Abstract
Recent studies manifest an increase of inflammatory diseases at an alarming rate due to gut microbiota dysbiosis, genetic and other environmental factors. Lactic acid bacteria (LAB) are known for their antimicrobial properties and their extensive applications in food and pharmaceutical industries. Cyclic peptides are receiving increased attention due to their remarkable stability to withstand variations in temperature and pH. LAB produces anti-inflammatory that can inhibit lipopolysaccharide-induced production of proinflammatory cytokines in macrophages. The structural backbones of cyclic peptides offer a promising approach for the treatment of chronic inflammatory conditions. The current review aims to present the overview of anti-inflammatory and wound healing properties of LAB-derived cyclic peptides.
Keywords: Lactic acid bacteria, Cyclic peptides, Anti-inflammatory, Wound healing, Nano-drug delivery system
Introduction
Lactic acid bacteria represent a cohort of microorganisms that are available in food sources, fermented foods, intestinal walls of humans and animals and have been consumed by humans without any side effects. Several selected strains, with well-defined characteristics, are included as probiotics in order to highlight the special advantages to consumers. Probiotic LAB has shown to aid in the prevention and treatment of diseases such as intestinal inflammation (LeBlanc et al. 2008). Inflammation is a consequent reaction of immune system to fight infections and heal wounds (Le Blanc et al. 2020). Numerous studies have shown that LAB strains and their associated peptides can modulate the host’s immune response by influencing the production of cytokines involved in the process of regulating and activating immune cells. They can exert immunomodulatory activities by stimulating the production of interleukin (IL)10 and/or lowering the levels of proinflammatory cytokines (Choi et al. 2019; La Manna et al. 2018; Dos Santos et al. 2016; Shadnoush et al. 2013). Studies have shown that the modulation of the host’s immune response is the main mechanism by which LAB implies benefits against inflammatory bowel diseases (IBD). Inflammatory bowel disease (IBD) is a group of disorders consisting of ulcerative colitis (UC) and Crohn’s disease (CD) (Tiwari 2022). LAB has proven to have known effects in treating paucities, UC, and CD (Florou-Paneri et al. 2013). The bacteriocins produced by LAB can repair the cell damage by modulating the expressions of anti-inflammatory cytokines (Hernández-González et al. 2021). Cyclic peptides exhibit better biological activity as compared to the other compounds due to their conformational rigidity. The rigidity of cyclic peptides decreases the entropy of Gibbs free energy, thereby allowing the enhanced binding toward target molecules. The cyclic structure renders them resistant to hydrolytic actions of enzymes such as exopeptidases and endopeptidases. Their structural rigidity, receptor selectivity, and biochemical stability also make them more membrane permeable as compared to the other counterparts. Novel cyclic peptides derived from LAB have sought attention due to their antibacterial activity making them applicable to use in the food and therapeutic industries. They have been extensively studied for other applications because of their heat stability, pH tolerance, and property to resist enzymatic actions. While most cyclic peptides derived from LAB are known to have antimicrobial properties against pathogenic microorganisms, certain peptides also have anti-inflammatory and wound healing properties.
The cyclic peptides from Lactobacillus reuteri inhibit enteropathogens like yeast, fungi, protozoa, and viruses and promote the growth of beneficial Gram-positive bacteria (Liu et al. 2020). They also play a major role in intestinal dysbiosis and immunopathogenesis of IBD. Cyclic peptides from Lacticaseibacillus casei, Lactiplantibacillus plantarum, Lacticaseibacillus rhamnosus, and Lactobacillus acidophilus competed with intestinal pathogens and lowered cholesterol level and improved IBD (Abdi et al. 2021). Recent studies reported that the topical application of formulations of cyclic LAB cyclic peptides can improve skin health and combat alteration of skin homeostasis. L. rhamnosus LR improves skin barrier function and is effective in improving the healing of infected chronic ischemic wound lesions (Venosi et al. 2019). Cyclic peptides from L. reuteri ATCC-55730 have been reported to have an anti-inflammatory effect on infected keratinocytes by reducing the transcription level of interleukin-8 (IL-8) and human-beta-defensin-2 (hBD) (Widyarman et al. 2018); L. plantarum K8 inhibits the tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ) expression (Jeon et al. 2016).
This review focuses on discussing the anti-inflammatory properties exhibited by LAB strains and the cyclic peptides derived from LAB. A summary about the nano-drug delivery systems of the peptides has also been included.
Pathogenesis of wound healing
Wound healing activity is a complicated and vital process that innervates the missing cellular structures and their respective tissue layers. Wounds can be classified into two types based on their time of healing, namely acute wounds and chronic wounds. Acute wounds restore themselves and heal normally by following a concise and efficient healing process, with the outcome of both cellular and tissue restoration. Chronic wounds do not heal properly or in a timely fashion and therefore do not progress through the normal healing stages (Martin and Nunan 2015). The process of wound healing can be distinguished into four overlapping, temporary phases namely (a) coagulation /hemostasis phase, (b) inflammatory phase, (c) proliferative phase, and (d) remodeling phase (Janis and Harrison 2016).
Hemostasis/coagulation phase
Hemostasis is the initial stage to appear following an injury. The damaged blood vessels form blood clot preventing blood loss from the site of damage. Platelet receptors interact with extracellular matrix (ECM) proteins (collagen, fibronectin) promoting adherence to the blood vessel wall. Platelet activation by thrombin triggers a conformational change and release of alpha and dense granules which reinforce coagulation. Platelet plug—an insoluble clot of fibrin, fibronectin, vitronectin, and thrombospondin—is formed to prevent bleeding. The platelet plug also shields the wound against bacterial invasion, providing a scaffold for the immune cells and cytokines to guide the early repair (Delavary et al. 2011). Platelets recruit immune cells by capturing them in the plug or by releasing chemokine attractants (Golebiewska and Poole 2015). Platelet also expresses a number of Toll-like receptors (TLRs) to regulate the production of antimicrobial peptides. Once sufficient clot is formed, coagulation stops, and platelet aggregation gets inhibited by prostacylin followed by inhibition of thrombin, coagulation factors V and VII by antithrombin III and activated protein C respectively (Mann 2003). The injured vessel wall is repaired by smooth muscle cells and endothelial cells that proliferate in response to PDGF (Kingsley et al. 2002).
Inflammatory phase
Innate inflammation is the primary defense against pathogenic wound invasion and is initiated by signals induced by the injury; damage-associated molecular patterns (DAMPs) released by the damaged tissue (Wilkinson and Hardman 2020). DAMPs and pathogen-associated molecular pattern (PAMP) activate mast cells, T cells, and macrophages by binding to suitable receptors and elicit inflammatory pathways (Chen and DiPietro 2017). This is followed by a subsequent release of proinflammatory cytokines and chemokines that result in an inflow of leukocytes to the site of injury (Martin and Leibovich 2005). Proinflammatory cytokines stimulate vasodilation which facilitates neutrophil and monocyte adhesion and diapedesis (Vestweber 2015). Chemoattractants like interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF- ∝), and bacterial lipopolysaccharides (LPS) recruit neutrophils into the wound (Kolaczkowska and Kubes 2013). Neutrophils release their own cytokines in response to proinflammatory signals and remove necrotic tissue and pathogens by phagocytosis (Segel et al. 2011). They also destroy pathogens using DNA coated with antimicrobial peptides (Brinkmann et al. 2004). In the absence of infection, the neutrophils are removed by innate clearance mechanisms like macrophage efferocytosis and apoptosis while the remaining neutrophils return to the circulation through transendothelial migration (Lin et al. 2011; Yoo and Huttenlocher 2011). Circulating monocytes are recruited after neutrophil and on reaching the site of injury these monocytes differentiate into macrophages (Rodero et al. 2014).
Macrophages induced by proinflammatory stimuli like LPS and interferon-gamma (IFN-γ) promote inflammation by releasing reactive oxygen species (ROS), inflammatory cytokines (IL-1, IL-6, and TNF-४), and growth factors (vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF)). These macrophages replace neutrophils as the main inflammatory mediators (Delavary et al. 2011). During the later stages of inflammation, in situ switching of existing macrophages to an anti-inflammatory phenotype is stimulated by miRNAs (Das et al. 2015), efferocytosis (Das et al. 2014), or changes in cytokines (Khallou-Laschet et al. 2010). Macrophages activated by alternate ways express anti-inflammatory cytokines (IL-4, IL-10, IL-13, IL-5) and arginase along with various growth factors that promote angiogenesis and re-epithelialization (Jetten et al. 2014; Barrientos et al. 2008; Eming et al. 2007). So the collective actions of macrophages promote scavenging of bacteria, proinflammatory cells, thereby promoting stabilization and remodeling of tissues and blood vessels.
Proliferative phase
The proliferative phase of wound healing begins with extensive activation of keratinocytes, fibroblasts, macrophages, and endothelial cells to promote wound closure, collagen deposition, and angiogenesis (Shaw and Martin 2016). Due to the changes in mechanical tension and electrical gradients, keratinocytes get activated and these activated keratinocytes undergo a partial epithelial-mesenchymal transition (Li et al. 2007a, b). Keratinocytes migrate across the wound to form epidermal layer (Wager and Leavesley 2015), and modulate their cell adhesion through protein kinase C-α (PKC-α)–mediated changes (Thomason et al. 2012) and ephrin (Eph)–mediated changes in adherens junctions (Nunan et al. 2015), resulting in the arrangement of their order in migrating epithelial sheets (Shaw and Martin 2016). The epidermal cells start growing from damaged appendages in shallow wounds. Specific stem cell compartments such as Lgr- and Lgr6-expressing cells from the hair follicle contribute to re-epithelialization (Joost et al. 2018). Keratinocytes, with the aid of matrix metalloproteinase (MMP-1 and MMP-9) and proteases (plasmin) (Rousselle et al. 2019), migrate through the necrotic tissue and debris of the wound bed. This migration terminates when keratinocytes from opposite edges meet and a thin epithelial layer is formed (Baum and Arpey 2005).
Fibroblasts are responsible for establishing the granulation tissue. These cells respond to signals from platelets, endothelial cells, and macrophages and either become profibrotic or differentiate into myofibroblasts (Li et al. 2007a). Fibroblast replaces the provisional matrix with a granulation tissue rich in fibronectin, immature collagens, and proteoglycans (Xue and Jackson 2015), with the aid of MMPs. This granulation tissue supports the formation of new blood vessels and deposition of mature ECM by acting as a scaffold for the migration and differentiation of wound cells.
The blood vessels formed during angiogenesis help to meet the metabolic requirements of the healing tissue. Angiogenesis is triggered by hypoxia which in turn triggers the expression of hypoxia-inducible factors (HIFs), cyclooxygenase 2 (COX 2), and release of VEGF (Huang et al. 2005). The endothelial cells in turn proliferate and migrate into the wound bed, forming new blood vessels to form a tubular network (Honnegowda et al. 2015). Macrophages aid in angiogenesis by producing MMPs to promote endothelial migration. Macrophages also guide the vessel’s tips together (Fantin et al. 2010) and phagocytosing vessels (Gurevich et al. 2018; Poche et al. 2015).
Remodeling phase
Remodeling phase starts with deposition of fibrin clot and collagen-I. Fibroblasts replace the initial fibrin clot with hyaluronan, fibronectin, and proteoglycans and form mature collagen fibrils (Darby et al. 2014). During the healing process, Collagen-III is replaced by collagen-I which increases the tensile strength of the scar. A major difference between the uninjured and healed skin is the orientation of collagen fibril. Collagen fibrils in uninjured skin have a basket weave orientation while the scarred tissue adopts a parallel bundle orientation (Young and McNaught 2011). A fine balance between collagen degradation and synthesis is achieved with regulation of matrix metalloproteinase (MMP), which cleave native collagens throughout the repair. Elastin reforms elastin fibers to retain skin elasticity (Darby et al. 2014; Duca et al. 2004). Myofibroblast contraction is facilitated by pseudopodial extensions, allowing cytoplasmic actin to bind to desmosomes, thereby binding to matrix fibrils and drawing the matrix together by a process termed as contracture. The wound healing response stops when macrophages, endothelial cells, and fibroblasts undergo apoptosis, leaving a scar (Larouche et al. 2018).
Pathogenesis of inflammation
Inflammation is a sequence of innate defense mechanisms that are generated by the vascular tissue against infectious and noninfectious stimuli such as damaged cells, irritants, or pathogens. The inflammatory processes are generally controlled and self-limiting while some progress as chronic inflammatory diseases (Cañedo-Dorantes and Cañedo-Ayala 2019; Mathews et al. 2015). Local and systemic inflammatory responses seek to eliminate the triggering stimuli while promoting tissue repair and healing processes, whereas, in the case of an infection, it tends to generate an immunological memory so that the host can respond more quickly and more effectively to a future encounter (Fullerton and Gilroy 2016).
