Introduction
The full potential of therapeutics for glioblastoma (GBM), the most aggressive and deadliest brain malignancy in adults, has yet to be reached. Malignant brain tumors have been well-characterized as immunologically cold, as defined by massive infiltration of immunosuppressive tumor-associated myeloid cells (TAMCs) unrivaled by any other types of solid tumors.1 TAMCs are a heterogeneous population of myeloid-derived immunosuppressive cells, including myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), and neutrophils. As a hallmark of GBM, TAMCs represent the predominant immune cell population, comprising up to 80% of the leukocytes in the brain tumor microenvironment.2,3 This unique TAMC-rich immune microenvironment is responsible for GBM resistance to emerging immunotherapies and existing standard of care treatments, such as chemotherapy and radiotherapy. The central role of TAMCs in brain tumor progression and therapeutic resistance has led to identifying the myeloid compartment as a promising therapeutic target for GBM.
This mini-review highlights recent progress in implementing nanotechnology in advancing TAMC-targeted therapies for GBM. One significant advantage of nanotechnology-mediated therapy is its versatility in encapsulating therapeutics with diverse physiochemical and/or pharmacological properties to overcome the challenges encountered through in vivo applications. These issues could be further solved by utilizing targeting mechanisms to enable precision therapeutic delivery to the organs/cells of interest. In addition, dual-/multi-functional nanoparticles that exert biological/pharmacological activities alone have also been discovered. Because TAMCs massively infiltrate into brain tumors and play a key role in causing immunosuppression-related therapy resistance of GBM, this review will be focused on two nanotherapeutic approaches that have been developed for harnessing TAMCs for GBM therapy, i.e., (i) immunomodulation of TAMCs to activate antitumor immune responses, and (ii) hijacking TAMCs as cellular carriers for GBM-targeted drug delivery.
Nanotherapeutic Targeting of TAMCs for GBM Immunomodulation
Recent discoveries revealing the immunological composition of the tumor microenvironment and the crosstalk between tumor and tumor-associated immune cells have identified the predominance of the myeloid compartment in GBM and its central role in affecting GBM progression and therapy outcome. Within the GBM microenvironment, TAMCs are programmed by tumor cells to exhibit a tumor-supporting, immunosuppressive (typically classified as M2 or M2-like) phenotype, which potently suppresses the antitumor activity of effector T cells, induces regulatory T and B cells, and supports tumor progression, migration, and therapy resistance.4 Therefore, therapeutic targeting of TAMCs to blunt their tumor-supportive activities and/or reinforce their intrinsic antitumor, proinflammatory immune activities is a promising immunotherapy branch that could work alone or synergistically improve existing treatments for GBM.
Targeting strategies for therapeutic delivery to TAMCs
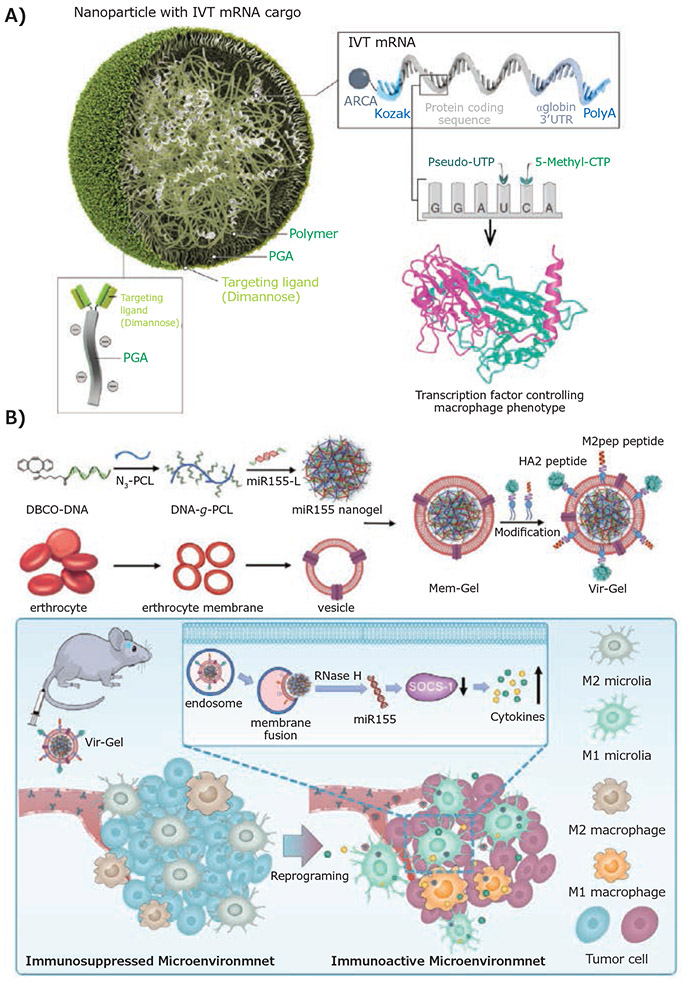
Precision targeting of TAMCs is challenging due to the high heterogeneity of the GBM microenvironment as well as the phenotypic plasticity of TAMCs. To overcome these obstacles, novel targeting strategies have been studied through the rational design of multifunctional nanomaterials with surface-decoration of targeting ligands that exhibit high binding affinity with overexpressed receptors on TAMCs. For example, to enable a robust genetic modulation of M2 TAMs, Stephan et al. chemically modified their poly (β-amino ester) (PbAE)-based nanoparticles with an