Abstract
Background
Postoperative recurrence [POR] of Crohn’s disease following ileocolonic resection is common. The impact of immediate postoperative intra-abdominal septic complications [IASC] on endoscopic and surgical recurrence has not been elucidated.
Aims
To evaluate if IASC is associated with an increased risk for endoscopic and surgical POR.
Methods
This was a retrospective study of adult Crohn’s disease patients undergoing ileocolonic resection with primary anastomosis between 2009 and 2020. IASC was defined as anastomotic leak or intra-abdominal abscess within 90 days of the date of surgery. Multivariable logistic and Cox proportional hazard modelling were performed to assess the impact of IASC on endoscopic POR [modified Rutgeerts’ score ≥ i2b] at index postoperative ileocolonoscopy and long-term surgical recurrence.
Results
In 535 Crohn’s disease patients [median age 35 years, 22.1% active smokers, 35.7% one or more prior resection] had an ileocolonic resection with primary anastomosis. A minority of patients [N = 47; 8.8%] developed postoperative IASC. In total, 422 [78.9%] patients had one or more postoperative ileocolonoscopies, of whom 163 [38.6%] developed endoscopic POR. After adjusting for other risk factors for postoperative recurrence, postoperative IASC was associated with significantly greater odds (adjusted odds ratio [aOR]: 2.45 [1.23–4.97]; p = 0.01) and decreased time (adjusted hazards ratio [aHR]: 1.60 [1.04–2.45]; p = 0.03] to endoscopic POR. Furthermore, IASC was associated with increased risk (aOR: 2.3 [1.04–4.87] p = 0.03) and decreased survival-free time [aHR: 2.53 [1.31–4.87]; p = 0.006] for surgical recurrence.
Conclusion
IASC is associated with an increased risk for endoscopic and surgical POR of Crohn’s disease. Preoperative optimization to prevent IASC, in addition to postoperative biological prophylaxis, may help reduce the risk for endoscopic and surgical POR.
Keywords: Crohn’s disease, postoperative recurrence, infection, postoperative complications
Graphical Abstract
1. Introduction
In the post-biologic era, ~20–30% of Crohn’s disease [CD] patients with stricturing or penetrating disease require ileocecal resection [ICR] for CD management within 10 years of diagnosis.1–6 However, ICR is not curative, with 15–20% of patients requiring surgical resection for postoperative recurrence [POR] of CD affecting the neoterminal ileum within 5 years of index ICR.6 Preceding both surgical and clinical recurrence, endoscopic recurrence occurs in 70–90% of patients within 1 year of index ICR.4–6 Seminal trials have shown the benefit of early endoscopic surveillance and medical prophylaxis, which are now recommended to guide postoperative CD management.7–10
Risk factors for POR may help guide endoscopic surveillance and medical prophylaxis to prevent surgical recurrence. Previous studies have shown that younger age [<30 years], active smoking, two or more prior surgeries, and penetrating or perianal disease behaviour correlate with ~50% clinical and 80% endoscopic POR rates.11–17 These risk strata have not been validated.
The pathogenesis of POR has not been elucidated and is thought to be multifactorial, including immune activation.18,19 In the peri- and post-operative periods, intra-abdominal complications such as leaks or abscesses may serve as a local and systemic immune-activating event rather than a ‘mechanical’ surgical complication. However, few reports have assessed the influence of immediate postoperative complications on subsequent POR.20,21 Furthermore, such complications may delay the initiation or interrupt continuation of postoperative prophylactic therapy in high-risk patients and may play an important role in the natural course of POR development. Postoperative complications including postoperative infections and intra-abdominal septic complications [IASC] have been found to be associated with early clinical POR in limited series.20,21 To our knowledge, the impact of IASC on endoscopic or surgical recurrence has not previously been studied. Here we aimed to assess the impact of postoperative IASC on endoscopic recurrence as well the impact on surgical recurrence.
2. Methods
2.1. Patient selection
We conducted a retrospective, observational cohort study of adult CD patients who underwent ICR for CD management between January 1, 2009 and January 1, 2020 at a multi-hospital, single institution healthcare system. Patients included in the study fulfilled the following inclusion criteria: [1] age ≥ 18 years; [2] CD diagnosis confirmed by two or more ICD-9 and ICD-10 codes [K50.90] entered by a gastroenterologist or colorectal surgeon; [3] ileocolonic resection with an indication for CD management [CPT codes: 44160, 44140, 44204, 44205] entered by a colorectal surgeon; and [4] primary ileocolonic anastomosis constructed at the time of ICR. Patients were excluded if any of the following criteria were met: [1] ICR for an indication other than CD [e.g. neoplasm, ischaemia]; [2] diverting ileostomy at the time of index ICR; and [3] absence of gastroenterology follow up [defined by at least one outpatient clinic visit postoperatively]. Eligible patients were consecutively reviewed, and selection criteria were confirmed via manual chart review [S.B., R.S.].
2.2. Demographic and clinical characteristics
Patient demographic, preoperative, operative and postoperative clinical characteristics were collected through manual chart review by two independent reviewers [S.B., R.S.]. Demographic clinical variables collected included gender, age at ICR, age at CD diagnosis, CD location and behaviour by Montreal classification, tobacco use history [never, former, active smoker], history of perianal disease involvement, history of preoperative biologic use [adalimumab, infliximab, certolizumab, vedolizumab, ustekinumab], number of prior ICRs, and history of upper gastrointestinal CD. Preoperative data included biologic use within 12 weeks of the date of surgery, systemic steroid use within 4 weeks of surgery, immunomodulator [azathioprine, mercaptopurine, methotrexate] use within 4 weeks of surgery and blood lab results (haemoglobin, white blood cell, platelet, albumin, C-reactive protein [CRP]) within 4 weeks of ICR. Operative data were obtained via the operative report and consisted of anastomosis configuration [side-to-side, end-to-side, end-to-end anastomosis] and technique [stapled or handsewn]. Postoperative data included development of IASC, defined as the development of anastomotic leak [as per surgeon clinical assessment and documentation], intra-abdominal abscess or fistulization detected on cross-sectional imaging, within 30 and 90 days of the date of surgery, postoperative biologic prophylaxis, defined as initiation of biologic treatment within 3 months of the date of surgery, all postoperative ileocolonoscopies, postoperative biologic exposure and repeat ileocolonic resections for CD indication.