An inflammatory response involves four components: inflammatory inducers, detecting sensors, downstream mediators, and affected target tissues (Freire and Van Dyke 2013). Following a wounding event, the main cells engaged in an inflammatory response are neutrophils, macrophages, mast cells, and lymphocytes. These cells are crucial in generating and stopping inflammation at the wound site, as well as facilitating subsequent stages of the healing process (Koh and DiPietro 2011). Cytokines are important mediators that help to coordinate the inflammation and repair processes in injured muscles. Proinflammatory cytokines not only initiate inflammation but also initiate repair, emphasizing the close relationship between inflammatory and repair/regeneration mechanisms (Oishi and Manabe 2018). Anti-inflammatory cytokines are molecules that are generally involved in the process that regulates the production of proinflammatory cytokine responses. Anti-inflammatory macrophages also release growth factors to promote re-epithelization, fibroplasia, and angiogenesis (Wilkinson and Hardman 2020). The collective behaviors of macrophages promote scavenging of bacteria and proinflammatory cytokines (Nosbaum et al. 2015). Cytokines that promote inflammation include IL1β, IL2, IL6, IL8, IL12, IL17, tumor necrosis factor (TNF)α, interferon (IFN) γ, and colony stimulating factor (CSF) 1, whereas anti-inflammatory cytokines include interleukin 4, interleukin 10, interleukin 13, and tumor growth factor TGF β (Ermakova 2018).
The type and severity of the inflammatory responses are determined by the nature of the inflammatory stimulus (bacterial, viral, or parasitic) as well as its persistence (Medzhitov 2010). Toll-like receptors (TLRs) expressed by tissue resident macrophages recognize bacterial pathogens. Inflammatory cytokines, chemokines, and proinflammatory lipid mediators like prostaglandins are released due to the binding of Toll-like receptors (Aderem and Ulevitch 2000).
There are 2 phases of inflammation that are acute and chronic phases, though there is overlap between these processes.
Acute inflammation
The acute inflammatory response is the initial response which is complicated but well-coordinated cascade of events involving cellular, molecular, and physiological modifications. It initiates from the production of soluble mediators which are vasoactive amines, complement, cytokines, chemokines, free radicals, and eicosanoids (prostaglandins) by resident cells, i.e., lymphocytes, tissue macrophages, dendritic cells, fibroblasts, endothelial cells, and mast cells in wounded or infected tissue (Fullerton and Gilroy 2016). These mediators elevate vascular permeability, allowing plasma containing antibodies and so many more soluble components, such as complement, which are essential for the humoral immune response (bacterial opsonization), and the dilution of harmful factors. Resident cell produces chemokines which establish gradients on the microvascular endothelium’s intraluminal surface, trapping and attracting neutrophils with the help of chemokine receptors. Activated neutrophils enter inflamed tissue via the endothelium and engage in intense antibacterial killing activities like degranulation and formation of reactive oxygen species via the oxidative burst. Monocytes follow neutrophils and link the adaptive and innate immune responses, deciding whether or not the damage can be healed with or without the support and help of the adaptive immune system (Headland and Norling 2015).
This acute cellular phase is enough to repair any injury depending upon the severity of the damage. Chronic inflammation can occur because of the prolonged exposure to inflammatory stimulus or an inappropriate response to self-molecules. During this phase, active immune cell populations can lead to the transition in to a mononuclear phenotype, causing tissue injury and fibrosis (Germolec et al. 2018).
Chronic inflammation
Inflammation that fails to heal for several months or years is referred to as chronic inflammation. In chronic inflammation, plasma cells, lymphocytes, and macrophages predominate over neutrophils, which predominate in acute inflammation (Pahwa et al. 2018). When inflammatory responses become chronic, cell mutation and proliferation can occur, often creating an environment favorable to cancer development (Singh et al. 2019).
Chronic inflammation has been linked to a wide range of diseases and disorders such as cancer, cardiovascular disease, diabetes, autoimmune diseases, arthritis, asthma, pulmonary diseases, atherosclerosis, Alzheimer’s disease, and aging-related conditions. Chronic inflammation has been linked to invasion, promotion, proliferation, cellular transformation, survival, angiogenesis, and metastasis, among other steps in tumorigenesis (Germolec et al. 2018; Pahwa et al. 2018; Singh et al. 2019).
Anti-inflammatory properties exhibited by strains of lactic acid bacteria
Notable characteristics such as wound healing and anti-inflammatory properties have been exhibited by Lactobacillus strains. Various mechanisms related to it have been discussed briefly.
Lactiplantibacillus plantarum
L. plantarum A41 in combination with L. fermentum SRK414 has the ability to downregulate the levels of cytokines that promote inflammation like TNFα, IL1b, and IL8. Furthermore, they were able to inhibit the expression of inflammatory mediators induced by LPS-stimulated inflammation (Lee and Kim 2020). When taken in conjunction with training, L. plantarum PS128 was able to decrease the proinflammatory cytokines like interleukin 6, 8, and TNFα and also showed to increase the production of anti-inflammatory cytokines (IL10) in athletes after intense exercise (Huang et al. 2019). The anti-inflammatory effect of L. plantarum Lp62 was first tested on HT 29 cell lines with the disease-causing microorganism Salmonella typhi 6539 acting as an inflammatory stimulus. L. plantarum Lp62 was shown to reduce the IL 8 production by S. typhi–stimulated HT 29 cells, thus showing its anti-inflammatory properties (Ferreira Dos Santos et al. 2016). In the mice model induced with colitis, L. plantarum Lp91 induced the inhibition of TNFα and cycloxygenase-2 (COX2). It was also found that anti-inflammatory markers such as IL10, COX1, IL4, and IL6 were drastically increased in mice that were induced with L. plantarum Lp91 (Duary et al. 2012). In addition, L. plantarum ZS2058 along with L. rhamnosus GG (LGG) pretreated in mice were able to significantly reduce the CRP (C-reactive protein) levels when infected with Salmonella infection. The reduced levels of CRP indicate that these probiotic strains alleviate the inflammatory responses (Liu et al. 2019a, b). L. plantarum has been shown to reduce the wound area in mice and also helps in accelerating the wound healing process (Nasrabadi et al. 2011). Studies showed that the application of L. plantarum gel not only elevated the wound healing process by elevating the synthesis of collagen but also increased the number of fibroblasts and TGF βLP levels, and reduced infection risks in diabetic rats. This shows that L. plantarum can be used in delayed wound healing (Salaran et al. 2019).
Lactococcus lactis
There are many research studies on the wound healing and anti-inflammatory activity of L. lactis. L. lactis NK34 has been seen inhibiting the nitric oxide production and proinflammatory cytokines like TNFα, interleukin 18, and COX2 in LPS (lipopolysaccharide)–induced RAW 264.7 cell lines, thereby possessing anti-inflammatory activity (Han et al. 2015). A recombinant strain of L. lactis with IL 35 was used to suppress collagen-induced arthritis, hence reducing IL 17 and IFN and increasing the production of proinflammatory cytokine IL 10 in mice (Maddaloni et al. 2018). Furthermore, L. lactis MG1363 FnBPA + not only enhanced the IL 10 production in mice but also reduced the disease severity and lowered the production of proinflammatory cytokines (Zurita-Turk et al. 2020). Mice fed with L. lactis NCDO 2118 had shown to reduce the IL 1β–induced IL 8 secretion and hence it exhibits potential anti-inflammatory effects (Luerce et al. 2014). In addition, L. lactis ML2018, in RAW264.7 cells, inhibited the nitric oxide release and the expression of inflammation induced by LPS. The in vivo anti-inflammation effects of L. lactis ML2018 were studied in an animal model of colitis induced by dextran sodium sulfate (DSS) (Liu et al. 2019a, b). L. lactis also exhibits wound healing properties. Studies show that Lactococcus in a heparin poloxamer hydrogel can be designed to bioengineer the wound microenvironment and boost angiogenesis which could further be helpful in diabetic wound healing (Lu et al. 2021). Furthermore, L. lactis thermosensitive hydrogel was able to heal the wound faster and also significantly helped to reduce the wounded area of inflammation in mice (Lu et al. 2020).
Lacticaseibacillus rhamnosus
L. rhamnosus is one of the strains with surface piliation (SpaCBA). L. rhamnosus was recombinantly engineered to generate native pili where it showed that SpaCBA pilus was able to modulate the synthesis of pro- and anti-inflammatory cytokines in dendritic cells procured from human monocytes (Ossowski et al. 2013). Studies show that SpaCBA regulates anti-inflammatory effects in murine macrophage RAW cell line by inducing IL10 mRNA and decreasing the IL6 mRNA (Vargas García et al. 2015). L. rhamnosus GG (LGG) has been demonstrated to be a promising tool in the treatment of chronic inflammation. Cancer may develop as a result of chronic inflammation caused by IL8. LGG has been shown to be successful in declining the manufacture of IL8 generated by Helicobacter pylori as well as the adherence on cells of gastric adenocarcinoma (Banna et al. 2017). Furthermore, L. rhamnosus LS 8 along with L. crustorum MN047 was shown to alleviate body weight gain and insulin resistance and obstruct proinflammatory cytokines like TNFα, IL1b, and IL6 in adipose tissue of mice treated with the two strains (Wang et al. 2020). In addition, sea buckthorn (Hippophae rhamnoides L.), malt, along with L. rhamnosus GG (ATCC 53103) decreased the LPS-induced inflammation in zebrafish. The findings revealed that there was a considerable decrease in the expression of proinflammatory cytokines like TNFα, Interleukin 1b, with high expression of IL10 (Sireswar et al. 2020). According to the studies, L. rhamnosus strain LDTM 7511 showed to decrease the expression of proinflammatory cytokines in the dextran sulfate sodium–induced colitis model of mice which can be potentially used in the treatment of inflammatory bowel disease (Yeo et al. 2020). Furthermore, the samples from ulcerative colitis patients incubated with L. rhamnosus GG showed lower expression of TNF and IL17 (Pagnini et al. 2018). L. rhamnosus also showed certain wound-healing properties. Studies showed that wounds treated with L. rhamnosus enhance skin wound closure and scar reduction, in addition to reducing inflammation and fibrogenesis thereby improving angiogenesis in the wounded skin (Moreira et al. 2021). Recently, an expedited gap closure using human keratinocytes in vitro using lysates of L. rhamnosus was observed.
Enterococcus
Enterococcus faecalis from infants was shown to inhibit the proinflammatory cytokine secretion, particularly IL8, via the JNK and p38 pathways (Wang et al. 2014). Enterococcus faecium HDRsEf1 strain could help decrease the expression of proinflammatory factors IL1, IL6, IL8, IL12p35, IL17, and TNFα while having no effect on anti-inflammatory factors IL10, PPAR, and TSLP in HT 29 cell lines (Tian et al. 2016). Studies also showed that E. faecium HDRsEf1 (Ef1) modulate the release of interleukin 8 by IPEC J2 cells Ef1 and its cell free supernatants were found to be capable of protecting enterocytes from an inflammatory condition. Furthermore, E. faecalis EF 2001 treatment in mice reduced the expression of various cytokines, consisting of cyclooxygenase, interferon α, interleukin 1, and IL6 in ulcerated bowel induced with DNBS and also inhibited DNBS-induced colonic tissue injury (Choi et al. 2019). In addition, E. faecalis was able to decrease the IL8 secretion and also increase the secretion of TGFβ in human intestinal Caco2, HT29, and HCT116. It was also found that this strain was able to modulate the inflammatory reactions via TLR3, TLR4, TLR9, and TRAF6 (Wang et al. 2008). In LPS-stimulated macrophage cell lines, E. faecium, Lactobacillus rhamnosus GG MTCC 1408, and LCS demonstrated a substantial anti-inflammatory effect by downregulating TNF aipha production and increasing the expression of IL 10 levels (Divyashri et al. 2015). Enterococcus also exhibits certain wound-healing properties. Studies found that treating skin wounds with the heat killed KH2 strain of E. faecalis elevated wound healing by increasing the production of inflammatory cytokines, which may be involved in the production of growth factors. Furthermore, increased growth factor production may hasten re-epithelialization, granulation tissue formation, and angiogenesis (Tanno et al. 2021).
Lactobacillus casei
L. casei 393 has been demonstrated decreasing the production of proinflammatory cytokines such as interleukin 10, interleukin 6, and TNF α in mice models induced with DMH (Casas-Solís et al. 2020). L. casei Zhang also showed to decrease the levels of proinflammatory cytokines in rat models, hence reducing inflammation (Wang et al. 2016). Furthermore, Lacticaseibacillus casei DG was able to decrease the levels of proinflammatory cytokines and upregulate the production of anti-inflammatory cytokines like interleukin 10 in an ex vivo model of irritable bowel syndrome (Compare et al. 2017). L. casei BL23 possessed immunomodulatory effects by downregulating the proinflammatory cytokine IL22 (Jacouton et al. 2017). In a mouse model with a high fat diet, L. casei CRL 431 was shown to decrease the expression of interleukin 6 and 17, and TNFα which are essential proinflammatory cytokines (Novotny Núñez et al. 2015) L. casei also possessed certain wound-healing properties. L. casei was shown to reduce inflammation and also increased the fibroblast cells, which further accelerated the wound healing process in an infection caused by Pseudomonas aeruginosa (Abootaleb et al. 2021). Studies show that L. casei was able to heal ulcers on the skin of diabetic rats, thereby reducing the area of the skin (Majid et al. 2016). L. casei shirota increased the number of fibroblasts which aided in accelerating the healing process of traumatic ulcers in rats (Kusumaningsih et al. 2021). Various other strains of lactic acid bacteria with anti-inflammatory property are shown in Table 1.
