Abstract
Introduction
Risdiplam is a survival of motor neuron 2 (SMN2) splicing modifier for the treatment of patients with spinal muscular atrophy (SMA). The JEWELFISH study (NCT03032172) was designed to assess the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of risdiplam in previously treated pediatric and adult patients with types 1–3 SMA. Here, an analysis was performed after all patients had received at least 1 year of treatment with risdiplam.
Methods
Patients with a confirmed diagnosis of 5q-autosomal recessive SMA between the ages of 6 months and 60 years were eligible for enrollment. Patients were previously enrolled in the MOONFISH study (NCT02240355) with splicing modifier RG7800 or treated with olesoxime, nusinersen, or onasemnogene abeparvovec. The primary objectives of the JEWELFISH study were to evaluate the safety and tolerability of risdiplam and investigate the PK after 2 years of treatment.
Results
A total of 174 patients enrolled: MOONFISH study (n = 13), olesoxime (n = 71 patients), nusinersen (n = 76), onasemnogene abeparvovec (n = 14). Most patients (78%) had three SMN2 copies. The median age and weight of patients at enrollment was 14.0 years (1–60 years) and 39.1 kg (9.2–108.9 kg), respectively. About 63% of patients aged 2–60 years had a baseline total score of less than 10 on the Hammersmith Functional Motor Scale–Expanded and 83% had scoliosis. The most common adverse event (AE) was upper respiratory tract infection and pyrexia (30 patients each; 17%). Pneumonia (four patients; 2%) was the most frequently reported serious AE (SAE). The rates of AEs and SAEs per 100 patient-years were lower in the second 6-month period compared with the first. An increase in SMN protein was observed in blood after risdiplam treatment and was comparable across all ages and body weight quartiles.
Conclusions
The safety and PD of risdiplam in patients who were previously treated were consistent with those of treatment-naïve patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-023-00444-1.
Keywords: Evrysdi, Pharmacodynamics, Risdiplam, Safety, Spinal muscular atrophy
Key Summary Points
| Why carry out this study? |
| Spinal muscular atrophy (SMA) is a genetic disease that affects infants to adults with varying degrees of disease severity. |
| With disease-modifying therapies available for SMA, it is imperative to understand the safety of these drugs when used successively or in combination. |
| The aim of this interim analysis was to determine the safety and pharmacodynamics of risdiplam in a heterogeneous population of patients with SMA who had been previously enrolled in an SMA clinical trial or treated with a previously approved SMA treatment. |
| What was learned from the study? |
| The JEWELFISH study population had a similar safety profile and increase in SMN protein levels after 12 months of treatment with risdiplam compared with treatment-naïve patients who were treated with risdiplam in the FIREFISH and SUNFISH clinical trials. |
| No safety signals were observed in the patient population after 12 months of treatment with risdiplam. |
| There were no deaths or drug-related safety findings that led to the withdrawal of any patients treated with risdiplam. |
Introduction
Spinal muscular atrophy (SMA) is a progressive neuromuscular disease caused by biallelic mutations of the survival of motor neuron 1 gene (SMN1) [1]. In humans, a second gene, SMN2, is present but produces only low levels of functional SMN protein which cannot fully compensate for the lack of SMN1 [2].
SMA is categorized into five clinical types (0–4), which are defined according to the maximum motor milestone achieved and the age of onset [2, 3]. Type 0 SMA is the most severe form of SMA, with a prenatal or neonatal onset and is usually fatal at birth [3]. Type 1 SMA generally manifests within 6 months of age. Infants with type 1 SMA are unable to sit, and the majority of untreated infants die before 2 years of age. Individuals with type 2 SMA typically have symptom onset between 6 and 18 months of age and may acquire the ability to sit or stand with support, but never walk independently. Individuals with type 3 SMA show symptom onset after 18 months of age. All patients are able to ambulate independently at some point, but those presenting symptoms before 3 years of age (type 3a) tend to lose ambulation earlier than those presenting after 3 years of age (type 3b) [4]. Type 4 SMA has an onset into adulthood and is the mildest form of the disease [5].
There are three disease-modifying therapies (DMTs) available for the treatment of SMA. Risdiplam (EVRYSDI®) is an oral small molecule designed to selectively modify splicing of SMN2 pre-mRNA and promote the inclusion of exon 7 to increase levels of functional SMN protein. Risdiplam has been approved in over 80 countries worldwide [5]. Nusinersen (SPINRAZA®), an antisense oligonucleotide, is an intrathecally administered SMN2 pre-mRNA splicing modifier [6, 7]. Onasemnogene abeparvovec (ZOLGENSMA®), a one-time intravenously administered gene transfer therapy, is designed to deliver a functional copy of the SMN1 gene [8, 9].
Prior to the approval of these DMTs, there were two small molecules, olesoxime (OLEOS, NCT02628743) and RG7800 (MOONFISH, NCT02240355) that were investigated for the treatment of SMA [10, 11]. Both treatments were discontinued: olesoxime showed no significant benefits to motor function over 130 weeks [12], and RG7800 was discontinued as a precaution due to findings observed in long-term preclinical animal models [13].
With multiple treatment options available, patients may request to switch treatments or to take them in combination for several reasons [14]. Some patients may not respond to their current therapy [15]. For some patients with reduced spinal access caused by severe scoliosis or scoliosis surgery (spinal fusion), receiving repeated intrathecally administered therapy may be challenging [16, 17]. Other patients may experience unwanted side effects either from the treatment drug or from its administration [18].
There are limited real-world data evaluating combination or sequential treatment with SMA DMTs [19–22] and as a result, the safety and efficacy of combination or sequential treatment are not well understood. To date, there is one report that describes the evaluation of onasemnogene abeparvovec followed by risdiplam, which was well tolerated in patients with type 1 SMA [22]. Another report from the risdiplam expanded access program showed patients treated with risdiplam who were either treatment-naïve or had been previously treated with nusinersen, onasemnogene abeparvovec, or both had a similar safety profile to those in pivotal risdiplam clinical trials [23]. Currently, there is one ongoing study (RESPOND; NCT04488133) evaluating the safety and efficacy of nusinersen in 60 patients following treatment with onasemnogene abeparvovec [24]. Herein, we describe the JEWELFISH study (NCT03032172) and report the safety and pharmacodynamics (PD) of risdiplam after 12 months of treatment.
Methods
Study Design
JEWELFISH is a multicenter, exploratory, non-comparative, open-label study of once-daily, orally administered risdiplam in pediatric and adult patients with SMA who have been previously treated in an SMA clinical trial or with a previously approved SMA treatment. Eligible patients were enrolled in the MOONFISH study (phase 1 trial of RG7800 versus placebo in adult and pediatric patients with SMA) or were treated with nusinersen, onasemnogene abeparvovec, or olesoxime.
The primary analysis of JEWELFISH will be performed after a 24-month period. For this interim analysis, safety and PD were evaluated in patients who received risdiplam for at least 12 months by the clinical cutoff date (CCOD) of 29 January 2021.
Patient Selection
Patients aged 6 months to 60 years of age at screening were eligible to enroll in the JEWELFISH study if they had a confirmed diagnosis of 5q-autosomal recessive SMA, including genetic confirmation of a biallelic mutation (homozygous deletion or heterozygosity) of the SMN1 gene predictive of loss of function, clinical history, or signs or symptoms attributable to SMA.
