Abstract
The dental pulp is highly vascularized and innervated tissue that is uniquely designed, being highly biologically active, while being enclosed within the calcified structure of the tooth. Is well established that the dental pulp vasculature is a key requirement for the functional performance of the tooth. Therefore, controlled regeneration of the dental pulp vasculature is a challenge that must be met for future regenerative endeavors in endodontics. In this short review, recent progress and challenges on the use of microengineering methods and biomaterials scaffolds to fabricate of the dental pulp vascular microenvironment are addressed. The conditions required to control the growth and differentiation of vascular capillaries are discussed, together with the conditions required for the formation of mature and stable pericyte-supported microvascular networks in 3D hydrogels and fabricated microchannels. Recent biofabrication, such as 3D printing and micromolding are also discussed. Moreover, recent advances in the field of organs-on-a-chip are discussed regarding their applicability to dental research and endodontic regeneration. Collectively, this short review offers future directions in the field that are presented with the objective of pointing towards successful pathways for successful clinical and translational strategies in regenerative endodontics, with especial emphasis on the dental pulp vasculature.
Keywords: Vascularization, Microengineering, Regeneration, Pericyte, 3D printing, Tooth on-a-chip
1. Introduction
The dental pulp is a highly specialized tissue, which differs from other craniofacial structures for being extensively vascularized while being enclosed in a highly calcified chamber (1). Recent data showing the distribution of the dental pulp vasculature and innervation in three-dimensions throughout the entire length of a human tooth illustrates the complexity of the vascular supply of pulp tissue (Figure 1) (2). Therefore, it is unquestionable that pulp vitality relies on the presence and function of vascular capillaries that extend coronally from the root apex to provide oxygen, nutrients, and removal of waste products to all cells throughout the pulp (1). In light of these functional requirements, it is tangible that the development of strategies that allow for controlled regeneration of the dental pulp vasculature is a challenge that must be met before the objective of dental pulp tissue engineering can be reproducibly achieved and translated into clinical use (3).
Figure 1.
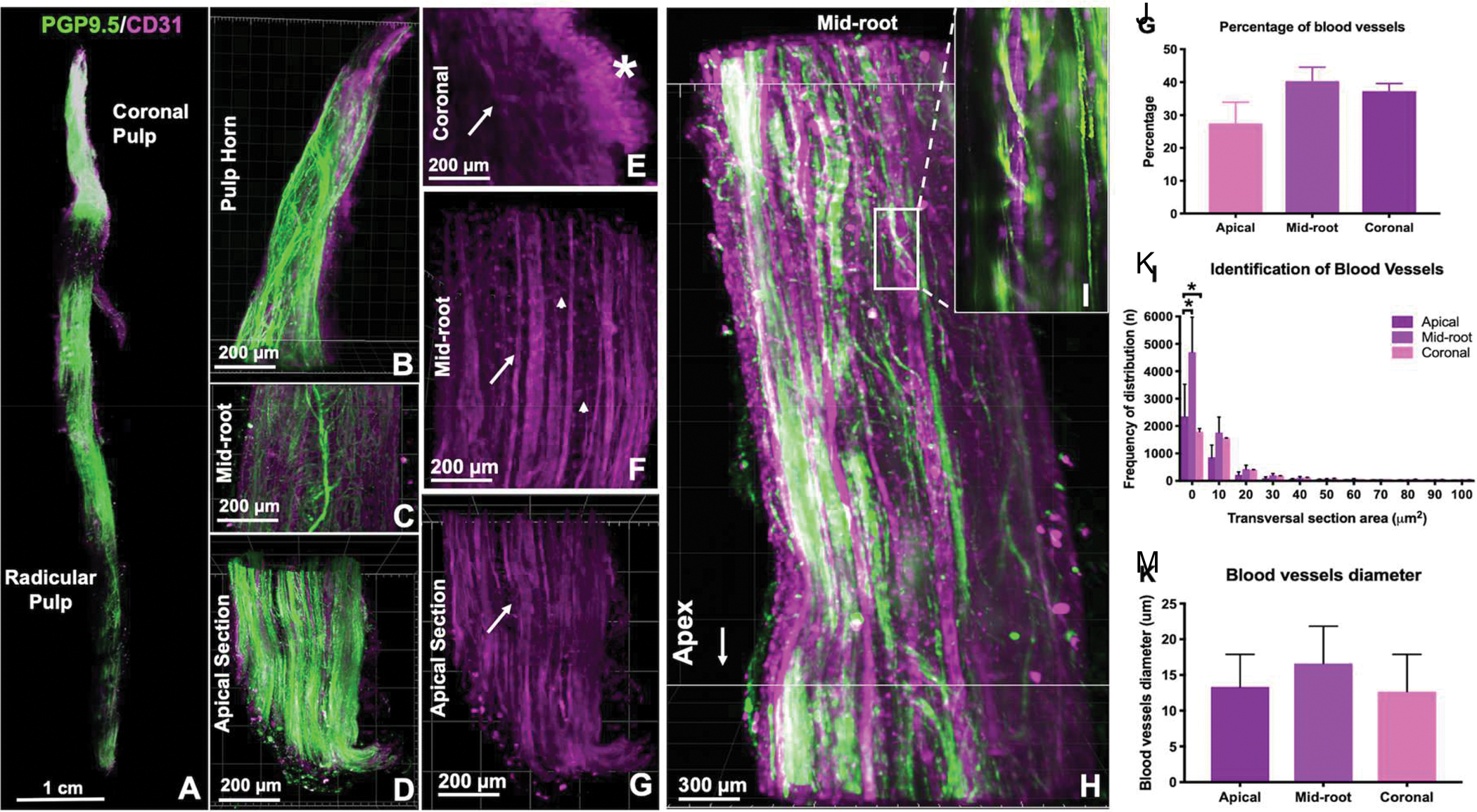
CLARITY microscopy of vasculature and innervation in the whole human tooth. A) PGP9.5 (innervation) and CD31 (vasculature) staining for cleared dental pulp, with higher magnification images of (B) pulp horn, (c) mid-root, and (d) apical sections of a canine. (E-G) CD31 staining for coronal, mid-root and apical sections, respectively, with (*) emphasis to the sub-odontoblastic vascular zone in the coronal region. (H) Interface of innervation and vasculature, where (I) nerve fibers appear to wrap around a vessel. (J) Quantification of percentage of blood vessels, (K) frequency of distribution in the three thirds of the root and (M) their diameters. Reproduced from (2).
As the field of regenerative endodontics establishes itself as an emerging reality in clinical dentistry, various forms of regenerative approaches have been proposed (4–6). The American Association of Endodontics (AAE) has defined the term ‘Regenerative Endodontics’ as “biologically-based procedures designed to physiologically replace damaged tooth structures, including dentin and root structures, as well as cells of the pulp-dentin complex” (7). The AAE definition considers a multitude of strategies that have been investigated, including root-canal revascularization (invoked apex bleeding), postnatal (adult) stem cell therapy, pulp implants, scaffold implants, three-dimensional cell printing, injectable scaffolds, and gene therapy (8). Invariably, many of these strategies benefit from the use of cell and biomaterials-based delivery approaches that can replace existing methods of obturation using inert materials, such as the conventional gutta-percha. Therefore, microengineering strategies that enable and stimulate the formation of a functional vascular supply in the dental pulp are highly desirable.
In this short review, recent progress and challenges on the emerging strategies to fabricate the dental pulp vascular microenvironment are addressed from a biomaterials and microengineering standpoint. Recent data on the conditions required to control the growth and differentiation of vascular capillaries are discussed, with a special emphasis on the conditions necessary for the formation of mature and functional pericyte-supported vessels in the tooth. Novel hydrogel biofabrication (i.e. 3D printing, microfluidics) methods are also discussed based upon their ability to replicate some of the complexity of the dental pulp vasculature. Moreover, recent advances from the field of organs-on-a-chip are discussed with regards to the ability of these methods to elucidated the influence of dentin and its embedded matrix molecules on the process of vasculature formation and regeneration in the tooth. Lastly, future directions in the field are presented with the objective of pointing towards successful pathways for clinical translation in regenerative endodontic approaches to re-establish the dental pulp vasculature with desirable functionality.
2. Vasculature formation
The vasculature is formed through a complex set of orchestrated biological events that involves the morphogenesis of endothelial cells into new hollow capillaries (vasculogenesis), the recruitment of perivascular mural cells (pericytes),(9, 10) and remodeling of the existing networks into a dense vascular plexus via angiogenic sprouting (11). These processes have been described in detail by our team and others previously, (12, 13) and it is recognized that both endothelial and perivascular mural cells are a pre-requisite for the formation of a functional (stable and non-permeable or “non-leaky”) vasculature in engineered tissues. Perhaps this is a critical discrepancy in much of the early methods of engineering of vascularized tissues, which focused primarily on the formation of endothelial capillaries without considering the complexity and need for perivascular mural support. Therefore, this review will pay particular attention to the conditions that are required to engineer microenvironments that are conducive to the adequate interaction between endothelial and perivascular mural cells, especially those from a mesenchymal origin.
