Abstract
Background:
Pre-pandemic psychological distress is associated with increased susceptibility to SARS-CoV-2 infection, but associations with COVID-19 severity are not established. The authors examined the associations between distress prior to SARS-CoV-2 infection and subsequent risk of hospitalization.
Methods:
Between April 2020 (baseline) and April 2021, we followed 54,781 participants from three ongoing cohorts: Nurses’ Health Study II (NHSII), Nurses’ Health Study 3 (NHS3), and the Growing Up Today Study (GUTS) who reported no current or prior SARS-CoV-2 infection at baseline. Chronic depression was assessed during 2010-2019. Depression, anxiety, worry about COVID-19, perceived stress, and loneliness were measured at baseline. SARS-CoV-2 infection and hospitalization due to COVID-19 was self-reported. Relative risks (RRs) were calculated by Poisson regression.
Results:
3,663 participants reported a positive SARS-CoV-2 test (mean age=55.0 years, standard deviation=13.8m) during follow-up. Among these participants, chronic depression prior to the pandemic (RR=1.72; 95% CI=1.20-2.46), and probable depression (RR=1.81, 95% CI=1.08-3.03), being very worried about COVID-19 (RR=1.79; 95% CI=1.12-2.86), and loneliness (RR=1.81, 95% CI=1.02-3.20) reported at baseline were each associated with subsequent COVID-19 hospitalization, adjusting for demographic factors and healthcare worker status. Anxiety and perceived stress were not associated with hospitalization. Depression, worry about COVID-19, and loneliness were as strongly associated with hospitalization as were high cholesterol and hypertension, established risk factors for COVID-19 severity.
Conclusions:
Psychological distress may be a risk factor for hospitalization in patients with SARS-CoV-2 infection. Assessment of psychological distress may identify patients at greater risk of hospitalization. Future work should examine whether addressing distress improves physical health outcomes.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has led to over 4.4 million hospital admissions in the United States (CDC), severely straining healthcare systems (Grimm, 2021). Identified risk factors for hospitalization due to severe COVID-19 include age, obesity, asthma, hypertension, and diabetes (Garg et al., 2020), in addition to behaviors such as smoking (Hamer et al., 2020). Various forms of distress, including depression, anxiety, and perceived stress, have also been implicated in elevated susceptibility to infections, including SARS-CoV-2 (Batty et al., 2020; Orlando et al., 2021; Taquet et al., 2021; H. Yang et al., 2020), as well as increased severity of infectious disease (Aiello et al., 2017; Cohen et al., 1995; Falagas et al., 2010; Janicki Deverts et al., 2017). Numerous prospective studies have additionally identified loneliness (LeRoy et al., 2017) and other factors associated with distress (e.g., small social network, low emotional support) (Cohen et al., 1997; Cohen et al., 2003) as potential contributors to a worse course of respiratory infections, although not consistently (Bu et al., 2020; Hamrick et al., 2002).
Better understanding of the links between psychological distress and risk of COVID-19-hospitalization may help prevent severe outcomes and strain on healthcare systems by identifying those at greatest risk. Although the Centers for Disease Control and Prevention have identified mental health conditions as risk factors for COVID-19 severity (People with Certain Medical Conditions 2021), our understanding of the association of distress with COVID-19 severity remains incomplete. Prior studies have primarily investigated mortality as the outcome (Lee et al., 2020; Li et al., 2020; Nemani et al., 2021; Wang et al., 2021), leaving association with hospitalization comparatively unexplored. Most studies used medical records of mental illness diagnoses to measure psychological distress (Lee et al., 2020; Li et al., 2020; Nemani et al., 2021; Orlando et al., 2021; Taquet et al., 2021; Wang et al., 2021; H. Yang et al., 2020). However, medical records have only moderate sensitivity to detect common mental disorders such as depression and anxiety (Beesley et al., 2020; Spiranovic et al., 2016; Townsend et al., 2012), which are frequently undiagnosed (Gwynn et al., 2008; Ko et al., 2012; Perruche et al., 2011). Additionally, some medical record studies examined diagnosis of any psychiatric disorder as a single exposure, reducing utility for intervention and prediction (Li et al., 2020; Wang et al., 2021). No studies have examined other common manifestations of distress, such as perceived stress and loneliness in relation to hospitalization from COVID-19.
In the present study, we prospectively examined whether various manifestations of psychological distress prior to infection with SARS-CoV-2 predicted hospitalization among individuals after SARS-CoV-2 infection, using data from three large longitudinal cohorts: the Nurses’ Health Study II (NHSII), the Nurses’ Health Study III (NHS3), and the Growing Up Today Study (GUTS) (Bao et al., 2016). We additionally examined the extent to which established risk factors for COVID-19 hospitalization (e.g., age, diabetes, asthma, hypertension, smoking, obesity) accounted for possible associations.
METHODS
Study design and population
Study participants were drawn from the Nurses Health cohorts, a set of cohorts initiated in the early 1970s to study the long-term health effects of exogenous hormone use in the early 1970s in a medically sophisticated group. The NHSII is a cohort of 116,429 female registered nurses living in the US, enrolled in 1989 at ages of 25-42 years and followed biennially. The NHSII was established to study the health effects of oral contraceptive use and other health risk factors among women of reproductive age. The NHS3, currently recruiting, was established in 2010 and includes more than 40,000 female nurses aged 18 years and older, living in the US or Canada and followed biannually using web-based questionnaires. NHS3 recruited more participants of different race/ethnicity groups. Recruitment was extended to male nurses in 2015 (N=856). GUTS began in 1996 when NHS II participants enrolled their offspring (N=27,793) aged 9-17 years to study the potential impacts of factors (e.g., diet and exercise) that influence weight change trajectory throughout life course.
From April 2020 to May 2020, we invited participants who returned the most recent main cohort questionnaire to complete a supplementary online COVID-19 survey. Of 105,662 invited participants, 58,612 (55%) responded to the first COVID-19 questionnaire from April 2020 to August 2020 (termed “baseline” henceforth). Respondents were then administered monthly surveys. Additional weekly surveys were administered to those who self-identified as frontline healthcare workers (n=23,053). In August 2020, the surveys changed to quarterly administration (Figure S1, Figure S2). The end of follow-up for the current analysis was April 27, 2021. Among 58,612 people who responded to the COVID-19 baseline survey, we further restricted analysis to 54,781 participants with no documented history of SARS-CoV-2 infection and not hospitalized at baseline who returned at least one follow-up questionnaire.
The study was approved by the Partners Healthcare System Institutional Review Board. Return of questionnaires constituted implied consent.
Measures
Forms of distress
Chronic depression in the 10 years prior to the COVID-19 pandemic was derived from multiple indicators queried at varying intervals on main cohort questionnaires, 2010-2019. These included self-reported physician-diagnosed depression, use of antidepressants, and depressive symptoms as reported on the Center for Epidemiologic Studies Depression Scale-10 (CESD-10) and the Patient Health Questionnaire-9 (PHQ-9) (Table S1). Participants were considered to have a history of chronic depression if they reported any indicator of depression at two or more time points.