Patients were previously enrolled in the MOONFISH study or had previous treatment with any of the following: (a) nusinersen (defined as having received at least four doses of nusinersen, provided that the last dose was received at least 90 days prior to screening); (b) olesoxime (provided that the last dose was received at most 18 months and at least 90 days prior to screening); (c) onasemnogene abeparvovec (provided that the time of treatment was at least 12 months prior to screening). Patients were enrolled across 24 different centers in Belgium, France, Germany, Italy, the Netherlands, Poland, Switzerland, the UK, and the USA.
Patient- and caregiver-reported reasons for switching from nusinersen or onasemnogene abeparvovec to risdiplam were collected during screening. A list of reasons has been provided in Table S1 of the Supplementary Material.
All patients received risdiplam once daily at the approved dosing regimen as follows: 0.2 mg/kg for infants aged 6 months to less than 2 years, 0.25 mg/kg for patients older than 2 years with a body weight less than 20 kg, and 5 mg for patients with a body weight of 20 kg or more.
Full inclusion/exclusion criteria are listed in the Supplementary Material. The target sample size was set at 180 patients. The target sample size was determined by practical considerations and not based on statistical power calculations. With 180 patients exposed to risdiplam, there is a 92% chance to detect an adverse event (AE) in at least one patient, assuming that the true underlying AE rate is 1.4%.
Outcomes
The primary objectives of the study were to evaluate the safety and tolerability of risdiplam and to investigate the pharmacokinetics (PK) of risdiplam and its metabolites. The secondary objectives were to investigate the PK/PD relationship of risdiplam. Investigation of PD includes the analyses of SMN mRNA splice forms and SMN protein in blood. The primary analysis of the JEWELFISH study was assessed after all patients received 24 months of treatment. In this interim analysis, all available safety data and SMN protein levels at month 12 are reported. A full list of safety outcomes and secondary and exploratory objectives can be viewed in the Supplementary Material.
Statistical Analysis
The intent-to-treat (ITT) population was defined as all enrolled patients, regardless of whether they received risdiplam or not. All patients who received at least one dose of risdiplam were included in the safety analysis population for this 12-month report.
Study Oversight
Approval from each site’s institutional review board or ethics committee was provided for this study. The clinical trial was conducted in accordance with the principles of the Helsinki Declaration of 1964 and its later amendments and following Good Clinical Practice guidelines, and was approved by an ethics committee at each site. An external independent data monitoring committee was established to monitor patient safety. Patients were required to be able and willing to provide written informed consent and to comply with the study protocol according to International Conference on Harmonization (ICH) guidelines and local regulations. Alternatively, a legally authorized representative must have been able to give consent for the patient according to ICH guidelines and local regulations and assent must have been given whenever possible. Written informed consent was provided by the patient or the patient’s legally authorized representative before their participation in the study.
Results
Study Population
A total of 174 patients were enrolled in the JEWELFISH study between March 2017 and January 2020. A total of 13 patients had previously enrolled in the MOONFISH study, 71 patients received olesoxime, 76 received nusinersen, and 14 received onasemnogene abeparvovec. Three patients who were previously enrolled in the MOONFISH study received placebo treatment and had not received RG7800. Three patients included in the nusinersen treatment group had also received olesoxime previously. One patient received treatment with onasemnogene abeparvovec first followed by nusinersen and was included in the onasemnogene abeparvovec group. One patient withdrew from the study at baseline prior to receiving risdiplam as a result of inadequate blood access; therefore, the safety of risdiplam in this interim report was assessed in 173 patients after all patients had been treated for at least 12 months (CCOD 29 January 2021).
Patient Characteristics
The JEWELFISH study population was broad, heterogeneous, and had a high degree of motor impairment at baseline (Table 1). The median age at enrollment was 14.0 years (1–60) and the median weight was 39.1 kg (9.2–108.9 kg). There were slightly more patients who were male (55%), and more than half of all patients had type 2 SMA (62%). The majority of patients (78%) had three SMN2 copies, with 13% of patients having four copies, and 7% who had 1–2 copies; the remainder were pending genotyping and were classified as unknown at the CCOD. Patients were categorized as non-sitters (34%; defined as a score of 0 on item 9 of the 32-item Motor Function Measure [MFM32]), non-ambulant sitters (57%; defined as a score ≥ 1 on item 9 of the MFM32 and unable to walk unassisted for 10 m or more), or walkers (9%). Overall, 89% of all patients were able to eat solid food. Additionally, 84% of patients weighed at least 20 kg at baseline with 79% of patients receiving 5 mg of risdiplam daily from day 1. The remaining patients enrolled before dose selection and, therefore, received 3 mg at the start of the study for a mean duration of 203 days (118–277 days) which was increased to 5 mg after dose confirmation from the SUNFISH trial (NCT02908685) [25]. Approximately half of all patients (53%) required non-invasive or invasive pulmonary care or bilevel positive airway pressure. Of patients aged 2–60 years (n = 168), 83% had scoliosis, 39% had severe scoliosis with a greater than 40° curvature, and 63% had a baseline total score of less than 10 on the Hammersmith Functional Motor Scale–Expanded (HFMSE).
Table 1.