α-D-mannopyranosyl-(1→2)-α-D-mannopyranose (Di-mannose) ligand through a polyglutamic acid (PGA) linker (Figure 1A)5 Di-mannose is a targeting ligand with high binding affinity to macrophage mannose receptor 1 (mrc1 or CD206) that are overexpressed on M2 TAMs. In addition to its role as a TAM-targeting ligand, Di-mannose also stabilized the nanoparticle by shielding the excess positive charge of the PbAE polymers post-mRNA encapsulation, which significantly reduced the nonspecific binding of nanoparticles to non-targeted cells through charge-charge interactions. As a result, substantial transfection of the mRNA therapeutics in TAMs was achieved using these TAM-targeting nanoparticles. Another cutting-edge TAM-targeting strategy by the Zhang group involves a dual-peptide functionalization of nanogels to target the miRNA-loading nanogel towards M2 TAMs and microglia in GBM by mimicking the process of virus infection (Figure 1B).6 Two functional peptides, M2pep and HA2, were covalently conjugated to the terminal maleimide group of 1,2-distearoylsn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (DSPE-PEG-Mal), and then inserted into the membrane of the nanogel formed by DNA-grafted polycaprolactone (DNA-g-PCL). These peptides played distinct roles in regulating the cellular uptake of the nanogel and drug release profiles. M2pep peptide was initially discovered through phage display7 and utilized as a targeting ligand to enhance the nanogel uptake by M2 immunosuppressive microglia and macrophage. In contrast, the function of influenza virus-derived HA2 peptide was shown to trigger drug release through an increased fusion of nanogel with the endosomal membrane.
Figure 1.
Ligand-receptor interaction-mediated nanoparticle targeting of tumor-associated macrophages. A) Schematic representation of M2 macrophage-targeting nanoparticles through binding to mannose receptor. Reproduced from reference 5, copyright 2019 Springer Nature. Open access article distributed under the terms of the Creative Commons CC BY license. B) Design of a dual-peptide modified, erythrocyte membrane-coated virus-mimicking nanogel for miRNA delivery. Reproduced with permission from reference 6, copyright 2021 John Wiley and Sons.
Besides targeting M2 TAMs, tremendous research efforts have also been put into creating innovative approaches that simultaneously enable efficacious therapeutic delivery to a heterogeneous population of immunosuppressive myeloid cells. Research findings from the Lesniak group have indicated that programmed death-ligand 1 (PD-L1) is overexpressed in different subsets of TAMCs in GBM models over other immune cell populations and tumor cells, and accordingly demonstrate the use of anti-PD-L1 therapeutic antibodies for functionalizing and targeting lipid nanoparticles to TAMCs.8 Despite being a standard of care treatment for newly diagnosed GBM, radiotherapy upregulated PD-L1 expression in TAMCs, thus potentiating the targeting and delivery efficiency of these nanoparticles. This is among the first studies to demonstrate the utility of PD-L1 as a targeting moiety for the delivery of therapy rather than solely as an immune checkpoint inhibitor. Similarly, Huang et al. have explored the possibility of using an innovative “three-birds-one-stone” multi-targeting strategy.9 An α7 nicotinic acetylcholine receptors (nAChRs)-binding peptide, DCDX (CGREIRTGRAERWSEKF), was utilized to functionalize a liposomal nanoparticle formulation for simultaneous drug delivery to multiple cell types that overexpress the same receptor nAChRs, including TAM, glioma, and glioma vessel endothelium. Their imaging results indicate a much-enhanced BBB-penetrating efficiency and brain tumor-specific accumulation of the peptide-functionalized liposomes.