2.3. Postoperative ileocolonoscopy grading
All postoperative ileocolonoscopies performed ≥ 3 months from the date of surgery were manually collected for review. Postoperative endoscopies performed prior to 3 months after ICR were excluded due to a likelihood these were related to operative complication [e.g. anastomotic bleeding] rather than disease activity monitoring. The first postoperative ileocolonoscopy after 3 months was designated as the index ileocolonoscopy. Endoscopic severity was assessed utilizing the modified Rutgeerts score. If the Rutgeerts score was not prospectively recorded, ileocolonoscopy was graded using a retrospective application of the modified Rutgeerts score based on available endoscopic images and procedure report. All retrospective Rutgeerts grading was performed by a single, independent grader [S.B.] who was blinded to all clinical data and outcomes. The grader was trained and validated [>90% accuracy] by an IBD gastroenterologist [B.H.C.] on a sample dataset prior to data collection. Postoperative ileocolonoscopies were excluded if the neoterminal ileum was not intubated or there was insufficient data to apply the Rutgeerts score. Endoscopic POR was defined as a modified Rutgeerts score of i2b disease or greater.22,23
2.4. Outcomes
Patients were grouped based on the development of IASC within 90 days of the date of ICR. The primary outcome of the study was development of endoscopic POR at time of index postoperative ileocolonoscopy. Secondary outcomes included: endoscopic POR over the entire postoperative follow-up, surgical POR within 1 year of ICR and over the entire postoperative period, and progression to severe endoscopic disease [Rutgeerts score of i3/i4] at both time of index postoperative ileocolonoscopy and over the entire postoperative follow-up period. Surgical recurrence was defined as repeat ICR for CD indication > 3 months from the index ICR. The time frame was chosen to omit surgical management of IASC complications. Additionally, surgical indications reported in the operative note for all repeat ICRs were manually collected to ensure surgery was not indicated for acute or chronic complications from IASC.
2.5. Statistical analyses
Data were described using counts and percentages for categorical variables, means and standard deviation for normally distributed continuous variables, and medians and quartiles for non-normally distributed continuous variables. Chi-square and Fisher’s exact tests were used to compare categorical variables. The Shapiro–Wilk normality test was used to determine normal distribution of continuous variables. Student’s t-test and Kruskal–Wallis test were applied to compare normal and non-normally distributed continuous variables, respectively. For the primary outcome, a multivariable logistic regression model controlling for independent variables of interest was performed. The number of independent variables included in the regression model was based on the rule of 10 to prevent model overfitting. Model covariates were selected utilizing established risk and protective factors for POR [two or more ICRs, active smoking, penetrating disease behaviour, perianal disease, postoperative biologic prophylaxis] in addition to including covariates with a p-value < 0.20 on univariate analysis. For secondary outcomes assessing an outcome over time, a Kaplan–Meier survival analysis and multivariable Cox proportional hazard regression model were conducted. Modelling adhered to the same methods as logistic model development. Sensitivity analyses were conducted on patients whose index postoperative ileocolonoscopy was conducted within 18 months of surgery to mitigate the effect of time on disease progression. This time frame was chosen to capture patients whose ICR was conducted prior to current postoperative surveillance recommendations. Additionally, to control for study design bias, a strength of association analysis was conducted limiting IASC to 30 days from the date of ICR.
2.6. Ethical considerations
The institutional review board approved the study at our study centre. All ethical principles laid out in the Declaration of Helsinki were followed.
3. Results
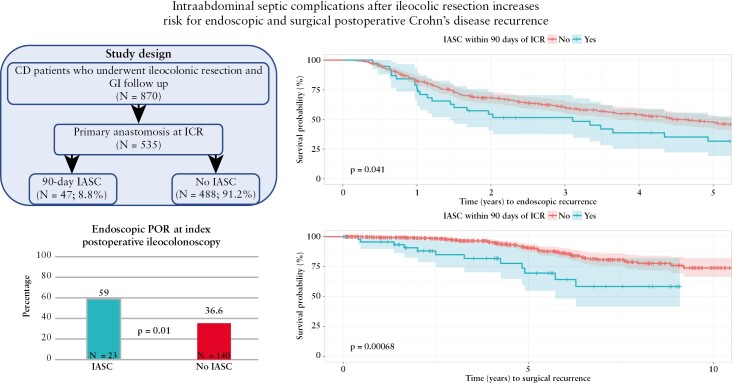
In total, 870 adult CD patients underwent ICR during the study time period, of whom 535 [61.5%] had construction of a primary anastomosis without diversion at the time of ICR and formed the study population. A total of 47 patients [8.8%] developed perioperative IASC (36 [6.7%] intra-abdominal abscess, 11 [2.1%] anastomotic leak), with a median [interquartile range, IQR] time to IASC of 13.4 [9.2, 29.3] days from the date of ICR. Nearly one-third of patients with IASC [N = 15; 31.9%] were managed surgically by drainage of abscess or fistula resection with construction of a diverting- or end-ileostomy. The remaining patients were treated non-surgically [N = 28 radiological drainage and antibiotics, N = 4 antibiotics only]. The median [IQR] age at the time of CD diagnosis and ICR was 23 [17, 30] and 35 [26, 49.5] years, respectively [Table 1]. Patients primarily had ileocolonic CD [52.1%] with stricturing [47.0%], penetrating [14.6%] or a combination of both disease behaviours [30.1%]. Approximately one-fifth of patients were actively smoking at the time of ICR and 35.7% had at least one prior ICR. Roughly one-quarter [23.2%] of patients were on systemic corticosteroids within 4 weeks of ICR [22.1% No IASC vs 34.0% IASC; p = 0.09]. A third of patients were on a biologic therapy within 12 weeks prior to ICR [33.2% No IASC vs 38.3% IASC; p = 0.48] and 17.9% [18.6% No IASC vs 10.6% IASC; p = 0.23] were started on postoperative biologic prophylaxis within 3 months of surgery, with a median time of 39 days to initiation of prophylaxis. In patients started on prophylaxis, those who experienced IASC had a median delay of 20 days compared to no IASC [58 vs 38 days; p = 0.28]. There was no association with IASC development across all demographic and clinical variables [Table 1].
Table 1.
Demographic and clinical characteristics of the study population.