Patient demographic and clinical characteristics at baseline
| Previous treatment | All patients | ||||
|---|---|---|---|---|---|
| Olesoxime | MOONFISH studyb | Nusinersen | Onasemnogene abeparvovec | (N = 174) | |
| (n = 71)a | (n = 13)a | (n = 76)c | (n = 14)d | ||
| Age at screening, years, median (range) | 16.0 (11–36) | 30.0 (16–58) | 12.0 (1–60) | 2.0 (1–5) | 14.0 (1–60) |
| ≥ 18 years, n (%) | 31 (44) | 11 (85) | 21 (28) | 0 | 63 (36) |
| Gender, female/male, n (%) | 36/35 (51/49) | 4/9 (31/69) | 36/40 (47/53) | 3/11 (21/79) | 79/95 (45/55) |
| SMA type, n (%) | |||||
| 1 | 2 (3) | 0 | 9 (12) | 4 (29) | 15 (9) |
| 2 | 50 (70) | 5 (39) | 43 (57) | 10 (71) | 108 (62) |
| 3 | 19 (27) | 8 (62) | 24 (32) | 0 | 51 (29) |
| SMN2 copy number, n (%) | |||||
| 1 | 0 | 0 | 0 | 1 (7) | 1 (1) |
| 2 | 0 | 0 | 9 (12) | 3 (21) | 12 (7) |
| 3 | 64 (90) | 6 (46) | 56 (74) | 10 (71) | 136 (78) |
| 4 | 5 (7) | 6 (46) | 11 (15) | 0 | 22 (13) |
| Unknowne | 2 (3) | 1 (8) | 0 | 0 | 3 (2) |
| Age of onset of initial symptoms, months, median (range) | 13 (0–258) | 9 (0–256) | 12 (0–188) | 8 (0–10) | 12 (0–258) |
| Motor function, n (%)f | |||||
| Non-sitters | 29 (41) | 7 (54) | 21 (28)g | 2 (14)g | 59 (34)g |
| Sitters | 42 (59) | 3 (23) | 42 (55)g | 12 (86)g | 99 (57)g |
| Walkers | 0 | 3 (23) | 13 (17) | 0 | 16 (9) |
| HFMSE total score < 10, n (%) | 59 (83) | 8 (62) | 35 (48)h,i | 3 (27)h,j | 105 (63)h,k |
| Scoliosis, n (%) | |||||
| Yes | 66 (93) | 9 (69) | 61 (84)h,i | 3 (27)h,j | 139 (83)h,k |
| > 40° curvature | 36 (51) | 3 (23) | 27 (37)h,i | 0 | 66 (39)h,k |
| Hip subluxation or dislocation, n (%) | 20 (28) | 2 (15) | 25 (34)h,i | 4 (36)h,j | 51 (30)h,k |
| Pulmonary carel, n (%) | |||||
| Yes | 39 (55) | 1 (8) | 43 (57) | 10 (71) | 93 (53) |
| Missing | 0 | 1 (8) | 0 | 0 | 1 (1) |
| Feeding status, n (%) | |||||
| Gastrostomy tube | 2 (3) | 0 | 7 (9) | 1 (7) | 10 (6) |
| Nasogastric food intake | 0 | 0 | 1 (1) | 0 | 1 (1) |
| Mixed (fluid/puree) oral intake | 1 (1) | 0 | 2 (3) | 1 (7) | 4 (2) |
| Modified oral food intake | 2 (3) | 0 | 2 (3) | 0 | 4 (2) |
| Solid food | 65 (93) | 13 (100) | 64 (84) | 12 (86) | 154 (89) |
| Median weight, all patients, kg (range) | 46.5 (17.7–85.0) | 56.5 (29.5–105.0) | 34.1 (9.3–108.9) | 12.1 (9.2–22.1) | 39.1 (9.2–108.9) |
| IQR | 33.3–57.7 | 46.0–65.0 | 22.9–50.0 | 10.4–14.1 | 26.0–56.0 |
| Weight-for-age percentile (≤ 10 years old), n (%)m | |||||
| ≤ 3rd percentile | 4 (14) | 3 (21) | 7 (17) | ||
| > 3rd to ≤ 5th percentile | 0 | 3 (21) | 3 (7) | ||
| > 5th to ≤ 10th percentile | 2 (7) | 1 (7) | 3 (7) | ||
| > 10th to ≤ 25th percentile | 7 (25) | 1 (7) | 8 (19) | ||
| > 25th to ≤ 50th percentile | 4 (14) | 3 (21) | 7 (17) | ||
| > 50th percentile | 11 (39) | 3 (21) | 14 (33) | ||
| BMI-for-age percentile, (≤ 19 years old), n (%)n | |||||
| ≤ 3rd percentile | 23 (54) | 3 (75) | 12 (21) | 2 (14) | 40 (34) |
| > 3rd to ≤ 5th percentile | 2 (5) | 0 | 1 (2) | 1 (7) | 4 (3) |
| > 5th to ≤ 10th percentile | 2 (5) | 1 (25) | 2 (4) | 3 (21) | 8 (7) |
| > 10th to ≤ 25th percentile | 3 (7) | 0 | 7 (12) | 3 (21) | 13 (11) |
| > 25th to ≤ 50th percentile | 1 (2) | 0 | 7 (12) | 3 (21) | 11 (9) |
| > 50th percentile | 12 (28) | 0 | 28 (49) | 2 (14) | 42 (36) |
| BMI category, (≥ 20 years old), n (%)o | |||||
| Underweight (< 18.5) | 12 (44) | 3 (33) | 6 (32) | 0 | 21 (38) |
| Normal (18.5 to < 25) | 12 (44) | 4 (44) | 8 (42) | 0 | 24 (44) |
| Overweight (25 to < 30) | 3 (11) | 1 (11) | 1 (5) | 0 | 5 (9) |
| Obese (≥ 30) | 0 | 1 (11) | 4 (21) | 0 | 5 (9) |
Data cutoff 29 January 2021. Intent-to-treat patients
BiPAP bilevel positive airway pressure, BMI body mass index, HFMSE Hammersmith Functional Motor Scale–Expanded, HINE-2 Hammersmith Infant Neurological Examination, Module 2, IQR interquartile range, MFM Motor Function Measure, SMA spinal muscular atrophy, SMN survival of motor neuron
aNo longer in clinical development
bThree patients who were previously enrolled in the MOONFISH study (NCT02240355) received placebo treatment and were never switched to RG7800
cThree patients in the nusinersen group had also received olesoxime (NCT01302600) previously
dOne patient in the onasemnogene abeparvovec group received treatment with onasemnogene abeparvovec first followed by nusinersen. Ten patients were enrolled in STRONG (NCT03381729), three patients in STR1VE (NCT03306277), and one patient in STR1VE-EU (NCT03461289) prior to enrollment in JEWELFISH
eUnknown SMN2 copy number is pending confirmation by genotyping
fNon-sitters are defined as scoring 0 on item 9 of the MFM32 while sitters are defined as scoring ≥ 1 on item 9 of the MFM32 but do not qualify as ambulant. Ambulant patients are defined as walkers. The MFM32 is a validated scale used to evaluate fine and gross motor function in people with neurologic disorders, including SMA [27]
gFor patients aged < 2 years, baseline motor milestones were evaluated by the HINE-2, a motor function measure for infants with SMA [28]
hOnly reported for patients aged 2–60 years
iPercentages calculated out of n = 73 eligible patients
jPercentages calculated out of n = 11 eligible patients
kPercentages calculated out of n = 168 eligible patients
lIncludes the use of invasive and non-invasive pulmonary care or BiPAP
mN at baseline: nusinersen = 28, onasemnogene abeparvovec = 14, all patients = 42
nN at baseline: olesoxime = 43, MOONFISH study = 4, nusinersen = 57, onasemnogene abeparvovec = 14, all patients = 118
oN at baseline: olesoxime = 27, MOONFISH study = 9, nusinersen = 19, all patients = 55
SMN Protein
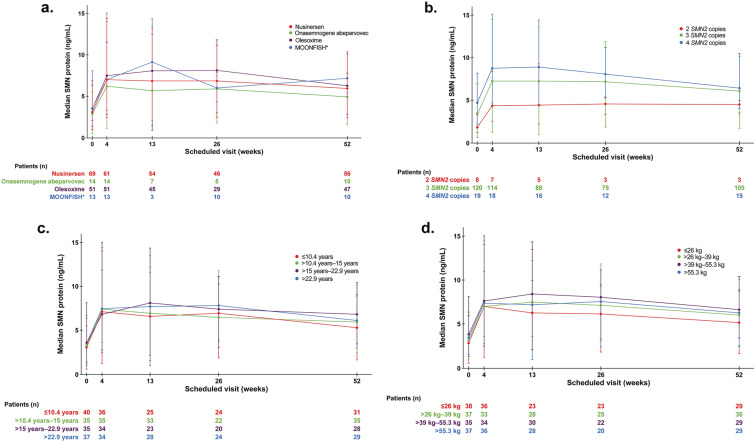
SMN protein levels in blood at baseline were comparable across groups independent of the previously received treatment (Table 2). Baseline median SMN protein levels in whole blood (minimum–maximum) were 3.55 ng/mL (2.16–8.16) in patients previously enrolled in the MOONFISH study and 2.86 ng/mL (0.53–5.53), 3.09 ng/mL (1.01–6.91), and 3.50 ng/mL (1.43–6.39) in patients previously treated with onasemnogene abeparvovec, nusinersen, or olesoxime, respectively. A sustained twofold increase from baseline was achieved for all groups after 4 weeks of risdiplam (Fig. 1a).