In the native pulp, vasculogenic events are regulated by a cascade of paracrine and angiocrine signaling molecules (vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), etc.) that are derived from the heterotypic interactions of endothelial cells, cells from the perivascular niche (smooth muscle cells, pericytes, undifferentiated stem cells),(14) and the surrounding dentin (15). Several approaches have attempted to mimic the presentation of growth factors involved in the pulp vascularization as a means to facilitate the engineering of functional pulp tissue. In an important effort towards engineering vascularized pulp, Sakai et al (2010) reported that stem cells from exfoliated deciduous teeth (SHEDs) differentiated into endothelial cell networks in a poly-L-lactic acid scaffold in the presence of recombinant VEGF in-vitro, and in untreated scaffolds in-vivo; although the specificity of the presence of mural cells and the non-permeable character of the engineered vessels was not fully described (16). In another key publication, Koike et al (2004) reported that the co-culture of endothelial cells and mesenchymal progenitor cells (10T1/2) in 3D fibronectin/type I collagen gels promoted differentiation of the mesenchymal cells into pericyte-like cells without the need for added growth factors,(17) while supporting the formation of perfusable and non-permeable blood capillaries that remained stable for at least one year in-vivo. A series of subsequent reports has demonstrated the feasibility of engineering functional vascular networks in 3D hydrogels by co-culturing endothelial cells and stem cells from different sources. This has included bone marrow mesenchymal stem cells (BM-MSCs), (18–22) dental pulp (SHEDs and dental pulp stem cells (DPSCs)) (23–25) and mesenchymal precursor cell lines (10T1/2), (26, 27) all with significant success. Yet, to date, the specific conditions required to controllably regenerate the dental pulp vasculature, while ensuring the functionality of the engineered vasculature in full-length root canals of mature teeth have remained elusive. Hence the translation of regenerative endodontics into a viable alternative for conventional root canal treatment of mature adult teeth has remained distant.
3. Engineering biomaterials to promote vasculature regeneration
A critical factor to regenerate vascularized tissues is to determine the conditions that enable the process of vascular morphogenesis that is necessary to occur between endothelial and perivascular mural cells. A large body of literature has demonstrated that the physical, compositional and mechanical properties of various biomaterial scaffolds play a critical role in influencing stem cell fate decisions and overall cell response (28–30). The influence of these regulatory mechanisms in the context of dental pulp vasculature formation have been far less investigated. One of the key aspects that for many years may have slowed down studies on the interface of dental pulp regeneration and the influence of physical properties, perhaps, was the lack of regenerative dental materials that allowed for direct control over substrate physical properties and were compatible with dental clinical protocols. For instance, much of the work performed in dental pulp regeneration to date has been focused on the use of biomaterial scaffolds that are difficult to have properties such as stiffness, or degradation carefully controlled. These have included conventional degradable biomaterials, such as collagen, collagen-like peptides (i.e. puramatrix), PLGA and so on (31).
Recent work on the development of photocrosslinkable scaffold materials (32, 33), such as methacrylated gelatin (GelMA) (34), hyaluronic acid (MeHA) (35), and even collagen (PhotoCol) (32), has opened up new avenues that connect the fields of clinical dentistry with the possibility of fine tuning the mechanics of the stem cell microenvironment. One example of this recent focus was the development of methacrylated gelatin hydrogels that can be photopolymerized using a conventional light-emitting diode (LED) dental curing light in the root canal space (Figure 2) (36), while allowing for careful control of hydrogel physical properties, as well as delivery of viable dental pulp cells. Recent data shows that hydrogel prepolymers encapsulated with odontoblast precursor cell lines (OD21) can be loaded into the root canal, crosslinked for as little as 3 seconds, and maintain cell viability values of nearly 90% over time (36). Importantly, the chemistry of these methacrylated materials is similar to the that used in the far majority of restorative dental materials. As such, the procedures and armamentarium of dental photopolymerization not only are compatible with methods of dental pulp regeneration, but also allow for a much more systematic and controllable manipulation of microenvironment physical properties. This, in turn, enables more precise mechanistic studies on the influence of physical properties on the engineering of mature and stable vascular capillaries in the dental pulp.
Figure 2.
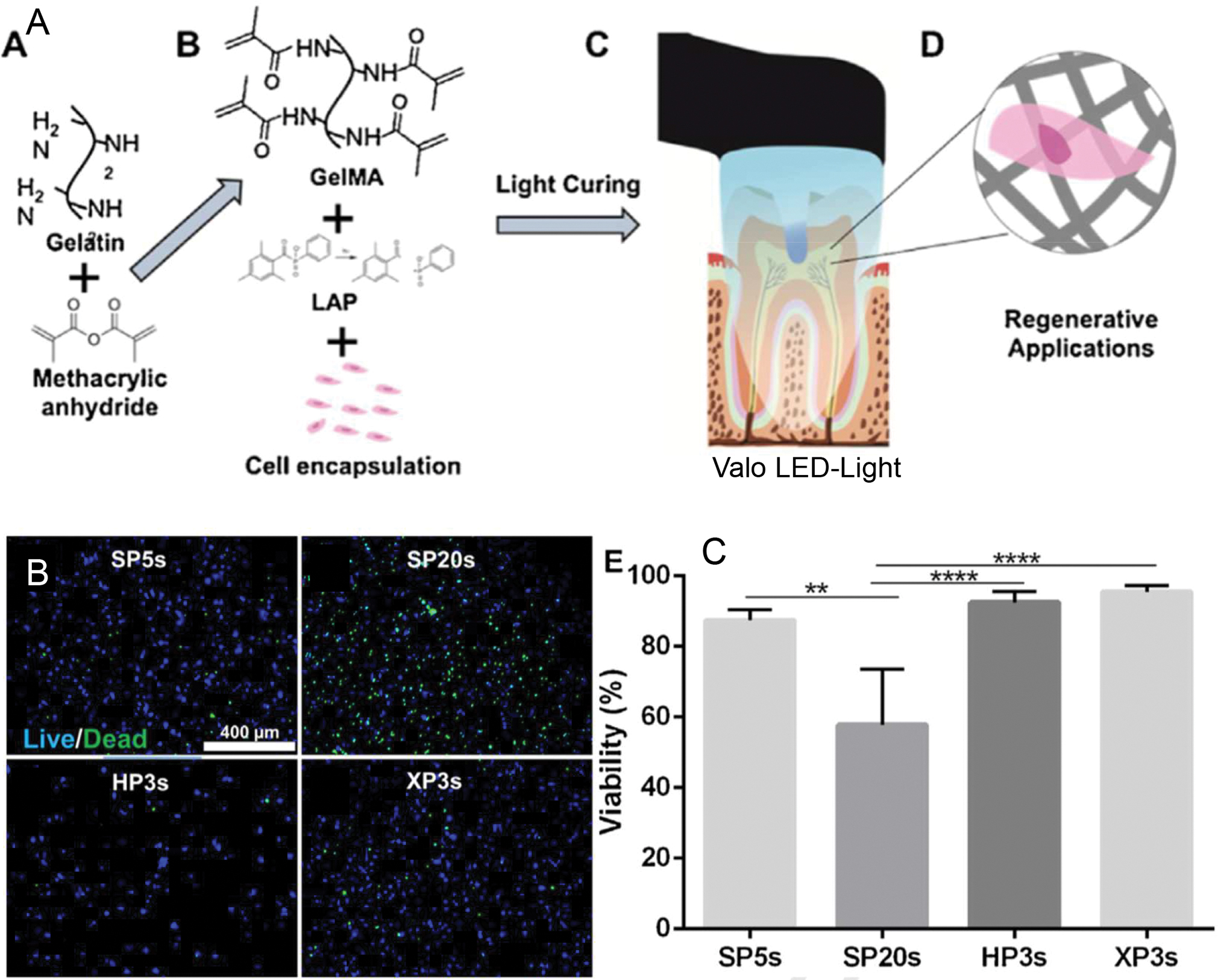
LED-Photocrosslinkable methacrylated gelatin (GelMA) for dental pulp regeneration. (A) Gelatin is chemically functionalized with methacrylate groups, combined with photoinitiators that are activated by visible light in the range of 405 nm of wavelength, such as conventional LED dental light curing units. (B) These hydrogels can be embedded with cells photopolymerized in as little as 3 seconds with (C) very high cell viability. (SP = Standard Power mode; HP = High Power mode; XP = Xtra Power mode). Reproduced from (36).