Table 1.
Anti-inflammatory properties of Lactic acid bacterial strains
| Strains | Anti-inflammatory properties | References |
|---|---|---|
| Ligilactobacillus salivarius ssp. salivarius CECT5713 |
Inflamed tissue recovery in rat colitis TNBS model, Improvement in the production of proinflammatory cytokines and iNOS (inducible NO synthase) expression |
(Peran et al. 2005) |
| Lactobacillus pentosus |
Alleviate ulcerative colonic inflammation caused by DSS in murine model Beneficial for regulation of intestinal Immunity Encourages the growth of beneficial bacterial metabolites like pantothenic acid |
(Ma et al. 2020) |
| Lactobacillus curvatus WiKim38 |
Increased IL 10 levels in bone marrow–derived dendritic cells Activation of NF-κB and ERK |
(Ma et al. 2020) |
| Pediococcus acidilactici | Suppressed the IL-8 production by Enterococcus faecalis in human intestinal epithelial cells | (Yoon and Kang 2020) |
| Bacillus amyloliquefaciens SCGB1 | Upregulated IL-10 production and downregulated IL-6 production in macrophage cell line induced with LPS | (Kang et al. 2021) |
| Lacticaseibacillus casei | Upregulation of anti-inflammatory cytokines and downregulation of proinflammatory cytokines on shigella induced human epithelial cells | (Tien et al. 2006) |
| Lactobacillus gasseri NCi501 | Reduced inflammation in IL-10 deficient mice | (Carroll et al. 2007) |
iNOS inducible NO synthase, NF-KB nuclear factor kappa light chain enhancer of activated B cells, ERK extracellular signal regulated kinase pathway, IL interleukin, LPS lipopolysaccharide, TNBS trinitrobenzenesulfonic acid
Lactic acid bacteria–derived cyclic peptides
Lactic acid bacteria are considered to be the most widely researched microorganisms over several years (Pasolli et al. 2020). Lactic acid bacteria are classified according to their morphological, metabolic, and physiological characteristics. Lactobacillus, Enterococcus, Lactococcus, Leuconostoc, Pediococcus, and Streptococcus comprise the core LAB group. Lactobacillus species are Gram positive, non-motile, non-spore forming, rod-shaped non-respiring (catalase negative), auxotrophic, aciduric, facultative anaerobes that release lactic acid as a major end product of carbohydrate fermentation (Bai and Ji 2017). They are widely used in the food industry for the production of fermented products and also as a food safety tool. Some LAB strains can produce bacteriocins, which are polypeptides exhibiting antimicrobial activity against a specific class of bacteria of the same or different species. Bacteriocins, in particular, are very desirable to the food sector because they do not influence the flavor or fragrance of the end products (Moraes et al. 2010). The bacteriocins from lactic acid bacteria are commonly divided into three groups based on their structure and properties as discussed in Table 2. They include the following: class I lantibiotics which are very small in size, i.e., > 5 kDa, include lactocin and nisin; class II non-lantibiotics are also small, i.e., < 10 kDa, cationic, heat stable, unmodified bacteriocins, comprising a double glycine leader being hydrophobic peptides, with plantaricin A, pediocin PA1, lactococcin G, and leucocin A as an example of class IIa and class IIb, respectively; and class III are larger in size, i.e., > 30 kDa, heat labile bacteriocins with helveticin J and enterolysin as constitutive (Nes and Holo 2000). Detection, purification, and characterization of several bacteriocins from lactic acid bacteria are done. However, the only bacteriocin in use is Nisin which is produced by Lactococcus lactis. It has a molecular weight of 3500 Da and consists of unusual amino acids. The utilization of cyclic peptides derived from LAB with probiotic potential has been demonstrated to be an effective way to improve one’s well-being. Cyclic peptides are immune to proteolytic enzymes as well as severe physiological stimuli with changes in pH and temperature. The LAB cyclic peptides have been known to be successful against gastrointestinal infections such as Helicobacter pylori, E. coli, and Salmonella species (Silpa and Rupachandra 2020). Cyclic peptides have a wide range of applications in the treatment of various diseases and disorders.
Table 2.
Different Classes of Bacteriocins derived from lactic acid bacteria and their characteristic features
| Class | Subclasses | Characteristic Features | Cyclic peptides | Reference |
|---|---|---|---|---|
| I lantibiotic | Lanthipeptides—Ia; globular and inflexible bacteriocins—IIb; sactpeptides—Iic | Small (< 5 kDa) peptides consisting of lanthionine and beta-methyllanthionine. | Nisin Z and Q, Nukacin ISK-1, Subtilin, Mersacidin | (Ahmad et al. 2017; Mokoena 2017) |
| II non-lantibiotic |
IIa—peptides like pediocin IIb two-peptides IIc leaderless Non-pediocin like single peptide—IId |
Small heat-stable, non-lanthionine-containing bacteriocins. Heat stable peptides active mainly against Listeria Monocytogenes. Two-peptide bacteriocins, Circular bacteriocins. |
Enterocin NRK-5- 3c, Enterocin A, Munditicin, Leucocin A, Lactococcin q, enterocin, nrk-5- 3az, Plantaricin Lactocyclin q, leucocyclin q |
(Alvarez-Sieiro et al. 2016; Bédard and Biron 2018; Borreoro et al. 2011; Fimland et al. 2005; Nissen-Meyer et al. 2010; Oppegard et al. 2007; Perez et al. 2018; Sawa et al. 2009) |
| III |
Bacteriolysins-IIIa, Nonlutic-IIIb |
High mol. weight bacteriocins > 30 kDa which are thermolabile and unmodified peptides. Destroys target cells by disrupting plasma membrane potential. |
Lysostaphin, Helveticin J |
(Sun et al. 2018) |
iNOS inducible NO synthase, NF-KB nuclear factor kappa light chain enhancer of activated B cells, ERK extracellular signal regulated kinase pathway, IL interleukin, LPS lipopolysaccharide
Anti-inflammatory cyclic peptides derived from LAB
Cyclic peptides from LAB are reported to be primarily active against closely related organisms (Zacharof and Lovitt 2012). Their application in controlling human pathogens is promising with a suitable mode of delivery. The emerging applications of LAB peptides in adjuvant therapy, vaccine development, and bioprotective agents are relevant in prevention of infection and amelioration (Mokoena 2017). They are typically composed of two or more amino acids linked together by peptide bonds and depict a broad spectrum of biological activities like antibacterial, antifungal, anti-inflammation, and so on (Zhang et al. 2019). Peptides that can mimic the activity of mediators involved in inflammation-related diseases are gaining popularity these days.
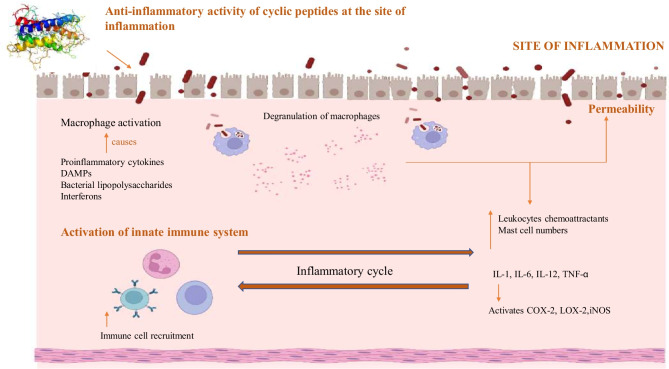
Following the injury, the vessels constrict rapidly to reduce bleeding from the wound. Platelet plug is formed which releases growth factors that are important in the phases of healing. DAMPs, hydrogen peroxide, LPS, and interferons activate macrophages at the site of injury. In the early stages of wound healing, macrophages are proinflammatory, expressing TNF-α and IL-6 and IL-1β. Proinflammatory macrophages recognize and engulf pathogens into phagosomes, rapidly killing them. Proinflammatory macrophages also recruit MMPs which aid in migration of new keratinocytes. During the later stages of inflammation, there is a transition of proinflammatory macrophages to anti-inflammatory macrophages which contribute to new vessel formation and re-epithelialization (Rodrigues et al. 2019). The cyclic peptide entering the site of injury helps in the downregulation of various proinflammatory cytokines. They also promote macrophage recruiting to the margin of wound area. Various peptides can also elevate the expression of transforming growth factor (TGF). Cyclic peptides can also promote wound contraction, expression of alpha-smooth muscle actin (α-SMA), and differentiation of fibroblast to myofibroblasts (Tang et al. 2014). Figure 1 depicts the mechanism by which cyclic peptides act upon its exposure toward the site of inflammation. Although some of them are found in nature, the majority of them are encoded in the structure of related proteins and can be released through various processes (La Manna et al. 2018). Lactic acid bacteria can be found in many sources. Cyclic peptides derived from LAB act as an immune modulating agent and showcase anti-inflammatory properties in affected tissues by promoting a reduction in the proinflammatory cytokines and a rise in the anti-inflammatory cytokines (Hernández-González et al. 2021).
Fig. 1.
Schematic representation of anti-inflammatory activity of cyclic peptides at the site of inflammation. Abbreviations used: TNFα, tumor necrosis factor α; DAMP, damage-associated molecular pattern; IL 1, 6, 12, interleukin 1, 6, 12; COX 2, cyclooxygenase 2; LOX 2, lipoxygenase 2; iNOS, inducible nitric oxide synthase
Nisin
Nisin is a lantibiotic produced by Gram-positive bacteria Lactococcus and Streptococcus species (Shin et al. 2016). It is an antibacterial peptide that consists of 34 amino acid residues and has a wide range of applications (Kitagawa et al. 2019). The class I lantibiotic consists of five methyl lanthionine rings that require various post-translational modifications that are then introduced in the precursor peptide (Reiners et al. 2020). Different variants of Nisin have been identified. Some of the natural variants are Nisin A, Z, Q, and U (Piper et al. 2011). There are many research studies on the anti-inflammatory and wound-healing activities of Nisin. Recent findings suggested that Nisin along with kanamycin helped in reducing the cytokines involved in inflammation and accelerated the production of anti-inflammatory cytokines in rats, which can be used in the treatment of Staphylococcus-induced endometriosis (Jia et al. 2019). Nisin along with different licorice polyphenols has been shown to inhibit NF κβ activation induced by E. faecalis implying that these combinations have anti-inflammatory properties (Grenier et al. 2020). Nisin Z modulated innate immune responses by inducing chemokine synthesis and suppressing LPS induced proinflammatory cytokines (Kindrachuk et al. 2013). In addition, Nisin can also be used in wound dressings. Nisin A has been proven to have immunomodulatory effects and also enhances the wound-healing process in human keratinocytes, human umbilical vein in vitro and porcine model ex vivo as it enhances skin cell mobility, impedes the effect of lipopolysaccharide, proinflammatory cytokines, and inhibits bacterial growth (Mouritzen et al. 2019). Studies also showed that Nisin preconditioned with mesenchymal stem cells on PLLA (poly-(l-lactide)-acid) scaffold helped in reducing the expression of inflammatory genes, making it useful in wound healing (Karimi et al. 2021).
Nisin is also reported to be effective in the treatment of various infections when administered via subcutaneous, intraperitoneal, intranasal and intravenous studies (Campion et al. 2013; Jabes et al. 2011; De Kwaadsteniet et al. 2009; Kruszewska et al. 2004). The half-life of nisin is reported to be 7–10 min due to the rapid adsorption on to surface of the microbiota within the intestine (Dobson et al. 2011). Enhanced nisin could be relevant if the conventional antibiotic therapy fails or safety issues predominate. Hagiwara et al. (2010) have established the safety of nisin in 90-day oral toxicity study involving rats where the rats were fed a diet containing nisin. No adverse effects were observed even at a dosage of 0.13mg/kg body weight/day. Reports also indicate the absence of hemolytic activity even at concentrations of 500 mg/L. S. aureus–infected mice treated with Nisin showed a rapid decrease in the bioluminescence due to the rapid cell lysis (Van Staden et al. 2016). Reports suggest that nisin inhibits cell wall biosynthesis by binding to cell wall precursor thereby disrupting synthesis of peptidoglycan. Binding of nisin leads to pore formation resulting in the leakage of cellular components (Van Staden 2012). A significant reduction of Clostridium difficille after nisin treatment was reported in a human colon model (Le Lay et al. 2015).
