | 100 | 41.3 | 100% CD | Outpatient | Use at least 1 opioid drug weekly for control of Crohn’s pain | NA |
| Targownik5 | 2014 | Canada | Cross-sectional | 4217 | — | 47% CD, 53% UC | Outpatient | Heavy opioid use defined as 50 MME/d for 30 d in any 1-y period AND at least 2 prescriptions in the same 1-y period | NA |
| Tinsley40 | 2015 | United States | Retrospective cohort | 229 | 37.28 | 100% UC | Outpatient | Opioids prescribed at discharge | 7 |
| Wren41 | 2018 | United States | Retrospective cohort | 93 668 | 23.1b | 52% CD, 40% UC, 8% IC | Outpatient | Chronic opioid use defined as 2 or more opioid prescriptions in a year; persistent chronic opioid use defined as 4 or more years of use | 6 |
Abbreviations: CD, Crohn’s disease; IBD, inflammatory bowel disease; IC, indeterminate colitis; IV, intravenous; NA, not applicable; NOS, Newcastle-Ottawa Scale; UC, ulcerative colitis.
Included only patients 65 years of age and older.
Included only patients 15-29 years of age.
A post hoc, sensitivity analysis was performed to assess the stability of our meta-analysis findings by using an alternative definition of opioid use, which included opioid use disorder. We were able to compare the RRs between our main and alternative definitions of opioid use to determine the influence of the individual dataset on the pooled analysis.
Statistical Analysis
Pooled rates and 95% confidence intervals (CIs) were calculated from the eligible studies using random-effects meta-analysis according to the methods described by Hartung-Knapp-Sidik-Jonkman.45 A random-effects model was used due to the heterogeneity in study setting, study population, and study design. The proportions and their 95% CIs were presented as forest plots. Statistical heterogeneity was assessed using the I2 statistic in which ≥30% signifies significant heterogeneity. We performed several subgroup analyses based on the setting and the timing of opioid administration. All analyses were performed using R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Selection
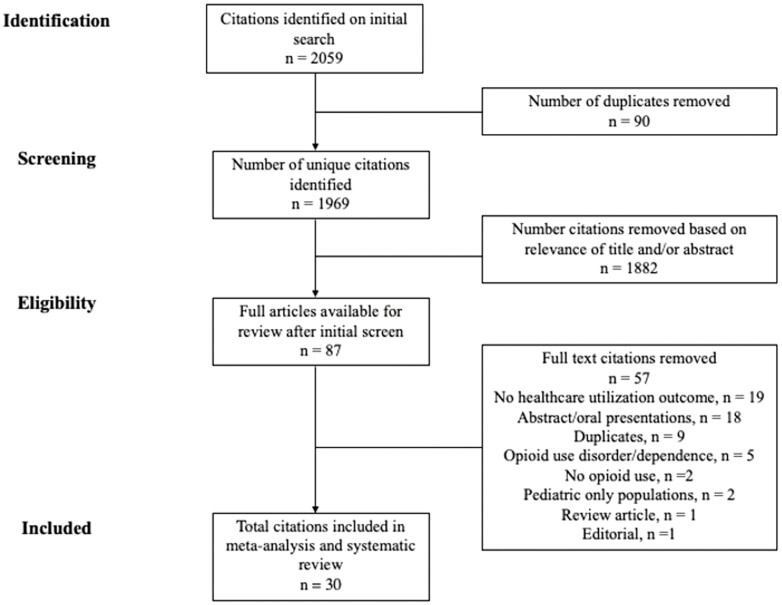
A total of 2059 studies were identified using our search strategy (Figure 1). Ninety studies were removed due to duplication, leaving 1969 unique citations. The titles and abstracts of these studies were screened, and 1882 were removed based on relevance. A total of 87 studies were fully reviewed, and an additional 57 were removed for the following reasons: lack of healthcare utilization outcome (n = 19), abstract only (n = 18), duplicate (n = 9), opioid use disorder or dependence (n = 5), not relevant to opioid use (n = 2), pediatric populations only (n = 2), review article (n = 1), or editorial (n = 1). A total of 30 unique studies were included in the final meta-analysis and systematic review.
Figure 1.
Search strategy flow diagram.
Study Characteristics
The 30 included citations comprised 20 retrospective cohorts, 3 prospective cohorts, 4 cross-sectional studies, and 3 case-control studies (Table 1). The average age of the study participants was 38 years. All studies included patients 18 years of age and older, except for Wren et al,41 who studied patients 15 to 29 years of age. Most studies included both patients with CD and UC (n = 19), with 8 studies including only patients with CD and 3 studies including only patients with UC. Most studies were conducted in the United States (n = 26), but 3 studies from Canada and 1 from the United Kingdom were included.
30-Day Readmissions and Hospitalizations
Seven studies including a combined 4688 patients evaluated 30-day readmission rates in patients with IBD receiving opioids.20,24,27,29,31,34,36 Pooled RR demonstrated no difference in readmission rates between patients with IBD who received opioids compared with those who did not (RR, 1.17; 95% CI, 0.86-1.61; I2 = 43%) (Supplemental Figure 2).