| Overall, N = 535 | No IASC, N = 488 | 90-day IASC, N = 47 | p | |
|---|---|---|---|---|
| Age at ICR, years (median [IQR]) | 35.00 [26.00, 49.50] | 35.00 [26.00, 50.00] | 39.00 [28.50, 49.00] | 0.36 |
| Age at CD diagnosis, years (median [IQR]) | 23.00 [17.00, 30.00] | 23.00 [17.00, 30.00] | 25.00 [17.00, 29.00] | 0.62 |
| CD location, n [%] | 0.39 | |||
| Colon | 12 [2.2] | 11 [2.3] | 1 [2.1] | |
| Ileocolonic | 279 [52.1] | 250 [51.2] | 29 [61.7] | |
| TI | 244 [45.6] | 227 [46.5] | 17 [36.2] | |
| CD behaviour, n [%] | 0.21 | |||
| Inflammatory | 44 [8.2] | 41 [8.4] | 3 [6.4] | |
| Penetrating | 78 [14.6] | 67 [13.8] | 11 [23.4] | |
| Stricturing + Penetrating | 161 [30.1] | 145 [29.8] | 16 [34.0] | |
| Stricturing | 251 [47.0] | 234 [48.0] | 17 [36.2] | |
| Tobacco use history, n [%] | 0.81 | |||
| Never | 324 [60.8] | 296 [60.9] | 28 [59.6] | |
| Former | 91 [17.1] | 84 [17.3] | 7 [14.9] | |
| Active | 118 [22.1] | 106 [21.8] | 12 [25.5] | |
| Prior ICR, n [%] | 0.07 | |||
| 0 | 342 [64.3] | 320 [66.0] | 22 [46.8] | |
| 1 | 111 [20.9] | 96 [19.8] | 15 [31.9] | |
| 2 | 46 [8.6] | 40 [8.2] | 6 [12.8] | |
| ≥ 3 | 33 [6.2] | 29 [6.0] | 4 [8.5] | |
| Upper GI CD, n [%] | 86 [16.1] | 78 [16.0] | 8 [17.0] | 1 |
| Gender [male], n [%] | 241 [45.0] | 217 [44.5] | 24 [51.1] | 0.48 |
| History of perianal CD, n [%] | 133 [25.0] | 122 [25.2] | 11 [23.4] | 0.93 |
| Exposure to biologic preoperatively, n [%] | 266 [50.0] | 239 [49.3] | 27 [57.4] | 0.36 |
| Perioperative biologic use, n [within 12 weeks] | 180 [33.7] | 162 [33.2] | 18 [38.3] | 0.48 |
| Anastomotic configuration, n [%] | 0.35 | |||
| End to End | 99 [18.5] | 94 [94.9] | 5 [5.1] | |
| End to Side | 148 [27.7] | 134 [90.5] | 14 [9.5] | |
| Side to Side | 288 [53.8] | 260 [90.3] | 28 [9.7] | |
| Postoperative biologic prophylaxis [3 months], n [%] | 96 [17.9] | 91 [18.6] | 5 [10.6] | 0.24 |
| Time to index postoperative ileocolonoscopy, days (median [IQR]) | 391.0 [242.3, 787.5] | 390.0 [241.5, 786.0] | 440.0 [275.5, 851.5] | 0.24 |
| Postoperative follow-up time, days (median [IQR]) | 1709.0 [766.0, 2641.0] | 1699.0 [762.3, 2632.0] | 2022.0 [822.5, 2834.0] | 0.45 |
Patients who received a diverting ileostomy at the time of ICR and were excluded from the study were more likely to have penetrating disease behaviour [p < 0.001], prior ICR [p = 0.006], hypoalbuminaemia prior to ICR [p < 0.001], were on biologic therapy within 12 weeks of ICR [p = 0.002], and were on systemic corticosteroids within 4 weeks prior to ICR [p = 0.02] [Supplementary Table 1].
Most patients [N = 422; 78.9%] underwent at least one postoperative ileocolonoscopy, a majority of whom underwent at least two [N = 282; 66.8%]. The median [IQR] time to index ileocolonoscopy was 391 [242.3, 787.5] days. Patients with IASC had a median 50-day delay to index postoperative ileocolonoscopy, though this was not significant [390 days No IASC vs 440 days IASC; p = 0.24]. Patients who underwent one or more postoperative ileocolonoscopies were more likely to have a history of perianal disease [p = 0.008]. The median postoperative follow-up time for the entire study population was 4.7 years.
3.1. Development of endoscopic POR at the time of index postoperative ileocolonoscopy and follow-up period
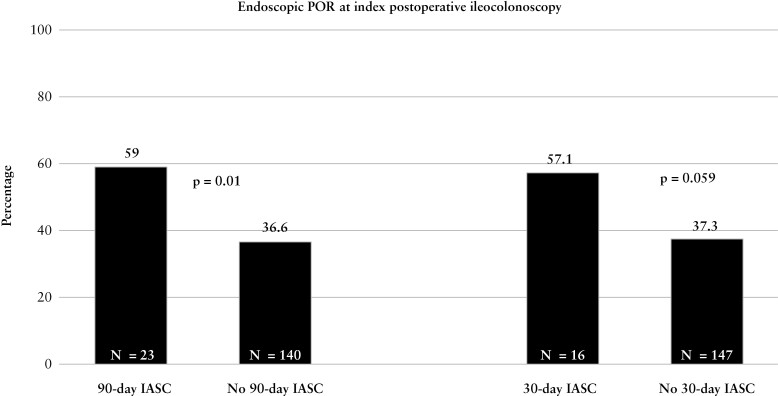
In patients who underwent postoperative ileocolonoscopy, 163 [38.6%] developed endoscopic POR at the time of index postoperative ileocolonoscopy [27.5% i0, 11.6% i1, 22.3% i2a, 18.2% i2b, 12.1% i3, 8.3% i4]. Significantly more patients with IASC [n = 23; 59.0%] developed endoscopic POR at index postoperative ileocolonoscopy compared to those who did not have IASC [n = 140; 36.6%, p = 0.01] [Figure 1]. On multivariable logistic regression controlling for risk factors of endoscopic POR, development of IASC within 90 days of ICR was associated with an increased odds of endoscopic POR at the time of index postoperative ileocolonoscopy compared to no IASC (adjusted odds ratio [aOR]: 2.45; 95% confidence interval [CI] [1.23–4.97]; p = 0.01) [Table 2]. Additionally, postoperative biologic prophylaxis was protective against endoscopic POR (aOR: 0.46; 95% CI [0.28–0.75]; p = 0.002). Recurrence rates by treatment were: 42.6% no prophylaxis [n = 133/312], 28.6% anti-tumour necrosis factor [anti-TNF] [n = 28/97], 22.2% ustekinumab [n = 2/9], 0% vedolizumab [n = 0/4]. On sensitivity analysis modifying IASC development to within 30 days from ICR, the association with an increased risk of endoscopic POR at the time of index ileocolonoscopy remained significant (aOR: 2.21; 95% CI [1.01–4.99]; p = 0.049) [Table 2].
Figure 1.
Rates of endoscopic POR at index postoperative ileocolonoscopy stratified by IASC.
Table 2.
Multivariable logistic regression assessing the risk of endoscopic POR at the time of index postoperative ileocolonoscopy.
| Adjusted OR [95% CI] | p value | Adjusted OR [95% CI] | p value | ||
|---|---|---|---|---|---|
| 90-day IASC | 2.45 [1.23–4.97] | 0.01 | 30-day IASC | 2.21 [1.01–4.99] | 0.05 |
| ≥ 2 ICRs [index ICR included] | 1.41 [0.91-2.18] | 0.12 | ≥ 2 ICR [index ICR included] | 1.47 [0.95-2.27] | 0.08 |
| Tobacco use [Reference: Never smoker] |
— | — | Tobacco use [Reference: Never smoker] |
— | — |
| Former | 1.51 [0.86–2.65 | 0.15 | Former | 1.49 [0.85–2.61] | 0.16 |
| Active | 1.22 [0.73–2.02] | 0.44 | Active | 1.23 [0.74–2.03] | 0.42 |
| Penetrating CD behaviour | 0.95 [0.61–1.47] | 0.80 | Penetrating CD behaviour | 0.98 [0.63–1.52] | 0.92 |
| Postoperative biologic prophylaxis | 0.46 [0.28–0.75] | 0.002 | Postoperative biologic prophylaxis | 0.46 [0.28–0.75] | 0.002 |
| History of perianal CD | 1.26 [0.77–2.05] | 0.35 | History of perianal CD | 1.23 [0.75–1.99] | 0.41 |
| Upper GI CD involvement | 0.93 [0.52–1.64] | 0.81 | Upper GI CD involvement | 0.93 [0.52–1.64] | 0.81 |
| Time to index postoperative ileocolonoscopy | 1.0 [0.99–1.01] | 0.64 | Time to index postoperative ileocolonoscopy | 1.0 [0.99–1.01] | 0.61 |
On sensitivity analysis in patients whose index postoperative ileocolonoscopy was within 18 months of the date of surgery, development of 90-day IASC remained associated with an increased odds of endoscopic POR (aOR: 2.80; 95% CI [1.09–7.60]; p = 0.035) [Table 3]. Additionally, history of prior ICR was associated with an increased odds of endoscopic POR (aOR: 2.10; 95% CI [1.19–7.60]; p = 0.01), while postoperative biologic prophylaxis was protective (aOR: 0.44; 95% CI [0.23–0.82]; p = 0.01) [Table 3]. On subgroup analysis in patients who underwent their first ICR, IASC remained associated with endoscopic POR at index ileocolonoscopy when controlling for tobacco use, penetrating CD, perianal disease, upper gastrointestinalCD, perioperative systemic corticosteroid use and postoperative biologic prophylaxis aOR: 2.90; 95% CI [1.11–7.89]; p = 0.03). However, on subgroup analysis in patients who had a history of ICR, controlling for risk factors, IASC was not associated with endoscopic POR at index postoperative ileocolonoscopy [p = 0.30].
