Abstract
Key message
The potential of seed priming is still not fully exploited. Our limited knowledge of the molecular dynamics of seed pre-germinative metabolism is the main hindrance to more effective new-generation techniques.
Abstract
Climate change and other recent global crises are disrupting food security. To cope with the current demand for increased food, feed, and biofuel production, while preserving sustainability, continuous technological innovation should be provided to the agri-food sector. Seed priming, a pre-sowing technique used to increase seed vigor, has become a valuable tool due to its potential to enhance germination and stress resilience under changing environments. Successful priming protocols result from the ability to properly act on the seed pre-germinative metabolism and stimulate events that are crucial for seed quality. However, the technique still requires constant optimization, and researchers are committed to addressing some key open questions to overcome such drawbacks. In this review, an update of the current scientific and technical knowledge related to seed priming is provided. The rehydration–dehydration cycle associated with priming treatments can be described in terms of metabolic pathways that are triggered, modulated, or turned off, depending on the seed physiological stage. Understanding the ways seed priming affects, either positively or negatively, such metabolic pathways and impacts gene expression and protein/metabolite accumulation/depletion represents an essential step toward the identification of novel seed quality hallmarks. The need to expand the basic knowledge on the molecular mechanisms ruling the seed response to priming is underlined along with the strong potential of applied research on primed seeds as a source of seed quality hallmarks. This route will hasten the implementation of seed priming techniques needed to support sustainable agriculture systems.
Keywords: Molecular hallmarks, Pre-germinative metabolism, Seed priming, Seed quality, Sustainability
Introduction
Plants and seeds are central in our life, being part of our cultures, religions, and medicines. The connection between seeds and humankind started with the domestication of wild species, when seeds were collected, sown, and then harvested from the best-performing plants, looking for the most favorable features. Since then, our knowledge of seeds has grown, revealing the impressive complexity of this biological system. Although this era provides researchers with breakthrough technological tools, many research questions are still open. Nowadays, seeds have gained immense economic value as the global seed market was worth 58.5 billion U.S. Dollars in 2020 and it is estimated to grow up to 105.3 billion U.S. Dollars by 2031 (www.worldseed.org/, www.euroseeds.eu/). To support such an impressive expansion—in line with the current global demand for increased food, feed, and biofuel production—continuous technological innovations should be provided to seed technologists, breeders, and farmers. There is strong pressure on these issues also because climate change is disrupting food security at the global level, a condition that has been further exacerbated by the coronavirus pandemic. The impact of COVID-19 on the production of certified seeds has been assessed by international institutions committed to developing strategies to preserve seed availability, accessibility, and quality (www.fao.org/family-farming/detail/ar/c/1331528/). The adverse effects of COVID-19 include a decreased production of seeds with certified quality due to the limited accessibility to the companies that supply certified seeds, along with challenges related to seed mobilization and field-based inspections (Nchanji et al. 2021).
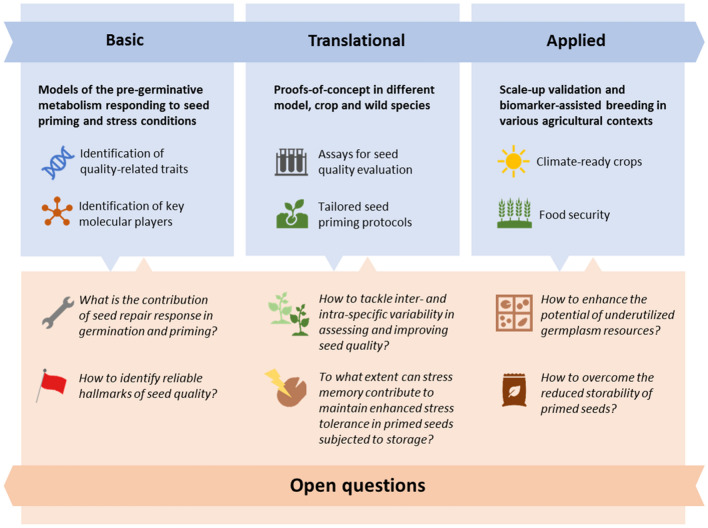
Farmers preferentially use commercial seeds with enhanced quality, resulting from the technological advances achieved by seed companies. High-vigor seeds offer several advantages, including high yields, improved nutritional value (Veena and Puthur 2022; Zrig et al. 2022), stress tolerance, and disease resistance. Additionally, different pre-sowing techniques, comprehensively defined as seed priming, are also applied to increase seed vigor. Within these methodologies, seed imbibition is carried out under controlled conditions, in water, or in solutions containing different types of priming agents, followed by desiccation. A successful seed priming requires that controlled hydration is stopped before the occurrence of radicle protrusion, otherwise, seeds will lose desiccation tolerance and thus viability (Paparella et al. 2015; Lutts et al. 2016). This simple definition describes the ability to act on the seed pre-germinative metabolism and stimulate events that are crucial for seed quality, required not only to enhance germination performance but also to boost seedling stress resilience. In this review, an update of the current scientific and technical knowledge related to seed priming is provided. Emphasis is given to the need to expand the basic knowledge of the molecular mechanisms underlying the seed response to priming. The literature so far available highlights the strong potential of basic research on primed seeds as a source of seed quality hallmarks. Looking at the seed metabolism with integrated high-throughput molecular approaches reveals key players (genes, proteins, metabolites) responsible for the ability to repair cellular damage, scavenge toxic radicals, and preserve genome integrity. The current state-of-the-art resulting from basic research must be expanded and, at the same time, fully exploited and translated into effective tools to improve seed priming technology. As shown in Fig. 1, the investigation of different models of pre-germinative metabolism challenged with optimal and/or suboptimal priming treatments represents a main pillar of basic research, whereas translational research requires proof-of-concept to validate the potential of novel seed quality hallmarks. The workflow outlined in Fig. 1 leads to the final step of applied research that is expected to provide reliable solutions for climate-ready crops and food security. Despite the efforts and encouraging results, the progression of this workflow is hampered by the numerous open questions that are still pending, listed in Fig. 1. All these aspects are discussed in different sections of this review.
Fig. 1.
Overview of the implications of seed priming technologies throughout basic, translational, and applied research, highlighting the most relevant deliverables of each phase and stating the main open questions driving future developments
Climate change, seeds, and crop productivity
Changes in global temperatures are dramatically altering the frequency and intensity of weather events, including heat waves, exposure to freezing conditions, drought periods, and precipitations. Extreme weather fluctuations are already affecting crop yields at the global scale. Indeed, the expected increase in agricultural production does not match the urgent need to feed the growing population of our planet, threatening future food security (Thiault et al. 2019; Calleja-Cabrera et al. 2020). The multifaced effects of climate change need to be considered at multiple levels, from seed/plant physiology to the socioeconomic factors involved in different agricultural realities.
Direct impact of greenhouse gases
Among the most severe challenges of climate change, the accumulation of greenhouse gases (CO2, O3, CH4) impacts crop physiology at different levels, including the occurrence of oxidative damage, decreased photosynthetic efficiency, and accelerated senescence (Wang et al. 2019). The increasing trends in atmospheric CO2 (25% higher since the levels recorded in 1959) have been suggested to alter carbon/nitrogen ratios, with consequences on nitrogen availability (Ziska et al. 2012; Lamichaney and Maity 2021).
Heat waves
The rising temperatures triggered by the accumulation of greenhouse gases cause severe effects on plants, mediated by the deterioration of macromolecules and by the exacerbation of oxidative stress. In this context, ROS accumulation associated with heat waves can alter hormonal signaling and the regulation of seed dormancy (Finch-Savage et al. 2017; Zhou et al. 2020; Farooq et al. 2021a, b). From an agricultural standpoint, such effects are particularly evident for cold-adapted crops, such as cool-season grain legumes, namely chickpeas (Cicer arietinum L.), lentils (Lens culinaris Medik.), and fava beans (Vicia faba L.), with substantial losses in terms of yields and nutrient content (Fahad et al. 2017; Kumar et al. 2021).
Drought events
Water availability is a major limiting factor for plant development and an economical issue for many farming systems. In plants at the reproductive stage, drought stress results in pollen sterility, ovary abortion, and reduced kernel number/biomass. Drought events associated with climate change are affecting crop yield worldwide, causing irreversible damage (Boyer and Westgate 2004), as yield losses under drought conditions have been estimated for many major crops, including maize (Zea mays L., 63–87%), wheat (Triticum aestivum L., 57%), rice (Oryza sativa L., 53–92%), and legumes, such as chickpea (Cicer arietinum L., 45–69%) and soybean (Glycine max L., 46–71%) (Fahad et al. 2017).
Flooding
Intense rainfall, often associated with floods occurring during key stages of crop growth, compromises yields and harvest quality since both plant biomass and seed size are reduced. Moreover, besides the stress caused by the anoxic conditions associated with prolonged submergence, flooding events occurring in coastal areas expose crops to osmotic and salt stress (Hanley et al. 2019).
Based on the available data on climate dynamics and crop productivity, Ray et al. (2019) used linear regression relationships to assess the impact of climate change on ten major crops, namely barley (Hordeum vulgare L.), cassava (Manihot esculenta L.), maize, oil palm (Elaeis guineensis L.), rapeseed (Brassica napus L.), rice, sorghum (Sorghum bicolor L.), soybean, sugarcane (Saccharum officinarum L.), and wheat. According to this study, the impact of global climate change on crop yields ranged from -13.4% (oil palm) to 3.5% (soybean), with variable geographical distribution. A negative impact was observed in Europe, Southern Africa, and Australia, mixed situations were reported in Asia and Northern and Central America, whereas Latin America was characterized by positive effects. Such a scenario has already led to a reduction in consumable food calories in these crops (Ray et al. 2019), underlining the centrality of seed quality improvement as a necessary strategy to adjust different farming systems in response to climate change. Hence, the advantages of seed priming as a versatile and resource-effective approach for seed quality improvement under different environmental conditions need to be highlighted, understood, and applied.
Priming technology: from traditional to innovative methods
From Theophrastus to nanoparticles: a time travel
In his pivotal writings “History of Plants” and “Causes of Plants”, Theophrastus of Eressus (371–287 B.C.), explored different aspects of seed biology, from seed production to germination and conservation (Evenari 1980, 1984; Thanos 1994, 2007), observing that cucumber (Cucumis sativus L.) seeds soaked in milk or water before sowing resulted in faster germination (Theophrastus, Enquiry into Plants, Book VII, I.6). The Roman naturalist Gaius Plinius Secundus (Pliny the Elder, A.D. 23–79) underlined in his Naturalis Historia the relevance of presoaking seeds to improve germination (Evenari 1984). The French agronomist and botanist Oliver de Serres (1539–1619) described the effectiveness of the treatment used by farmers on grains (Triticum, Secale, and Hordeum spp.) in which seeds were soaked for 2 days in manure water and then dried in the shade before sowing. Even Charles Darwin (1809–1882) conducted several experiments on seed germination, resulting in observations that contributed to his theory on the evolution of living organisms (Black 2009). He tested osmopriming by submerging in salty sea water the seeds from different plant species. The treatment was able to enhance germination for some of the tested seeds (Darwin 1857). The seed ability to survive in salt water suggested to Darwin that long-distance dispersal was a possible explanation for the geographical distribution of species (Black 2009).
Results from subsequent studies available at the beginning of the nineteenth century contributed to formulating the modern concept of seed priming. Kidd and West (1918, 1919) initially defined the dangerous practice of soaking dwarf bean (Phaseolus vulgaris L.) and pea (Pisum sativum L.) seeds before sowing. They also underlined that, despite this evidence, gardeners were still soaking their pea and bean seeds "to help them", taking advantage of their empirical ability to adjust the proper amount of water (Kidd and West 1918, 1919). The transition from this empirical awareness to a well-defined concept of seed priming, as a reproducible technique at the service of people working with seeds, started in the 1960s, with the early work of May et al. (1962) who evidenced that treated seeds dried under controlled conditions displayed fast germination under physiological and stress conditions. Then Ellis (1963) reported that the emergence of tomato (Solanum lycopersicum L.) seedlings was improved when seeds were treated with solutions containing different salts (K3PO4, KNO3, NaCl). Heydecker and his coworkers imbibed the seeds of several horticultural and ornamental crops using a solution of the osmotic agent polyethylene glycol (PEG), obtaining accelerated and uniform germination (Heydecker et al. 1973). The impact of the treatments was analyzed based on osmotic potential, temperature, duration, and the efficacy of their combinations. The successful output prompted the authors to comment on the “fascinating physiological implications” of this technique (Heydecker et al. 1973), subsequently named the “priming” of seeds (Heydecker and Gibbins 1978).
Since then, the use of seed priming has progressively increased, and different types of protocols have been developed. Among them, hydro-, osmo-, hormo-, chemo-, and bio-priming are widely applied and declined in many variants, tailored on plant genotype and seed lot, envisaged as tools to fight the current challenges of agriculture and ecosystems (Paparella et al. 2015; Marthandan et al. 2020; Paul et al. 2021). Our time travel continues nowadays with the noteworthy technological revolution brought by nanotechnology in agricultural research, in terms of seed quality enhancement and the ongoing discussion on the sustainability of nanomaterials (Chandrasekaran et al. 2020; Shelar et al. 2021; Amritha et al. 2021, do Espirito Santo Pereira et al. 2021).
Main features and priming treatments
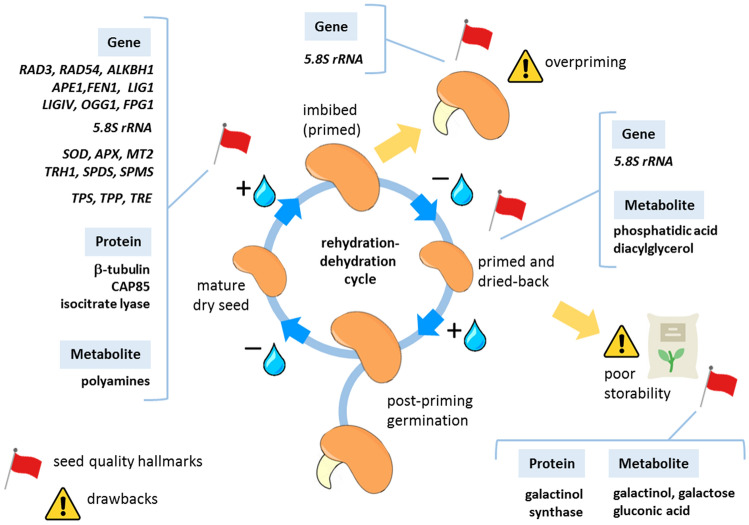
When seed priming is applied, controlled water uptake allows a boost of seed metabolism and accelerates germination, but it is mandatory to avoid the reach of the radicle protrusion stage (Soeda et al. 2005). If the seeds exceed this critical threshold, the germination process becomes irreversible, desiccation tolerance is lost and the dry‐back step will result in the death of these desiccation-sensitive seeds (Bradford et al. 1990). The concept of rehydration–dehydration cycle (Fig. 2) is used to represent the treatment (controlled seed imbibition followed by desiccation or dry-back) routinely applied in standard seed vigorization protocols. Such a concept represents an ideal biological context to explore some critical issues of the technique, related to loss and gain of desiccation tolerance (Fabrissin et al. 2021; Farooq et al. 2019; Paparella et al. 2015; Pagano et al. 2022a, 2022b). Some of the main conventional seed priming techniques (hydropriming, solid matrix priming, osmopriming, chemopriming, hormopriming, thermopriming) are briefly described in this paragraph along with hybrid methodologies and the most recent applications based on nanomaterials.
Fig. 2.
The rehydration–dehydration cycle as a schematic representation of the treatment (controlled seed imbibition followed by desiccation or dry-back) routinely applied in standard seed vigorization protocols. Primed seeds undergo post-priming germination. The different steps of the rehydration–dehydration cycle can be regarded as potential sources of novel seed quality hallmarks, specified as genes, proteins, metabolites
Hydropriming
This technique represents a cost-effective, and eco-friendly priming approach that requires soaking seeds in water only and the subsequent dry-back to their original weight. Each crop genotype shows a distinctive critical time threshold for soaking, below a safe limit that must be analytically assessed to gain the best impact on seed vigor (Harris et al. 2001). The treatment improves germination performance, seedling emergence, crop yield, and stress resilience (Farooq et al. 2010; Damalas et al. 2019; Khan et al. 2020a). Hydropriming has been tested in several climate-sensitive regions (Harris et al. 2001; Binang et al. 2012; Matsushima et al. 2013; Nakao et al. 2018, 2020; Adhikari et al. 2021). Yield improvement reached 28% in dicot plants, especially legumes, and 15% in cereals, respectively. However, treatments turned out to be more advantageous under abiotic stress conditions (22–28% yield enhancement) (Carrillo-Reche et al. 2018). Uncontrolled water uptake by the seeds represents the weakness point of hydropriming, since imbibition depends on the affinity of the seed tissues to water. For this reason, it is crucial to understand the optimal water volume, temperature conditions, and duration of the treatment to prevent radicle protrusion and the subsequent loss of desiccation tolerance. Despite the evident need to optimize protocols on a genotype scale or even seed lot scale, hydropriming remains the preferred option for seed technologists, breeders, and farmers, particularly when facing harsh agro-climatic conditions.
Solid matrix priming (SMP)
Seeds are mixed with a solid carrier that is water moistened to establish the desired water potential for effective priming, in such a way that imbibition is slowed down resembling the natural rehydration process in the soil. After the treatment, the solid matrix material needs to be mechanically separated without damaging the seeds (Damalas et al. 2019). Different types of solid insoluble matrices are available, e.g., charcoal, Cocopeat, Sphagnum moss, perlite, vermiculite, Celie or Micro Cell, diatomaceous earth clay, and sand. They can be combined to strengthen the priming effect as reported by Madsen et al. (2018), who tested a mixture containing calcium bentonite clay, diatomaceous earth, compost, worm castings, non-ionic alkyl terminated block copolymer surfactant, a plant growth regulator, a fungicide, a liquid fertilizer, and tap water. SMP carried out under enriched O2 conditions turned out to be effective on low-quality leek (Allium ampeloprasum L.) seeds (Ozden et al. 2018). SMP improves the pre-germinative metabolism, field emergence, and establishment, especially in horticultural crops (Grzesik and Nowak 1998; Lutts et al. 2016), cereals (Hacisalihoglu et al. 2018), and native grasses (Madsen et al. 2018). SMP provides the advantage of exerting a more effective control on moisture content and water potential during seed imbibition. However, some upgrades and optimization are still needed. A significant improvement in this technique has been achieved by developing novel and sustainable procedures that do not require separating seeds from the matrix material (Madsen et al. 2018).
Osmopriming
Seeds are subjected to osmopriming using aerated solutions containing potassium nitrate (KNO3), potassium phosphate (K3PO4), potassium chloride (KCl) salts, NaCl, CaCl2, MgSO4, or polyethylene glycol (PEG) with different water potentials, applied for different periods (Biswas et al. 2019; Feghhenabi et al. 2020; Lei et al. 2021, Hussain et al. 2022). In presence of osmotic agents, the amount of water entering the seeds is limited but sufficient to trigger the pre-germinative metabolism. In some cases, germination rate and percentage are more responsive to soaking time rather than water potential imposed by the osmotic agent (Abdallah et al. 2016; Mirmazloum et al. 2020). Salt priming enhances tolerance to salinity (Janda et al. 2016), heat (Qiu et al. 2003), and chilling (Cheng et al. 2014). PEG-primed seeds show improved germination and seedling growth rates, as well as stand establishment under drought (Goswami et al. 2013; Zhang et al. 2015), temperature stress (Murray et al. 1993; Parera and Cantliffe 1992; Bush et al. 2000; Nascimento et al. 2013; Lei et al. 2021, Hussain et al. 2022), or in presence of multiple stresses (Bittencourt et al. 2004; Chen et al. 2010). PEG is generally described as a successful priming agent (Moosavi et al. 2009; Patanè et al. 2009; Pradhan et al. 2015; Patade et al. 2015; Moreno et al. 2017; Abid et al. 2018; Hussain et al. 2019a; Nadeem et al. 2019), with some exceptions (Shahi-Gharahlar et al. 2009). Despite the huge number of reports describing the molecular and physiological mechanisms underlying the seed response to osmopriming under specific stress conditions, the current knowledge concerning the potential of osmopriming to strengthen the response against multiple stress factors is still scanty (Lei et al. 2021).
Halopriming
Priming with salt solutions is also referred to as halopriming. Seeds are soaked in solutions containing inorganic salts, e.g., NaCl, KNO3, CaCl2, CaSO4. Halopriming with NaCl was reported to enhance germination and seedling establishment in milk thistle (Silybum marianum L., Sedghi et al. 2010), as well as to increase salt tolerance of melon (Cucumis melo L., Sivritepe et al. 2005), canola (Brassica napus L., Farhoudi et al. 2007), sugarcane (Patade et al. 2009), and Vigna radiata L. (Jisha and Puthur 2014a). Halopriming applied to rice seeds resulted in enhanced protein, carbohydrate, and photosynthetic pigment content, along with antioxidant enzyme activities associated with reduced lipid peroxidation levels under salt stress (Jisha and Puthur 2014b). Halopriming treatments of maize seeds with NaCl improved germination and seedling biomass, leading to higher grain yield and water use efficiency in the field, particularly under drought stress conditions (El-Sanatawy et al. 2021). Halopriming applied to seeds triggers plant stress memory, preventing the deleterious impact of abiotic stresses such as drought and salinity (El-Sanatawy et al. 2021). This intriguing issue, currently under investigation, might provide novel insights into the molecular networks of seed priming and maximize the hidden potential of this technique (Srivastava et al. 2021).
Chemopriming
Exogenous and plant-derived chemicals are used as priming agents. An increasing range of molecules playing a signaling role in the plant stress response can act as priming agents. The list includes H2S, NO, and natural compounds (e.g., chitosan, melatonin, ascorbic acid, alpha-tocopherol, trehalose, and polyamines), and plant extracts. Their efficacy to enhance salt tolerance has been reported in different crop species, and mechanisms underlying the impact of these priming agents on the seed pre-germinative metabolism are also documented (Zulfiqar et al. 2022). Silicon-mediated seed priming gained attention as a strategy to induce stress adaptation (Farooq et al. 2021a; El-Serafy et al. 2021). The range of potential new agents for chemopriming is expanding, as reported for sodium nitroprusside (Hameed et al. 2021), 2,6 dichloro-isonicotinic acid (Martinez-Aguilar et al. 2021), pineapple stem-derived protease (Perez et al. 2021), polyamines and humic acid (Sheteiwy et al. 2017; Mridha et al. 2021; Hongna et al. 2021). Plant extracts containing bioactive molecules with high antioxidant potential can be effective priming agents, as reported for rice seeds treated with a carrot root extract rich in carotenoids, phenolic compounds, tocopherols, nitrogen compounds, and vitamins (Bigolin Teixeira et al. 2021). Chemopriming will benefit from the expanding technology for waste recycling since the valorization of by-products (e.g., from the processing of fruits, vegetables, tubers, cereals, and legumes) is expected to provide novel ingredients for sustainable seed priming formulations.
ROS-mediated priming
The beneficial effects of exogenous hydrogen peroxide (H2O2) have been documented (Wahid et al. 2007; Khan et al. 2015; Wojtyla et al. 2016; Dufková et al. 2019). H2O2 promotes germination within a proper dose range whereas it becomes toxic at high levels (Bailly et al. 2008). H2O2 is a signal molecule involved in phytohormone metabolism, such as SA-mediated signaling pathways. The synergistic action of H2O2 and SA was able to boost germination in maize seeds by promoting the antioxidant response and energy metabolism, particularly under chilling (Luo et al. 2017) and in kidney beans subjected to salt stress (Tania et al. 2022). A similar protective role of H2O2-mediated priming was highlighted in rice seeds and seedlings under drought stress in rice (Jira-Anunkul and Pattanagul 2020), as well as cauliflower seeds and seedlings challenged with salt stress (Ellouzi et al. 2021). Furthermore, H2O2 could improve the photosynthetic efficiency in sunflower plants developed from primed seeds, under salt stress (Silva et al. 2022). The complex cellular and molecular networks in which H2O2 is an active player cover a range of developmental and stress responses that require cross-talk with phytohormones and antioxidant mechanisms, sometimes ruled by genetic features. Such complexity should be more extensively investigated to provide knowledge useful to optimize the H2O2-based treatments.