Additionally, subgroup analysis demonstrated no difference in readmission rates in those who received opioids prior to admission (RR, 1.81; 95% CI, 0.56-5.8; I2 = 0%), during the admission (RR, 1.03; 95% CI, 0.75-1.43; I2 = 10%), or at discharge (RR, 1.29; 95% CI, 0.97-1.71; I2 = 0%) (Supplemental Figure 3). Similarly, Tinsley et al40 demonstrated no difference in 30-day readmission rates between those who received opioids at discharge and those who did not (OR, 1.82; 95% CI, 0.48-6.93), but this study was not eligible for inclusion in the meta-analysis.
When examining hospitalization rate, 7 studies found a statistically significant association between opioid use and frequency of hospitalizations.9,11,21,35,38,39,41 In contrast, Targownik et al5 showed no difference in the hospitalization rate between heavy opioid users and those not taking opioids.
Length of Stay
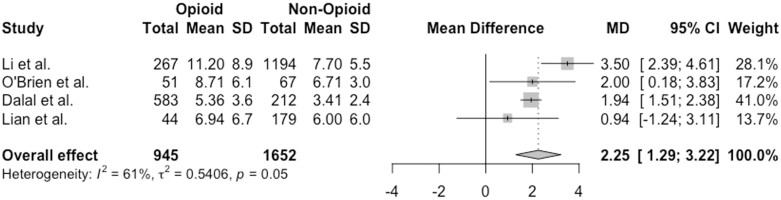
Four studies including 2597 patients examined the relationship between hospital LOS and opioid use in patients with IBD.27,31,32,36 Pooled mean differences (MDs) demonstrated that IBD patients who received opioids during the hospitalization had a higher LOS compared with those who did not (MD, 2.25 days; 95% CI, 1.29-3.22 days; I2 = 61%) (Figure 2). One additional study by Kelso et al30 showed that patients with an LOS >4 days were more likely to have received intravenous opioids compared with those with a shorter LOS. In contrast, Berry et al20 demonstrated no difference in the LOS in patients who were prescribed opioids compared with those who were not.
Figure 2.
Association between hospital length of stay and opioid use among patients with inflammatory bowel disease. CI, confidence interval; MD, mean difference.
Healthcare Costs
Five studies demonstrated an association between opioid use in patients with IBD and increased healthcare spending.9,11,13,18,41 Two studies demonstrated that opioid users were more likely to be in the top quartile of spending, while Click et al12 showed that opioid users were more likely to be in the top 5% of spenders.11,13,18 Wren et al41 demonstrated that 28.8% of patients prescribed opioids for 4 or more years spent >50 000 healthcare dollars a year compared with 9.2% of nonusers (P < .001).
ED and Outpatient Visits
Six studies examined the relationship between ED visits and opioid use in patients with IBD.9,11,18,35,38,41 Five of these studies demonstrated that patients taking opioids had a greater number of ED visits compared with those not using opioids.9,11,18,35,38 Wren et al41 demonstrated that opioid users were more likely to have at least 1 ED visit in a given year.
The data examining the relationship between outpatient visits and opioid use in patients with IBD was limited to 2 studies.21,41 Wren et al41 showed that patients with chronic opioid use had a greater number of outpatient visits during the study period. This result was more pronounced in those who had been using chronic opioids for 4 or more years. Similarly, Buckley et al21 demonstrated that patients with IBD using opioids had a greater frequency of outpatient visits compared with those not using opioids.
Polypharmacy
Four studies examined the relationship between polypharmacy and opioid use in IBD patients.21,26,37,41 Three studies demonstrated a statistically significant association between opioid use and polypharmacy in IBD patients,21,26,41 while Parian et al37 found no significant difference between opioid users and nonusers on mild, moderate, or severe polypharmacy.
Insurance Coverage
Three studies examined the relationship between opioid use in patients with IBD and use of public vs private health insurance.21,23,33 Chitnavis et al23 failed to show a significant difference in opioid use among patients using disability insurance or Medicaid insurance compared with those with commercial insurance (OR, 1.77; 95% CI, 0.49-6.38). Similarly, Long et al33 showed no difference in opioid prescriptions between those with and without insurance. In contrast, Buckley et al21 showed that 48% of patients with IBD with commercial insurance or Medicare were prescribed at least 1 opioid, compared with 73% of those with Medicaid.