All other forms of distress were assessed at COVID-19 study baseline. Current depression and anxiety symptoms were assessed with the PHQ-4, which combines a 2-item measure of depression (PHQ-2) and a 2-item measure of anxiety (Generalized Anxiety Disorder-2 (GAD-2)) (Kroenke et al., 2009). The PHQ-4 queries frequency of symptoms, with response options 0 (“not at all”) to 3 (“nearly every day”). A summed score of 3 or greater on each subscale indicates probable major depressive disorder or anxiety disorder (Kroenke et al., 2003; Kroenke et al., 2007). To create reference groups with no symptoms, and to examine possible dose-dependent relations, we divided these scores into three levels for analysis: 0 points (reference), 1-2 points (subclinical symptoms), ≥3 points (probable depression or anxiety). The PHQ-2 and GAD-2 have been validated against clinical diagnosis (Kroenke et al., 2003; Kroenke et al., 2009; Kroenke et al., 2007; Lowe et al., 2010). Worry about COVID-19 was assessed with a single question, ‘How worried are you about COVID-19?’ with response options: “not at all”, “not very”, “somewhat”, and “very worried” (YouGov, 2020). “Not at all” and “not very worried” were combined to serve as the reference group, as only 5.9% participants responded “not at all worried”.
Due to concerns about participant burden, two additional forms of distress were assessed only among non-healthcare workers. The 4-item Perceived Stress Scale (PSS-4) (Cohen, 1988), a shortened version of the well-validated 14-item PSS (Cohen et al., 1983), assesses ability to cope with existing stressors. Total scores range from 0-16, with higher scores indicating higher levels of perceived stress, with satisfactory psychometrics (Cohen, 1988; Mitchell et al., 2008; Vallejo et al., 2018; Warttig et al., 2013). Loneliness was assessed with the 3-item UCLA Loneliness Scale (Hughes et al., 2004) which has demonstrated good internal consistency reliability and validity (Hughes et al., 2004; Steptoe et al., 2013). Participants reported how often they felt: (i) lack of companionship; (ii) left out; and (iii) isolated from others. Responses were coded as 1 (“hardly ever”), 2 (“some of the time”), and 3 (“often”), with higher scores indicating greater loneliness. We divided the score into three levels for analysis, 3 points (loneliness hardly ever, reference), 3-5 points (less than some of the time), and ≥6 (some of the time or often) (Steptoe et al., 2013). For all continuous scores, effects associated with an increase of one interquartile range (IQR) were estimated. Because the distribution of the scores were skewed, IQRs were approximate.
COVID-19 infection and hospitalization
Past 7-, 30-, and 90-day positive SARS-CoV-2 diagnostic test (antibody, antigen, or PCR), COVID-19 symptoms, and hospitalization due to COVID-19 occurring since March 1, 2020, were self-reported on all questionnaires. Participants were asked specifically, “Have you been hospitalized because of COVID-19?” and the treatments they received, including intravenous fluid, oxygen through nasal prong or facial mask, ICU admission, and invasive ventilation.
Covariates
All covariates were assessed prior to the pandemic in main cohort questionnaires, using the most recent data available (Table S2). Demographic factors included age, race/ethnic identity, sex, and educational attainment of the participant (GUTS) or their spouse/partner (NHS2 and NHS3). Risk factors for COVID-19 severity included body mass index (BMI), smoking, and history of clinician-diagnosed: diabetes, hypertension, high cholesterol, asthma, and cancer (yes/no for each of the diseases).
Statistical analysis
To examine the association of distress and risk of SARS-CoV-2 infection, we compared level of distress among participants with and without a positive SARS-CoV-2 diagnostic test during follow-up. Among participants who reported a positive SARS-CoV-2 test, we compared the prevalence of established risk factors for severe COVID-19 and demographic factors by forms of distress at baseline. To examine the association of distress with risk of subsequent hospitalization for COVID-19, we fit Poisson regression models with hospitalization as the dependent variable with each form of distress as the independent variable in separate models adjusted for demographic factors, educational attainment, and healthcare worker status. To examine whether health risk factors that commonly co-occur with distress might account for possible associations, we further adjusted for: 1) health behavioral factors including BMI and smoking; and 2) health behavioral factors and history of hypertension, diabetes, high cholesterol, asthma, and cancer.
We conducted several sensitivity analyses. First, as frontline healthcare workers have higher risk of infection and increased psychological distress than non-healthcare workers (Chou et al., 2020; Preti et al., 2020; Sasaki et al., 2020; Zheng et al., 2020), we investigated the association of forms of distress with COVID-19 hospitalization stratified by healthcare worker status and tested a distress-by-healthcare-worker-status interaction term. Second, we further adjusted for month using indicator variables, to account for the different phases of the pandemic. Third, although we excluded participants who reported a positive SARS-CoV-2 test at baseline, some participants reported COVID-19-related symptoms at baseline. Therefore, to ensure that distress preceded SARS-COV-2 infection, we further excluded 175 respondents who were identified as possible cases using a symptom-based predictor of SARS-CoV-2 infection (Rich-Edwards et al., 2021). Fourth, we excluded 104 participants who reported a positive SARS-COV-2 test within 30 days of return of the baseline questionnaire, to minimize the possibility that prodromal symptoms were causing psychological distress. Fifth, to remove possible effects of vaccination on SARS-CoV-2 susceptibility and severity, we excluded people who reported SARS-COV-2 infections after participants could have been fully vaccinated (Feb 1, 2021 for active healthcare workers, N excluded=225, and April 1, 2021 for non-healthcare workers, N excluded=21). Sixth, we further adjusted for cohort and number of questionnaires. Seventh, to examine the possible difference in sex, we stratified analysis by sex. As only 1 male participant was hospitalized, models restricted to males did not convergence. We presented analyses to female participants (n=3,535). Eighth, we fit models using the most recent measures of pre-infection psychological distress as independent variables. Ninth, to evaluate whether loneliness might be a proxy for low availability of getting care when sick, we adjusted for living situation reported at baseline (living alone or not).
For all models, relative risks (RRs) were estimated using generalized linear models(Zou, 2004) in SAS 9.4 (SAS Institute). A 2-sided P <0.05 was considered statistically significant.
RESULTS
The 54,781 participants who returned at least one follow-up questionnaire were primarily female (96.6%) and White (96.5%), with mean age=57.5 years (SD=13.8). More than one third of participants (38.0%) were frontline healthcare workers. We documented 3,663 incident cases of SARS-CoV-2 (6.7% of participants) from May 1, 2020, to April 1, 2021. At baseline, the mean age of participants who had a positive SARS-CoV-2 test during follow-up was 55.0 years (SD=13.8); 96.5% were female, 128 (3.5%) were male, 96.7% were White, and 52.1% were frontline healthcare workers. The mean (SD) age of male participants was 33.4 (4.9). 34 (26.6%) of male participants were active healthcare workers. The median time from return of baseline questionnaire to positive SARS-CoV-2 test was 30 weeks (range, 1-47 weeks). Active healthcare workers had elevated level of depression and anxiety at baseline compared to non-active healthcare workers (depressive symptoms: 1.18 vs 1.06; anxiety symptoms: 1.60 vs 1.35). The prevalence of all forms of distress at baseline was similar among respondents who reported a positive SARS-CoV-2 test during follow up and those who did not (Table S3).