Hormopriming
The use of different phytohormones as priming agents has become an established approach with proven beneficial effects in promoting plant stress tolerance (Rhaman et al. 2021). Successful seed priming has been reported using auxin (indole-acetic acid, IAA) (Eisvand et al. 2010; Fahad et al. 2015), cytokinins (CKs) (Bryksova et al. 2020), gibberellins (GAs) (Ghobadi et al. 2012; Ma et al. 2018), and abscisic acid (ABA) (Gurmani et al. 2011, 2013; Wei et al. 2015; Zongshuai et al. 2017; Safari et al. 2018). Seed priming with ethylene-related compounds was applied to lettuce (Lactuca sativa L.) to increase germination under high temperatures (Nascimento et al. 2004). Successful salicylic acid (SA)-mediated priming was also reported (Rehman et al. 2011; Li et al. 2017a; Khan et al. 2019; Karalija et al. 2021; Zhu et al. 2021). Jasmonic acid (JA) has been used as a priming agent to treat tomato seeds, enhancing the seedling ability to withstand nematode attacks (Bali et al. 2020). Priming with brassinosteroids (BRs) significantly enhanced tolerance to heavy metal toxicity (Basit et al. 2021, 2022), and drought (Huang et al. 2020), whereas the use of melatonin contributed to improved cold tolerance (Cao et al. 2019; Kołodziejczyk et al. 2021). Due to the complexity of phytohormone metabolism and regulatory networks, the selection of suitable conditions and effective molecules for hormopriming depends on a deeper knowledge of several key molecular players and the way they could contribute to boosting germination (Bryksova et al. 2020).
Thermopriming
Heat priming can be applied to hydrating or germinating seeds that are exposed to low or high temperatures. Heat priming provided advantages to Arabidopsis (Serrano et al. 2019) and Triticum aestivum L. (Fan et al. 2018) exposed to high temperatures. The stimulating impact of raising temperature on bread wheat seed germination was reported by Gerna et al. (2018), who showed how a commercial hot steam treatment was able to advance seed metabolism and redox shifts associated with germination and seedling growth. Ahmad et al. (2020) reported that thermopriming applied to V. radiata L. seeds, by treating at 4 °C for about 1 h followed by drying, had a significant impact on chlorophyll, carotenoids, protein, and proline content. Thermopriming at 60 °C for 6 and 10 h was successfully applied to safflower (Carthamus tinctorius L.) seeds resulting in improved yield and percentage of seed oil in the field (Barazandeh et al. 2019). Lentil (Lens culinaris Medik.) seeds that underwent heat priming for 6 h at 35 °C displayed beneficial effects, including synthesis of osmolytes and increased photosynthetic performance, particularly evident in heat-sensitive genotypes (Bhardwaj et al. 2021). The potential of thermopriming should be better exploited given the current drawbacks of global warming that affect crop productivity. Mechanisms that link heat priming at the seed level with the plant ability to withstand heat stress in the field should be better clarified.
Biostimulants
Complex mixtures derived from raw materials, e.g., waste from food and paper industries, safe for the environment and possessing a broad spectrum of biological activities, are used as biostimulants to improve seed germination (Bulgari et al. 2019; Gupta et al. 2022) and promote the plant defense response (Alzahrani and Rady 2019). Biostimulants based on protein hydrolysates, natural products derived from agricultural waste, can help reduce the use of chemical fertilizers. Given the complexity and heterogeneity of the starting materials, high-throughput automated phenotyping is used to speed up the selection of the best-performing formulations (Sorrentino et al. 2021). Biostimulants restored the oxidative balance in cucumber seeds exposed to heat stress, triggering the expression of ICL gene coding for isocitrate lyase, a key enzyme in seed germination (Campobenedetto et al. 2020). Flavonoids extracted from citrus fruits combined with a cell-free supernatant from a novel bacteria Devosia sp. SL-43 proved to be an effective priming agent (flavopriming) when applied to soybean and canola seeds under salt stress (Shah et al. 2022). Biostimulants represent a promising avenue in the context of sustainable agriculture, not only for their broad range of beneficial effects on germination and stress tolerance but also for their potential in the implementation of circular economy policies.
Nanopriming
Nanoparticles (NPs) of metal oxides, widely used in industries, appear as emerging tools for seed priming purposes in agriculture. The application of selenium and zinc oxide nanoparticles (SeNPs, ZnONPs) during B. napus seed imbibition under salinity stress showed how nanopriming was able to modulate the expression of ABA-related genes (El-Badri et al. 2021). Seed priming with titanium dioxide nanoparticles (TiO2-NPs) resulted in beneficial effects on biochemical, morphological, and physiological characteristics of coriander (Coriandrum sativum L.) plants under Cd stress (Sardar et al. 2022). MgO-based NPs promoted seed germination, growth, and photosynthetic efficiency of maize (Shinde et al. 2020), and V. radiata (Anand et al. 2020) seedlings, similar to what was reported for Ca-based NPs in rice (Yugandhar and Savithramma 2013). ZnO-based NPs applied to rice (Prerna et al. 2019) and wheat (Nadeem et al. 2019; Rai-Kalal and Jajoo 2021) could improve productivity. According to Li et al. (2021), ZnO NPs could mitigate Cd toxicity in rice by promoting an increase in seedling weight associated with changes in antioxidant response and metabolic pathways related to DNA/RNA synthesis. Similarly, Salam et al. (2022) reported a higher content of nutrients and antioxidant enzymes in ZnO NPs-primed maize seeds and seedlings able to withstand Co toxicity, with enhanced growth and yield. Promising results were reported when ZnO-based NPs were used to increase seed germination in lettuce (Rawashdeh et al. 2020). NPs containing SiO2 used as seed priming agents improved drought tolerance in wheat (Rai-Kalal et al. 2021). Nanoscale micronutrient iron (α-Fe2O3), prepared via co-precipitation with the marine macroalga Turbinaria ornata and used as a priming agent, could enhance seed germination in rice and maize (Prerna et al. 2021). Si-based NPs might help increase plants' biomass and yield while reducing oxidative stress and Cd uptake in wheat grains (Hussain et al. 2019b). Seed priming with commercially available silver nanoparticles (AgNPs) enhanced salinity tolerance in pearl millet (Pennisetum glaucum L.) (Khan et al. 2020b). AgNPs-mediated seed priming in Chinese cabbage (Brassica rapa subsp. Pekinensis) increased crop yield and nutritional quality with the added value of biosafety, as Ag did not bioaccumulate in edible tissues (Zhou et al. 2021). Nanoparticulate systems represent a sustainable approach to convey bioactive compounds for agricultural applications, as in the case of successful seed priming mediated by alginate/chitosan (nanoALG/CS) and chitosan/tripolyphosphate (nanoCS/TPP) containing GA3 performed in tomato (do Espírito Santo Pereira et al. 2019). Multi-walled carbon nanotubes (MWCNTs) were used to prime wheat seeds, resulting in accelerated germination, enhanced growth, and higher yield, providing a new opportunity based on cost-effective nanomaterials for boosting crop performance (Joshi et al. 2018). Despite the recent advances, there is still a gap of knowledge concerning the way nanoparticles might affect seed physiology and microenvironments, e.g., it has been suggested that dry-back can alter the properties of nanomaterials and seed viability (Shelar et al. 2021). For this reason, additional studies are required to mitigate such risk.
‘Green’ priming
Synthesis of plant-based nanoparticles is a further refinement of nanotechnology that uses sustainable manufacturing processes to produce safe and innocuous nanoscale biomaterials for agricultural applications (Singh et al. 2018; Amritha et al. 2021). AgNPs synthesis by laser ablation, irradiation, thermal treatment, or chemical reduction shows several drawbacks, such as energy requirement and use of organic solvents, resulting in hazardous wastes and difficulty in scale-up. Differently, green synthesis is low cost, safe, and eco-friendly since nanoparticles contain organic materials (lipids, proteins, polysaccharides) with unique physical and chemical properties (Masukar et al. 2011, Mochochoko et al. 2013). Ag-based ‘green’ nanoparticles have an added protective advantage because of Ag anti-bactericidal and anti-fungicidal properties. Polysaccharides extracted from Chlorella vulgaris were used to produce AgNPs showing antimicrobial activity against Bacillus sp., Erwinia sp., Candida sp., and effective seed priming in Triticum vulgare and P. vulgaris (El-Naggar et al. 2020). Turmeric oil nanoemulsions (TNE) and AgNPs synthesized from agro-industrial byproducts (curcumin-removed turmeric oleoresin combined with onion (Allium cepa L.) peel extract as reducing agent) were used as nanopriming agents for watermelon (Citrullus lanatus) seeds. This eco-friendly and sustainable nanotechnological approach enhanced seed germination, growth, and yield while maintaining fruit quality (Acharya et al. 2020). Biocompatible FeO NPs synthesized using Cassia occidentalis L. flower extracts were tested as nanopriming agents and shown to promote germination of Pusa basmati rice seeds, by enhancing α-amylase activity, iron acquisition, and elevated soluble sugar levels (Afzal et al. 2021). The effects of nanopriming with galactomannan-stabilized phyto-complexed calcium hydroxide (Ca(OH)2), selenium oxyanion-calcium hydroxide SeO-(Ca(OH)2), and selenium-calcium hydroxide Se-(Ca(OH)2) nanocomposites were tested in V. radiata seeds. Seed extracts from Cassia angustifolia, rich in galactomannan and other biomolecules, enabled their terminal oxygen and hydroxide groups to bind Ca and Se ions. The porous Se-(Ca(OH)2) nanocomposite showed high efficacy in interacting with seed embryos and stimulating germination (Antony et al. 2022). Nano-scale zero-valent iron (G-nZVI), synthesized using fruit peel waste of Punica granatum L., could increase the germination percentage and seedling vigour of rice (Guha et al. 2021). Nanostructured lignin microparticles (LNP), obtained from alkaline lignin by acid treatment, were tested on maize seeds resulting in beneficial effects (Del Buono et al. 2021). ZnO NPs obtained through green synthesis using Senna occidentalis leaf extract were used to prime-aged Pusa basmati rice seeds (Sharma et al. 2021). With ‘green’ priming, the opportunity to meet higher and better yields with sustainability is further enhanced, while the continuous advances in the field of nanotechnology will contribute to fully exploiting the potential of this technique.
Hybrid priming
Multiple priming agents are combined and used synergically to boost multiple levels of stress tolerance in crop plants. Hydro-electro hybrid priming (HEHP) was successfully applied to onion (Zhao et al. 2018) and tomato (Garcia et al. 2021) seeds by combining hydropriming with exposure to electrostatic field irradiation. Positive outputs were also reported when combining hormopriming with osmopriming in broccoli (Hassini et al. 2017) and with hydropriming in sunflower (Górnik and Lahuta 2017). GA and H2O2 were combined to treat tobacco (Nicotiana tabacum L.) seeds using diphenylene iodonium chloride and uniconazole, as inhibitors of H2O2 and GA synthesis, respectively. The study evidenced that GA and H2O2 were essential for seed germination by decreasing ABA/GA ratio and stimulating reserve mobilization (Li et al. 2018). Priming Sulla carnosa seeds with SA and H2O2 significantly improved the growth performance and the rhizosphere acidification of iron-deficient plants (Jelali et al. 2021). Benefits were observed with the combined application of phytohormones and other growth regulators (Sharma et al. 2020), as in the case of lentil seeds exposed to γ-aminobutyric acid (GABA) and heat priming (Bhardwaj et al. 2021). The diverseness of seed priming protocols provides the opportunity to combine different approaches in the attempt to generate a synergic positive impact on the seed pre-germinative metabolism. Possibly, dealing with richer assortments of priming agents will require more complex and time-consuming procedures, although this challenge would result in targeted and sustainable hybrid priming protocols.
Pre-germinative metabolism: the key to understanding seed priming
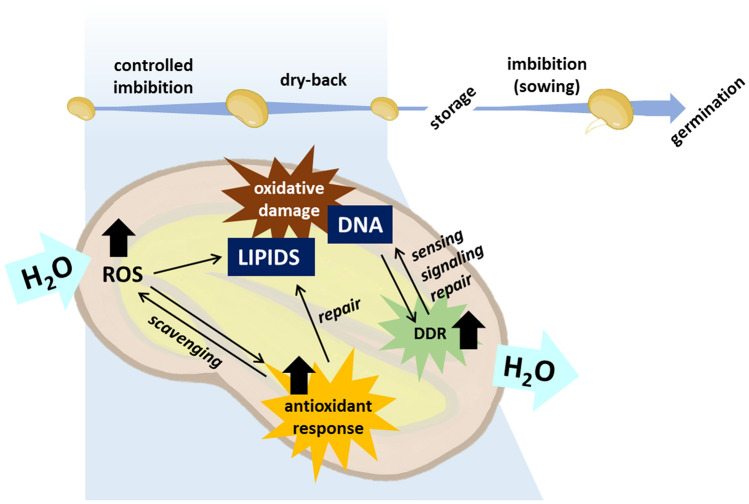
To provide an overall description of the germination process while also taking into account inter-specific variability, Bewley (1997) proposed a triphasic pattern based on the dynamics of water uptake. At the onset of the germination process (Phase I), seed coat permeability and tissue capillarity physically drive the water uptake required for the resumption of seed metabolism. During the subsequent phase (Phase II), the seed water potential is balanced, leading to a reduction in the rate of water uptake as metabolism transitions toward germination. With post-germination (Phase III), further water uptake is associated with radicle protrusion, with the consequent increase in water content associated with seedling establishment. From a metabolic perspective, the transition from imbibition to radicle emergence implies the consequential activation of energy metabolism, DNA and membrane repair mechanisms, turnover of stored transcripts, de novo transcription and translation, cell elongation, and reservoir mobilization (Bewley and Black 1994; Bewley 1997; Nonogaki et al. 2010).
The first wave of metabolic activities triggers energy production and reserve remobilization, the translation mechanism, and protects against oxidative stress by promoting antioxidant and repair pathways (Rajjou et al. 2012; Domergue et al. 2019). This highly complex and sophisticated plethora of molecular and metabolic pathways confined within the early germination temporal frame has been termed ‘pre-germinative metabolism’ (Paparella et al. 2015; Macovei et al. 2017). To date the scientific literature has provided increasing evidence of the role played by different components of the pre-germinative metabolism in the seed response to priming, strengthening the idea that a deeper understanding of such mechanisms represents a fundamental path toward “next-generation” priming tools. The main pathways contributing to the seed response to priming, and currently regarded as potential sources of novel seed quality molecular hallmarks, are described below whereas a graphical representation of their cellular and subcellular localization is provided in Fig. 3 and Fig. 4, respectively.
Fig. 3.
Molecular mechanisms of seed priming. The main drawbacks of the technique, overpriming and reduced shelf-life of primed seeds are also represented
Fig. 4.
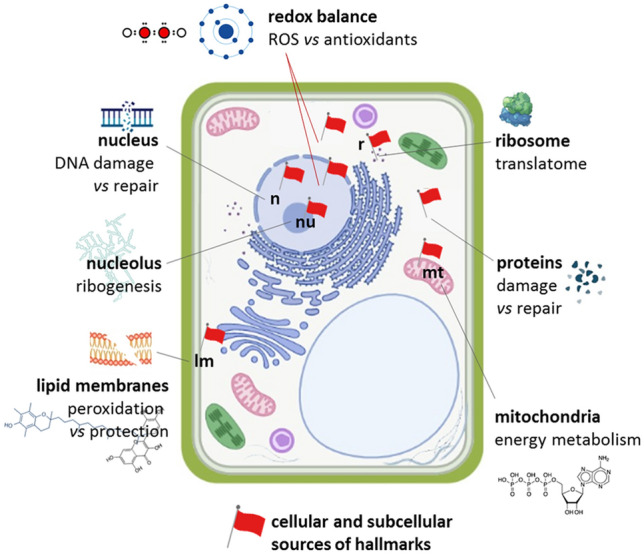
Representation of the cellular and subcellular sources of the different seed quality hallmarks currently under investigation. ROS reactive oxygen species, n nucleus, nu nucleolus, mt mitochondria, lm lipid membrane, r ribosome
Energy metabolism as an indicator to assess the seed's physiological response to priming
Respiration is a key process during early seed imbibition, dedicated to fulfilling the energy requirements of the developing heterotrophic embryo (Bewley 1997). ATP synthesis and oxygen consumption occur rapidly upon rehydration, and this requires the presence of intact and functional mitochondria in the desiccated embryo cells (Raveneau et al. 2017; Paszkiewicz et al. 2017; Nietzel et al. 2020), although other reports underlined the need for maturation of protomitochondria before respiration can start (Law et al. 2012). In a recent study, Nietzel et al. (2020) were able to define the kinetics of reactivation of mitochondrial energy and identify the target cysteine residues acting as thiol switches in early germination, highlighting the impact of redox metabolism and its link to energy-related pathways. Depending on both seed type and available substrates, the respiration activity significantly differs. Raymond et al. (1985) carried out a comparative study on starchy and fatty seeds, evidencing that under aeration ATP was predominantly generated by respiration in all the tested seeds whereas fermentation significantly contributed only in some species. Under anoxia, starchy seeds (e.g., peas and maize) performed better in terms of energy metabolism than fatty seeds (e.g., lettuce) (Raymond et al. 1985). The fermentative pathway is less effective in ATP production and excess accumulation of the related products (e.g., acetaldehyde and ethanol) compromises seed germination (Zhang et al. 1995). ATP is required to support high germination rates (Ching 1973; Lunn and Madsen 1981; Kibinza et al. 2006; He et al. 2019; Qu et al. 2020), to help seeds withstand deterioration (Anderson 1977), and to accomplish successful seed priming (Corbineau et al. 2000) although reports are underlining how high ATP content (Perl 1986; Corbineau et al. 2002), as well as extracellular ATP levels (Wang et al. 2022), can affect germination. Despite contrasting reports, energy metabolism—measured, e.g., in terms of energy charge and ATP:ADP ratio—could be an informative target to explore the impact of priming treatments on seed physiology (Corbineau et al. 2002; Marthandan et al. 2020) (Fig. 3).
The seed translatome provides insights into the mechanisms ruling germination and priming
Translatome (the global picture of ribosome-associated mRNA) has been recently used to assess changes in protein levels during seed germination (Bai et al. 2021). By looking at the translatome, it is possible to capture and integrate those multiple and variable regulatory events that modulate protein synthesis and define cell physiology. Besides the traditional approach of polysomal profiling, more recent technological advances allow to define the position of ribosomes at the level of codons (King and Gerber 2016), thus providing a closer perspective on the translatome dynamics. Messenger RNAs accumulated during seed maturation are translated during early seed imbibition (Rajjou et al. 2004). Selected stored mRNAs, loaded into polysomes, include those involved in redox processes, glycolysis, and protein synthesis (Sano et al. 2020). The comparison between proteome, transcriptome, and polysome changes, along with the different germination phases, has allowed to integrate multi-level data and gain novel insights into the translational control of seed germination. This can be envisaged either as a strategy evolved to limit energy consumption or as a sort of checkpoint for germination to occur (Bai et al. 2021). The direct recruitment of ribosomal subunits on cis-acting elements (internal ribosome entry sites-IRES), mediated by IRES-specific trans-acting factors (ITAFs), is a key step that triggers m7G cap-independent translation of mRNAs investigated in the context of seed germination (Sano et al. 2020). One of these ITAF players, the ErbB3-binding protein (EBP1), was found to over-accumulate in primed sugarbeet (Beta vulgaris L.) seeds (Catusse et al. 2011). De novo protein synthesis resulted in the significant production of antioxidant enzymes involved in ROS (reactive oxygen species) scavenging (Galland et al. 2014), driving attention to the complex role of ROS in seed germination and priming. The seed translatome, a crucial element to understand the complex regulatory pathways of germination, can be also regarded as a potential source of seed quality hallmarks (Fig. 3 and Fig. 4), respectively.
The seed antioxidant response and its impact on successful priming
The dual role of ROS in early seed germination has been extensively dissected (Bailly et al. 2004, 2008; Kranner et al. 2010; Jeevan Kumar et al. 2015; Bailly 2019). ROS generated during the rehydration of seeds represents a major source of cellular damage (Kibinza et al. 2006; Kurek et al. 2019). On the other hand, successful germination relies on the well-known ‘oxidative window’, in which ROS act as signal molecules and trigger germination (Oracz et al. 2007, 2016, Wojtyla et al. 2016; Barba-Espin et al. 2011; Bailly 2019). The oxidative window is established thanks to the essential and tightly regulated ROS-scavenging activity of both enzymatic and non-enzymatic players (Bailly et al. 2008; Bailly 2019; Bailly and Merendino 2021; Li et al. 2022a). Besides ROS, also RNS (reactive nitrogen species) perform as signaling molecules to promote germination within the oxidative window (Jeevan Kumar et al. 2021; Farooq et al. 2021b). Overall, the connection between a high antioxidant performance observed in the pre-germinative metabolism window and the seed's ability to display improved germination and seedling growth under environmental stresses is consolidated by an increasing body of literature. Such a correlation has become a reference point for the validation of novel formulations that combine conventional and innovative priming agents applied to a wide range of crop varieties. Bioregulators, e.g., auxins, gibberellins, cytokinins, abscisic acid, brassinosteroids, polyamines, strigolactones, and ascorbic acid, provide effective protection against oxidative stress due to their ability to modulate the plant antioxidant system. Given this relevant role, they can be applied as priming agents alone, or in combination with other treatments (Zulfiqar and Ashraf 2021). The dynamics of ROS accumulation versus the seed antioxidant response are envisaged as sources of potential seed quality hallmarks (Fig. 3). The impact of different seed priming techniques has been evaluated in terms of antioxidant response in cereals (Hameed et al. 2019; Cao et al. 2019; Sen and Puthur 2020; Khan et al. 2020a; Shah et al. 2021; Guo et al. 2022), legumes (Kesharvarz et al. 2017; Lilya et al. 2019; Forti et al. 2020b; Chen et al. 2021; Pagano et al. 2022a, 2022b), Solanaceae (Anand et al. 2019; Ali et al. 2019; Bali et al. 2020; Forti et al. 2020a, 2021), and tree species (Zhai et al. 2022). The beneficial effects of seed priming can extend beyond germination and seedling development since these treatments contribute to enhancing the antioxidant defense in field-grown plants (Fig. 4). Seed priming with ascorbic acid could enhance salt tolerance, boosting the antioxidant response of tomato Micro-Tom plants and improving growth and fruit yield (Alves et al. 2021). Furthermore, the enhanced antioxidant activity promoted by seed priming contributes to plant recovery from stress (Aswathi et al. 2022).
DNA damage response: the key to preserving genome integrity in seeds
A key aspect of the pre-germinative metabolism is the molecular network underlying DNA damage sensing, signaling, and repair, namely the highly conserved DNA damage response (DDR), essential for genome maintenance (Bray and West 2005; Nikitaki et al. 2018; Waterworth et al. 2010, 2015, 2016, 2019, 2022; Kiran et al. 2020). The entire set of the genetic information stored inside the DNA double helix must be preserved by removing any deleterious change resulting in oxidative stress induced by either exogenous factors or endogenous by-products accumulated along the seed life cycle (Jeevan Kumar et al. 2015; Waterworth et al. 2019; Kurek et al. 2019). Pioneering work has set the starting scenario for disclosing the issues of DNA repair in germinating seeds (Cheah and Osborne 1978, Osborne 1980, 1983; Zlatanova et al. 1987; Dandoy et al. 1987; Elder and Osborne 1993). Rehydration is required for the resumption of the entire metabolic network, including DNA repair that takes place during early imbibition when the embryo cells are at the G1 stage (Bewley 1997; Osborne 2000). In the desert plant Artemisias sphaerocephala, imbibed seeds release pectinaceous mucilage that preserves the seed moisture during germination. Interestingly these authors hypothesize that such conditions might promote DNA repair under environmental stress (Yang et al. 2001).