Nanoparticle-enabled Immunomodulation of TAMCs
TAMCs plasticity enables the diverse functionality of these innate immune cells and therefore their multifaceted roles in the GBM microenvironment. TAMCs could either exhibit an immunosuppressive or anti-inflammatory (typically classified as M2-like or alternative) phenotype or an immunostimulatory or proinflammatory (typically classified as M1-like or classical) phenotype. Consequently, TAMC-targeted therapy in cancer treatment has emphasized how to blunt the immunosuppressive functions of TAMCs and/or how to induce their antitumor activities through triggered repolarization. Including but not limited to the abovementioned TAMC-targeting efficiency, nanomedicine approaches have exhibited undoubted advantages in immunomodulation of TAMC functionality over using free therapeutics.
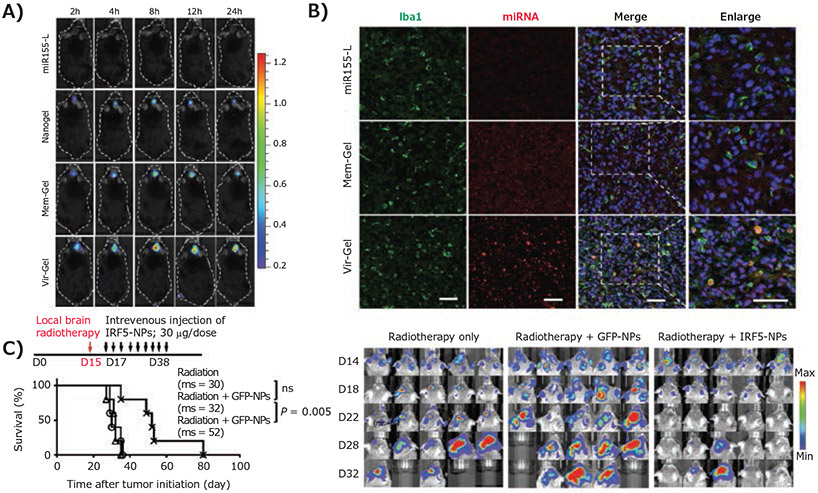
One significant advantage of nanotechnology-mediated drug delivery is that nanoparticles are a versatile platform capable of encapsulating and delivering different types of therapeutics to overcome the challenges that therapeutic molecules encounter upon transition to in vivo applications, including insufficient solubility, stability, in vivo circulation, tumor-specific accumulation, cell uptake, and/or therapeutic efficacy.10 This is particularly true for nucleic acid therapeutics, whose clinical transition has been greatly limited by their unfavorable physiochemical properties.11 To address such issues, Zhang et al. have demonstrated that their virus-mimicking nanogel carried miR155, a micro-RNA therapeutic that inhibits the expression of anti-inflammatory factor suppressor of cytokine signaling 1 (SOCS-1), whereby inducing proinflammatory cytokine production in TAMCs.6 The nanogel was formulated by a DNA-grafted polycaprolactone (DNA-g-PCL) polymer, capable of encapsulating miRNA molecules through crosslinking, and coated by an erythrocyte membrane to provide additional protection to the loaded miRNA therapeutics and to increase in vivo brain tumor accumulation (Figure 1B). Accordingly, the nanogel significantly extended the miRNA lifetime in vivo and, combined with the peptide modification as abovementioned, improved its myeloid cell-targeting and uptake efficiency (Figure 2A), which led to effective repolarization of the M2-like immunosuppressive TAMs and microglia to an M1 phenotype. Another example of nanoparticle-enabled TAM-targeted delivery of nucleic acid therapeutics was reported by the Stephan group.5 mRNA therapeutics encoding interferon regulatory factor 5 (IRF5) together with IKKβ, an IRF5-phosphorylating/activating kinase, were generated for repolarization of M2 macrophages. The mRNAs were encapsulated through charge-charge interactions with nanoparticles formed by cationic PbAE polymers. The IRF5/IKKβ mRNA nanoparticle treatment impaired immunosuppressive activity of M2 TAMs. Brain tumor regression was observed in tumor-bearing animals when mRNA nanoparticle treatment was combined with radiation (Figure 2B), suggesting the potential use of TAM-targeted therapy in combination therapy for GBM. Extending this line of thought, nanoparticles have also been widely used in combination therapy based on their capability to simultaneously co-deliver multiple therapeutics with distinct physiochemical and pharmacokinetic properties to ensure maximized synergistic effects. For example, Huang et al. developed a brain-targeted liposomal formulation for the co-delivery of honokiol combined with disulfiram/copper to synergistically target the mammalian target of rapamycin (mTOR) pathway in the GBM microenvironment.9 Using blood-brain barrier (BBB)-penetrating liposomes in GBM models reshaped the mTOR-mediated glucose metabolism and boosted the antitumor immune system by activating antitumor M1 macrophage and dendritic cells and priming effector T and NK cells.