But why should one worry about these materials related questions when engineering the dental pulp vasculature? There is an increasing body of evidence that points toward to the fact that not only the composition of the extracellular matrix will interfere with the process of vascular morphogenesis (37), but also that matrix mechanical properties will influence virtually all interactions that are required to happen during the process of vasculogenesis and angiogenesis (13). Both human umbilical vein endothelial cells (HUVECs) and primary endothelial colony forming cells (ECFCs) seeded on substrates of increasing stiffness, have been reported to show a pattern of faster proliferation and a shift from the formation of vasculogenic ring-like structures, to evenly distributed endothelial monolayers fully covering the substrate (38) (Figure 3A,B). Counterintuitively, the exact same cells when encapsulated in 3D hydrogels show an opposite pattern, with lower stiffness enhancing vasculogenesis (Figure 3C,D). Accordingly, recent data shows that HUVECs three-dimensionally embedded in LED-photopolymerized GelMA hydrogels simply do not form vascular capillaries when the hydrogel stiffness reaches values above ~3 kPa (39). Obviously other conditions, such as the type of hydrogel, presence of perivascular cells, medium conditions, will play a key role. But it is evident that microenvironment mechanics significantly affects the process of engineered vasculogenesis, and that process is not exclusive to the dental pulp. Moreover, preliminary data from our group also appears to suggest that while substrates of high stiffness promote endothelial cell spreading and proliferation, it also prevents cells from migrating into the matrix to initiate endothelial sprouting.
Figure 3.
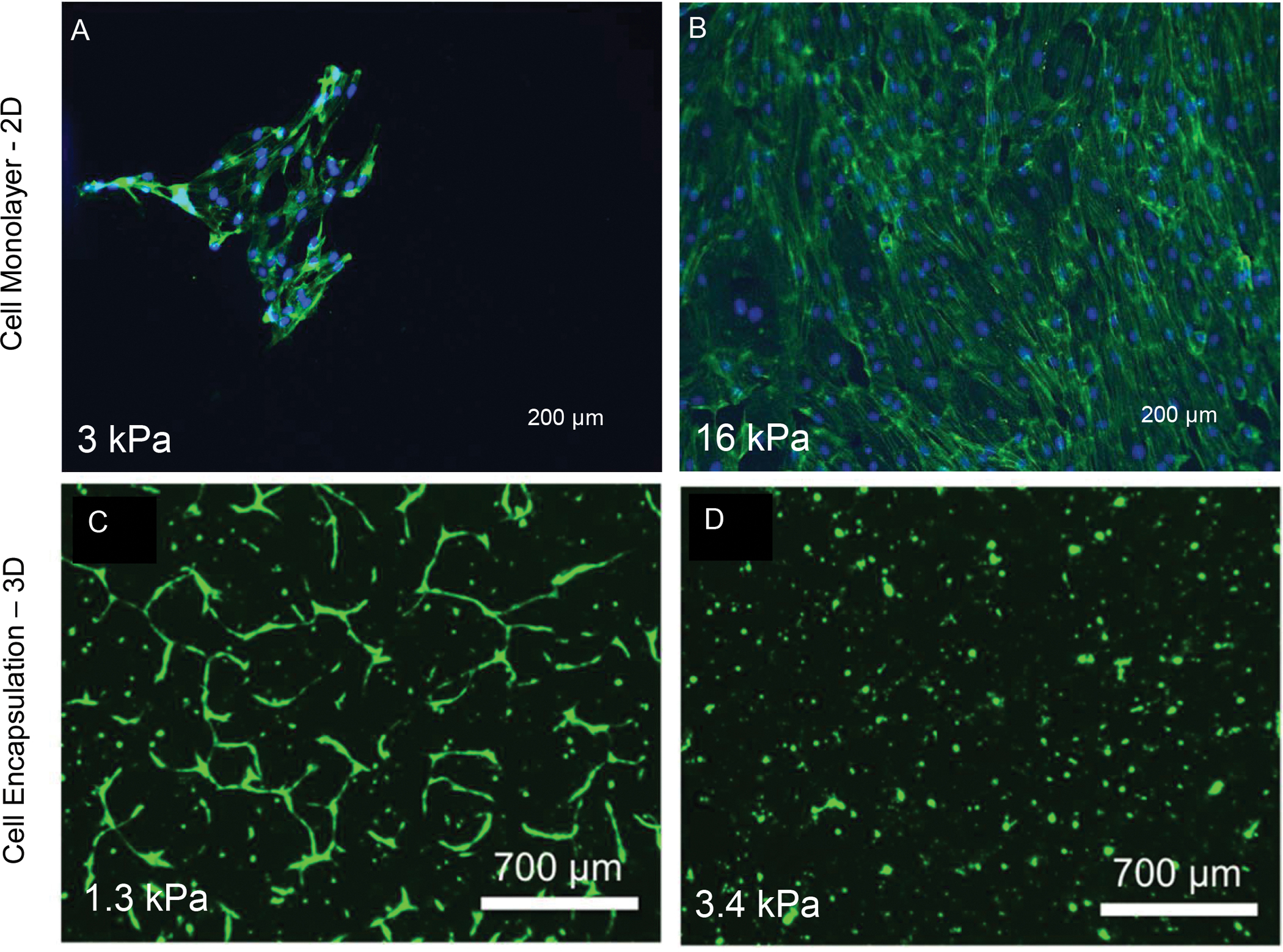
Endothelial cells seeded on 2D substrates of (A) low and (B) high stiffness show a trend where higher stiffness is conducive to faster endothelial monolayer formation. Reproduced from (38). (B) Co-cultures of endothelial cells with bone marrow mesenchymal stromal cells embedded in LED-photopolymerized GelMA hydrogels for (C) lower (1.3 kPa) and higher (3.4 kPa) of stiffness show an opposing trend, where stiffness values above 3 kPa appear to inhibit vasculogenesis. Reproduced from (39).
Similar to the evidence regarding the influence of mechanics on the behavior of endothelial cells, the process of stem cell differentiation into alpha smooth muscle actin expressing perivascular mural lineages, which are consistent with pericytes, is also significantly affected by matrix mechanics. First, it must be highlighted that different populations of dental and mesenchymal stem cells appear to have a different ability to differentiate into pericyte-like cells and give rise to stable and functional vascular capillaries. A direct comparison of bone marrow derived mesenchymal stem cells, dental pulp stem cells and stem cells from the apical papilla co-cultured with HUVECs under the exact same conditions show a distinct pattern of differentiation, where BM-MSCs appear to be more prone to the formation of stable and well-established vascular capillaries (Figure 4).
Figure 4.