Table 2.
Baseline median protein levels in whole blood
| Category | Baseline median SMN protein (ng/mL) | Min–max (ng/mL) |
|---|---|---|
| SMA type | ||
| Type 1 | 2.02 | 0.76–3.45 |
| Type 2 | 3.30 | 0.53–6.39 |
| Type 3 | 4.02 | 1.01–8.16 |
| Copy number | ||
| 2 copies | 1.76 | 1.21–3.45 |
| 3 copies | 3.25 | 0.53–6.91 |
| 4 copies | 4.69 | 2.16–8.16 |
| Previous treatment | ||
| Onasemnogene abeparvovec | 2.86 | 0.53–5.53 |
| MOONFISH study | 3.55 | 2.16–8.16 |
| Nusinersen | 3.09 | 1.01–6.91 |
| Olesoxime | 3.50 | 1.43–6.39 |
| Age quartile | ||
| ≤ 10.4 years | 3.06 | 0.53–5.53 |
| > 10.4 years–15 years | 3.26 | 1.21–6.39 |
| > 15 years–22.9 years | 3.56 | 1.01–6.68 |
| > 22.9 years | 3.55 | 1.36–8.16 |
| Weight quartile | ||
| ≤ 26 kg | 2.86 | 0.53–6.39 |
| > 26 kg–39 kg | 3.14 | 1.01–5.98 |
| > 39 kg–55.3 kg | 3.45 | 1.58–6.02 |
| > 55.3 kg | 3.84 | 1.36–8.16 |
SMA spinal muscular atrophy, SMN survival of motor neuron
Fig. 1.
Median SMN protein in blood by a previous treatment, b SMN2 copy number, c age quartiles (years), d weight quartiles (kg). *Three patients who were previously enrolled in the MOONFISH study received placebo treatment and were never switched to RG7800. SMN survival of motor neuron
Median baseline levels tended to be higher in patients with type 2 (3.30 ng/mL [0.53–6.39]) and 3 SMA (4.02 ng/mL [1.01–8.16]) compared with patients with type 1 SMA (2.02 ng/mL [0.76–3.45]; Fig. S1 of the Supplementary Material). Similarly, baseline SMN protein concentration correlated with SMN2 copy number (Fig. 1b), with higher levels observed in patients with more SMN2 copies. The observed increase in SMN protein after risdiplam treatment was comparable across all ages (Fig. 1c) and body weight quartiles (Fig. 1d).
Treatment Discontinuation and Safety
In the safety population, eight patients discontinued the study at the time of the CCOD (29 January 2021). Five patients (n = 5/8) withdrew from the study between months 0 and 12. Of these five patients, three withdrew as a result of patient decision, one withdrew because of patient-reported lack of improvement, and one patient withdrew from the study because of irritable bowel syndrome and panic attack; both AEs were deemed unrelated to risdiplam. Two patients (n = 2/8) withdrew from the study after they had received 12 months of treatment as a result of patient decision. Lastly, one patient (n = 1/8) chose to withdraw because of the COVID-19 pandemic during the safety follow-up period.
As of the CCOD, the mean duration (standard deviation) of exposure to risdiplam was 17.0 (7.1) months (0.9–47.0). Overall, 92% of patients reported at least one AE (Table 3). The most common AEs were upper respiratory tract infection and pyrexia (17%; in both). Approximately 14% of patients reported a serious AE (SAE). Pneumonia (2%) was reported as the most observed SAE. A complete list of AEs and SAEs can be viewed in Table S2 of the Supplementary Material.
Table 3.
Adverse events observed in the JEWELFISH population
| Previous treatment | All patients (N = 173)b |
||||
|---|---|---|---|---|---|
| Olesoxime (n = 70) |
MOONFISH studya (n = 13) |
Nusinersen (n = 76) |
Onasemnogene abeparvovec (n = 14) |
||
| Patients with at least one AE, n (%) | 63 (90) | 12 (92) | 71 (93) | 13 (93) | 159 (92) |
| Total number of AEs | 357 | 66 | 450 | 50 | 923 |
| Total number of deaths | 0 | 0 | 0 | 0 | 0 |
| Total number of patients with at least one, n (%) | |||||
| SAE | 8 (11) | 3 (23) | 11 (15) | 2 (14) | 24 (14) |
| Treatment-related SAE | 1 (1)c | 0 | 0 | 0 | 1 (1)c |
| SAE leading to dose modification/interruption | 2 (3) | 1 (8) | 3 (4) | 0 | 6 (4) |
| AE leading to withdrawal from treatment | 0 | 0 | 1 (1)d | 0 | 1 (1)d |
| Treatment-related AE | 8 (11) | 6 (46) | 19 (25) | 0 | 33 (19) |
| Related AE leading to withdrawal from treatment | 0 | 0 | 0 | 0 | 0 |
| Most common AEse n (number of patients [%]) | |||||
| Upper respiratory tract infection | 14 (20) | 0 | 14 (18) | 2 (14) | 30 (17) |
| Pyrexia | 8 (11) | 1 (8) | 17 (22) | 4 (29) | 30 (17) |
| Headache | 12 (17) | 1 (8) | 15 (20) | 0 | 28 (16) |
| Nausea | 5 (7) | 0 | 14 (18) | 1 (7) | 20 (12) |
| Diarrhea | 4 (6) | 0 | 14 (18) | 1 (7) | 19 (11) |
| Nasopharyngitis | 6 (9) | 2 (15) | 7 (9) | 2 (14) | 17 (10) |
| Vomiting | 6 (9) | 1 (8) | 5 (7) | 2 (14) | 14 (8) |
| Most common SAEsf n (number of patients [%]) | |||||
| Pneumonia | 1 (1) | 0 | 2 (3) | 1 (7) | 4 (2) |
| Lower respiratory tract infection | 2 (3) | 0 | 1 (1) | 0 | 3 (2) |
| Upper respiratory tract infection | 0 | 0 | 3 (4) | 0 | 3 (2) |
| Respiratory failure | 0 | 0 | 3 (4) | 0 | 3 (2) |
As follow-up duration is different between groups, the overall rate of AEs and SAEs cannot be compared. Multiple occurrences of the same AE in one individual are counted only once except for the “Total number of AEs” row, for which multiple occurrences of the same AE are counted separately
AE adverse event, SAE serious AE
aThree patients who were previously enrolled in the MOONFISH study (NCT02240355) received placebo treatment and were never switched to RG7800
bOne patient withdrew from the study at baseline and therefore 173 patients received risdiplam
cAn SAE of supraventricular tachycardia was considered related to risdiplam treatment by the Investigator (in the context of hypoxia) and resolved with ongoing treatment with risdiplam
dIrritable bowel syndrome and panic attack, which were unrelated to risdiplam, led to the withdrawal of one patient who was previously treated with nusinersen
eAEs reported in ≥ 14 patients
fSAEs reported in ≥ 3 patients. Includes AEs with onset from first dose of study drug up to the clinical cutoff date (29 January 2021)
A total of six patients (MOONFISH study [n = 1], nusinersen [n = 3], and olesoxime [n = 2]) had at least one SAE that led to dose modification or interruption; none were related to risdiplam. At least one treatment-related AE was reported in 33 patients (MOONFISH study [n = 6], nusinersen [n = 19], and olesoxime [n = 8]). One patient, previously treated with olesoxime, had an SAE of supraventricular tachycardia (in the context of hypoxia), which was considered related to risdiplam treatment by the investigator and resolved without changes to the study drug.
