Abstract
Background
Native Americans (NAs) are more likely to experience chronic pain than non-Hispanic Whites (NHWs); however, the proximate causes predisposing NAs to chronic pain remain elusive. Likely due to centuries of adversity, discrimination, and marginalization, NAs report greater psychological stress than NHWs, which may place them at risk for sleep problems, a well-established risk factor for chronic pain onset.
Purpose
This study examined the effects of psychological stress and sleep problems on subjective and physiological measures of pain processing in NAs and NHWs.
Methods
Structural equation modeling was used to determine whether ethnicity (NA or NHW) was associated with psychological stress or sleep problems and whether these variables were related to conditioned pain modulation of pain perception (CPM-pain) and the nociceptive flexion reflex (CPM-NFR), temporal summation of pain (TS-pain) and NFR (TS-NFR), and pain tolerance in a sample of 302 (153 NAs) pain-free participants.
Results
NAs experienced more psychological stress (Estimate = 0.027, p = .009) and sleep problems (Estimate = 1.375, p = .015) than NHWs. When controlling for age, sex, physical activity, BMI, and general health, NA ethnicity was no longer related to greater sleep problems. Psychological stress was also related to sleep problems (Estimate = 30.173, p = <.001) and psychological stress promoted sleep problems in NAs (indirect effect = 0.802, p = .014). In turn, sleep problems were associated with greater TS-pain (Estimate = 0.714, p = .004), but not other pain measures.
Conclusions
Sleep problems may contribute to chronic pain risk by facilitating pain perception without affecting facilitation of spinal neurons or endogenous inhibition of nociceptive processes. Since psychological stress promoted pain facilitation via enhanced sleep problems, efforts to reduce psychological stress and sleep problems among NAs may improve health outcomes.
Keywords: Sleep, Chronic pain risk, Nociceptive flexion reflex, Temporal summation, Health disparities
Psychological stress was associated with sleep problems that in turn were associated with pain facilitation processes in Native Americans.
Introduction
Population studies suggest that chronic pain affects 11%–40% of Americans [1]. Beyond the physical and emotional toll, the estimated annual national costs associated with chronic pain (e.g., treatment, disability services, lost productivity) range from $560 to $635 billion [2]. Relative to the general US population, Native Americans (NAs) are more likely to develop chronic pain [3–8]. Despite this, there is a dearth of research seeking to identify the proximate and ultimate mechanisms predisposing NAs to chronic pain.
In the wake of centuries of adversity (e.g., genocide, colonization, forced relocation), NAs disproportionately face several psychosocial stressors relative to non-Hispanic Whites (NHWs). For example, NAs are more likely to experience childhood and adult trauma [3, 9], food insecurity [10], poverty [11, 12], and report greater experiences of discrimination [13]. Together, these stressors faced by NAs may contribute to enhanced psychological stress, a state characterized as feeling that one’s ability to cope is outweighed by environmental demands [14]. Psychological stress has been hypothesized to predict chronic pain onset [15], and it is a risk factor for myriad adverse health outcomes (e.g., stroke, cancer, cardiovascular disease, and diabetes) [16–19]. Results from studies attempting to link psychological stress with specific pronociceptive (i.e., pain-promoting) mechanisms, however, have been mixed [20, 21], suggesting that this relationship may be influenced by other pain-promoting factors [22].
One such factor linking psychological stress and chronic pain is impaired sleep. Higher psychological stress predicts poorer outcomes on multiple sleep parameters, including lower sleep duration [23], increased physiological arousal during nonrapid eye movement sleep [24], and diminished sleep quality [25, 26]. Moreover, there is evidence suggesting that NAs may experience more sleep problems than NHWs, perhaps as a function of increased psychological stress [27]. In one study [27], NAs reported insufficient sleep more frequently than NHWs, and this relationship remained statistically significant when controlling for potentially confounding variables, including education, sex, obesity, and lifestyle factors. After controlling for frequent mental distress (i.e., subjective reports of stress, depression, and emotional difficulties on 14 or more of the past 30 days), however, the difference between NHWs and NAs on insufficient sleep was no longer statistically significant. In contrast, somewhat contradictory findings were reported by another study [28]. They found lifetime discrimination was associated with poorer self-reported sleep in NAs even when controlling for variables similar to psychological stress, including perceived stress and depressive symptoms [28]. Together, this literature suggests that NAs may experience worse sleep than NHWs, although the extent to which psychological stress may promote sleep impairment remains unclear.
Over time, people who experience impaired sleep are more likely to develop chronic pain [29]. The comorbidity between sleep problems and chronic pain is also well established, with studies finding that between 45% and 88% of chronic pain patients also report significant sleep complaints [30–33]. Early research linking impaired sleep to chronic pain hypothesized a reciprocal relationship [32], with recent studies finding that sleep impairment predicts chronic pain onset above and beyond chronic pain predicting sleep impairment [30, 34–36], implicating insufficient sleep as a salient pain-promoting factor.
Indeed, people with impaired sleep demonstrate similar patterns of psychophysiological functioning as people with chronic pain. For instance, a study of healthy, pain-free participants found that sleep restriction was associated with impaired pain inhibition during a quantitative sensory testing task called conditioned pain modulation (CPM), suggesting that sleep restriction disrupts the body’s descending inhibitory pain circuits [37], perhaps via reduced endogenous opioid responsivity [38]. CPM measures the degree to which a response to a noxious test stimulus (e.g., a painful electric stimulation delivered over the sural nerve) is inhibited by the concomitant administration of another noxious stimulus, called the conditioning stimulus (e.g., placing one’s hand in painfully cold water) [39]. Pain inhibition during CPM can be measured using pain ratings (CPM-pain) in response to the test stimulus or by physiological correlates of pain-processing signals in the spinal cord (i.e., spinal nociception) in response to the test stimulus, including the nociceptive flexion reflex (NFR; CPM-NFR), a spinally-mediated withdrawal reflex. Although correlated, CPM-pain and CPM-NFR may diverge due in part to multiple modulatory networks that can be engaged by the CPM task [40]. Relative to individuals without chronic pain, impaired CPM-pain inhibition has been observed across individuals with various chronic pain conditions [39, 41]. Moreover, less CPM-pain inhibition has been found to predict greater postoperative pain [42, 43], implicating impaired CPM as a promising predictor of chronic pain risk [44].
In addition to impairing pain inhibition, sleep problems may confer chronic pain risk via facilitation of pain amplification at the spinal level (i.e., wind-up), which is believed to assess processes that contribute to central sensitization [45]. Central sensitization is a prolonged state of neuronal hyperexcitability and synaptic efficiency that can lead to enhanced pain sensitivity (i.e., hyperalgesia), and it is believed to underlie many chronic pain conditions [46]. It has been hypothesized that sleep problems may promote central sensitization via prolonged low-grade inflammation that increases spinal neuronal excitability [47]. Although unstudied in NAs, a previous study of African Americans and NHWs found that reduced sleep efficiency (related to increased depressive symptoms) mediated ethnic differences on a temporal summation (TS) task [48], which is the psychophysical correlate of wind-up. During TS, a series of identical, painful noxious stimuli are presented in quick succession. Because the series of stimuli evoke hyperexcitability of spinal neurons, later stimuli in the series produce greater pain compared to the first stimulus [49]. Although most healthy individuals show summation during TS [50], greater summation has been observed in chronic pain populations when compared to pain-free populations [51]. Additionally, greater preoperative TS predicts postoperative pain [52, 53]; together, these findings suggest that heightened TS may be associated with chronic pain risk. Like CPM, TS can be measured using self-report pain ratings (TS-pain) or by measurement of NFR magnitude (TS-NFR); the two measures can diverge because TS-pain can be affected by supraspinal processes (e.g., pain catastrophizing) that do not affect TS-NFR [54, 55].
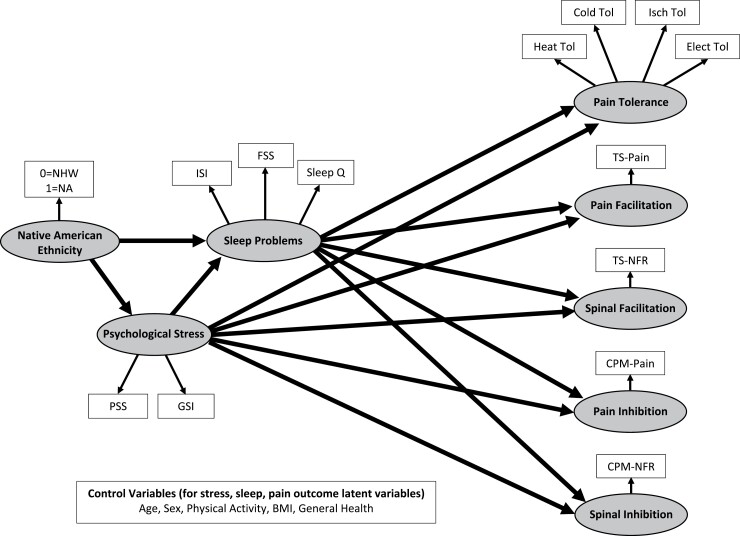
Together, there is ample evidence linking sleep problems to physiological mechanisms related to chronic pain risk. Thus, identification of psychosocial variables that contribute to the onset and maintenance of sleep problems may yield a more comprehensive understanding of the mechanisms by which chronic pain disparities across racial and ethnic groups exist. As such, structural equation modeling (SEM) will be used to analyze the relationships between ethnicity (NA or NHW), psychological stress, sleep problems, and performance on experimental pain tasks. Figure 1 depicts the hypothesized model that will be tested in the current study. We predict that NAs will experience greater psychological stress and sleep problems that will in turn increase pain and spinal facilitation (enhanced TS-pain and TS-NFR, respectively), impair inhibition of pain and spinal nociception (reduced CPM-pain and CPM-NFR, respectively), and promote hyperalgesia (reduced pain tolerance).
Fig. 1.
Proposed measurement and structural model for the current study.
Methods
Participants
The study’s sample consisted of 302 healthy, chronic pain-free participants from the Oklahoma Study of Native American Pain Risk (OK-SNAP). Till date, OK-SNAP remains the largest and most comprehensive assessment of pain processing in NAs, and data were collected between March 2014 and October 2018. Participants were recruited from tribal and nontribal newspapers ads, fliers, personal communications with NA groups, email announcements, online platforms, and word of mouth. Interested participants then completed a phone screen with study researchers to preliminarily determine eligibility. Participants deemed eligible following the phone screen were scheduled for the first of two laboratory testing days, which began with an additional screening for eligibility criteria.