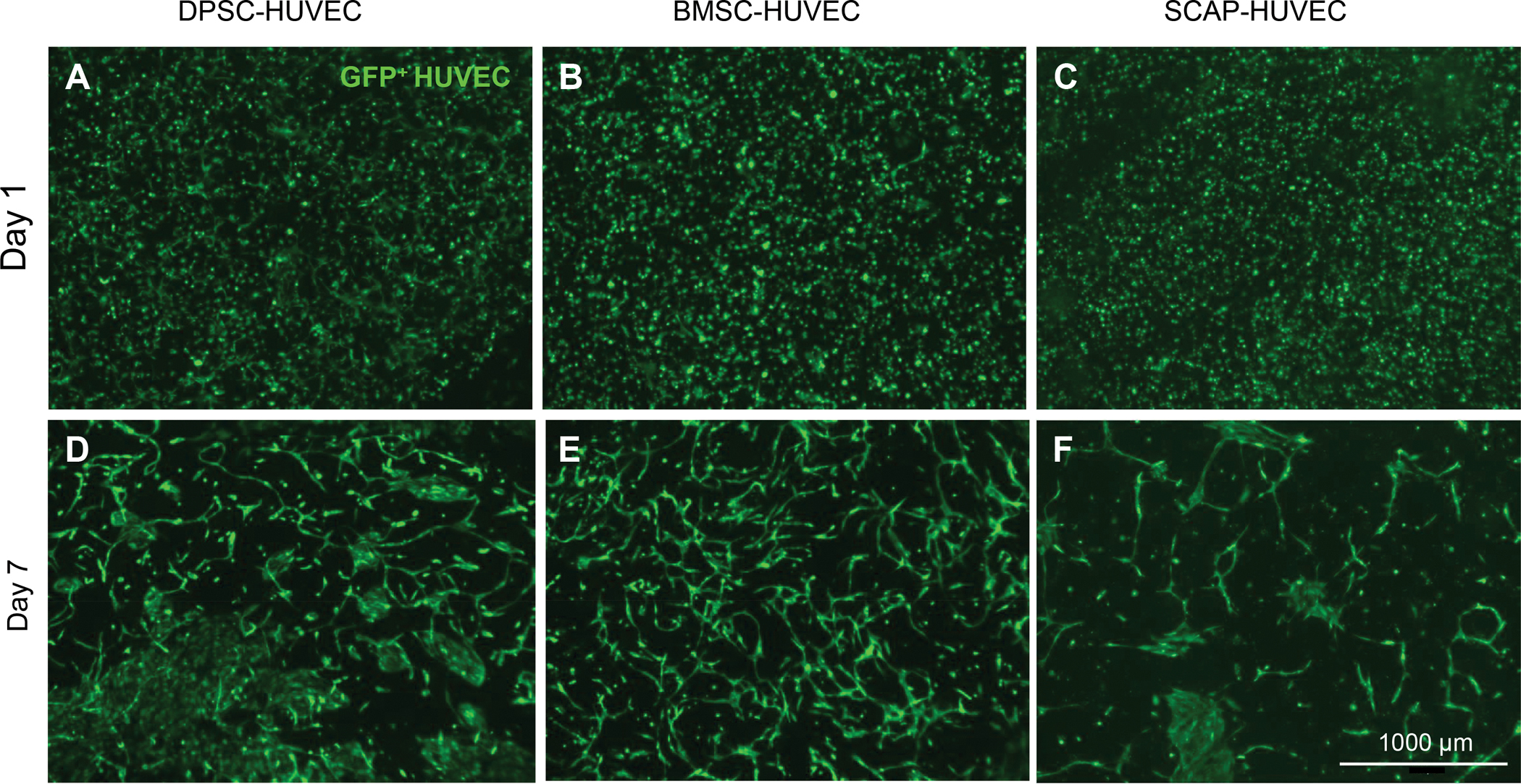
Vasculogenesis in cell-laden GelMA hydrogels using co-cultures of endothelial cells with (A,D) dental pulp stem cells (DPSCs), (B,E) bone marrow mesenchymal stromal cells (BM-MSC), or (C,F) stem cells from the apical papilla (SCAP) in GelMA hydrogels. Results from day 1 to 7 show the enhanced formation of vascular capillaries when BM-MSCs are used.
When co-cultures of ECs and BM-MSCs are seeded onto 2D microenvironments of different mechanical properties, stem cells present a contrasting pattern to those observed in endothelial cells alone. Different from endothelial cells, which proliferate rapidly in substrates of high stiffness, BM-MSCs have a reduction in differentiation toward a pericyte lineage. These mechanically-regulated events are corroborated by further experiments showing that the effects observed due to the stiffness increase are nearly lost when cells ate treated with blebbistatin, a pharmacological inhibitor of mechanotrasduction pathways in contractile cells (29) (Figure 5). When these same cells are cultured in 3D, a more consistent trend is observed between endothelial and mesenchymal cells. A recent report from our group shows that cells have a greater tendency of differentiating and forming pericyte-supported endothelial capillaries when hydrogel stiffness is also kept below ~ 3 kPa (Figure 5) (39). Interestingly, it was also reported that vasculature formation is only observed when cells are culture at a density above 5×106 cells ml of hydrogel (39).
Figure 5.
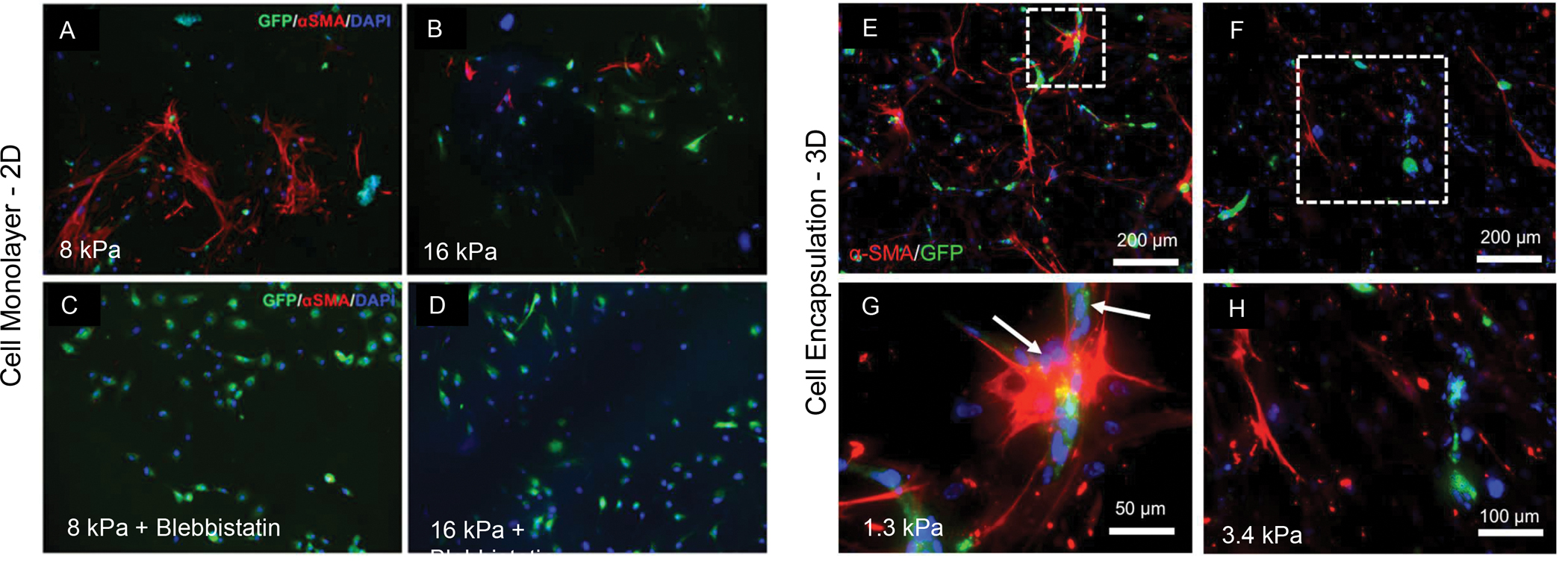
Endothelial cells (HUVECs) co-cultured with bone marrow mesenchymal stromal cells (BM-MSC) either in 2D or in 3D. A visible increase the expression of the pericyte-related marker (alpha SMA) is seen in stem cells cultured on hydrogels of (A) low stiffness versus substrates of (B) higher elastic modulus. The effect is virtually lost when (C and D) cells are treated with blebbistatin, a pharmacological inhibitor of mechanotransduction effect. A similar trend of pericyte differentiation potential by stem cells co-cultured with endothelial cells is seen when cells are cultured in 3D, where (E, G) lower stiffness enhances cell differentiation in comparison to (F, H) higher stiffness hydrogels. Of note, what is considered low and high stiffness for 2D and 3D cell culture is visibly different. Reproduced from (31)
It is also noteworthy that when hydrogels are engineered with adequate vasculature formation and substantial perivascular support in-vitro prior to implantation, these human-cell derived vessels go on to anastomose with the host vasculature and enable blood transport without apparent leakage for at least 3 weeks in-vivo after implantation (40). Recent data from our group shows that vessels expressing human-specific CD31, a marker for endothelial cell communication in the vasculature, and alpha smooth muscle actin, as a marker for pericyte-like phenotype, are distributed across the thickness of a cell-laden hydrogel, and show traces of red blood cells which indicate the anastomosis with the host. Others have reported similar outcomes in engineered hydrogels (41), so it appears that the field is moving at a fast pace towards enabling the engineering of fully vascularized tissue constructs that can exert the required function upon implantation.
4. Microengineering and 3D printing strategies to fabricate the dental pulp vasculature
One of the emerging areas in the field of tissue engineering that has gained significant momentum in the past decade is that of biofabrication. Despite recent controversy with respect to terminology,(42–44) biofabrication has been defined as “the automated generation of biologically functional products with structural organization from living cells, bioactive molecules, biomaterials, cell aggregates such as micro-tissues, or hybrid cell-material constructs, through bioprinting or bioassembly and subsequent tissue maturation processes ”(45). The need to engineer tissues with increasing complexity in 3D, encompassing multiple cell lineages and functions, has demanded researchers to come up with an increasing number of strategies to facilitate the engineering of tissue constructs that are more complex than simple scaffolds loaded with cells and growth factors, which originally defined the field of tissue engineering nearly 3 decades ago. While many strategies under the broader umbrella of biofabrication have been explored for engineering of vascular capillaries,(13) the area that deserves particular attention is that of 3D printing (46, 47). And while recent examples have not focused the dental pulp vasculature, there has been examples of pulp regeneration strategies that have benefitted directly from 3D printing inspired strategies.