DNA repair pathways triggered by seed priming
Controlled rehydration carried out during priming triggers specific DNA repair mechanisms, such as the base excision repair (BER) pathway in which DNA glycosylases remove oxidized bases, generating abasic sites that are subsequently filled through DNA synthesis. The most frequent oxidation product is 8-oxoguanine, removed by either the 8-oxoguanine DNA glycosylase/lyase (OGG1) or formamidopyrimidine-DNA glycosylase (FPG) (Córdoba-Cañero et al. 2014). Upregulation of OGG1 and FPG genes has been reported in hydroprimed and bioprimed M. truncatula seeds (Forti et al. 2020a, b; Pagano et al. 2022a), and osmoprimed eggplant (Solanum melongena L.) seeds (Kiran et al. 2020). Seed priming triggers DSBs repair through non-homologous end joining (NHEJ) and homologous recombination (HR), as well as mismatch repair (MMR) pathways (Kiran et al. 2020). Upregulation of SOG1 gene, encoding the master regulator of DDR in plants, was reported along dry-back in hydroprimed M. truncatula seeds, indicating active DNA damage-dependent signaling (Pagano et al. 2022a). Despite these pieces of evidence, the current knowledge about DNA repair and genome maintenance mechanisms activated by seed priming still needs to be deeply explored.
DNA repair genes as early indicators of the seed response to priming
Highly conserved, specific repair pathways target the different types of DNA lesions such as base- and nucleotide-excision repair (BER, NER) acting on base damage/single-strand breaks (SSBs) and bulky lesions, as well as non-homologous end joining (NHEJ), alternative NHEJ pathways (alt-NHEJ), and homologous repair (HR) involved in the repair of double-strand breaks (DSBs). Such mechanisms have been investigated in the context of pre-germinative metabolism to assess their impact on seed quality, their involvement in the response to priming, and their potential as sources of seed quality hallmarks (Fig. 3). The highly conserved DNA damage checkpoint kinases ATAXIA TELANGIECTASIA MUTATED (ATM) and ATM AND RAD3-RELATED (ATR) are now regarded as crucial determinants of seed viability and their function can be modulated in response to seed dormancy and environmental signals (Waterworth et al. 2016, 2019). DSBs are highly cytotoxic and can cause major karyotypic instability leading to cell death (Amiard et al. 2013).The seed repair response for removing DSBs is triggered during early imbibition, even in high-quality seeds, with a significant role played by DNA ligases (Waterworth et al. 2010). Along with seed imbibition, increased levels of 7,8-dihydro-8-oxoguanine (8-oxodG) can be detected (Macovei et al. 2010, 2011a, 2011b; Balestrazzi et al. 2011). Upregulation of several BER genes, e.g., OGG1 (8-oxoguanine DNA glycosylase), FPG1 (formamidopyrimidine-DNA glycosylase), and TDP1 (tyrosyl-DNA phosphodiesterase) associated with metabolic resumption has been reported in imbibed seeds of Medicago truncatula (Pagano et al. 2017, 2019, 2022a; Forti et al. 2020b), Arabidopsis (Chen et al. 2012a; Cordoba-Canero et al. 2014), and eggplant (Forti et al. 2020a; Kiran et al. 2020). To date, reports that investigate the use of the expression profiles of DNA repair genes, e.g., BER genes, as early indicators of the seed response to priming have highlighted both genotype- and seed lot-dependent variability (Forti et al. 2020a, 2021).
Seed proteome integrity and protein repair/removal: another perspective toward molecular hallmarks
Water uptake triggers the resumption of the functional seed proteome; however, damaged proteins resulting from the desiccation–rehydration cycle need to be repaired or removed. It has been reported that proteins involved in the translation process are preferred targets of the seed protective and repair mechanisms. Issues regarding proteome integrity could disclose new seed quality hallmarks (Fig. 3). The ‘Job’s rule’ states that any other protein can be replaced in imbibed seeds except for those belonging to the translational machinery (Rajjou et al. 2008). Furthermore, proteins associated with the translational apparatus, e.g., ribosomal proteins, are significantly long lived due to peculiar protective mechanisms. They associate with the multi-task LEA (Late Embryogenesis Abundant) proteins acting as shields to prevent damage to the cellular components (Battaglia et al. 2008; Dirk and Downie 2018). Accumulation of LEA proteins has been reported in primed seeds of different species, such as sugarbeet (Capron et al. 2000) and rapeseed (Kubala et al. 2015a). Recurrent damage, resulting from oxidative stress and detected in desiccated as well as aged tissues, is represented by isoaspartate formation. This is the preferred substrate of isoaspartyl methyltyransferase, a repair enzyme active during seed imbibition, particularly on those proteins involved in the processing of the ribosomal RNAs in the nucleolus (Galland and Rajjou 2015; Dirk and Downie 2018). Osmopriming applied to aged tomato seeds was able to restore the activity of isoaspartyl methyltyransferase, suggesting a role of this enzyme in cellular repair during the treatment (Kester et al. 1997). More recently, such a role has been further dissected, revealing that isoaspartyl methyltyransferase repairs isoaspartyl damage occurring in the antioxidant enzymes SOD and CAT, contributing to protection against oxidative stress (Ghosh et al. 2020). Due to the intrinsic properties of seed priming, and the need to keep the balance between stimulation of germination and the undesired side effects caused by excess oxidative injury, proteins involved in repair mechanisms represent promising indicators. The proteome integrity in rehydrating seeds, and specifically the translational machinery components, is also under the control of methionine sulfoxide reductase. The latter prevents the accumulation of oxidized methionine which contributes to decreased translation fidelity (Nelson et al. 2014; Dirk and Downie 2018). The chaperonin peptidyl-prolyl cis–trans isomerase, required for proper folding through proline isomerization, and heat shock proteins that protect against misfolding, are included in the set of relevant repair proteins active in germinating seeds (Dirk and Downie 2018). In vivo protein oxidation generates carbonyl groups, an irreversible process leading to the selective 20S proteosome-mediated degradation of carbonylated proteins (Nyström 2005). ROS-induced oxidation of storage proteins during seed imbibition triggers reserve mobilization by promoting proteolytic cleavage, thus supporting the germination process (Job et al. 2005; Barba-Espín et al. 2011; El-Maarouf-Bouteau et al. 2013). Similarly, oxidative modifications of mRNAs are implicated in the early step of seed germination, since the occurrence of modified bases in selected transcripts impairs translation, possibly facilitating dormancy release (El-Maarouf-Bouteau et al. 2013).
The emerging contribution of nucleolus to seed priming issues
Metabolic resumption and recovery of translation at the onset of germination are linked to the nucleolar functional restoration since the plant nucleolus is the site of ribosomal RNA synthesis and ribosome biogenesis (Kalinina et al. 2018). Morphological changes (e.g., vacuolization) associated with nucleolar activation have been reported in early studies on germinating embryos (Deltour and de Barsy 1985); however, the current knowledge concerning the role of nucleolus in the context of the pre-germinative metabolism is still scanty. Evidence has been so far provided about the involvement of plant nucleolus in stress signaling pathways, and in the maintenance of genome integrity (Kalinina et al. 2018). Given such findings, the molecular processes occurring within the nucleolus might significantly contribute to seed vigor and take part in the complex response to seed priming (Pagano et al. 2022a). DDR genes implicated in the seed repair response during rehydration have been correlated with nucleolar functions as reported for TDPs (Donà et al. 2013; Macovei et al. 2018). A deeper investigation of those processes connecting ribogenesis and DDR will provide valuable knowledge for applied research purposes, as discussed below (see overpriming). It is worth noting that the knowledge on nucleolar function has been so far gathered using Arabidopsis as a model plant, whereas a few studies dealing with pre-germinative metabolism have been performed in the model legume M. truncatula. Although promising, such data need to be validated in a larger panel of model and crop plants. Furthermore, the potential of the nucleolus as a source of novel seed quality hallmarks (Fig. 3) still needs to be deeply explored.
The current scientific literature reflects the efforts made by the plant research community to dissect the plethora of molecular events underpinning the seed response to priming and clarify those mechanisms that promote the seed's ability to repair stress-induced damage. The complexity of the pre-germinative metabolism is delaying the current research addressing one of the main open questions listed in Fig. 1: What is the contribution of seed repair response in germination and priming? The seed priming techniques remain too empirical and the frequency of undesired side effects, that compromise the successful outputs of treatments, is far from being acceptable in terms of economic value and competitiveness of the final product. The availability of additional experimental systems will help expand the current knowledge of the drawbacks of seed priming.
Exploring the drawbacks of seed priming
When does seed priming fail? A frequent drawback is overpriming that compromises survival to desiccation (dry-back step), but a relevant issue is also the reduced shelf-life of the primed seeds (Wang et al. 2018, Zulfiquar 2021, Tu et al. 2022). A systematic analysis of the molecular events occurring in seeds subjected to non-optimal priming treatment is missing. In addition, the strong genotype- and seed lot-dependent variability represents a major concern since it delays the search for common hallmarks of seed quality. This section provides a critical review of the current literature dedicated to overpriming and post-priming storage.
Overpriming compromises survival to dry-back
Among the parameters that should be kept under control, there is the duration of the rehydration step. When the treatment is prolonged, the resulting metabolic acceleration can easily bring seeds to overcome the critical threshold that leads to irreversible germination (Fig. 2). When such a condition occurs, desiccation tolerance is lost and embryos will not survive the dry-back step. This process, known as overpriming, can significantly compromise the efficacy and economic value of priming protocols. Interestingly, it is also known that desiccation tolerance can be rescued within a short developmental window between germination and seedling establishment when seeds are challenged with osmotic stress (Bruggink and van der Toorn 1995; Peng et al. 2017). It has been suggested that the resumption of desiccation tolerance can help seeds facing unpredicted drying conditions soon after germination (Dekkers et al. 2015). In M. truncatula, this crucial developmental window exists only when the length of the protruding radicle ranges between 1 and 3 mm (Buitink et al. 2003; Maia et al. 2011), and it has been exploited to establish an experimental system to investigate the role of antioxidant response and DNA repair pathways in primed versus overprimed M. truncatula seeds as well as the molecular events associated with the progressive loss of desiccation tolerance (Pagano et al. 2022a,b). The focus on the role of nucleolus and ribogenesis in such a context of enhanced oxidative stress and genotoxic damage allowed disclosing of novel potential molecular players linked to dehydration-induced genotoxic stress. Nucleolar perturbation resulted in increased accumulation of precursor and mature rRNAs, detected only in overprimed embryos along the dry-back step. Among the tested rRNA, precursor and mature 5.8S rRNA showed an early peak at 2 h of dry‐back only in overprimed embryos, suggesting a potential role as a stress hallmark concerning overpriming (Pagano et al. 2022a). Although a similar profile was also reported by the same authors in overprimed seeds of the closely related legume alfalfa (Medicago sativa L.), validation of 5.8S rRNA as a stress hallmark, at least in legumes, will necessitate testing across a wider range of species and/or genotypes.
Conditions leading to overpriming change with species, cultivars, and seed lots
The use of different model systems becomes a necessary premise to gain insights into the associated events. Cotyledons, hypocotyls, and radicles display different levels of desiccation tolerance, while the aerial parts are the most desiccation tolerant (Reisdorph and Koster 1999; Dekkers et al. 2015). It is also known that desiccation tolerance can be improved by applying osmotic agents or phytohormones (Reisdorph and Koster 1999; Buitink et al. 2003). Furthermore, it is essential to consider the variability of different subpopulations within a seed lot in their response to priming and dry-back. Such issues are reflected in one of the main open questions listed in Fig. 1: How to tackle inter- and intra-specific variability in assessing and improving seed quality? Pagano et al. (2022b) investigated the occurrence of mild/severe overpriming in M. truncatula seeds, testing kinetin-mediated hormopriming as a tool to counteract overpriming and using ROS accumulation during dry-back as a potential hallmark of post-priming seedling establishment performances. A significant increase in the percentage of seedlings with aberrant morphology positively correlated with the increased radicle length measured before dry-back. Kinetin-mediated hormopriming was not able to rescue overpriming; however, it could accelerate and synchronize germination and this effect could represent an alternative route to decrease the exposure to desiccation stress within a tailored priming protocol (Pagano et al. 2022b). On the other hand, prolonged exposure to kinetin results in reduced radicle growth, associated with global metabolomic depletion and accumulation of DSBs (Araujo et al. 2019). A direct correlation was evidenced between radicle length measured before dry-back, ROS levels quantified at the end of dry-back, and the occurrence of ‘aberrant’ seedling morphology. Both spectrophotometric assays and DAB staining highlighted ROS accumulation in M. truncatula embryos during post-priming dry-back, and similar profiles were also observed in M. sativa (Pagano et al. 2022b). Such findings suggest the possibility of using ROS as hallmarks of seed priming progression, at least in these two closely related legumes. Such a case study could be reproduced in horticultural model/crop species and/or related CWRs, according to the need to explore different models of pre-germinative metabolism.
Storage potential is decreased in primed seeds
Another major drawback of seed priming is the limited shelf-life of the treated seeds (Fig. 2), observed in most cases (Nath et al. 1991; Owen and Pill 1994; Bray 1995; Hussain et al. 2015; Yan 2017), although beneficial effects on seed storability are also documented (Burgass and Powell 1984; Georghiou et al. 1987; Dearman et al. 1986; Abnavi and Ghobadi 2012; Pandey and Pati 2017; Das and Dutta 2022). Specific case studies are reported for cotton seeds subjected to mannitol-mediated osmopriming able to maintain enhanced germination performance for at least 12 months of storage at room temperature, thus having an added value for commercial purposes (Toselli and Casenave 2014). Interestingly, priming applied to low-vigor seeds boosts the repair of damaged structures and improves the storage potential whereas the physiological acceleration triggered in high-vigor seeds can be deleterious to longevity (Powell et al. 2000). Both dry-back and storage conditions affect post-priming seed longevity; a rapid dehydration step may alter the levels of soluble carbohydrates, impairing desiccation tolerance whereas slow dehydration may improve the storability of primed seed (Bruggink et al. 1999). Several environmental factors, namely oxygen availability, moisture, and temperature, are regarded as key players in seed deterioration. Increased oxygen levels promote cellular respiration and, combined with high temperature and relative humidity, accelerate seed deterioration (Ellis et al. 2008; Schwember and Bradford 2011). Such conditions trigger ROS accumulation leading to oxidative damage, loss of membrane integrity, depletion of seed reserves, and loss of seed viability (Liu et al. 2016). The impact of these factors on the longevity of primed seeds is still poorly investigated and this reminds that there is still an open question in applied research: How to overcome the reduced storability of primed seeds? (Fig. 1).
How to maintain the priming benefits in stored seeds?
Improved knowledge of the cellular and molecular events associated with the different storage conditions applied to primed seeds is required to support the technology. Primed seeds are more prone to oxidative damage compared to non-primed seeds (Hussain et al. 2015). Reduced starch metabolism in primed rice seeds has been correlated with decreased germination; however, it has been suggested that, despite reserve depletion, seeds still retain metabolic energy for germination (Justice and Bass 1978). Vacuum storage is beneficial to primed seeds possibly because they are kept under low moisture content and limited respiration (Chiu et al. 2003; Yeh et al. 2005; Hu et al. 2006; Feng et al. 2017; Wang et al. 2018). The metabolic acceleration imposed by priming and the related repair activities triggers cell cycle progression. Indeed, the screening of biologically active compounds able to suppress Arabidopsis seed deterioration revealed that treatments with the cell cycle inhibitor mimosine and other molecules with similar roles (aphidicolin, hydroxyurea, and oryzalin) were able to improve seed storability (Sano and Seo 2019). Priming with antioxidants has been also tested as a strategy to prolong rice and oat seed viability during storage (Xu et al. 2020; Xia et al. 2020). The poor performance of primed, desiccation-tolerant orthodox seeds under storage resembles the response of desiccation-sensitive, recalcitrant seeds. It has been reported that recalcitrant seeds do not produce/activate components/mechanisms that typically provide desiccation tolerance in orthodox seeds (Berjak and Pammenter 2013). For instance, the absence of LEA proteins in recalcitrant seeds of Castanospermun australe turned out to be a crucial determinant of desiccation tolerance (Delahaie et al. 2013). Looking at the molecular dynamics occurring in recalcitrant seeds might bring knowledge useful to unravel the response to storage observed in primed orthodox seeds. In this context, basic research should be expanded in both model and crop species.
Farmers need to know how long the primed seeds can retain high germinability before planting to properly manage their seed lots and companies are committed to providing such information. However, the seed potential to survive long-term storage differs among cultivars and species; thus, one possible strategy to overcome this drawback would be the selective use of genotypes with enhanced storage potential. Improving such a trait will increase the commercial value of primed seeds as the farmers will be able to manage sustainably their field activities when challenged by deleterious climate conditions or unexpected drawbacks. Moreover, decoding the genetic and physiological traits underlying the storability of primed seeds may lead to the development of targeted assays to monitor and prevent seed deterioration in different crop species, with beneficial effects on seed marketing and distribution.
Protocols for effective storage of primed seeds
It is difficult to list guidelines for improving the viability of stored primed seeds. Several parameters should be considered carefully, e.g., storage time should be defined based on the seed ageing profile, priming and conservation conditions should be adjusted to buffer genetic and environmental variability. Tu et al. (2022) reported that the priming effects were maintained in pepper seeds preserved in sealed plastic bags at a low temperature. Priming with ascorbic acid and sodium nitroprusside allowed an improved performance of stored sunflower seeds, possibly due to a boost in the antioxidant defense (Pereira et al. 2022). Priming with water and spermidine, followed by drying in presence of silica gel and a saturated saline solution was applied to tobacco seeds that were cryopreserved in liquid nitrogen for 24 h without loss of germinability (Lopes et al. 2018). These findings suggest that the longevity of primed seeds can be improved by selecting optimal priming parameters, storage, and post-storage conditions (Fabrissin et al. 2021).
Priming memory in seeds
The concept of priming memory has been used to explain the beneficial effects of seed priming in terms of enhanced stress tolerance at the seed/seedling level (Srivastava and Kumar 2021). Seeds are brought toward an advanced physiological stage while experiencing abiotic stresses that trigger stress-responsive mechanisms. This provides the basis for cross-tolerance, a feature maintained notwithstanding dry-back (Chen and Arora 2013). There is increasing evidence that seed priming stimulates the plant's immune memory, a feature held through development or even across generations (Yang et al. 2022). The molecular events associated with stress memory in planta have been explored at the level of chromatin remodeling, alternative transcript splicing, metabolite accumulation, and autophagy; thus, similar processes might also contribute to establishing stress memory during seed priming (Liu et al. 2022). Given that stress memory is a valuable feature ascribed to seed priming, another research question arises: To what extent can stress memory contribute to maintaining enhanced stress tolerance in primed seeds subjected to storage? (Fig. 1). Priming memory is influenced by several factors, including priming agents, dry-back, and storage conditions, as well as seed lot quality (Sano et al. 2017; Fabrissin et al. 2021). Primed seeds can survive storage but lose the high germination capacity acquired with treatments. Different profiles describing the persistence of such benefits during storage have been so far reported, revealing variable responses that make it difficult to figure out guidelines useful for seed operators. Osmoprimed leek seeds maintained the added value of the treatment during storage in silica gel for up to 15 months (Corbineau et al. 1994) whereas osmoprimed B. napus seeds showed superior germination performance after 6 months of storage at 8 °C (Basra et al. 2003). Capsicum frutescens seeds primed with liquid seaweed sap of Kappaphycus alvarezii and Gracilaria edulis maintained their high vigour index after 12 months of storage at room temperature (Dutta et al. 2019), similar to what was reported in pepper (Sivritepe and Sivritepe 2008) and tomato (Hérnadez-Herrera et al. 2014). It has been suggested that the enhanced mineral content and phytohormone levels of seaweed extracts might contribute to preserving the priming benefits along storage (Layek et al. 2015). These benefits were lost rapidly in the same seeds subjected to hydropriming (Corbineau et al. 1994; Basra et al. 2003; Dutta et al. 2019). The molecular and physiological bases that support the ability of primed seeds to keep their enhanced vigour under storage are still poorly investigated, although there is evidence that different protective mechanisms are triggered in response to different priming treatments (Fabrissin et al. 2021).
Can failure help us understand the keys to successful treatments? For sure there is still much to learn from seeds challenged with irreversible damage. In this context, open questions can be better assessed using and/or integrating up-to-date tools that often need to be adapted to the unique features of the seed tissues.
Tools to investigate the impact of seed priming on the pre-germinative metabolism
This paragraph provides an update on the techniques currently used to monitor seed vigor at different levels, by capturing key parameters from the morphological to the molecular point of view. Considering the complexity of the seed vigor trait, all these approaches offer unique advantages and, in most cases, they should be integrated into multidisciplinary strategies to address some of the main open research questions listed in Fig. 1: How to identify reliable hallmarks of seed quality? How to tackle inter- and intra-specific variability in assessing and improving seed quality? How to enhance the potential of underutilized germplasm resources?
Digital phenotyping
The first level for assessing the impact of seed priming is the accurate phenotyping of the germination process. Scoring can be carried out by operators through visual observation; however, this affects accuracy and for this reason, several tools, e.g., Germinator (Joosen et al. 2010), phenoSeeder (Jahnke et al. 2016), MultiSense (Keil et al. 2017), SeedGerm (Colmer et al. 2020), ScreenSeed (Merieux et al. 2021), have been so far developed to automatize seed diagnostics and associated phenotypic analysis. Spatiotemporal dynamics of seed germination can be measured through digital analysis based on seed color, texture, morphology, and growth patterns, with the added value of standardization. The Germinator software package allows high-throughput scoring of germinating seeds in transparent trays stacked in an incubator (Joosen et al. 2010), whereas the phenoSeeder system relies on a pick-and-place robot and a modular setup of sensors that provide biometric traits from individual seeds and calculate three-dimensional data (Jahnke et al. 2016). The technological platform known as MultiSense tool allows the parallel monitoring of respiration in imbibing seeds (up to 100 samples) over an extended period, tracking oxygen (O2), carbon dioxide (CO2), and/or pH (Keil et al. 2017). The ScreenSeed technology, developed for Arabidopsis, provides a fast procedure allowing to handle thousands of seeds without compromising the repeatability or accuracy of the germination measurements (Merieux et al. 2021). An approach for determining seed quality was developed using FT-NIR spectroscopy and X-ray imaging data by FT-NIR spectroscopy that can be used in conjunction with machine learning algorithms to improve seed germination and vigor prediction (Dantas de Medeiros et al. 2020).
Genotoxicity assessment
As previously highlighted, the issues of genotoxic stress arising along the seed life cycle and peaking during rehydration are crucial in terms of seed vigor. The ability to remove DNA damage is regarded as an excellent component of such a complex trait; thus, the availability of reproducible, standardized tests for genotoxicity assessment in germinating seeds is highly desirable. Such tools are regarded as extremely valuable when exploring the seed response to stress conditions possibly imposed by novel priming treatments. Comet assay (single-cell gel electrophoresis, SCGE) is widely used to measure not only DNA damage accumulation (SSDs, DSBs, oxidized base lesions) but also the dynamics of DNA repair (Olive and Banath 2006, Ventura et al. 2013; Collins 2015). This is a sensitive and low-cost method, widely utilized for both basic and applied research purposes, and diagnostics. The comet assay can be performed under alkaline conditions to detect total DNA strand breaks, including those generated at the apurinic or apyrimidinic sites (AP sites, alkali-labile sites, ALS) whereas the neutral version specifically detects the presence of DSBs (Collins et al. 2008). The technique has been optimized for its application on seed tissues, e.g., at the radicle protrusion stage, as reported by Pagano et al. (2017, 2019) who evaluated the genotoxic effects of the histone deacetylase inhibitors trichostatin A and sodium butyrate in M. truncatula by performing alkaline comet assay. The same approach was also used by de Sousa Araujo et al. (2019) to disclose the genotoxic impact of prolonged kinetin-based seed priming in M. truncatula. Genomic instability was evaluated in seeds of rice and beans stored in gene banks using comet assay to investigate the molecular bases of seed deterioration (Dantas et al. 2018). Comet assay has been tested on embryos or embryo axes isolated from eggplant (Kiran et al. 2020), M. truncatula (Pagano et al. 2022a), and Acer pseudoplatanus L. (Plitta-Michalak et al. 2022) seeds.