IBD-Related Surgeries
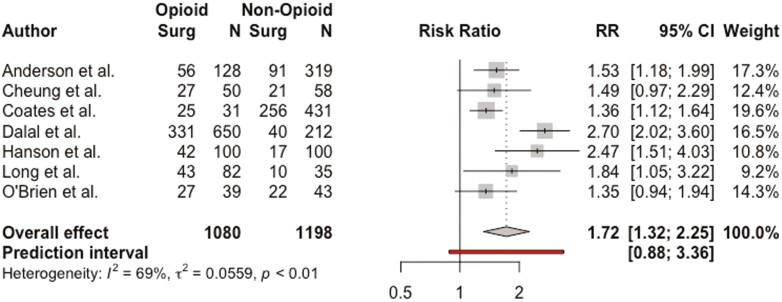
Seven studies including 2278 patients examined the relationship between a prior history of gastrointestinal surgery and opioid use in patients with IBD.19,22,25,27,28,33,36 Pooled RR demonstrated that patients with a history of prior IBD-related surgery were more likely to be prescribed opioid medications (RR, 1.72; 95% CI, 1.32-2.25; I2 = 69%) (Figure 3). On subgroup analysis, patients with a history of IBD-related surgery were more likely to have received outpatient opioid prescriptions (RR, 1.53; 95% CI, 1.17-1.99; I2 = 24%) but not inpatient opioid prescriptions (RR, 2.39; 95% CI, 0.24-23.36; I2 = 30%) (Supplemental Figure 3).
Figure 3.
Association between prior inflammatory bowel disease–related surgery and opioid use among patients with inflammatory bowel disease. CI, confidence interval; RR, relative risk.
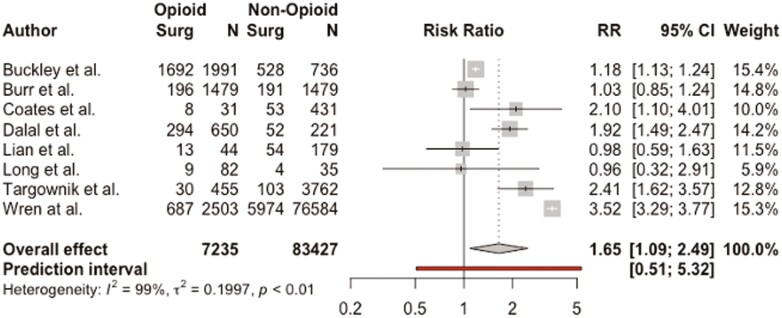
An additional 8 studies including 90 665 patients examined the relationship between gastrointestinal surgery during the study period and opioid use in patients with IBD.4,5,21,25,27,32,33,41 Pooled RR demonstrated that patients who underwent IBD-related surgery during the study period were more likely to be taking opioid medications (RR, 1.65; 95% CI, 1.09-2.49; I2 = 99%) (Figure 4). However, the results no longer reached statistical significance (with a much smaller sample size) when separated by outpatient opioid use (RR, 1.83; 95% CI, 0.95-3.53; I2 = 99%) and inpatient opioid use (RR, 1.37; 95% CI, 0.49-3.87; I2 = 69%) (Supplemental Figure 3).
Figure 4.
Association between inflammatory bowel disease–related surgery during the study period and opioid use among patients with inflammatory bowel disease. CI, confidence interval; RR, relative risk.
Medications
Biologics
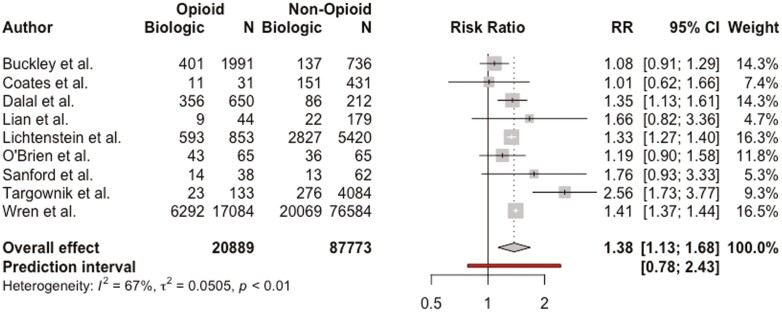
Nine studies including 108 662 patients examined the relationship between biologic use (infliximab, adalimumab, certolizumab pegol, golimumab, vedolizumab, and ustekinumab) and opioid use in patients with IBD.3,5,21,25,27,32,36,39,41 Pooled RR demonstrated that patients receiving biologic therapy were more likely to be taking opioid medications (RR, 1.38; 95% CI, 1.13-1.68; I2 = 67%) (Figure 5).
Figure 5.
Association between biologic use and opioid use among patients with inflammatory bowel disease. CI, confidence interval; RR, relative risk.
Immunomodulators
Seven studies including 104 994 patients examined the relationship between immunomodulator use (azathioprine, 6-mercaptapurine, and methotrexate) and opioid use in patients with IBD.4,5,21,25,27,39,41 Pooled RR demonstrated that patients receiving immunomodulator therapy were not more likely to be taking opioid medications (RR, 1.13; 95% CI, 0.89-1.44; I2 = 84%).
Steroids
Seven studies including 102 961 patients examined the relationship between steroid use and opioid use in patients with IBD.5,21,25,27,36,39,41 Pooled RR demonstrated that patients receiving steroid therapy were not more likely to be taking opioid medications (RR, 1.36; 95% CI, 0.88-2.10; I2 = 96%).