Plantaricin
Plantaricin is a bacteriocin produced by Lactobacillus plantarum that is known to possess anti-microbial activity. The major types of plantaricin are A, C, W, and S that first began as a precursor which consists of a double glycine moiety, later synthesized by L. plantarum via the PlnE and PlnF genes. The PlnG and PlnH proteins then export and process these peptides (Kareem and Razavi 2020). Various other strains of plantaricin have also been identified and are known to have great importance in different areas. There are many research studies on the anti-inflammatory and wound healing activities of Plantaricin. Plantaricin NC8 αβ showed to suppress the proinflammatory mediators of S. aureus and also increased the keratinocyte’s inflammatory responses when infected with S. aureus infection (Musa et al. 2021). L. plantarum strains have shown potential in the treatment of irritable bowel syndrome using probiotics to decrease abdominal discomfort and gas (Li et al. 2020). They also dampen inflammatory reactions in the host’s gut by modulating the immune response, improving the barrier function of the epithelium (Hirao et al. 2014) and reducing the translocation of native microbes (Pavan et al. 2010). L. plantarum ability to synthesize PlnEFI contributes anti-inflammatory potential. The TNBS persuaded wild type strain NCIMB8826 R will not be restricted to colitis. The pathogenesis of Crohn’s disease in people is comparable to that of severe colitis in acute TNBS model (Scheiffele and Fuss 2002). Evidence suggests that a single colony isolation of NCIMB8826 (L. plantarum WCFS1) notably reduced TNBS-induced inflammation (Yin et al. 2018) yet no confirmation is established for disease attenuation, non-significant reduction in inflammatory responses. L. plantarum NCIMB8826 R and LM0419 were detected in equal amounts in the stool and in the appendix contents of mice according to 16 s rRNA sequencing, q PCR. This indicates that in both healthy and diseased GI tracts, PlnEFI is not a primary determinant of L. plantarum (Ilott et al. 2015). Native strains of lactobacilli are able to withstand inflammatory diseases in the gastrointestinal tract, including dextran sulfate sodium–induced colitis (Tachon et al. 2014) and Salmonella infection. L. plantarum WCFS1 showed promising effects in gut microbiota whereas both L. plantarum NCIMB8826 R and LM0419 showed minor effects on the bacterial composition of the fecal and appendix (Yin et al. 2018). An abundance of certain intestinal bacterial groups has been detected for other class IIb bacteriocin producing, lactic acid bacteria including other L. plantarum strains, L. salivarus, Lactobacillus sake, Pediococcus acidilactici, Enterococcus faecium, and Lactococcus garveiae (Feller et al. 2007). Many studies have repeatedly found a substantial link between Crohn’s disease and mycobacterium, which can be blocked by specific bacteriocins like L. plantarum WCFS1 (Singh et al. 2018). The biosynthesis of plantaricin is a distinctive feature that is pertinent to host microbe interaction in the digestive tract. Synthesis of PlnEFI produced by plantaricin is proven to be highly beneficial in maintaining a healthy GI tract (Ilott et al. 2015).
The mechanism of action of plantaricins on conditions like IBD is not well explored. The in vivo studies report that L. plantarum can induce secretion of IL-10 in splenocytes and mesentric lymphocytes, and blocks proinflammatory cytokine expression (IL-1β, IL-6, TNF-α, COX-2 etc.). Another report suggests that L. plantarum decreased expression of IL-1a and IL-8 while increasing the expression of IFN-c and IL-10 (Le and Yang 2018). With respect to dosages, the most optimal dose for plantaricins like plantaricin E and plantaricin F was reported to be 250 mg/kg/BMW and 500 mg/kg/BMW respectively. The plantaricins were found to maintain leukocyte level and reduce mononuclear infiltration within the intestinal track (Hanny et al. 2019). Plantaricin A was also reported to induce keratinocyte and fibroblast migration and differentiation and collagen formation eventually leading to rapid wound healing. It also shortened the inflammatory phase of wound healing (Heydari et al. 2011), and complete re-epithelization of the wound was observed within 12 days in male Wistar rats (Moysidis et al. 2022).
Enterocin
Enterococci are a Gram-positive, facultative anaerobic-based LAB that produces a bacteriocin named Enterocin (Szabóová et al. 2011). Different types of Enterocin have been identified; some include Enterocin A, B, Q, P, and RJ (Fimland et al. 2005). Recent studies showed that the TNF α levels were reduced by enterocin OE 342 from LPS-stimulated PBMC cells by increasing the percentage of IFN γ to 72.2% post-treatment (Franz et al. 2007). LAB modify the expression of different cytokines by increasing phagocytosis thus preventing inflammatory responses in the intestine. Also in the mouse experimental model, they restore the synthesis of IgA and replace Th1/Th2 balance (Al-Fakharany et al. 2018). Apart from being immunomodulators, this strain of LAB has close relationships with human infections. Enterocin has been shown to inhibit bacteria from wounds and while also assisting in physiological wound healing (Fang et al. 2000).
Salivaricin
Lactobacillus salivarius UC118 is known for its probiotic properties and constitution of intestinal microbiota (Riboulet-Bisson et al. 2012). It is a type II bacteriocin that normally occurs in human oral cavities, vagina, and intestine. Majority of L. salivarius isolates belonged to the bacteriocin producers of subclasses IIa, IIb, and IId (O’Shea et al. 2011). A probiotic mixture containing LAB has been shown to improve intestinal epithelial integrity and protection, in case of inflammatory luminal elements which are food and bacteria derived. Few strains also have immunomodulatory action. During recent research, CECT5713 a strain of L. salivarius, obtained from breast milk, was shown to promote host immunity by generating IL 10 and specific immunoglobulins, and also shows a rise in monocyte count and natural killer cells (Sierra et al. 2010). Additional evidence suggests Lactobacillus salivarius B1 enhances Interleukin 6 gene expression and population of immunocompetent cells in the intestines of pigs (Zhang et al. 2011). Probiotic microorganisms such as L. acidophilus, L. rhamnosus, and Lactobacillus salivarius DPC6005 can incite changes in host microbiota activity or composition since few microbial colonies are related to intestinal disorders such as inflammatory bowel disease (IBD) and hemorrhoids (Ley 2010). L. salivarius UCC118 exhibits probiotic properties and provides protection against infections from Salmonella typhimurium UK1 and LO28 and Listeria monocytogenes EGDe due to the production of the bacteriocin Abp118 (Corr et al. 2007). Several strains of Lactobacilli have been identified which can modify host immunity (Walsh et al. 2008). Research conducted on L. salivarius UCC118 concluded that the strain had no effect on cytokine levels of IL 8 and IL 10 in pigs, but there is a possibility in the development of various cytokines like interleukin 6 and 12 by modulation of UCC118 as seen by other L. salivarius strain (Peran et al. 2005; Riboulet-Bisson et al. 2012). L. salivarius and its derived peptide, salivaricin LHM, are of therapeutic use by stimulating the immune system through overproduction of proinflammatory cytokines like interleukin 10 and interleukin 4. Lactobacillus salivarius and Salivaricin LHM exhibit antimicrobial activity as well as immunomodulatory properties by increasing the production of proinflammatory cytokines (Mahdi et al. 2019).
Nano delivery systems of LAB-derived cyclic peptides for treating inflammatory diseases
Because of their small dimensions and flexible surface chemistry, nanoparticulate (NP) drug delivery devices are of special interest among inflammation targeting methods in IBD. Recently, new drug delivery methods for IBD therapy that selectively targets the site of inflammation have been developed (Zhang et al. 2017). Nanoparticle-mediated drug delivery systems have been widely used to treat chronic inflammatory diseases such as IBD (Yang and Merlin 2019). A drug that is nanoparticle loaded may have the same/better efficacy at a smaller dose than the same drug rendered in a traditional formulation (Melero et al. 2017). Nanoparticles may enhance the pharmacokinetics of the drug particles, reducing toxicity and dosing frequency. The physiological changes indicated by the inflamed tissues are often the target approaches employed for NP-based drug delivery in the treatment of IBD (Dahan et al. 2010). These short peptide NPs are efficient in conquering several limitations associated with the conventional treatment methods by reducing adverse health effects and increasing drug accumulation at the tumor site (Saravanan and Barabadi 2021), thereby resulting in enhanced efficacy of the cancer therapeutics (Rayatpour and Javan 2021). Studies showed that Nisin was electro spun into nano fibers which then significantly reduced the S. aureus infections in murine models and also helped in accelerating the wound healing process (Jia et al. 2019). We have mainly discussed the potential examples of nano delivery systems of LAB-derived cyclic peptides in Table 3.
Table 3.
Examples of bacteriocins formulated using nano technological approach with its applications
| Cyclic peptide | Isolated strain | Method of purification | Nano-drug delivery system series | Type of nanocarrier | Application in nano-delivery system | References |
|---|---|---|---|---|---|---|
| Nisin | Lactococcus lactis |
Capture method; Cation-exchange Chromatography |
Nanofiber electrospinning with metallic nanoparticles | Silver nanoparticles—PEO 50–PDLLA 50 nano-fibers | (i) Production of anti-microbial wound dressing material to target against S. aureus skin infections | (Heunis et al. 2013) |
| Enterocin FH-99 | Enterococcus spp. | Nanoencapsulation method | Metallic nanoparticles | Silver nanoparticles—Enterocin-capped-silver nanoparticles (EnSNPs) |
(i) Proinflammatory activities in burnt wounds, meshed skin grafts (ii) Activation of apoptosis in inflammatory cells of the dermis |
(Bhol and Schechter 2005; Fan and Bard 2002; Nadworny et al. 2008) |
|
(iii) Decline in the expression of IL-8 and Transforming Growth Factor (TGF)- β |
||||||
| Plantaricin 432 | Lactobacillus plantarum 432 | Amberlite XAD 2, cation exchange chromatography, gel chromatography | (i) Nanofiber electrospinning; metallic nanoparticles | (i) Gold nanoparticles—solid lipid nanoparticles (SLNPs) | (i) Bacterial inhibition of Lactococcus murei and E. coli | (Gomashe and Dharmik 2012; Heunis et al. 2010) |
| (ii) Polymeric nanoparticles | (ii) Plantaricin encapsulated into polyethylene oxide (PEO) nanofibers | (ii) Inhibition of Lactobacillus sakei | ||||
| Lacticin 3147 | Lactococcus lactis DPC6577 | Amberlite XAD 16 N resin, C18 solid phase extraction, reversed phase HPLC; confirmed by MALDI-TOF mass spectrometry | Solid lipid nanoparticle delivery system (SLNPs) | Microemulsion templating technique |
(i) Alternative for treating topical infected wounds (ii) Defend against bacterial infections in colon |
(Rea et al. 2007) |
|
(iii) Protection of LTNA1 and LTNA2 from digestive enzymes |
||||||
| Bacteriocin produced by Lactobacillus acidophilic CH1 | Lactobacillus gasseri | Ammonium sulfate precipitation, sequential cation exchange on carboxymethylcellulose and reverse phase chromatography | Metallic nanoparticles | Gold nanoparticles | Increased the anti-microsporidial effect without significant cell toxicity | (Fahim et al. 2016; Mossallam et al. 2014) |
PEO poly (ethylene oxide), PDLLA poly (D,L-lactide), MALDI-TOF matrix-assisted laser desorption /ionization-time of flight, LTNA1 and LTNA2 lacticin A1 and lacticin A2
Conclusion and future prospects
This review provides an overview of the current knowledge on anti-inflammatory and wound-healing properties of lactic acid bacteria and its derived peptides which is very essential in combating modern day diseases. Certain LAB strains and their cyclic peptides downregulated the expression of proinflammatory cytokines and upregulated the production of anti-inflammatory cytokines respectively thereby aiding in the wound-healing process.
The rise in pathogenic organisms and mortality rate remains a major concern around the world, necessitating the development of a new approach for the prevention and treatment of infectious diseases. Lactic acid Bacteria has attracted a lot of attention due to their unique properties and ability to produce bacteriocins. They have the ability to reduce the perpetual demand for natural products and functional food which appears to be an emerging field of research. Bacteriocins have diverse applications in various fields which have led them to gain a lot of importance in the scientific world and are also considered to provide therapeutic properties to cure inflammation-related diseases like ulcerative colitis. Previous studies have explored the potential of LAB strains in food industries without harmful effects. Cyclic peptides derived from lactic acid bacteria produce inhibitory effects against food-borne pathogens and elevate the levels of serum anti-inflammatory cytokines. Novel cyclic peptides can decrease drug dependence and also provide aid in disease resistance. The role of cyclic peptides in areas other than the food industry has to be explored further. Nano-drug delivery systems of LAB-derived cyclic peptides can have the potential to deliver drugs to a specific site of action effectively. Peptide-incorporated nanomaterials can open opportunities against the treatment of disorders like cancer. The advantages of LAB and its derived peptides being evaluated with the help of in vivo and in vitro assays provide substantial support for their great application in a variety of medical therapies.