Detection of reactive oxygen species
The pivotal role of ROS as players able to influence the delicate balance between stimulation of the germination process (signal molecules) and oxidative damage (cytotoxic effect) makes their detection and quantification a relevant step in the assessment of seed quality, especially in the context of seed priming. ROS detection is challenging due to its low concentration and short half-life (Steffens et al. 2013). Indirect measurement can be carried out by quantifying lipid peroxidation expressed as malondialdehyde (MDA) content in dry and imbibed seeds, as reported in studies describing the impact of seed priming in sunflower (Bailly et al. 2000), cucumber (Krainart et al. 2015), barley (Junhaeng et al. 2018), wheat (Hameed et al. 2019), rice (Akhila and Puthur 2020), Solanum villosum (Forti et al. 2021). Histochemical approaches are also used to detect ROS, e.g., 3,3-diaminobenzidine (DAB) (Thordal-Christensen et al. 1997) that reacts with H2O2 in a peroxidase-catalyzed reaction producing an oxidized insoluble brown precipitate, and nitroblue tetrazolium (NBT) useful to detect the superoxide radical (Fryer et al. 2002). DAB and NBT assays have been used to characterize the response of seeds treated with osmopriming (Kiran et al. 2020), hydropriming and hormopriming (Pagano et al. 2022b; Lee et al. 2022), nanopriming (Mahakham et al. 2017; Li et al. 2022b). Fluorescent probes, such as derivates of dichlorodihydrofluorescein diacetate (DCFH-DA) can be used as a non-invasive approach to detect ROS through live-cell imaging (Kristiansen et al. 2009; Bailly and Merendino 2021) or specific fluorimetric assays (Doria et al. 2019; Macovei et al. 2019; Forti et al. 2020a, 2021; Pagano et al. 2022a, b). ROS are detected by EPR using spin traps or spin probes with different properties including lipophilicity, reaction kinetic, and stability of adducts (Buitink et al. 1999, Bacic et al. 2008, Araujo et al. 2016, 2019; Pagano et al. 2017, 2022a). EPR has proved an informative approach when used to explore the seed response to priming (Roqueiro et al. 2012; Pagano et al. 2022a).
Omics
Omics-based analyses can speed up the search for reliable indicators since they provide global, enlarged datasets, where bioinformaticians dig deeply working side by side with seed biologists (Macovei et al. 2017). Proteomic analyses, used to assess the impact of priming on seed metabolism from a global perspective, have highlighted the functional networks boosted through controlled rehydration, to support accelerated germination. Enzymes of the glycolysis pathway and TCA cycle are activated to provide the required energy as well as antioxidant enzymes and enzymes involved in the synthesis of antioxidant compounds, along with enzymes required for fatty acid, amino acid, and protein synthesis (Yacoubi et al. 2011; Lv et al. 2016). Proteins participating in water transport, cell wall modification, cytoskeletal organization, and cell division were also differentially accumulated in primed seeds (Kubala et al. 2015b). Findings resulting from metabolomics-based investigation reflect the high variability in sugar metabolic pools and interconversion pathways existing in phylogenetic distant plant families and underline a relevant issue. The dynamic changes of the seed pre-germinative metabolism should be taken into account, more that the profile of every single metabolite (He et al. 2019; Domergue et al. 2019). Omics are also used to investigate the impact of cumulative rehydration–dehydration cycles. Analysis of protein accumulation in Ferocactus peninsulae seeds challenged with rehydration–dehydration cycles revealed differential expression of proteins involved in primary metabolism, ubiquitination pathways, and reserve protein. Changes in total RNA stability were also observed (López-Urrutia et al. 2014). A link between seed respiration, energy metabolism, fatty acid β-oxidation, nitrogen mobilization, membrane permeability, and enhanced germination performance was highlighted in seeds of the Arabidopsis ecotype Ler subjected to recurrent rehydration–dehydration cycles (Bai et al. 2012). Noteworthy, although single-case studies bring valuable information, only systematic and expanded investigations covering a wide range of species will contribute to significant advances with broad implications.
Hallmarks of successful seed priming
The concept of seed quality hallmark
At the basis of priming treatments, the rehydration–dehydration cycle can be described in terms of metabolic pathways that are triggered, modulated, or turned off, depending on the seed's physiological stage. Understanding the way seed priming affects, either positively or negatively, such metabolic pathways and impacts gene expression and protein/metabolite accumulation/depletion represents an essential step toward the identification of novel seed quality hallmarks. The awareness that enhanced seed vigor stands as the starting point of a robust climate-resilient agrifood chain has prompted researchers to look at the basic aspects of the seed pre-germinative metabolism through fresh eyes, building an updated concept of seed quality biomarkers or hallmarks (Corbineau 2012; Jeevan Kumar et al. 2016; Domuerge et al. 2019). The development of effective hallmarks of the seed response to priming, nowadays one of the main issues for seed companies, can be achieved through a deeper understanding of the molecular events associated with the seed pre-germinative metabolism. Such events need to be dissected at different levels with integrated experimental approaches, to provide a full picture from which crucial players could be retrieved and classified as potential quality hallmarks. Once again, the main open questions to be considered are: What is the contribution of seed repair response in germination and priming? How to identify reliable hallmarks of seed quality? How to tackle inter- and intra-specific variability in assessing and improving seed quality? (Fig. 1). It is generally acknowledged that such potential seed quality biomarkers can be found only by looking at the plethora of metabolic pathways (e.g., antioxidant response, DNA repair, RNA- and protein-related processes), activated by imbibition, that contribute to defining seed quality (Rajjou et al. 2012). Seed quality hallmarks can be represented by the presence/absence or specific accumulation/depletion patterns of proteins and metabolites, as well as by peculiar gene expression profiles. They can be assigned to the different steps along the rehydration–dehydration cycle (Fig. 2).
Do we have reliable seed quality hallmarks?
Once a potential hallmark has been identified, validation should follow by testing the occurrence of that specific biomarker in a range of low- and high-quality seed lots of different genotypes of the same species or even in different species. However, the strong genotype-dependent variability frequently observed during validation assays significantly delays the process. A list of potential hallmarks of seed vigor is provided in Table 1 and discussed. Enhancement of DNA repair pathways during priming can be traced by monitoring the expression levels of DDR genes, such as RAD3 and RAD54 (Sharma and Maheshwari 2015), OGG1, and FPG genes (Forti et al. 2020a, 2020b; Pagano et al. 2022a, 2022b). Accumulation of β tubulin has been used as an indicator of priming-mediated metabolic acceleration (Lanteri et al. 2000) whereas the group 2 LEA protein dehydrin has been proposed as a potential biochemical marker of priming-mediated stress tolerance (Chen et al. 2012b). Comparative proteomics carried out on hydroprimed sugarbeet seeds has revealed distinctive proteins upregulated during priming and downregulated during aging, revealing the glyoxylate enzyme isocitrate lyase as a putative contributor to seed vigor (Catusse et al. 2011). Galactinol levels increase during seed maturation and subsequently decline during germination in chickpeas (Salvi et al. 2016), whereas galactinol accumulation results in detrimental effects on seed quality in maize (Li et al. 2017b). The role of galactinol as a marker for seed longevity was also proven (De Souza Vidìgidal et al. 2016). Metabolic biomarkers of seed quality include galactose and gluconic acid, negatively correlated with seed vigor in rice (Chen et al. 2022). Changes in polyamine accumulation, reported in M. truncatula seeds facing genotoxic stress, highlight their potential as metabolic signatures of seed germination under adverse conditions (Pagano et al. 2019). Hu et al. (2018) analyzed the effects of rehydration–dehydration cycles by looking at membrane glycerolipids; total glycerolipids remained constant across the cycles but phosphatidic acid and diacylglycerol showed opposite accumulation profiles. As the number of cycles increased, deleterious metabolic changes (increased lipid unsaturation, decreased levels of plastidic lipids, and phosphatidylserine acyl chains lengthened) were concomitant with loss of seed viability (Hu et al. 2018).
Table 1.
Putative molecular hallmarks of the seed response to priming
| Type | Name | Function | Plant species | Seed priming | Stress treatment | Methodology | References |
|---|---|---|---|---|---|---|---|
| G | CAP85 | Response to desiccation stress | S. oleracea L | Osmopriming | – | QRT-PCR | Chen et al. (2012a, b) |
| P5CS | Proline biosynthesis | B. napus L | Osmopriming | – | Kubala et al. (2015a, b) | ||
| RAD3, RAD54 | DNA repair (NER, HR) | C. arietinum L |
Hydropriming Osmopriming |
– | Sharma & Maheshwari (2015) | ||
| OGG1, LIGIV | DNA repair (BER) | M. truncatula L | – | TSA-induced genotoxic stress | Pagano et al. (2017) | ||
|
ALKBH1, APE1, FEN1, LIG1 SOD, MT2, TRH1 SPDS, SPMS |
DNA repair (BER) ROS scavenging Polyamine biosynthesis |
M. truncatula L | – | NaB-induced genotoxic stress | Pagano et al. (2019) | ||
| TPS1, TPS7, TPS10, TPPA, TPPI, TRE | Threalose metabolism | M. truncatula L | – | Osmotic/salt stress | Macovei et al. (2019) | ||
|
OGG1, FPG1 SOD, APX |
DNA repair (BER) ROS scavenging |
S. melongena L |
Hydropriming Osmopriming |
– |
Forti et. al 2020a Kiran et al. (2020) |
||
|
FPG1 SOD, APX, MT2 |
DNA repair (BER) ROS scavenging |
M. truncatula L |
Hydropriming Biopriming |
– | Forti et al. (2020b) | ||
| 5.8S rRNA | Ribogenesis |
M. truncatula L M. sativa L |
Hydropriming |
Desiccation stress Overpriming |
Pagano et al. (2022a, b) | ||
| P | β-Tubulin | Microtubules, DNA replication | C. annuum L | Osmopriming | – | Immunoblotting | Lanteri et al. (2000) |
| Dehydrin CAP85 | Response to desiccation stress | S. oleracea L | Osmopriming | – | Chen et al. (2012a, b) | ||
| Isocitrate lyase | Reserve mobilization | B. vulgaris L | Hydropriming | – | Proteomics | Catusse et al. (2011) | |
| Galactinol synthase | RFOs synthesis | A. thaliana | – | Accelerated aging | Enzyme assay | Li et al. (2017a, b) | |
| M | Galactinol | Osmoprotectant | C. arietinum L | – | Storage | GC-FID, GFC | Salvi et a. (2016) |
| Galactinol | Osmoprotectant | Z. mays L | – | Accelerated aging | Li et al. (2017a, b) | ||
| Phosphatidic acid, diacylglycerol | Membrane integrity |
A. thaliana L. perenne L N. tabacum L |
– | Desiccation stress | Lipidomics | Hu et al. (2018) | |
| Polyamines | ROS scavenging | M. truncatula L | – | NaB-induced genotoxic stress | Metabolomics | Pagano et al. (2019) | |
| Galactose, gluconic acid | RFOs metabolism | O. sativa L | – | Accelerated aging | Chen et al. (2022) |
For each hallmark, the corresponding function and the plant species investigated are shown. The seed priming technique and/or stress treatment imposed to seeds that allowed to disclose the hallmark, as well as the methodology used are shown
ALKBH alkylation repair homolog, APE apurinic/apyrimidinic endonuclease, APX ascorbate peroxidase, FEN flap endonuclease, FPG DNA formamidopyrimidine DNA glycosylase, G gene, GC-FID gas chromatography-flame ionization detector, GFC gel filtration chromatography, LEA late embryogenesis abundant, LIG ligase, M metabolite, MT metallothionein, NaB sodium butyrate, OGG oxoguanine DNA glycosylase, P protein, P5CS pyrroline-5-carboxylate synthase, QRT-PCR quantitative real-time polymerase chain reaction, RAD radiation-sensitive, RFO raffinose family oligosaccharides, SOD superoxide dismutase, SPDS spermidine synthase, SPMS spermine/spermidine synthase, TRH thioredoxin, TSA trichostatin, TPS trehalose phosphate synthase, TPP threalose phosphate phosphatase, TRE trehalase
Conclusion
The renewed potential of seed priming to overcome severe drawbacks is particularly visible when seeds are used in fragile agroecosystems (Singh et al. 2020; Devika et al. 2021). This leads to further open questions: How to enhance the potential of underutilized germplasm resources? (Fig. 1). Seed priming promotes the repair of drought-induced damage, supporting the recovery of drought-stressed plants when water becomes available (Marthandan et al. 2020; Aswathi et al. 2021). The use of “on-farm” seed priming is effective against soil crusting and limited soil moisture, typical of the semi-arid areas of developing countries, and improves disease tolerance (Harris et al. 2005; Carrillo-Reche et al. 2018). Cost-effective and easy-to-manage “on-farm” seed priming represents a unique route toward food security in the poorest regions of the planet.
Despite this promising scenario, certain relevant research questions need to be assessed to ensure the transition of seed priming toward a new technological phase. In this review, such open questions have been underlined in association with the different topics and/or problematics that constitute state-of-the-art research on seed priming. The complexity of seed physiology, the genetic background, and the influence of the environment are the main sources of variability when the technique is applied. Researchers must investigate such variability to find the key players contributing to the delicate balance between the stimulation of seed metabolism and the undesired side effects (e.g., oxidative damage and genotoxic stress) caused by suboptimal treatments. Would it be possible to identify universal hallmarks of the seed response to priming useful or would be more reasonable to think about indicators restricted to specific taxonomic groups, e.g., legumes? To what extent storability of primed seeds can be improved? These are the challenges of seed priming, already taken up by the scientific community. Such challenges should be taken up and addressed, also considering the feasibility of seed priming at the field level. When dealing with fragile ecosystems, the choice of the most appropriate priming approach should be carefully handled to maximize the benefits. Participatory trials with local farmers should help seed technologists and researchers in the validation of candidate treatments.
Given the current advances in seed priming and the efforts made to understand the molecular dynamics underlying either the benefits or the drawbacks of this technique, which recommendations should be given to the seed operators (e.g., technologists, breeders) of the private and public sectors? Is it possible to draw some guidelines? Although promising results need to be consolidated through the extensive validation of novel hallmarks, seed technologists should be more focused on finding out novel treatments, working on combinations of priming agents, and exploiting new types of materials.
Author contributions
AP, AM, and AB conceptualized and wrote the manuscript. AB supervised and finalized the manuscript. All the authors have read and approved the final version of the manuscript.
Funding
Open access funding provided by Università degli Studi di Pavia within the CRUI-CARE Agreement. This work was supported by the Italian Ministry of Education, University and Research (MIUR): Dipartimenti di Eccellenza Program (2018–2022) – Dept. of Biology and Biotechnology “L. Spallanzani”, University of Pavia (to A.M. and A. B.).
Availability of data and materials
Not applicable.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdallah EH, Musa Y, Mustafa M, Sjahril R, Riadi M. Comparison between hydro-and osmo-priming to determine period needed for priming indicator and its effect on germination percentage of aerobic rice cultivars (Oryza sativa L.) J Agr Sci. 2016;38:222–230. doi: 10.17503/agrivita.v38i3.886. [DOI] [Google Scholar]
- Abid M, Hakeem A, Shao Y, Liu Y, Zahoor R, Fan Y, Suyu J, Ata-Ul-Karim ST, Tian Z, Jiang D, Snider JL, Dai T. Seed osmopriming invokes stress memory against post-germinative drought stress in wheat (Triticum aestivum L.) Environ Exp Bot. 2018;145:12–20. doi: 10.1016/j.envexpbot.2017.10.002. [DOI] [Google Scholar]
- Abnavi MS, Ghobadi M. The effects of source of priming and post-priming storage duration on seed germination and seedling growth characteristics in wheat (Triticum aestivum L.) J Agr Sci. 2012;4:256–268. doi: 10.5539/jas.v4n9p256. [DOI] [Google Scholar]
- Acevedo M, Pixley K, Zinyengere N, Meng S, Tufan H, Cichy K, Bizikova L, Isaacs K, Ghezzi-Kopel K, Porciello J. A scoping review of adoption of climate-resilient crops by small-scale producers in low- and middle-income countries. Nat Plants. 2020;6:1231–1241. doi: 10.1038/s41477-020-00783-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya P, Jayaprakasha GK, Crosby KM, Jifon JL, Patil BS. Nanoparticle-mediated seed priming improves germination, growth, yield, and quality of watermelons (Citrullus lanatus) at multi-locations in Texas. Sci Rep. 2020;10:5037. doi: 10.1038/s41598-020-61696-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari B, Dhital PR, Ranabhat S, Poudel H. Effect of seed hydro-priming durations on germination and seedling growth of bitter gourd (Momordica charantia) PloS One. 2021;16:e0255258. doi: 10.1371/journal.pone.0255258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal S, Sharma D, Singh NK. Eco-friendly synthesis of phytochemical-capped iron oxide nanoparticles as nano-priming agent for boosting seed germination in rice (Oryza sativa L.) Environ Sci Pollut Res. 2021;28:40275–40287. doi: 10.1007/s11356-020-12056-5. [DOI] [PubMed] [Google Scholar]
- Ahmad I, Ullah S, Nafees M. Effect of osmopriming and thermopriming on amelioration of mercuric chloride stress tolerance in mungbean (Vigna radiata L.) Plant Physiol Rep. 2020;25:516–528. doi: 10.1007/s40502-020-00535-3. [DOI] [Google Scholar]
- Akhila S, Puthur JS. Influence of different seed priming techniques on oxidative and antioxidative responses during the germination of Oryza sativa varieties. Physiol Mol Biol Plants. 2020;26:551–565. doi: 10.1007/s12298-019-00750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M, Hayat S, Ahmad H, Ghani MI, Amin B, Atif MJ, Cheng Z. Priming of Solanum melongena L. seeds enhances germination, alters antioxidant enzymes, modulates ROS, and improves early seedling growth: indicating aqueous garlic extract as seed-priming bio-stimulant for eggplant production. Appl Sci. 2019;9:2203. doi: 10.3390/app9112203. [DOI] [Google Scholar]
- Alves R, Rossatto DR, da Silva J, Checchio MV, de Oliveira KR, Oliveira F, de Queiroz SF, da Cruz MCP, Gratão PL. Seed priming with ascorbic acid enhances salt tolerance in micro-tom tomato plants by modifying the antioxidant defense system components. Biocatalysis Agr Biotech. 2021;31:101927. doi: 10.1016/j.bcab.2021.101927. [DOI] [Google Scholar]
- Alzahrani Y, Rady MM. Compared to antioxidants and polyamines, the role of maize grain-derived organic biostimulants in improving cadmium tolerance in wheat plants. Ecotoxicol Environ Saf. 2019;182:109378. doi: 10.1016/j.ecoenv.2019.109378. [DOI] [PubMed] [Google Scholar]
- Amiard S, Gallego ME, White CI. Signaling of double strand breaks and deprotected telomeres in Arabidopsis. Front Plant Sci. 2013;4:405. doi: 10.3389/fpls.2013.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amritha MS, Sridharan K, Puthur JT, Dhankher OM. Priming with nanoscale materials for boosting abiotic stress tolerance in crop plants. J Agric Food Chem. 2021;69:10017–10035. doi: 10.1021/acs.jafc.1c03673. [DOI] [PubMed] [Google Scholar]
- Anand A, Kumari A, Thakur M, Archana K. Hydrogen peroxide signaling integrates with phytohormones during the germination of magnetoprimed tomato seeds. Sci Rep. 2019;9:8814. doi: 10.1038/s41598-019-45102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand KV, Anugraga AR, Kannan M, Singaravelu G, Govindaraju K. Bio-engineered magnesium oxide nanoparticles as nano-priming agent for enhancing seed germination and seedling vigour of green gram (Vigna radiata L.) Mater Lett. 2020;271:127792. doi: 10.1016/j.matlet.2020.127792. [DOI] [Google Scholar]
- Anderson JD. Adenylate metabolism of embryonic axes from deteriorated soybean seeds. Plant Physiol. 1977;59:610–614. doi: 10.1104/pp.59.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony D, Yadav R, Kalimuthu R, Kumuthan MS. Phyto-complexation of galactomannan-stabilized calcium hydroxide and selenium-calcium hydroxide nanocomposite to enhance the seed-priming effect in Vigna radiata. Int J Biol Macromol. 2022;194:933–944. doi: 10.1016/j.ijbiomac.2021.11.148. [DOI] [PubMed] [Google Scholar]
- Araújo SS, Paparella S, Dondi D, Bentivoglio A, Carbonera D, Balestrazzi A. Physical methods for seed invigoration: advantages and challenges in seed technology. Front Plant Sci. 2016;7:646. doi: 10.3389/fpls.2016.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo S, Pagano A, Dondi D, Lazzaroni S, Pinela E, Macovei A, Balestrazzi A. Metabolic signatures of germination triggered by kinetin in Medicago truncatula. Sci Rep. 2019;9:10466. doi: 10.1038/s41598-019-46866-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswathi KPR, Kalaji HM, Puthur JT. Seed priming of plants aiding in drought stress tolerance and faster recovery: a review. Plant Growth Reg. 2022 doi: 10.1007/s10725-021-00755-z. [DOI] [Google Scholar]
- Bacic G, Spasojevic I, Šecerov B, Mojovic M. Spin-trapping of oxygen free radicals in chemical and biological systems: new traps, radicals and possibilities. Spectrochim Acta A Mol Biomol Spectrosc. 2008;69:1354–1366. doi: 10.1016/j.saa.2007.09.047. [DOI] [PubMed] [Google Scholar]