Small molecules
No studies examining the relationship between opioids and tofacitinib were identified.
Risk-of-Bias Assessment and Sensitivity Analysis
Twelve of the 30 included studies scored <7 on the NOS, indicating greater risk for bias and potentially lower-quality studies.42,43 Visual inspection of the funnel plots for each outcome study in the meta-analysis demonstrated relative symmetry, indicating low risk for publication bias.44
A post hoc, sensitivity analysis was performed using an alternative definition of opioid use, which included patients with opioid use disorders as well. This analysis added 487 729 patients from the Nationwide Readmissions Database.46 Pooled RR continued to demonstrate a non–statically significant trend toward 30-day readmissions in patients with IBD who received opioids compared with those who did not (RR, 1.26; 95% CI, 0.94-1.67; I2 = 78%) (Supplemental Figure 2). No additional utilization outcomes were available to perform additional analysis.
Discussion
To our knowledge, this is the first meta-analysis to demonstrate several important links between healthcare utilization and opioid use in patients with IBD. In this review, we identify that (1) there is a non–statistically significant trend toward increased 30-day readmission rates in patients with IBD who use opioids, regardless of whether opioids were given prior to admission, during the admission, or on discharge; (2) opioid use was associated with a longer inpatient LOS compared with patients with IBD who did not receive opioids; (3) prior IBD-related surgery is a risk factor for outpatient opioid use; and (4) inpatient opioid use did not increase risk for IBD-related surgery during admission. Similar to a recent meta-analysis, we found that biologics but not immunomodulators were associated with opioid use; however, unlike Niccum et al,8 we did not find that use of corticosteroids was associated with increased opioid use.
On systematic review of the literature, we found that opioid use in patients with IBD was associated with increased hospitalizations, healthcare costs, ED visits, outpatient visits, and polypharmacy. The association between opioid use and increased outpatient visits can be expected due to the need for controlled substance monitoring and may not necessarily reflect a negative effect on utilization attributable to opioids themself. One could argue that increased outpatient visits could provide the opportunity for tight symptom monitoring, which may lead to improved patient outcomes as demonstrated in the CALM (Effect of Tight Control Management on Crohn’s Disease) trial.47 However, the increase in ED visits and hospitalizations indicates that patients using opioids are also more likely to interact with the healthcare system in the form of unplanned acute care.
With several studies indicating higher levels of healthcare utilization in patients prescribed opioids for musculoskeletal conditions,48–51 it is perhaps not surprising that high utilization is also seen in patients with IBD who take opioids. What may be more intriguing is that we did not identify a statistically significant association between opioids and increased 30-day readmissions. Known risk factors for readmissions in patients with IBD include chronic pain, anxiety and depression, and medical complexity,52,53 many of which are also listed as risk factors for opioid use.54–56 The lack of association between opioid use and 30-day readmissions suggests that other factors such as mental health conditions and chronic pain may be driving the increased healthcare utilization independent of opioid use seen in patients with IBD. However, given that publicly available readmission databases typically do not include prescription claims data, the lack of direct association between opioid use and 30-day readmissions may simply be due to lack of available data. To test this hypothesis, we performed a sensitivity analysis to include studies using an alternative definition for opioid use, including patients with a diagnosis of substance use disorder, comparing the relationship between opioid use and 30-day readmissions. While this analysis added over 400 000 additional patients, the trend toward increased 30-day readmissions in patients using opioids remained nonsignificant. Furthermore, the addition of opioid use disorder to the analysis significantly increased the heterogeneity, indicating that studies using opioid use disorder as their exposure may be fundamentally different from studies looking at prescription opioid use. The findings from our sensitivity analysis suggests that the result and conclusions drawn from our study were not affected by the alternative definitions that could be made during the review process, and that results of our review can be regarded with a higher degree of certainty. The varying definitions among studies highlights the complexity involved in answering this question using retrospective and claims data, which often rely on surrogate markers of opioid use such as opioid use disorder or chronic pain and are prone to multiple confounding factors and biases.
A notable strength of this study was the subgroup analysis of inpatient vs outpatient opioid use. These data were particularly helpful in determining the relationship between IBD-related surgery and the use of opioids in the acute inpatient phase vs the more chronic outpatient phase of care. Many clinicians avoid the use of opioids in an acute IBD exacerbation due to the concern that opioid-induced bowel dysfunction, a well-established entity in which gastrointestinal transit time is delayed due to binding of mu-receptor agonists in the enteric nervous system,57,58 could lead to obstruction, ileus, or perforation. However, the results of our analysis showed no increased risk for surgery in patients who received opioids while admitted to the hospital. These results may indicate that a short course of opioids while admitted for an acute IBD flare may not be as dangerous as is generally regarded, though opioid use was associated with an increased LOS.