Exclusion criteria for OK-SNAP were: (1) individuals <18 years of age, (2) self-reported history of cardiovascular, neuroendocrine, musculoskeletal, or neurological disorders, (3) self-reported chronic pain conditions or current acute pain, (4) BMI ≥ 35 (due to difficulty in obtaining electromyogram signals for NFR), (5) current psychotic symptoms (assessed by self-report and by the Psychosis Screening Questionnaire), (6) current/recent use of anti-depressants, anxiolytic, analgesic, stimulant, or anti-hypertensive medication, (7) substance use problems, and/or (8) an inability to read and speak English. NA ethnicity was verified for all NA participants by Certificate Degree of Indian Blood or tribal membership cards. NA participants were predominately from southern plains and eastern Oklahoma tribes. All participants provided verbal and written informed consent, including an overview of study procedures and the reminder that they could withdraw at any time. Participants were compensated with $100 for the completion of each testing day for their time and effort. OK-SNAP was approved by IRBs of The University of Tulsa, Cherokee Nation, and the Indian Health Service Oklahoma City Area Office.
Of the 329 participants initially eligible for OK-SNAP, 247 completed both testing days, 41 completed one testing day, and 39 completed partial tasks during one testing day. A computer malfunction resulted in the loss of two participants’ data. Additionally, 22 participants were neither NA nor NHW, and were subsequently excluded from the current analyses. Three additional participants were excluded due to a diagnosis of Type 1 or Type 2 diabetes. Because the analyses did not use listwise deletion, data from 302 participants (153 NA and 149 NHW) were available for use in the current study (Table 1). Rhudy et al. [56] provide a full description of participants in this sample.
Table 1.
Means, Standard Deviations, and Intercorrelations for all Study Variables (N = 302).
| Variable | M | SD | NA Ethn | Stress | Psych Dis | Insomn | Fatigue | SleepQ | Heat Tol | Cold Tol | Isch Tol | Elec Tol | TS-Pain | TS-NFR | CPM-Pain | CPM-NFR | Age | Sex | Phys Act | BMI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NA Ethn | — | — | 1 | |||||||||||||||||
| Stress | 13.848 | 5.971 | 0.123* | 1 | ||||||||||||||||
| Psych Dis# | 0.122 | 0.087 | 0.146* | 0.706* | 1 | |||||||||||||||
| Insomn | 6.752 | 4.717 | 0.203* | 0.405* | 0.543* | 1 | ||||||||||||||
| Fatigue | 27.354 | 9.576 | 0.218* | 0.467* | 0.506* | 0.576* | 1 | |||||||||||||
| SleepQ | 1.129 | 0.711 | 0.198* | 0.338* | 0.426* | 0.782* | 0.473* | 1 | ||||||||||||
| Heat Tol | 45.610 | 1.743 | −0.085 | −0.109 | −0.009 | 0.025 | 0.027 | 0.057 | 1 | |||||||||||
| Cold Tol# | 1.707 | 0.358 | −0.216* | 0.061 | 0.068 | 0.015 | −0.043 | 0.054 | 0.438* | 1 | ||||||||||
| Isch Tol# | 2.147 | 0.413 | −0.114* | −0.022 | 0.023 | 0.038 | −0.009 | 0.013 | 0.461* | 0.394* | 1 | |||||||||
| Elec Tol | 30.768 | 12.342 | 0.023 | 0.094 | 0.085 | 0.118* | 0.030 | 0.129* | 0.404* | 0.408* | 0.379* | 1 | ||||||||
| TS-Pain | 11.997 | 13.063 | 0.098 | 0.091 | 0.162* | 0.234* | 0.152* | 0.175* | −0.083 | −0.080 | −0.109 | −0.055 | 1 | |||||||
| TS-NFR | 0.555 | 0.489 | 0.076 | 0.014 | 0.063 | 0.066 | 0.040 | 0.044 | 0.111 | −0.047 | 0.082 | 0.075 | 0.198* | 1 | ||||||
| CPM-Pain | −6.750 | 7.351 | −0.118* | 0.026 | 0.068 | 0.106 | −0.070 | 0.072 | −0.063 | 0.130* | 0.016 | 0.102 | 0.015 | −0.085 | 1 | |||||
| CPM-NFR | −0.007 | 0.370 | 0.067 | 0.070 | 0.088 | 0.062 | 0.074 | 0.063 | 0.001 | −0.187* | −0.165* | −0.112 | 0.044 | 0.271* | −0.011 | 1 | ||||
| Age# | 1.437 | 0.165 | 0.106 | −0.201* | −0.083 | 0.020 | −0.029 | −0.084 | 0.039 | −0.190* | −0.051 | −0.007 | 0.180* | 0.327* | 0.158* | 0.160* | 1 | |||
| Sex | — | — | 0.085 | −0.012 | −0.021 | −0.033 | −0.087 | −0.057 | −0.372* | −0.147* | −0.180* | −0.052 | 0.062 | −0.121* | 0.042 | −0.154* | 0.045 | 1 | ||
| Phys Act# | 3.762 | 0.447 | 0.085 | 0.062 | 0.076 | 0.151* | 0.076 | 0.144* | 0.086 | 0.050 | 0.037 | 0.120* | 0.173* | 0.126* | 0.116* | 0.115* | 0.066 | −0.117* | 1 | |
| BMI | 25.017 | 4.209 | 0.204* | 0.000 | 0.040 | 0.081 | 0.060 | 0.012 | 0.017 | −0.126* | −0.180* | 0.077 | −0.006 | 0.126* | 0.034 | 0.142* | 0.346* | −0.081 | 0.061 | 1 |
| Gen Hlth | 79.497 | 13.397 | −0.062 | −0.316* | −0.320* | −0.263* | −0.355* | −0.198* | 0.025 | 0.074 | −0.037 | −0.062 | 0.021 | −0.013 | 0.042 | 0.014 | 0.082 | 0.003 | 0.011 | −0.059 |
Note: All values in the table were created after transformations, winsorizing outliers, and multiple imputation. Native American (NA) ethnicity was coded 0 = non-Hispanic white, 1 = Native American Sex was coded 0 = male, 1 = female. Psych Dis = psychological distress. SleepQ = sleep quality. TS = temporal summation. NFR = nociceptive flexion reflex. CPM = conditioned pain modulation. CPM variables were coded such that lower (negative) scores were associated with greater inhibition, whereas higher (positive) scores were associated with facilitation. Phys Act = physical activity. BMI = body mass index. Gen Hlth = general health perception. Tol = tolerance.
Log10 transformed.
p < .05.
The sample size estimates needed for structural equation modeling are typically based on participant-to-parameter ratios. Acceptable ratios have been suggested to vary between 5:1 [57] and 20:1 [58]. In the present study (N = 302), the model predicting pain outcomes without control variables had a ratio of 8.4:1. The addition of control variables, however, reduced the ratio to 3.3:1 (a samples size of 455–1,820 would be needed to achieve a ratio of 5:1 up to 20:1). Thus, interpretation of the model with controls should be made with caution and will be used primarily to examine whether results were similar with statistical controls in place.
Brief Overview of Procedures
OK-SNAP was conducted over two testing sessions, with each session lasting approximately 4–6 h. As mentioned earlier, informed consent and screening of eligibility criteria were conducted prior to the performance of study procedures. The order of the testing days for each participant was randomized (blocking for sex and ethnicity) to reduce the potential of order effects. One testing day included assessing pain tolerance, including electric pain tolerance, heat tolerance, cold tolerance, and ischemic tolerance. On the other testing day, measures of pain modulation (CPM-pain/CPM-NFR, TS-pain/TS-NFR) were assessed. Between tasks, participants had breaks to minimize the potential for carryover effects. For a full description, see [56].
Testing Environment and Apparatus
Participants were seated in a comfortable reclining chair (Perfect Chair Zero Gravity Recliner, Human Touch, Long Beach, CA) in a sound-attenuated and electrically shielded room during testing. Participants were monitored by an experimenter in a nearby room via audio and video equipment. Questionnaires were completed on a computer. Experimental procedures were controlled using custom-built software (LabVIEW; National Instruments, Austin, TX).
To deliver electric pain stimuli, a constant current stimulator (Digitimer DS7A; Hertfordshire, England) and a bipolar electrode (Nicolet; 30 mm inter-electrode distance) filled with a conductive gel (EC60, Grass Technologies) were attached to the participant’s left ankle. Electric stimulations were only delivered to participants’ ankles throughout the study. A Contact Heat Evoked Potential Stimulator (CHEPS) thermode from a Medoc (Haifa, Israel) Pathway device was used to deliver heat stimuli. For measuring cold pain tolerance and the conditioning stimulus for CPM tasks, a circulating water bath (Thermo Fisher Scientific, Pittsburgh, PA) was used. The water level within the cold-water bath was kept constant (6 in. deep) for all participants to maximize the standardization of procedures and to ensure that participants received similar cold exposure to their hands/forearms.
To prepare the electromyogram (EMG) for NFR recording, the skin was first cleaned with alcohol and exfoliated using Nuprep gel (Weaver and Company, Aurora, CO) to achieve impedances <5 kΩ. Prior to electrode application, electrodes were filled with a conductive gel (EC60; Grass Technologies, West Warwick, RI). The electrodes were then placed over the left biceps femoris muscle to assess the magnitude of the nociceptive flexion reflex (NFR) via EMG recording. A Grass Technologies Model 15LT amplifier with AC module 15A54 was used to filter (10–300 Hz) and amplify (×10,000) the signal. The signal was then sampled and digitized (1,000 Hz) using a National Instruments (Austin, TX) analog-to-digital converter. Recordings were then stored on a computer hard drive.