The AE rate for the first 6 months of treatment was 648.0 events per 100 patient-years (PY [95% confidence interval; CI 595.2–704.3; total PY at risk 85.5 years]). For the 6–12-month period, the AE rate was 270.2 events (95% CI 235.8–308.2; total PY at risk 82.2 years). The rate of SAEs was 33.9 events per 100 PY (95% CI 22.7–48.7; total PY at risk 85.5 years) from 0 to 6 months. The rate of SAEs between 6 and 12 months was 18.3 events per 100 PY (95% CI 10.2–30.1; total PY at risk 82.2 years).
All available safety laboratory results, vital signs, and electrocardiograms did not show any clinically significant adverse findings compared with baseline. There have been no clinically significant safety findings in patients reflective of potential risks previously identified from non-clinical toxicology studies (effects on epithelial tissues, retinal toxicity, or hematologic effects). Ophthalmologic monitoring did not show any evidence of retinal toxicity. Ophthalmologic AEs were not suggestive of risdiplam-induced effects and resolved without change of treatment with risdiplam. No drug-related safety findings led to the withdrawal of any patients exposed to risdiplam and no deaths were reported.
Discussion
This interim analysis of the JEWELFISH study examined the safety of risdiplam after 12 months of treatment in patients who were previously enrolled in a trial of an investigational SMA therapy or had received an approved SMA DMT. Based on this study design, the enrolled cohort was highly heterogenous as reflected by their broad age range (1–60 years), SMA type (types 1–3), and advanced progression of SMA (a majority of patients were profoundly weak as indicated by low baseline total HFMSE scores and high rates of severe scoliosis and hip subluxation or dislocation). Patients in JEWELFISH study were also heavier (i.e., up to 108.9 kg) compared with patient populations in other SMA clinical trials of DMTs [26, 27].
Risdiplam treatment led to a median twofold increase in blood SMN protein levels after 4 weeks of treatment which was sustained for at least 12 months. This is consistent with results from the patients from the FIREFISH (NCT02913482) [28, 29] and SUNFISH studies who were treatment-naïve. There were no differences observed in the median-fold increase in SMN protein across nusinersen, onasemnogene abeparvovec, or the other treatment groups. Baseline SMN protein levels in the onasemnogene abeparvovec group were slightly lower compared with the other treatment subgroups; this may be a reflection of the patient population consisting of a greater proportion of patients with lower SMN2 copy numbers and type 1 SMA. Although median baseline levels tended to be lower among patients with type 1 SMA, the proportional increase of SMN protein was comparable across all patient subgroups analyzed, including among those who were older and heavier.
SMN protein is the direct PD marker that confirms risdiplam’s mode of action. Risdiplam addresses the underlying cause of the disease by promoting the inclusion of exon 7 to generate full-length SMN2 mRNA and subsequently increasing the production of functional SMN protein from the SMN2 gene. In preclinical studies risdiplam showed good distribution throughout the body, including the CNS, and the risdiplam-induced SMN protein increase in the brain mirrored what was observed in blood [30].
Risdiplam was well tolerated in patients (N = 173) who were treated for 12 months. AEs and SAEs were reflective of the underlying disease. The most frequently reported AEs (≥ 8%) were upper respiratory tract infection, pyrexia, headache, nausea, diarrhea, nasopharyngitis, and vomiting. The most common SAE (≥ 2%) was pneumonia.
A greater than 50% decline in rates of AEs per 100 PY was observed in between the first and second 6-month periods. There was a similar trend toward decreasing rates of overall SAEs per 100 PY in the second 6-month period. The progressive decrease of AEs during the course of the study suggests good safety and tolerability of treatment and may indicate that the general health of enrolled patients is improving. Similar results have been observed in the FIREFISH (type 1 SMA) and SUNFISH (types 2 and 3) population following treatment with risdiplam. No treatment-related safety findings have led to withdrawal, and there were low rates of treatment discontinuation in the study [25, 29].
The oral, “at-home” daily dosing of risdiplam was beneficial particularly during the COVID-19 pandemic when patients were limited or restricted from traveling to clinical sites. Taking risdiplam at home also reduced the need to travel to hospitals to receive treatment, which decreased the burden on patients and caregivers [31]. Additionally, risdiplam was a viable treatment option for individuals with SMA with advanced scoliosis who may have difficulty receiving treatment intrathecally. Of note, roughly a third of patients in JEWELFISH study who were previously treated with nusinersen reported tolerability concerns over the intrathecal administration (Table S1 of the Supplementary Material). Tolerability concerns generally referred to challenges associated with intrathecal administration in patients with scoliosis or those who have undergone spinal surgery and the inability to receive a lumbar puncture.
Overall, the COVID-19 pandemic did not significantly impact the ability to monitor and manage patient safety during the study. For many patients who enrolled towards the end of the recruitment period, the 6-month visit fell during the first peak of the pandemic and so attendance was affected because of restriction measures at site and national levels. On-site visit attendance improved as demonstrated by the high percentage of patients (91%) who completed visits at month 12, despite travel restrictions and challenges associated with the pandemic. As such, the safety results of the study and safety profile of risdiplam were not significantly impacted and remain scientifically valid.
Limitations
One limitation of this report is that the results are from an interim analysis performed at 12 months of risdiplam treatment. The results provide evidence on the safety of risdiplam for patients who have been previously treated with DMTs. The primary analysis at 24 months will be reported when the data are available. Another limitation is the high variability observed in the measurements of SMN protein in whole blood, which was likely introduced as a result of slightly differing conditions in blood collection and sample handling procedures across clinical sites, patients, and time-points. Furthermore, SMN protein levels are correlated with patient age and SMN2 copy number (and SMN2 copy number is correlated with SMA type and disease severity). Therefore, the interpretation and comparison of SMN protein across patient groups with different age, SMA type, and/or SMN2 copy number should be approached with caution, in particular when comparing small groups with few patients and samples.