Interestingly, the subgroup analysis on outpatient opioid use did not show an association with increased IBD-related surgeries. However, only 2 of these studies, Targownik et al5 and Wren et al,41 specified the “heavy” and chronic use of opioids, respectively. Both of these studies demonstrated a strong association between opioid use and need for surgery. The remaining 3 studies defined opioid use more broadly, even including a single outpatient prescription. Therefore, while our subgroup analysis did not identify outpatient opioid use as a predictor of surgery, chronic, outpatient opioid use may still be a significant risk factor for future surgery.
Additionally, our subgroup analysis showed that prior IBD-related surgery was a risk factor for outpatient opioid prescriptions. With the ongoing opioid epidemic and increasing number of opioid-related deaths, it is vital that we identify individuals who are at risk for chronic opioid use, misuse, and addiction.59 Patients with IBD are already at risk for developing chronic abdominal pain, particularly if they have severe disease or concomitant anxiety and depression.60 Our analysis suggests that a history of prior IBD-related surgery as a potential risk factor for chronic pain and chronic opioid use, and clinicians should strongly consider nonopioid alternatives in patients with prior bowel surgery.
Our analysis had 2 main limitations. The first was the quality of studies included in our review. Currently there are no prospective, controlled trials dedicated to the study of the effects of opioid use in patients with IBD, and as result, the majority of included studies were retrospective cohorts, making it difficult to determine causality. Additionally, of the studies included, 40% scored <7 on the NOS (Table 1), indicating potential for bias related to study quality. Therefore, it remains unclear whether opioid use itself is driving higher rates of healthcare utilization or if high rates of healthcare utilization put patients with IBD at risk for exposure to opioids. Furthermore, some research suggests that covariates such as quality of life,19,39 mental health conditions,54–56 substance abuse or dependence,46,56,61 and functional gastrointestinal disorders55,62,63 could be driving the relationship between opioid use and healthcare utilization.
The second significant limitation is this the degree of heterogeneity. The I2 value in this study ranged from 24% to 99%, which was consistent with the I2values reported in the recently published meta-analysis by Niccum et al.8 The high I2 values seen in our analysis and Niccum et al suggest a large degree of variability between included studies and call into question the accuracy of these meta-analyses. However, it also highlights the need for more rigorous research studies focused on potential outcomes of opioid use in the IBD population. Much of the data were collected from patient characteristic tables of studies not specifically focused on opioid use, which likely accounts for the observed degree of variability between studies. Despite the I2 values, our analysis and the analysis performed by Niccum et al are the only pooled data available on the topic of opioid use and IBD.
In summary, the results of this systematic review and meta-analysis indicate that opioid use in IBD patients is a risk factor for high healthcare utilization. However, it is unclear if opioid use is the cause of this increased utilization or is merely an indicator of more severe disease. Regardless, with the ongoing opioid epidemic and rising healthcare costs, clinicians should continue to make all efforts to reduce opioid use in this population. There remains a critical need to identify nonopioid alternatives for pain in patients with IBD.
Supplementary Material
Contributor Information
Jessica L Sheehan, Department of Internal Medicine, Michigan Medicine, Ann Arbor, MI, USA.
Janson Jacob, Department of Internal Medicine, Michigan Medicine, Ann Arbor, MI, USA.
Elliot M Berinstein, Department of Medicine, St. Joseph Mercy Ann Arbor Hospital, Ypsilanti, MI, USA.
LaVana Greene-Higgs, Department of Internal Medicine, Michigan Medicine, Ann Arbor, MI, USA.
Calen A Steiner, Division of Gastroenterology and Hepatology, Department of Internal Medicine, University of Colorado, Aurora, Colorado, USA.
Sameer K Berry, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Michigan Medicine, Ann Arbor, MI, USA.
Carol Shannon, Taubman Health Sciences Library, University of Michigan, Ann Arbor, Michigan, USA.
Shirley A Cohen-Mekelburg, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Michigan Medicine, Ann Arbor, MI, USA; VA Center for Clinical Management Research, VA Ann Arbor Health Care System, Ann Arbor, MI, USA; Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, MI, USA.
Peter D R Higgins, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Michigan Medicine, Ann Arbor, MI, USA.
Jeffrey A Berinstein, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Michigan Medicine, Ann Arbor, MI, USA; Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, MI, USA.
Funding
JAB was supported by National Institute for Diabetes and Digestive and Kidney Diseases grant T32 DK062708 at the time this research was conducted. PDRH is supported by National Institute for Diabetes and Digestive and Kidney Disease grants R01 DK125687, R01 DK118154, R01 DK109032, and T32 DK062708.
Conflicts of Interest
P.D.R.H. has received consulting fees from AbbVie and Pfizer. J.A.B. has received consulting fees from Buhlmann Diagnostics Corp. All other authors report no relevant disclosures.