Pain Modulation: TS and CPM
Determination of Electric Stimulus Intensity for TS and CPM
The intensity of electric stimulations (in mA) required to reliably elicit an NFR and moderate pain for each participant was determined using three tasks: NFR threshold, Pain30, and 3-stimulation threshold. NFR threshold testing consisted of three ascending and descending staircases of electric stimulations to the participants’ sural nerve to determine the minimum stimulation intensity required to reliably elicit NFR (assessed by biceps femoris EMG). During NFR threshold testing, participants also rated their pain due to the stimulations on a 0–100 visual analog scale (VAS), which ranged from “no pain sensation” to “the most intense pain sensation imaginable.” If the stimulations during NFR threshold did not yield a pain rating of >30 on the VAS, an ascending series of stimulations was given until the rating was >30. The stimulus intensity necessary for a VAS rating >30 (i.e., Pain30) was recorded. Finally, an ascending staircase consisting of trains of three stimuli, given at a frequency of 2 Hz, were given to determine the intensity required to elicit NFR on the third stimulus in the train (3-stimulation threshold).
Once NFR threshold, Pain30, and 3-stimulation threshold were determined, stimulus intensity was set for the TS-NFR and CPM tasks. For TS, stimulus intensity was set at the higher value (in mA) of 1.2× NFR threshold or 1.2 × 3-stimulation threshold. CPM test stimulus intensity was set at the highest value (in mA) of 1.2× NFR threshold, 1× Pain30, or 1.2 × 3-stimulation threshold.
TS-NFR
The TS-NFR paradigm used in OK-SNAP assessed the amount of NFR summation (the increase in NFR magnitude) following a series of three suprathreshold electric stimuli. To assess this, five series of three suprathreshold electric stimuli (0.5 s inter-stimulation intervals) were delivered. Once each series was finished, participants were prompted by a computer-presented VAS to indicate the pain intensity for each of the three stimulations. After the participant completed the ratings, there was an inter-series interval of 8–12 s before the next series of stimulations began. Given the potential for signal contamination caused by tensing in the biceps femoris during this task, baseline EMG in the 60 ms prior to the third stimulus for each series was visually inspected by the experimenter for excessive muscle tension or voluntary movement. If there was excessive noise in the mean rectified EMG (>5 µV), the series was repeated. NFR magnitude was then calculated in d-units. To do this, the average biceps femoris EMG activity in the 60 ms prior to the first stimulation in each series was subtracted from the average biceps femoris EMG activity in the 70–150 ms after each stimulus in the series. This difference was then divided by the average of the standard deviations of the rectified EMG during the two intervals. Given that TS tasks measure the change in pain perception/nociception over a period of successive stimuli at the same intensity, TS-NFR was defined as the difference in the NFR magnitude between the first stimulus in a series subtracted from the third stimulus in the same series. This TS-NFR change score was then averaged across the five series to produce the final TS-NFR score.
TS-Pain
Assessing TS-pain from the 3-stimulus series noted above is not ideal, because the 2 Hz series is too fast for participants to make pain ratings immediately following each stimulus. Thus, all three ratings must be made after the last stimulus, which can be affected by recall bias, especially for the first two stimuli in the series. Therefore, we took a different approach similar to other studies of electric and mechanical TS-pain [59, 60]. Five single electric stimuli were delivered at the same intensity as TS-NFR (8–12 s interstimulus interval). Participants were prompted to make pain ratings using the same VAS immediately after each stimulation in the series. The TS-pain variable was created by taking the average pain rating from the five single stimuli and subtracting that value from the average pain rating of the third stimulus in each series in TS-NFR.
CPM
The CPM paradigm in OK-SNAP included measures of pain and NFR in response to electric test stimuli (delivered to the ankle) before and during the presentation of a tonic conditioning stimulus. The conditioning stimulus was a circulating, 10 ± 0.1°C water bath. Each CPM phase lasted 2 min total, beginning with a 20 s wait period (to allow engagement of descending modulatory circuits), followed by five electric test stimuli delivered at random over an 8–12 s interstimulus interval. There was a 2-min break between the baseline and conditioning phase of CPM. Using a numerical rating scale presented on the computer screen (0 “no pain”, 20 “mild pain”, 40 “moderate pain”, 60 “severe pain”, 80 “very severe pain”, and 100 “worst possible pain”), participants verbally stated their pain ratings in response to the electrical test stimulations. The experimenter, who could hear the verbal pain ratings, recorded the ratings from the study control room. For CPM-NFR, NFR magnitude was calculated as a d-score. To calculate this, the mean rectified biceps femoris EMG activity in the 90–150 ms following the electric stimulation was subtracted by the mean rectified biceps femoris EMG activity in the 60 ms preceding the stimulation. This value was then divided by the average standard deviation of the rectified EMG activity in these two intervals. Approximately 3% of CPM-NFR trials had average EMG baselines >3.0 μV and were thus excluded from analyses. Both CPM-pain and CPM-NFR variables were calculated by subtracting the average pain ratings/NFR magnitude during the conditioning phase by the average pain rating/NFR magnitude during the baseline phase.
Pain Tolerance
Different stimulus modalities are known to impact different nociceptive processes [61]. Nonetheless, different pain tolerance measures should assess a common latent construct associated with the capacity to tolerate noxious stimuli. Indeed, pain tolerance measures were all correlated about r = .40 (see Table 1). During these tasks, participants made ratings on a VAS with the anchors 0 (“no pain”) and 100 (“maximum tolerable pain”).
Cold Tolerance
Cold tolerance was assessed using a cold pressor. Participants were asked to submerge their hand and forearm with fingers spread apart into a 6 ± 0.1°C circulating water bath. During submersion, participants were asked to continually provide pain ratings on the computer using the VAS described earlier. Participants were instructed to remove their hand from the water once they reached the maximum VAS rating. The time in seconds that they kept their hand in the cold-water bath was used to calculate pain tolerance. If participants had not removed their hand by 300 s (5 min), they were prompted via computer instruction to remove their hand from the water bath.
Ischemia Tolerance
Ischemic pain tolerance was assessed using a forearm tourniquet test. Using a dynamometer (Lafayette Instrument Company, IN), participants performed hand exercises at 50% grip strength at one second intervals for 2 min before raising their arm for 15 s to allow for desanguination. A blood pressure cuff was then placed over the biceps on that arm and inflated to a pressure of 220 mmHg to reduce blood flow. During occlusion, participants continuously rated their pain on the computer-presented VAS. The time (in seconds) to achieve a maximum tolerance rating on the VAS was defined as ischemic pain tolerance. Alternatively, the participant was prompted by computer instruction to end the task if the maximum duration (25 min) was reached.
Electric Tolerance
To calculate electric pain tolerance, a single ascending staircase of stimulations starting at 0 mA (and increasing by intervals of 2 mA) was given. Following each stimulus, participants were instructed to rate the pain they experienced using the computer-presented VAS. The stimulations increased until the participant rated the stimulus as the maximum tolerable pain (or they reached the maximum intensity of 50 mA), at which point the stimulus intensity that evoked that rating was recorded.
Heat Tolerance
Heat pain tolerance was assessed five times (one practice trial, four averaged trials). To measure heat tolerance, the CHEPS thermode was attached to the participants’ left volar forearm [62]. Each trial began at 32°C and heated up at a rate of 0.5°C/s until the participant pressed a button to end the trial once the pain became intolerable. The maximum intensity was set to 51°C to ensure participant safety.
Sleep Problems
A latent variable of sleep problems, reflecting dysfunction associated with a lack of sleep, was created using the sleep quality item of the Pittsburgh Sleep Quality Index (PSQI), the total score of the Insomnia Severity Index (ISI), and the total score from the Fatigue Severity Scale (FSS).
Pittsburgh Sleep Quality Index (PSQI) Sleep Quality Item
Although the PSQI is considered a reliable and valid measure of insomnia [63], participants did not reliably report AM versus PM on their bedtime and wake up time, thus the overall PSQI score could not be calculated. So, the perceived sleep quality item from the PSQI was used instead. This item asks participants to rate their overall sleep quality in the past month on a four-point scale ranging from “very good” to “very bad” [64].
Insomnia Severity Index (ISI)
Like the PSQI, the ISI is widely utilized as a screener for insomnia and other sleep disorders associated with a lack of sleep. The ISI is a 7-item, self-report questionnaire assessing difficulty in falling asleep, staying asleep, or waking up too early [65]. In addition, the ISI measures one’s satisfaction, concern, and perceived interference in daily activities due to sleep pattern [65]. Like the PSQI, the ISI has demonstrated good reliability and validity, and it is one of the most widely used measures of sleep impairment [63]. The ISI displayed good internal consistency in this sample for NA participants (α = 0.872), NHW participants (α = 0.859), and the overall sample (α = 0.872).
Fatigue Severity Scale (FSS)
Finally, the current study used the total score from the FSS, a 9-item self-report measure of fatigue, a symptom of myriad medical and psychological disorders associated with significant functional impairment [66]. Although this scale does not directly assess fatigue associated with sleep problems, people with sleep problems score significantly higher on the FSS than people without sleep problems [67], suggesting that it may be used as a measure of sleep impairment. Moreover, because OK-SNAP included only healthy, pain-free participants, differences in fatigue in this sample were conceptualized as related to sleep problems. Additionally, the formation of a latent variable from the three measures should only extract variance from the FSS related to sleep problems. In this sample, the FSS had good internal consistency for NA participants (α = 0.878), NHW participants (α = 0.879), and the overall sample (α = 0.882).
Psychological Stress
The present study used a latent psychological stress variable built from the total score of the Perceived Stress Scale (PSS) and the Global Severity Index (GSI) of the Symptom Checklist-90-Revised (SCL-90-R).
Perceived Stress Scale
The PSS is a 10-item self-report questionnaire assessing an individual’s stress in the past month, with greater scores indicating higher perceived stress [68]. Items are measured on a 5-point ordinal scale; example items include: In the last month, how often have you been upset because of something that happened unexpectedly? In the last month, how often have you felt nervous and “stressed”? The PSS demonstrated good internal consistency for NA participants (α = 0.869), NHW participants (α = 0.866), and the overall sample (α = 0.868).
Global Severity Index of the Symptom Checklist-90-Revised
Like the PSS, the GSI of the SCL-90-R is well-validated and widely used as a comprehensive measure of psychological distress [69, 70]. The SCL-90-R is a 90-item self-report questionnaire, with the GSI reflecting the average score on all items answered by a participant [71]. Previous research has found that psychiatric inpatients score higher on the GSI at admission than at discharge, and score higher on the GSI than controls, supporting its use as a measure of psychological stress [71]. The GSI demonstrated good internal consistency in our sample for NA participants (α = 0.974), NHW participants (α = 0.969), and the overall sample (α = 0.972).