Table 3.
Multivariable logistic regression assessing the risk of endoscopic POR at the time of index postoperative ileocolonoscopy [<18 months from the date of surgery].
| Adjusted OR [95% CI] | p value | Adjusted OR [95% CI] | p value | ||
|---|---|---|---|---|---|
| 90-day IASC | 2.80 [1.09–7.60] | 0.04 | 30-day IASC | 3.06 [0.98–10.65] | 0.06 |
| ≥ 2 ICRs [index ICR included] | 2.1 [1.19–7.60] | 0.01 | ≥ 2 ICRs [index ICR included] | 2.21 [1.26–3.91] | 0.006 |
| Tobacco use [Reference: Never smoker] |
— | — | Tobacco use [Reference: Never smoker] |
— | — |
| Former | 2.28 [1.13–4.65] | 0.02 | Former | 2.28 [1.14–4.63] | 0.02 |
| Active | 1.28 [0.66–2.46] | 0.46 | Active | 1.33 [0.69–2.54] | 0.39 |
| Penetrating CD behaviour | 0.94 [0.52–1.70] | 0.85 | Penetrating CD behaviour | 0.98 [0.54–1.76] | 0.94 |
| Postoperative biologic prophylaxis | 0.44 [0.23–0.82] | 0.01 | Postoperative biologic prophylaxis | 0.44 [0.23–0.82] | 0.01 |
| History of perianal CD disease | 1.12 [0.58–2.15] | 0.73 | History of perianal CD disease | 1.05 [0.54–2.00] | 0.89 |
| Upper GI CD involvement | 0.69 [0.28–1.57] | 0.39 | Upper GI CD involvement | 0.66 [0.27–1.52] | 0.35 |
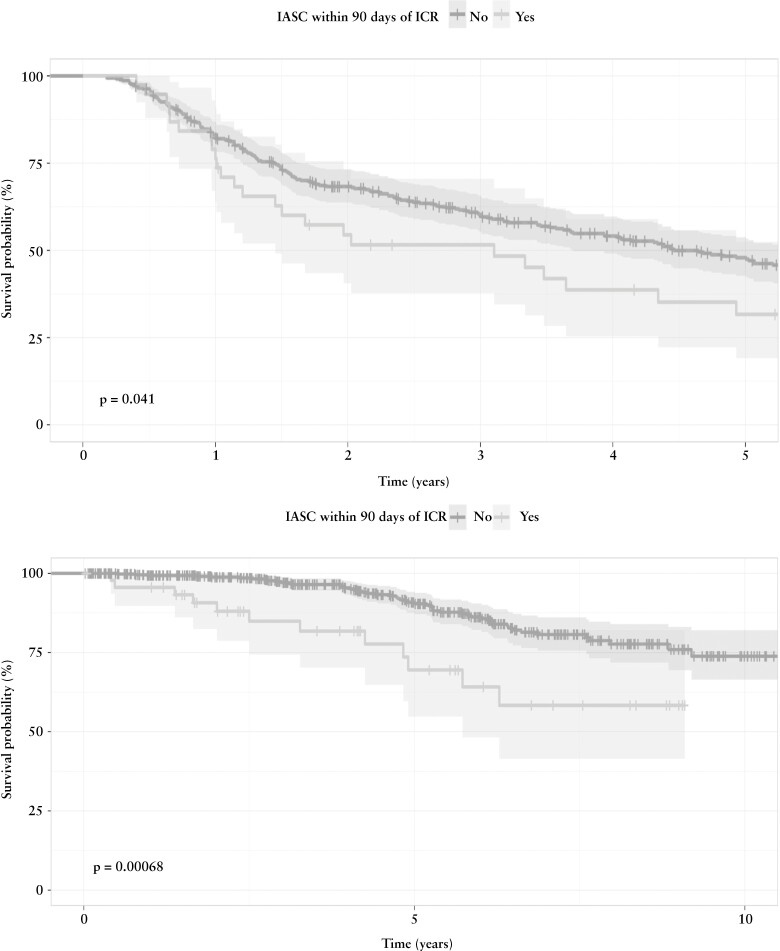
When assessing for development of endoscopic POR during the first five postoperative years, 206 patients [48.8%] experienced endoscopic POR with a median time of 1.5 years from ICR. Patients with IASC experienced endoscopic POR at higher rates than those who did not develop IASC [65.8% vs 47.9%; p = 0.05]. On Kaplan–Meier survival analysis, we found that 90-day IASC was associated with decreased time to endoscopic POR detection [p = 0.04] [Figure 2a]. This association remained on multivariable Cox proportional hazard modelling (adjusted hazard ratio [aHR]: 1.6; 95% CI [1.04–2.45]; p = 0.03) [Table 4]. Additionally, postoperative biologic prophylaxis was associated with delayed endoscopic POR (aHR: 0.65; 95% CI [0.45–0.92; p = 0.016). When observing patients who did not have endoscopic POR at the time of index endoscopy and at least one subsequent endoscopy [N = 173; 163 no IASC and 10 IASC], 42.9% of patients without IASC [N = 70] went on to develop endoscopic POR compared to 30% [N = 3] of patients with IASC [p = 0.52].
Figure 2.
[a] Time [years] to endoscopic POR stratified by IASC. [b] Time [years] to surgical POR stratified by IASC.
Table 4.
Multivariable Cox proportional hazard regression assessing the risk of endoscopic POR in a 5-year postoperative follow-up.
| Adjusted HR [95% CI] | p value | |
|---|---|---|
| 90-day IASC | 1.60 [1.04–2.45] | 0.03 |
| ≥ 2 ICRs [index ICR included] | 1.11 [0.82–1.52] | 0.49 |
| Tobacco use [Reference: Never smoker] |
— | — |
| Former | 1.39 [0.94–2.03] | 0.10 |
| Active | 1.24 [0.88–1.74] | 0.21 |
| Penetrating CD behaviour | 0.84 [0.92–1.14] | 0.27 |
| Postoperative biologic prophylaxis | 0.65 [0.45–0.92] | 0.02 |
| History of perianal CD disease | 1.25 [0.90–1.75] | 0.19 |
| Upper GI CD involvement | 0.78 [0.52–1.16] | 0.22 |
| Age < 30 years at time of ICR | 1.05 [0.75–1.48] | 0.76 |
Though IASC was associated with endoscopic POR, it was not associated with severe endoscopic POR [i3/i4] at the time of index postoperative ileocolonoscopy [p = 0.82] or throughout the entire postoperative follow-up period [p = 0.89]. Additionally, 114 [27.0%] patients developed any degree of anastomotic stricturing observed endoscopically that was not associated with IASC [27.3% No IASC vs 23.1% IASC; p = 0.71].