References
- 1. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361(21):2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bielefeldt K, Davis B, Binion DG. Pain and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15(5):778–788. doi: 10.1002/ibd.20848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREATTM Registry. Am J Gastroenterol. 2012;107(9):1409–1422. doi: 10.1038/ajg.2012.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burr NE, Smith C, West R, Hull MA, Subramanian V. Increasing prescription of opiates and mortality in patients with inflammatory bowel diseases in England. Clin Gastroenterol Hepatol. 2018;16(4):534–541.e6. doi: 10.1016/j.cgh.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 5. Targownik LE, Nugent Z, Singh H, Bugden S, Bernstein CN. The prevalence and predictors of opioid use in inflammatory bowel disease: a population-based analysis. Am J Gastroenterol. 2014;109(10):1613–1620. doi: 10.1038/ajg.2014.230 [DOI] [PubMed] [Google Scholar]
- 6. Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66(5):839–851. doi: 10.1136/gutjnl-2015-311079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen-Mekelburg S, Rosenblatt R, Gold S, et al. The impact of opioid epidemic trends on hospitalised inflammatory bowel disease patients. J Crohns Colitis. 2018;12(9):1030–1035. doi: 10.1093/ecco-jcc/jjy062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Niccum B, Moninuola O, Miller K, Khalili H. Opioid use among patients with inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19(5):895–907.e4. doi: 10.1016/j.cgh.2020.08.041 [DOI] [PubMed] [Google Scholar]
- 9. Park KT, Ehrlich OG, Allen JI, et al. The cost of inflammatory bowel disease: an initiative from the Crohn’s & Colitis Foundation. Inflamm Bowel Dis. 2020;26(1):1–10. doi: 10.1093/ibd/izz104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park KT, Bass D. Inflammatory bowel disease-attributable costs and cost-effective strategies in the United States: a review. Inflamm Bowel Dis. 2011;17(7):1603–1609. doi: 10.1002/ibd.21488 [DOI] [PubMed] [Google Scholar]
- 11. Limsrivilai J, Stidham RW, Govani SM, Waljee AK, Huang W, Higgins PDR. Factors that predict high health care utilization and costs for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2017;15(3):385–392.e2. doi: 10.1016/j.cgh.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rao BB, Click BH, Koutroubakis IE, et al. The Cost of Crohn’s disease: varied healthcare expenditure patterns across distinct disease trajectories. Inflamm Bowel Dis. 2017;23(1):107–115. doi: 10.1097/MIB.0000000000000977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Click B, Ramos Rivers C, Koutroubakis IE, et al. Demographic and clinical predictors of high healthcare use in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22(6):1442–1449. doi: 10.1097/MIB.0000000000000763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269, W64. doi: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 15. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 16. Rethlefsen ML, Kirtley S, Waffenschmidt S, et al. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev. 2021:10(1):39. doi: 10.1186/s13643-020-01542-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Orbell S, Schneider H, Esbitt S, et al. Health care utilization. In: Gellman MD, Turner JR, eds. Encyclopedia of Behavioral Medicine. Springer; 2013:909–910. doi: 10.1007/978-1-4419-1005-9_885 [DOI] [Google Scholar]
- 18. Alley K, Singla A, Afzali A. Opioid use is associated with higher health care costs and emergency encounters in inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(12):1990–1995. doi: 10.1093/ibd/izz100 [DOI] [PubMed] [Google Scholar]
- 19. Anderson A, Click B, Ramos-Rivers C, et al. The association between sustained poor quality of life and future opioid use in inflammatory bowel disease. Inflamm Bowel Dis. 2018;24(7):1380–1388. doi: 10.1093/ibd/izy040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berry SK, Takakura W, Bresee C, Melmed GY. Pain in inflammatory bowel disease is not improved during hospitalization: the impact of opioids on pain and healthcare utilization. Dig Dis Sci. 2020;65(6):1777–1783. doi: 10.1007/s10620-019-05906-x [DOI] [PubMed] [Google Scholar]
- 21. Buckley JP, Kappelman MD, Allen JK, Van Meter SA, Cook SF. The burden of comedication among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(13):2725–2736. doi: 10.1097/01.MIB.0000435442.07237.a4 [DOI] [PubMed] [Google Scholar]