3D printing of biomaterials and cellularized tissue constructs on the microscale has had an immense impact in the field of tissue engineering (33, 48) Three recent reviews authored by our group discuss specific applications of 3D printing specifically for dental pulp regeneration (49), the broader scope of regenerative dentistry (50), and the intricacies of 3D printing vascular capillaries (47). The reader is encouraged to refer to these papers for further details on these respective topics. There is a multitude of variations in existing 3D printing methods, but the majority of successful 3D printing approaches that have had an impact in the engineering of vascularized constructs have utilized extrusion processes (46, 47). In these methods, typically a sacrificial template material is dispensed in pre-determined positions in X-Y-Z using the motorized stages of the printing apparatus. The sacrificial template material is often composed of a dissolvable or removable material, which can be extruded and subsequently covered either with a cell-laden hydrogel, or dense aggregate of cells (51). Miller et al first reported the method to 3D print a sacrificial template fiber composed of a dissolvable carbohydrate glass that was covered with a variety of cell-laden hydrogel materials, and subsequently dissolved, giving rise to hollow capillary like structures that were then loaded with human endothelial cells (52). In a subsequent report, our group developed a method to 3D print an agarose template fiber which did not need to be dissolved, and could simply be removed from a cell-laden hydrogel material, also forming complex 3D branching capillary-like structures populated with human endothelial cells (53) (Figure 6A,B). Recent efforts from our team have demonstrated the possibility of 3D printing similar channel like structures in combination of other printable bioinks to form perfusable and pericyte-supported vascular capillaries (Figure 6G).
Figure 6. 3D printing and microengineering of the dental pulp vasculature.
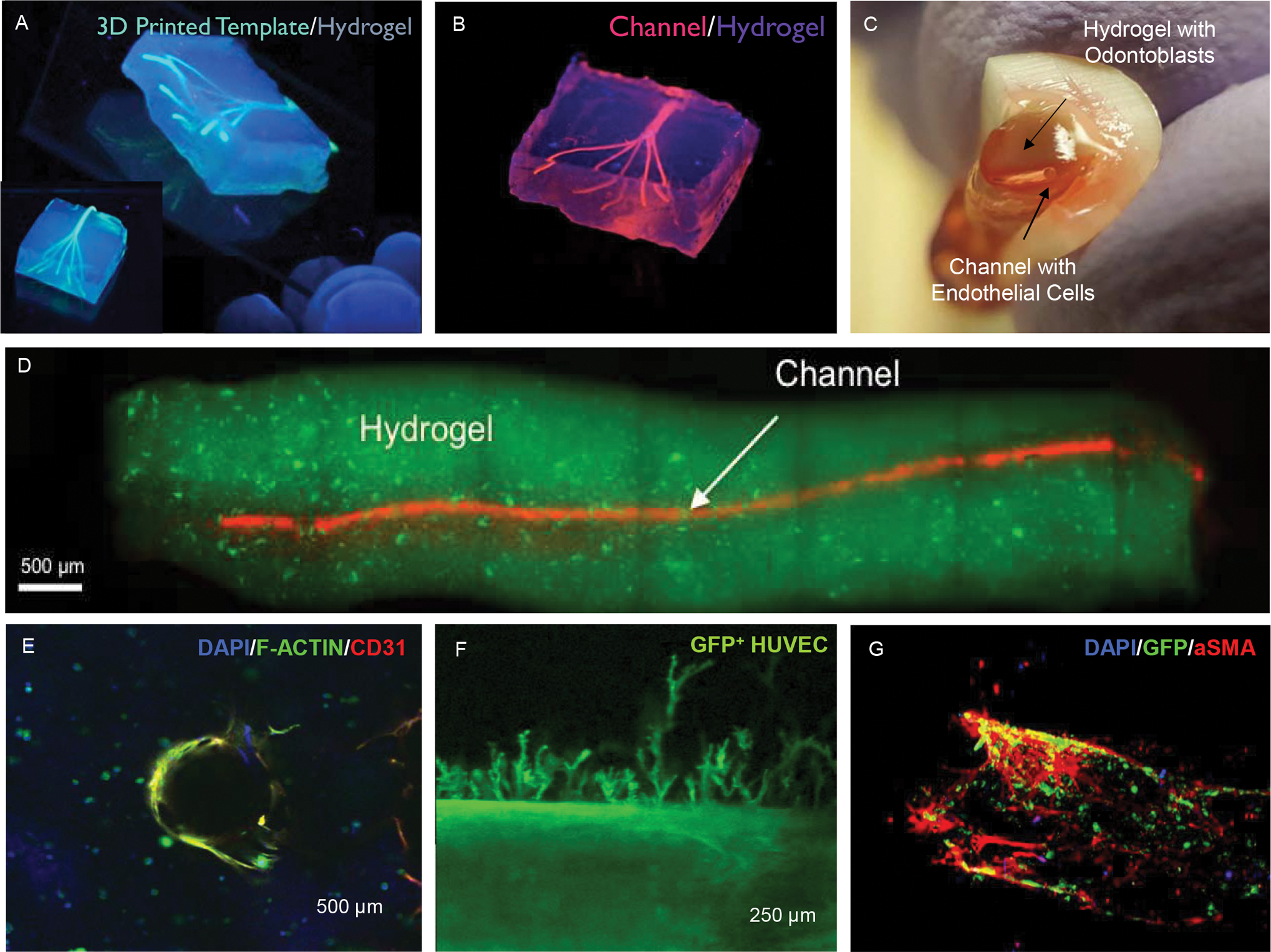
(A) Extrusion 3D printing of sacrificial template fibers embedded in cell-laden hydrogels followed by (B) template fiber removal resulting in the formation of bifurcating channels in the range of 100–1000 μm. Reproduced from (53) (C-D) A similar strategy of sacrificial templating was adapted to microengineer hollow conduits embedded in odontoblast-laden photocrosslinkable hydrogels loaded into the root canal space of full-length human teeth. Reproduced from (38). (E) These hydrogel conduits can be loaded with endothelial cells to form a monolayer that can (F) lead to endothelial sprouting into the hydrogel matrix. (G) When endothelial cells (HUVECs) are co-cultured with bone marrow mesenchymal stromal cells in the right conditions, a pericyte-supported vascular channel can also be formed.
More recently, two distinct examples of 3D printing have been utilized to regenerate vessel like structures with remarkable success. Lee et al reported the use of the so-called freeform reversible embedding of suspended hydrogels (FRESH) method for the fabrication of complex vascular-like structures in including a full human neonatal heart (54). In this method, a high-density collagen hydrogel precursor was dispensed in a bath of gelatin microparticles, which allow the material to undergo fibrillogenesis overtime, as the setting material ‘floats’ in a liquid-like supporting bath. This method has allowed for significant developments in printing of large-scale tissues and organs, and is likely to have ramifications to the field of the dental pulp regeneration where vascular density and branching complexity increases as they extend upwards from small arterioles in the apical foramen. In another example, a digital light processing (DLP) 3D printing approach, which differs from the conventional extrusion method in that it uses light polymerization as the printing mode, was used to pattern complex vascular systems, including the vasculature in a model of 3D printed breathing lung (55). In this method, a photocrosslinkable hydrogel pre-polymer loaded onto a vat is photocrosslinked with light onto a build platform, which is moved upward, as individual layers of the material are built one after another. Although this method has not been utilized to regenerate the dental pulp vasculature, it does allow for resolution values that would be favorable to engineering microscale structures. These advances represent the emerging solutions that can address the specific challenges associated with recreation of the microscale morphologies and complex, multilayered cellular structures of the dental pulp and its vascular supply.