- Bai B, Sikron N, Gendler T, Kazachkova Y, Barak S, Grafi G, Khozin-Goldberg I, Fait A. Ecotypic variability in the metabolic response of seeds to diurnal hydration–dehydration cycles and its relationship to seed vigor. Plant Cell Physiol. 2012;53:38–52. doi: 10.1093/pcp/pcr169. [DOI] [PubMed] [Google Scholar]
- Bai B, van der Horst N, Cordewener JH, America AHP, Nijveen H, Bentsink L. Delayed protein changes during seed germination. Front Plant Sci. 2021;12:735719. doi: 10.3389/fpls.2021.735719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly C. Active oxygen species and antioxidants in seed biology. Seed Sci Res. 2004;14:93–107. doi: 10.1079/SSR2004159. [DOI] [Google Scholar]
- Bailly C. The signalling role of ROS in the regulation of seed germination and dormancy. Biochem J. 2019;476:3019–3032. doi: 10.1042/BCJ20190159. [DOI] [PubMed] [Google Scholar]
- Bailly C, Merendino L. Oxidative signalling in seed germination and early seedling growth: an emerging role for ROS trafficking and inter-organelle communication. Biochem J. 2021;478:1977–1984. doi: 10.1042/BCJ20200934. [DOI] [PubMed] [Google Scholar]
- Bailly C, Benamar A, Corbineau F, Côme D. Antioxidant systems in sunflower (Helianthus annuus L.) seeds as affected by priming. Seed Sci Res. 2000;10:35–42. doi: 10.1017/S0960258500000040. [DOI] [Google Scholar]
- Bailly C, El-Maarouf-Bouteau H, Corbineau F. From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. Comptes Rendus Biol. 2008;331:806–814. doi: 10.1016/j.crvi.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Balestrazzi A, Confalonieri M, Macovei A, Carbonera D. Seed imbibition in Medicago truncatula Gaertn.: expression profiles of DNA repair genes in relation to PEG-mediated stress. J Plant Physiol. 2011;168:706–713. doi: 10.1016/j.jplph.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Bali S, Kaur P, Jamwal VL, Gandhi SG, Sharma A, Ohri P, Bhardwaj R, Ali MA, Ahmad P. Seed priming with jasmonic acid counteracts root knot nematode infection in tomato by modulating the activity and expression of antioxidative enzymes. Biomolecules. 2020;10:98. doi: 10.3390/biom1001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barazandeh F, Sadzalian MR, Rahimmalek M, Karami S. Effect of thermo-priming on germination, agronomic characteristics and seed oil of safflower (Carthamus tinctorius) cultivar. J Plant Prod. 2019;26:107–122. doi: 10.22069/JOPP.2019.14401.2291. [DOI] [Google Scholar]
- Barba-Espín G, Diaz-Vivancos P, Job D, Belghazi M, Job C, Hernandez JA. Understanding the role of H2O2 during pea seed germination: a combined proteomic and hormone profiling approach. Plant Cell Environ. 2011;34:1907–1919. doi: 10.1111/j.1365-3040.2011.02386.x. [DOI] [PubMed] [Google Scholar]
- Basit F, Chen M, Ahmed T, Shahid M, Noman M, Liu J, An J, Hashem A, Fahad Al-Arjani A-B, Alqarawi AA, et al. Seed priming with brassinosteroids alleviates chromium stress in rice cultivars via improving ROS metabolism and antioxidant defense response at biochemical and molecular levels. Antioxidants. 2021;10:1089. doi: 10.3390/antiox10071089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basit F, Liu J, An J, Chen M, He C, Zhu X, Li Z, Hu J, Guan Y. Seed priming with brassinosteroids alleviates aluminum toxicity in rice via improving antioxidant defense system and suppressing aluminum uptake. Environ Sci Pollut Res. 2022;29:10183–10197. doi: 10.1007/s11356-021-16209-y. [DOI] [PubMed] [Google Scholar]
- Basra SMA,Ullah E, Warraich EA, Cheema MA, Afzal I (2003) Effect of storage on growth and yield of primed canola (Brassica napus) seeds. Int J Agric Biol 5: 117–120. http://www.ijab.org
- Battaglia M, Olvera-Carrillo Y, Garciarrubio A, Campos F, Covarrubias AA. The enigmatic LEA proteins and other hydrophilins. Plant Physiol. 2008;148:6–24. doi: 10.1104/pp.108.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berjak P, Pammenter NW. Implications of the lack of desiccation tolerance in recalcitrant seeds. Front Plant Sci. 2013;4:478. doi: 10.3389/fpls.2013.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD. Seed germination and dormancy. Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD, Black M. Seeds: physiology of development and germination. 2. New York, London: Plenum Press; 1994. [Google Scholar]
- Bhardwaj A, Sita K, Sehgal A, Bhandari K, Kumar S, Prasad PVV, Jha U, Kumar J, Siddique KHM, Nayyar H. Heat priming of lentil (Lens culinaris Medik.) seeds and foliar treatment with γ-aminobutyric acid (GABA), confers protection to reproductive function and yield traits under high-temperature stress environments. Int J Mol Sci. 2021;22:5825. doi: 10.3390/ijms22115825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigolin Teixeira S, Nunes Pires S, Espinel Ávila G, Paschoal Silva BE, Novo Schmitz V, Deuner C, da Silva AR, da Silva MD, Deuner S. Application of vigor indexes to evaluate the cold tolerance in rice seeds germination conditioned in plant extract. Sci Rep. 2021;11:11038. doi: 10.1038/s41598-021-90487-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binang WB, Shiyam JO, Ntia JD. Effect of seed priming method on agronomic performance and cost effectiveness of rainfed, dry-seeded NERICA rice. Res J Seed Sci. 2012;5:136–143. doi: 10.3923/rjss.2012.136.143. [DOI] [Google Scholar]
- Biswas S, Rasal-Monir M, Islam M, Modak S, Kabir MH. Induction of salt tolerance in tomato through seed priming. Plant. 2019;7:47. doi: 10.11648/j.plant.20190703.14. [DOI] [Google Scholar]
- Bittencourt M, Dias D, Dias L, Araújo E. Effects of priming on asparagus seed germination and vigour under water and temperature stress. Seed Sci Technol. 2004;32:607–616. doi: 10.15258/sst.2004.32.2.29. [DOI] [Google Scholar]
- Black M. Darwin and seeds. Seed Sci Res. 2009;19:193–199. doi: 10.1017/S0960258509990171. [DOI] [Google Scholar]
- Boyer JS, Westgate M. Grain yields with limited water. J Exp Bot. 2004;55:2385–2394. doi: 10.1093/jxb/erh219. [DOI] [PubMed] [Google Scholar]
- Bradford KJ, Steiner JJ, Trawatha SE. Seed priming influence on germination and emergence of pepper seed lots. Crop Sci. 1990;30:718–721. doi: 10.2135/cropsci1990.0011183X003000030049x. [DOI] [Google Scholar]
- Bray CM. Biochemical processes during the osmopriming of seeds. In: Kigel J, Galili G, editors. Seed development and germination. New York, NY, USA: Marcel Dekker Inc; 1995. pp. 767–789. [Google Scholar]
- Bray CM, West CE. DNA repair mechanisms in plants: crucial sensors and effectors for the maintenance of genome integrity. New Phytol. 2005;168:511–528. doi: 10.1111/j.1469-8137.2005.01548.x. [DOI] [PubMed] [Google Scholar]
- Bruggink T, van der Toorn P. Induction of desiccation tolerance in germinated seeds. Seed Sci Res. 1995;5:1–4. doi: 10.1017/S096025850000252X. [DOI] [Google Scholar]
- Bruggink GT, Ooms JJJ, van der Toorn P. Induction of longevity in primed seeds. Seed Sci Res. 1999;9:49–53. doi: 10.1017/S0960258599000057. [DOI] [Google Scholar]
- Bryksová M, Hybenová A, Hernándiz AE, Novák O, Pencík A, Spíchal L, De Diego N, Doležal K. Hormopriming to mitigate abiotic stress effects: a case study of N9 -substituted cytokinin derivatives with a fluorinated carbohydrate moiety. Front Plant Sci. 2020;11:599228. doi: 10.3389/fpls.2020.599228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitink J, Hemminga MA, Hoekstra FA. Characterization of molecular mobility in seed tissues: an electron paramagnetic resonance spin probe study. Biophys J. 1999;76:3315–3322. doi: 10.1016/S0006-3495(99)77484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitink J, Ly VuB, Satour P, Leprince O. The re-establishment of desiccation tolerance in germinated radicles of Medicago truncatula Gaertn. Seeds Seed Sci Res. 2003;13:273–286. doi: 10.1079/SSR2003145. [DOI] [Google Scholar]
- Bulgari R, Franzoni G, Ferrante A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy. 2019;9:306. doi: 10.3390/agronomy9060306. [DOI] [Google Scholar]
- Burgass RW, Powell AA (1984) Evidence for repair processes in the invigoration of seeds by hydration. Ann Bot 53:753–757. https://www.jstor.org/stable/42757439
- Bush EW, Wilson P, Shepard DP, McClure G (2000) Enhancement of seed germination in common carpetgrass and centipedegrass seed. HortSci 35: 769–770. 10.21273/HORTSCI.35.4.769
- Calleja-Cabrera J, Boter M, Oñate-Sánchez L, Pernas M. Root growth adaptation to climate change in crops. Front Plant Sci. 2020;11:544. doi: 10.3389/fpls.2020.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campobenedetto C, Grange E, Mannino G, van Arkel J, Beekwilder J, Karlcva R, Garabello C, Contartese V, Bertea CM. A biostimulant seed treatment improved heat stress tolerance during cucumbers seed germination by acting on the antioxidant system and glyoxylate cycle. Front Plant Sci. 2020;11:836. doi: 10.3389/fpls.2020.00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Li G, Cui Z, Yang F, Jiang X, Diallo L, Kong F. Seed priming with melatonin improves the seed germination of waxy maize under chilling stress via promoting the antioxidant system and starch metabolism. Sci Rep. 2019;9:15044. doi: 10.1038/s41598-019-51122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron I, Corbineau F, Dacher F, Job C, Côme D, Job D. Sugarbeet seed priming: effects of priming conditions on germination, solubilization of 11-S globulin and accumulation of LEA proteins. Seed Sci Res. 2000;10:243–254. doi: 10.1017/S0960258500000271. [DOI] [Google Scholar]
- Carrillo-Reche J, Vallejo-Marín M, Quilliam RS. Quantifying the potential of ‘on-farm’ seed priming to increase crop performance in developing countries. A meta-analysis. Agron Sustain Dev. 2018;38:64. doi: 10.1007/s13593-018-0536-0. [DOI] [Google Scholar]
- Catusse J, Meinhard J, Job C, Strub J-M, Fischer U, Peststova E, Westhoff P, Van Dorsselaer A, Job D. Proteomics reveals potential biomarkers of seed vigor in sugarbeet. Proteomics. 2011;11:1–12. doi: 10.1002/pmic.201000586. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran U, Luo X, Wang Q, Shu K. Are there unidentified factors involved in the germination of nanoprimed seeds? Front Plant Sci. 2020;11:832. doi: 10.3389/fpls.2020.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah KS, Osborne DJ. DNA lesions occur with loss of viability in embryos of ageing rye seed. Nature. 1978;272:593–599. doi: 10.1038/272593a0. [DOI] [PubMed] [Google Scholar]
- Chen K, Arora R. Priming memory invokes seed stress-tolerance. Environ Exp Bot. 2013;94:33–45. doi: 10.1016/j.envexpbot.2012.03.005. [DOI] [Google Scholar]
- Chen K, Arora R, Arora U. Osmopriming of spinach (Spinacia oleracea L. cv Bloomsdale) seeds and germination performance under temperature and water stress. Seed Sci Technol. 2010;38:36–48. doi: 10.15258/sst.2010.38.1.04. [DOI] [Google Scholar]
- Chen H, Chu P, Zhou Y, Li Y, Liu J, Ding Y, Tsang EWT, Jiang L, Wu K, Huang S. Overexpression of AtOGG1, a DNA glycosylase/AP lyase, enhances seed longevity and abiotic stress tolerance in Arabidopsis. J Exp Bot. 2012;63:4107–4121. doi: 10.1093/jxb/ers093. [DOI] [PubMed] [Google Scholar]
- Chen K, Fessehaie A, Arora R. Dehydrin metabolism is altered during seed osmopriming and subsequent germination under chilling and desiccation in Spinacia oleracea L. cv. Bloomsdale: possible role in stress tolerance. Plant Sci. 2012;183:27–36. doi: 10.1016/j.plantsci.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Chen Z, Cao X-L, Niu J-P. Effects of exogenous ascorbic acid on seed germination and seedling salt-tolerance of alfalfa. PloS One. 2021;16:e0250926. doi: 10.1371/journal.pone.0250926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BX, Fu H, Gao JD, Zhang YX, Huang WJ, Chen ZJ, Qi-Zhang Yan SJ, Liu J. Identification of metabolomic biomarkers of seed vigor and aging in hybrid rice. Rice. 2022;15:7. doi: 10.1186/s12284-022-00552-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Yang Z, Wang M, Song J, Sui N. Salinity improves chilling resistance in Suaeda salsa. Acta Physiol Plant. 2014;36:1823–1830. doi: 10.1007/s11738-014-1555-3. [DOI] [Google Scholar]
- Ching TM. Adenosine triphosphate content and seed vigor. Plant Physiol. 1973;51:400–402. doi: 10.1104/pp.51.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu KY, Chen CL, Sung JM. Partial vacuum storage improves the longevity of primed sh-2 sweet corn seeds. Horti Sci. 2003;98:99–111. doi: 10.1016/S0304-4238(02)00206-6. [DOI] [Google Scholar]
- Collins AR. The comet assay: a heavenly method! Mutagenesis. 2015;30:1–4. doi: 10.1093/mutage/geu079. [DOI] [PubMed] [Google Scholar]
- Collins AR, Oscoz AA, Brunborg G, Gaivão I, Giovannelli L, Kruszewski M, Smith CC, Stetina R. The comet assay: topical issues. Mutagenesis. 2008;23:143–151. doi: 10.1093/mutage/gem051. [DOI] [PubMed] [Google Scholar]
- Colmer J, O’Neill CM, Wells R, Bostrom A, Reynolds D, Websdale D, Shiralagi G, Lu W, Lou Q, Le Cornu T, Ball J, Renema J, Andalus GF, Benjamins R, Penfield S, Zhou J. SeedGerm: a cost-effective phenotyping platform for automated seed imaging and machine-learning based phenotypic analysis of crop seed germination. New Phytol. 2020;228:778–793. doi: 10.1111/nph.16736. [DOI] [PubMed] [Google Scholar]
- Corbineau F. Markers of seed quality: from present to future. Seed Sci Res. 2012;22:61–68. doi: 10.1017/S0960258511000419. [DOI] [Google Scholar]
- Corbineau F, Picard MA, Côme D. Germinability of leek seeds and its improvement by osmopriming. Acta Hortic. 1994;371:45–52. doi: 10.17660/ActaHortic.1994.371.4. [DOI] [Google Scholar]
- Corbineau F, Ozbingol N, Vinel D, Côme D. Improvement of tomato seed germination by osmopriming as related to energy metabolism. In: Black M, Bradford KJ, Vazquez-Ramos J, editors. Seed biology: advances and applications: proceedings of the sixth international workshop on seeds. Mérida (Mexico) CABI Publishing; 2000. pp. 467–476. [Google Scholar]
- Corbineau F, Gay-Mathieu C, Vinel D, Côme D. Decrease in sunflower (Helianthus annuus) seed viability caused by high temperature as related to energy metabolism, membrane damage and lipid composition. Physiol Plant. 2002;116:489–496. doi: 10.1034/j.1399-3054.2002.1160407.x. [DOI] [Google Scholar]
- Cordoba-Canero D, Roldan-Arjona T, Ariza RR. Arabidopsis ZDP DNA 30-phosphatase and ARP endonuclease function in 8-oxoG repair initiated by FPG and OGG1 DNA glycosylases. Plant J. 2014;79:824–834. doi: 10.1111/tpj.12588. [DOI] [PubMed] [Google Scholar]
- Damalas CA, Koutroubas SD, Fotiadis S. Hydro-priming effects on seed germination and field performance of faba bean in spring oowing. Agriculture. 2019;9:201. doi: 10.3390/agriculture9090201. [DOI] [Google Scholar]
- Dandoy E, Schyns R, Deltour L, Verly WG. Appearance and repair of apurinic/apyrimidinic sites in DNA during early germination. Mutat Res. 1987;181:57–60. doi: 10.1016/0027-5107(87)90287-9. [DOI] [Google Scholar]
- Dantas AF, Lopes RM, Fascineli ML, José S, Pádua JG, Gimenes MA, Grisolia CK. Comet and cytogenetic tests as tools for evaluating genomic instability in seeds of Oryza sativa L. and Phaseolus vulgaris L. from gene banks. Gen Mol Biol. 2018;41:145–153. doi: 10.1590/1678-4685-GMB-2017-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas de Medeiros A, Silva L, Ribeiro JP, Ferreira K, Fim Rosas J, Santos A, Barboza da Silva C. Machine learning for seed quality classification: an advanced approach using merger data from FT-NIR spectroscopy and X-ray imaging. Sensors. 2020;20:4319. doi: 10.3390/s20154319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin CR. On the action of sea-water on the germination of seeds. J Proc Linn Soc Lond (Botany) 1857;1:130–140. [Google Scholar]
- Das G, Dutta P. Effect of nanopriming with zinc oxide and silver nanoparticles on storage of chickpea seeds and management of wilt disease. J Agr Sci Tech. 2022;24:213–226. [Google Scholar]
- de Espírito Santo Pereira A, Caixeta Oliveira H, Fraceto LF. Polymeric nanoparticles as an alternative for application of gibberellic acid in sustainable agriculture: a feld study. Sci Rep. 2019;9:7135. doi: 10.1038/s41598-019-43494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza VD, Willems L, van Arkel J, Dekkers BJW, Hilhorst HWM, Bentsink L. Galactinol as marker for seed longevity. Plant Sci. 2016;246:112–118. doi: 10.1016/j.plantsci.2016.02.015. [DOI] [PubMed] [Google Scholar]
- De Sousa AS, Pagano A, Dondi D, Lazzaroni S, Pinela E, Macovei A, Balestrazzi A. Metabolic signatures of germination triggered by kinetin in Medicago truncatula. Sci Rep. 2019;9:10466. doi: 10.1038/s41598-019-46866-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearman J, Brocklehurst PA, Drew RLK. Effects of osmotic priming and aging on onion seed germination. Ann Appl Biol. 1986;108:639–648. doi: 10.1111/j.1744-7348.1986.tb02003.x. [DOI] [Google Scholar]
- Dekkers BJW, Costa MCD, Maia J, Bentsink L, Ligterink W, Hilhorst HMW. Acquisition and loss of desiccation tolerance in seeds: from experimental model to biological relevance. Planta. 2015;241:563–577. doi: 10.1007/s00425-014-2240-x. [DOI] [PubMed] [Google Scholar]
- Del Buono D, Luzi F, Puglia D. Lignin nanoparticles: a promising tool to improve maize physiological, biochemical, and chemical traits. Nanomaterials. 2021;11:846. doi: 10.3390/nano11040846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahaie J, Hundertmark M, Bove J, Leprince O, Rogniaux H, Buitink J. LEA polypeptide profiling of recalcitrant and orthodox legume seeds reveals ABI3-regulated LEA protein abundance linked to desiccation tolerance. J Exp Bot. 2013;64:4559–4573. doi: 10.1093/jxb/ert274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deltour R, de Barsy T. Nucleolar activation and vacuolation in embryo radicle cells during early germination. J Cell Sci. 1985;76:67–83. doi: 10.1242/jcs.76.1.67. [DOI] [PubMed] [Google Scholar]
- Devika OS, Singh S, Sarkar D, Barnwal P, Suman J, Rakshit A. Seed priming: a potential supplement in integrated resource management under fragile intensive ecosystems. Front Sustain Food Syst. 2021;5:654001. doi: 10.3389/fsufs.2021.654001. [DOI] [Google Scholar]
- Dirk LMA, Downie AB. An examination of job’s rule: protection and repair of the proteins of the translational apparatus in seeds. Seed Sci Res. 2018;28:168–181. doi: 10.1017/S0960258518000284. [DOI] [Google Scholar]
- do Espirito Santo Pereira A, Caixeta Oliveira H, Fernandes Fraceto L, Santaella C. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials. 2021;11:267. doi: 10.3390/nano11020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domergue J-B, Abadie C, Limami A, Way D, Tcherkez G. Seed quality and carbon primary metabolism. Plant Cell Environ. 2019;42:2776–2788. doi: 10.1111/pce.13618. [DOI] [PubMed] [Google Scholar]
- Donà M, Confalonieri M, Minio A, Biggiogera M, Buttafava A, Balestrazzi A. RNA-Seq analysis discloses early senescence and nucleolar dysfunction triggered by Tdp1α depletion in Medicago truncatula. J Exp Bot. 2013;64:1941–1951. doi: 10.1093/jxb/ert063. [DOI] [PubMed] [Google Scholar]
- Doria E, Pagano A, Ferreri C, Larocca AV, Macovei A, Araújo S, Balestrazzi A. How does the seed pre-germinative metabolism fight against imbibition damage? Emerging roles of fatty acid cohort and antioxidant defence. Front Plant Sci. 2019;10:1–13. doi: 10.3389/fpls.2019.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufková H, Berka M, Luklová M, Rashotte AM, Brzobohatý B, Cerný M. Eggplant germination is promoted by hydrogen peroxide and temperature in an independent but overlapping manner. Molecules. 2019;24:4270. doi: 10.3390/molecules24234270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta SK, Layek J, Akoijam RS, Boopathi T, Vanlalhmangaiha SS, Singh SB, Lungmuana PN. Seaweed extract as natural priming agent for augmenting seed quality traits and yield in Capsicum frutescens L. J Appl Phycol. 2019;31:3803–3813. doi: 10.1007/s10811-019-01871-0. [DOI] [Google Scholar]
- Eisvand HR, Tavakkol-Afshari R, Sharifzadeh F, Maddah Arefi H, Hesamzadeh Hejazi SM. Effects of hormonal priming and drought stress on activity and isozyme profiles of antioxidant enzymes in deteriorated seed of tall wheatgrass (Agropyron elongatum Host) Seed Sci Technol. 2010;38:280–297. doi: 10.15258/sst.2010.38.2.02. [DOI] [Google Scholar]
- El-Badri AM, Batool M, Wang C, Hashem AM, Tabl KM, Nishawy E, Kuai J, Zhou G, Wang B. Selenium and zinc oxide nanoparticles modulate the molecular and morpho-physiological processes during seed germination of Brassica napus under salt stress. Ecotoxic Environ Safety. 2021;225:112695. doi: 10.1016/j.ecoenv.2021.:112695. [DOI] [PubMed] [Google Scholar]
- Elder R, Osborne D. Function of DNA synthesis and DNA repair in the survival of embryos during early germination and in dormancy. Seed Sci Res. 1993;3:43–53. doi: 10.1017/S0960258500001550. [DOI] [Google Scholar]
- Ellis RH, Hong TD, Roberts EH. Seed moisture content, storage, viability and vigor. Seed Sci Res. 2008;1:275–279. doi: 10.1017/S0960258500001008. [DOI] [Google Scholar]
- Ellouzi H, Oueslati S, Hessini K, Rabhi M, Abdelly C. Seed-priming with H2O2 alleviates subsequent salt stress by preventing ROS production and amplifying antioxidant defense in cauliflower seeds and seedlings. Sci Hort. 2021;288:110360. doi: 10.1016/j.scienta.2021.110360. [DOI] [Google Scholar]
- Ells JE. The influence of treating tomato seed with nutrient solution on emergence rate and seedling growth. Proc Am Soc Hort Sci. 1963;83:684–687. [Google Scholar]
- El-Maarouf-Bouteau H, Meimoun P, Job C, Job D, Bailly C. Role of protein and mRNA oxidation in seed dormancy and germination. Front Plant Sci. 2013;4:77. doi: 10.3389/fpls.2013.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Naggar NE-A, Hussein MH, Shaaban-Dessuuki SA, Dalal SR. Production, extraction and characterization of Chlorella vulgaris soluble polysaccharides and their applications in AgNPs biosynthesis and biostimulation of plant growth. Sci Rep. 2020;10:3011. doi: 10.1038/s41598-020-59945-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sanatawy AM, Ash-Shormillesy SMAI, Qabil N, Awad MF, Mansour E. Seed halo-priming improves seedling vigor, grain yield, and water use efficiency of maize under varying irrigation regimes. Water. 2021;13:2115. doi: 10.3390/w13152115. [DOI] [Google Scholar]