- 22. Cheung M, Khan S, Akerman M, et al. Clinical markers of Crohn’s disease severity and their association with opiate use. J Clin Med Res. 2015;7(1):33–36. doi: 10.14740/jocmr1969w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chitnavis MV, Baray M, Northup PG, Tuskey AG, Behm BW. Opioid use and misuse in ulcerative colitis. World J Gastrointest Pharmacol Ther. 2019;10(1):22–28. doi: 10.4292/wjgpt.v10.i1.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Christian KE, Jambaulikar GD, Hagan MN, et al. Predictors of early readmission in hospitalized patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23(11):1891–1897. doi: 10.1097/MIB.0000000000001213 [DOI] [PubMed] [Google Scholar]
- 25. Coates MD, Seth N, Clarke K, et al. Opioid analgesics do not improve abdominal pain or quality of life in Crohn’s disease. Dig Dis Sci. 2020;65(8):2379–2387. doi: 10.1007/s10620-019-05968-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cross RK, Wilson KT, Binion DG. Narcotic use in patients with Crohn’s disease. Am J Gastroenterol. 2005;100(10):2225–2229. doi: 10.1111/j.1572-0241.2005.00256.x [DOI] [PubMed] [Google Scholar]
- 27. Dalal RS, Palchaudhuri S, Snider CK, Lewis JD, Mehta SJ, Lichtenstein GR. Exposure to intravenous opioids is associated with future exposure to opioids in hospitalized patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2020;18(10):2269–2278.e3. doi: 10.1016/j.cgh.2019.12.024 [DOI] [PubMed] [Google Scholar]
- 28. Hanson KA, Loftus EV, Harmsen SW, Diehl NN, Zinsmeister AR, Sandborn WJ. Clinical features and outcome of patients with inflammatory bowel disease who use narcotics: a case-control study. Inflamm Bowel Dis. 2009;15(5):772–777. doi: 10.1002/ibd.20847 [DOI] [PubMed] [Google Scholar]
- 29. Hazratjee N, Agito M, Lopez R, Lashner B, Rizk MK. Hospital readmissions in patients with inflammatory bowel disease. Am J Gastroenterol. 2013;108(7):1024–1032. doi: 10.1038/ajg.2012.343 [DOI] [PubMed] [Google Scholar]
- 30. Kelso M, Weideman RA, Cipher DJ, Feagins LA. Factors associated with length of stay in veterans with inflammatory bowel disease hospitalized for an acute flare. Inflamm Bowel Dis. 2017;24(1):5–11. doi: 10.1093/ibd/izx020 [DOI] [PubMed] [Google Scholar]
- 31. Li Y, Stocchi L, Cherla D, Liu X, Remzi FH. Association of preoperative narcotic use with postoperative complications and prolonged length of hospital stay in patients with Crohn disease. 2016:9. [DOI] [PubMed]
- 32. Lian L, Fazio VW, Hammel J, Shen B. Impact of narcotic use on the requirement for colectomy in inpatients with ulcerative colitis. Dis Colon Rectum. 2010;53(9):1295–1300. doi: 10.1007/DCR.0b013e3181e7562c [DOI] [PubMed] [Google Scholar]
- 33. Long MD, Barnes EL, Herfarth HH, Drossman DA. Narcotic use for inflammatory bowel disease and risk factors during hospitalization. Inflamm Bowel Dis. 2012;18(5). doi: 10.1002/ibd.21806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mudireddy P, Scott F, Feathers A, Lichtenstein GR. Inflammatory bowel disease predictors and causes of early and late hospital readmissions. Inflamm Bowel Dis. 2017;23(10):1832–1839. doi: 10.1097/MIB.0000000000001242 [DOI] [PubMed] [Google Scholar]
- 35. Nugent Z, Singh H, Targownik LE, Strome T, Snider C, Bernstein CN. Predictors of emergency department use by persons with inflammatory bowel diseases: a population-based study. Inflamm Bowel Dis. 2016;22(12):2907–2916. doi: 10.1097/MIB.0000000000000965 [DOI] [PubMed] [Google Scholar]
- 36. O’Brien SJ, Chen RC, Stephen VT, et al. Preoperative opioid prescription is associated with major complications in patients with Crohn’s disease undergoing elective ileocolic resection. Dis Colon Rectum. 2020;63(8):1090–1101. doi: 10.1097/DCR.0000000000001571 [DOI] [PubMed] [Google Scholar]
- 37. Parian A, Ha CY. Older age and steroid use are associated with increasing polypharmacy and potential medication interactions among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(6):1392–1400. doi: 10.1097/MIB.0000000000000391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pauly NJ, Michailidis L, Kindred MG, et al. Predictors of chronic opioid use in newly diagnosed Crohn’s disease. Inflamm Bowel Dis. 2017;23(6):1004–1010. doi: 10.1097/MIB.0000000000001087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sanford D, Thornley P, Teriaky A, Chande N, Gregor J. Opioid use is associated with decreased quality of life in patients with Crohn’s disease. Saudi J Gastroenterol. 2014;20(3):182–187. doi: 10.4103/1319-3767.133020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tinsley A, Naymagon S, Mathers B, Kingsley M, Sands BE, Ullman TA. Early readmission in patients hospitalized for ulcerative colitis: incidence and risk factors. Scand J Gastroenterol. 2015;50(9):1103–1109. doi: 10.3109/00365521.2015.1020862 [DOI] [PubMed] [Google Scholar]