Figure 2.
Macrophage-targeting nanoparticles stimulate anti-GBM immunity and prolong survival of GBM-bearing mice. A) In vivo fluorescence imaging and confocal laser scanning microscopy imaging indicates enhanced brain distribution and macrophage/microglia uptake of miRNA in the brain through virus-mimic nanoparticle-mediated delivery post-intravenous injection. Reproduced with permission from reference 6, copyright 2021 John Wiley and Sons. B) Kaplan-Meier animal survival curves and sequential bioluminescence imaging of brain tumors indicate the therapy efficacy of IRF5-inducing nanoparticles when combined with radiotherapy. Reproduced from reference 5, copyright 2019 Springer Nature. Open access article distributed under the terms of the Creative Commons CC BY license.
Other than an effective drug delivery carrier, dual-functional therapeutic nanoparticles that exert biological activities alone have also been developed as synergistic combinations to enhance the efficacy of their cargo.12 The Lesniak group has recently engineered a TAMC-targeting lipid nanoparticle by functionalizing nanoparticles with an anti-PD-L1 antibody.8 The use of this lipid nanoparticle to deliver a CDK (cyclin-dependent kinase) inhibitor effectively inhibited the interferon-gamma (IFNγ)-stimulated de novo production of PD-L1 in TAMCs, which impaired the immunosuppressive activity of TAMCs in the GBM microenvironment. In addition to a drug carrier to enable the TAMC-targeted delivery of CDK inhibitor, the nanoparticles alone induced lysosomal degradation of existing PD-L1 in TAMCs, which served as an additional mechanism to synergize with the CDK inhibitor for the PD-L1 blockade. Another study by the Lesniak group demonstrates the repolarization effects of magnetic zinc-doped iron oxide nanoparticles on MDSCs, which sensitized GBM-bearing mice to radiation therapy.13. These solid nanoparticles coated with a cationic polymer, polyethylenimine (PEI), were efficiently taken up by MDSCs in GBM, which triggered a proinflammatory-like polarization of immunosuppressive MDSCs, as evidenced by increased expression of M1-associated genes, such as inducible nitric oxide synthase (iNOS) and tumor necrosis factor α (TNFα), and decreased M2-associated genes Arginase (Arg1) and IL10.