Author contribution
S. Rupachandra had the idea for the article and critically revised the final version of the manuscript. Parikhshith Saravanan, Pooja R, Nanditaa Balachander, Kesav Ram Singh K, and Silpa S collected the data and contributed toward the initial draft of the manuscript.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
2/28/2023
A Correction to this paper has been published: 10.1007/s12223-023-01044-0
References
- Abdi M, Lohrasbi V, Asadi A, Esghaei M, Jazi FM, Rohani M, Talebi M. Interesting probiotic traits of mother’s milk Lactobacillus isolates; from bacteriocin to inflammatory bowel disease improvement. Microb Pathog. 2021;158:104998. doi: 10.1016/j.micpath.2021.104998. [DOI] [PubMed] [Google Scholar]
- Abootaleb M, Mohammadi Bandari N, Arbab Soleimani N. Interference of Lactobacillus casei with Pseudomonas aeruginosa in the treatment of infected burns in Wistar rats. Iran J Basic Med Sci. 2021;24:143–149. doi: 10.22038/IJBMS.2020.47447.10920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- Ahmad V, Khan MS, Jamal QMS, Alzohairy MA, Al Karaawi MA, Siddiqui MU. Antimicrobial potential of bacteriocins: in therapy, agriculture and food preservation. Int J Antimicrob Agents. 2017;49:1–11. doi: 10.1016/j.ijantimicag.2016.08.016. [DOI] [PubMed] [Google Scholar]
- Al-Fakharany OM, Abdel Aziz AA, El-Banna TES, Sonbol FI. Immunomodulatory and anticancer activities of Enterocin Oe-342 produced by Enterococcus Feacalis isolated from stool. J Clin Immunol. 2018;09:1000558. [Google Scholar]
- Alvarez-Sieiro P, Montalbán-López M, Mu D, Kuipers OP. Bacteriocins of lactic acid bacteria: extending the family. Appl Microbiol Biotechnol. 2016;100:2939–2951. doi: 10.1007/s00253-016-7343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banna GL, Torino F, Marletta F, Santagati M, Salemi R, Cannarozzo E, Falzone L, Ferraù F, Libra M. Lactobacillus rhamnosus GG: an overview to explore the rationale of its use in cancer. Front Pharmacol. 2017;8:603. doi: 10.3389/fphar.2017.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Ji S. Isolation and identification of lactic acid bacteria from koumiss in Eastern Inner Mongolia of China. In AIP Conf Proc. 2017;1794:05005. [Google Scholar]
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surge. 2005;31:674–686. doi: 10.1097/00042728-200506000-00011. [DOI] [PubMed] [Google Scholar]
- Bédard F, Biron E. Recent progress in the chemical synthesis of class II and S-glycosylated bacteriocins. Front Microbiol. 2018;9:1048. doi: 10.3389/fmicb.2018.01048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhol KC, Schechter PJ. Topical nanocrystalline silver cream suppresses inflammatory cytokines and induces apoptosis of inflammatory cells in a murine model of allergic contact dermatitis. Br J Dermatol. 2005;152:1235–1242. doi: 10.1111/j.1365-2133.2005.06575.x. [DOI] [PubMed] [Google Scholar]
- Borrero J, Brede DA, Skaugen M, Diep DB, Herranz C, Nes IF, Cintas LM, Hernández PE. Characterization of Garvicin ML, a novel circular bacteriocin produced by Lactococcus garvieae DCC43 Appl. Environ Microbiol. 2011;77:369–373. doi: 10.1128/aem.01173-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. J Sci. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Campion A, Casey PG, Field D, Cotter PD, Hill C, Ross RP. In vivo activity of 484 nisin A and nisin V against Listeria monocytogenes in mice. BMC Microbiol. 2013;13:23. doi: 10.1186/1471-2180-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cañedo-Dorantes L, Cañedo-Ayala M (2019) Skin acute wound healing: a comprehensive review. Int J Inflam [DOI] [PMC free article] [PubMed]
- Carroll IM, Andrus JM, Bruno-Bárcena JM, Klaenhammer TR, Hassan HM, Threadgill DS. Anti-inflammatory properties of Lactobacillus gasserie expressing manganese superoxide dismutase using the interleukin 10-deficient mouse model of colitis. Am J of Physiol Gastrointest Liver Physiol. 2007;293:729–738. doi: 10.1152/ajpgi.00132.2007. [DOI] [PubMed] [Google Scholar]
- Casas-Solís J, Huizar-López MDR, Irecta-Nájera CA, Pita-López ML, Santerre A. Immunomodulatory effect of Lactobacillus casei in a murine model of colon carcinogenesis. Probiotics Antimicrob Proteins. 2020;12:1012–1024. doi: 10.1007/s12602-019-09611-z. [DOI] [PubMed] [Google Scholar]
- Chen L, DiPietro LA. Toll-like receptor function in acute wounds. Adv Wound Care. 2017;6:344–355. doi: 10.1089/wound.2017.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E-J, Lee HJ, Kim WJ, Han KI, Iwasa M, Kobayashi K, Debnath T, Tang Y, Kwak YS, Yoon JH, Kim EK. Enterococcus faecalis EF-2001 protects DNBS-induced inflammatory bowel disease in mice model. PLoS ONE. 2019;14:0210854. doi: 10.1371/journal.pone.0210854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compare D, Rocco A, Coccoli P, Angrisani D, Sgamato C, Iovine B, Salvatore U, Nardone G. Lactobacillus casei DG and its postbiotic reduce the inflammatory mucosal response: an ex-vivo organ culture model of post-infectious irritable bowel syndrome. BMC Gastroenterol. 2017;17:53. doi: 10.1186/s12876-017-0605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corr SC, Li Y, Riedel CU, O’Toole PW, Hill C, Gahan CGM. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci USA. 2007;104:7617–7621. doi: 10.1073/pnas.0700440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Amidon GL, Zimmermann EM. Drug targeting strategies for the treatment of inflammatory bowel disease: a mechanistic update. Expert Rev Clin Immunol. 2010;6:543–550. doi: 10.1586/eci.10.30. [DOI] [PubMed] [Google Scholar]
- Darby IA, Laverdet B, Bonté F, Desmoulière A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol. 2014;7:301. doi: 10.2147/CCID.S50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Ganesh K, Khanna S, Sen CK, Roy S. Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. J Immun J. 2014;192:1120–1129. doi: 10.4049/jimmunol.1300613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Sinha M, Datta S, Abas M, Chaffee S, Sen CK, Roy S. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Clin Pathol. 2015;185:2596–2606. doi: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kwaadsteniet M, Doeschate KT, Dicks LMT. Nisin F in the treatment of respiratory tract infections caused by Staphylococcus aureus. Lett Appl Microbiol. 2009;48:65–70. doi: 10.1111/j.1472-765X.2008.02488.x. [DOI] [PubMed] [Google Scholar]
- Delavary BM, van der Veer WM, van Egmond M, Niessen FB, Beelen RH. Macrophages in skin injury and repair. Immunobiology. 2011;216:753–762. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Divyashri G, Krishna G, Muralidhara PSG. Probiotic attributes, antioxidant, anti-inflammatory and neuromodulatory effects of Enterococcus faecium CFR 3003: in vitro and in vivo evidence. J Med Microbiol. 2015;64:1527–1540. doi: 10.1099/jmm.0.000184. [DOI] [PubMed] [Google Scholar]
- Dobson A, Crispie F, Rea MC, O'Sullivan O, Casey PG, Lawlor PG, Cotter PD, Ross P, Gardiner GE, Hill C. Fate and efficacy of lacticin 3147-producing Lactococcus lactis in the mammalian gastrointestinal tract. FEMS Microbiol Ecol. 2011;76:602–614. doi: 10.1111/j.1574-6941.2011.01069.x. [DOI] [PubMed] [Google Scholar]
- Dos Santos TF, Melo TA, Santos DS, Rezende RP, Dias JCT, Romano CC. Efficacy of oral administration of lactic acid bacteria isolated from cocoa in a fermented milk preparation: reduction of colitis in an experimental rat model. Genet Mol Res. 2016;15:3. doi: 10.4238/gmr.15038097. [DOI] [PubMed] [Google Scholar]
- Duary RK, Bhausaheb MA, Batish VK, Grover S. Anti-inflammatory and immunomodulatory efficacy of indigenous probiotic Lactobacillus plantarum Lp91 in colitis mouse model. Mol Biol Rep. 2012;39:4765–4775. doi: 10.1007/s11033-011-1269-1. [DOI] [PubMed] [Google Scholar]
- Duca L, Floquet N, Alix AJ, Haye B, Debelle L. Elastin as a matrikine. Crit Rev Oncol/hematol. 2004;49:235–244. doi: 10.1016/j.critrevonc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- Ermakova NA (2018) The role of inflammation in age-related macular degeneration. Vestn Oftalmol 134:116. 10.17116/oftalma2018134061116 [DOI] [PubMed]
- Fahim HA, Khairalla AS, El-Gendy AO. Nanotechnology: a valuable strategy to improve bacteriocin formulations. Front Microbiol. 2016;7:1385. doi: 10.3389/fmicb.2016.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan FRF, Bard AJ. Chemical, electrochemical, gravimetric, and microscopic studies on antimicrobial silver films. J Phy Chem A. 2002;106:79–287. doi: 10.1021/jp012548d. [DOI] [Google Scholar]
- Fang H, Elina T, Heikki A, Seppo S. Modulation of humoral immune response through probiotic intake. FEMS Microbiol Immunol. 2000;29:47–52. doi: 10.1111/j.1574-695X.2000.tb01504.x. [DOI] [PubMed] [Google Scholar]
- Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Am J Hematol. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller M, Huwiler K, Stephan R, Altpeter E, Shang A, Furrer H, Pfyffer GE, Jemmi T, Baumgartner A, Egger M. Mycobacterium avium subspecies paratuberculosis and Crohn’s disease: a systematic review and meta-analysis. Lancet Infect Dis. 2007;7:607–613. doi: 10.1016/S1473-3099(07)70211-6. [DOI] [PubMed] [Google Scholar]
- Ferreira Dos Santos T, Alves Melo T, Almeida ME, Passos Rezende R, Romano CC (2016) Immunomodulatory effects of Lactobacillus plantarum Lp62 on intestinal epithelial and mononuclear cells. BioMed Res Int 8404156 [DOI] [PMC free article] [PubMed]
- Fimland G, Johnsen L, Dalhus B, Nissen-Meyer J. Pediocin-like antimicrobial peptides (class IIa bacteriocins) and their immunity proteins: biosynthesis, structure, and mode of action. J Pept Sci an Official Publication of the European Peptide Society. 2005;11:688–696. doi: 10.1002/psc.699. [DOI] [PubMed] [Google Scholar]
- Florou-Paneri P, Christaki E, Bonos E. Lactic acid bacteria as source of functional ingredients. Lactic Acid Bacteria - R & D for Food, Health and Livestock Purposes. 2013 doi: 10.5772/47766. [DOI] [Google Scholar]
- Franz CMAP, Franz CMA, Van Belkum MJ, Holzapfel WH, Abriouel H, Gálvez A. Diversity of enterococcal bacteriocins and their grouping in a new classification scheme. FEMS Microbiol Rev. 2007;31:293–310. doi: 10.1111/j.1574-6976.2007.00064.x. [DOI] [PubMed] [Google Scholar]
- Freire MO, Van Dyke TE. Natural Resolution of Inflammation Periodontol. 2013;2000(63):149–164. doi: 10.1111/prd.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15:551–567. doi: 10.1038/nrd.2016.39. [DOI] [PubMed] [Google Scholar]
- Germolec DR, Shipkowski KA, Frawley RP, Evans E. Markers of inflammation. Methods Mol Biol. 2018;1803:57–79. doi: 10.1007/978-1-4939-8549-4_5. [DOI] [PubMed] [Google Scholar]
- Golebiewska EM, Poole AW. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 2015;29:153–162. doi: 10.1016/j.blre.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomashe AV, Dharmik PG (2012) Synergistic effect of gold nanoparticles and bacteriocin against food blemishing microbes: a novel approach for food packaging material preparation. Glob J Res Anal 3:1–3. 10.15373/22778160/may2014/55