- 41. Wren AA, Bensen R, Sceats L, et al. Starting young: trends in opioid therapy among US adolescents and young adults with inflammatory bowel disease in the Truven MarketScan database between 2007 and 2015. Inflamm Bowel Dis. 2018;24(10):2093–2103. doi: 10.1093/ibd/izy222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ottawa Hospital Research Institute. Accessed May 19, 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 43. Lo CKL, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14(1):25. doi: 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Charilaou P, Mohapatra S, Joshi T, et al. Opioid use disorder increases 30-day readmission risk in inflammatory bowel disease hospitalizations: a nationwide matched analysis. J Crohns Colitis. 2020;14(5):636–645. doi: 10.1093/ecco-jcc/jjz198 [DOI] [PubMed] [Google Scholar]
- 47. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390(10114):2779–2789. doi: 10.1016/S0140-6736(17)32641-7 [DOI] [PubMed] [Google Scholar]
- 48. Jain N, Sharma M, Wang D, Ugiliweneza B, Drazin D, Boakye M. Burden of preoperative opioid use and its impact on healthcare utilization after primary single level lumbar discectomy. Spine J. 2021;21(10):1700–1710. doi: 10.1016/j.spinee.2021.04.013 [DOI] [PubMed] [Google Scholar]
- 49. Zhao X, Shah D, Gandhi K, et al. The association of pain interference and opioid use with healthcare utilization and costs, and wage loss among adults with osteoarthritis in the United States. J Med Econ. 2019;22(11):1192–1201. doi: 10.1080/13696998.2019.1658590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wilson JM, Farley KX, Bradbury TL, Erens GA, Guild GN. Preoperative opioid use is a risk factor for complication and increased healthcare utilization following revision total knee arthroplasty. Knee. 2020;27(4):1121–1127. doi: 10.1016/j.knee.2020.05.013 [DOI] [PubMed] [Google Scholar]
- 51. Rhon DI, Cook CE, Cleland JA, Snodgrass SJ. The influence of prior opioid use on healthcare utilization and recurrence rates for non-surgical patients seeking initial care for patellofemoral pain. Clin Rheumatol. 2021;40(3):1047–1054. doi: 10.1007/s10067-020-05307-w [DOI] [PubMed] [Google Scholar]
- 52. Cohen-Mekelburg S, Rosenblatt R, Wallace B, et al. Inflammatory bowel disease readmissions are associated with utilization and comorbidity. Am J Manag Care. 2019;25(10):474–481. [PubMed] [Google Scholar]
- 53. Barnes EL, Kochar B, Long MD, et al. Modifiable risk factors for hospital readmission among patients with inflammatory bowel disease in a Nationwide Database. Inflamm Bowel Dis. 2017;23(6):875–881. doi: 10.1097/MIB.0000000000001121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mantzouranis G, Fafliora E, Saridi M, et al. Alcohol and narcotics use in inflammatory bowel disease. Ann Gastroenterol. 2018;31(6):649–658. doi: 10.20524/aog.2018.0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Edwards JT, Radford-Smith GL, Florin TH. Chronic narcotic use in inflammatory bowel disease patients: prevalence and clinical characteristics. J Gastroenterol Hepatol. 2001;16(11):1235–1238. doi: 10.1046/j.1440-1746.2001.02468.x [DOI] [PubMed] [Google Scholar]
- 56. Noureldin M, Higgins PDR, Govani SM, et al. Incidence and predictors of new persistent opioid use following inflammatory bowel disease flares treated with oral corticosteroids. Aliment Pharmacol Ther. 2019;49(1):74–83. doi: 10.1111/apt.15023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Müller-Lissner S, Bassotti G, Coffin B, et al. Opioid-induced constipation and bowel dysfunction: a clinical guideline. Pain Med. 2017;18(10):1837–1863. doi: 10.1093/pm/pnw255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Camilleri M. Opioid-induced constipation: challenges and therapeutic opportunities. Am J Gastroenterol. 2011;106(5):835–842. doi: 10.1038/ajg.2011.30 [DOI] [PubMed] [Google Scholar]
- 59. Gomes T, Tadrous M, Mamdani MM, Paterson JM, Juurlink DN. The burden of opioid-related mortality in the United States. JAMA Network Open. 2018;1(2):e180217. doi: 10.1001/jamanetworkopen.2018.0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Morrison G, Van Langenberg DR, Gibson SJ, Gibson PR. Chronic pain in inflammatory bowel disease: characteristics and associations of a hospital-based cohort. Inflamm Bowel Dis. 2013;19(6):1210–1217. doi: 10.1097/MIB.0b013e318280e729 [DOI] [PubMed] [Google Scholar]
- 61. Micic D, Gaetano JN, Rubin JN, et al. Factors associated with readmission to the hospital within 30 days in patients with inflammatory bowel disease. PLoS One. 2017;12(8):e0182900. doi: 10.1371/journal.pone.0182900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Crocker JA, Yu H, Conaway M, Tuskey AG, Behm BW. Narcotic use and misuse in Crohnʼs disease. Inflamm Bowel Dis. 2014;20(12):2234–2238. doi: 10.1097/MIB.0000000000000194 [DOI] [PubMed] [Google Scholar]
- 63. Abdalla MI, Sandler RS, Kappelman MD, et al. Prevalence and impact of inflammatory bowel disease-irritable bowel syndrome on patient-reported outcomes in CCFA partners. Inflamm Bowel Dis. 2017;23(2):325–331. doi: 10.1097/MIB.0000000000001017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.