Harnessing GBM-infiltrating TAMCs as Cellular Carriers for Nanoparticle Delivery
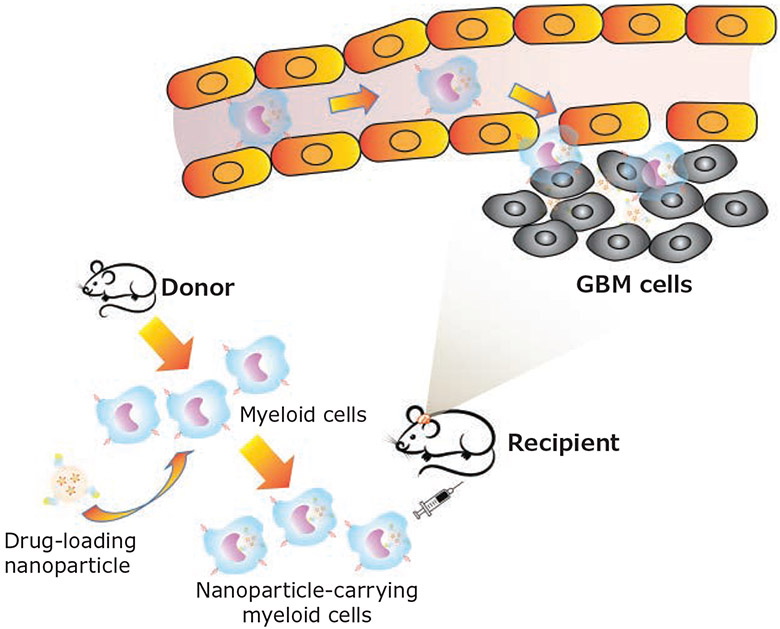
Inefficient delivery of therapeutics to brain tumors remains a significant hurdle to efficacious GBM treatments. The substantial physiological barriers and pathological characteristics of GBM significantly limit sufficient drug distribution to the tumor bed.14 To overcome such obstacles, the use of endogenous cells as cellular carriers has emerged as a promising strategy to enable efficient brain tumor-targeted delivery of therapeutics. As a hallmark of GBM, TAMCs are primarily recruited and have an intrinsic capability to overcome the biological barriers and migrate deep into the tumor mass. Thus, harnessing GBM-infiltrating myeloid cells as cellular carriers, or cellular “Trojan Horses”, to enhance GBM-targeted nanoparticle delivery has captured tremendous research interest (Figure 3).
Figure 3.
Schematic illustration of harnessing GBM-infiltrating myeloid cells as cellular “Trojan horse” for brain-targeted nanoparticle delivery. Drug-encapsulated nanoparticles are engulfed by myeloid cells isolated from donor mice and injected into GBM-bearing recipient mice intravenously. Upon reaching brain tumors through myeloid migration, nanoparticle/drug are released from cellular carriers to tumor cells/tumor microenvironment for antitumor effects.
Macrophage-mediated Nanoparticle Delivery
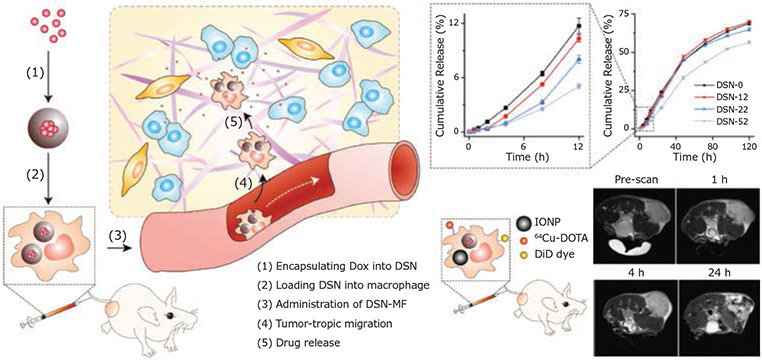
Macrophages are one of the most widely studied cellular carriers for loading large amounts of nanoparticle/drug complexes. In addition to the tumor-homing efficiency, the inherent phagocytic activity of macrophages also makes such cells an ideal candidate as cellular “Trojan Horses” for nanoparticle delivery. However, rational design of nanoparticle formulations that ensure the stability of nanoparticles during migration and avoid premature drug release and unwanted toxicity to carrier cells is highly desired. To address this issue, Xie et al. constructed a nanocapsule with a core nanocomplex formed by silica and doxorubicin (Dox), a first-line chemotherapeutic reagent, through electrostatic interaction. The nanocapsule core was further protected by a solid silica sheath to reduce the premature release of Dox.15 The thicknesses of the silica coating were optimized for extended drug release to attenuate the potential Dox toxicity to macrophages after the engulfment of the nanocapsule. A high drug loading capacity of 16.6 pg Dox/cell and two-phase drug release kinetics were achieved without affecting cell function. After intravenous injection, macrophages effectively maintained the intracellular nanocapsule and their tumor-homing ability (Figure 4), which largely induced tumor cell apoptosis and contributed to suppressed tumor growth in vivo.
Figure 4.
Schematic representation of harnessing macrophages as cellular carrier for GBM-targeted delivery. The silica matrix and coating of nanoparticle led to a controlled two-phase drug release of doxorubicin. Axial T2 MR images indicate a tumor-targeting capability in vivo. Reproduced with permission from reference 15, copyright 2018 John Wiley and Sons.