- Grenier D, Marcoux E, Azelmat J, Ben Lagha A, Gauthier P. Biocompatible combinations of nisin and licorice polyphenols exert synergistic bactericidal effects against Enterococcus faecalis and inhibit NF-κB activation in monocytes. AMB Express. 2020;10:120. doi: 10.1186/s13568-020-01056-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich DB, Severn CE, Twomey C, Greenhough A, Cash J, Toye AM, Mellor H, Martin P. Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression. EMBO J. 2018;37:e97786. doi: 10.15252/embj.201797786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara A, Imai N, Nakashima H, Toda Y, Kawabe M, Furukawa F, Delves-Broughton J, Yasuhara K, Hayashi SM. A 90-day oral toxicity study of nisin A, an anti-microbial peptide derived from Lactococcus lactis subsp. lactis, in F344 rats. Food Chem Toxicol. 2010;489:2421–2428. doi: 10.1016/j.fct.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Han KJ, Lee NK, Park H, Paik HD. Anticancer and anti-inflammatory activity of probiotic Lactococcus lactis NK34. J Microbiol Biotechnol. 2015;25:1697–1701. doi: 10.4014/jmb.1503.03033. [DOI] [PubMed] [Google Scholar]
- Hanny ELL, Mustopa AZ, Budiarti S, Darusman HS, Ningrum RA. Efficacy, toxicity study and antioxidant properties of plantaricin E and F recombinants against enteropathogenic Escherichia coli K1. 1 (EPEC K1. 1) Mol Biol Rep. 2019;46:6501–6512. doi: 10.1007/s11033-019-05096-9. [DOI] [PubMed] [Google Scholar]
- Headland SE, Norling LV. The resolution of inflammation: principles and challenges. Semin Immunol. 2015;27(3):149–160. doi: 10.1016/j.smim.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Hernández-González JC, Martínez-Tapia A, Lazcano-Hernández G, García-Pérez BE, Castrejón-Jiménez NS (2021) Bacteriocins from lactic acid bacteria. A powerful alternative as antimicrobials, probiotics, and immunomodulators in veterinary medicine. Animals 11: 979. 10.3390/ani11040979 [DOI] [PMC free article] [PubMed]
- Heunis TDJ, Botes M, Dicks LMT. Encapsulation of Lactobacillus plantarum 423 and its bacteriocin in nanofibers. Probiotics Antimicrob Proteins. 2010;2:46–51. doi: 10.1007/s12602-009-9024-9. [DOI] [PubMed] [Google Scholar]
- Heunis TDJ, Smith C, Dicks LMT. Evaluation of a nisin-eluting nanofiber scaffold to treat Staphylococcus aureus-induced skin infections in mice. Antimicrob Agents Chemother. 2013;57:3928–3935. doi: 10.1128/AAC.00622-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydari N, Tajabadi E, Dehghan B, Torabi K. Study of cutaneous wound healing in rats treated with Lactobacillus plantarum on days 1, 3, 7, 14 and 21. Afr J Pharm Pharmacol. 2011;5:2395–2401. [Google Scholar]
- Hirao LA, Grishina I, Bourry O, Hu WK, Somrit M, Sankaran-Walters S, Gaulke CA, Fenton AN, Li JA, Crawford RW, Chuang F, Tarara R, Marco ML, Bäumler AJ, Cheng H, Dandekar S. Early mucosal sensing of SIV infection by paneth cells induces IL-1β production and initiates gut epithelial disruption. PLoS Pathog. 2014;10:e1004311. doi: 10.1371/journal.ppat.1004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honnegowda TM, Kumar P, Udupa EGP, Kumar S, Kumar U, Rao P. Role of angiogenesis and angiogenic factors in acute and chronic wound healing. Plastic Aesthetic Res. 2015;2:243–249. doi: 10.4103/2347-9264.165438. [DOI] [Google Scholar]
- Huang SP, Wu MS, Shun CT, Wang HP, Hsieh CY, Kuo ML, Lin JT. Cyclooxygenase-2 increases hypoxia-inducible factor-1 and vascular endothelial growth factor to promote angiogenesis in gastric carcinoma. J Biomed Sci. 2005;12:229–241. doi: 10.1007/s11373-004-8177-5. [DOI] [PubMed] [Google Scholar]
- Huang WC, Wei CC, Huang CC, Chen WL, Huang HY. The beneficial effects of Lactobacillus plantarum PS128 on high-intensity, exercise-induced oxidative stress, inflammation, and performance in triathletes. Nutrients. 2019;11:326. doi: 10.3390/nu11020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilott NE, Heward JA, Roux B, Tsitsiou E, Fenwick PS, Lenzi L, Goodhead I, Hertz-Fowler C, Heger A, Hall N, Donnelly LE, Sims D, Lindsay MA. Corrigendum: long non-coding RNAs and enhancer RNAs regulate the lipopolysaccharide-induced inflammatory response in human monocytes. Nat Commun. 2015;6:6814. doi: 10.1038/ncomms7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabés D, Brunati C, Candiani G, Riva S, Romanó G, Donadio S. Efficacy of the new lantibiotic NAI-107 in experimental infections induced by multidrug-resistant Gram-positive pathogens. Antimicrob Agents Chemother. 2011;55:1671–1676. doi: 10.1128/AAC.01288-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacouton E, Chain F, Sokol H, Langella P, Bermúdez-Humarán LG. Probiotic strain Lactobacillus casei BL23 prevents colitis-associated colorectal cancer. Front Immunol. 2017;8:1553. doi: 10.3389/fimmu.2017.01553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janis JE, Harrison B (2016) Wound Healing: Part I. Basic Science. Plast Reconst Surg 138(3):9S–17S [DOI] [PubMed]
- Jeon B, Kim HR, Kim H, Chung DK. In vitro and in vivo downregulation of C3 by lipoteichoic acid isolated from Lactobacillus plantarum K8 suppressed cytokine-mediated complement system activation. FEMS Microbiol Lett. 2016;363:14. doi: 10.1093/femsle/fnw140. [DOI] [PubMed] [Google Scholar]
- Jetten N, Roumans N, Gijbels MJ, Romano A, Post MJ, de Winther MP, van der Hulst RR, Xanthoulea S. Wound administration of M2-polarized macrophages does not improve murine cutaneous healing responses. PLoS ONE. 2014;9:e102994. doi: 10.1371/journal.pone.0102994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, He M, Wang C, Chen A, Zhang X, Xu J, Fu H, Liu B. Nisin reduces uterine inflammation in rats by modulating concentrations of pro- and anti-inflammatory cytokines. Am J Reprod Immunol. 2019;81:13096. doi: 10.1111/aji.13096. [DOI] [PubMed] [Google Scholar]
- Joost S, Jacob T, Sun X, Annusver K, La Manno G, Sur I, Kasper M. Single-cell transcriptomics of traced epidermal and hair follicle stem cells reveals rapid adaptations during wound healing. Cell Rep. 2018;25:585–597. doi: 10.1016/j.celrep.2018.09.059. [DOI] [PubMed] [Google Scholar]
- Kang M, Choi HJ, Yun B, Lee J, Yoo J, Yang HJ, Jeong DY, KimY OhS. Bacillus amyloliquefaciens SCGB1 alleviates dextran sulfate sodium-induced colitis in mice through immune regulation. J Med Food. 2021;24:709–719. doi: 10.1089/jmf.2021.K.0044. [DOI] [PubMed] [Google Scholar]
- Kareem RA, Razavi SH. Plantaricin bacteriocins: as safe alternative antimicrobial peptides in food preservation—a review. J Food Saf. 2020;40:12735. doi: 10.1111/jfs.12735. [DOI] [Google Scholar]
- Karimi M, Maghsoud Z, Halabian R. Effect of preconditioned mesenchymal stem cells with nisin prebiotic on the expression of wound healing factors such as TGF-β1, FGF-2, IL-1, IL-6, and IL-10. Regen Eng Transl Med. 2021;7:30–40. doi: 10.1007/s40883-021-00194-2. [DOI] [Google Scholar]
- Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston AT, Clement M, Dussiot M, Levillain O, Graff-Dubois S, Nicoletti A, Caligiuri G. Macrophage plasticity in experimental atherosclerosis. PLoS ONE. 2010;5:8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindrachuk J, Jenssen H, Elliot M, Nijnik A, Magrangeas-Janot L, Pasupuleti M, Thorson L, Ma S, Easton DM, Bains M, Finlay B, Breukink EJ, Georg-Sahl H, Hancock REW. Manipulation of innate immunity by a bacterial secreted peptide: lantibiotic nisin Z is selectively immunomodulatory. Innate Immun. 2013;19:315–327. doi: 10.1177/1753425912461456. [DOI] [PubMed] [Google Scholar]
- Kingsley K, Huff JL, Rust WL, Carrol IK, Martinez AM, Fitchmun M, Plopper GE. ERK1/2 mediates PDGF-BB stimulated vascular smooth muscle cell proliferation and migration on laminin-5. Biochem Biophys Res Commun. 2002;29:31000–31006. doi: 10.1016/S0006-291X(02)00331-5. [DOI] [PubMed] [Google Scholar]
- Kitagawa N, Otani T, Inai T. Nisin, a food preservative produced by Lactococcus lactis, affects the localization pattern of intermediate filament protein in HaCaT cells. Anat Sci Int. 2019;94:163–171. doi: 10.1007/s12565-018-0462-x. [DOI] [PubMed] [Google Scholar]
- Koh TJ, Di Pietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- Kruszewska D, Sahl HG, Bierbaum G, Pag U, Hynes SO, Ljungh Å. Mersacidin eradicates methicillin-resistant Staphylococcus aureus (MRSA) in a mouse rhinitis model. J Antimicrob Chemother. 2004;54:648–653. doi: 10.1093/jac/dkh387. [DOI] [PubMed] [Google Scholar]
- Kusumaningsih T, Irmawati A, Ernawati DS, Prahasanti C, Aljunaid M, Amelia S. The differences in the number of fibroblasts and blood vessels after the topical and systemic administration of Lactobacillus casei Shirota probiotics for the treatment of traumatic ulcers in Wistar rats (Rattus norvegicus) Vet World. 2021;14:1279–1283. doi: 10.14202/vetworld.2021.1279-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Manna S, Di Natale C, Florio D, Marasco D. Peptides as therapeutic agents for inflammatory-related diseases. Int J Mol Sci. 2018;19:2174. doi: 10.3390/ijms19092714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larouche J, Sheoran S, Maruyama M, Martino MM. Immune regulation of skin wound healing: mechanisms and novel therapeutic targets. Adv Wound Caref. 2018;7:209–231. doi: 10.1089/wound.2017.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Lay C, Fernandez B, Hammami R, Ouellette M, Fliss I. On Lactococcus lactis UL719 competitivity and nisin (Nisaplin®) capacity to inhibit Clostridium difficile in a model of human colon. Front Microbiol. 2015;6:1020. doi: 10.3389/fmicb.2015.01020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le B, Yang SH. Efficacy of Lactobacillus plantarum in prevention of inflammatory bowel disease. Toxicol Rep. 2018;5:314–317. doi: 10.1016/j.toxrep.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc JG, Levit R, Savoy de Giori G, de Moreno de LeBlanc A. Application of vitamin-producing lactic acid bacteria to treat intestinal inflammatory diseases. Appl Microbiol Biotechnol. 2020;104:3331–3337. doi: 10.1007/s00253-020-10487-1. [DOI] [PubMed] [Google Scholar]
- Lee CS, Kim SH. Anti-inflammatory and anti-osteoporotic potential of Lactobacillus plantarum A41 and L. fermentum SRK414 as probiotics. Probiotics Antimicrob Proteins. 2020;12:623–634. doi: 10.1007/s12602-019-09577-y. [DOI] [PubMed] [Google Scholar]
- LeBlanc J, de LeBlanc A, Perdigon G, Miyoshi A, Rochat T, Bermudez-Humaran L, Langella P, Sesma F, Azevedo V. Anti-inflammatory properties of lactic acid bacteria: current knowledge, applications and prospects. Antiinfect Agents Med Chem. 2008;7:148–154. doi: 10.2174/187152108784911287. [DOI] [Google Scholar]
- Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010;26:5–11. doi: 10.1097/mog.0b013e328333d751. [DOI] [PubMed] [Google Scholar]
- Li B, Liang L, Deng H, Guo J, Shu H, Zhang L. Efficacy and safety of probiotics in irritable bowel syndrome: a systematic review and meta-analysis. Front Pharmacol. 2020;11:332. doi: 10.3389/fphar.2020.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen J, Kirsne R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Lin Q, Fang D, Fang J, Ren X, Yang X, Wen F, Su SB. Impaired wound healing with defective expression of chemokines and recruitment of myeloid cells in TLR3-deficient mice. J Immunol. 2011;186:3710–3717. doi: 10.4049/jimmunol.1003007. [DOI] [PubMed] [Google Scholar]
- Liu J, Gu Z, Song F, Zhang H, Zhao J, Chen W. Lactobacillus plantarum ZS2058 and Lactobacillus rhamnosus GG use different mechanisms to prevent Salmonella infection in vivo. Front Microbiol. 2019;10:299. doi: 10.3389/fmicb.2019.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Zhang X, Hao Y, Ding J, Shen J, Xue Z, Qi W, Li Z, Song Y, Zhang T, Wang N. Protective effects of a novel probiotic strain, Lactococcus lactis ML2018, in colitis: in vivo and in vitro evidence. Food Funct. 2019;10:1132–1145. doi: 10.1039/C8FO02301H. [DOI] [PubMed] [Google Scholar]
- Liu Q, Yu Z, Tian F, Zhao J, Zhang H, Zhai Q, Chen W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb Cell Fact. 2020;19:1–11. doi: 10.1186/s12934-020-1289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YF, Deng J, Wang J, Luo GX. Effects and mechanism of Lactococcus lactis thermo-sensitive hydrogel on the wound healing of full-thickness skin defects in diabetic mice. Chin J Burns. 2020;36:1117–1129. doi: 10.3760/cma.j.cn501120-20201004-00427. [DOI] [PubMed] [Google Scholar]