Conclusions
The safety profile of risdiplam in patients treated for 12 months (CCOD 29 January 2021) reported in this interim analysis is consistent with results reported in treatment-naïve individuals in the FIREFISH [23, 24] and SUNFISH studies [26]. There were no safety signals observed across the whole JEWELFISH study population, including patients previously treated with nusinersen or onasemnogene abeparvovec. The median twofold increases in SMN protein levels were consistent across the different subpopulations of JEWELFISH and were consistent with levels reported in the FIREFISH and SUNFISH studies. The JEWELFISH study is ongoing, with the primary analysis to be assessed after 24 months.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
JEWELFISH Study Group: Nicolas Deconinck, Irina Balikova, Inge Joniau, Valentine Tahon, Sylvia Wittevrongel, Nathalie Goemans, Catherine Cassiman, Lies Prove, Lisa Vancampenhout, Marleen van den Hauwe, Annelies Van Impe, Claude Cances, Vincent Soler, Lauriane Maillard De La Morandais, Delphine Vovan, Pascal Cintas, Françoise Auriol, Marianne Mus, Gwennaelle Alphonsa, Valerie Bellio, Olaia Gil Mato, Florence Flamein, Cécile Evrard, Amina Ziouche, Ikram Bouacha-Allou, Philippe Debruyne, Gilles Derlyn, Sabine Defoort, Florian Leroy, Loïc Danjoux, Isabelle Desguerre, Dominique Bremond-Gignac, Maxence Rateuax, Elodie Deladrière, Carole Vuillerot, Quentin Veillerot, Bénédicte Sibille-Dabadi, Aurélie Barrière, Marie Tinat, Manel Saidi, Stephanie Fontaine, Camille De Montferrand, Laure Le-Goff, Aurélie Portefaix, Ulrike Walther Louvier, Pierre-André Duval, Pascale Caradec, Souad Touati, Alberto Zamora Herranz, Janbernd Kirschner, Jan Bollig, Fanni Molnár, Sibylle Vogt, Astrid Pechmann, David Schorling, Sabine Wider, Heike Kölbel, Ulrike Schara, Frederik Braun, Andrea Gangfuss, Tim Hagenacker, Anja Eckstein, Dirk Dekowski, Michael Oeverhaus, Mareile Stoehr, Barbara Andres, Karin Smuda, Enrico Bertini, Adele D’Amico, Sergio Petroni, Paola Valente, Anna Maria Bonetti, Adelina Carlesi, Irene Mizzoni, Claudio Bruno, Marina Pedemonte, Noemi Brolatti, Enrico Priolo, Giuseppe Rao, Lorenza Sposetti, Simone Morando, Giacomo Comi, Silvia Osnaghi, Valeria Minorini, Francesca Abbati, Federica Fassini, Michaela Foà, Maria Amalia Lopopolo, Francesca Magri, Alessandra Govoni, Megi Meneri, Valeria Parente, Eugenio Mercuri, Laura Antonaci, Maria Carmela Pera, Marika Pane, Giulia Maria Amorelli, Costanza Barresi, Guglielmo D’Amico, Lorenzo Orazi, Giorgia Coratti, Roberto De Sanctis, Giuseppe Vita, Maria Sframeli, Gian Luca Vita, Pasquale Aragona, Leandro Inferrera, Elisa Imelde Postorino, Daniela Montanini, Vincenzo Di Bella, Concetta Donato, Elisabetta Calà, Ludo Van der Pol, Jos Aalbers, Joke de Boer, Saskia Imhof, Pascale Cooijmans, Thijs Ruyten, Danny Van Der Woude, Anna Kostera-Pruszczyk, Beata Klimaszewska, Dominika Romańczak, Zuzanna Gierlak-Wójcicka, Malwina Kępa, Adam Sikorski, Marcin Sobieraj, Anna Lusakowska, Biruta Kierdaszuk, Karolina Czeczko, Dirk Fischer, Bettina Henzi, Konstantin Gugleta, Akos Kusnyerik, Patricia Siems, Sabina Akos, Nora Frei, Christine Seppi, Christine Wondrusch Haschke, Michela Guglieri, Volker Straub, Richard Bell, Mahmoud Nassar, Stuart Page, Michael Patrick Clarke, Aedheen Regan, Anna Mayhew, Robert Muni Lofra, Deepak Parasuraman, Simone Bruschi, Abdul-Jabbar Ghauri, Andrew Castle, Saima Naqvi, Nicola Patt, Mariacristina Scoto, Federica Trucco, Robert H Henderson, Roopen Kukadia, Will Moore, Evelin Milev, Catherine Rye, Victoria Selby, Amy Wolfe, Basil Darras, Anna Maria Baglieri, Anne Fulton, Courtney Lucken, Elizabeth Maczek, Amy Pasternak, Claudia A Chiriboga, Steven Kane, Ma Edylin M. Bautista, Eileen Frommer, Noelle Pensec, Rachel Salazar, Cara Yochai, Rafael Rodrigues-Torres, Manroop Chawla, John Day, Shannon Beres, Richard Gee, Sally Dunaway Young, Richard Finkel, Aledie Navas Nazario, Airaj Fasiuddin, Julie A. Wells, Jennifer Wilson, Debbie Berry, Virgina Rizzo, Julie Duke, Migvis Monduy, Jorge Collado.
Funding
This study, Rapid Service Fee and the Open Access fee was sponsored by F. Hoffmann-La Roche Ltd.
Medical Writing and Other Assistance
The authors thank Michelle B. Kim, PhD, of MediTech Media for providing medical writing support, which was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
The authors would like to thank all the patients and families who participated in the JEWELFISH clinical trial, the clinical trial sites, and staff members. This program was sponsored by F. Hoffmann-La Roche Ltd.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Claudia A. Chiriboga, Birgit Jaber, Carmen Martin, Francis Warren, Heidemarie Kletzl, Imogen Carruthers, Ksenija Gorni, Renata S. Scalco, and Kathryn R. Wagner contributed to the study conception and design. Material preparation, data collection and analysis were performed by all authors. The first draft of the manuscript was written by Dr. Michelle B. Kim and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Birgit Jaber, Carmen Martin, Francis Warren, Heidemarie Kletzl, Ksenija Gorni, Renata S. Scalco, and Kathryn R. Wagner are employees and shareholders of F. Hoffmann-La Roche Ltd, Basel, Switzerland. At the time of the study, Imogen Carruthers was an employee of F. Hoffmann-La Roche Ltd, Basel, Switzerland and is now an employee of CS Genetics. Claudia A. Chiriboga has taken part in advisory boards for AveXis/Novartis, Biogen, PTC Therapeutics, Genentech, and F. Hoffmann-La Roche, received educational speaker fees from Biogen, F. Hoffmann-La Roche and Genentech and received research support grants from AveXis/Novartis, Biogen, F. Hoffmann-La Roche, and the National Institutes of Health. Claudio Bruno has received honoraria for scientific advisory boards from Biogen, Novartis, F. Hoffmann-La Roche, and Sarepta Therapeutics, and has received research support grants from Biogen. Tina Duong has received honoraria for scientific advisory boards or consultancy from Biogen, Novartis, F. Hoffmann-La Roche, Genentech, Pfizer, Sarepta Therapeutics, Audentes, Astellas, and Dyne. Dirk Fischer has received honoraria for scientific advisory boards or consultancy from F. Hoffmann-La Roche. Eugenio Mercuri has received honoraria for scientific advisory boards and educational speaker fees from Sarepta Therapeutics, Santhera, PTC Therapeutics, Pfizer, F. Hoffmann-La Roche, Biogen, Avexis, Novartis, Scholar Rock, and Cytokinetics, and has received research support grants from Biogen. Janbernd Kirschner has received honoraria for scientific advisory boards from Biogen, Novartis, PTC Therapeutics, Pfizer, F. Hoffmann-La Roche, Sarepta Therapeutics and reports grants from Biogen, Novartis, and F. Hoffmann-La Roche. Anna Kostera-Pruszczyk has received compensation for participation at symposia, lectures, and scientific advisory boards from Biogen, F. Hoffmann-La Roche, AveXis/Novartis and PTC Therapeutics. She has also received institutional grant support from Biogen and is an investigator in SMA trials sponsored by F. Hoffmann-La Roche. Francesco Muntoni has received honoraria for scientific advisory boards from Novartis Gene Therapies, Inc., Biogen, PTC Therapeutics, Sarepta Therapeutics, F. Hoffmann-La Roche and reports grants and personal fees from Novartis Gene Therapies, Inc., Biogen, Sarepta Therapeutics and F. Hoffmann-La Roche. FM is member of the Pfizer Rare Disease Advisory Board and of Dyne Therapeutic SAB.