3.2. Surgical recurrence in first postoperative year and entire postoperative follow-up
Surgical recurrence occurred in 64 [11.9%] of patients with a median time of 4.4 years from index ICR. Repeat ICR was indicated for severe endoscopic disease or penetrating disease identified on imaging [N = 41; 64.1%], small bowel obstruction secondary to anastomotic stricturing [31.3%], or stricturing or active disease in the large bowel [4.7%]. A quarter [25.5%] of patients with IASC [n = 12] experienced surgical recurrence [indication: 50% POR, 33.3% penetrating disease, 16.7% anastomotic stricturing] compared to 10.7% [n = 52] of those who did not develop IASC [indication: 46.2% POR, 19.2% penetrating disease, 34.6% anastomotic stricturing] [p = 0.007]. When controlling for risk factors of POR, IASC (aOR: 2.31; 85% CI [1.04–4.87]; p = 0.03], history of ICR (aOR: 2.93; 95% CI [1.67–5.20]; p < 0.001) and perianal disease (aOR: 2.38; 95% CI [1.29–4.39]; p = 0.005) were associated with an increased risk for surgical recurrence. On Kaplan–Meier survival analysis, IASC was associated with decrease time to surgical recurrence [p < 0.001] [Figure 2b]. On multivariable Cox proportional hazard regression, development of IASC was associated with an increased hazard risk for repeat ICR compared to no IASC (aHR: 2.53; 95% CI [1.31–4.87]; p = 0.006) [Table 5].
Table 5.
Multivariable Cox proportional hazard regression assessing the risk of surgical recurrence across the entire postoperative period.
| Adjusted HR [95% CI] | p value | |
|---|---|---|
| 90-day IASC | 2.53 [1.31–4.87] | 0.006 |
| ≥ 2 ICRs [index ICR included] | 2.45 [1.47–4.07] | < 0.001 |
| Tobacco use [Reference: Never smoker] |
— | — |
| Former | 0.65 [0.29–1.44] | 0.29 |
| Active | 1.38 [0.78–2.43] | 0.26 |
| Penetrating CD behaviour | 1.17 [0.68–2.01] | 0.57 |
| Postoperative biologic or immunomodulator exposure | 0.80 [0.45–1.43] | 0.45 |
| History of perianal CD disease | 1.66 [0.98–2.83] | 0.06 |
3.3. Sensitivity analysis: including patients with diverting ileostomy created at the time of ICR
In patients who underwent ICR with primary anastomosis or diverting ileostomy with subsequent ileostomy takedown and bowel continuity restoration [N = 820; 89 IASC, 723 No IASC] and at least one subsequent postoperative ileocolonoscopy [N = 597; 66 IASC, 531 No IASC], the development of IASC was associated with an increased odds for endoscopic POR at the time of index postoperative ileocolonoscopy (aOR: 2.2; 95% CI [1.31, 3.79]; p = 0.003) when controlling for risk factors of POR. Additionally, IASC was associated with decreased time to surgical recurrence on Kaplan–Meier survival analysis [p < 0.001] and multivariable Cox proportional hazard regression (aHR: 2.37; 95% CI [1.50–3.76]; p < 0.001) [Supplementary Figure 1; Supplementary Table 2].
4. Discussion
In this retrospective, observational study of adult CD patients who underwent ICR with primary anastomosis, we found that development of IASC within 90 days of ICR was associated with an increased risk of and more rapid time to both endoscopic and surgical POR. Initiation of post-operative biologic prophylaxis was associated with decreased endoscopic POR, suggesting a potential mitigating strategy. To our knowledge, this is the largest study demonstrating post-operative IASC as a risk factor for endoscopic and surgical POR.
In the current study, we observed that 90-day postoperative IASC led to a significantly higher risk and decreased time to endoscopic POR. This association was robust to sensitivity analyses to control for study design bias, including reclassifying IASC to the 30-day postoperative period, limiting to those whose index postoperative ileocolonoscopy occurred within 18 months of ICR or if diverting ileostomy was made at the time of ICR. This study, to our knowledge, is the first to evaluate and demonstrate the impact of IASC on endoscopic POR of CD. The role of enteric and intra-abdominal infections and subsequent inflammatory response in inflammatory bowel disease, and specifically CD, has not been fully elucidated. Previous studies have suggested a relationship between enteric infections and intestinal dysbiosis as a factor for the subsequent development of IBD.24–29 Furthermore, in non-operative IBD patients, enteric infections have been observed at higher prevalence during IBD flares, suggesting a potential causative or at least associated interaction.30–32 However, the role of infection and the pathogenesis of POR is less well described. Macrophages play a key role in response to infections during the acute inflammatory process through the production of pro-inflammatory cytokines and serving as an antigen-presenting cell. A previous study showed that infiltration of macrophages into the neoterminal ileal mucosa in patients in endoscopic remission may lead to the development of endoscopic lesions.33 Additionally, macrophages migrate and traffic bacteria to mesenteric lymph nodes, which may increase the risk for POR through decreased diversification of intestinal microbiota and the activation of memory T cells leading to high expression of TNF-α and subsequent induction of the Th1/Th17 inflammatory cascade.34–42 Furthermore, the systemic cytokine response to infection, including upregulation of interleukin 6 [IL-6], CRP and interferon-gamma, and the down-regulation of anti-inflammatory cytokines such as IL-10, has also been observed in CD flares and recurrence.36,43–46 This may explain why the mesentery has been implicated in the pathophysiology of luminal CD development.47,48 When observing patients who did not have endoscopic POR at the time of index ileocolonoscopy, there was no difference in subsequent development of endoscopic POR between patients with or without IASC. Though this sample size is small, this finding suggests that the increased risk that IASC presents may be a short-term risk due to exacerbation of the inflammatory cascade. Additionally, the significant protective effect of postoperative biologic prophylaxis, when adjusted for IASC development, observed in the current study suggests that the increased risk with IASC is consistent with the inflammatory cascade that increases the risk for POR. The use of agents targeted at decreasing TNF-α levels, lymphocyte trafficking, regulating innate immunity, and modulating Th1/Th17 function through binding of IL-12 and IL-23 suggests that treating the inflammation caused by intra-abdominal infectious processes is vitally important in preventing endoscopic POR.49–51
Beyond endoscopic POR, the current study found an increased risk and decreased survival-free time to repeat surgical resection for CD management in patients who had IASC after index ICR. Furthermore, IASC was associated with an increased risk for early surgical recurrence within the first postoperative year, though this was a rare event and may have been underpowered for significance. However, we did not find an association with IASC and severe endoscopic disease. This discrepancy may be due to repeat ICR indicated for penetrating disease complications detected on cross-sectional imaging and not reported on endoscopy, though the latter may be rare, with only five patients who did not have correlating severe endoscopic disease in the current study. The impact of postoperative IASC on subsequent surgical recurrence has been infrequently studied, but previous literature has also shown this relationship in both adult and paediatric patient populations.13,20,21,52–54 It can be theorized that patients with severe CD are at high risk for postoperative IASC and POR, although in our study cohort there were no differences in high-risk factors such as young age, penetrating disease behaviour, active smoking, perianal disease and two or more ICRs between those who developed IASC and those who did not. Additionally, these risk factors were incorporated into our multivariable modelling to reduce confounding. Previously, our study group has shown an association with hypoalbuminaemia [<3.5 g/dL] and postoperative IASC in adult CD patients receiving ICR with or without diverting ileostomy.55,56 This association remained in the current study, with patients with hypoalbuminaemia having approximately double the rate of IASC. However, hypoalbuminaemia was not associated with endoscopic or surgical POR, pointing towards IASC’s independent impact on POR.