To take full advantage of macrophages as carriers for nanoparticle delivery, some research groups have paid attention to the impact of macrophage plasticity on macrophage-mediated delivery efficiency. Wang et al. have reported the use of M1-polarized macrophages to deliver Dox-loaded poly(lactide-co-glycolide) (PLGA) nanoparticles.16 They indicated that M1 macrophages exhibited enhanced phagocytic capability and thus were more efficient in engulfing large amounts of nanoparticles. Moreover, the antitumor activity of M1 macrophages may add more value to the therapy with the potential to synergistically inhibit tumor growth. The results demonstrated that the PLGA nanoparticles mainly resided in the lysosome of bone marrow-derived M1 macrophages. This system showed good tumor targeting capability when injected intravenously in a U87 nude mice model.
It is worth noting that, instead of using live cells, some efforts have been put into improving nanoparticle delivery by decorating nanoparticles with the ligands originally expressed on the macrophage cell membrane to enhance tumor cell binding/uptake. For instance, when delivered by oligopeptide hydrogel together with C-X-C motif chemokine ligand 10 (CXCL10),17 α4β1 integrin, a ligand that directs the migration of glioma-associated macrophages (GAM) towards brain tumor cells, was used as camouflage to improve GBM cell uptake of a tumor-homing immune nanoregulator (THINR). This nanoregulator was synthesized by co-loading of mitoxantrone (MIT) and siRNA targeting indoleamine 2,3-dioxygenase 1 (IDO1) into a Zinc 2-methylimidazole (ZIF-8)-based nano-metal-organic framework, followed by a coating with GAM membrane. The tumor-homing activity of the GAM membrane facilitated the THINR targeting of the remaining infiltrating tumor cells post-surgical resection. Moreover, activated T cells were recruited to the surgical site via CXCL10-CXCR3 (CXC motif chemokine receptor 3) axis to induce higher levels of immunogenic cancerous cell death, resulting in tumor recurrence inhibition.
Neutrophil and Dendritic Cell-mediated Delivery
Apart from macrophages, neutrophils also show remarkable tumor tropism with the ability to penetrate inflamed brain tumors. Proinflammatory cytokines like interleukin-8 (IL8) and TNFα are known to direct neutrophil migration and are commonly upregulated in the postoperative brain. Nanoparticle-loaded neutrophils can thus be recruited to the inflammatory site and release the cargo through NETosis. This process entails bursting out net-like intracellular structures comprising DNA and protein when activated by cytokine signals in the surrounding environment.18 The Zhang group adopted such a method to construct paclitaxel (PTX)-encapsulated liposomes, formulated by soy phosphatidylcholine (SPC), cholesterol, and HG2C18. These were carried and delivered by neutrophils trafficked towards the inflammatory signals released from the surgical site of the brain.19 They demonstrated that the neutrophil-mediated nanoparticle delivery effectively penetrates across endothelial cells and brain parenchyma cells, energetically releasing PTX to the remaining invading GBM cells post-tumor resection. The capability of the method to overcome multiple biological barriers, respond to inflammatory factors, and stimulate drug release, enabled a precision GBM-specific chemotherapy through intravenous injection, and resulted in a dramatic inhibition of tumor recurrence post-surgery.
Furthermore, the fundamental role that dendritic cells (DCs) play in antitumor immunity makes these cells another ideal candidate for tumor-targeted nanoparticle delivery. The research findings from Chen et al. highlighted the dual role of DCs when they were used to deliver Dox-loaded nanodiamonds.20 The polyglycerol-coated nanodiamonds were decorated with a cyclic RGD peptide (tripeptides of L-arginine, glycine, and L-aspartic acid), a GBM-targeting ligand with high binding affinity to the overexpressed integrin αvβ3 receptor. The chemotherapeutic payload, Dox, loaded in the nanodiamonds, is known to induce tumor cell cytotoxicity and the emission of damage-associated molecular patterns (DAMPs). They demonstrated that DCs efficiently infiltrated the GBM and released Dox to tumor cells. Subsequently, the Dox-induced immunogenic GBM cell death activated the carrier DCs, activating lymphocytes downstream via stimulating tumor-derived antigen presentation.