Respondents with probable depression or anxiety at baseline were younger, had higher BMI, had a higher prevalence of asthma, and were more likely to be frontline healthcare workers than those who had no symptoms of these disorders (Table 1). Compared with participants who reported not being worried or not very worried about COVID-19, those reporting being very worried were more likely to be female, to be racial or ethnic minorities, to be frontline healthcare workers, to have a higher BMI, and to have a comorbidity. Lonely participants were younger than participants who were hardly ever lonely. Chronic depression and all forms of distress at baseline were correlated (Table 2, all P<0.001), with depression and anxiety most strongly correlated (Spearman correlation=0.63). Despite appearing to be similar constructs, each of the 2 items that comprised the GAD-2 anxiety measure was only modestly correlated with worry about COVID-19 (rho=0.29, 0.26, P<0.001).
Table 1.
Health and demographic characteristics by forms of distress at baseline (April-August 2020), among participants who ever tested positive for SARS-CoV-2 between April 2020 - April 2021, N=3,663
| Depressive symptoms | Anxiety symptoms | Worry about COVID-19 | Loneliness | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| No symptoms | Probable depression | No symptoms | Probable anxiety | No | High | Hardly ever | Yes | ||
| N=1790 | N=477 | N=1349 | N=767 | N=1105 | N=597 | N=748 | N=501 | ||
|
|
|||||||||
| Age, years | mean (SD) | 57.4 (12.8) | 49.9 (14.6) | 59.4 (12.0) | 50.2 (14.3) | 56.4 (13.6) | 55.3 (13.7) | 57.7 (14.5) | 56.8 (15.1) |
| Race | |||||||||
| White | N (%) | 1737 (97.0) | 462 (96.9) | 1304 (96.7) | 744 (97.0) | 1080 (97.7) | 566 (94.8) | 730 (97.6) | 490 (97.6) |
| Sex | |||||||||
| Female | N (%) | 1727 (96.5) | 460 (96.4) | 1297 (96.2) | 743 (96.9) | 1049 (94.9) | 587 (98.3) | 704 (94.1) | 473 (94.4) |
| Male | N (%) | 63 (3.5) | 17 (3.6) | 52 (3.9) | 24 (3.1) | 56 (5.1) | 10 (1.7) | 44 (5.9) | 28 (5.6) |
| Frontline healthcare worker, yes | N (%) | 892 (49.8) | 284 (59.5) | 605 (44.9) | 449 (58.5) | 504 (45.6) | 310 (51.9) | - | - |
| Partner’s educationb | |||||||||
| High school or lower | N (%) | 227 (12.7) | 40 (8.4) | 204 (15.1) | 61 (8.0) | 138 (12.5) | 56 (9.4) | 92 (12.3) | 49 (9.8) |
| College | N (%) | 732 (40.9) | 146 (30.6) | 525 (38.9) | 275 (35.9) | 450 (40.7) | 187 (31.3) | 286 (38.2) | 171 (34.1) |
| Graduate school | N (%) | 300 (46.8) | 76 (15.9) | 228 (16.9) | 130 (17.0) | 178 (16.1) | 103 (17.3) | 137 (18.3) | 77 (15.4) |
| Unmarried | N (%) | 471 (26.3) | 200 (41.9) | 343 (25.4) | 278 (36.3) | 303 (27.4) | 229 (38.4) | 194 (25.9) | 184 (36.7) |
| BMI, kg/m2 | |||||||||
| <18.5 | N (%) | 25 (1.4) | 5 (1.1) | 17 (1.3) | 8 (1.0) | 19 (1.7) | 8 (1.3) | 9 (1.2) | 9 (1.8) |
| 18.5 to <25 | N (%) | 607 (33.9) | 123 (25.8) | 427 (31.7) | 225 (29.3) | 379 (34.3) | 162 (27.1) | 235 (31.4) | 141 (28.1) |
| 25 to <30 | N (%) | 526 (29.4) | 123 (25.8) | 412 (30.5) | 222 (28.9) | 335 (30.3) | 170 (28.5) | 221 (29.6) | 136 (27.2) |
| 30 to <35 | N (%) | 346 (19.3) | 86 (18.0) | 282 (20.9) | 127 (16.6) | 199 (18.0) | 94 (15.8) | 122 (16.3) | 98 (19.6) |
| ≥35 | N (%) | 221 (12.6) | 98 (20.6) | 172 (12.8) | 136 (17.7) | 127 (11.5) | 126 (21.1) | 116 (15.5) | 87 (17.4) |
| Smoking status | |||||||||
| Current | N (%) | 44 (2.5) | 18 (3.8) | 40 (3.0) | 25 (3.3) | 30 (2.7) | 22 (3.7) | 17 (2.3) | 16 (3.2) |
| Former | N (%) | 449 (25.1) | 113 (23.7) | 388 (28.8) | 178 (23.4) | 269 (24.3) | 181 (30.3) | 180 (24.1) | 125 (25.0) |
| Never | N (%) | 1297 (72.5) | 346 (72.5) | 921 (68.3) | 564 (73.5) | 808 (72.9) | 394 (66.0) | 551 (73.7) | 360 (71.9) |
| Diabetes, ever | N (%) | 94 (5.3) | 23 (4.8) | 83 (6.2) | 35 (4.6) | 55 (5.0) | 32 (5.4) | 46 (6.2) | 36 (7.2) |
| Hypertension, ever | N (%) | 354 (39.8) | 100 (21.0) | 296 (21.9) | 157 (20.5) | 224 (20.3) | 146 (24.5) | 182 (24.3) | 133 (26.6) |
| High cholesterol, ever | N (%) | 439 (24.5) | 125 (26.2) | 355 (26.3) | 194 (25.3) | 286 (25.9) | 165 (27.6) | 202 (27.0) | 147 (29.3) |
| Asthma, ever | N (%) | 196 (11.0) | 103 (21.6) | 126 (9.3) | 145 (18.9) | 119 (10.8) | 97 (16.3) | 89 (11.9) | 70 (14.0) |
| Cancer, ever | N (%) | 88 (4.9) | 23 (4.8) | 68 (5.0) | 33 (4.3) | 51 (4.6) | 33 (5.5) | 43 (5.8) | 26 (5.2) |
Numbers do not add to 100% because mid-levels of variables, i.e., subclinical depressive and anxious symptoms, somewhat worried, and somewhat lonely, are not shown due to space constraints; UCLA loneliness information was collected only in non-active health care workers.
Participants’ own education attainment in GUTS (unmarried category did not apply)
Table 2.
Spearman correlations between forms of distress among persons with a positive SARS-CoV-2 test from April 2020 to April 2021, NHS II, NHS3, and GUTS, N=3,663
| History of depression | Depressive symptoms | Anxiety symptoms | Worry about COVID | Perceived stress | Loneliness | |
|---|---|---|---|---|---|---|
|
|
||||||
| History of depression | 1.0 | - | - | - | - | - |
| Depressive symptoms | ρ=0.20* | 1.0 | - | - | - | - |
| Anxiety symptoms | ρ=0.13* | ρ=0.63* | 1.0 | - | - | - |
| Worry about COVID | ρ=0.05* | ρ=0.23* | ρ=0.30* | 1.0 | - | - |
| Perceived stress | ρ=0.10* | ρ=0.54* | ρ=0.54* | ρ=0.23* | 1.0 | - |
| Loneliness | ρ=0.15* | ρ=0.47* | ρ=0.32* | ρ=0.13* | ρ=0.39* | 1.0 |
Note. NHS = Nurses’ Health Study; GUTS = Growing Up Today Study. Perceived stress and loneliness were queried in non-active health care workers only.