- Lu Y, Li H, Wang J, Yao M, Peng Y, Liu T, Li Z, Luo G, Deng J. Engineering bacteria-activated multifunctionalized hydrogel for promoting diabetic wound healing. Adv Funct Mater. 2021;31:2105749. doi: 10.1002/adfm.202105749. [DOI] [Google Scholar]
- Luerce TD, Gomes-Santos AC, RochaC S, MoreiraTG CN, Lemos L, Sousa AL, Pereira VB, de Azevedo M, Moraes K, Cara DC, LeBlanc JG, Azevedo V, Faria AMC, Miyoshi A. Anti-inflammatory effects of Lactococcus lactis NCDO 2118 during the remission period of chemically induced colitis. Gut Pathog. 2014;6:33. doi: 10.1186/1757-4749-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hu C, Yan W, Jiang H, Liu G (2020) Lactobacillus pentosus increases the abundance of Akkermansia and Affects the serum metabolome to alleviate DSS-induced colitis in a murine model. Front Cell Dev Biol 8:591408 [DOI] [PMC free article] [PubMed]
- Maddaloni M, Kochetkova I, Hoffman C, Pascual DW. Delivery of IL-35 by Lactococcus lactis ameliorates collagen-induced arthritis in mice. Front Immunol. 2018;9:2691. doi: 10.3389/fimmu.2018.02691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdi LH, Jabbar HS, Auda IG. Antibacterial immunomodulatory and antibiofilm triple effect of Salivaricin LHM against Pseudomonas aeruginosa urinary tract infection model. Int J Biol Macromol. 2019;134:1132–1144. doi: 10.1016/j.ijbiomac.2019.05.181. [DOI] [PubMed] [Google Scholar]
- Majid SA, Khorasgani MR, Emami SH, Talebi A, Gholami AM (2016) Study of diabetic cutaneous wound healing in rats treated with Lactobacillus Casei and its Exopolysaccharide. Int J Adv Biotechnol Res 7:2083–2092
- Mann KG. Factor VII-activating protease: coagulation, fibrinolysis, and atherothrombosis? Circulation. 2003;107:654–655. doi: 10.1161/01.CIR.0000057382.68508.3D. [DOI] [PubMed] [Google Scholar]
- Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173:370–378. doi: 10.1111/bjd.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews S, Panicker S, Arunkumar G (2015) Detection of anti-inflammatory activity of Lactobacilli producing bacteriocins. Int J Curr Microbiol Appl Sci 4:598–606. https://www.cabdirect.org/globalhealth/abstract/20153129861
- Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Melero A, Draheim C, Hansen S, Giner E, Carreras JJ, Talens-Visconti R, Garrigues TM, Peris JE, Recio MC, Giner R, Lehr CM. Targeted delivery of Cyclosporine A by polymeric nanocarriers improves the therapy of inflammatory bowel disease in a relevant mouse model. Eur J Pharm Biopharm. 2017;119:361–371. doi: 10.1016/j.ejpb.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Mokoena MP. Lactic acid bacteria and their bacteriocins: classification, biosynthesis and applications against uropathogens: a mini-review. Molecules. 2017;22(8):1255. doi: 10.3390/molecules22081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes PM, Perin LM, Ortolani MBT, Yamazi AK, Viçosa GN, Nero LA. Protocols for the isolation and detection of lactic acid bacteria with bacteriocinogenic potential. LWT - Food Sci Technol. 2010;43:1320–1324. doi: 10.1016/j.lwt.2010.05.005. [DOI] [Google Scholar]
- Moreira CF, Cassini-Vieira P, Canesso MCC, Felipetto M, Ranfley H, Teixeira MM, Nicoli JR, Martins FS, Barcelos LS. Lactobacillus rhamnosus CGMCC 1.3724 (LPR) improves skin wound healing and reduces scar formation in mice. Probiotics Antimicrob Proteins. 2021;13:709–719. doi: 10.1007/s12602-020-09713-z. [DOI] [PubMed] [Google Scholar]
- Mossallam SF, Amer EI, Diab RG. Potentiated anti-microsporidial activity of Lactobacillus acidophilus CH1 bacteriocin using gold nanoparticles. Exp Parasitol. 2014;144:14–21. doi: 10.1016/j.exppara.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Mouritzen MV, Andrea A, Qvist K, Poulsen SS, Jenssen H. Immunomodulatory potential of Nisin A with application in wound healing. Wound Repair Regen. 2019;27:650–660. doi: 10.1111/wrr.12743. [DOI] [PubMed] [Google Scholar]
- Moysidis M, Stavrou G, Cheva A, Deka IA, Tsetis JK, Birba V, Kapoukranidou D, Ioannidis A, Tsaousi G, Kotzampassi K. The 3-D configuration of excisional skin wound healing after topical probiotic application. Injury. 2022;53:1385–1393. doi: 10.1016/j.injury.2022.02.006. [DOI] [PubMed] [Google Scholar]
- Musa A, Wiman E, Selegård R, Aili D, Bengtsson T, Khalaf H. Plantaricin NC8 αβ prevents Staphylococcus aureus-mediated cytotoxicity and inflammatory responses of human keratinocytes. Sci Rep. 2021;11:12514. doi: 10.1038/s41598-021-91682-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NadwornyP L, Wang J, TredgetE E, Burrell RE. Anti-inflammatory activity of nanocrystalline silver in a porcine contact dermatitis model. Nanomed: Nanotechnol. Biol Med. 2008;4:241–251. doi: 10.1016/j.nano.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Nasrabadi H, Ebrahimi T, BanadakiD (2011) Study of cutaneous wound healing in rats treated with Lactobacillus plantarum on days 1, 3, 7, 14 and 21. Afr J Pharm Pharmacol 5:2395–2401. https://academicjournals.org/journal/AJPP/article-abstract/1CB0A1436018.
- Nes IF, Holo H. Class II antimicrobial peptides from lactic acid bacteria. Biopolymers. 2000;55:50–61. doi: 10.1002/1097-0282(2000)55:1<50::3. [DOI] [PubMed] [Google Scholar]
- Nissen-Meyer J, Oppegård C, Rogne P, Haugen HS, Kristiansen PE. Structure and mode-of-action of the two-peptide (Class-IIb) bacteriocins. Probiotics Antimicrob Proteins. 2010;2:52–60. doi: 10.1007/s12602-009-9021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosbaum A, Ettinger M, Truong HA, Pauli ML, Gearty SV, Abbas AK, Rosenblum MD. Regulatory T cells facilitate cutaneous wound healing. J Invest Dermatol. 2015;135:S3. doi: 10.4049/jimmunol.1502139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny Núñez I, Maldonado Galdeano C, de Moreno de LeBlanc A, Perdigón G (2015) Lactobacillus casei CRL 431 administration decreases inflammatory cytokines in a diet-induced obese mouse model. Nutrition 31:1000–1007 [DOI] [PubMed]
- Nunan R, Campbell MR, Pitulescu ME, Jiang WG, Harding KG, Adams RH, Nobes CD, Martin P. Ephrin-Bs drive junctional downregulation and actin stress fiber disassembly to enable wound re-epithelialization. Cell Rep. 2015;13:1380–1395. doi: 10.1016/j.celrep.2015.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea EF, O’Connor PM, Raftis EJ, O’Toole PW, Stanton C, Cotter PD, Ross RP, Hill C. Production of multiple bacteriocins from a single locus by gastrointestinal strains of Lactobacillus salivarius. J Bacteriol. 2011;193:6973–6982. doi: 10.1128/JB.06221-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi Y, Manabe I. Macrophages in inflammation, repair and regeneration. Int Immunol. 2018;30:511–528. doi: 10.1093/intimm/dxy054. [DOI] [PubMed] [Google Scholar]
- Oppegård C, Rogne P, Emanuelsen L, Kristiansen PE, Fimland G, Nissen-Meyer J. The two-peptide class II bacteriocins: structure, production, and mode of action. J Mol Microbiol Biotechnol. 2007;13:210–219. doi: 10.1159/000104750. [DOI] [PubMed] [Google Scholar]
- Pagnini C, Corleto VD, Martorelli M, Lanini C, D’Ambra G, Di Giulio E, Delle Fave G. Mucosal adhesion and anti-inflammatory effects of Lactobacillus rhamnosus GG in the human colonic mucosa: a proof-of-concept study. World J Gastroenterol. 2018;24:4652–4662. doi: 10.3748/wjg.v24.i41.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahwa R, Goyal A, Bansal P, Jialal I (2018) Chronic inflammation 12: 370. https://europepmc.org/article/nbk/nbk493173 [PubMed]
- Pasolli E, De Filippis F, Mauriello IE, Cumbo F, Walsh AM, Leech J, Cotter PD, Segata N, Ercolini D. Large-scale genome-wide analysis links lactic acid bacteria from food with the gut microbiome. Nat Commun. 2020;11:2610. doi: 10.1038/s41467-020-16438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan S, Jacobsen E, VisserR GF, Bai Y. Loss of susceptibility as a novel breeding strategy for durable and broad-spectrum resistance. Mol Breed. 2010;25:1–12. doi: 10.1007/s11032-009-9323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peran L, CamuescoD ComaladaM, Nieto A, Concha A, Diaz-Ropero MP, Olivare M, Xaus J, Zarzuel A, Galvez J. Preventative effects of a probiotic, Lactobacillus salivarius ssp. salivarius, in the TNBS model of rat colitis. World J Gastroenterol. 2005;11:5185–5192. doi: 10.3748/wjg.v11.i33.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez RH, Zendo T, Sonomoto K. Circular and leaderless bacteriocins: biosynthesis, mode of action, applications, and prospects. Front Microbiol. 2018;9:2085. doi: 10.3389/fmicb.2018.02085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper C, Hill C, Cotter PD, Ross RP. Bioengineering of a Nisin A-producing Lactococcus lactis to create isogenic strains producing the natural variants Nisin F. Q and Z. Microb. biotechnol. 2011;4(3):375–382. doi: 10.1111/j.1751-7915.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poché RA, Hsu CW, McElwee ML, Burns AR, Dickinson ME. Macrophages engulf endothelial cell membrane particles preceding pupillary membrane capillary regression. Dev Biol. 2015;403:30–42. doi: 10.1016/j.ydbio.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayatpour A, Javan M. Targeting the brain lesions using peptides: a review focused on the possibility of targeted drug delivery to multiple sclerosis lesions. Pharmacol Res. 2021;167:105441. doi: 10.1016/j.phrs.2021.105441. [DOI] [PubMed] [Google Scholar]
- Rea MC, Clayton E, O’Connor PM, Shanahan F, Kiely B, Paul Ross R, Hill C. Antimicrobial activity of lacticin 3147 against clinical Clostridium difficile strains. J Med Microbiol. 2007;56:940–946. doi: 10.1099/jmm.0.47085-0. [DOI] [PubMed] [Google Scholar]
- Reiners J, Lagedroste M, Gottstein J, Adeniyi ET, Kalscheuer R, Poschmann G, Stühler K, Smits SHJ, Schmitt L. Insights in the antimicrobial potential of the natural nisin variant Nisin H. Front Microbiol. 2020;11:573614. doi: 10.3389/fmicb.2020.573614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riboulet-Bisson E, Sturme MHJ, Jeffery IB, O’Donnell MM, Neville BA, Forde BM, Claesson MJ, Harris H, Gardiner GE, Casey PG, Lawlor PG, O’Toole PW, Ross RP. Effect of Lactobacillus salivarius bacteriocin Abp118 on the mouse and pig intestinal microbiota. PLoS ONE. 2012;7:e31113. doi: 10.1371/journal.pone.0031113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodero MP, Licata F, Poupel L, Hamon P, Khosrotehrani K, Combadiere C, Boissonnas A. In vivo imaging reveals a pioneer wave of monocyte recruitment into mouse skin wounds. PLoS ONE. 2014;9:108212. doi: 10.1371/journal.pone.0108212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99:665–706. doi: 10.1152/physrev.00067.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle P, Braye F, Dayan G. Re-epithelialization of adult skin wounds: cellular mechanisms and therapeutic strategies. Adv Drug Deliv Rev. 2019;14:6344–6365. doi: 10.1016/j.addr.2018.06.019. [DOI] [PubMed] [Google Scholar]
- Silpa S, Rupachandra S. Cyclic peptide production from lactic acid bacteria (LAB) and their diverse applications. Crit Rev Food Sci Nutr. 2020;11:2909–2927. doi: 10.1080/10408398.2020.1860900. [DOI] [PubMed] [Google Scholar]
- Salaran M, Oryan A, Nikahval B, Kamali A, Ghaemi M, Abbasi-Teshnizi F, Azizzadeh M. Topical application of Lactobacillus plantarum on burn wound healing in diabetic rats. Iran J Vet Surg. 2019;14:60–72. [Google Scholar]
- Saravanan M, Barabadi H. Cancer Nanotheranostics. 2021;1:2. [Google Scholar]