- El-Serafy RS, El-Sheshtawy A-NA, Atteya AK, Al-Hashimi A, Abbasi AM, Al-Ashkar I. Seed priming with silicon as a potential to increase salt stress tolerance in Lathyrus odoratus. Plants. 2021;10:2140. doi: 10.3390/plants10102140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenari M. The history of germination research and the lesson it contains for today. Isr J Bot. 1980;29:4–21. doi: 10.1080/0021213X.1980.10676873. [DOI] [Google Scholar]
- Evenari M (1984) Seed physiology: its history from antiquity to the beginning of the 20th century. Bot Rev 50:119–142. https://www.jstor.org/stable/4354032
- Fabrissin I, Sano N, Seo M, North HM. Ageing beautifully: can the benefits of seed priming be separated from a reduced lifespan trade-off? J Exp Bot. 2021;72:2312–2333. doi: 10.1093/jxb/erab004. [DOI] [PubMed] [Google Scholar]
- Fahad S, Hussain S, Bano A, Saud S, Hassan S, Shan D, Khan FA, Khan F, Chen YT, Wu C, Tabassum MA, Chun MX, Afzal M, Jan A, Jan MT, Huang J. Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: consequences for changing environment. Environ Sci Pollut Res. 2015;22:4907–4921. doi: 10.1007/s11356-014-3754-2. [DOI] [PubMed] [Google Scholar]
- Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S, Ihsan MZ, Alharby H, Wu C, Wang D, Huang J. Crop production under drought and heat stress: plant responses and management options. Front Plant Sci. 2017;8:1147. doi: 10.3389/fpls.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Ma C, Huang Z, Abid M, Jiang S, Dai T, Han X. Heat priming during early reproductive stages enhances thermotolerance to post-anthesis heat stress via improving photosynthesis and plant productivity in winter wheat (Triticum aestivum L.) Front Plant Sci. 2018;9:805. doi: 10.3389/fpls.2018.00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (2021) Strategic framework 2022–31, pp. 1–39. https://www.fao.org/3/cb7099en/cb7099en.pdf
- Farhoudi R, Sharifzadeh F, Poustini K, Makkizadeh MT, Kochakpor M. The effects of NaCl priming on salt tolerance in canola (Brassica napus) seedlings grown under saline conditions. Seed Sci Technol. 2007;35:754–759. doi: 10.15258/sst.2007.35.3.23. [DOI] [Google Scholar]
- Farooq M, Wahid A, Ahmad N, Asad SA. Comparative efficacy of surface drying and re-drying seed priming in rice: changes in emergence, seedling growth and associated metabolic events. Paddy Water Environ. 2010;8:15–22. doi: 10.1007/s10333-009-0170-1. [DOI] [Google Scholar]
- Farooq M, Usman M, Nadeem F, Rehman H, Wahid A, Basra SMA, Siddique KHM. Seed priming in field crops: potential benefits, adoption and challenges. Crop Pasture Sci. 2019;70:731771. doi: 10.1071/CP18604. [DOI] [Google Scholar]
- Farooq HA, Hameed A, Sheikh MA. Silicon-mediated priming induces acclimation to mild water-deficit stress by altering physio-biochemical attributes in wheat plants. Front Plant Sci. 2021;12:625541. doi: 10.3389/fpls.2021.625541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq MA, Zhang X, Zafar MM, Ma W, Zhao J. Roles of reactive oxygen species and mitochondria in seed germination. Front Plant Sci. 2021;12:781734. doi: 10.3389/fpls.2021.781734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feghhenabi F, Hadi H, Khodaverdiloo H, van Genuchten MT. Seed priming alleviated salinity stress during germination and emergence of wheat (Triticum aestivum L.) Agr Water Manag. 2020;231:106022. doi: 10.1016/j.agwat.2020.106022. [DOI] [Google Scholar]
- Feng J, Zhao Z, Liu H, Wang H, Li J. Effects of seed moisture content and storage condition on Platycodon grandiflorum seed quality. Guizhou Agr Sci. 2017;45:24–27. doi: 10.1100/2012/128105. [DOI] [Google Scholar]
- Finch-Savage WE, Footitt S. Seed dormancy cycling and the regulation of dormancy mechanisms to time germination in variable field environments. J Exp Bot. 2017;68:843–856. doi: 10.1093/jxb/erw477. [DOI] [PubMed] [Google Scholar]
- Forti C, Ottobrino V, Bassolino L, Toppino L, Rotino GL, Pagano A, Macovei A, Balestrazzi A. Molecular dynamics of pre-germinative metabolism in primed eggplant (Solanum melongena L.) seeds. Hort Res. 2020;207:87. doi: 10.1038/s41438-020-0310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forti C, Shankar A, Singh A, Balestrazzi A, Prasad V, Macovei A. Seed priming improves germination on heavy-metal contaminated soil by inducing upregulation of genes involved in DNA repair and antioxidant response. Genes. 2020;11:242. doi: 10.3390/genes11030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forti C, Ottobrino V, Doria E, Bassolino L, Toppino L, Rotino GL, Pagano A, Macovei A, Balestrazzi A. Hydropriming applied on fast germinating Solanum villosum miller seeds: impact on pre-germinative metabolism. Front Plant Sci. 2021;12:639336. doi: 10.3389/fpls.2021.639336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MJ, Oxborough K, Mullineaux PM, Baker NR. Imaging of photooxidative stress responses in leaves. J Exp Bot. 2002;53:1249–1254. doi: 10.1093/JEXBOT/53.372.1249. [DOI] [PubMed] [Google Scholar]
- Galland M, Rajjou L. Regulation of mRNA translation controls seed germination and is critical for seedling vigor. Front Plant Sci. 2015;6:284. doi: 10.3389/fpls.2015.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland M, Huguet R, Arc E, Cueff G, Job D, Rajjou L. Dynamic proteomics emphasizes the importance of selective mRNA translation and protein turnover during Arabidopsis seed germination. Mol Cell Prot. 2014;1:252–268. doi: 10.1074/mcp.M113.032227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia D, Zhao Y, Zhao S, Ming LC, Huang D. Hydroelectrostatic hybrid priming stimulates germination performance via ABA and GA regulation: New promising evidence for tomato gene expression. Curr Plant Biol. 2021;27:100215. doi: 10.1016/j.cpb.2021.100215. [DOI] [Google Scholar]
- Georghiou K, Thanos CA, Passam HC. Osmoconditioning as a means of counteracting the aging of pepper seeds during hightemperature storage. Ann Bot. 1987;60:279–285. doi: 10.1093/oxfordjournals.aob.a087445. [DOI] [Google Scholar]
- Gerna D, Roach T, Arc E, Stöggl W, Limonta M, Vaccino P, Kranner I. Redox poise and metabolite changes in bread wheat seeds are advanced by priming with hot steam. Biochem J. 2018;475:3725–3743. doi: 10.1042/BCJ20180632. [DOI] [PubMed] [Google Scholar]
- Ghobadi M, Abnavi MS, Honarmand SJ, Ghobadi ME, Mohammadi RM. Effect of hormonal priming (GA3) and osmopriming on behaviour of seed germination in wheat (Triticum aestivum L.) J Agric Sci. 2012;4:244–250. doi: 10.5539/jas.v4n9p244. [DOI] [Google Scholar]
- Ghosh S, Kamble NU, Verma P, Salvi P, Petla BP, Roy S, Rao V, Hazra A, Varshney V, Kaur H, Majee M. Arabidopsis protein I-ISOASPARTYL METHYLTRANSFERASE repairs isoaspartyl damage to antioxidant enzymes and increases heat and oxidative stress tolerance. Plant Biol. 2020;295:783–799. doi: 10.1016/S0021-9258(17)49935-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Górnik K, Lahuta LB. Application of phytohormones during seed hydropriming and heat shock treatment on sunflower (Helianthus annuus L.) chilling resistance and changes in soluble carbohydrates. Acta Physiol Plant. 2017;39:1–12. doi: 10.1007/s11738-017-2413-x. [DOI] [Google Scholar]
- Goswami A, Banerjee R, Raha S. Drought resistance in rice seedlings conferred by seed priming. Protoplasma. 2013;250:1115–1129. doi: 10.1007/s00709-013-0487-x. [DOI] [PubMed] [Google Scholar]
- Grzesik M, Nowak J. Effects of matriconditioning and hydropriming on (Helichrysum bracteatum L.) seed germination, seedling emergence and stress tolerance. Seed Sci Technol. 1988;26:363–376. [Google Scholar]
- Guha T, Gopal G, Das H, Mukherjee A, Kundu R. Nanopriming with zero-valent iron synthesized using pomegranate peel waste: A “green” approach for yield enhancement in Oryza sativa L. cv. Gonindobhog. Plant Physiol Biochem. 2021;163:261–275. doi: 10.1016/j.plaphy.2021.04.006. [DOI] [PubMed] [Google Scholar]
- Guo X, Zhi W, Feng Y, Zhou G, Zhu G. Seed priming improved salt-stressed sorghum growth by enhancing antioxidative defense. PloS One. 2022;17:e0263036. doi: 10.1371/journal.pone.0263036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Doležal K, Kulkarni MG, Balazs E, van Staden J. Role of non-microbial biostimulants in regulation of seed germination and seedling establishment. Plant Growth Regul. 2022;97:271–313. doi: 10.1007/s10725-021-00794-6. [DOI] [Google Scholar]
- Gurmani A, Bano A, Khan S, Din J, Zhang J. Alleviation of salt stress by seed treatment with abscisic acid (ABA), 6-benzylaminopurine (BA) and chlormequat chloride (CCC) optimizes ion and organic matter accumulation and increases yield of rice (‘Oryza sativa’ L.) Aust J Crop Sci. 2011;5:1278–1285. [Google Scholar]
- Gurmani AR, Bano A, Ullah N, Khan H, Jahangir M, Flowers TJ. Exogenous abscisic acid (ABA) and silicon (Si) promote salinity tolerance by reducing sodium (Na+) transport and bypass flow in rice (Oryza sativa indica) Aust J Crop Sci. 2013;7:1219–1226. [Google Scholar]
- Hacisalihoglu G, Kantanka S, Miller N, Gustin JL, Settles AM. Modulation of early maize seedling performance via priming under sub-optimal temperatures. PloS One. 2018;13:e0206861. doi: 10.1371/journal.pone.0206861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed A, Hameed A, Farooq T, Noreen R, Javed S, Batool S, Ahmad A, Gulzar T, Ahmad M. Evaluation of structurally different benzimidazoles as priming agents, plant defence activators and growth enhancers in wheat. BMC Chem. 2019;13:29. doi: 10.1186/s13065-019-0546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed A, Farooq T, Hameed A, Sheikh MA. Sodium nitroprusside mediated priming memory invokes water-deficit stress acclimation in wheat plants through physio-biochemical alterations. Plant Physiol Biochem. 2021;160:329–340. doi: 10.1016/j.plaphy.2021.01.037. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Hartley FC, Hayes L, Franco M. Simulated seawater flooding reduces oilseed rape growth, yield and progeny performance. Ann Bot. 2019;125:247–254. doi: 10.1093/aob/mcz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D, Pathan AK, Gothkar P, Joshi A, Chivasa W, Nyamudeza P. On-farm seed priming: using participatory methods to revive and refine a key technology. Agric Syst. 2001;69:151–164. doi: 10.1016/S0308-521X(01)00023-3. [DOI] [Google Scholar]
- Harris D, Breese WA, Rao JVDKK (2005) The improvement of crop yield in marginal environments using “on-farm” seed priming: nodulation, nitrogen fixation and disease resisitance. Aust J Agric Res 56: 1211–1218. http://oar.icrisat.org/id/eprint/5096
- Hassini I, Martinez-Ballesta MC, Boughanmi N, Moreno DA, Carvajal M. Improvement of broccoli sprouts (Brassica oleracea L. var. italica) growth and quality by KCl seed priming and methyl jasmonate under salinity stress. Sci Hort. 2017;226:141–151. doi: 10.1016/j.scienta.2017.08.030. [DOI] [Google Scholar]
- He Y, Cheng J, He Y, Yang B, Cheng Y, Yang C, Zhang H, Wang Z. Influence of isopropylmalate synthase OsIPMS1 on seed vigour associated with amino acid and energy metabolism in rice. Plant Biotech J. 2019;17:322–337. doi: 10.1111/pbi.12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Herrera RM, Santacruz-Ruvalcaba F, Ruiz-López MA, Norrie J, Hernández-Carmona G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.) J Appl Phycol. 2014;26:619–628. doi: 10.1007/s10811-013-0078-4. [DOI] [Google Scholar]
- Heydecker W, Gibbins BM. The ‘priming’ of seeds. Acta Hort. 1978;83:213–215. doi: 10.17660/ActaHortic.1978.83.29. [DOI] [Google Scholar]
- Heydecker W, Higgins J, Gulliver RL. Accelerated germination by osmotic seed treatment. Nature. 1973;246:42–44. doi: 10.1038/246042a0. [DOI] [Google Scholar]
- Hongna C, Junmei S, Leyuan T, Xiaori H, Guolin L, Xianguo C. Exogenous spermidine priming mitigates the osmotic damage in germinating seeds of Leymus chinensis under salt-alkali stress. Front Plant Sci. 2021;12:701538. doi: 10.3389/fpls.2021.701538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Tao M, Lu X, Xin P. Studies on the respiration and ethylene released amount of ultradried seed stored at different temperature. Seed. 2006;25:13–16. doi: 10.16590/j.cnki.1001-4705.2006.02.039. [DOI] [Google Scholar]
- Hu X-L, Yu X-M, Chen H-Y, Li W-Q. Turnover of glycerolipid metabolite pool and seed viability. Int J Mol Sci. 2018;19:1417. doi: 10.3390/ijms19051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Zhang L, Zeng R, Wang X, Zhang H, Wang L, Liu S, Wang X, Chen T. Brassinosteroid priming improves peanut drought tolerance via eliminating inhibition on genes in photosynthesis and hormone signaling. Genes. 2020;11:919. doi: 10.3390/genes11080919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S, Zheng M, Khan F, Khaliq A, Fahad S, Peng S, Huang J, Cui K, Nie L. Benefits of rice seed priming are offset permanently by prolonged storage and the storage conditions. Sci Rep. 2015;5:8101. doi: 10.1038/srep08101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S, Shaukat M, Ashraf M, Zhu C, Jin Q, Zhang J (2019a) Salinity stress in arid and semi arid climates: effects and management in field crops. Climate change and Agriculture.In: Hussain S (ed) IntechOpen. 10.5772/interchopen.87982
- Hussain A, Rizwan M, Ali Q, Ali S. Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ Sci Pollut Res. 2019;26:7579–7588. doi: 10.1007/s11356-019-04210-5. [DOI] [PubMed] [Google Scholar]
- Jahnke S, Roussel J, Hombach T, Kochs J, Fischbach A, Huber G, Scharr H. phenoSeeder – a robot system for automated handling and phenotyping of individual seeds. Plant Physiol. 2016;172:1358–1370. doi: 10.1104/pp.16.01122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda T, Darko È, Shehata S, Kovacs V, Pal M, Szalai G. Salt acclimation processes in wheat. Plant Physiol Biochem. 2016;101:68–75. doi: 10.1016/j.plaphy.2016.01.025. [DOI] [PubMed] [Google Scholar]
- Jeevan Kumar SP, Rajendra Prasad S, Banerjee R, Thammineni C. Seed birth to death: dual functions of reactive oxygen species in seed physiology. Ann Bot. 2015;116:663–668. doi: 10.1093/aob/mcv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeevan Kumar SP, Rajendra Prasad S, Kumar M, Singh C, Sinha AK, Pathak A. Seed quality markers: a review. RRJBS. 2016;S3:13–17. [Google Scholar]
- Jeevan Kumar SP, Chintagunta AD, Reddy YM, Rajjou L, Garlapati VK, Agarwal DK, Prasad SR, Simal-Gandara J. Implications of reactive oxygen and nitrogen species in seed physiology for sustainable crop productivity under changing climate conditions. Curr Plant Biol. 2021;26:100197. doi: 10.1016/j.cpb.2021.100197. [DOI] [Google Scholar]
- Jelali N, Youssef RB, Boukari N, Zorrig W, Dhifi W, Abdelly C. Salicylic acid and H2O2 seed priming alleviates Fe deficiency through the modulation of growth, root acidification capacity and photosynthetic performance in Sulla carnosa. Plant Physiol Biochem. 2021;159:392–399. doi: 10.1016/j.plaphy.2020.11.039. [DOI] [PubMed] [Google Scholar]
- Jira-Anunkul W, Pattanagul W. Effects of hydrogen peroxide application on agronomic traits of rice (Oryza sativa L.) under drought stress. Plant Soil Environ. 2021;67:221–229. doi: 10.17221/628/2020-PSE. [DOI] [Google Scholar]
- Jisha KC, Puthur JT. Halopriming of seeds imparts tolerance to NaCl and PEG induced stress in Vigna radiata (L.) Wilczek varieties. Physiol Mol Biol Plants. 2014;20:303–312. doi: 10.1007/s12298-014-0234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jisha KC, Puthur JT. Seed haloprimingoOutdo hydropriming in enhancing seedling vigor and osmotic stress tolerance potential of rice varieties. J Crop Sci Biotech. 2014;17:209–219. doi: 10.1007/s12892-014-0077-2. [DOI] [Google Scholar]
- Job C, Kersulec A, Ravasio L, Chareyer S, Pepin R, Job D (1997) The solubilization of the basic subunit of sugarbeet seed 11-S globulin during priming and early germination. Seed Sci Res 7:225–244. 10.1017/S0960258500003585
- Job C, Rajjou L, Lovigny Y, Belghazi M, Job D. Patterns of protein oxidation in Arabidopsis seeds and during germination. Plant Physiol. 2005;138:790–802. doi: 10.1104/pp.105.062778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosen RVL, Kodde J, Willems LAJ, Ligterink W, van der Plas LHW, Hilhorst HWM. GERMINATOR: a software package for high-throughput scoring and curve fitting of Arabidopsis seed germination. Plant J. 2010;62:148–159. doi: 10.1111/j.1365-313X.2009.04116.x. [DOI] [PubMed] [Google Scholar]
- Joshi A, Kaur S, Dharamvir K, Nayyar H, Verma G. Multi-walled carbon nanotubes applied through seed-priming influence early germination, root hair, growth and yield of bread wheat (Triticum aestivum L.) J Sci Food Agric. 2018;98:3148–3160. doi: 10.1002/jsfa.8818. [DOI] [PubMed] [Google Scholar]
- Junhaeng P, Thobunluepop P, Chanprasert W, Onwimol D, Nakasathien S, Pawelzik E. The seed water sorption isotherm and antioxidant-defensive mechanisms of Hordeum vulgare L. primed seeds. Am J Plant Sci. 2018;9:2385–2407. doi: 10.4236/ajps.2018.912173. [DOI] [Google Scholar]
- Justice OL, Bass LN. Agriculture handbook. Washington, DC: USDA Printing Office; 1978. Principles and practices of seed storage; pp. 25–198. [Google Scholar]
- Kalinina NO, Makarova S, Makhotenko A, Love AJ, Taliansky M. The multiple functions of the nucleolus in plant development, disease and stress responses. Front Plant Sci. 2018;9:132. doi: 10.3389/fpls.2018.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalija E, Selovic A, Dahija S, Demir A, Samardzic J, Vrobel O, Zeljkovic SC, Paric A. Use of seed priming to improve Cd accumulation and tolerance in Silene sendtneri, novel Cd hyper-accumulator. Ecotox Environ Safety. 2021;210:111882. doi: 10.1016/j.ecoenv.2020.111882. [DOI] [PubMed] [Google Scholar]
- Keil P, Liebsch G, Borisjuk L, Rolletschek H. MultiSense: a multimodal sensor tool enabling the high-throughput analysis of respiration. Methods Mol Biol. 2017;1670:47–56. doi: 10.1007/978-1-4939-7292-0_5. [DOI] [PubMed] [Google Scholar]
- Keshavarz H, Sadegh R, Moghadam G. Seed priming with cobalamin (vitamin B12) provides significant protection against salinity stress in the common bean. Rhizosphere. 2017;3:143–149. doi: 10.1016/j.rhisph.2017.04.010. [DOI] [Google Scholar]
- Kester ST, Geneve RL, Houtz RL. Priming and accelerated ageing affect L-isoaspartyl methyltransferase activity in tomato (Lycopersicon esculentum Mill.) seed. J Exp Bot. 1997;48:943–949. doi: 10.1093/jxb/48.4.943. [DOI] [Google Scholar]
- Khan TA, Yusuf M, Fariduddin Q. Seed treatment with H2O2 modifies net photosynthetic rate and antioxidant system in mung bean (Vigna radiata L. Wilczek) plants. Israel J Plant Sci. 2015;62:167–175. doi: 10.1080/07929978.2015.1060806. [DOI] [Google Scholar]
- Khan A, Kamran M, Imran M, Al-Harrasi A, Al-Rawahi A, Al-Amri I, Lee I-J, Khan AL. Silicon and salicylic acid confer high-pH stress tolerance in tomato seedlings. Sci Rep. 2019;9:19788. doi: 10.1038/s41598-019-55651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F, Hussain S, Khan S, Geng M. Seed priming improved antioxidant defense system and alleviated Ni-induced adversities in rice seedlings under N, P, or K deprivation. Front Plant Sci. 2020;11:565647. doi: 10.3389/fpls.2020.565647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I, Ali Raza MA, Awan SA, Shah GAM, Ali B, Tariq R, Hassan MJ, Alyemeni MN, Brestic M, Zhang X, Ali S, Huang L. Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (AgNPs): the oxidative damage, antioxidant enzymes and ions uptake are major determinants of salt tolerant capacity. Plant Physiol Biochem. 2020;156:221–232. doi: 10.1016/j.plaphy.2020.09.018. [DOI] [PubMed] [Google Scholar]
- Kibinza S, Vinel D, Côme D, Bailly C, Corbineau F. Sunflower seed deterioration as related to moisture content during ageing, energy metabolism and active oxygen species scavenging. Physiol Plant. 2006;128:496–506. doi: 10.1111/j.1399-3054.2006.00771.x. [DOI] [Google Scholar]
- Kidd RA, West C. Physiological predetermination: the influence of the physiological conditions of seed upon the course of subsequent growth and upon yield, I-v. Ann Appl Biol. 1918;5:1–251. doi: 10.1111/j.1744-7348.1918.tb05277.x. [DOI] [Google Scholar]
- Kidd RA, West C. Physiological predetermination: the influence of the physiological conditions of seed upon the course of subsequent growth and upon yield, I-v. Ann Appl Biol. 1919;6:1–26. doi: 10.1111/j.1744-7348.1919.tb05298.x. [DOI] [Google Scholar]
- King HA, Gerber AP. Translatome profiling: methods for genome-scale analysis of mRNA translation. Brief Funct Gen. 2016;15:22–31. doi: 10.1093/bfgp/elu045. [DOI] [PubMed] [Google Scholar]
- Kiran KR, Deepika VB, Swathy PS, Prasad K, Kabekkodu SP, Murali TS, Satyamoorthy K, Muthusamy A. ROS-dependent DNA damage and repair during germination of NaCl primed seeds. J Photochem Photobiol B. 2020;213:112050. doi: 10.1016/j.jphotobiol.2020.112050. [DOI] [PubMed] [Google Scholar]
- Kołodziejczyk I, Kazmierczak A, Posmyk MM. Melatonin application modifies antioxidant defense and induces endoreplication in maize seeds exposed to chilling stress. Int J Mol Sci. 2021;22:8628. doi: 10.3390/ijms22168628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krainart C, Siri B, Vichitphan K (2015) Effects of accelerated aging and subsequent priming on seed quality and biochemical change of hybrid cucumber (Cucumis sativa Linn.) seeds. Int J Agr Tech 11: 165–179. http://www.ijat-aatsea.com. ISSN2630-0192
- Kranner I, Minibayeva FV, Beckett RP, Seal CE. What is stress? Concepts, definitions and applications in seed science. New Phytol. 2010;188:655–673. doi: 10.1111/j.1469-8137.2010.03461.x. [DOI] [PubMed] [Google Scholar]
- Kristiansen KA, Jensen PE, Møller IA, Schulz A. Monitoring reactive oxygen species formation and localization in living cells by use of the fluorescent probe CM-H2DCFDA and confocal laser microscopy. Physiol Plant. 2009;136:369–383. doi: 10.1111/j.1399-3054.2009.01243.x. [DOI] [PubMed] [Google Scholar]