Control Variables
Control variables in the current study were: age, which is associated with impaired CPM and heightened TS [72], biological sex, with males demonstrating more CPM inhibition than females [73], body mass index (BMI), which is associated with greater self-reported sleep problems [74], physical activity level (as measured by the International Physical Activity Questionnaire [IPAQ]) [75], with greater physical activity being associated with less hyperalgesia and more efficient CPM [76, 77], and general health perception (subscale of the Medical Outcomes Study 36-item Short Form Health Survey [SF-36] [78]), which may contribute to psychological stress [79]. Models were run with and without control variables that might confound results due to known effects on variables in the model.
Data Analysis
Structural equation models were conducted with LISREL 8.8 [80]. Ethnicity was dummy coded (NHW = 0, NA = 1) in the models. A few observations were removed prior to analysis due to participants failing to follow instructions (e.g., poor effort, pain tolerance lower than pain threshold) [for a full description, see 48]. Moreover, there was 15.23% of observations missing (11.48% in model with controls), mostly due to participants not all showing up for and completing day 2 of testing. We have reported elsewhere that differences between completers and non-completers of the study is minimal (e.g., completers were slightly older, reported fewer psychological problems, and reported slightly more body pain [81]); moreover, results were nearly identical if these people were removed from the analysis rather than being imputed. However, LISREL uses full information maximum likelihood (FIML), which means the models are run with all available data, even if there are missing values (the current state-of-the-art method for handling missingness) [82].
Cold tolerance, ischemia tolerance, age, physical activity scores (IPAQ), and psychological distress (GSI) were log10 transformed to reduce positive skew. Outliers were identified using Wilcox’s MAD-median procedure and then winsorized to the next nearest non-outlier value [83]. All pain outcome variables, except electric tolerance, were winsorized, as were insomnia severity, perceived stress (PSS), psychological distress (GSI), general health (SF-36), and physical activity (IPAQ).
Indicators for each latent variable were chosen based on theory (e.g., sleep-related variables were used to assess sleep problems). Measurement models for the latent variables were tested simultaneous with the structural model. Thus, a good fitting overall model reflects adequate measurement and structural models.
Model fit was assessed from the root mean square error of approximation (RMSEA). As noted by Kline [18], values ≤0.05 are considered “close-fit”, values between 0.05 and 0.08 are considered a “reasonable approximate fit,” and values ≥0.10 are considered “poor fit.” Moreover, the “Test of Close Fit” provided by LISREL was also reported. The Test of Close Fit assesses the null hypothesis (H0) that RMSEA < 0.05. Thus, if H0 is rejected, the model is not close fitting. The chi-square goodness of fit is also reported; however, this metric is highly sensitive to sample size and thus is significant in most large samples. These are the only measures of model fit provided by LISREL 8.80.
We have shown elsewhere that ethnic group differences in pain outcomes are not present in this study [56], so the direct pathways between NA ethnicity and pain outcomes were not included in the structural model. Indirect tests of mediation were tested using the Sobel test within LISREL 8.80. Significance was set at p < .05 (two-tailed) for all analyses.
Results
Background Characteristics
In the full sample of 302 participants, 153 were NA (58% female) and 149 were NHW (50% female). There were no group differences on biological sex, χ2 = 2.20, p = .138. Table 1 presents means, standard deviations (SD), and intercorrelations for all study variables. Means and standard deviations for variables that were log10 transformed are presented here: The average participant age was 29.596 years (SD = 13.116), the average psychological distress (GSI) was.373 (SD = 0.366), the average ischemic tolerance was 257.26 s (SD = 327.080), the average cold tolerance was 86.48 s (SD = 97.184), and the average level of physical activity was 8888.6890 metabolic equivalent (MET) minutes per week (SD = 7908.696).
Sleep and Pain
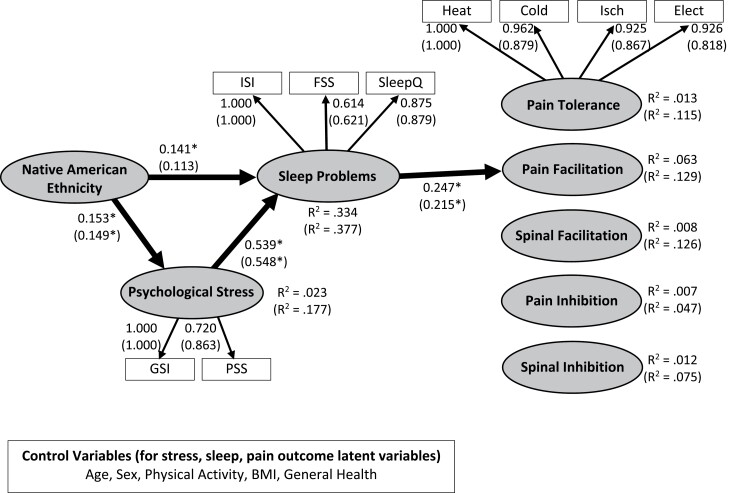
Figure 2 depicts the final model with standardized loadings and coefficients. Table 2 presents the results of the analyses with and without control variables. The model without control variables demonstrated a reasonable fit according to the raw value of the RMSEA = 0.0507 (90% CI: 0.0357, 0.0651), but the test of close fit (p =0.45) indicated a close fit. There was a significant chi-square (χ2 = 122.57, df = 69 p < .001). As shown in Fig. 2, NAs experienced more psychological stress and sleep problems. In turn, sleep problems were associated with greater pain facilitation (enhanced TS-Pain). No other structural path to pain outcomes was significant. Moreover, there were significant indirect pathways indicating that NAs experience more sleep problems as a result of experiencing greater psychological stress (unstandardized indirect effect = 0.802, SE = 0.327, p = .014), and NAs have increased pain facilitation as a result of increased psychological stress and sleep problems (unstandardized indirect effect = 1.579, SE = 0.620, p = .011). Moreover, there was a significant indirect pathway linking greater psychological stress to greater pain facilitation (unstandardized indirect effect = 21.548, SE = 8.117, p = .008). No other indirect effect was significant.
Fig. 2.
Structural equation model linking Native American ethnicity (coded 0 = non-Hispanic White [NHW], 1 = Native American [NA]) to psychological stress, sleep problems, and pain outcomes (pain tolerance, temporal summation of pain [TS-pain], temporal summation of the nociceptive flexion reflex [TS-NFR], conditioned pain modulation of pain [CPM-pain], conditioned pain modulation of NFR [CPM-NFR]. Standardized coefficients, standardized loadings, and R-squared values depicted without parentheses are from the model that did not include control variables. Standardized coefficients, standardized loadings, and R-squared values in parentheses are results from the model that included age, biological sex, physical activity, body mass index (BMI), and general health perceptions as control variables. The latent variable for psychological stress was estimated from the total score of the Perceived Stress Scale and the Global Severity Index of the SCL-90. The latent variable for sleep problems was estimated from the total score of the Insomnia Severity Index, the Fatigue Severity Scale, and the Sleep Quality subscale from the PSQI. Heat tolerance (Heat Tol), cold pressor tolerance (Cold Tol), ischemic tourniquet test tolerance (Isch Tol), and electric tolerance (Elect Tol) were used to estimate the pain tolerance latent variable. *p < .05.
Table 2.