- Sawa N, Zendo T, Kiyofuji J, Fujita K, Himeno K, Nakayama J, Sonomoto K. Identification and characterization of Lactocyclicin Q, a novel cyclic bacteriocin produced by Lactococcus sp. strain QU 12. Appl Environ Microbiol. 2009;75:1552–1558. doi: 10.1128/aem.02299-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele F, Fuss IJ. Induction of TNBS colitis in mice. Curr Protoc Immunol. 2002;49:1. doi: 10.1002/0471142735.im1519s49. [DOI] [PubMed] [Google Scholar]
- Segel GB, Halterman MW, Lichtman MA. The paradox of the neutrophil’s role in tissue injury. J Leukoc Biol. 2011;89:359–372. doi: 10.1189/jlb.0910538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadnoush M, Shaker Hosseini R, Mehrabi Y, Delpisheh A, Alipoor E, Faghfoori Z, Mohammadpour N, Zaringhalam Moghadam J. Probiotic yogurt affects pro- and anti-inflammatory factors in patients with inflammatory bowel disease. Iran J Pharm Res. 2013;12:929–936. [PMC free article] [PubMed] [Google Scholar]
- Shaw TJ, Martin P (2016) Wound repair: a showcase for cell plasticity and migration. Curr Opin Cell Biol 4229–37 [DOI] [PubMed]
- Shin JM, Gwak JW, Kamarajan P, Fenno JC, Rickard AH, Kapila YL. Biomedical applications of nisin. J Appl Microbiol. 2016;120:1449–1465. doi: 10.1111/jam.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra S, Lara-Villoslada F, Sempere L, Olivares M, Boza J, Xaus J. Intestinal and immunological effects of daily oral administration of Lactobacillus salivarius CECT5713 to healthy adults. Anaerobe. 2010;16:195–200. doi: 10.1016/j.anaerobe.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Singh A, Walia D, Batra N. Fresh-cut fruits: microbial degradation and preservation. Microbial Contamination and Food Degradation. 2018;10:149–176. doi: 10.1016/b978-0-12-811515-2.00006-8. [DOI] [Google Scholar]
- Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and Cancer Ann Afr Med. 2019;18:121–126. doi: 10.4103/aam.aam_56_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sireswar S, Biswas S, Dey G. Adhesion and anti-inflammatory potential of Lactobacillus rhamnosus GG in a sea buckthorn based beverage matrix. Food Funct. 2020;11:2555–2572. doi: 10.1039/C9FO02249J. [DOI] [PubMed] [Google Scholar]
- Sun Z, Wang X, Zhang X, Wu H, Zou Y, Li P, Sun C, Xu W, Liu F, Wang D. Class III bacteriocin Helveticin-M causes sublethal damage on target cells through impairment of cell wall and membrane. J Ind Microbiol Biotechno. 2018;45:213–227. doi: 10.1007/s10295-018-2008-6. [DOI] [PubMed] [Google Scholar]
- Szabóová R, Lauková A, Chrastinová L, Strompfová V, Simonová MP, Vasilková Z, Cobanová K, Plachá I, Chrenková M. Effect of combined administration of enterocin 4231 and sage in rabbits. Pol J Vet Sci. 2011;14:359–366. doi: 10.2478/v10181-011-0054-3. [DOI] [PubMed] [Google Scholar]
- Tachon G, Harrois A, Tanaka S, Kato H, Huet O, Pottecher J, Vicaut E, Duranteau J. Microcirculatory alterations in traumatic hemorrhagic shock. Crit Care Med. 2014;42:1433–1441. doi: 10.1097/CCM.0000000000000223. [DOI] [PubMed] [Google Scholar]
- Tang J, Liu H, Gao C, Mu L, Yang S, Rong M, Zhang Z, Liu J, Ding Q, Lai R. A small peptide with potential ability to promote wound healing. PLoS. 2014;9:92082. doi: 10.1371/journal.pone.0092082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno H, Kanno E, Kurosaka S, Oikawa Y, Watanabe T, Sato K, Kasamatsu J, Miyasaka T, Ishi S, Shoji M, Takagi N, Imai Y, Ishii K, Tachi M, Kawakami K. Topical administration of heat-killed Enterococcus faecalis strain KH2 promotes re-epithelialization and granulation tissue formation during skin wound-healing. Biomedicines. 2021;9:20–25. doi: 10.3390/biomedicines9111520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason HA, Cooper NH, Ansell DM, Chiu M, Merrit AJ, Hardman MJ, Garrod DR. Direct evidence that PKCα positively regulates wound re-epithelialization: correlation with changes in desmosomal adhesiveness. J Pathol. 2012;227:346–356. doi: 10.1002/path.4016. [DOI] [PubMed] [Google Scholar]
- Tian Z, Yang L, Li P, Xiao Y, Peng J, Wang X, Li Z, Liu M, Bi D, Shi D. The inflammation regulation effects of Enterococcus faecium HDRsEf1 on human enterocyte-like HT-29 cells. Anim Cells Sys. 2016;20:70–76. doi: 10.1080/19768354.2016.1160955. [DOI] [Google Scholar]
- Tien MT, Girardin SE, Regnault B, Le Bourhis L, Dillies MA, Coppée JY, Bourdet-Sicard R, Sansonetti PJ, Pédron T. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J Immunol. 2006;176:1228–1237. doi: 10.4049/jimmunol.176.2.1228. [DOI] [PubMed] [Google Scholar]
- Tiwari SK. Bacteriocin-producing probiotic lactic acid bacteria in controlling dysbiosis of the gut microbiota. Front Cell Infect Microbiol. 2022;14:415. doi: 10.3389/fcimb.2022.851140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Staden AD, Brand AM, Dicks LMT. Nisin F-loaded brushite bone cement prevented the growth of Staphylococcus aureus in vivo. J Appl Microbiol. 2012;112:831–840. doi: 10.1111/j.1365-2672.2012.05241.x. [DOI] [PubMed] [Google Scholar]
- Van Staden ADP, Heunis T, Smith C, Deane S, Dicks LM. Efficacy of lantibiotic treatment of Staphylococcus aureus-induced skin infections, monitored by in vivo bioluminescent imaging. Antimicrob Agents Chemother. 2016;60:3948–3955. doi: 10.1128/AAC.02938-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas García CE, Petrova M, Claes IJJ, De Boeck I, Verhoeven TLA, Dilissen E, von Ossowski I, Palva A, Bullens DM, Vanderleyden J, Lebeer S. Piliation of Lactobacillus rhamnosus GG promotes adhesion, phagocytosis, and cytokine modulation in macrophages. Appl Environ Microbiol. 2015;81:2050–2062. doi: 10.1128/AEM.03949-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venosi S, Ceccarelli G, De Angelis M, Laghi L, Bianchi L, Martinelli O, Maruca D, Cavallari EN, Toscanella F, Vassalini P, Trinchieri V. Infected chronic ischemic wound topically treated with a multi-strain probiotic formulation: a novel tailored treatment strategy. J Transl Med. 2019;17:1–9. doi: 10.1186/s12967-019-2111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D. How leukocytes cross the vascular endothelium. Nat Rev Immunol. 2015;15:692–704. doi: 10.1038/nri3908. [DOI] [PubMed] [Google Scholar]
- von Ossowski I, Pietilä TE, Rintahaka J, Nummenmaa E, Mäkinen VM, Reunanen J, Satokari R, de Vos WM, Palva I, Palva A. Using recombinant Lactococci as an approach to dissect the immunomodulating capacity of surface piliation in probiotic Lactobacillus rhamnosus GG. PLoS ONE. 2013;8:64416. doi: 10.1371/journal.pone.0064416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager LJ, Leavesley DI. MicroRNA regulation of epithelial-to-mesenchymal transition during re-epithelialisation: assessing an open wound. Wound Pract Res. 2015;23:132–142. [Google Scholar]
- Walsh MC, Gardiner GE, Hart OM, Lawlor PG, Daly M, Lynch B, Richert BT, Radcliffe S, Giblin L, Hill C, Fitzgerald GF, Stanton C, Ross P. Predominance of a bacteriocin-producing Lactobacillus salivarius component of a five-strain probiotic in the porcine ileum and effects on host immune phenotype. FEMS Microbiol Ecol. 2008;64:317–327. doi: 10.1111/j.1574-6941.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- Wang S, Hibberd ML, Pettersson S, Lee YK. Enterococcus faecalis from healthy infants modulates inflammation through MAPK signaling pathways. PLoS ONE. 2014;9:97523. doi: 10.1371/journal.pone.0097523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Ng LHM, Chow WL, Lee YK (2008) Infant intestinal Enterococcus faecalis down-regulates infl ammatory responses in human intestinal cell lines. World J Gastroenterol 14(7):1067. 10.3748/wjg.14.1067 [DOI] [PMC free article] [PubMed]
- Wang T, Yan H, Lu Y, Li X, Wang X, Shan Y, Yi Y, Liu B, Zhou Y, Lü X. Anti-obesity effect of Lactobacillus rhamnosus LS-8 and Lactobacillus crustorum MN047 on high-fat and high-fructose diet mice base on inflammatory response alleviation and gut microbiota regulation. Eur J Nutr. 2020;59:2709–2728. doi: 10.1007/s00394-019-02117-y. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xie J, Li Y, Dong S, Liu H, Chen J, Wang Y, Zhao S, Zhang Y, Zhang H. Probiotic Lactobacillus casei mreduces pro-inflammatory cytokine production and hepatic inflammation in a rat model of acute liver failure. Eur J Nutr. 2016;55:821–831. doi: 10.1007/s00394-015-0904-3. [DOI] [PubMed] [Google Scholar]
- Widyarman AS, Drestia AM, Bachtiar EW, Bachtiar BM. The anti-inflammatory effects of glycerol-supplemented probiotic Lactobacillus reuteri on infected epithelial cells In vitro. Contemp Clin Dent. 2018;9:298. doi: 10.4103/ccd.ccd_53_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10:200223. doi: 10.1098/rsob.200223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Jackson CJ. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care. 2015;4:119–136. doi: 10.1089/wound.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Merlin D. Nanoparticle-mediated drug delivery systems for the treatment Of IBD: Current Perspectives. Int J Nanomedicine. 2019;14:8875–8889. doi: 10.2147/IJN.S210315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo S, Park H, Seo E, Kim J, KimB K, Choi IS, Huh CS. Anti-inflammatory and gut microbiota modulatory effect of Lactobacillus rhamnosus strain LDTM 7511 in a dextran sulfate sodium-induced colitis murine model. Microorganisms. 2020;8:6. doi: 10.3390/microorganisms8060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Heeney D, Srisengfa Y, Golomb B, Griffey S, Marco M. Bacteriocin biosynthesis contributes to the anti-inflammatory capacities of probiotic Lactobacillus plantarum. Benef Microbes. 2018;9:333–344. doi: 10.3920/bm2017.0096. [DOI] [PubMed] [Google Scholar]
- Yoo SK, Huttenlocher A. Spatiotemporal photolabeling of neutrophil trafficking during inflammation in live zebrafish. J Leukoc Biol. 2011;89:661–667. doi: 10.1189/jlb.1010567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JW, Kang SS. In Vitro Antibiofilm and anti-inflammatory properties of bacteriocins produced by Pediococcus acidilactici against Enterococcus faecalis. Foodborne Pathog Dis. 2020;17:764–771. doi: 10.1089/fpd.2020.2804. [DOI] [PubMed] [Google Scholar]
- Young A, McNaught CE. The Physiology of Wound Healing Surgery (oxford) 2011;29:475–479. [Google Scholar]
- Zacharof MP, Lovitt RW. Bacteriocins produced by lactic acid bacteria a review article. APCBEE Proc. 2012;2:50–56. doi: 10.1016/j.apcbee.2012.06.010. [DOI] [Google Scholar]
- Zhang J, Deng J, Wang Z, Che C, Li YF, Yang Q. Modulatory effects of Lactobacillus salivarius on intestinal mucosal immunity of piglets. Curr Microbiol. 2011;62:1623–1631. doi: 10.1007/s00284-011-9906-4. [DOI] [PubMed] [Google Scholar]
- Zhang M, Sunaba T, Sun Y, Sasaki K, Isoda H, Kigoshi H, Kita M. Anti-inflammatory marine cyclic peptide stylissatin A and its derivatives inhibit differentiation of murine preadipocytes. Chem Commun. 2019;55:5471–5474. doi: 10.1039/c9cc02517k. [DOI] [PubMed] [Google Scholar]
- Zhang S, Langer R, Traverso G. Nanoparticulate drug delivery systems targeting inflammation for treatment of inflammatory bowel disease. Nano Today. 2017;16:82–96. doi: 10.1016/j.nantod.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurita-Turk M, Mendes Souza B, Prósperi de Castro C, Bastos Pereira V, Pecini da Cunha V, Melo Preisser T, Caetano de Faria AM, Carmona Cara Machado D, Miyoshi A. Attenuation of intestinal inflammation in IL-10 deficient mice by a plasmid carrying Lactococcus lactis strain. BMC Biotechnol. 2020;20:38. doi: 10.1186/s12896-020-00631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study