Given the findings of this study, IASC should be considered as an independent risk factor for endoscopic and surgical POR of CD. Clinical management should focus on preoperative and perioperative optimization prior to ICR, including improved nutritional status [enteral preferred], albumin monitoring, smoking cessation within 3 months of ICR, weaning and avoidance of perioperative systemic corticosteroids, and optimizing elective surgical timing to avoid emergent ICR.57,58 Perioperative biologic therapy should be continued to reduce relapse of CD prior to ICR without major concern for increased risk of IASC.55,59–62 In patients who develop IASC, further studies are required to determine the most appropriate approach to reduction of POR. In the meantime, it is our suggestion that timely initiation of postoperative biologics should be considered after treatment of IASC in collaboration between gastroenterologists and surgical counterparts to mitigate the risk of subsequent endoscopic and surgical POR.
This study is not without limitations. The retrospective nature of the study introduces limitations to data collection and inherent study design bias. Following index ileocolonoscopy, all postoperative endoscopies were clinically indicated and not standardized, which may introduce detection bias, in addition to residual confounding despite robust multivariable regression modelling and sensitivity analyses. We did not collect malnutrition data to correspond to hypoalbuminaemia. The presence of anastomotic stricturing was assessed, but the degree of stricturing or requirement of intervention at the time of endoscopy was not included, which limits the ability to assess progression to surgical recurrence. Utilization of antibiotics in the postoperative course was not collected. The study was conducted at a quaternary referral centre, which may limit the generalizability and external validity of results.
5. Conclusion
Postoperative intra-abdominal septic complications are associated with increased overall risk and decreased time to endoscopic and surgical POR of CD. Although this study cannot determine a causative relationship, immune activation to enteric infection may play an independent role in the pathogenesis of POR. Optimization of pre- and perioperative medical and surgical management, including nutritional status, avoidance of perioperative steroids and continued biologic therapy, may aid in preventing IASC. In patients who experience IASC, initiation of postoperative biologics following treatment of IASC may mitigate the increased risk of endoscopic and surgical postoperative recurrence.
Supplementary Material
Acknowledgments
The authors would like to acknowledge John McMichael, Tana Zimmer and Theresa Hanslik for their assistance in data capture and study organization.
Contributor Information
Salam P Bachour, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH, USA.
Ravi S Shah, Cleveland Clinic Department of Gastroenterology, Hepatology, and Nutrition, Cleveland, OH, USA.
Florian Rieder, Cleveland Clinic Department of Gastroenterology, Hepatology, and Nutrition, Cleveland, OH, USA; Department of Inflammation and Immunity, Lerner Research Institute, Cleveland, OH, USA.
Taha Qazi, Cleveland Clinic Department of Gastroenterology, Hepatology, and Nutrition, Cleveland, OH, USA.
Jean Paul Achkar, Cleveland Clinic Department of Gastroenterology, Hepatology, and Nutrition, Cleveland, OH, USA.
Jessica Philpott, Cleveland Clinic Department of Gastroenterology, Hepatology, and Nutrition, Cleveland, OH, USA.
Bret Lashner, Cleveland Clinic Department of Gastroenterology, Hepatology, and Nutrition, Cleveland, OH, USA.
Stefan D Holubar, Cleveland Clinic Department of Colorectal Surgery, Cleveland, OH, USA.
Amy L Lightner, Cleveland Clinic Department of Colorectal Surgery, Cleveland, OH, USA.
Edward L Barnes, University of North Carolina at Chapel Hill, Division of Gastroenterology and Hepatology, Chapel Hill, NC, USA.
Jordan Axelrad, New York University Department of Gastroenterology and Hepatology, New York, NY, USA.
Miguel Regueiro, Cleveland Clinic Department of Gastroenterology, Hepatology, and Nutrition, Cleveland, OH, USA.
Benjamin Click, Cleveland Clinic Department of Gastroenterology, Hepatology, and Nutrition, Cleveland, OH, USA.
Benjamin L Cohen, Cleveland Clinic Department of Gastroenterology, Hepatology, and Nutrition, Cleveland, OH, USA.
Funding
This work was supported in part by a Cleveland Clinic Lerner Research Institute Research Program Committee grant.
Conflict of Interest
There are no disclosures relevant to the data presented in this paper. Professional disclosures are listed: Florian Rieder reports consulting and advisory boards for Adnovate, Agomab, Allergan, AbbVie, Boehringer-Ingelheim, Celgene/BMS, CDISC, Cowen, Galmed, Genentech, Gilead, Gossamer, Guidepoint, Helmsley, Index Pharma, Jannsen, Koutif, Mestag, Metacrine, Morphic, Organovo, Origo, Pfizer, Pliant, Prometheus Biosciences, Receptos, RedX, Roche, Samsung, Surmodics, Surrozen, Takeda, Techlab, Theravance, Thetis, UCB, Ysios and 89Bio and research funding from the NIH, Helmsley Charitable Trust, Crohn’s and Colitis Foundation, UCB, Pliant, BMS, AbbVie, Pfizer, Boehringer Ingelheim, Morphic and Kenneth Rainin Foundation. Jessica Philpott reports serving as a speaker for Abbvie. Edward Barnes reports consulting for AbbVie, Gilead, Pfizer and TARGET-RWE. Jordan Axelrad reports receiving research grants from BioFire Diagnostics; consultancy fees or honorarium from BioFire Diagnostics and Janssen; and holds U.S. patent 2012/0052124A1. Stefan Holubar reports consulting fees for Shionogi, Takeda and Guidepoint; research grant support from Crohn’s & Colitis Foundation. Amy Lightner reports serving as a consultant for Takeda. Miguel Regueiro reports serving on the advisory board or consultant for Abbvie, Janssen, UCB, Takeda, Pfizer, Miraca Labs, Amgen, Celgene, Seres, Allergan, Genentech, Gilead, Salix, Prometheus, Lilly, TARGET Pharma Solutions, ALFASIGMA, S.p.A. and Bristol Meyer Squibb. Benjamin Click reports consulting fees for TARGET-RWE, and speakers bureau for Takeda. Benjamin Cohen receives financial support for advisory boards and consultant for Abbvie, Celgene-Bristol Myers Squibb, Lilly, Pfizer, Sublimity Therapeutics, Takeda, TARGET RWE; CME Companies: Cornerstones, Vindico; speaking: Abbvie. All other authors report no conflicts of interests.