- Kubala S, Garnczarska M, Wojtyla L, Clippe A, Kosmala A, Żmieńko A, Lutts S, Quinet M. Deciphering priming-induced improvement of rapeseed (Brassica napus L.) germination through an integrated transcriptomic and proteomic approach. Plant Sci. 2015;231:94–113. doi: 10.1016/j.plantsci.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Kubala S, Wojtyla Ł, Quinet M, Lechowska K, Lutts S, Garnczarska M. Enhanced expression of the proline synthesis gene P5CSA in relation to seed osmopriming improvement of Brassica napus germination under salinity stress. J Plant Physiol. 2015;183:1–12. doi: 10.1016/j.jplph.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Kumar J, Mir RR, Shafi S, Sen Gupta D, Djalovic I, Miladinovic J, Kumar R, Kumar S, Kumar R. Genomics associated interventions for heat stress tolerance in cool season adapted grain legumes. Int J Mol Sci. 2021;23:399. doi: 10.3390/ijms23010399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurek K, Plitta-Michalak B, Ratajczak E. Reactive oxygen species as potential drivers of the seed aging process. Plants (basel) 2019;8:174. doi: 10.3390/plants8060174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichaney A, Maity A. Implications of rising atmospheric carbon dioxide concentration on seed quality. Int J Biometeorol. 2021;65:805–812. doi: 10.1007/s00484-020-02073-x. [DOI] [PubMed] [Google Scholar]
- Lanteri S, Portis E, Bergervoet HW, Groot SPC. Molecular markers for the priming of pepper seeds (Capsicum annuum L.) J Hort Sci Biotech. 2000;75:607–611. doi: 10.1080/14620316.2000.11511294. [DOI] [Google Scholar]
- Law SR, Narsai R, Taylor NL, Delannoy E, Carrie C, Giraud E, Millar AH, Small I, Whelan J. Nucleotide and RNA metabolism prime translational initiation in the earliest events of mitochondrial biogenesis during Arabidopsis germination. Plant Physiol. 2012;158:1610–1627. doi: 10.1104/pp.111.192351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layek J, Das A, Ramkrushna GI, Trivedi K, Yesuraj D, Chandramohan M, Kubavat D, Agarwal PK, Ghosh A. Seaweed sap: a sustainable way to improve productivity of maize in North-east India. Int J Environ Stud. 2015;72:305–315. doi: 10.1080/00207233.2015.1010855. [DOI] [Google Scholar]
- Lee HY, Back K. 2-Hydroxymelatonin promotes seed germination by increasing reactive oxygen species production and gibberellin synthesis in Arabidopsis thaliana. Antioxidants. 2022;11:37. doi: 10.3390/antiox11040737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei C, Bagavathiannan M, Wang H, Sharpe SM, Meng W, Yu J. Osmopriming with polyethylene glycol (PEG) for abiotic stress tolerance in germinating crop seeds: a review. Agronomy. 2021;11:2194. doi: 10.3390/agronomy11112194. [DOI] [Google Scholar]
- Li Z, Xu J, Gao Y, Wang C, Guo G, Luo Y, Huang Y, Hu W, Sheteiwy MS, Guan Y, Hu J. The synergistic priming effect of exogenous salicylic acid and H2O2 on chilling tolerance enhancement during maize (Zea mays L.) seed germination. Front Plant Sci. 2017;8:1153. doi: 10.3389/fpls.2017.01153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Zhang Y, Wang D, Liu Y, Dirk LMA, Goodman J, Downie AB, Wang J, Wang G, Zhao T. Regulation of seed vigor by manipulation of raffinose family oligosaccharides in maize and Arabidopsis thaliana. Mol Plant. 2017;10:1540–1555. doi: 10.1016/j.molp.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Li Z, Gao Y, Zhang Y, Lin C, Gong D, Guan Y, Hu J. Reactive oxygen species and gibberellin acid mutual induction to regulate tobacco seed germination. Front Plant Sci. 2018;9:1279. doi: 10.3389/fpls.2018.01279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liang L, Li W, Ashraf U, Ma L, Tang X, Pan S, Tian H, Mo Z. ZnO nanoparticle-based seed priming modulates early growth and enhances physio-biochemical and metabolic profles of fragrant rice against cadmium toxicity. J Nanobiotechnol. 2021;19:75. doi: 10.1186/s12951-021-00820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Niu Y, Zheng Y, Wang Z. Advances in the understanding of reactive oxygen species-dependent regulation on seed dormancy, germination, and deterioration in crops. Front Plant Sci. 2022;13:826809. doi: 10.3389/fpls.2022.826809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu J, Fu C, Khan MN, Hu J, Zhao F, Wu H, Li Z. CeO2 nanoparticles modulate Cu–Zn superoxide dismutase and lipoxygenase-IV isozyme activities to alleviate membrane oxidative damage to improve rapeseed salt tolerance. Environ Sci Nano. 2022;9:1116–1132. doi: 10.1039/D1EN00845E. [DOI] [Google Scholar]
- Lilya B, Réda D, Ouzna A-B. Vigna unguiculata seed priming is related to redox status of plumule, radicle and cotyledons. Funct Plant Biol. 2019;46:584–594. doi: 10.1071/FP18202. [DOI] [PubMed] [Google Scholar]
- Liu J, Gui J, Gao W, Ma J, Wang Q. Review of the physiological and biochemical reactions and molecular mechanisms of seed aging. Acta Ecol Sinica. 2016;36:4997–5006. doi: 10.5846/stxb201502040289. [DOI] [Google Scholar]
- Liu X, Quan W, Bartels D. Stress memory responses and seed priming correlate with drought tolerance in plants: an overview. Planta. 2022;255:45. doi: 10.1007/s00425-022-03828-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes CA, de Carvalho MLM, de Souza AC, Oliveira JA, de Andrade DB. Cryopreservation of primed tobacco seeds. J Seed Sci. 2018;40:415–420. doi: 10.1590/2317-1545v40n4207216. [DOI] [Google Scholar]
- López-Urrutia E, Martínez-García M, Monsalvo-Reyes A, Salazar-Rojas V, Montoya R, Campos J. Differential RNA- and protein-expression profiles of cactus seeds capable of hydration memory. Seed Sci Res. 2014;24:91–99. doi: 10.1017/S0960258513000317. [DOI] [Google Scholar]
- Lunn G, Madsen E. ATP-levels of germinating seeds in relation to vigor. Physiol Plant. 1981;53:164–169. doi: 10.1111/j.1399-3054.1981.tb04127.x. [DOI] [Google Scholar]
- Lutts S, Paolo B, Lukasz WSKS, Robert P. New challenges in seed biology-basic and translational research driving seed technology. Rijeka, Croatia: Intech Open; 2016. Seed priming: new comprehensive approaches for an old empirical technique; pp. 1–46. [Google Scholar]
- Lv Y, Zhang S, Wang J, Hu Y. Quantitative proteomic analysis of wheat seeds during artificial ageing and priming using the isobaric tandem mass tag labeling. PloS One. 2016;11:e0162851. doi: 10.1371/journal.pone.0162851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HY, Zhao DD, Ning QR, Wei JP, Li Y, Wang MM, Liu XL, Jiang CJ, Liang ZW. A multi-year beneficial effect of seed priming with gibberellic acid-3 (GA3) on plant growth and production in a perennial grass, Leymus chinensis. Sci Rep. 2018;8:1–9. doi: 10.1038/s41598-018-31471-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macovei A, Balestrazzi A, Confalonieri M, Carbonera D. The Tdp1 (Tyrosyl-DNA phosphodiesterase) gene family in barrel medic (Medicago truncatula Gaertn.): bioinformatic investigation and expression profiles in response to copper- and PEG-mediated stress. Planta. 2010;232:393–407. doi: 10.1007/s00425-010-1179-9. [DOI] [PubMed] [Google Scholar]
- Macovei A, Balestrazzi A, Confalonieri M, Faè M, Carbonera D. New insights on the barrel medic MtOGG1 and MtFPG functions in relation to oxidative stress response in planta and during seed imbibition. Plant Physiol Biochem. 2011;49:1040–1050. doi: 10.1016/j.plaphy.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Macovei A, Balestrazzi A, Confalonieri M, Buttafava A, Carbonera D. The TFIIS and TFIIS-like genes from Medicago truncatula are involved in oxidative stress response. Gene. 2011;470:20–30. doi: 10.1016/j.gene.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Macovei A, Pagano A, Leonetti P, Carbonera D, Balestrazzi A, Araújo SS. Systems biology and genome-wide approaches to unveil the molecular players involved in the pre-germinative metabolism: implications on seed technology traits. Plant Cell Rep. 2017;36:669–688. doi: 10.1007/s00299-016-2060-5. [DOI] [PubMed] [Google Scholar]
- Macovei A, Faè M, Biggiogera M, de Sousa AS, Carbonera D, Balestrazzi A. Ultrastructural and molecular analyses reveal enhanced nucleolar activity in Medicago truncatula cells overexpressing the MtTdp2alpha gene. Front Plant Sci. 2018;9:596. doi: 10.3389/fpls.2018.00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macovei A, Pagano A, Cappuccio M, Gallotti L, Dondi D, Araújo S, Fevereiro P, Balestrazzi A. A snapshot of the trehalose pathway during seed imbibition in Medicago truncatula reveals temporal-and stress-dependent shifts in gene expression patterns associated with metabolite changes. Front Plant Sci. 2019;10:1590. doi: 10.3389/fpls.2019.01590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen MD, Svejcar L, Radke J, Hulet A. Inducing rapid seed germination of native cool season grasses with solid matrix priming and seed extrusion technology. PloS One. 2018;13:e0204380. doi: 10.1371/journal.pone.0204380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahakham W, Sarmah A, Maensiri S, Theerakulpisut P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci Rep. 2017;7:8263. doi: 10.1038/s41598-017-08669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia J, Dekkers BJW, Provart NJ, Ligterink W, Hilhorst HWM. The re-establishment of desiccation tolerance in germinated Arabidopsis thaliana seeds and its associated transcriptome. PloS One. 2011;6:e29123. doi: 10.1371/journal.pone.0029123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthandan V, Geetha R, Kumutha K, Renganathan VG, Karthikeyan A, Ramalingam J. Seed priming: a feasible strategy to enhance drought tolerance in crop plants. Int J Mol Sci. 2020;21:8258. doi: 10.3390/ijms21218258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Aguilar K, Hernández-Chávez JL, Alvarez-Venegas R. Priming of seeds with INA and its transgenerational effect in common bean (Phaseolus vulgaris L.) plants. Plant Sci. 2021;305:110834. doi: 10.1016/j.plantsci.2021.110834. [DOI] [PubMed] [Google Scholar]
- Masurkar SA, Chaudhari PR, Shidore VB, Kamble SP. Rapid biosynthesis of silver nanoparticles using Cymbopogan citratus (lemongrass) and its antimicrobial activity. Nano-Micro Lett. 2011;3:189–194. doi: 10.1007/BF03353671. [DOI] [Google Scholar]
- Matsushima K-I, Sakagami J-I. Effects of seed hydropriming on germination and seedling vigor during emergence of rice under different soil moisture conditions. Am J Plant Sci. 2013;04:1584–1593. doi: 10.4236/ajps.2013.48191. [DOI] [Google Scholar]
- May LHE, Milthorpe EJ, Milthorpe FL. Pre-sowing hardening of plants to drought. An appraisal of the contributions by P. A. Gekel. Field Crop Abst. 1962;15:93–98. [Google Scholar]
- Merieux N, Cordier P, Wagner MH, Ducournau S, Aligon S, Job D, Grappin P, Grappin E. ScreenSeed as a novel high throughput seed germination phenotyping method. Sci Rep. 2021;11:1404. doi: 10.1038/s41598-020-79115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirmazloum I, Kiss A, Erdélyi E, Ladányi M, Németh EZ, Radácsi P. The effect of osmopriming on seed germination and early seedling characteristics of Carum carvi L. Agriculture. 2020;10:94. doi: 10.3390/agriculture10040094. [DOI] [Google Scholar]
- Mochochoko T, Oluwafemi OS, Jumbam DN, Songca SP. Green synthesis of silver nanoparticles using cellulose extracted from an aquatic weed; water hyacinth. Carbohyd Polym. 2013;98:290–294. doi: 10.1016/j.carbpol.2013.05.038. [DOI] [PubMed] [Google Scholar]
- Moosavi A, Tavakkol Afshari R, Sharif-Zadeh F, Aynehband A. Seed priming to increase salt and drought stress tolerance during germination in cultivated species of Amaranth. Seed Sci Technol. 2009;37:781–785. doi: 10.15258/sst.2009.37.3.26. [DOI] [Google Scholar]
- Moreno C, Seal CE, Papenbrock J. Seed priming improves germination in saline conditions for Chenopodium quinoa and Amaranthus caudatus. J Agr Crop Sci. 2018;204:40–48. doi: 10.1111/jac.12242. [DOI] [Google Scholar]
- Mridha D, Paul I, De A, Ray I, Das A, Joardar M, Chowdhury NR, Bhadoria PBS, Roychowdhury T. Rice seed (IR64) priming with potassium humate for improvement of seed germination, seedling growth and antioxidant defense system under arsenic stress. Ecotoxicol Environ Safety. 2021;219:1–10. doi: 10.1016/j.ecoenv.2021.112313. [DOI] [PubMed] [Google Scholar]
- Murray G, Swensen JB, Gallian JJ. Emergence of sugar beet seedlings at low soil temperature following seed soaking and priming. HortSci. 1993;28:31–32. doi: 10.21273/HORTSCI.28.1.31. [DOI] [Google Scholar]
- Nadeem M, Li J, Yahya M, Wang M, Ali A, Cheng A, Wang X, Ma C. Grain legumes and fear of salt stress: focus on mechanisms and management strategies. Int J Mol Sci. 2019;20:799. doi: 10.3390/ijms20040799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao Y, Asea G, Minoru Y, Nobuki K, Hiroyuki H, Kisho M, Yabuta S, Rieko K, Jun-Ichi S. Development of hydropriming techniques for sowing seeds of upland rice in Uganda. Am J Plant Sci. 2018;9:2170–2182. doi: 10.4236/ajps.2018.911157. [DOI] [Google Scholar]
- Nakao Y, Sone C, Sakagami J-I. Genetic diversity of hydro priming effects on rice seed emergence and subsequent growth under different moisture conditions. Genes. 2020;11:994. doi: 10.3390/genes1109099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento WM, Cantliffe DJ, Huber DJ. Ethylene evolution and endo-beta-mannanase activity during lettuce seed germination at high temperature. Sci Agric. 2004;61:156–163. doi: 10.1590/S0103-90162004000200006. [DOI] [Google Scholar]
- Nascimento W, Huber D, Cantliffe D. Carrot seed germination and respiration at high temperature in response to seed maturity and priming. Seed Sci Technol. 2013;41:164–169. doi: 10.15258/sst.2013.41.1.19. [DOI] [Google Scholar]
- Nath S, Coolbear P, Hampton JG. Hydration-dehydration treatments to protect or repair stored “karamu” wheat seeds. Crop Sci. 1991;31:822–826. doi: 10.2135/cropsci1991.0011183X003100030055x. [DOI] [Google Scholar]
- Nchanji EB, Lutomia CK, Chirwa R, Templer N, Rubyogo JC, Onyango P. Immediate impacts of COVID-19 pandemic on bean value chain in selected countries in sub-Saharan Africa. Agric Syst. 2021;188:103034. doi: 10.1016/j.agsy.2020.103034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CJ, Alexova R, Jacoby RP, Millar AH. Proteins with high turnover rate in barley leaves estimated by proteome analysis combined with in planta isotope labeling. Plant Physiol. 2014;166:91–108. doi: 10.1104/pp.114.243014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nietzel T, Mostertz J, Rubertia C, Née G, Fuchsa P, Wagner S, Moseler A, Müller-Schüsselec SJ, Benamar A, Poschet G, Büttnerg M, Møller IM, Lilli CH, Macherel D, Wirtz M, Hell R, Finkemeier I, Meyer AJ, Hochgräfe F, Schwarzländer M. Redox-mediated kick-start of mitochondrial energy metabolism drives resource-efficient seed germination. Proc Natl Acad Sci USA. 2020;117:741–751. doi: 10.1073/pnas.1910501117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitaki Z, Holá M, Donà M, Pavlopoulou A, Michalopoulos I, Angelis KJ, Georgakilas AG, Macovei A, Balestrazzi A. Integrating plant and animal biology for the search of novel DNA damage biomarkers. Mut Res-Rev Mut Res. 2018;775:21–38. doi: 10.1016/j.mrrev.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Nonogaki H, Bassel GW, Bewley JD. Germination—still a mystery. Plant Sci. 2010;179(6):574–581. doi: 10.1016/j.plantsci.2010.02.010. [DOI] [Google Scholar]
- Nyström T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005;24:1311–1317. doi: 10.1038/sj.emboj.7600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive PL, Banáth JP. The comet assay: a method to measure DNA damage in individual cells. Nat Protoc. 2006;1:23–29. doi: 10.1038/nprot.2006.5. [DOI] [PubMed] [Google Scholar]
- Oracz K, Bouteau HEM, Farrant JM, Cooper K, Belghazi M, Job C, Job D, Corbineau F, Bailly C. ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. Plant J. 2007;50:452–465. doi: 10.1111/j.1365-313X.2007.03063.x. [DOI] [PubMed] [Google Scholar]
- Osborne DJ. Biochemical control systems operating in the early hours of germination. Can J Bot. 1983;61:3568–3577. doi: 10.1139/b83-406. [DOI] [Google Scholar]
- Osborne DJ. Hazards of a germinating seed: available water and the maintenance of genomic integrity. Isr J Plant Sci. 2000;48:173–179. doi: 10.1560/JNXX-M1TH-2UM3-5UF6. [DOI] [Google Scholar]
- Osborne DJ, Sharon R, Ben-Ishai R. Studies on DNA integrity and DNA repair in germinating embryos of rye (Secale cereale) Israel J Plant Sci. 1980;29:259–272. doi: 10.1080/0021213X.1980.10676892. [DOI] [Google Scholar]
- Owen PL, Pill WG. Germination of osmotically primed asparagus and tomato seeds after storage up to 3 months. J Am Soc Hortic Sci. 1994;119:636–641. doi: 10.21273/JASHS.119.3.636. [DOI] [Google Scholar]
- Ozden E, Ermis S, Sahin O, Taskin MB, Demir I. Solid matrix priming treatment with O2 enhanced quality of leek seed lots. Not Bot Horti Agrobot Cluj-Napoca. 2018;46:371–375. doi: 10.15835/nbha46110749. [DOI] [Google Scholar]
- Pagano A, Susana Araújo S, Macovei A, Leonetti P, Balestrazzi A. The seed repair response during germination: disclosing correlations between DNA repair, antioxidant response, and chromatin remodeling in Medicago truncatula. Front Plant Sci. 2017;8:1972. doi: 10.3389/fpls.2017.01972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano A, De Sousa AS, Macovei A, Dondi D, Lazzaroni S, Balestrazzi A. Metabolic and gene expression hallmarks of seed germination uncovered by sodium butyrate in Medicago truncatula. Plant Cell Environ. 2019;42:259–269. doi: 10.1111/pce.13342. [DOI] [PubMed] [Google Scholar]
- Pagano A, Folini G, Pagano P, Sincinelli F, Rossetto A, Macovei A, Balestrazzi A. ROS accumulation as a hallmark of dehydration stress in primed and overprimed Medicago truncatula seeds. Agronomy. 2022;12:268. doi: 10.3390/agronomy12020268. [DOI] [Google Scholar]
- Pagano A, Zannino L, Pagano P, Doria E, Dondi D, Macovei A, Biggiogera M, de Sousa AS, Balestrazzi A. Changes in genotoxic stress response, ribogenesis and PAP (3’-phosphoadenosine 5’-phosphate) levels are associated with loss of desiccation tolerance in overprimed Medicago truncatula seeds. Plant Cell Environ. 2022 doi: 10.1111/pce.14295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey V, Pati AK. Limitation of improvement in germination by osmopriming of differentially aged non-orthodox neem (Azadirachta indica) seeds. Biochem Physiol. 2017;6:2. doi: 10.4172/2168-9652.1000217. [DOI] [Google Scholar]
- Paparella S, Araujo SS, Rossi G, Wijayasinghe M, Carbonera D, Balestrazzi A. Seed priming: state of the art and new perspectives. Plant Cell Rep. 2015;34:1281–1293. doi: 10.1007/s00299-015-1784-y. [DOI] [PubMed] [Google Scholar]
- Parera CA, Cantliffe DJ. Priming leek seed for improved germination and emergence at high temperature. Hort Sci. 1992;27:1077–1079. doi: 10.21273/HORTSCI.27.10.1077. [DOI] [Google Scholar]
- Paszkiewicz G, Gualberto JM, Benamar A, Macherel D, Logan DC. Arabidopsis seed mitochondria are bioenergetically active immediately upon imbibition and specialize via biogenesis in preparation for autotrophic growth. Plant Cell. 2017;29:109–128. doi: 10.1105/tpc.16.00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patade VY, Sujata B, Suprasanna P. Halopriming imparts tolerance to salt and PEG induced drought stress in sugarcane. Agric Ecosyst Environ. 2009;134:24–28. doi: 10.1016/j.agee.2009.07.003. [DOI] [Google Scholar]
- Patade VY, Bhargava S, Suprasanna P. Better osmotic adjustment mediates salt and PEG stress tolerance in primed plants of contrasting cultivars of sugarcane. Sugar Tech. 2015;17:348–355. doi: 10.1007/s12355-014-0350-1. [DOI] [Google Scholar]
- Patanè C, Cavallaro V, Cosentino SL. Germination and radicle growth in unprimed and primed seeds of sweet sorghum as affected by reduced water potential in NaCl at different temperatures. Indust Crop Prod. 2009;30:1–8. doi: 10.1016/J.INDCROP.2008.12.005. [DOI] [Google Scholar]
- Paul S, Dey S, Kundu R. Seed priming: an emerging tool towards sustainable agriculture. Plant Growth Regul. 2021 doi: 10.1007/s10725-021-00761-1. [DOI] [Google Scholar]
- Peng L, Lang S, Wang Y, Pritchard HW, Wang XF. Modulating role of ROS in reestablishing desiccation tolerance in germinating seeds of Caragana korshinskii Kom. J Exp Bot. 2017;68:3585–3601. doi: 10.1093/jxb/erx172. [DOI] [PubMed] [Google Scholar]
- Pereira AAS, Nery FC, Ferreira RA, da Silva VN, Bernardes MM, dos Santos HO, Bicalho EM. Can priming with ascorbic acid or nitric oxide improve the germinability of stored sunflower seeds? J Seed Sci. 2022;44:1–11. doi: 10.1590/2317-1545v44256600. [DOI] [Google Scholar]
- Pérez L, Acosta Y, Nápoles L, Carvajal C, Linares C, Sershen C, Lorenzo JC, Pérez A. Pineapple stem-derived bromelain based priming improves pepper seed protein reserve mobilization, germination, emergence and plant growth. Physiol Mol Biol Plants. 2021;27:1651–1657. doi: 10.1007/s12298-021-01038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl M. ATP synthesis and utilization in the early stage of seed germination in relation to seed dormancy and quality. Physiol Plant. 1986;66:177–182. doi: 10.1111/j.1399-3054.1986.tb01253.x. [DOI] [Google Scholar]
- Plitta-Michalak BP, Ramos AA, Pupel P, Michalak M. Oxidative damage and DNA repair in desiccated recalcitrant embryonic axes of Acer pseudoplatanus L. BMC Plant Biol. 2022;22:40. doi: 10.1186/s12870-021-03419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell AA, Yule LJ, Jing H-C, Groot SPC, Bino RJ, Pritchard HW. The influence of aerated hydration seed treatment on seed longevity as assessed by the viability equations. J Exp Bot. 2000;51:2031–2043. doi: 10.1039/jexbot/51.353.2032. [DOI] [PubMed] [Google Scholar]
- Pradhan N, Prakash P, Manimurugan C, Tiwari SK, Sharma R, Singh P (2015) Screening of tomato genotypes using osmopriming with PEG 6000 under salinity stress. Res Environ Life Sci 8: 245–250. http://rels.comxa.com
- Prerna DI, Govindaraju K, Tamilselvan S, Kannan M. Seaweed-based biogenic ZnO nanoparticles for improving agro-morphological characteristics of rice (Oryza sativa L.) J Plant Growth Regul. 2019;5:1–2. doi: 10.1007/s00344-019-10012-3. [DOI] [Google Scholar]
- Prerna DI, Govindaraju K, Tamilselvan S, Kannan M, Vasantharaja R, Chaturvedi S, Shkolnik D. Influence of nanoscale micro-nutrient α-Fe2O3 on seed germination, seedling growth, translocation, physiological effects and yield of rice (Oryza sativa) and maize (Zea mays) Plant Physiol Biochem. 2021;162:564–580. doi: 10.1016/j.plaphy.2021.03.023. [DOI] [PubMed] [Google Scholar]