Unstandardized Coefficients for the Structural Equation Model Predicting Pain Outcomes (N = 302)
| Measurement model | Model without control variables | Model with control variables | R² | |||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | Z-test | R² | Estimate | SE | Z-test | ||
| Native American Status --> Ethnicity | 1.000 | — | — | — | 1.000 | — | — | — |
| Psychological Stress--> GSI | 1.000 | — | — | 0.980 | 59.030 | 5.277 | 11.187 | 0.605 |
| Psychological Stress--> PSS | 49.638 | 5.671 | 8.752 | 0.509 | 1.000 | — | — | 0.825 |
| Sleep Problems--> ISI | 1.000 | — | — | 0.905 | 1.000 | 0.899 | ||
| Sleep Problems--> FSS | 1.238 | 0.129 | 9.568 | 0.342 | 1.252 | 0.130 | 9.610 | 0.345 |
| Sleep Problems--> SleepQ | 0.130 | 0.009 | 14.913 | 0.692 | 0.131 | 0.009 | 14.861 | 0.692 |
| Pain Tolerance--> Heat Tolerance | 1.000 | 0.449 | 1.000 | 0.511 | ||||
| Pain Tolerance--> Cold Tolerance | 0.196 | 0.027 | 7.297 | 0.415 | 0.179 | 0.024 | 7.556 | 0.395 |
| Pain Tolerance--> Ischemia Tolerance | 0.218 | 0.031 | 7.151 | 0.384 | 0.205 | 0.027 | 7.494 | 0.384 |
| Pain Tolerance--> Electric Tolerance | 6.563 | 0.917 | 7.157 | 0.385 | 5.799 | 0.804 | 7.209 | 0.342 |
| Pain Facilitation --> TS-pain | 1.000 | — | — | — | 1.000 | — | — | — |
| Spinal Facilitation --> TS-NFR | 1.000 | — | — | — | 1.000 | — | — | — |
| Pain Inhibition --> CPM of Pain | 1.000 | — | — | — | 1.000 | — | — | — |
| Spinal Inhibition --> CPM of NFR | 1.000 | — | — | — | 1.000 | — | — | — |
| Error in PSS | 17.977 | 2.364 | 7.605 | 14.476 | 2.035 | 7.115 | ||
| Error in GSI | 0.000 | 0.001 | 0.210 | 0.001 | 0.0004 | 2.788 | ||
| Error in ISI | 2.473 | 1.154 | 2.143 | 2.622 | 1.145 | 2.291 | ||
| Error in FSS | 69.948 | 6.855 | 10.204 | 69.408 | 6.819 | 10.179 | ||
| Error in SleepQ | 0.179 | 0.026 | 6.989 | 0.178 | 0.026 | 6.965 | ||
| Error in Heat Tol | 1.876 | 0.242 | 7.763 | 1.668 | 0.236 | 7.077 | ||
| Error in Cold Tol | 0.082 | 0.010 | 8.223 | 0.085 | 0.010 | 8.631 | ||
| Error in Ischemia Tol | 0.117 | 0.014 | 8.615 | 0.117 | 0.013 | 8.766 | ||
| Error in Electric Tol | 105.262 | 12.236 | 8.603 | 112.712 | 12.257 | 9.196 | ||
| Structural model | Estimate | SE | Z-test | R² | Estimate | SE | Z-test | R² |
|---|---|---|---|---|---|---|---|---|
| Predicting Psychological Stress | 0.023 | 0.177 | ||||||
| Race --> Psychological Stress | 0.027 | 0.010 | 2.620 | 0.024 | 0.010 | 2.485 | ||
| Age --> Psychological Stress | -0.064 | 0.030 | -2.127 | |||||
| Sex --> Psychological Stress | -0.002 | 0.009 | -0.223 | |||||
| Physical Activity --> Psychological Stress | 0.014 | 0.011 | 1.307 | |||||
| BMI --> Psychological Stress | 0.0004 | 0.001 | 0.323 | |||||
| Gen Health --> Psychological Stress | -0.002 | 0.0004 | -5.876 | |||||
| Predicting Sleep Problems | 0.334 | 0.377 | ||||||
| Race --> Sleep Problems | 1.375 | 0.566 | 2.427 | 1.090 | 0.580 | 1.879 | ||
| Psychological Stress --> Sleep Problems | 30.173 | 4.445 | 6.789 | 33.210 | 4.758 | 6.981 | ||
| Age --> Sleep Problems | — | — | — | 1.299 | 1.827 | 0.711 | ||
| Sex --> Sleep Problems | — | — | — | -0.256 | 0.565 | -0.453 | ||
| Physical Activity --> Sleep Problems | — | — | — | 0.865 | 0.631 | 1.371 | ||
| BMI --> Sleep Problems | — | — | — | 0.016 | 0.072 | 0.219 | ||
| Gen Health -->Sleep Problems | — | — | — | -0.026 | 0.027 | -0.954 | ||
| Predicting Pain Tolerance | 0.013 | 0.115 | ||||||
| Psychological Stress --> Pain Tolerance | 1.222 | 1.332 | 0.917 | 0.819 | 1.751 | 0.468 | ||
| Sleep Problems --> Pain Tolerance | 0.010 | 0.025 | 0.388 | 0.012 | 0.028 | 0.448 | ||
| Age --> Pain Tolerance | — | — | — | -0.007 | 0.610 | -0.012 | ||
| Sex --> Pain Tolerance | — | — | — | -0.817 | 0.195 | -4.187 | ||
| Physical Activity --> Pain Tolerance | — | — | — | 0.180 | 0.212 | 0.848 | ||
| BMI --> Pain Tolerance | — | — | — | -0.028 | 0.024 | -1.182 | ||
| Gen Health ---> Pain Tolerance | — | — | — | 0.005 | 0.009 | 0.573 | ||
| Predicting Pain Facilitation (TS-Pain) | 0.063 | 0.129 | ||||||
| Psychological Stress --> Pain Facilitation | 0.886 | 12.966 | 0.068 | 8.406 | 16.197 | 0.519 | ||
| Sleep Problems --> Pain Facilitation | 0.714 | 0.249 | 2.863 | 0.624 | 0.261 | 2.392 | ||
| Age --> Pain Facilitation | — | — | — | 17.738 | 5.634 | 3.149 | ||
| Sex --> Pain Facilitation | — | — | — | 1.638 | 1.731 | 0.946 | ||
| Physical Activity --> Pain Facilitation | — | — | — | 4.886 | 1.952 | 2.504 | ||
| BMI --> Pain Facilitation | — | — | — | -0.338 | 0.219 | -1.544 | ||
| Gen Health --> Pain Facilitation | — | — | — | 0.066 | 0.083 | 0.790 | ||
| Predicting Spinal Facilitation (TS-NFR) | 0.008 | 0.126 | ||||||
| Psychological Stress --> Spinal Facilitation | 0.289 | 0.473 | 0.612 | 0.718 | 0.578 | 1.241 | ||
| Sleep Problems --> Spinal Facilitation | 0.006 | 0.009 | 0.614 | -0.001 | 0.009 | -0.117 | ||
| Age --> Spinal Facilitation | — | — | — | 0.980 | 0.200 | 4.904 | ||
| Sex --> Spinal Facilitation | — | — | — | -0.120 | 0.061 | -1.947 | ||
| Physical Activity --> Spinal Facilitation | — | — | — | 0.089 | 0.069 | 1.279 | ||
| BMI --> Spinal Facilitation | — | — | — | 0.001 | 0.008 | 0.174 | ||
| Gen Health --> Spinal Facilitation | — | — | — | 0.000 | 0.003 | -0.062 | ||
| Predicting Pain Inhibition (CPM-Pain) | 0.007 | 0.047 | ||||||
| Psychological Stress --> Pain Inhibition | 1.555 | 7.349 | 0.212 | 5.336 | 9.353 | 0.570 | ||
| Sleep Problems --> Pain Inhibition | 0.122 | 0.139 | 0.879 | 0.106 | 0.148 | 0.718 | ||
| Age --> Pain Inhibition | — | — | — | 6.822 | 3.265 | 2.090 | ||
| Sex --> Pain Inhibition | — | — | — | 1.046 | 1.003 | 1.043 | ||
| Physical Activity --> Pain Inhibition | — | — | — | 1.735 | 1.131 | 1.534 | ||
| BMI --> Pain Inhibition | — | — | — | -0.042 | 0.127 | -0.329 | ||
| Gen Health --> Pain Inhibition | — | — | — | 0.054 | 0.048 | 1.123 | ||
| Predicting Spinal Inhibition (CPM-NFR) | 0.012 | 0.075 | ||||||
| Psychological Stress --> Spinal Inhibition | 0.455 | 0.373 | 1.221 | 0.765 | 0.467 | 1.639 | ||
| Sleep Problems --> Spinal Inhibition | 0.001 | 0.007 | 0.200 | -0.003 | 0.007 | -0.382 | ||
| Age --> Spinal Inhibition | — | — | — | 0.357 | 0.161 | 2.210 | ||
| Sex --> Spinal Inhibition | — | — | — | -0.096 | 0.050 | -1.936 | ||
| Physical Activity --> Spinal Inhibition | — | — | — | 0.065 | 0.056 | 1.155 | ||
| BMI --> Spinal Inhibition | — | — | — | 0.008 | 0.006 | 1.198 | ||
| Gen Health --> Spinal Inhibition | — | — | — | 0.001 | 0.002 | 0.481 | ||
| Residual for Psychological Stress | 0.007 | 0.001 | 7.596 | 0.005 | 0.001 | 7.640 | ||
| Residual for Sleep Problems | 15.774 | 2.053 | 7.685 | 14.549 | 1.934 | 7.522 | ||
| Residual for Pain Tolerance | 1.509 | 0.304 | 4.966 | 1.541 | 0.290 | 5.311 | ||
| Residual for Pain Facilitation | 185.019 | 17.089 | 10.827 | 172.007 | 15.866 | 10.841 | ||
| Residual for Spinal Facilitation | 0.262 | 0.023 | 11.288 | 0.232 | 0.021 | 11.268 | ||
| Residual for Pain Inhibition | 61.942 | 5.599 | 11.062 | 59.528 | 5.383 | 11.058 | ||
| Residual for Spinal Inhibition | 0.153 | 0.014 | 11.001 | 0.143 | 0.013 | 10.953 |
Note: z-tests ≥ 1.96 or ≤ −1.96 are statistically significant at p < .05. NHW = non-Hispanic White; NA = Native American; BMI = body mass index; HRV = heart rate variability; TS = temporal summation; NFR = nociceptive flexion reflex; CPM = conditioned pain modulation; SleepQ = Sleep Quality Index from PSQI; Gen Health = General Health subscale from the SF-36; PSS = Perceived Stress Scale; GSI = Global Severity Index from the SCL = −90; Tol = Tolerance.
The model with control variables (Table 2, results in parentheses of Fig. 2) had a slightly worse fit than the model without the controls, but the RMSEA = 0.0536 (90% CI: 0.042, 0.066) and test of close fit (p = 0.29) still suggested a close fit to these data. There was a significant chi-square (χ2 = 184.837, df = 99, p < .001). Even after controlling for age, sex, physical activity, BMI, and general health, NAs still experienced greater psychological stress, but the direct path from NA ethnicity to sleep problems was no longer significant. Results of all other pathways were identical to the model without controls.
Discussion
Seeking to elucidate mechanisms that might place NAs at higher risk for chronic pain than NHWs, this study found that NAs experienced more psychological stress and more sleep problems than NHWs, which in turn promoted enhanced pain facilitation (TS-pain). When including control variables (i.e., age, sex, physical activity, BMI, and general health); however, NAs no longer experienced more sleep problems than NHWs. In models with and without control variables added, sleep problems were associated with pain facilitation (TS-pain) but not facilitation of spinal neurons (TS-NFR), impaired endogenous inhibition of pain (CPM-pain) or spinal nociception (CPM-NFR), or hyperalgesia. Indirect paths in the models found that NAs experienced more sleep problems due to higher psychological stress. Similarly, enhanced pain facilitation (TS-pain) in NAs was due to greater psychological stress and more sleep problems. The implications of these findings are twofold: first, by linking psychological stress and sleep problems under normal conditions to a specific pain-promoting mechanism, these findings broaden our understanding of pathways that could promote pain in NAs, although additional research would be necessary to conclusively establish this pathway in NA chronic pain risk. Next, the findings underscore the role of sleep as a modifiable factor that may contribute to chronic pain risk [44].
Psychological Stress and Sleep Problems Promoted Pain Facilitation, but not Hyperexcitability of Spinal Neurons
Although highly correlated, pain facilitation (TS-pain) and spinal facilitation (TS-NFR) can be modulated by separate circuits and are thus dissociable in certain circumstances [54, 55]. For instance, TS-NFR, which assesses spinal nociception, is unaffected by certain processes in the brain (e.g., pain catastrophizing) that contribute to TS-pain [54]. When both are measured, discrepancies between TS-pain and TS-NFR can be cautiously used to infer whether spinal or supraspinal processes contribute to enhanced temporal summation. In the current study, sleep problems promoted greater pain facilitation (TS-pain) without also promoting hyperexcitability of spinal neurons (TS-NFR), and this pathway accounted for greater TS-pain in NAs. This suggests that sleep problems contribute to sensitization of pain perception, ostensibly via supraspinal circuits that amplify pain signals after they reach the brain. TS-pain is frequently enhanced in chronic pain samples [44] and has substantial theoretical support as a proxy measure of central sensitization, a state of hypersensitivity to potentially painful stimuli believed to be important in the development and maintenance of many chronic pain conditions [84]. Therefore, these findings support the hypothesis that psychosocial stress and sleep problems may amplify pain processing in the brain without altering pain processing at the spinal level.