Data Availability Statement
The data forming this article will be shared at reasonable request to the corresponding author [B.L.C.].
References
- 1. Bernstein CN, Loftus EV, Ng SC, et al. Hospitalisations and surgery in Crohn’s disease. Gut 2012;61:622–9. [DOI] [PubMed] [Google Scholar]
- 2. Nguyen GC, Nugent Z, Shaw S, et al. Outcomes of patients with Crohn’s disease improved from 1988 to 2008 and were associated with increased specialist care. Gastroenterology 2011;141:90–7. [DOI] [PubMed] [Google Scholar]
- 3. Vind I, Riis L, Jess T, et al. Increasing incidences of inflammatory bowel disease and decreasing surgery rates in Copenhagen City and County, 2003–2005: a population-based study from the Danish Crohn colitis database. Am J Gastroenterol 2006;101:1274–82. [DOI] [PubMed] [Google Scholar]
- 4. Ramadas AV, Gunesh S, Thomas GAO, et al. Natural history of Crohn’s disease in a population-based cohort from Cardiff (1986–2003): a study of changes in medical treatment and surgical resection rates. Gut. 2010;59:1200–6. [DOI] [PubMed] [Google Scholar]
- 5. Murthy SK, Begum J, Benchimol EI, et al. Introduction of anti-TNF therapy has not yielded expected declines in hospitalisation and intestinal resection rates in inflammatory bowel diseases: a population-based interrupted time series study. Gut 2020;69:274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsai L, Ma C, Dulai PS, et al. Contemporary risk of surgery in patients with ulcerative colitis and crohn’s disease: a meta-analysis of population-based cohorts. Clin Gastroenterol Hepatol 2021;19:2031–2045.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Cruz P, Kamm MA, Hamilton AL, et al. Crohn’s disease management after intestinal resection: a randomised trial. Lancet 2015;385:1406–17. [DOI] [PubMed] [Google Scholar]
- 8. De Cruz P, Kamm MA, Hamilton AL, et al. Efficacy of thiopurines and adalimumab in preventing Crohn’s disease recurrence in high-risk patients - a POCER study analysis. Aliment Pharmacol Ther 2015;42:867–79. [DOI] [PubMed] [Google Scholar]
- 9. Regueiro M, Feagan BG, Zou B, et al. Infliximab reduces endoscopic, but not clinical, recurrence of crohn’s disease after ileocolonic resection. Gastroenterology 2016;150:1568–78. [DOI] [PubMed] [Google Scholar]
- 10. Regueiro M, Schraut W, Baidoo L, et al. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology 2009;136:441–450.e1; quiz 716. [DOI] [PubMed] [Google Scholar]
- 11. Reese GE, Nanidis T, Borysiewicz C, et al. The effect of smoking after surgery for Crohn’s disease: a meta-analysis of observational studies. Int J Colorectal Dis 2008;23:1213–21. [DOI] [PubMed] [Google Scholar]
- 12. De Cruz P, Kamm MA, Prideaux L, et al. Postoperative recurrent luminal Crohn’s disease: a systematic review. Inflamm Bowel Dis 2012;18:758–77. [DOI] [PubMed] [Google Scholar]
- 13. Yang KM, Yu CS, Lee JL, et al. Risk factors for postoperative recurrence after primary bowel resection in patients with Crohn’s disease. World J Gastroenterol 2017;23:7016–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shah R, Nakamura T, Bachour S, et al. S0825 Prior surgical history is the strongest risk factor for postoperative crohn’s disease recurrence: a guideline-based risk-stratified analysis. Am J Gastroenterol 2020;115:S424–S424. [Google Scholar]
- 15. Simillis C, Yamamoto T, Reese GE, et al. A meta-analysis comparing incidence of recurrence and indication for reoperation after surgery for perforating versus nonperforating Crohn’s disease. Am J Gastroenterol 2008;103:196–205. [DOI] [PubMed] [Google Scholar]
- 16. Regueiro M, Velayos F, Greer JB, et al. American Gastroenterological Association Institute technical review on the management of Crohn’s disease after surgical resection. Gastroenterology 2017;152:277–95.e3. [DOI] [PubMed] [Google Scholar]
- 17. Nguyen GC, Loftus EV, Hirano I, et al. American Gastroenterological Association Institute Guideline on the management of Crohn’s disease after surgical resection. Gastroenterology 2017;152:271–5. [DOI] [PubMed] [Google Scholar]
- 18. Geremia A, Biancheri P, Allan P, et al. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev 2014;13:3–10. [DOI] [PubMed] [Google Scholar]
- 19. Lee SH, Kwon JE, Cho M-L. Immunological pathogenesis of inflammatory bowel disease. Intest Res 2018;16:26–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo Z, Cao L, Guo F, et al. The presence of postoperative infectious complications is associated with the risk of early postoperative clinical recurrence of Crohn’s disease. World J Surg 2017;41:2371–7. [DOI] [PubMed] [Google Scholar]
- 21. Iesalnieks I, Kilger A, Glaß H, et al. Intraabdominal septic complications following bowel resection for Crohn’s disease: detrimental influence on long-term outcome. Int J Colorectal Dis 2008;23:1167–74. [DOI] [PubMed] [Google Scholar]
- 22. Ollech JE, Aharoni-Golan M, Weisshof R, et al. Differential risk of disease progression between isolated anastomotic ulcers and mild ileal recurrence after ileocolonic resection in patients with Crohn’s disease. Gastrointest Endosc 2019;90:269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bachour SP, Shah RS, Lyu R, et al. S699 Isolated anastomotic lesions do not increase risk for severe endoscopic disease progression in postoperative Crohn’s disease. Off J Am Coll Gastroenterol. ACG 2021;116:S316. [Google Scholar]
- 24. Jostins L, Ripke S, Weersma RK, et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Hertogh G, Geboes K. Crohn’s disease and infections: a complex relationship. Medscape Gen Med 2004;6:14. [PMC free article] [PubMed] [Google Scholar]
- 26. Porter CK, Tribble DR, Aliaga PA, et al. Infectious gastroenteritis and risk of developing inflammatory bowel disease. Gastroenterology 2008;135:781–6. [DOI] [PubMed] [Google Scholar]
- 27. Keithlin J, Sargeant J, Thomas MK, et al. Systematic review and meta-analysis of the proportion of Campylobacter cases that develop chronic sequelae. BMC Public Health 2014;14:1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gradel KO, Nielsen HL, Schønheyder HC, et al. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology 2009;137:495–501. [DOI] [PubMed] [Google Scholar]
- 29. Axelrad JE, Joelson A, Green PHR, et al. Enteric infections are common in patients with flares of inflammatory bowel disease. Am J Gastroenterol 2018;113:1530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Axelrad JE, Joelson A, Nobel YR, et al. Enteric infection in relapse of inflammatory bowel disease: the utility of stool microbial PCR testing. Inflamm Bowel Dis 2017;23:1034–9. [DOI] [PubMed] [Google Scholar]
- 31. Mylonaki M, Langmead L, Pantes A, et al. Enteric infection in relapse of inflammatory bowel disease: importance of microbiological examination of stool. Eur J Gastroenterol Hepatol 2004;16:775–8. [DOI] [PubMed] [Google Scholar]
- 32. Lobatón T, Domènech E. Bacterial intestinal superinfections in inflammatory bowel diseases beyond Clostridum difficile. Inflamm Bowel Dis 2016;22:1755–62. [DOI] [PubMed] [Google Scholar]