- Qiu N, Lu C. Enhanced tolerance of photosynthesis against high temperature damage in salt-adapted halophyte atriplex centralasiatica plants. Plant Cell Environ. 2003;26:1137–1145. doi: 10.1046/j.1365-3040.2003.01038.x. [DOI] [Google Scholar]
- Qu C, Zhang S, Zhao H, Chen J, Zuo Z, Sun X, Cheng Y, Xu Z, Liu G. Analysis of the energy source at the early stage of poplar seed germination: verification of Perl’s pathway. Biotech. 2020;10:418. doi: 10.1007/s13205-020-02413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai-Kalal P, Jajoo A. Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol Biochem. 2021;160:341–351. doi: 10.1016/j.plaphy.2021.01.032. [DOI] [PubMed] [Google Scholar]
- Rai-Kalal P, Tomar RS, Jajoo A. Seed nanopriming by silicon oxide improves drought stress alleviation potential in wheat plants. Funct Plant Biol. 2021;48:905–915. doi: 10.1071/FP21079. [DOI] [PubMed] [Google Scholar]
- Rajjou L, Gallardo K, Debeaujon I, Vandekerckhove J, Job C, Job D. The effect of alpha-amanitin on the Arabidopsis seed proteome highlights the distinct roles of stored and neosynthesized mRNAs during germination. Plant Physiol. 2004;134:1598–1613. doi: 10.1104/pp.103.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajjou L, Lovigny Y, Groot SP, Belghazi M, Job C, Job D. Proteome-wide characterization of seed aging in Arabidopsis: a comparison between artificial and natural aging protocols. Plant Physiol. 2008;148:620–641. doi: 10.1104/pp.108.123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajjou L, Duval M, Gallardo K, Catusse J, Bally J, Job C, Job D. Seed germination and vigor. Annu Rev Plant Biol. 2012;63:507–533. doi: 10.1146/annurev-arplant-042811-05550. [DOI] [PubMed] [Google Scholar]
- Raveneau MP, Benamar A, Macherel D. Water content, adenylate kinase, and mitochondria drive adenylate balance in dehydrating and imbibing seeds. J Exp Bot. 2017;68:3501–3512. doi: 10.1093/jxb/erx182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawashdeh RY, Harb AM, AlHasan AM. Biological interaction levels of zinc oxide nanoparticles; lettuce seeds as case study. Heliyon. 2020;6:e03983. doi: 10.1016/j.heliyon.2020.e03983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray DK, West PC, Clark M, Gerber JS, Prishchepov AV, Chatterjee S. Climate change has likely already affected global food production. PLoS One. 2019;14:e0217148. doi: 10.1371/journal.pone.0217148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond P, Al-Ani A, Pradet A. ATP production by respiration and fermentation, and energy charge during aerobiosis and anaerobiosis in twelve fatty and starchy germinating seeds. Plant Physiol. 1985;79:879–884. doi: 10.1104/pp.79.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman H, Farooq M, Basra SM, Afzal I. Hormonal priming with salicylic acid improves the emergence and early seedling growth in cucumber. J Agric Soc Sci. 2011;7:109–113. [Google Scholar]
- Reisdorph NA, Koster K. Progressive loss of desiccation tolerance in germinating pea (Pisum sativum) seed. Physiol Plant. 1999;105:266–271. doi: 10.1034/j.1399-3054.1999.105211.x. [DOI] [Google Scholar]
- Rhaman MS, Imran S, Rauf F, Khatun M, Baskin CC, Murata Y, Hasanuzzaman M. Seed priming with phytohormones: an effective approach for the mitigation of abiotic stress. Plants. 2021;10:37. doi: 10.3390/plants10010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roqueiro G, Maldonado S, del Carmen RM, Maroder H. Fluctuation of oxidative stress indicators in Salix nigra seeds during priming. J Exp Bot. 2012;63:3631–3642. doi: 10.1093/jxb/ers030. [DOI] [PubMed] [Google Scholar]
- Safari H, Hosseini SM, Azari A, Rafsanjani MH. Effects of seed priming with ABA and SA on seed germination and seedling growth of sesame (Sesamum indicum L.) under saline condition. Aust J Crop Sci. 2018;12:1385. doi: 10.21475/ajcs.18.12.09.pne940. [DOI] [Google Scholar]
- Saha P, Chatterjee P, Biswas AK. NaCl pretreatment alleviates salt stress by enhancement of antioxidant defense system and osmolyte accumulation in mungbean (Vigna radiata L. Wilczek) Indian J Exp Biol. 2010;48:593–600. [PubMed] [Google Scholar]
- Salam A, Khan R, Liu L, Yang S, Azhar W, Ulhassan Z, Zeedìshan M, Wu J, Fan X, Gan Y. Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. J Haz Mat. 2022;423:127021. doi: 10.1016/j.jhazmat.2021.127021. [DOI] [PubMed] [Google Scholar]
- Salvi P, Saxena SC, Petla BP, Kamble NU, Kaur H, Verma P, Rao V, Ghosh S, Majee M. Differentially expressed galactinol synthase(s) in chickpea are implicated in seed vigor and longevity by limiting the age induced ROS accumulation. Sci Rep. 2016;6:35088. doi: 10.1038/srep35088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano N, Seo M. Cell cycle inhibitors improve seed storability after priming treatments. J Plant Res. 2019;132:263–271. doi: 10.1007/s10265-018-01084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano N, Kim JS, Onda Y, Nomura T, Mochida K, Okamoto M, Seo M. RNA-Seq using bulked recombinant inbred line populations uncovers the importance of brassinosteroid for seed longevity after priming treatments. Sci Rep. 2017;7:8095. doi: 10.1038/s41598-017-08116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano N, Rajjou L, North HM. Lost in translation: physiological roles of stored mRNAs in seed germination. Plants. 2020;9:347. doi: 10.3390/plants9030347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardar R, Ahmed S, Yasin NA. Titanium dioxide nanoparticles mitigate cadmium toxicity in Coriandrum sativum L. through modulating antioxidant system, stress markers and reducing cadmium uptake. Environ Pollut. 2022;292:118373. doi: 10.1016/j.envpol.2021.118373. [DOI] [PubMed] [Google Scholar]
- Schwember AR, Bradford KJ. Oxygen interacts with priming, moisture content and temperature to affect the longevity of lettuce and onion seeds. Seed Sci Res. 2011;21:175–185. doi: 10.1017/S0960258511000080. [DOI] [Google Scholar]
- Sedghi M, Nemati A, Amanpour-Balaneji B, Gholipouri A. Influence of different priming materials on germination and seedling establishment of milk thistle (Silybum marianum) under salinity stress. World Appl Sci J. 2010;11:604–609. [Google Scholar]
- Sen A, Puthur JT. Influence of different seed priming techniques on oxidative and antioxidative responses during the germination of Oryza sativa varieties. Physiol Mol Biol Plants. 2020;226:551–565. doi: 10.1007/s12298-019-00750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano N, Ling Y, Bahieldin A, Mahfouz MM. Thermopriming reprograms metabolic homeostasis to confer heat tolerance. Sci Rep. 2019;9:1–14. doi: 10.1038/s41598-018-36484-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah T, Latif S, Saeed F, Ali I, Ullah S, Alsahli AA, Jan S, Ahmad P. Seed priming with titanium dioxide nanoparticles enhances seed vigor, leaf water status, and antioxidant enzyme activities in maize (Zea mays L.) under salinity stress. J King Saud Univ Sci. 2021;33:101207. doi: 10.1016/j.jksus.2020.10.004. [DOI] [Google Scholar]
- Shah A, Subramanian S, Smith DL. Seed priming with Devosia sp. cell-free supernatant (CFS) and Citrus bioflavonoids enhance canola and soybean seed germination. Molecules. 2022;27:3410. doi: 10.3390/molecules27113410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahi-Gharahlar A, Farhoudi R, Mosavi M (2009) Effect of seed pretreatment on summer squash (Cucurbita pepo) seed germination and seedling characteristics under salinity condition. Seed Sci Biotechnol 3: 15–23. http://www.globalsciencebooks.info/Online/GSBOnline/images/0906/SSB_3(1&2)/SSB_3(2)60-63o.pdf
- Sharma SN, Maheshwari A. Expression patterns of DNA repair genes associated with priming small and large chickpea (Cicer arietinum) seeds. Seed Sci Tech. 2015;43:1–12. doi: 10.15258/sst.2015.43.2.11. [DOI] [Google Scholar]
- Sharma L, Priya M, Kaushal N, Bhandhari K, Chaudhary S, Dhankher OP, Nayyar H. Plant growth-regulating molecules as thermoprotectants: functional relevance and prospects for improving heat tolerance in food crops. J Exp Bot. 2020;71:569–594. doi: 10.1093/jxb/erz333. [DOI] [PubMed] [Google Scholar]
- Sharma D, Afzal S, Singh NK. Nanopriming with phytosynthesized zinc oxide nanoparticles for promoting germination and starch metabolism in rice seeds. J Biotechnol. 2021;336:64–75. doi: 10.1016/j.jbiotec.2021.06.014. [DOI] [PubMed] [Google Scholar]
- Shelar A, Singh AV, Maharjan RS, Laux P, Luch A, Gemmati D, Tisato V, Singh SP, Santilli MF, Shelar A, Chaskar M, Patil R. Sustainable agriculture through multidisciplinary seed nanopriming: prospects of opportunities and challenges. Cells. 2021;10:428. doi: 10.3390/cells10092428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheteiwy M, Shen H, Xu J, Guan Y, Song W, Hu J. Seed polyamines metabolism induced by seed priming with spermidine and 5-aminolevulinic acid for chilling tolerance improvement in rice (Oryza sativa L.) seedlings. Environ Exp Bot. 2017;137:58–72. doi: 10.1016/j.envexpbot.2017.02.007. [DOI] [Google Scholar]
- Shinde S, Paralikar P, Ingle AP, Rai M. Promotion of seed germination and seedling growth of Zea mays by magnesium hydroxide nanoparticles synthesized by the fltrate from Aspergillus niger. Arab J Chem. 2020;13:3172–3182. doi: 10.1016/j.arabic.2018.10.001. [DOI] [Google Scholar]
- Silva PCC, Azevedo Neto AD, Gheyi HR, Ribas RF, Silva CRR, Cova AMW. Seed priming with H2O2 improves photosynthetic efficiency and biomass production in sunflower plants under salt stress. Arid Land Res Manag. 2022;36:283–297. doi: 10.1080/15324982.2021.1994482. [DOI] [Google Scholar]
- Singh J, Dutta T, Kim KH, Rawat M, Samddar P, Kumar P. ‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnol. 2018;16:84. doi: 10.1186/s12951-018-0408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Singh R, Tripathi S, Devi RS, Srivastava P, Singh P, Kumar A, Bhadouria R. Seed priming: state of the art and new perspectives in the era of climate change. In: Prasad MNP, Pietrzykowski M, editors. Climate change and soil interactions. Elsevier; 2020. pp. 143–170. [Google Scholar]
- Sivritepe HO, Sivritepe N, Eris A, Turhan E. The effects of NaCl pre-treatments on salt tolerance of melons grown under long-term salinity. Sci Hortic. 2005;106:568–581. doi: 10.1016/j.scienta.2005.05.011. [DOI] [Google Scholar]
- Soeda Y, Konings MCJM, Vorst O, van Houwelingen AMML, Stoopen GM, Maliepaard CA, Kodde J, Bino RJ, Groot SPC, van der Geest AHMAHM. Gene expression programs during Brassica oleracea seed maturation, osmopriming, and germination are indicators of progression of the germination grocess and the stress tolerance level. Plant Physiol. 2005;137:354–368. doi: 10.1104/pp.104.051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino M, De Diego N, Ugena L, Spíchal L, Lucini L, Miras-Moreno B, Zhang L, Rouphael Y, Colla G, Panzarová K. Seed priming with protein hydrolysates improves Arabidopsis growth and stress tolerance to abiotic stresses. Front Plant Sci. 2021;12:626301. doi: 10.3389/fpls.2021.626301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava AK, Kumar JS, Suprasanna P. Seed ‘primeomics’: plants memorize their germination under stress. Biol Rev. 2021;96:1723–1743. doi: 10.1111/brv.12722. [DOI] [PubMed] [Google Scholar]
- Steffens B, Steffen-Heins A, Sauter M. Reactive oxygen species mediate growth and death in submerged plants. Front Plant Sci. 2013;4:179. doi: 10.3389/fpls.2013.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tania SS, Rhaman MS, Rauf F, Rahaman MM, Kabir MH, Hoque MA, Murata Y. Alleviation of salt-inhibited germination and seedling growth of kidney bean by seed priming and exogenous application of aalicylic acid (SA) and hydrogen peroxide (H2O2) Seeds. 2022;1:87–98. doi: 10.3390/seeds1020008. [DOI] [Google Scholar]
- Thanos CA. Aristotle and Theophrastus on plant-animal interactions. In: Arianoutsou M, Groves RH, editors. Plant-animal interactions in Mediterranean-type ecosystems. Dordrecht: Kluwer Academic Publishers; 1994. pp. 3–11. [Google Scholar]
- Thanos CA (2007) Theophrastus, the Founder of Seed Science - his accounts on seed preservation. ENSC news. https://www.researchgate.net/publication/258133215
- Theophrastus. Enquiry into plants, Volume II: Book 7. Of herbaceous plants, other than coronary plants: pot herbs and similar wild herbs. Loeb Classical Library 79. Cambridge, MA: Harvard University Press, 1916. 10.4159/DLCL.theophrastus-enquiry_plants.1916
- Thiault L, Mora C, Cinner JE, Cheung WWL, Graham NAJ, Januchowski-hartley FA, et al. Escaping the perfect storm of simultaneous climate change impacts on agriculture and marine fisheries. Science. 2019;5:eaaw9976. doi: 10.1126/sciadv.aaw9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. doi: 10.1046/j.1365-313X.1997.11061187.x. [DOI] [Google Scholar]
- Toselli ME, Casenave EC. Is the enhancement produced by priming in cottonseeds maintained during storage? Bragantia, Campinas. 2014;73:372–376. doi: 10.1590/1678-4499.259. [DOI] [Google Scholar]
- Tu K, Cheng Y, Pan T, Wang J, Sun Q. Effects of seed priming on vitality and preservation of pepper seeds. Agriculture. 2022;12:603. doi: 10.3390/agriculture12050603. [DOI] [Google Scholar]
- Veena M, Puthur JT. Seed nutripriming with zinc is an apt tool to alleviate malnutrition. Environ Geochem Health. 2022;44:2355–2373. doi: 10.1007/s10653-021-01054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura L, Giovannini A, Savio M, Donà M, Macovei A, Buttafava A, Carbonera D, Balestrazzi A. Single cell gel electrophoresis (Comet) assay with plants: research on DNA repair and ecogenotoxicity testing. Chemosphere. 2013;92:1–9. doi: 10.1016/j.chemosphere.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Wahid A, Perveen M, Gelani S, Basra SM. Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J Plant Physiol. 2007;3:283–294. doi: 10.1016/j.jplph.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Wang W, He A, Peng S, Huang J, Cui K, Nie L. The effect of storage condition and duration on the deterioration of primed rice seeds. Front Plant Sci. 2018;9:172. doi: 10.3389/fpls.2018.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hu Z, Islam ARMT, Chen S, Shang D, Xue Y. Effect of warming and elevated O3 concentration on CO2 emissions in a wheat-soybean rotation cropland rotation cropland. Int J Environ Res Public Health. 2019;16:1755. doi: 10.3390/ijerph16101755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang Y, Shi Z, Pang H, Jia L, Feng H. Extracellular ATP is involved in regulating rabidopsis seed germination. Planta. 2022;255:66. doi: 10.1007/s00425-022-03839-w. [DOI] [PubMed] [Google Scholar]
- Waterworth WM, Masnavi G, Bhardwaj RM, Jiang Q, Bray CM, West CE. A plant DNA ligase is an important determinant of seed longevity. Plant J. 2010;63:848–860. doi: 10.1111/j.1365-313X.2010.04285.x. [DOI] [PubMed] [Google Scholar]
- Waterworth WM, Bray CM, West CE. The importance of safeguarding genome integrity in germination and seed longevity. J Exp Bot. 2015;66:3549–3558. doi: 10.1093/jxb/erv080. [DOI] [PubMed] [Google Scholar]
- Waterworth MW, Footitt S, Bray CM, Finch-Savage WE, West CE. DNA damage checkpoint kinase ATM regulates germination and maintains genome stability in seeds. Proc Natl Acad Sci USA. 2016;113:9647–9652. doi: 10.1073/pnas.1608829113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterworth WM, Bray CM, West CE. Seeds and the art of genome maintenance. Front Plant Sci. 2019;10:706. doi: 10.3389/fpls.2019.00706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterworth WM, Latham L, Wang D, Alsharif M, West CE. Seed DNA damage response promote germination and growth in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2022;119:1–8. doi: 10.1073/pnas.2202172119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LX, Lv BS, Wang MM, Ma HY, Yang HY, Liu XL, Jiang CJ, Liang ZW. Priming effect of abscisic acid on alkaline stress tolerance in rice (Oryza sativa L.) seedlings. Plant Physiol Biochem. 2015;90:50–57. doi: 10.1016/j.plaphy.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Wojtyla Ł, Lechowska K, Kubala S, Garnczarska M. Different modes of hydrogen peroxide action during seed germination. Front Plant Sci. 2016;7:66. doi: 10.3389/fpls.2016.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia F, Cheng H, Chen L, Zhu H, Mao P, Wang M. Influence of exogenous ascorbic acid and glutathione priming on mitochondrial structural and functional systems to alleviate aging damage in oat seeds. BMC Plant Biol. 2020;20:104. doi: 10.1186/s12870-020-2321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Xin X, Yin G, Zhou J, Zhou Y, Lu X. Timing for antioxidant-priming against rice seed ageing: optimal only in non-resistant stage. Sci Rep. 2020;10:13294. doi: 10.1038/s41598-020-70189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoubi R, Job C, Belghazi M, Chaibi W, Job D. Toward characterizing seed vigor in alfalfa through proteomic analysis of germination and priming. J Prot Res. 2011;10:3891–3903. doi: 10.1021/pr101274f. [DOI] [PubMed] [Google Scholar]
- Yan M. Prolonged storage reduced the positive effect of hydropriming in Chinese cabbage seeds stored at different temperatures. South Afr J Bot. 2017;111:313–315. doi: 10.1016/j.sajb.2017.04.005. [DOI] [Google Scholar]
- Yang X, Zhang W, Dong M, Boubriak I, Huang Z. The achene mucilage hydrated in desert dew assists seed cells in maintaining DNA integrity: adaptive strategy of desert plant Artemisia sphaerocephala. PLoS One. 2011;6:e24346. doi: 10.1371/journal.pone.0024346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Zhi P, Chang C. Priming seeds for the future: Plant immune memory and application in crop protection. Front Plant Sci. 2022;13:961840. doi: 10.3389/fpls.2022.961840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh YM, Chiu KY, Chen CL, Sung JM. Partial vacuum extends the longevity of primed bitter gourd seeds by enhancing their anti-oxidative activities during storage. Sci Hort. 2005;104:101–112. doi: 10.1016/j.scienta.2004.08.006. [DOI] [Google Scholar]
- Yugandhar P, Savithramma N (2013) Green synthesis of calcium carbonate nanoparticles and their efects on seed germination and seedling growth of Vigna mungo (L.) Hepper. Int J Adv Res 1: 89–103. https://www.journalijar.com/
- Zhai K, Ji Z, Jiang D, Zhao G, Zhong T. Sand priming promotes seed germination, respiratory metabolism and antioxidant capacity of Pinus massoniana lamb. Agriculture. 2022;12:455. doi: 10.3390/agriculture12040455. [DOI] [Google Scholar]
- Zhang M, Yoshiyama M, Nagashima T, Nakagawa Y, Yoshioka T, Esashi Y. Aging of soybean seeds in relation to metabolism at different relative humidities. Plant Cell Physiol. 1995;36:1189–1195. doi: 10.1093/oxfordjournals.pcp.a078875. [DOI] [Google Scholar]
- Zhang F, Yu J, Johnston CR, Wang Y, Zhu K, Lu F, Zhang Z, Zou J. Seed priming with polyethylene glycol induces physiological changes in sorghum (Sorghum bicolor L. Moench) seedlings under suboptimal soil moisture environments. PLoS One. 2015;10:e0140620. doi: 10.1371/journal.pone.0140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Hu M, Gao Z, Chen X, Huang D. Biological mechanisms of a novel hydro-electro hybrid priming recovers potential vigor of onion seeds. Environ Exp Bot. 2018;150:260–271. doi: 10.1016/j.envexpbot.2018.04.002. [DOI] [Google Scholar]
- Zhou W, Chen F, Luo X, Dai Y, Yang Y, Zheng C, Yang W, Shu K. A matter of life and death: molecular, physiological, and environmental regulation of seed longevity. Plant Cell Environ. 2020;43:293–302. doi: 10.1111/pce.13666. [DOI] [PubMed] [Google Scholar]
- Zhou X, Jia X, Zhang Z, Chen K, Wang L, Chen H, Yang Z, Li C, Zhao L. AgNPs seed priming accelerated germination speed and altered nutritional profile of Chinese cabbage. Sci Total Environ. 2021;808:151896. doi: 10.1016/j.scitotenv.2021.151896. [DOI] [PubMed] [Google Scholar]
- Zhu ZH, Sami A, Xu QQ, Wu LL, Zheng WY, Chen ZP, Jin XZ, Zhang H, Li Y, Yu Y, Zhou KJ. Effects of seed priming treatments on the germination and development of two rapeseed (Brassica napus L.) varieties under the co-influence of low temperature and drought. PLoS One. 2021;16:e0257236. doi: 10.1371/journal.pone.0257236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziska LH, Bunce JA, Shimono H, Gealy DR, Baker JT, Newton PC, Reynolds MP, Jagadish KS, Zhu C, Howden M, Wilson LT. Food security and climate change: on the potential to adapt global crop production by active selection to rising atmospheric carbon dioxide. Proc Biol Sci. 2012;279:4097–4105. doi: 10.1098/rspb.2012.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatanova JS, Ivanov PV, Stoilov LM, Chimshirova KV, Stanchev BS. DNA repair precedes replicative synthesis during early germination in maize. Plant Mol Biol. 1987;10:139–144. doi: 10.1007/BF00016151. [DOI] [PubMed] [Google Scholar]
- Zongshuai W, Xiangnan L, Xiancan Z, Shengqun L, Fengbin S, Fulai L, Yang W, Xiaoning Q, Fahong W, Zhiyu Z, Duan P, Yang A, Cai J, Jiang D. Salt acclimation induced salt tolerance is enhanced by abscisic acid priming in wheat. Plant Soil Environ. 2017;63:307–314. doi: 10.17221/287/2017-PSE. [DOI] [Google Scholar]
- Zrig A, Saleh A, Hamouda F, Okla MK, Al-Qahtani WH, Alwasel YA, Al-Hashimi A, Hegab MY, Hassan AHA, AbdElgawad H. Impact of sprouting under potassium nitrate priming on nitrogen assimilation and bioactivity of three Medicago species. Plants (basel) 2022;11:71. doi: 10.3390/plants11010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulfiqar F. Effect of seed priming on horticultural crops. Sci Hort. 2021;286:110197. doi: 10.1016/j.scienta.2021.110197. [DOI] [Google Scholar]
- Zulfiqar F, Ashraf M. Bioregulators: unlocking their potential role in regulation of the plant oxidative defense system. Plant Mol Biol. 2021;105:11–41. doi: 10.1007/s11103-020-01077-w. [DOI] [PubMed] [Google Scholar]
- Zulfiqar F, Nafees M, Chen J, Darras A, Ferrante A, Hancock JT, Ashraf M, Zaid A, Latif N, Corpas FJ, Altaf MA, Siddique KHM. Chemical priming enhances plant tolerance to salt stress. Front Plant Sci. 2022;13:946922. doi: 10.3389/fpls.2022.946922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.