In a direct extension of the above-mentioned 3D printing methods, a recent report by our team described an adaptation of the 3D printing method that we developed earlier, which enabled the translation of the technology into the scope of dental pulp vascular regeneration. Athirasala et al reported the fabrication of agarose hydrogel ‘cones’, which could be positioned and stabilized inside of an empty root canal, surrounded with an odontoblast-laden hydrogel biomaterial, photocrosslinked using a dental curing light, and then sacrificed to form a conduit-like microchannel that traversed the entire length of the canal space (38) (Figure 6C–F). This strategy for coronal loading of both the hydrogel and the endothelial cells into the root canal is particularly relevant in a translational context, however, it requires careful optimization of the hydrogel precursor loading and curing times to ensure homogenous and complete photopolymerization over the entire length of the root canal. Still, one would expect that the coronal region of the photocrosslinkable hydrogel would be more crosslinked than the apical region, which would invariably result in a gradient in degradation resistance that increases from the apical side to the coronal side, which is a desirable effect. The report described the optimization of the conditions that were conducive to the spreading and proliferation of odontoblast cells in the matrix, as well as the loading of endothelial cells inside the fabricated conduits, thus forming an endothelialized microchannel in an odontoblast-laden hydrogel in a long root segment. Of note, these conduits establish the communication between the highly vascularized apex with the long axis of the engineered pulp tissue throughout the root canal space, and enables the improved oxygenation of the cells embedded in the construct. Importantly, as we described above, endothelialized vessel-like structures such as these have been shown to anastomose with the host vasculature and form chimeric vessels with the host in animal models. (40) Moreover, preliminary data (data not shown) from our team has shown that much like engineered conduits that facilitate nerve guidance in regenerated spinal cord injuries and other neural regeneration approaches, the dental pulp conduits can also facilitate and guide the projection of neurites from the apex of a tooth model into the core of the engineered pulp.
5. The tooth on-a-chip and microdevices to study the dental pulp and vasculature
While the previous sections described the physical and mechanical considerations that may promote the regeneration of vascular capillaries in the dental pulp, one important aspect that remains elusive is the specific participation of dentin in the process of pulp regeneration. Extensive research, including some of our own (56), has been published demonstrating that the dentin matrix is rich in molecules that have strong biological activity (57). These include a multitude of growth factors that are known to play an important role in development and tooth mineralization, and also molecules with potent angiogenic activity such as VEGF and TGF beta, which have also been reported in significant concentrations (15). Since these molecules are diffusible, it is reasonable to hypothesize that their diffusion into the extracellular matrix may form a gradient of factors that could regulate the formation of the pulp vasculature. Anatomically, the formation of a well-established odontoblastic layer, a cell-free zone, and an underlying region rich with a vascular plexus corroborate the formation of this diffusible zone of factors that may play a role in regulating the regenerative process in a healthy pulp. The specifics of these interactions, however, have remained poorly understood.
Understanding the participation of dentin on the formation of the dental pulp vasculature is an important step to enable the controllable regeneration of a functional tissue. One of the recent developments that seeks to address this goal, among other objectives, is the fabrication of a microfluidic device that our team has recently referred to as the tooth-on-a-chip (58). The tooth on a chip is a controllable model system that enables investigation of pulp-related mechanisms in-vitro. This recent development has emerged from the growing field of organs-on-a-chip (59, 60), which seeks to replicate specific functions of tissues and organs by taking advantage of micro-engineered substrates with microfluidics technologies that replicate levels of functionality that are difficult to achieve with conventional 2D or 3D cell culture models (61, 62). These microdevices allow for straightforward experimental control over multifactorial questions that are too difficult to systematically study in-vivo (61, 62), and enable one to systematically and reproducibly replicate multicellular architectures, cell-cell/cell-matrix interactions, tissue mechanics, and fluid-flow conditions that are naturally present in complex tissues such as the dental pulp.
Our group has recently described the development and characterization of the first tooth on-a-chip model in a recent paper (58), and a few characteristics are noteworthy. For instance, the model can be engineered to enable the formation of a controllable interface between the tooth and exogenous oral components (i.e. bacteria, dental materials, saliva, oral care products), just like in a real cavity, where the dental pulp forms a direct interface with the dentin and indirect contact with the oral cavity via the dentin tubules. These components can be put in direct contact with the native dentin, which will function as a permeable barrier, establishing the indirect interface with the underlying living dental pulp. Granted that many of these interfaces have been replicated to some extent in existing models of the dental pulp (63–66). However, the tooth on a chip enables one to assess and quantify the immediate response of the pulp tissue to exogenous components in real time, continuously, and under controllable conditions, which is a unique advantage. Moreover, since the device is smaller than a conventional USB drive, the volumes required to assess a response are very small, which further reduces the costs of experiments and increases throughput (Figure 7).
Figure 7. The tooth on-a-chip.
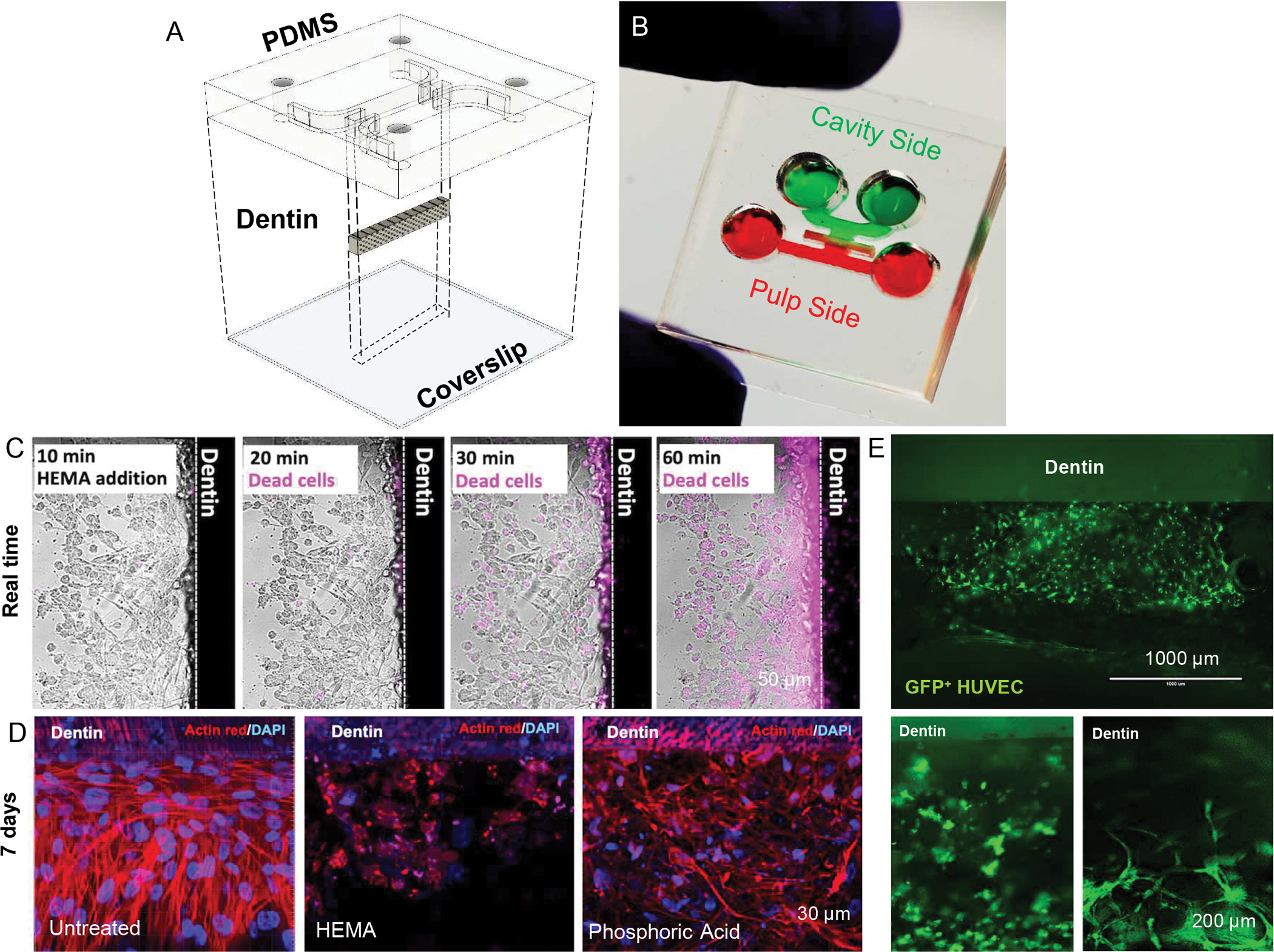
(A) Design and (B) photograph of the tooth on-a-chip microdevice. (C) The device allows for real-time imaging of cell response to injury, as illustrated by the increase in the apoptotic response (pink staining) of differentiated odontoblast seeded on a dentin fragment over time. (D) Conventional staining methods on fixed samples are also possible after several days, and illustrate the remarkable effect of dental materials on pulp cells even after 7 days from their application. Reproduced from (58). (E) Endothelial cells cultured on the tooth on-a-chip microdevice for 24 h, either with (bottom right) or without (bottom left) the perfusion of EDTA through the dentin tubules for 1 min, showing a clear vasculogenic effect due to the presence of dentin matrix molecules and flow. PDMS = Polydimethylsiloxane.