- 33. Zorzi F, Monteleone I, Sarra M, et al. Distinct profiles of effector cytokines mark the different phases of Crohn’s disease. PLoS One 2013;8:e54562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sokol H, Brot L, Stefanescu C, et al. Prominence of ileal mucosa-associated microbiota to predict postoperative endoscopic recurrence in Crohn’s disease. Gut 2020;69:462–72. [DOI] [PubMed] [Google Scholar]
- 35. Sakuraba A, Sato T, Kamada N, et al. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn’s disease. Gastroenterology 2009;137:1736–45. [DOI] [PubMed] [Google Scholar]
- 36. Sensi B, Siragusa L, Efrati C, et al. The role of inflammation in Crohn’s disease recurrence after surgical treatment. J. Immunol. Res 2020;2020:e8846982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bsat M, Chapuy L, Rubio M, et al. Differential pathogenic Th17 profile in mesenteric lymph nodes of Crohn’s disease and ulcerative colitis patients. Front Immunol 2019;10:1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chapman CG, Yamaguchi R, Tamura K, et al. Characterization of T-cell receptor repertoire in inflamed tissues of patients with crohn’s disease through deep sequencing. Inflamm Bowel Dis 2016;22:1275–85. [DOI] [PubMed] [Google Scholar]
- 39. Sathaliyawala T, Kubota M, Yudanin N, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 2013;38:187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Camus M, Esses S, Pariente B, et al. Oligoclonal expansions of mucosal T cells in Crohn’s disease predominate in NKG2D-expressing CD4 T cells. Mucosal Immunol 2014;7:325–34. [DOI] [PubMed] [Google Scholar]
- 41. Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol 2013;13:309–20. [DOI] [PubMed] [Google Scholar]
- 42. Machiels K, Pozuelo del Río M, Martinez-De la Torre A, et al. Early postoperative endoscopic recurrence in Crohn’s disease is characterised by distinct microbiota recolonisation. J. Crohns Colitis 2020;14:1535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yamamoto T, Umegae S, Kitagawa T, et al. Mucosal cytokine production during remission after resection for Crohn’s disease and its relationship to future relapse. Aliment Pharmacol Ther 2004;19:671–8. [DOI] [PubMed] [Google Scholar]
- 44. Iaculli E, Agostini M, Biancone L, et al. C-reactive protein levels in the perioperative period as a predictive marker of endoscopic recurrence after ileo-colonic resection for Crohn’s disease. Cell Death Discov 2016;2:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ruffolo C, Scarpa M, Faggian D, et al. Subclinical intestinal inflammation in patients with Crohn’s disease following bowel resection: a smoldering fire. J Gastrointest Surg 2010;14:24–31. [DOI] [PubMed] [Google Scholar]
- 46. Gonsky R, Fleshner P, Deem RL, et al. Association of ribonuclease T2 gene polymorphisms with decreased expression and clinical characteristics of severity in Crohn’s disease. Gastroenterology 2017;153:219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Coffey CJ, Kiernan MG, Sahebally SM, et al. Inclusion of the mesentery in ileocolic resection for Crohn’s disease is associated with reduced surgical recurrence. J. Crohns Colitis 2018;12:1139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Holubar SD, Gunter RL, Click BH, et al. Mesenteric excision and exclusion for ileocolic crohn’s disease: feasibility and safety of an innovative, combined surgical approach with extended mesenteric excision and Kono-S anastomosis. Dis Colon Rectum 2022;65:e5–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arijs I, Hertogh GD, Lemmens B, et al. Effect of vedolizumab (anti-α4β7-integrin) therapy on histological healing and mucosal gene expression in patients with UC. Gut 2018;67:43–52. [DOI] [PubMed] [Google Scholar]
- 50. Zeissig S, Rosati E, Dowds CM, et al. Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease. Gut 2019;68:25–39. [DOI] [PubMed] [Google Scholar]
- 51. Benson JM, Peritt D, Scallon BJ, et al. Discovery and mechanism of ustekinumab. mAbs 2011;3:535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Holzheimer RG, Molloy RG, Wittmann DH. Postoperative complications predict recurrence of Crohn’s disease. Eur J Surg Acta Chir 1995;161:129–35. [PubMed] [Google Scholar]
- 53. Riss S, Schuster I, Papay P, et al. Repeat intestinal resections increase the risk of recurrence of Crohn’s disease. Dis Colon Rectum 2013;56:881–7. [DOI] [PubMed] [Google Scholar]
- 54. Abdelaal K, Jaffray B. Colonic disease site and perioperative complications predict need for later intestinal interventions following intestinal resection in pediatric Crohn’s disease. J Pediatr Surg 2016;51:272–6. [DOI] [PubMed] [Google Scholar]
- 55. Shah RS, Bachour S, Jia X, et al. Hypoalbuminemia, not biologic exposure, is associated with postoperative complications in crohn’s disease patients undergoing ileocolic resection. J. Crohns Colitis 2021;15:1142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Neary PM, Aiello AC, Stocchi L, et al. High-risk ileocolic anastomoses for Crohn’s disease: when is diversion indicated?. J Crohns Colitis 2019;13:856–63. [DOI] [PubMed] [Google Scholar]
- 57. Morar PS, Hodgkinson JD, Thalayasingam S, et al. Determining predictors for intra-abdominal septic complications following ileocolonic resection for Crohn’s disease—considerations in pre-operative and peri-operative optimisation techniques to improve outcome. J Crohns Colitis 2015;9:483–91. [DOI] [PubMed] [Google Scholar]
- 58. Huang W, Tang Y, Nong L, et al. Risk factors for postoperative intra-abdominal septic complications after surgery in Crohn’s disease: A meta-analysis of observational studies. J Crohns Colitis 2015;9:293–301. [DOI] [PubMed] [Google Scholar]
- 59. Colombel JF, Loftus EV, Tremaine WJ, et al. Early postoperative complications are not increased in patients with Crohn’s disease treated perioperatively with infliximab or immunosuppressive therapy. Am J Gastroenterol 2004;99:878–83. [DOI] [PubMed] [Google Scholar]
- 60. Kunitake H, Hodin R, Shellito PC, et al. Perioperative treatment with infliximab in patients with Crohn’s disease and ulcerative colitis is not associated with an increased rate of postoperative complications. J Gastrointest Surg 2008;12:1730–1736; discussion 1736–7. [DOI] [PubMed] [Google Scholar]
- 61. Marchal L, D’Haens G, Van Assche G, et al. The risk of post-operative complications associated with infliximab therapy for Crohn’s disease: a controlled cohort study. Aliment Pharmacol Ther 2004;19:749–54. [DOI] [PubMed] [Google Scholar]
- 62. Canedo J, Lee S-H, Pinto R, et al. Surgical resection in Crohn’s disease: is immunosuppressive medication associated with higher postoperative infection rates?. Colorectal Dis 2011;13:1294–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data forming this article will be shared at reasonable request to the corresponding author [B.L.C.].