P<0.001
All subsequent analyses were conducted among the 3,663 respondents with a positive SARS-CoV-2 test during follow-up. Of these, 132 (4%) were hospitalized due to COVID-19, of whom 75% received oxygen and 20% reported ICU admission. Chronic depression prior to the pandemic was significantly associated with COVID-19 hospitalization (risk ratio (RR)=1.72; 95% confidence interval (CI)=1.20-2.46, Figure 1) adjusted for demographic factors. Probable depression at baseline was also associated with COVID-19 hospitalization (RR=1.81; 95% CI=1.08-3.03, p=0.02, Table 3). In analyses examining depressive symptoms as a continuous measure, an IQR increase in depressive symptoms was associated with 45% increased risk of COVID-19 hospitalization (95% CI=17%-81%, p<0.001).
Figure 1.
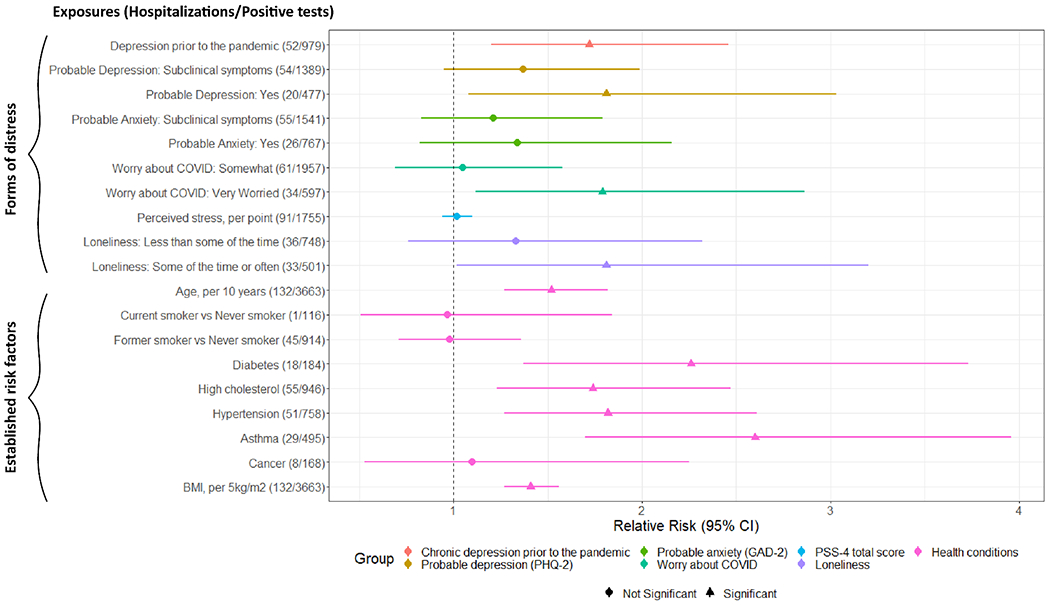
Relative Risks (RRs) and 95% Confidence Intervals (CIs) of the associations between forms of distress at baseline and established risk factors for severe COVID-19 in relation to COVID-19 hospitalization, April 2020 - April 2021, N=3,663
Multivariable model adjusted for age, sex, racial identity, healthcare worker status and partner’s education (participants’ own education attainment in GUTS). Perceived stress and loneliness were queried in non-active health care workers only.
Note. PHQ-2 = 2-item Patient Health Questionnaire; GAD-2 = 2-item Generalized Anxiety Disorder.
Table 3.
Association of forms of distress prior to infection and risk of COVID-19 hospitalization among persons testing positive for SARS-CoV-2, April 2020 - April 2021, N=3,663
| Hospitalized/Positive tests (N) | Model 1: age, sex, and racial identity, healthcare worker status, and partner’s education | Model 2: Model 1 further adjusted for smoking and BMI | Model 3: Model 2 further adjusted for hypertension, diabetes, asthma, high cholesterol, and cancer | |
|---|---|---|---|---|
|
|
||||
| Risk ratio (95% confidence interval) | ||||
|
|
||||
| Prior to the pandemic | ||||
| Chronic depression in the decade prior to the pandemic | 52/979 | 1.72 (1.20-2.46) | 1.47 (1.02-2.11) | 1.34 (0.93-1.93) |
| At COVID-19 study baseline | ||||
| Probable depression (PHQ-2) | ||||
| No (0 points) | 58/1790 | 1.0 [Reference] | 1.0 [Reference] | 1.0 [Reference] |
| Subclinical symptoms (1-2 points) | 54/1389 | 1.37 (0.95-1.99) | 1.30 (0.90-1.89) | 1.26 (0.87-1.83) |
| Yes (3-6 points) | 20/477 | 1.81 (1.08-3.03) | 1.59 (0.95-2.67) | 1.46 (0.87-2.46) |
| Depressive symptoms, continuous, per approximate IQR | 1.45 (1.17-1.81) | 1.37 (1.10-1.71) | 1.32 (1.06-1.65) | |
| Probable anxiety (GAD-2) | ||||
| No (0 points) | 51/1349 | 1.0 [Reference] | 1.0 [Reference] | 1.0 [Reference] |
| Subclinical symptoms (1-2) | 55/1541 | 1.21 (0.83-1.79) | 1.20 (0.81-1.76) | 1.18 (0.80-1.74) |
| Yes (3-6 points) | 26/767 | 1.34 (0.82-2.16) | 1.23 (0.76-2.00) | 1.16 (0.71-1.88) |
| Anxiety symptoms, continuous, per approximate IQR | 1.18 (0.94-1.48) | 1.14 (0.91-1.43) | 1.11 (0.88-1.39) | |
| Worry about COVID | ||||
| Not at all/not very worried | 37/1105 | 1.0 [Reference] | 1.0 [Reference] | 1.0 [Reference] |
| Somewhat worried | 61/1957 | 1.05 (0.69-1.58) | 0.98 (0.65-1.47) | 0.97 (0.64-1.46) |
| Very worried | 34/597 | 1.79 (1.12-2.86) | 1.55 (0.96-2.48) | 1.51 (0.94-2.42) |
| P trend* | 0.02 | 0.10 | 0.12 | |
| Among participants who indicated they were not currently frontline healthcare workers (n=1,754) | ||||
| Perceived stress, per approximate IQR | 88/1754 | 1.09 (0.79-1.49) | 1.04 (0.76-1.41) | 1.00 (0.73-1.36) |
| Loneliness | ||||
| Hardly ever (3 points) | 19/489 | 1.0 [Reference] | 1.0 [Reference] | 1.0 [Reference] |
| Less than some of the time (4-5 points) | 36/748 | 1.33 (0.76-2.32) | 1.26 (0.72-2.20) | 1.22 (0.70-2.14) |
| Some of the time or often (6-9 points) | 33/501 | 1.81 (1.02-3.20) | 1.67 (0.95-2.97) | 1.58 (0.89-2.81) |
| Loneliness score, continuous, per approximate IQR | 1.29 (1.01-1.65) | 1.23 (0.96-1.56) | 1.20 (0.94-1.53) | |
Note. PHQ-2 = 2-item Patient Health Questionnaire-2; GAD-2 = 2-item Generalized Anxiety Disorder; perceived stress and loneliness were queried in non-active health care workers only. IQR, interquartile range
P trend analysis used indicator levels as a continuous variable.