In our recent report, we demonstrated the functionality of the tooth-on-a-chip by replicating the step by step process of a restorative treatment, and show the direct response of odontoblasts in the pulp to acid etching, different monomer molecules and the whole process of a 2-step adhesive system (58), some which are illustrated in Figure 7. Results offer a unique window of the dental pulp response to injury, and show a diverse set of morphological changes as the materials are interfaced with the tooth, which were previously unknown. In another interesting example of the applications of the tooth on a chip, we sought to determine the vasculogenic activity of the diffusible matrix factors that are embedded in dentin. To that end, we utilized the tooth on-a-chip in an experiment where the dentin was treated with EDTA for 1 minute using fluid flow directed against the dentin and into a hydrogel loaded with a co-culture of endothelial and mesenchymal stem cells. When the data were compared with samples that did not have fluid flow (i.e. where dentin matrix molecules were not forced through the hydrogel) we observed that the vasculature formed better and faster in the presence of the matrix molecules (Figure 7D).
Another set of observations that deserves significant attention in the context of vascular pulp regeneration and repair is the influence of the microbiome in the process of vascular morphogenesis. It is highly unlikely that any regenerative effort in the dental pulp will not be influenced by the presence of bacteria or bacterial by-products that are either abundant within the dental tubules or left behind as trace elements after the root canal is medicated and instrumented(67). Interesting evidence has emerged using vasculature on-a-chip microdevices that can be adapted to the tooth on a chip model, showing that even the presence of bacteria-derived lipopolysaccharide (LPS) or TNF-alpha alone can lead to the formation of leaky vessels (67). This forms the basis for future experiments. Despite these interesting observations, the tooth on-a-chip has important limitations. Although relevant cell components are present in this model, it is very difficult to mimic the multiple biological phenomena occurring in the native pulp, especially the interface of different tissue types (i.e. (a) innervation regulating (b) vessel function, which (c) enables flow of inflammatory cells to a site of injury). Therefore, the tooth on-a-chip remains a reductionist approach with several desirable features.
6. Conclusion and future directions
The implications of engineering the dental pulp vasculature for the translation of regenerative endodontics in adult and mature teeth are wide-ranging. Recent progress in the basic understanding of the conditions that enable controllable formation of vascularized tissues in-vitro has been tangible. Biomaterials that allow for controllable behavior of endothelial and stem cells appear to open up important avenues that will lead to more predictable and reproducible clinical outcomes. Of those, photocrosslinkable cell-laden hydrogels with controllable stiffness appear to play a key role in the process of vascular morphogenic through mechanotransduction-regulated pathways. Similarly, different microengineering strategies for dental pulp regeneration, such as 3D bioprinting and intracanal tissue fabrication, are likely to speed up the process of engineering vascularized pulp-like tissues. Moreover, controllable and systematic in-vitro approaches that take advantage of microscale technologies, such as microfluidics and organs on a chip have opened up new possibilities to elucidate complex mechanisms linking the dental pulp, the dentin matrix and the exogenous components interfacing with the tooth. Collectively, these recent developments and technologies point toward new directions in the field of dental pulp regeneration that can be accelerated into translation into clinical practices with patient benefits through fostering active collaborations between research and clinical scientists.
Clinical relevance:
Regeneration of the dental pulp vasculature is a fundamental part of any strategy aiming to regenerate the pulp. Here we review recent approaches and relevant criteria that enable successful engineering of functional vascular capillaries in the context of dental pulp tissue engineering. Several successful methods may speed up the translation of dental pulp regeneration beyond what is currently possible, including in fully formed adult teeth.
7. Acknowledgement
The author acknowledges Avathamsa Athriasala for assistance with reviewing this manuscript. Funding from the National Institute of Dental and Craniofacial Research (R01DE026170 and 3R01DE026170–03S1 to LEB), the Oregon Clinical & Translational Research Institute (OCTRI) – Biomedical Innovation Program (BIP), the IADR-GSK Innovation in Oral Care Awards sponsored by the International Association for Dental Research (IADR), the Michigan-Pittsburgh-Wyss Resource Consortium – Regenerative Medicine Resource Center (MPW-RM) are also acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. References
- 1.About I Pulp Vascularization and Its Regulation by the Microenvironment. In: Goldberg M, About I, editors. The Dental Pulp. Berlin, Heidelberg: Springer; 2014. [Google Scholar]
- 2.Franca CM, Riggers R, Muschler JL, Widbiller M, Lococo PM, Diogenes A, et al. 3D-Imaging of Whole Neuronal and Vascular Networks of the Human Dental Pulp via CLARITY and Light Sheet Microscopy. Sci Rep 2019;9(1):10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith AJ, Cooper PR. Regenerative Endodontics: Burning Questions. J Endod 2017;43(9S):S1–S6. [DOI] [PubMed] [Google Scholar]
- 4.Diogenes A, Ruparel NB, Shiloah Y, Hargreaves KM. Regenerative endodontics: A way forward. J Am Dent Assoc 2016;147(5):372–380. [DOI] [PubMed] [Google Scholar]
- 5.Diogenes AR, Ruparel NB, Teixeira FB, Hargreaves KM. Translational science in disinfection for regenerative endodontics. J Endod 2014;40(4 Suppl):S52–57. [DOI] [PubMed] [Google Scholar]
- 6.Hargreaves KM, Diogenes A, Teixeira FB. Treatment options: biological basis of regenerative endodontic procedures. J Endod 2013;39(3 Suppl):S30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eleazer PD, Glickman GN, McClanahan SB, Webb TD, Justman BC. Glossary of Endodontic Terms. Chicago: American Association of Endodontists; 2012. [Google Scholar]
- 8.Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod 2007;33(4):377–390. [DOI] [PubMed] [Google Scholar]
- 9.Wanjare M, Kusuma S, Gerecht S. Perivascular cells in blood vessel regeneration. Biotechnol J 2013;8(4):434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol 2005;7(4):452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011;473(7347):298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barabaschi GD, Manoharan V, Li Q, Bertassoni LE. Engineering Pre-vascularized Scaffolds for Bone Regeneration. Advances in experimental medicine and biology 2015;881:79–94. [DOI] [PubMed] [Google Scholar]
- 13.Bae H, Puranik AS, Gauvin R, Edalat F, Carrillo-Conde B, Peppas NA, et al. Building vascular networks. Science translational medicine 2012;4(160):160ps123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saghiri MA, Asatourian A, Sorenson CM, Sheibani N. Role of angiogenesis in endodontics: contributions of stem cells and proangiogenic and antiangiogenic factors to dental pulp regeneration. J Endod 2015;41(6):797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang R, Cooper PR, Smith G, Nor JE, Smith AJ. Angiogenic activity of dentin matrix components. J Endod 2011;37(1):26–30. [DOI] [PubMed] [Google Scholar]
- 16.Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado MA, Shi S, et al. SHED differentiate into functional odontoblasts and endothelium. J Dent Res 2010;89(8):791–796. [DOI] [PubMed] [Google Scholar]
- 17.Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK. Tissue engineering: creation of long-lasting blood vessels. Nature 2004;428(6979):138–139. [DOI] [PubMed] [Google Scholar]
- 18.Jeon JS, Bersini S, Whisler JA, Chen MB, Dubini G, Charest JL, et al. Generation of 3D functional microvascular networks with human mesenchymal stem cells in microfluidic systems. Integr Biol (Camb) 2014;6(5):555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S, Lee H, Chung M, Jeon NL. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 2013;13(8):1489–1500. [DOI] [PubMed] [Google Scholar]
- 20.Kuo KC, Lin RZ, Tien HW, Wu PY, Li YC, Melero-Martin JM, et al. Bioengineering vascularized tissue constructs using an injectable cell-laden enzymatically crosslinked collagen hydrogel derived from dermal extracellular matrix. Acta biomaterialia 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin RZ, Melero-Martin JM. Bioengineering human microvascular networks in immunodeficient mice. J Vis Exp 2011(53):e3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin RZ, Moreno-Luna R, Li D, Jaminet SC, Greene AK, Melero-Martin JM. Human endothelial colony-forming cells serve as trophic mediators for mesenchymal stem cell engraftment via paracrine signaling. Proceedings of the National Academy of Sciences of the United States of America 2014;111(28):10137–10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goncalves SB, Dong Z, Bramante CM, Holland GR, Smith AJ, Nor JE. Tooth slice-based models for the study of human dental pulp angiogenesis. J Endod 2007;33(7):811–814. [DOI] [PubMed] [Google Scholar]