Conclusion
The unmet clinical needs in treating patients with brain malignancy have highlighted the urgency of developing novel antitumor therapeutic modalities. Although patients with certain types of cancers have benefited from the emerging immunotherapy strategies, such progress has yet to be experienced by GBM patients. Recent research findings have revealed the vital role that TAMCs play in inducing GBM immunosuppression, progression, metastasis, and therapy resistance. However, targeted therapy for this predominant population of immune cells in GBM remains understudied to date. Compelling preclinical and clinical studies have identified therapeutic modulation of immunosuppressive TAMCs as a promising new branch to potentiate the anti-GBM immunity and thus improve the therapeutic effects of both immunotherapy and conventional standard treatments. Thanks to the well-documented functionality, versatility, and feasibility, nanotechnology has emerged as a powerful toolbox to enable and improve a wide range of innovative therapeutic strategies; from eliminating immunosuppressive TAMCs to reeducating these myeloid cells to be “good” immune cells or harnessing them as cellular “Trojan horses” for GBM-targeted delivery. Manufactured by rationally designed multifunctional materials, TAMC-targeted nanoparticles have demonstrated their ability to specifically target and effectively modulate TAMCs, either through the pharmacological activity of payload therapeutics or the biological functions exerted by nanoparticles themselves. Undoubtedly, nanotechnology will continuously and significantly propel cancer therapy advances and hold great hope as a promising treatment option for GBM patients.
PLGA Nanoparticles and Microspheres
| Name | Form | Average Diameter | Cat. No |
|---|---|---|---|
| PLGA microspheres | pellets | 1 μm | LG1000-50MG-A |
| powder | 2 μm | 805130-50MG | |
| pellets | 5 μm | LG5000-50MG-A | |
| pellets | 10 μm | LG10K-50MG-A | |
| pellets | 20 μm | LG20K-50MG-A | |
| powder | 25 μm | 805114-50MG | |
| pellets | 30 μm | LG30K-50MG-A | |
| pellets | 40 μm | LG40K-50MG-A | |
| powder | 50 μm | 805122-50MG | |
| PLGA nanoparticles | powder | 100 nm | 805092-50MG |
| powder | 500 nm | 805149-50MG |
Fluorescent PLGA Nanoparticles and Microspheres
| Name | Excitation/Emission | Average Diameter | Cat. No. |
|---|---|---|---|
| Near-IR Fluorescent PLGA microspheres | 780/820 nm | 1 μm | LGFN1000-50MG-A |
| 780/820 nm | 2 μm | LGFN2000-50MG-A | |
| 780/820 nm | 5 μm | LGFN5000-50MG-A | |
| 780/820 nm | 10 μm | LGFN10K-50MG-A | |
| 780/820 nm | 20 μm | LGFN20K-50MG-A | |
| 780/820 nm | 30 μm | LGFN30K-50MG-A | |
| 780/820 nm | 40 μm | LGFN40K-50MG-A | |
| Near-IR Fluorescent PLGA nanoparticles | 780/820 nm | 100 nm | LGFN100-50MG-A |
| 780/820 nm | 200 nm | LGFN200-50MG-A | |
| 780/820 nm | 500 nm | LGFN500-50MG-A | |
| Red Fluorescent PLGA microspheres | 647/670 nm | 1 μm | LGFR1000-50MG-A |
| 647/670 nm | 10 μm | LGFR10K-50MG-A | |
| 647/670 nm | 20 μm | LGFR20K-50MG-A | |
| 647/670 nm | 50 μm | LGFR50K-50MG-A | |
| Red Fluorescent PLGA nanoparticles | 647/670 nm | 100 nm | LGFR100-50MG-A |
| 647/670 nm | 200 nm | LGFR200-50MG-A | |
| 647/670 nm | 500 nm | LGFR500-50MG-A | |
| Orange Fluorescent PLGA microspheres | 530/582 nm | 1 μm | LGFO1000-50MG-A |
| 530/582 nm | 10 μm | LGFO10K-50MG-A | |
| 530/582 nm | 20 μm | LGFO20K-50MG-A | |
| 530/582 nm | 50 μm | LGFO50K-50MG-A | |
| Orange Fluorescent PLGA nanoparticles | 530/582 nm | 100 nm | LGFO100-50MG-A |
| 530/582 nm | 200 nm | LGFO200-50MG-A | |
| Green Fluorescent PLGA microspheres | 460/500 nm | 1 μm | LGFG1000-50MG-A |
| 460/500 nm | 2 μm | 805181-50MG | |
| 460/500 nm | 5 μm | LGFG10K-50MG-A | |
| 460/500 nm | 20 μm | LGFG20K-50MG-A | |
| 460/500 nm | 25 μm | 805173-50MG | |
| 460/500 nm | 30 μm | LGFG30K-50MG-A | |
| 460/500 nm | 40 μm | LGFG40K-50MG-A | |
| 460/500 nm | 50 μm | 805165-50MG | |
| Green Fluorescent PLGA nanoparticles | 460/500 nm | 100 nm | 805157-50MG |
| 460/500 nm | 200 nm | 805211-50MG | |
| 460/500 nm | 500 nm | 805300-50MG |
Acknowledgments
This work was supported by NIH/NCI grant (R01CA266487) to P.Z., NCI Outstanding Investigator Award (R35CA197725) and NIH grants (P50CA221747, R01NS115955) to M.S.L.