Worry about COVID-19 was significantly associated with COVID-19 hospitalization (very worried, RR=1.79; 95% CI=1.12-2.86; P trend=0.03), as was loneliness (RR=1.81, 95% CI=1.02-3.20, p=0.04). An IQR increase in the continuous loneliness score was associated with 29% increased risk of COVID-19 hospitalization (95% CI=1%-65%, p=0.04). Anxiety and perceived stress were not associated with hospitalization, although perceived stress could only be assessed in non-healthcare workers. Figure 1 shows risk ratios for hospitalization associated with forms of distress and, for comparison, several established risk factors for severe COVID-19. The magnitude of the associations with COVID-19 hospitalization was as strong for chronic depression prior to the pandemic and worry about COVID-19 at baseline as for hypertension and high cholesterol.
In models further adjusted for smoking and BMI (Table 3, model 2), associations between chronic depression, depression at baseline, worry, and loneliness and COVID-19 hospitalization were attenuated (attenuation range, 13-29%). Higher BMI among persons with distress accounted for most of the attenuation. Associations were further attenuated after adjustment for comorbidities (additional attenuation, 6-25%; total attenuation due to all covariates, 23-46%), primarily due to higher prevalence of asthma among persons with distress. Depressive symptoms coded continuously remained significantly associated with increased risk for COVID-19 hospitalization in the model fully adjusted for health behaviors and comorbidities (RR per IQR=1.32, 95% CI=1.06-1.65, p=0.01).
Associations of distress with COVID-19 hospitalization were generally somewhat stronger in non-healthcare workers than healthcare workers, though these differences were not statistically significant (data not shown). Results were comparable in models further adjusted for month, excluding probable baseline SARS-CoV-2 cases, excluding cases diagnosed within 30 days after return of baseline questionnaire, further adjusting for cohort number, restricting analysis to female, and using the most recent distress measures prior to infection. In analyses of loneliness, results were similar in models further adjusted for living alone (Table S4).
DISCUSSION
In this prospective study of 54,809 people who reported no history of SARS-CoV-2 infection, in demographically adjusted models, chronic depression prior to the pandemic, and probable depression, worry about COVID-19, and loneliness early in the pandemic were associated with increased risk for hospitalization due to COVID-19, among the 3,663 individuals who subsequently became infected with SARS-CoV-2. There was no association of anxiety or perceived stress with risk of hospitalization, although we were not able to assess perceived stress among healthcare workers. Depression, worry about COVID-19, and loneliness were as strongly associated with hospitalization as were established risk factors for COVID-19 severity, such as high cholesterol and hypertension.
Higher prevalence of risk factors for COVID-19 severity, in particular, higher BMI and asthma accounted for 23-46% of these associations. In fully adjusted models accounting for all comorbidities, only depressive symptoms at baseline remained significantly associated with risk of COVID-19 hospitalization. We did not have information about whether distress preceded weight gain and asthma or the reverse. Evidence from longitudinal studies suggest a bi-directional association of depression and high BMI, with depression increasing risk of subsequent obesity and obesity increasing risk of subsequent depression. Longitudinal and Mendelian randomization studies also indicate bi-directional effects between depression and adult-onset asthma, although evidence that asthma predicts incident depression is not consistent (Gao et al., 2015; Kim et al., 2019; Lehto et al., 2019; Lu et al., 2018; Zhu et al., 2019). Mendelian randomization studies show causal effects of both BMI and body fat on loneliness, but not loneliness on BMI (Abdellaoui et al., 2019; Day et al., 2018); however, the direction of causality with asthma has not been well studied. Therefore, depression and loneliness may have increased risk of obesity and asthma, and obesity and asthma may also have increased risk of depression and loneliness.
A single prior study using data from the UK Biobank assessed depressive and anxious symptoms with the PHQ-4 in 2006-2010 and found an association between these symptoms and susceptibility to SARS-CoV-2 infection and COVID-19 hospitalization over an 1-year period in age- and sex-adjusted models, but not after further adjustment for ethnicity, comorbidity, and lifestyle factors (Batty et al., 2020). However, the study conflated risk of infection and risk of hospitalization, as the reference group included people uninfected with SARS-CoV-2 and people infected with SARS-CoV-2 who were not hospitalized. As risk factors for SARS-CoV-2 infection and COVID-19 severity may differ (R. Yang et al., 2020), the implications of these findings are unclear.
Chronic depression is pro-inflammatory and immunosuppressive (Leonard, 2010). Psychological distress, more generally, may increase risk of severe clinical outcomes in respiratory infections (Aiello et al., 2017; Bu et al., 2020; Falagas et al., 2010; LeRoy et al., 2017), possibly through dysregulation of stress signaling pathways. Sustained stress leads to protracted HPA-axis stimulation and subsequent chronic immune suppression (Thomas & Lena, 2010) as well as elevated levels of circulating cortisol (Hannibal & Bishop, 2014), which leads to susceptibility to infection and more severe clinical outcomes among those infected (Tian et al., 2014). Chronic exposure to elevated cortisol may additionally result in an inability to attenuate levels of pro-inflammatory cytokines post-infection (Chi et al., 2013; Cohen et al., 1997; Tian et al., 2014), which further compounds disease severity (Del Valle et al., 2020; Short et al., 2014; Zhao et al., 2018) and increases risk of hospitalization (Yende et al., 2005). Despite common pathways linking depression, anxiety, stress, and loneliness to immune dysregulation, we did not find associations of anxiety and perceived stress with hospitalization risk. Of note, perceived stress has not been consistently associated with physical health outcomes (Macleod et al., 2002; Väänänen et al., 2009).
The present study was conducted in a primarily female sample. Prior studies have suggested men with COVID-19 may face higher rates of hospitalization than women. A systematic review of COVID-19 outcomes in Europe found that men with COVID-19 experienced higher rates of hospitalization than women (Gebhard et al., 2020). Multicenter cohort studies have similarly found higher rates of COVID-19 hospitalization in men versus women, although these studies were not adjusted for factors which may affect hospitalization, such as age and health comorbidities (Garg et al., 2020; Gomez et al., 2021). Past studies of SARS-CoV and MERS-CoV outbreaks have also found men to have higher case fatality rates than women (Ebrahim et al., 2021; Karlberg et al., 2004). Additional research is therefore needed to investigate the association of distress with risk of COVID-19 hospitalization in men.