Psychological Stress Promotes Sleep Problems in Native Americans
Both with and without control variables, the models tested in the current study found that higher levels of the latent psychological stress variable promoted sleep problems. In the model without controls, there was also a direct path linking NA ethnicity to sleep problems. When controlling for potentially confounding variables (i.e., age, sex, physical activity, BMI, and general health), though, NAs only experienced more sleep problems than NHWs as a consequence of greater psychological stress. Therefore, the absence of a direct pathway between NA ethnicity and sleep problems in the model with control variables indicates that these factors (i.e., age, sex, physical activity, BMI, and general health) promoted greater sleep problems in NAs. Given the role of psychological stress in promoting sleep problems, these results underscore the importance of systematic efforts to successfully reduce the heightened experience of psychosocial stressors for NAs that could ostensibly enhance psychological stress. Indeed, our operationalization of the psychological stress variable encompassed only perceived stress and global psychological distress, and thus would not account for other psychosocial risk factors that could predispose NAs to chronic pain. For instance, previous findings from OK-SNAP suggest that other psychosocial stressors disproportionately experienced by NAs, such as adverse life events [81, 85] and discrimination [86], may further contribute to the NA pain disparity.
Pain Facilitation: A Possible Link Between Sleep Problems and Chronic Pain Risk
Other studies examining the role of sleep problems on experimental pain have prospectively linked sleep impairment to multiple pain-promoting processes, including greater pain sensitivity (hyperalgesia) [87–89], pain facilitation (TS-pain) [88], and reduced pain inhibition (CPM-pain) [37, 88, 90]. However, findings across these studies are somewhat inconsistent, likely due to methodological differences in both the procedures for assessing sleep impairment (i.e., forced awakening, sleep deprivation) as well as the procedures and sensory modalities used in the pain paradigms [30, 89]. As noted by others [30, 32], it is plausible that methods of inducing sleep impairment have distinct effects on pain modulation. For instance, Smith et al. found that disrupted sleep continuity was associated with impaired pain inhibition (CPM-pain), whereas sleep restriction was not [37]. Given that the descending inhibitory effects of CPM are mediated by endogenous opioids [39], repeated nighttime awakenings may reduce endogenous opioid activity, leading to less efficient descending pain modulation (CPM-pain). In contrast to these studies, which utilized experimental methods to induce sleep problems, the current study measured sleep problems using a latent variable of self-reported sleep problems and daytime fatigue in the weeks prior to pain testing. While self-report measures of sleep problems are less reliable and more prone to overreporting than objective measures of sleep (e.g., polysomnography, wrist actigraphy) [91–93], assessing the effect of sleep problems under normal conditions also provides evidence for sleep problems conferring chronic pain risk with a greater degree of ecological validity.
Although unexplored in the current study, additional examination of mediators by which sleep problems contribute to pain facilitation is warranted. Sleep problems are linked to changes in immune system function (i.e., enhanced concentration of pro-inflammatory cytokines [38, 94]), hyperresponsivity of the hypothalamic–pituitary–adrenal (HPA) axis [95], reduced efficiency of endogenous opioids [37, 96], greater negative affect and pain catastrophizing [97], and reduced positive affect [30, 98]. Thus, the successful amelioration of sleep problems may have a widespread protective effect on chronic pain risk. Moreover, sleep problems are well-established risk factors for other medical conditions associated with significant disability, morbidity, and suffering (e.g., heart attack, diabetes, stroke, obesity) [99]. Sleep problems have also been found to vary across ethnic groups in the United States and may contribute to observed health disparities across ethnic groups [100–104]. For example, a study of over 29,000 Americans found that African Americans were more likely to experience both short (<5 h) and long (>9 h) sleep duration than NHWs [102]. Another study of ethnic minorities found that experiences of racial discrimination were associated with reduced sleep quality [104]. Relative to other ethnic groups, NAs have also been found to experience frequent insufficient sleep at higher rates [27]. Therefore, sleep problems may be an important proximate target for interventions aiming to promote overall health and reduce ethnic differences in disease burden. Fortunately, extant psychological treatments aimed at improving sleep, like cognitive behavioral therapy for insomnia (CBT-I), are brief, highly efficacious, and cost efficient [105].
Strengths and Limitations
The current study utilized the largest known sample of NAs who underwent quantitative sensory testing to determine the effects of psychological stress and sleep impairment on physiological processes related to chronic pain risk (i.e., TS and CPM) [44]. Additionally, the study benefitted from using structural equation modeling (SEM) to analyze these relationships and assess model fit. Modeling the constructs using latent variables reduces error variance associated with measurement [58] and SEM was able to determine paths and indirect paths linking psychosocial variables with sleep and pain outcomes [58]. Given the debate within the sleep and pain literature in determining whether pain precedes sleep problems or vice versa [30], analyses that can establish potential pathways between sleep and pain make useful contributions to the literature. Despite its strengths, this study also faced some limitations.
First, the current study only included healthy, chronic pain-free individuals who were not taking centrally active medications. Because of this, the generalizability of the study to other populations, including populations with chronic pain or other chronic health conditions (e.g., diabetes, hypertension, obesity), is limited. Since these conditions are bidirectionally related to sleep problems [106, 107] and are highly prevalent among NA groups [9, 108–110], generalizability of these findings to broader NA populations is also limited. In addition, the present analyses excluded members of non-NA racial and ethnic minorities; thus, caution in generalizing these findings to other racial/ethnic groups is warranted. Furthermore, NA participants in OK-SNAP largely came from northeastern Oklahoma, such that generalizability to NAs in other locations may be limited. Given that there were participants who did not complete all of OK-SNAP, there is a potential for selection effects. However, we have previously shown that there are few differences between study completers and non-completers [111, 112]. As mentioned earlier, the self-report measures that comprise the sleep problems latent variable are prone to symptom overreporting and may not be as valid as objective measures of sleep. Nevertheless, self-report sleep measures used in the study are significantly related to objective sleep measures [63], suggesting that they are adequate for the current study. And finally, all study variables were collected over multiple days; thus, the data are largely cross-sectional and causal inferences are limited. For example, it is not possible to determine whether stress is a precursor or a consequence of sleep problems.
Summary
This study found that Native Americans experienced more psychological stress and more sleep problems than non-Hispanic Whites. In turn, sleep problems promoted pain facilitation (temporal summation of pain) but not facilitation of spinal neurons (temporal summation of the nociceptive flexion reflex), impaired endogenous inhibition of pain (conditioned pain modulation) or spinal nociception (conditioned pain modulation of the nociceptive flexion reflex), or hyperalgesia. These findings provide additional evidence of sleep problems promoting chronic pain risk and identify psychological stress and sleep problems as a mechanism that may contribute to the higher prevalence of chronic pain in Native Americans.
Acknowledgements
The authors would like to thank Bethany L. Kuhn, Mara J. Demuth, Natalie Hellman, Tyler A. Toledo, Edward W. Lannon, Shreela Palit, Michael F. Payne, Cassandra A. Sturycz, Yvette Guereca, Burkhart J. Hahn, Heather B. Coleman, Kathryn A. Thompson, Jessica M. Fisher, Samuel P. Herbig, Ky’Lee B. Barnoski, Garrett Newsom, and Lucinda Chee for their help with data collection, as well as Dr. John M. Chaney for his consultation on the project. This research was supported by the National Institute on Minority Health and Health Disparities of the National Institute of Health under Award Number R01MD007807. Edward Lannon (DGE - 1546597) and Shreela Palit (DGE-1546597) were supported by the National Science Foundation Graduate Research Fellowship Program. The content is solely the responsibility of the authors and does not necessarily reflect the views of the National Institutes of Health, National Science Foundation, Indian Health Service, or the Cherokee Nation. The authors report no conflicts of interest.
Contributor Information
Parker A Kell, Department of Psychology, The University of Tulsa, Tulsa, OK, USA.
Felicitas A Huber, Department of Psychology, The University of Tulsa, Tulsa, OK, USA.
Erin N Street, Department of Psychology, The University of Tulsa, Tulsa, OK, USA.
Joanna O Shadlow, Department of Psychology, The University of Tulsa, Tulsa, OK, USA.
Jamie L Rhudy, Department of Psychology, The University of Tulsa, Tulsa, OK, USA.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards Authors Parker A. Kell, Felicitas A. Huber, Erin N. Street, Joanna O. Shadlow, Jamie L. Rhudy declare that they have no conflict of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Primary Data XXXXX.
Authors’ Contributions Parker Kell: Writing—Original Draft, Writing—Review & Editing, Visualization, Conceptualization, Formal Analysis. Felicitas Huber: Writing—Review & Editing, Investigation. Erin Street: Writing—Review & Editing. Joanna Shadlow: Writing—Review & Editing, Investigation, Project Administration, Funding Acquisition. Jamie Rhudy: Conceptualization, Methodology, Software, Formal Analysis, Investigation, Data Curation, Writing—Original Draft, Writing—Review & Editing, Visualization, Project Administration, Funding Acquisition.
Ethical Approval All procedures were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its subsequent amendments.
Informed Consent Informed consent was obtained from all individual participants included in the study.
Transparency Open Science Statement
1. Study Registration: This study was not formally registered.
2. Analytic Plan Registration: The analysis plan was not formally pre-registered.
3. Availability of Data: De-identified data from this study are not available in an a public archive. De-identified data from this study will be made available (as allowable according to institutional IRB standards) by emailing the corresponding author.
4. Availability of Analytic Code: Analytic code used to conduct the analyses presented in this study are not available in a public archive. They may be available by emailing the corresponding author.
5. Availability of Materials: Materials used to conduct the study are not publicly available.
References
- 1. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018; 67:1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 3. Beals J, Manson SM, Whitesell NR, Spicer P, Novins DK, Mitchell CM. Prevalence of DSM-IV disorders and attendant help-seeking in 2 American Indian reservation populations. Arch Gen Psychiatry. 2005; 62:99–108. [DOI] [PubMed] [Google Scholar]
- 4. Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine. 2006; 31:2724–2727. [DOI] [PubMed] [Google Scholar]
- 5. Ferucci ED, Templin DW, Lanier AP. Rheumatoid arthritis in American Indians and Alaska Natives: a review of the literature. Semin Arthritis Rheum. 2005; 34:662–667. [DOI] [PubMed] [Google Scholar]
- 6. Leake J, Jozzy S, Uswak G. Severe dental caries, impacts and determinants among children 2–6 years of age in Inuvik Region, Northwest Territories, Canada. J Can Dent Assoc. 2008; 74:519–519. [PubMed] [Google Scholar]
- 7. Mauldin J, Cameron HD, Jeanotte D, Solomon G, Jarvis JN. Chronic arthritis in children and adolescents in two Indian health service user populations. BMC Musculoskelet Disord. 2004; 5:30–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rhee H. Prevalence and predictors of headaches in US adolescents. Headache. 2000; 40:528–538. [DOI] [PubMed] [Google Scholar]
- 9. Jimenez N, Garroutte E, Kundu A, Morales L, Buchwald D. A review of the experience, epidemiology, and management of pain among American Indian, Alaska Native, and Aboriginal Canadian peoples. J Pain. 2011; 12:511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O’Connell M, Buchwald DS, Duncan GE. Food access and cost in American Indian communities in Washington State. J Am Diet Assoc. 2011; 111:1375–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Semega JL, Fontenot KR, Kollar MA. Income and poverty in the United States: 2016. Curr Popul Rep. 2017, P60–259. [Google Scholar]
- 12. U. S. Census Bureau. American Community Survey; 2018. [Google Scholar]
- 13. Findling MG, Casey LS, Fryberg SA, et al. Discrimination in the United States: experiences of Native Americans. Health Serv Res. 2019; 54:1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007; 298:1685–1687. [DOI] [PubMed] [Google Scholar]
- 15. Burke NN, Finn DP, McGuire BE, Roche M. Psychological stress in early life as a predisposing factor for the development of chronic pain: clinical and preclinical evidence and neurobiological mechanisms. J Neurosci Res. 2017; 95:1257–1270. [DOI] [PubMed] [Google Scholar]
- 16. Booth J, Connelly L, Lawrence M, et al. Evidence of perceived psychosocial stress as a risk factor for stroke in adults: a meta-analysis. BMC Neurol. 2015; 15:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sklar LS, Anisman H. Stress and cancer. Psychol Bull. 1981; 89:369–406. [PubMed] [Google Scholar]
- 18. Steptoe A, Kivimäki M. Stress and cardiovascular disease. Nat Rev Cardiol. 2012; 9:360–370. [DOI] [PubMed] [Google Scholar]
- 19. Tiedt JA, Brown LA. Allostatic load: the relationship between chronic stress and diabetes in Native Americans. J Theo Constr Test. 2014; 18:22. [Google Scholar]
- 20. Heymen S, Maixner W, Whitehead WE, Klatzkin RR, Mechlin B, Light KC. Central processing of noxious somatic stimuli in patients with irritable bowel syndrome compared with healthy controls. Clin J Pain. 2010; 26:104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilder-Smith CH, Song G, Yeoh KG, Ho KY. Activating endogenous visceral pain modulation: a comparison of heterotopic stimulation methods in healthy controls. Eur J Pain. 2009; 13:836–842. [DOI] [PubMed] [Google Scholar]
- 22. Hassett AL, Clauw DJ. Does psychological stress cause chronic pain? Psychiatr Clin North Am. 2011; 34:579–594. [DOI] [PubMed] [Google Scholar]
- 23. Hicks RA, Garcia ER. Level of stress and sleep duration. Percept Mot Skills. 1987; 64:44–46. [DOI] [PubMed] [Google Scholar]
- 24. Hall M, Thayer JF, Germain A, et al. Psychological stress is associated with heightened physiological arousal during NREM sleep in primary insomnia. Behav Sleep Med. 2007; 5:178–193. [DOI] [PubMed] [Google Scholar]
- 25. Åkerstedt T, Orsini N, Petersen H, Axelsson J, Lekander M, Kecklund G. Predicting sleep quality from stress and prior sleep–a study of day-to-day covariation across six weeks. Sleep Med. 2012; 13:674–679. [DOI] [PubMed] [Google Scholar]
- 26. Da Costa D, Zummer M, Fitzcharles M. Determinants of sleep problems in patients with spondyloarthropathy. Musculoskelet Care. 2009; 7:143–161. [DOI] [PubMed] [Google Scholar]
- 27. Chapman DP, Croft JB, Liu Y, Perry GS, Presley-Cantrell LR, Ford ES. Excess frequent insufficient sleep in American Indians/Alaska natives. J Environ Public Health. 2013; 2013:259645–259645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Danyluck C, Blair IV, Manson SM, Laudenslager ML, Daugherty SL, Brondolo E. Discrimination and sleep impairment in American Indians and Alaska Natives. Ann Behav Med. 2021. [DOI] [PubMed] [Google Scholar]
- 29. Nitter AK, Pripp AH, Forseth KO. Are sleep problems and non-specific health complaints risk factors for chronic pain? A prospective population-based study with 17 year follow-up. Scand J Pain. 2012; 3:210–217. [DOI] [PubMed] [Google Scholar]
- 30. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013; 14:1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jank R, Gallee A, Boeckle M, Fiegl S, Pieh C. Chronic pain and sleep disorders in primary care. Pain Res Treat. 2017, 9081802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004; 8:119–132. [DOI] [PubMed] [Google Scholar]
- 33. Smith MT, Perlis M, Smith M, Giles D, Carmody T. Sleep quality and presleep arousal in chronic pain. J Behav Med. 2000; 23:1–13. [DOI] [PubMed] [Google Scholar]
- 34. Boardman H, Thomas E, Millson D, Croft P. The natural history of headache: predictors of onset and recovery. Cephalalgia. 2006; 26:1080–1088. [DOI] [PubMed] [Google Scholar]
- 35. Davies KA, Macfarlane G, Nicholl B, et al. Restorative sleep predicts the resolution of chronic widespread pain: results from the EPIFUND study. Rheumatology. 2008; 47:1809–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bonvanie IJ, Oldehinkel AJ, Rosmalen JG, Janssens KA. Sleep problems and pain: a longitudinal cohort study in emerging adults. Pain. 2016; 157:957–963. [DOI] [PubMed] [Google Scholar]
- 37. Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007; 30:494–505. [DOI] [PubMed] [Google Scholar]
- 38. Haack M, Simpson N, Sethna N, Kaur S, Mullington J. Sleep deficiency and chronic pain: potential underlying mechanisms and clinical implications. Neuropsychopharmacology. 2020; 45:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010; 23:611–615. [DOI] [PubMed] [Google Scholar]
- 40. Roy M, Piche M, Chen J-I, Peretz I, Rainville P. Cerebral and spinal modulation of pain by emotions. Proc Natl Acad Sci USA. 2009; 106:20900–20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain. 2012; 13:936–944. [DOI] [PubMed] [Google Scholar]
- 42. Yarnitsky D, Crispel Y, Eisenberg E, et al. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain. 2008; 138:22–28. [DOI] [PubMed] [Google Scholar]
- 43. Wilder-Smith OH, Schreyer T, Scheffer GJ, Arendt-Nielsen L. Patients with chronic pain after abdominal surgery show less preoperative endogenous pain inhibition and more postoperative hyperalgesia: a pilot study. J Pain Palliat Care Phamacother. 2010; 24:119–128. [DOI] [PubMed] [Google Scholar]
- 44. Yarnitsky D, Granot M, Granovsky Y. Pain modulation profile and pain therapy: between pro-and antinociception. Pain. 2014; 155:663–665. [DOI] [PubMed] [Google Scholar]
- 45. Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993; 52:259–285. [DOI] [PubMed] [Google Scholar]
- 46. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011; 152:S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nijs J, Loggia ML, Polli A, et al. Sleep disturbances and severe stress as glial activators: key targets for treating central sensitization in chronic pain patients? Expert Opin Ther Targets. 2017; 21:817–826. [DOI] [PubMed] [Google Scholar]
- 48. Bulls HW, Lynch MK, Petrov ME, et al. Depressive symptoms and sleep efficiency sequentially mediate racial differences in temporal summation of mechanical pain. Ann Behav Med. 2017; 51:673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Price DD. Characteristics of second pain and flexion reflexes indicative of prolonged central summation. Exp Neurol. 1972; 37:371–387. [DOI] [PubMed] [Google Scholar]
- 50. Arendt-Nielsen L, Brennum J, Sindrup S, Bak P. Electrophysiological and psychophysical quantification of temporal summation in the human nociceptive system. Eur J Appl Physiol. 1994; 68:266–273. [DOI] [PubMed] [Google Scholar]
- 51. Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ. Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003; 102:87–95. [DOI] [PubMed] [Google Scholar]
- 52. Petersen KK, Arendt-Nielsen L, Simonsen O, Wilder-Smith O, Laursen MB. Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. Pain. 2015; 156:55–61. [DOI] [PubMed] [Google Scholar]
- 53. Izumi M, Petersen KK, Laursen MB, Arendt-Nielsen L, Graven-Nielsen T. Facilitated temporal summation of pain correlates with clinical pain intensity after hip arthroplasty. Pain. 2017; 158:323–332. [DOI] [PubMed] [Google Scholar]
- 54. Rhudy JL, Martin SL, Terry EL, et al. Pain catastrophizing is related to temporal summation of pain, but not temporal summation of the nociceptive flexion reflex. Pain. 2011; 152:794–801. [DOI] [PubMed] [Google Scholar]
- 55. France CR, France JL, al’Absi M, Ring C, McIntyre D. Catastrophizing is related to pain ratings, but not nociceptive flexion reflex threshold. Pain. 2002; 99:459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rhudy JL, Lannon EW, Kuhn BL, et al. Assessing peripheral fibers, pain sensitivity, central sensitization, and descending inhibition in Native Americans: main findings from the Oklahoma Study of Native American Pain Risk. Pain. 2020; 161:388–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bentler PM, Chou CP. Practical issues in structural modeling. Sociol Methods Res. 1987; 16:78–117. [Google Scholar]
- 58. Kline RB. Structural Equation Modeling. New York, NY: The Guilford Press; 1998. [Google Scholar]
- 59. Farrell M, Gibson S. Age interacts with stimulus frequency in the temporal summation of pain. Pain Med. 2007; 8:514–520. [DOI] [PubMed] [Google Scholar]
- 60. Goodin BR, Bulls HW, Herbert MS, et al. Temporal summation of pain as a prospective predictor of clinical pain severity in adults aged 45 years and above with knee osteoarthritis: ethnic differences. Psychosom Med. 2014; 76:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rainville P, Feine JS, Bushnell MC, Duncan GH. A psychophysical comparison of sensory and affective responses to four modalities of experimental pain. Somatosens Mot Res. 1992; 9:265–277. [DOI] [PubMed] [Google Scholar]
- 62. Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 2005; 113:20–26. [DOI] [PubMed] [Google Scholar]
- 63. Smith MT, Wegener ST. Measures of sleep: the Insomnia Severity Index, Medical Outcomes Study (MOS) Sleep Scale, Pittsburgh Sleep Diary (PSD), and Pittsburgh Sleep Quality Index (PSQI). Arthritis Care Res. 2003; 49:S184–S196. [Google Scholar]
- 64. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index (PSQI): A new instrument for psychiatric research and practice. Psychiatry Res. 1989; 28:193–213. [DOI] [PubMed] [Google Scholar]
- 65. Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011; 34:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989; 46:1121–1123. [DOI] [PubMed] [Google Scholar]
- 67. Kim SJ, Kim S, Jeon S, Leary EB, Barwick F, Mignot E. Factors associated with fatigue in patients with insomnia. J Psych Res. 2019; 117:24–30. [DOI] [PubMed] [Google Scholar]
- 68. Cohen S, Kamarck T, Mermelstein R. Perceived stress scale. Measuring Stress: A Guide for Health and Social Scientists; 1994. [Google Scholar]
- 69. Derogatis LR. SCL-90-R: Symptom Checklist-90-R: Administration, Scoring & Procedures Manual. Minneapolis, Minn: National Computer Systems, Inc.; 1994. [Google Scholar]
- 70. Prinz U, Nutzinger DO, Schulz H, Petermann F, Braukhaus C, Andreas S. Comparative psychometric analyses of the SCL-90-R and its short versions in patients with affective disorders. BMC Psychiatry 2013; 13:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rytilä-Manninen M, Fröjd S, Haravuori H, et al. Psychometric properties of the symptom checklist-90 in adolescent psychiatric inpatients and age- and gender-matched community youth. Child Adolesc Psychiatry Ment Health. 2016; 10:23–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hackett J, Naugle KE, Naugle KM. The decline of endogenous pain modulation with aging: a meta-analysis of temporal summation and conditioned pain modulation. J Pain. 2020; 21:514–528. [DOI] [PubMed] [Google Scholar]
- 73. Hermans L, Van Oosterwijck J, Goubert D, et al. Inventory of personal factors influencing conditioned pain modulation in healthy people: a systematic literature review. Pain Pract. 2016; 16:758–769. [DOI] [PubMed] [Google Scholar]
- 74. Bixler EO, Vgontzas AN, Lin H-M, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005; 90:4510–4515. [DOI] [PubMed] [Google Scholar]
- 75. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003; 35:1381–1395. [DOI] [PubMed] [Google Scholar]
- 76. Umeda M, Lee W, Marino CA, Hilliard SC. Influence of moderate intensity physical activity levels and gender on conditioned pain modulation. J Sports Sci. 2016; 34:467–476. [DOI] [PubMed] [Google Scholar]
- 77. Lima LV, DeSantana JM, Rasmussen LA, Sluka KA. Short-duration physical activity prevents the development of activity-induced hyperalgesia through opioid and serotoninergic mechanisms. Pain. 2017; 158:1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. Boston: The Health Institute New England Medical Center; 1993. [Google Scholar]
- 79. Farmer MM, Ferraro KF. Distress and perceived health: mechanisms of health decline. J Health Soc Behav. 1997; 38:298–311. [PubMed] [Google Scholar]
- 80. Jöreskog KG, Sörbom D. LISREL 8.80. Chicago: Scientific Software International; 2006. [Google Scholar]
- 81. Kell PA, Hellman N, Huber FA, et al. The relationship between adverse life events and endogenous inhibition of pain and spinal nociception: findings from the Oklahoma Study of Native American Pain Risk (OK-SNAP). J Pain. 2021; 22: 1097–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Little TD, Lang KM, Wu W, Rhemtulla M. Missing data. In: Cicchetti D, ed. Dev Psychopathol. 2016:1–37. 10.1002/9781119125556.devpsy117. [DOI] [Google Scholar]
- 83. Wilcox RR. Understanding and Applying Basic Statistical Methods Using R. John Wiley & Sons, 2016. [Google Scholar]
- 84. Eide PK. Wind-up and the NMDA receptor complex from a clinical perspective. Eur J Pain. 2000; 4:5–15. [DOI] [PubMed] [Google Scholar]
- 85. Sturycz CA, Hellman N, Payne MF, et al. Race/Ethnicity does not moderate the relationship between adverse life experiences and temporal summation of the nociceptive flexion reflex and pain: results from the Oklahoma Study of Native American Pain Risk. J Pain. 2019; 20:941–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Güereca YM, Kell PA, Kuhn BL, et al. The relationship between experienced discrimination and pronociceptive processes in Native Americans: results from the Oklahoma Study of Native American Pain Risk. J Pain. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001; 10:35–42. [DOI] [PubMed] [Google Scholar]
- 88. Staffe AT, Bech MW, Clemmensen SLK, Nielsen HT, Larsen DB, Petersen KK. Total sleep deprivation increases pain sensitivity, impairs conditioned pain modulation and facilitates temporal summation of pain in healthy participants. PLoS One. 2019; 14:e0225849–e0225849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006; 10:357–369. [DOI] [PubMed] [Google Scholar]
- 90. Edwards RR, Grace E, Peterson S, Klick B, Haythornthwaite JA, Smith MT. Sleep continuity and architecture: associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain. 2009; 13:1043–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008; 19:838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. O’Donnell D, Silva EJ, Münch M, Ronda JM, Wang W, Duffy JF. Comparison of subjective and objective assessments of sleep in healthy older subjects without sleep complaints. J Sleep Res. 2009; 18:254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang L, Zhao Z-X. Objective and subjective measures for sleep disorders. Neurosci Bull. 2007; 23:236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. 2010; 24:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Goodin BR, Smith MT, Quinn NB, King CD, McGuire L. Poor sleep quality and exaggerated salivary cortisol reactivity to the cold pressor task predict greater acute pain severity in a non-clinical sample. Biol Psychol. 2012; 91:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hicks RA, Moore JD, Findley P, Hirshfield C, Humphrey V. REM sleep deprivation and pain thresholds in rats. Percept Mot Skills. 1978; 47:848–850. [DOI] [PubMed] [Google Scholar]
- 97. Campbell CM, Buenaver LF, Finan P, et al. Sleep, pain catastrophizing, and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res. 2015; 67:1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hamilton NA, Catley D, Karlson C. Sleep and the affective response to stress and pain. Health Psychol. 2007; 26:288–295. [DOI] [PubMed] [Google Scholar]
- 99. Colten HR, Altevogt BM; Institute of Medicine (US) Committee on Sleep Medicine and Research, eds. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington (DC): National Academies Press (US); 2006. [PubMed] [Google Scholar]
- 100. Adenekan B, Pandey A, McKenzie S, Zizi F, Casimir GJ, Jean-Louis G. Sleep in America: role of racial/ethnic differences. Sleep Med Rev. 2013; 17:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep. 2015; 38:877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Nunes J, Jean-Louis G, Zizi F, et al. Sleep duration among black and white Americans: results of the National Health Interview Survey. J Natl Med Assoc. 2008; 100:317–322. [DOI] [PubMed] [Google Scholar]
- 103. Williams NJ, Grandner MA, Snipes SA, et al. Racial/ethnic disparities in sleep health and health care: importance of the sociocultural context. Sleep Health. 2015; 1:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ong AD, Williams DR. Lifetime discrimination, global sleep quality, and inflammation burden in a multiethnic sample of middle-aged adults. Cultur Divers Ethnic Minor Psychol. 2019; 25:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mitchell MD, Gehrman P, Perlis M, Umscheid CA. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Fam Pract. 2012; 13:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Aurora RN, Punjabi NM. Obstructive sleep apnoea and type 2 diabetes mellitus: a bidirectional association. Lancet Respir Med. 2013; 1:329–338. [DOI] [PubMed] [Google Scholar]
- 107. Lam JCM, Mak JCW, Ip MSM. Obesity, obstructive sleep apnoea and metabolic syndrome. Respirology. 2012; 17:223–236. [DOI] [PubMed] [Google Scholar]
- 108. Fretts AM, Howard BV, Kriska AM, et al. Physical activity and incident diabetes in American Indians: the Strong Heart Study. Am J Epidemiol. 2009; 170:632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Castor ML, Smyser MS, Taualii MM, Park AN, Lawson SA, Forquera RA. A nationwide population-based study identifying health disparities between American Indians/Alaska Natives and the general populations living in select urban counties. Am J Pub Health. 2006; 96:1478–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Grossman DC, Krieger JW, Sugarman JR, Forquera RA. Health status of urban American Indians and Alaska Natives: a population-based study. JAMA. 1994; 271:845–850. [PubMed] [Google Scholar]
- 111. Toledo TA, Kuhn BL, Payne MF, et al. The effect of pain catastrophizing on endogenous inhibition of pain and spinal nociception in Native Americans: results from the Oklahoma Study of Native American Pain Risk. Ann Behav Med. 2020; 54:575–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Rhudy JL, Kuhn BL, Demuth MJ, et al. Are cardiometabolic markers of allostatic load associated with pronociceptive processes in Native Americans? A structural equation modeling analysis from the Oklahoma Study of Native American Pain Risk. J Pain. 2021; 22(11):1429–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]