Biographies





References
- (1).Thorsson V; Gibbs DL; Brown BSD; Wolf D; Bortone DS; Ou Yang TH et al. Immunity 2018, 48 (4), 812–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Friebel E; Kapolou K; Unger S; Nunez NG; Utz S; Rushing EJ; et al. Cell 2020, 181 (7), 1626–1642. [DOI] [PubMed] [Google Scholar]
- (3).Klemm F; Maas RR; Bowman RL; Kornete M; Soukup K; Nassiri S et al. Cell 2020, 181 (7):1643–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Quail DF; Joyce JA Cancer Cell 2017, 31 (3),326–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Zhang F; Parayath NN; Ene CI; Stephan SB; Koehne AL; Coon ME; et al. Nat COmmun. 2019, 10 (1), 3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Gao XH; Li S; Ding F; Liu XL; Wu YJ; Li J; et al. Adv. Mater 2021, 33 (9), e2006116. [DOI] [PubMed] [Google Scholar]
- (7).Conde J; Bao C; Tan Y; Cui D, Edelman ER; Azevedo HS; et al. Adv. Funct. Mater 2015, 25 (27),4183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Zhang P; Miska J; Lee-Chang C; Rashidi A; Panek WK; An SJ; et al. P. Natl. Acad. Sci. U.S.A 2019, 116 (47), 23714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Zheng Z; Zhang J; Jiang J; He Y; Zhang W; Mo X; et al. J. Immunother. Cancer 2020, 8 (2), 6e000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Mitchell MJ; Billingsley MM; Haley RM; Wechsler ME; Peppas NA; Langer R Nat. Rev. Drug Discov 2021, 20 (2), 101–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Paunovska K; Loughrey D; Dahlman JE; Nat. Rev. Genet 2022, 23, 265–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Zhang P; Xu JN; Gao SE; Li S Dual-function nanocarriers with interfacial drug-interactive motifs for improved delivery of chemotherapeutic agents. Nanobiomaterials in Cancer Therapy: Applications of Nanobiomaterials. Elsevier, 2016; 367–394. [Google Scholar]
- (13).Wu C; Muroski ME; Miska J; Lee-Chang C; Shen Y; Rashidi A; et al. Nanomed. 2019, 16, 126–37. [DOI] [PubMed] [Google Scholar]
- (14).Deeken J; Loscher W; Clin. Cancer Res 2007, 13 (6):1663–74. [DOI] [PubMed] [Google Scholar]
- (15).Zhang W; Wang M; Tang W; Wen R; Zhou S; Lee C; et al. Adv. Mater 2018, 30 (50), e1805557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Pang L; Zhu Y; Qin J; Zhao W; Wang J Drug Deliv. 2018, 25 (1), 1922–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Zhang J; Chen C; Li AN; Jing WQ; Sun P; Huang XY, et al. Nat. Nanotechnol 2021, 16 (5), 538–548. [DOI] [PubMed] [Google Scholar]
- (18).Masucci MT; Minopoli M; Del Vecchio S; Carriero MV Front Immunol. 2020, 11, 1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Xue J; Zhao Z; Zhang L; Xue L; Shen S; Wen Y; et al. Nat Nanotechnol. 2017, 12 (7), 692–700. [DOI] [PubMed] [Google Scholar]
- (20).Li TF; Li K; Zhang Q; Wang C; Yue Y; Chen Z; et al. Biomaterials 2018, 181, 35–52. [DOI] [PubMed] [Google Scholar]