Compliance with Ethics Guidelines
Approval from each site’s institutional review board or ethics committee was provided for this study. The study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. All subjects provided informed consent to participate in the study.
Data Availability
For eligible studies, qualified researchers may request access to individual patient level clinical data through a data request platform. The datasets generated during and/or analyzed during the current study are available via Vivli (https://vivli.org/ourmember/roche/). For up to date details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification.
Footnotes
The original online version of this article was revised to include the JEWELFISH Study Group members and to correct table footnote cue g in Table 1.
The members of the JEWELFISH Study are listed online in “Acknowledgements”.
Change history
7/3/2023
A Correction to this paper has been published: 10.1007/s40120-023-00503-7
Contributor Information
Claudia A. Chiriboga, Email: cac3@cumc.columbia.edu
the JEWELFISH Study Group:
Nicolas Deconinck, Irina Balikova, Inge Joniau, Valentine Tahon, Sylvia Wittevrongel, Nathalie Goemans, Catherine Cassiman, Lies Prove, Lisa Vancampenhout, Marleen van den Hauwe, Annelies Van Impe, Claude Cances, Vincent Soler, Lauriane Maillard De La Morandais, Delphine Vovan, Pascal Cintas, Françoise Auriol, Marianne Mus, Gwennaelle Alphonsa, Valerie Bellio, Olaia Gil Mato, Florence Flamein, Cécile Evrard, Amina Ziouche, Ikram Bouacha-Allou, Philippe Debruyne, Gilles Derlyn, Sabine Defoort, Florian Leroy, Loïc Danjoux, Isabelle Desguerre, Dominique Bremond-Gignac, Maxence Rateuax, Elodie Deladrière, Carole Vuillerot, Quentin Veillerot, Bénédicte Sibille-Dabadi, Aurélie Barrière, Marie Tinat, Manel Saidi, Stephanie Fontaine, Camille De Montferrand, Laure Le-Goff, Aurélie Portefaix, Ulrike Walther Louvier, Pierre-André Duval, Pascale Caradec, Souad Touati, Alberto Zamora Herranz, Janbernd Kirschner, Jan Bollig, Fanni Molnár, Sibylle Vogt, Astrid Pechmann, David Schorling, Sabine Wider, Heike Kölbel, Ulrike Schara, Frederik Braun, Andrea Gangfuss, Tim Hagenacker, Anja Eckstein, Dirk Dekowski, Michael Oeverhaus, Mareile Stoehr, Barbara Andres, Karin Smuda, Enrico Bertini, Adele D’Amico, Sergio Petroni, Paola Valente, Anna Maria Bonetti, Adelina Carlesi, Irene Mizzoni, Claudio Bruno, Marina Pedemonte, Noemi Brolatti, Enrico Priolo, Giuseppe Rao, Lorenza Sposetti, Simone Morando, Giacomo Comi, Silvia Osnaghi, Valeria Minorini, Francesca Abbati, Federica Fassini, Michaela Foà, Maria Amalia Lopopolo, Francesca Magri, Alessandra Govoni, Megi Meneri, Valeria Parente, Eugenio Mercuri, Laura Antonaci, Maria Carmela Pera, Marika Pane, Giulia Maria Amorelli, Costanza Barresi, Guglielmo D’Amico, Lorenzo Orazi, Giorgia Coratti, Roberto De Sanctis, Giuseppe Vita, Maria Sframeli, Gian Luca Vita, Pasquale Aragona, Leandro Inferrera, Elisa Imelde Postorino, Daniela Montanini, Vincenzo Di Bella, Concetta Donato, Elisabetta Calà, Ludo Van der Pol, Jos Aalbers, Joke de Boer, Saskia Imhof, Pascale Cooijmans, Thijs Ruyten, Danny Van Der Woude, Anna Kostera-Pruszczyk, Beata Klimaszewska, Dominika Romańczak, Zuzanna Gierlak-Wójcicka, Malwina Kępa, Adam Sikorski, Marcin Sobieraj, Anna Lusakowska, Biruta Kierdaszuk, Karolina Czeczko, Dirk Fischer, Bettina Henzi, Konstantin Gugleta, Akos Kusnyerik, Patricia Siems, Sabina Akos, Nora Frei, Christine Seppi, Christine Wondrusch Haschke, Michela Guglieri, Volker Straub, Richard Bell, Mahmoud Nassar, Stuart Page, Michael Patrick Clarke, Aedheen Regan, Anna Mayhew, Robert Muni Lofra, Deepak Parasuraman, Simone Bruschi, Abdul-Jabbar Ghauri, Andrew Castle, Saima Naqvi, Nicola Patt, Mariacristina Scoto, Federica Trucco, Robert H Henderson, Roopen Kukadia, Will Moore, Evelin Milev, Catherine Rye, Victoria Selby, Amy Wolfe, Basil Darras, Anna Maria Baglieri, Anne Fulton, Courtney Lucken, Elizabeth Maczek, Amy Pasternak, Claudia A Chiriboga, Steven Kane, Ma Edylin M. Bautista, Eileen Frommer, Noelle Pensec, Rachel Salazar, Cara Yochai, Rafael Rodrigues-Torres, Manroop Chawla, John Day, Shannon Beres, Richard Gee, Sally Dunaway Young, Richard Finkel, Aledie Navas Nazario, Airaj Fasiuddin, Julie A. Wells, Jennifer Wilson, Debbie Berry, Virgina Rizzo, Julie Duke, Migvis Monduy, and Jorge Collado
References
- 1.Lefebvre S, Burglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80(1):155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 2.Munsat T. Workshop report: international SMA collaboration. Neuromuscul Disord. 1991;1:81. doi: 10.1016/0960-8966(91)90052-T. [DOI] [Google Scholar]
- 3.Wadman RI, Stam M, Gijzen M, et al. Association of motor milestones, SMN2 copy and outcome in spinal muscular atrophy types 0–4. J Neurol Neurosurg Psychiatry. 2017;88(4):365–367. doi: 10.1136/jnnp-2016-314292. [DOI] [PubMed] [Google Scholar]
- 4.Zerres K, Rudnik-Schoneborn S. Natural history in proximal spinal muscular atrophy. Clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch Neurol. 1995;52(5):518–23. doi: 10.1001/archneur.1995.00540290108025. [DOI] [PubMed] [Google Scholar]
- 5.New three-year data for Roche’s Evrysdi (risdiplam) show long-term improvements in survival and motor milestones in babies with type 1 spinal muscular atrophy (SMA) 2022. https://www.roche.com/media/releases/med-cor-2022-04-29. Accessed Nov 2022.