- 24.Rosa V, Zhang Z, Grande RH, Nor JE. Dental pulp tissue engineering in full-length human root canals. J Dent Res 2013;92(11):970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dissanayaka WL, Hargreaves KM, Jin L, Samaranayake LP, Zhang C. The interplay of dental pulp stem cells and endothelial cells in an injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo. Tissue engineering. Part A 2015;21(3–4):550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuchiara MP, Gould DJ, McHale MK, Dickinson ME, West JL. Integration of Self-Assembled Microvascular Networks with Microfabricated PEG-Based Hydrogels. Adv Funct Mater 2012;22(21):4511–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saik JE, Gould DJ, Watkins EM, Dickinson ME, West JL. Covalently immobilized platelet-derived growth factor-BB promotes angiogenesis in biomimetic poly(ethylene glycol) hydrogels. Acta biomaterialia 2011;7(1):133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science 2005;310(5751):1139–1143. [DOI] [PubMed] [Google Scholar]
- 29.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science 2009;324(5935):1673–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006;126(4):677–689. [DOI] [PubMed] [Google Scholar]
- 31.Albuquerque MT, Valera MC, Nakashima M, Nor JE, Bottino MC. Tissue-engineering-based strategies for regenerative endodontics. J Dent Res 2014;93(12):1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caliari SR, Burdick JA. A practical guide to hydrogels for cell culture. Nat Methods 2016;13(5):405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C, et al. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv Mater 2014;26(1):85–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010;31(21):5536–5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khademhosseini A, Eng G, Yeh J, Fukuda J, Blumling J, 3rd, Langer R, et al. Micromolding of photocrosslinkable hyaluronic acid for cell encapsulation and entrapment. Journal of biomedical materials research. Part A 2006;79(3):522–532. [DOI] [PubMed] [Google Scholar]
- 36.Monteiro N, Thrivikraman G, Athirasala A, Tahayeri A, Franca CM, Ferracane JL, et al. Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry. Dent Mater 2018;34(3):389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao RR, Peterson AW, Ceccarelli J, Putnam AJ, Stegemann JP. Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis 2012;15(2):253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Athirasala A, Lins F, Tahayeri A, Hinds M, Smith AJ, Sedgley C, et al. A Novel Strategy to Engineer Pre-Vascularized Full-Length Dental Pulp-like Tissue Constructs. Sci Rep 2017;7(1):3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monteiro N, He W, Franca CM, Athirasala A, Bertassoni LE. Engineering Microvascular Networks in LED Light-Cured Cell-Laden Hydrogels. ACS Biomaterials Science & Engineering 2018;4(7). [DOI] [PubMed] [Google Scholar]
- 40.Thrivikraman G, Athirasala A, Gordon R, Zhang L, Bergan R, Keene DR, et al. Rapid fabrication of vascularized and innervated cell-laden bone models with biomimetic intrafibrillar collagen mineralization. Nat Commun 2019;10(1):3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baranski JD, Chaturvedi RR, Stevens KR, Eyckmans J, Carvalho B, Solorzano RD, et al. Geometric control of vascular networks to enhance engineered tissue integration and function. Proceedings of the National Academy of Sciences of the United States of America 2013;110(19):7586–7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groll J, Burdick JA, Cho DW, Derby B, Gelinsky M, Heilshorn SC, et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication 2018;11(1):013001. [DOI] [PubMed] [Google Scholar]
- 43.Moroni L, Boland T, Burdick JA, De Maria C, Derby B, Forgacs G, et al. Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol 2018;36(4):384–402. [DOI] [PubMed] [Google Scholar]
- 44.Groll J, Boland T, Blunk T, Burdick JA, Cho DW, Dalton PD, et al. Biofabrication: reappraising the definition of an evolving field. Biofabrication 2016;8(1):013001. [DOI] [PubMed] [Google Scholar]
- 45.Mironov V, Trusk T, Kasyanov V, Little S, Swaja R, Markwald R. Biofabrication: a 21st century manufacturing paradigm. Biofabrication 2009;1(2):022001. [DOI] [PubMed] [Google Scholar]
- 46.Kinstlinger IS, Miller JS. 3D-printed fluidic networks as vasculature for engineered tissue. Lab Chip 2016;16(11):2025–2043. [DOI] [PubMed] [Google Scholar]
- 47.Jurney P, Parthiban SP, Athirasala A, Franca C, Tahayeri A, Menezes P, et al. 3D Bioprinting of Blood Vessels and Vascular Networks: Progress and Challenges Toward Biofabrication of Functional Vascularized Tissues and Organs’. In: Demirci U, El Assal R, Chen P, editors. Emerging Technologies for Biofabrication and Biomanufacturing. World Scientific Publishing.(2019). 2019. [Google Scholar]
- 48.Ha M, Athirasala A, Tahayeri A, Menezes PP, Bertassoni LE. Micropatterned hydrogels and cell alignment enhance the odontogenic potential of stem cells from apical papilla in-vitro. Dent Mater 2020;36(1):88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franca CM, Sercia A, Parthiban SP, Bertassoni LE. Current and Future Applications of 3D Bioprinting in Endodontic Regeneration — A Short Review. California Dental Association Journal 2019;47(10). [Google Scholar]
- 50.Obregon F, Vaquette C, Ivanovski S, Hutmacher DW, Bertassoni LE. Three-Dimensional Bioprinting for Regenerative Dentistry and Craniofacial Tissue Engineering. J Dent Res 2015;94(9 Suppl):143S–152S. [DOI] [PubMed] [Google Scholar]
- 51.Skylar-Scott MA, Uzel SGM, Nam LL, Ahrens JH, Truby RL, Damaraju S, et al. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci Adv 2019;5(9):eaaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater 2012;11(9):768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 2014;14(13):2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee A, Hudson AR, Shiwarski DJ, Tashman JW, Hinton TJ, Yerneni S, et al. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019;365(6452):482–487. [DOI] [PubMed] [Google Scholar]
- 55.Grigoryan B, Paulsen SJ, Corbett DC, Sazer DW, Fortin CL, Zaita AJ, et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019;364(6439):458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Athirasala A, Tahayeri A, Thrivikraman G, Franca CM, Monteiro N, Tran V, et al. A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication 2018;10(2):024101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith AJ, Scheven BA, Takahashi Y, Ferracane JL, Shelton RM, Cooper PR. Dentine as a bioactive extracellular matrix. Arch Oral Biol 2012;57(2):109–121. [DOI] [PubMed] [Google Scholar]
- 58.Franca CM, Tahayeri A, Rodrigues NS, Ferdosian S, Puppin Rontani RM, Sereda G, et al. The tooth on-a-chip: a microphysiologic model system mimicking the biologic interface of the tooth with biomaterials. Lab Chip 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bertassoni LE. Dentin on the nanoscale: Hierarchical organization, mechanical behavior and bioinspired engineering. Dent Mater 2017;33(6):637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol 2014;32(8):760–772. [DOI] [PubMed] [Google Scholar]
- 61.Beebe DJ, Ingber DE, den Toonder J. Organs on Chips 2013. Lab Chip 2013;13(18):3447–3448. [DOI] [PubMed] [Google Scholar]
- 62.Huh D, Torisawa YS, Hamilton GA, Kim HJ, Ingber DE. Microengineered physiological biomimicry: organs-on-chips. Lab Chip 2012;12(12):2156–2164. [DOI] [PubMed] [Google Scholar]
- 63.Hume WR. An analysis of the release and the diffusion through dentin of eugenol from zinc oxide-eugenol mixtures. J Dent Res 1984;63(6):881–884. [DOI] [PubMed] [Google Scholar]
- 64.Ersev H, Schmalz G, Bayirli G, Schweikl H. Cytotoxic and mutagenic potencies of various root canal filling materials in eukaryotic and prokaryotic cells in vitro. J Endod 1999;25(5):359–363. [DOI] [PubMed] [Google Scholar]
- 65.Schmalz G, Schuster U, Nuetzel K, Schweikl H. An in vitro pulp chamber with three-dimensional cell cultures. J Endod 1999;25(1):24–29. [DOI] [PubMed] [Google Scholar]
- 66.Camilleri J, Laurent P, About I. Hydration of Biodentine, Theracal LC, and a prototype tricalcium silicate-based dentin replacement material after pulp capping in entire tooth cultures. J Endod 2014;40(11):1846–1854. [DOI] [PubMed] [Google Scholar]
- 67.Alimperti S, Mirabella T, Bajaj V, Polacheck W, Pirone DM, Duffield J, et al. Three-dimensional biomimetic vascular model reveals a RhoA, Rac1, and N-cadherin balance in mural cell-endothelial cell-regulated barrier function. Proceedings of the National Academy of Sciences of the United States of America 2017;114(33):8758–8763. [DOI] [PMC free article] [PubMed] [Google Scholar]