Our study has several strengths. Periodic surveys were sent to three large cohorts prospectively measuring distress, incident infection, and hospitalization over a 1-year period during an active stage of the COVID-19 pandemic. Distress was measured early in the pandemic, which may have more accurately captured recent distress compared with studies examining pre-pandemic medical records of mental illness. Findings were also robust to exclusion of persons with COVID-19 diagnosis within 30 days of the baseline questionnaire. We examined some largely unexplored forms of distress, such as loneliness, perceived stress, and worry about COVID-19.
Our study has several limitations. Baseline anxiety and depression were measured with brief screeners rather than clinician diagnoses and may been misclassified. Worry about COVID-19 was measured with a single question, which may have lower reliability than a multi-item measure (Diamantopoulos et al., 2012). SARS-CoV-2 infection and hospitalization due to COVID-19 were self-reported, although self-reported health outcomes have had good validity in these cohorts (Forman et al., 2008; Troy et al., 1995). We were not able to capture COVID-19-related mortality. Finally, our sample of individuals infected with COVID-19 was relatively small, limiting statistical power, and was comprised primarily of white female nurses, limiting generalizability.
Conclusions
In this prospective study of adults, we found chronic depression prior to the pandemic and probable depression, worry about COVID-19, and loneliness predicted hospitalization due to COVID-19, which was in part accounted for by poorer physical health in persons with these forms of distress. Depressive symptoms early in the pandemic remained associated with COVID-19 hospitalization even after adjusting for health-related behaviors and comorbidities. Our findings suggest the need to consider psychological health in addition to physical health as risk factors of severe COVID-19. Future research should examine whether reducing distress, in addition to other medical interventions, improves outcomes in patients with SARS-CoV-2 and other infectious diseases.
Supplementary Material
Funding:
This research was supported by NIH NICHD grant 3R01HD094725-02S1 (to ALR). Other support includes grants U01HL145386, R24ES028521, U01 CA176726, R01 CA67262, and R01 HD057368 from the National Institutes of Health; the Dean’s Fund for Scientific Advancement Acceleration Award from the Harvard T. H. Chan School of Public Health; and the Massachusetts Consortium on Pathogen Readiness Evergrande COVID-19 Response Fund Award.
Competing Interests:
WBE is the site PI for a COVID-19 therapeutics study funded by Gilead pharmaceuticals (funds to institution). Views expressed are those of the authors and do not necessarily represent those of the US Federal Government.
References:
- Abdellaoui A, Sanchez-Roige S, Sealock J, Treur JL, Dennis J, Fontanillas P, … Boomsma DI (2019). Phenome-wide investigation of health outcomes associated with genetic predisposition to loneliness. Human molecular genetics, 28(22), 3853–3865. 10.1093/hmg/ddz219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello, Simanek AM, Stebbins RC, & Dowd JB (2018). Infectious diseases. In The Routledge International Handbook of Psychosocial Epidemiology (1st ed., Vol. 1, pp. 281–300). Routledge. 10.4324/9781315673097-13 [DOI] [Google Scholar]
- Bao Y, Bertoia ML, Lenart EB, Stampfer MJ, Willett WC, Speizer FE, & Chavarro JE (2016). Origin, Methods, and Evolution of the Three Nurses’ Health Studies. Am J Public Health, 106(9), 1573–1581. 10.2105/AJPH.2016.303338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty GD, Deary IJ, Luciano M, Altschul D, Kivimäki M, & Gale CR (2020). Psychosocial factors and hospitalisations for COVID-19: Prospective cohort study based on a community sample. Brain, Behavior, and Immunity, 89. 10.1016/j.bbi.2020.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesley LJ, Salvatore M, Fritsche LG, Pandit A, Rao A, Brummett C, … Mukherjee B (2020). The emerging landscape of health research based on biobanks linked to electronic health records: Existing resources, statistical challenges, and potential opportunities. Statistics in Medicine, 39(6), 773–800. 10.1002/sim.8445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu F, Philip K, & Fancourt D (2020). Social isolation and loneliness as risk factors for hospital admissions for respiratory disease among older adults. Thorax, 75(7), 597. 10.1136/thoraxjnl-2019-214445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, White House COVID-19 Team, Data Strategy and Execution Workgroup. (2022). New Amissions of Patients with Confirmed COVID-19 per 100,000 Population by Age Group, United States: Aug 01, 2020 – Feb 11, 2022. Unified Hospital Dataset. https://covid.cdc.gov/covid-data-tracker/#new-hospital-admissions [Google Scholar]
- Chi Y, Zhu Y, Wen T, Cui L, Ge Y, Jiao Y, … Zhou M (2013). Cytokine and Chemokine Levels in Patients Infected With the Novel Avian Influenza A (H7N9) Virus in China. The Journal of Infectious Diseases, 208(12), 1962–1967. 10.1093/infdis/jit440 [DOI] [PubMed] [Google Scholar]
- Chou R, Dana T, Buckley DI, Selph S, Fu R, & Totten AM (2020). Epidemiology of and Risk Factors for Coronavirus Infection in Health Care Workers: A Living Rapid Review. Annals of internal medicine, 173(2), 120–136. 10.7326/M20-1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S (1988). Perceived stress in a probability sample of the United States. In (Vol. In Spacapan S & Oskamp S (Eds.), pp. 31–67). Sage Publications, Inc. [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP, Fireman P, Gwaltney JM Jr, & Newsom JT (1995). State and trait negative affect as predictors of objective and subjective symptoms of respiratory viral infections. Journal of Personality and Social Psychology, 68(1), 159–169. 10.1037/0022-3514.68.1.159 [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP, Rabin BS, & Gwaltney JM Jr. (1997). Social Ties and Susceptibility to the Common Cold. JAMA, 277(24), 1940–1944. 10.1001/jama.1997.03540480040036 [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Turner R, Alper CM, & Skoner DP (2003). Sociability and Susceptibility to the Common Cold. Psychological Science, 14(5), 389–395. 10.1111/1467-9280.01452 [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. J Health Soc Behav, 24(4), 385–396. [PubMed] [Google Scholar]
- Day FR, Ong KK, & Perry JRB (2018). Elucidating the genetic basis of social interaction and isolation. Nature communications, 9(1), 2457. Retrieved 2018/07//, from 10.1038/s41467-018-04930-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle DM, Kim-Schulze S, Huang H-H, Beckmann ND, Nirenberg S, Wang B, … Gnjatic S (2020). An inflammatory cytokine signature predicts COVID-19 severity and survival. Nature Medicine, 26(10), 1636–1643. 10.1038/s41591-020-1051-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantopoulos A, Sarstedt M, Fuchs C, Wilczynski P, & Kaiser S (2012). Guidelines for choosing between multi-item and single-item scales for construct measurement: a predictive validity perspective. Journal of the Academy of Marketing Science, 40(3), 434–449. 10.1007/s11747-011-0300-3 [DOI] [Google Scholar]
- Ebrahim SH, Maher AD, Kanagasabai U, Alfaraj SH, Alzahrani NA, Alqahtani SA, … Memish ZA (2021). MERS-CoV Confirmation among 6,873 suspected persons and relevant Epidemiologic and Clinical Features, Saudi Arabia - 2014 to 2019. EClinicalMedicine, 41, 101191. 10.1016/j.eclinm.2021.101191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas ME, Karamanidou C, Kastoris A, Karlis G, & Rafailidis P (2010). Psychosocial factors and susceptibility to or outcome of acute respiratory tract infections [Review article]. The International Journal of Tuberculosis and Lung Disease, 14, 141–148. [PubMed] [Google Scholar]
- Forman JP, Curhan GC, & Taylor EN (2008). Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension (Dallas, Tex. : 1979), 52(5), 828–832. 10.1161/hypertensionaha.108.117630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y-H, Zhao H-S, Zhang F-R, Gao Y, Shen P, Chen R-C, & Zhang G-J (2015). The Relationship between Depression and Asthma: A Meta-Analysis of Prospective Studies. PloS one, 10(7), e0132424–e0132424. 10.1371/journal.pone.0132424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S, Kim L, Whitaker M, O’Halloran A, Cummings C, Holstein R, … Fry A (2020). Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR. Morbidity and Mortality Weekly Report, 69(15), 458–464. 10.15585/mmwr.mm6915e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, & Klein SL (2020). Impact of sex and gender on COVID-19 outcomes in Europe. Biology of sex differences, 11(1), 29. 10.1186/s13293-020-00304-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez J, Du-Fay-de-Lavallaz JM, Fugar S, Sarau A, Simmons JA, Clark B, Sanghani RM, Aggarwal NT, Williams KA, Doukky R, & Volgman AS Sex Differences in COVID-19 Hospitalization and Mortality. Journal of women’s health (2021), 30(5), 646–653. 10.1089/jwh.2020.8948 [DOI] [PubMed] [Google Scholar]
- Grimm C (2021). Hospitals Reported That the COVID-19 Pandemic Has Significantly Strained Health Care Delivery: Results of a National Pulse Survey, Feb. 22-26, 2021; 2021 ASI 4006-11.45844;OEI-09-21-00140.
- Gwynn RC, McQuistion HL, McVeigh KH, Garg RK, Frieden TR, & Thorpe LE (2008). Prevalence, Diagnosis, and Treatment of Depression and Generalized Anxiety Disorder in a Diverse Urban Community. Psychiatric Services, 59(6), 641–647. 10.1176/ps.2008.59.6.641 [DOI] [PubMed] [Google Scholar]
- Hamer M, Kivimäki M, Gale CR, & Batty GD (2020). Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: A community-based cohort study of 387,109 adults in UK. Brain, behavior, and immunity, 87, 184–187. 10.1016/j.bbi.2020.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrick N, Cohen S, & Rodriguez MS (2002). Being popular can be healthy or unhealthy: Stress, social network diversity, and incidence of upper respiratory infection. Health Psychology, 21(3), 294–298. 10.1037/0278-6133.21.3.294 [DOI] [PubMed] [Google Scholar]
- Hannibal KE, & Bishop MD (2014). Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Physical therapy, 94(12), 1816–1825. 10.2522/ptj.20130597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ME, Waite LJ, Hawkley LC, & Cacioppo JT (2004). A Short Scale for Measuring Loneliness in Large Surveys: Results From Two Population-Based Studies. Res Aging, 26(6), 655–672. 10.1177/0164027504268574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicki Deverts D, Cohen S, & Doyle WJ (2017). Dispositional Affect Moderates the Stress-Buffering Effect of Social Support on Risk for Developing the Common Cold. J Pers, 85(5), 675–686. 10.1111/jopy.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlberg J, Chong DS, & Lai WY (2004). Do men have a higher case fatality rate of severe acute respiratory syndrome than women do?. American journal of epidemiology, 159(3), 229–231. 10.1093/aje/kwh056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Min C, Oh DJ, Lim J-S, & Choi HG (2019). Bidirectional association between asthma and migraines in adults: Two longitudinal follow-up studies. Scientific reports, 9(1), 18343. Retrieved 2019/12//, from 10.1038/s41598-019-54972-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JY, Farr SL, Dietz PM, & Robbins CL (2012). Depression and Treatment Among U.S. Pregnant and Nonpregnant Women of Reproductive Age, 2005–2009. Journal of Women’s Health, 21(8), 830–836. 10.1089/jwh.2011.3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2003). The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care, 41(11), 1284–1292. 10.1097/01.MLR.0000093487.78664.3C [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB, & Lowe B (2009). An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics, 50(6), 613–621. 10.1176/appi.psy.50.6.613 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB, Monahan PO, & Lowe B (2007). Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med, 146(5), 317–325. 10.7326/0003-4819-146-5-200703060-00004 [DOI] [PubMed] [Google Scholar]
- Lee SW, Yang JM, Moon SY, Yoo IK, Ha EK, Kim SY, … Yon DK (2020). Association between mental illness and COVID-19 susceptibility and clinical outcomes in South Korea: a nationwide cohort study. The Lancet Psychiatry, 7(12), 1025–1031. 10.1016/S2215-0366(20)30421-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto K, Pedersen NL, Almqvist C, Lu Y, & Brew BK (2019). Asthma and affective traits in adults: a genetically informative study. The European respiratory journal, 53(5). 10.1183/13993003.02142-2018 [DOI] [PubMed] [Google Scholar]
- Leonard BE (2010). The concept of depression as a dysfunction of the immune system. Current immunology reviews, 6(3), 205–212. 10.2174/157339510791823835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoy AS, Murdock KW, Jaremka LM, Loya A, & Fagundes CP (2017). Loneliness predicts self-reported cold symptoms after a viral challenge. Health psychology : official journal of the Division of Health Psychology, American Psychological Association, 36(5), 512–520. 10.1037/hea0000467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li F, Fortunati F, & Krystal JH (2020). Association of a Prior Psychiatric Diagnosis With Mortality Among Hospitalized Patients With Coronavirus Disease 2019 (COVID-19) Infection. JAMA Network Open, 3(9), e2023282–e2023282. 10.1001/jamanetworkopen.2020.23282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe B, Wahl I, Rose M, Spitzer C, Glaesmer H, Wingenfeld K, … Brahler E (2010). A 4-item measure of depression and anxiety: validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. J Affect Disord, 122(1-2), 86–95. 10.1016/j.jad.2009.06.019 [DOI] [PubMed] [Google Scholar]
- Lu Z, Chen L, Xu S, Bao Q, Ma Y, Guo L, … Ruan L (2018). Allergic disorders and risk of depression: A systematic review and meta-analysis of 51 large-scale studies. Annals of Allergy, Asthma & Immunology, 120(3), 310–317.e312. 10.1016/j.anai.2017.12.011 [DOI] [PubMed] [Google Scholar]
- Macleod J, Davey Smith G, Heslop P, Metcalfe C, Carroll D, & Hart C (2002). Psychological stress and cardiovascular disease: empirical demonstration of bias in a prospective observational study of Scottish men. BMJ (Clinical research ed.), 324(7348), 1247–1251. 10.1136/bmj.324.7348.1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AM, Crane PA, & Kim Y (2008). Perceived stress in survivors of suicide: psychometric properties of the Perceived Stress Scale. Res Nurs Health, 31(6), 576–585. 10.1002/nur.20284 [DOI] [PubMed] [Google Scholar]
- Nemani K, Li C, Olfson M, Blessing EM, Razavian N, Chen J, … Goff DC (2021). Association of Psychiatric Disorders With Mortality Among Patients With COVID-19. JAMA Psychiatry, 78(4), 380–386. 10.1001/jamapsychiatry.2020.4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando V, Rea F, Savaré L, Guarino I, Mucherino S, Perrella A, … Corrao G (2021). Development and validation of a clinical risk score to predict the risk of SARS-CoV-2 infection from administrative data: A population-based cohort study from Italy. PloS one, 16(1), e0237202–e0237202. 10.1371/journal.pone.0237202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- People with Certain Medical Conditions (2021). Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html [Google Scholar]
- Perruche F, Elie C, Ussel M, Ray P, Thys F, Bleichner G, … Claessens Y-E (2011). Anxiety and depression are unrecognised in emergency patients admitted to the observation care unit. Emergency Medicine Journal, 28(8), 662. 10.1136/emj.2009.089961 [DOI] [PubMed] [Google Scholar]
- Preti E, Di Mattei V, Perego G, Ferrari F, Mazzetti M, Taranto P, … Calati R (2020). The Psychological Impact of Epidemic and Pandemic Outbreaks on Healthcare Workers: Rapid Review of the Evidence. Current psychiatry reports, 22(8), 43–43. 10.1007/s11920-020-01166-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich-Edwards JW, Ding M, Rocheleau CM, Boiano JM, Kang JH, Becene I, … Lawson CC (2021). American Frontline Healthcare Personnel’s Access to and Use of Personal Protective Equipment Early in the COVID-19 Pandemic. Journal of Occupational and Environmental Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N, Kuroda R, Tsuno K, & Kawakami N (2020). The deterioration of mental health among healthcare workers during the COVID-19 outbreak: A population-based cohort study of workers in Japan. Scandinavian journal of work, environment & health, 46(6), 639–644. 10.5271/sjweh.3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Kroeze EJBV, Fouchier RAM, & Kuiken T (2014). Pathogenesis of influenza-induced acute respiratory distress syndrome. The Lancet Infectious Diseases, 14(1), 57–69. 10.1016/S1473-3099(13)70286-X [DOI] [PubMed] [Google Scholar]
- Spiranovic C, Matthews A, Scanlan J, & Kirkby KC (2016). Increasing knowledge of mental illness through secondary research of electronic health records: opportunities and challenges. Advances in Mental Health, 14(1), 14–25. 10.1080/18387357.2015.1063635 [DOI] [Google Scholar]
- Steptoe A, Shankar A, Demakakos P, & Wardle J (2013). Social isolation, loneliness, and all-cause mortality in older men and women [ 10.1073/pnas.1219686110]. Proc Natl Acad Sci U S A, 110(15), 5797–5801. 10.1073/pnas.1219686110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M, Luciano S, Geddes JR, & Harrison PJ (2021). Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. The Lancet Psychiatry, 8(2), 130–140. 10.1016/S2215-0366(20)30462-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GG, & Lena E (2010). Chronic stress and the hpa axis: clinical assessment and therapeutic considerations. A review of natural and nutraceutical therapies for clinical practice, 9. [Google Scholar]
- Tian R, Hou G, Li D, & Yuan T-F (2014). A possible change process of inflammatory cytokines in the prolonged chronic stress and its ultimate implications for health. The Scientific World Journal, 2014, 780616–780616. 10.1155/2014/780616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend L, Walkup JT, Crystal S, & Olfson M (2012). A systematic review of validated methods for identifying depression using administrative data. Pharmacoepidemiology and Drug Safety, 21(S1), 163–173. 10.1002/pds.2310 [DOI] [PubMed] [Google Scholar]
- Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, & Willett WC (1995). The validity of recalled weight among younger women. International journal of obesity and related metabolic disorders : Journal of the International Association for the Study of Obesity, 19(8), 570–572. [PubMed] [Google Scholar]
- Vallejo MA, Vallejo-Slocker L, Fernandez-Abascal EG, & Mananes G (2018). Determining Factors for Stress Perception Assessed with the Perceived Stress Scale (PSS-4) in Spanish and Other European Samples. Front Psychol, 9, 37. 10.3389/fpsyg.2018.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väänänen A, Murray M, Koskinen A, Vahtera J, Kouvonen A, & Kivimäki M (2009). Engagement in cultural activities and cause-specific mortality: prospective cohort study. Preventive medicine, 49(2-3), 142–147. 10.1016/j.ypmed.2009.06.026 [DOI] [PubMed] [Google Scholar]
- Wang Q, Xu R, & Volkow ND (2021). Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry, 20(1), 124–130. 10.1002/wps.20806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warttig SL, Forshaw MJ, South J, & White AK (2013). New, normative, English-sample data for the Short Form Perceived Stress Scale (PSS-4). J Health Psychol, 18(12), 1617–1628. 10.1177/1359105313508346 [DOI] [PubMed] [Google Scholar]
- Yang H, Chen W, Hu Y, Chen Y, Zeng Y, Sun Y, … Song H (2020). Pre-pandemic psychiatric disorders and risk of COVID-19: a UK Biobank cohort analysis. The Lancet Healthy Longevity, 1(2), e69–e79. 10.1016/S2666-7568(20)30013-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Gui X, & Xiong Y (2020). Comparison of Clinical Characteristics of Patients with Asymptomatic vs Symptomatic Coronavirus Disease 2019 in Wuhan, China. JAMA Network Open, 3(5), e2010182–e2010182. 10.1001/jamanetworkopen.2020.10182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yende S, Tuomanen EI, Wunderink R, Kanaya A, Newman AB, Harris T, … Kritchevsky SB (2005). Preinfection systemic inflammatory markers and risk of hospitalization due to pneumonia. American journal of respiratory and critical care medicine, 172(11), 1440–1446. 10.1164/rccm.200506-888OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- YouGov. (2020). Yahoo! News Coronavirus - April 8, 2020. Y. News. https://docs.cdn.yougov.com/1ayt0i64g6/20200408_yahoo_coronavirus.pdf [Google Scholar]
- Zhao CL, Huang JW, Zhang L, Zhang QR, Li QM, & Zhou M (2018). Respiratory virus infections and inflammatory cytokines in hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chinese journal of tuberculosis and respiratory diseases, 41(12), 942–948. 10.3760/cma.j.issn.1001-0939.2018.12.009 [DOI] [PubMed] [Google Scholar]
- Zheng L, Wang X, Zhou C, Liu Q, Li S, Sun Q, … Wang W (2020). Analysis of the Infection Status of Healthcare Workers in Wuhan During the COVID-19 Outbreak: A Cross-sectional Study. Clinical Infectious Diseases, 71(16), 2109–2113. 10.1093/cid/ciaa588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Zhu X, Liu C-L, Shi H, Shen S, Yang Y, … Liang L (2019). Shared genetics of asthma and mental health disorders: a large-scale genome-wide cross-trait analysis. The European respiratory journal, 54(6). 10.1183/13993003.01507-2019 [DOI] [PubMed] [Google Scholar]
- Zou G (2004). A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol, 159(7), 702–706. 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