- 6.Biogen Inc. SPINRAZA® (nusinersen) US prescribing information 2016. Updated December 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/209531lbl.pdf. Accessed Nov 2022.
- 7.Biogen Inc. SPINRAZA® (nusinersen) EMA prescribing information 2017. Updated December 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004312/WC500229704.pdf. Accessed Nov 2022.
- 8.AveXis Inc. ZOLGENSMA® (onasemnogene abeparvovec-xioi) US prescribing information 2019. Updated May 2019. https://www.fda.gov/media/126109/download. Accessed Nov 2022.
- 9.European Medicines Agency. ZOLGENSMA® (onasemnogene abeparvovec-xioi). Updated May 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/zolgensma. Accessed Nov 2022.
- 10.ClinicalTrials.gov. NCT02628743: A study to evaluate long term safety, tolerability, and effectiveness of olesoxime in patients with spinal muscular atrophy. Updated Aug 2019. https://clinicaltrials.gov/ct2/show/NCT02628743. Accessed Nov 2022.
- 11.ClinicalTrials.gov. NCT02240355: A study of RO6885247 in adult and pediatric patients with spinal muscular atrophy (MOONFISH). Updated April 2015. https://clinicaltrials.gov/ct2/show/NCT02240355. Accessed Nov 2022.
- 12.Muntoni F, Bertini E, Comi G, et al. Long-term follow-up of patients with type 2 and non-ambulant type 3 spinal muscular atrophy (SMA) treated with olesoxime in the OLEOS trial. Neuromuscul Disord. 2020;30(12):959–969. doi: 10.1016/j.nmd.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Ratni H, Ebeling M, Baird J, et al. Discovery of risdiplam, a selective survival of motor neuron-2 (SMN2) gene splicing modifier for the treatment of spinal muscular atrophy (SMA) J Med Chem. 2018;61(15):6501–6517. doi: 10.1021/acs.jmedchem.8b00741. [DOI] [PubMed] [Google Scholar]
- 14.Chaytow H, Faller KME, Huang Y-T, Gillingwater TH. Spinal muscular atrophy: from approved therapies to future therapeutic targets for personalized medicine. Cell Rep Med. 2021;2(7):100346. doi: 10.1016/j.xcrm.2021.100346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agosto C, Salamon E, Divisic A, et al. Do we always need to treat patients with spinal muscular atrophy? A personal view and experience. Orphanet J Rare Dis. 2021;16(1):78. doi: 10.1186/s13023-020-01593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johannsen J, Weiss D, Schlenker F, Groth M, Denecke J. Intrathecal administration of nusinersen in pediatric SMA patients with and without spine deformities: experiences and challenges over 3 years in a single center. Neuropediatrics. 2021;52(3):179–185. doi: 10.1055/s-0040-1718916. [DOI] [PubMed] [Google Scholar]
- 17.Stolte B, Totzeck A, Kizina K, et al. Feasibility and safety of intrathecal treatment with nusinersen in adult patients with spinal muscular atrophy. Ther Adv Neurol Disord. 2018;11:1756286418803246. doi: 10.1177/1756286418803246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moshe-Lilie O, Visser A, Chahin N, Ragole T, Dimitrova D, Karam C. Nusinersen in adult patients with spinal muscular atrophy: observations from a single center. Neurology. 2020;95(4):e413–e416. doi: 10.1212/WNL.0000000000009914. [DOI] [PubMed] [Google Scholar]
- 19.Harada Y, Rao VK, Arya K, et al. Combination molecular therapies for type 1 spinal muscular atrophy. Muscle Nerve. 2020;62(4):550–554. doi: 10.1002/mus.27034. [DOI] [PubMed] [Google Scholar]
- 20.Yang M, Georgieva M, Wu E, et al. Outcomes of single-agent onasemnogene abeparvovec or nusinersen, and of nusinersen switching to onasemnogene abeparvovec, in patients with spinal muscular atrophy: results of a provider survey in the United States (2346) Neurology. 2021;96(15):2346. [Google Scholar]
- 21.Mirea A, Shelby E-S, Axente M, et al. Combination therapy with nusinersen and onasemnogene abeparvovec-xioi in spinal muscular atrophy type I. J Clin Med. 2021;10(23):5540. doi: 10.3390/jcm10235540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oechsel KF, Cartwright MS. Combination therapy with onasemnogene and risdiplam in spinal muscular atrophy type 1. Muscle Nerve. 2021;64(4):487–490. doi: 10.1002/mus.27375. [DOI] [PubMed] [Google Scholar]
- 23.Kwon JM, Arya K, Kuntz N, et al. An expanded access program of risdiplam for patients with type 1 or 2 spinal muscular atrophy. Ann Clin Transl Neurol. 2022;9(6):810–818. doi: 10.1002/acn3.51560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinicaltrials.gov. NCT04488133: A study of nusinersen among participants with spinal muscular atrophy who received onasemnogene abeparvovec (RESPOND) 2022. https://clinicaltrials.gov/ct2/show/NCT04488133. Accessed Nov 2022.
- 25.Mercuri E, Deconinck N, Mazzone ES, et al. Safety and efficacy of once-daily risdiplam in type 2 and non-ambulant type 3 spinal muscular atrophy (SUNFISH part 2): a phase 3, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2022;21(1):42–52. doi: 10.1016/S1474-4422(21)00367-7. [DOI] [PubMed] [Google Scholar]
- 26.Day JW, Finkel RS, Chiriboga CA, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20(4):284–293. doi: 10.1016/S1474-4422(21)00001-6. [DOI] [PubMed] [Google Scholar]
- 27.Acsadi G, Crawford TO, Müller-Felber W, et al. Safety and efficacy of nusinersen in spinal muscular atrophy: the EMBRACE study. Muscle Nerve. 2021;63(5):668–677. doi: 10.1002/mus.27187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baranello G, Darras BT, Day JW, et al. Risdiplam in type 1 spinal muscular atrophy. N Engl J Med. 2021;384(10):915–923. doi: 10.1056/NEJMoa2009965. [DOI] [PubMed] [Google Scholar]
- 29.Darras BT, Masson R, Mazurkiewicz-Bełdzińska M, et al. Risdiplam-treated infants with type 1 spinal muscular atrophy versus historical controls. N Engl J Med. 2021;385(5):427–435. doi: 10.1056/NEJMoa2102047. [DOI] [PubMed] [Google Scholar]
- 30.Poirier A, Weetall M, Heinig K, et al. Risdiplam distributes and increases SMN protein in both the central nervous system and peripheral organs. Pharmacol Res Perspect. 2018;6:e00447. doi: 10.1002/prp2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paracha N, Hudson P, Mitchell S, Sutherland CS. Systematic literature review to assess the cost and resource use associated with spinal muscular atrophy management. Pharmacoeconomics. 2022;40(1):11–38. doi: 10.1007/s40273-021-01105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For eligible studies, qualified researchers may request access to individual patient level clinical data through a data request platform. The datasets generated during and/or analyzed during the current study are available via Vivli (https://vivli.org/ourmember/roche/). For up to date details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification.