Abstract
Protection of stalled replication forks is crucial for cells to respond to replication stress and maintain genome stability. Genome instability and replication stress have been linked to immune activation. Here we show a role of Abro1 and FANCD2 in linking replication fork protection with restriction of innate immune response. We reveal that stalled replication fork degradation induced by Abro1- or FANCD2-deficiency leads to accumulation of cytosolic single-stranded DNA (ssDNA) and activation of cGAS/STING-dependent innate immune response that is dependent on DNA2 nuclease. We further show that increased cytosolic ssDNA contains ribosomal DNA that can bind to cGAS. In addition, Abro1 and FANCD2 limits replication stress induced P-bodies formation and P-bodies are capable of modulating activation of the innate immune response after prolonged replication stress. Our study demonstrates a connection between replication stress and activation of the innate immune response that may be targeted for therapeutic purpose.
Introduction
Recent research has provided strong evidences that genome instability can trigger inflammatory response when chromosomal or short stranded nuclear DNA are released into the cytoplasm and detected by a cytosolic DNA sensing pathway, the cyclic guanosine 5′-monophosphate–adenosine 5′-monophosphate (cGAMP) synthase (cGAS) - adaptor stimulator of interferon genes (STING) –dependent pathway 1–3. Upon binding cytosolic DNA, cGAS catalyzes the production of cGAMP from adenosine 5′-triphosphate (ATP) and guanosine 5′-triphosphate (GTP), which in turn binds and activates STING 1, 4–10. The activated STING triggers a signaling cascade that lead to activation of innate immune response with induction of type I interferons and other immune molecules 1, 4–10. Several studies have shown that DNA damage and accumulation of micronuclei or cytoplasmic DNA activate cGAS-STING pathway leading to induction of innate immune signaling 2, 3, 11.
Protection of stalled replication fork integrity is crucial for cells to respond to replication stress and maintain genome stability 12–14. Emerging evidences indicate that DNA repair or replication fork processing in the replication stress response is connected with activation of the innate immune response. Depletion of several factors regulating DNA end resection results in accumulation of cytosolic ssDNA and activation of innate immune response 15–20, suggesting a link between stalled replication fork end protection and innate immune response.
FANCD2 and BRCA1/BRCA2-dependent protection of stalled replication forks inhibit MRE11 nuclease access to newly synthesized DNA 21, 22. FANCD2 also has been shown required for fork protection in BRCA1/2-deficient cells 23, 24. In addition, Abro1 is an important player in the protection of stalled replication fork integrity. Previously, we have shown that Abro1 protects stalled replication fork through inhibiting DNA2 nuclease/WRN helicase-dependent degradation of RAD51-mediated reversed forks 25. Lack of Abro1 leads to increased degradation of newly synthesized DNA and accumulation of ssDNA and depletion of DNA2, WRN or RAD51 restores fork integrity in Abro1-deficient cells.
P-bodies are cytoplasmic granules that form in response to various stresses. The core components of the P-bodies are involved in translational repression and/or mRNA decay, including the de-capping enzyme DCP1a/DCP2, the deadenylation complex Ccr4-Not, Lsm1–7 and various de-capping activators such as Edc3, Pat1, DDX6, EDC4, and the 5’−3’ exoribonuclease 1 (XRN1) 26–29. Several P-bodies component proteins such as DCP1a, DCP2 and XRN1 directly regulate the stability of transcripts involved in the inflammatory response 30. Formation and function of P-bodies have been shown involved in the response to replication stress in yeast 31, 32.
Here we show that Abro1- and FANCD2-deficiency in replication fork protection leads to accumulation of cytosolic ssDNA and triggers cGAS-STING-dependent innate immune signaling. Restoring fork integrity reduces cytosolic ssDNA and eliminates the activation of innate immune response. We further show that ribosomal (rDNA) fragments accumulate as cytosolic ssDNA in Abro1- or FANCD2-deficient cells that can be detected by cGAS. In addition, Abro1 and FANCD2 limit replication stress-induced P-bodies and P-bodies play a role in the activation of fork degradation-induce innate immune response. Finally, loss of Abro1 or FANCD2 promotes secretion of proinflammatory cytokines/chemokines and immune cell migration. Thus, our study has provided a mechanistic link between Abro1- or FANCD2- dependent replication fork protection and inhibition of innate immune response.
Results
Abro1 limits cytosolic ssDNA and cGAS-dependent signaling
We tested whether fork degradation due to Abro1-deficiency leads to accumulation of cytosolic DNA and triggers innate immune response. We found that knockout of Abro1 in U2OS (KO) cells results in a marked increase of cytosolic DNA that can be detected by picogreen staining and immunofluorescence (IF) with ssDNA antibody upon treatment of replication stressing agent hydroxyurea (HU) (Fig. 1a, Extended Data Fig. 1a). Treatment with S1 nuclease which digests ssDNA eliminated ssDNA staining (Extended Data Fig. 1b). In addition, isolated cytosolic DNA from cells are susceptible to S1 nuclease but not RNaseH, RNaseT1 or RNase III degradation, confirming the presence of ssDNA in the cytosol (Extended Data Fig. 1c). By pulse-labeling cells with BrdU and native BrdU staining which detects ssDNA, it showed that cytosolic BrdU intensity (ssDNA) was much enhanced in Abro1 KO cells treated with HU, indicating that cytosolic ssDNA could be generated from degradation of newly synthesized DNA at stalled forks due to Abro1-deficiency (Extended Data Fig.1d).
Fig. 1. Abro1 limits cytosolic ssDNA and replication stress-induced cGAS-STING-dependent innate immune response.
(a) Increased cytosolic ssDNA in Abro1 KO cells in response to HU. Immunofluorescence of ssDNA in cells untreated (UT) or treated with 4 mM HU (4h) using ssDNA antibody. Scale bars, 10 μm. Cytosolic ssDNA quantification of mean fluorescence intensity (MFI) per cell was shown with mean ± SD, WT (n=170), KO (n=183), WT HU (n=184), KO HU (n=184). One way Anova was used for statistics. ***p<0.0001 ****p<0.0001
(b) Abro1-deficiency leads to increased pSTAT1 in cells. WT or Abro1 KO U2OS cells were treated with HU (4 mM). Western blots were carried out with lysates of cells at indicated times after HU treatment.
(c) Induction of innate immune genes IL6 and CXCL10 expression in Abro1 KO cells. Cells were treated with 4 mM HU. Levels of IL6 and CXCL10 mRNAs were detected by real-time qPCR and relative fold change was quantified with mean ± SD (n=4 technical replicates). Biological replicates are shown in Extended Data Fig. 1e.
(d) Increased pSTAT1 levels in Abro1−/− MEFs in response to HU. Abro1 +/+ and −/− MEFs were treated with HU (4mM).
(e) Induction of IL6 and CXCL10 expression in Abro1−/− MEFs treated with HU. Levels of IL6 and CXCL10 mRNAs were detected by real-time qPCR and relative fold change was quantified with mean ± SD (n=4 technical replicates). Biological replicates are shown in Extended Data Fig. 1g.
(f) cGAS knockdown decreases the elevated pSTAT levels in Abro1 KO cells upon treatment of HU.
(g) cGAS knockdown decreases the induction of IL6 and CXCL10 upon HU treatment. Relative fold change of real time qPCR was quantified with mean ± SD (n=3 biological replicates). Two way Anova was used for statistics. ****p<0.0001
Loss of Abro1 in U2OS cells or MEFs led to an induction of innate immune response, especially when cells were treated with HU, marked by elevated phosphorylation of STAT1 (pSTAT1) and upregulation of the innate immune response genes IL6 and CXCL10 mRNAs (Fig. 1b–e). The normal pSTAT1 levels were restored when GFP-tagged WT Abro1 was expressed in the KO cells (Extended Data Fig. 1e). Treatment with other DNA replication stressing agent, such as low-dose camptothecin (CPT), induced a similar response with increased pSTAT1 (Extended Data Fig. 1f). We confirmed that the increased innate immune response due to Abro1-deficiency is dependent on cGAS-STING pathway since knockdown of cGAS diminished the increased pSTAT1 level and reduced expression of IL6 and CXCL10 (Fig. 1f, g). Knockdown of STING also reduced the elevated pSTAT1 levels in Abro1 KO cells (Extended Data Fig. 1g). Similar reduction of pSTAT1 levels was observed in Abro1−/− MEFs when cGAS was depleted (Extended Data Fig. 1h).
Abro1 loss-elicited innate immune signaling depends on DNA2
Abro1 protects stalled fork stability through inhibiting DNA2-mediated fork degradation 25. If the increased innate immune response in Abro1 KO cells is due to fork degradation, restoring fork stability by depleting DNA2 should decrease cytosolic DNA and eliminate the induced innate immune response. This is indeed the case. Knockdown of DNA2 abolished the accumulation of cytosolic ssDNA and reduced the elevated pSTAT1 levels and the induction of IL6 and CXCL10 in Abro1 KO cells (Fig. 2a–d). Depletion of DNA2 in Abro1 −/− MEFs also reduced pSTAT1 levels and decreased IL6, CXCL10 mRNA expression (Fig. 2e, f). On the other hand, treatment of mirin, an inhibitor of Mre11 nuclease, had little effect on cytosolic DNA accumulation and pSTAT1 levels (Extended Data Fig. 2a, b). These are correlated with our previous findings that inhibition of DNA2 but not Mre11 restores fork stability in Abro1-deficient cells 25. WRN helicase works with DNA2 in degrading RAD51-mediated reversed forks in Abro1-deficient cells 25. Restoring fork stability by depleting WRN or RAD51 in Abro1 KO cells also reduced the elevated pSTAT1 levels (Fig. 2g and Extended Data Fig. 2c). Thus, DNA2/WRN-mediated fork degradation in Abro1-deficient cells likely triggers innate immune response.
Figure 2. Increased innate immune response in Abro1-deficient cells depends on DNA2/WRN.
(a) (b) Knockdown of DNA2 decreases cytosolic ssDNA in Abro1 KO cells. Cells were untreated or treated with HU (4 mM, 4h). Immunofluorescence was carried out with ssDNA antibody. Scale bars, 10 μm. Mean fluorescence intensity (MFI) per cell was quantified and shown with mean ± SD, WT (n=171), KO (n=174), WT HU (n=170), KO HU (n=170), WT_siDNA2 (n=182), KO_siDNA2 (n=170), WT HU_siDNA2 (n=170), KO HU_siDNA2 (n=177). One way Anova was used for statistics. ****p<0.0001
(c) Knockdown of DNA2 decreases pSTAT1 in Abro1 KO cells upon treatment of HU.
(d) Knockdown of DNA2 abolishes the induction of IL6 and CXCL10 in Abro1 KO cells upon HU. Levels of IL6 and CXCL10 mRNAs were detected by real-time qPCR and relative fold change was quantified as mean ± SD (n=3 biological replicates). Two way Anova was used for statistics. ****p<0.0001
(e) Knockdown of DNA2 decreases pSTAT1 in Abro1−/− MEFs upon treatment of HU.
(f) Knockdown of DNA2 eliminates induction of IL6 and CXCL10 in Abro1−/− MEFs cells upon HU. Levels of IL6 and CXCL10 mRNAs were detected by real-time qPCR and relative fold change was quantified as mean ± SD (n=3 biological replicates). Two way Anova was used for statistics.
(g) Knockdown of WRN decreases pSTAT1 in Abro1 KO cells upon treatment of HU.
Cytosolic rDNA in Abro1-deficient cells are sensed by cGAS
We observed increased ssDNA staining in nuclear regions that appears to be nucleolus in HU-treated Abro1 KO cells (Fig. 2a). This is confirmed by the overlap of ssDNA staining with that of nucleolin, a component of the nucleolus (Extended Data Fig. 3a). Treatment with Leptomycin B (LMB), an inhibitor that blocks nuclear export, eliminated the accumulation of cytosolic ssDNA but enhanced the accumulation of ssDNA in the nucleolus region (Extended Data Fig. 3b). Treatment of LMB also decreased the upregulated pSTAT levels in Abro1 KO cells (Extended Data Fig. 3c). These suggest to us that a great portion of ssDNA detected in the cytoplasm may come from nucleolus. The nucleolus is primarily the site of ribosome biogenesis, where multiple repeats of rDNAs are organized in clusters of tandem repeats and transcribed by RNA polymerase I (RNAPI) to produce 18S, 5.8S and 28S rRNA. rDNA are the most abundant and highly transcribed genes in eukaryotes and the rDNA loci are among the major difficult-to-replicate regions across the genome 33, 34. DNA2 has been shown to play an important role in the replication of rDNA and maintaining rDNA stability 35, 36.
We first examined whether rDNA fragments are present in cytosol. We isolated cytosolic DNA and performed qPCR using primers targeting 18S and 28S regions of the rDNA, which detected a robust increased amount of rDNA in HU-treated Abro1 KO cells (Fig. 3a). We also used slot blot to detect rDNA using biotin-labeled 18S and 28S rDNA probes. Cytosolic rDNA amount was minimal in WT cells but much elevated in Abro1 KO cells and further enhanced when the cells were treated with HU (Fig. 3b, Extended Data Fig. 3d, e). Furthermore, fluorescence in situ hybridization (FISH) detected a robust increase of rDNA staining in the cytoplasm in Abro1 KO cells treated with HU, which was susceptible to S1 nuclease but not RNase H, or RNaseT1/RNaseIII digestion (Fig. 3c, d and Extended Data Fig. 3f). Therefore, a majority of cytosolic rDNA exist as ssDNA.
Figure 3. Ribosomal DNA (rDNA) fragments accumulate in the cytoplasm in Abro1- deficient cells and are detected by cGAS.
(a) Increased cytosolic rDNA in Abro1 KO cells detected by qPCR using isolated cytosolic DNA and primers targeting 18S (18S-1 and 18S-2) and 28S rDNA region (28S) (n=3 biological replicates).
(b) Detection of rDNA in the cytoplasm of Abro1 KO cells by slot blot with biotin-labeled rDNA 28S probe. “Nuclear DNA” (from 2 × 105 cells) and “Cytosolic DNA” (from 5 × 105 cells) were loaded. Band intensity (a.u.) is quantified using Image J.
(c) Detection of rDNA by FISH in Abro1 KO cells with biotin-labelled rDNA 28S probe.
(d) Treatment with S1, but not RNaseH, or RNaseT1/RNaseIII reduces cytosolic rDNA in HU (4mM, 4h)-treated Abro1 KO cells. FISH was carried out using biotin-labeled rDNA 28S probe. Scale bars, 20 μm.
(e) RNAPI inhibitor CX-5461 (1μM, 1h) reduces the amount of cytosolic ssDNA in Abro1 KO cells treated with HU (4mM, 4h) when added before the end of the HU treatment. Mean fluorescence intensity (MFI) per cell was quantified with mean ± SD.
(f) Treatment with CX5461 (1μM, 1h) reduces the elevated pSTAT1 levels in Abro1 KO cells. Cells were treated with HU for 16 h.
(g) A schematic of isolation of nuclear, cytosolic and cGAS bound DNA.
(h) Cytosolic rDNA is detected bound to cGAS by slot blot with biotin-labelled rDNA 28S probe. “Nuclear DNA” (from 2 × 105 cells); “Cytosolic DNA” (from 5 × 105 cells), or “cGAS bound DNA” (prepared from 1.25 × 106 cells) were loaded. Band intensity (a.u.) is quantified using Image J.
(i) Detection of cGAS bound rDNA by qPCR using 28S primers. cGAS bound DNA extracted from cGAS IP was used (n=3 biological replicates).
(j) Cytosolic ssDNA activates cGAS in cGAMP synthesis. Isolated cytosolic DNA was incubated with recombinant cGAS. The amount of cGAMP produced from cGAS reaction with cytosolic DNA isolated from one million cells was measured by ELISA (mean from triplicates of each experiment, n=3 independent experiments)
One way Anova was used for statistics for a-j. * p=0.02, **p=0.006, ****p<0.0001
We then determined whether the accumulation of cytosolic rDNA is dependent on DNA2. Using cell fractionation and slot blot, we found that knockdown of DNA2 eliminated the increased amount of cytosolic rDNA in Abro1 KO cells, indicating that DNA2 plays a critical role in limiting rDNA fragments accumulation in the cytoplasm (Fig. 3b and Extended Data Fig. 3e). rDNA are the most heavily transcribed genes. The robust transcription can lead to transcription-replication conflicts that stall replication 37, 38. We investigated whether inhibition of transcription of rDNA affects the accumulation of cytosolic ssDNA and activation of the cGAS pathway. We treated the cells with cx5461, a RNAPI inhibitor, and found that the treatment reduced the accumulation of cytosolic ssDNA, pSTAT1 levels, as well as the induction of IL6 and CXCL10 in Abro1 KO cells (Fig. 3e, f, and Extended Data Fig. 3g, h). Thus, rDNA accumulation in the cytosol likely plays a significant role in triggering activation of the cGAS-mediated innate immune signaling.
Finally, we determined whether cytosolic rDNA can be sensed by cGAS. We first examined whether cGAS binds to cytosolic rDNA by immunoprecipitating cGAS from cytoplasmic fraction of cells followed by DNA extraction and detection of rDNA using slot blot (Fig. 3g, h and Extended Data Fig. 4a–c). It showed that immunoprecipitation of cGAS pulled down rDNA and that the amount of cGAS-bound rDNA increased in HU-treated Abro1 KO cells (Fig. 3h and Extended Data Fig. 4c). This is confirmed by qPCR using rDNA primers (Fig. 3i and Extended Data Fig. 4d). We then determined whether cytosolic ssDNA can activate cGAS. We incubated isolated cytosolic DNA with recombinant cGAS followed by measurement of cGAMP synthesis in vitro. It showed that cytosolic DNA isolated from Abro1 KO cells, especially after HU treatment, activated cGAS for cGAMP synthesis (Fig. 3j). Importantly, treatment with S1 nuclease largely decreased cGAMP production, indicating that cytosolic ssDNA activates cGAS (Fig. 3j). PCR products from 28S or 18S rDNA also can activate cGAS for cGAMP synthesis (Extended Data Fig. 4e). Together, the increased cytosolic rDNA fragments due to Abro1-deficiency are likely sensed by cGAS in triggering the innate immune response.
P-bodies modulate Abro1 loss-elicited innate immune response
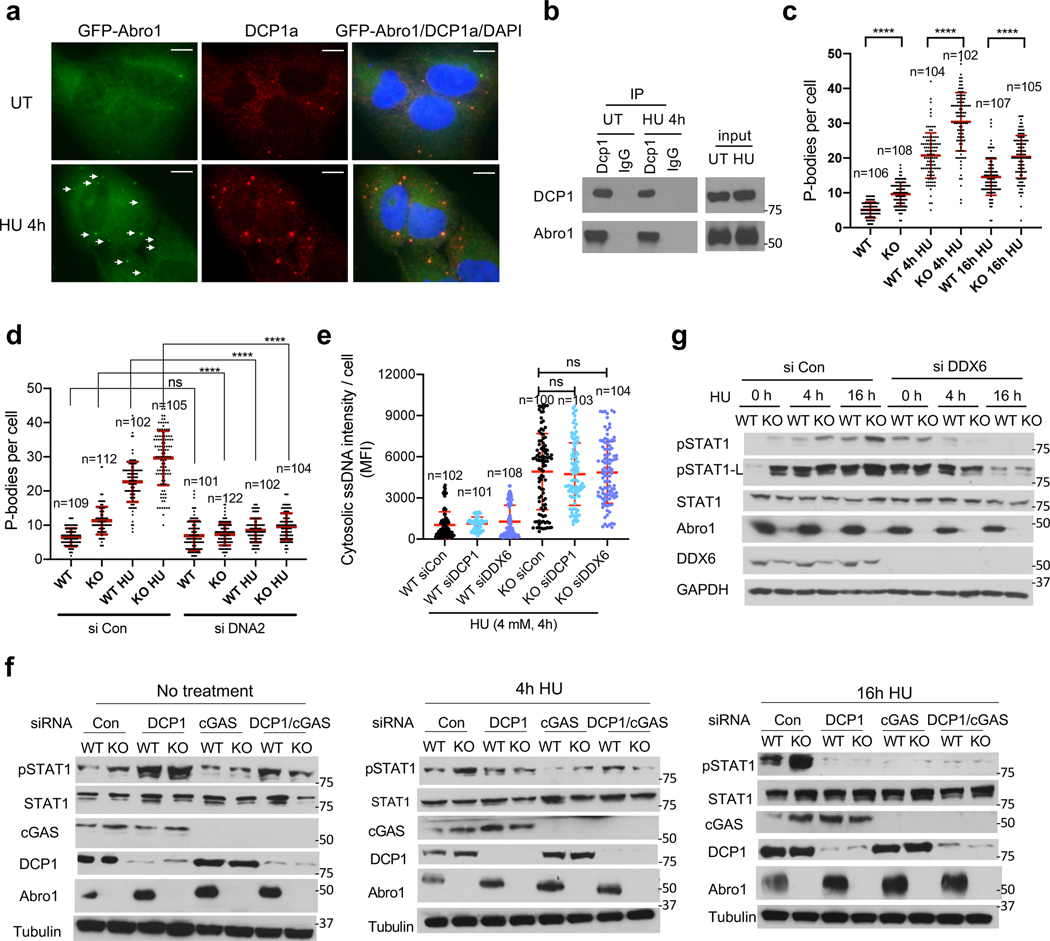
We found that Abro1 forms cytoplasmic foci, the number of which increases upon cells treated with different genotoxic agents (Extended Data Fig. 5a). Using P-body component DCP1a as a marker, we found that Abro1 colocalizes with P-bodies and the number of P-bodies increases in response to HU (Fig. 4a). In addition, Abro1 interacts with DCP1a, although the interaction appears not to be regulated upon HU treatment (Fig. 4b). Lack of Abro1 leads to increased number of P bodies in Abro1 KO U2OS and −/− MEFs (Fig. 4c and Extended Data Fig. 5b). This is likely due to the stress response caused by increased ssDNA since knocking down DNA2 which eliminates ssDNA accumulation decreased the number of P-bodies in Abro1 KO cells (Fig. 4d). We also examined whether disruption of P-bodies influence the level of cytosolic ssDNA in Abro1-deficient cells. Depletion of DCP1a or DDX6, another component of the P-bodies critical for their assembly 39, disrupted P-bodies formation and abolished GFP-Abro1 cytoplasmic foci (Extended Data Fig. 5c), but did not have much effect on the levels of cytosolic ssDNA or cytosolic rDNA accumulation in HU-treated Abro1 KO cells (Fig. 4e and Extended Data Fig. 5d). Thus, the increased P-bodies formation upon replication stress is likely a downstream event of ssDNA accumulation in Abro1-deficient cells.
Figure 4. Replication stress induced P-bodies are involved in modulating Abro1-deficiency-elicited innate immune response.
(a) GFP-Abro1 forms cytoplasmic foci that colocalize with P-bodies marked by immunofluorescence staining with DCP1a antibodies. Scale bars, 10 μm.
(b) Abro1 interacts with DCP1a. Immunoprecipitation of DCP1a antibody or IgG was carried out followed by western blots with indicated antibodies.
(c) Quantification of the number of P-bodies in Abro1 WT and KO U2OS cells upon HU treatment. Number of P-bodies per cell was shown with mean ± SD. One-way Anova was used for statistics. ****p<0.0001
(d) Knockdown of DNA2 decreases P-bodies number in Abro1 KO cells. Number of P-bodies per cell was quantified and shown with mean ± SD. One-way Anova was used for statistics. ****p<0.0001
(e) Knockdown of DCP1a or DDX6 does not reduce cytosolic ssDNA accumulation in Abro1 KO cells. Cells treated with indicated siRNAs were treated with HU (4mM, 4h). Mean fluorescence intensity (MFI) of cytosolic ssDNA per cell was quantified and shown with mean ± SD. One way Anova was used for statistics.
(f) DCP1a knockdown or DCP1a/cGAS double knockdown decreases pSTAT1 levels in Abro1 KO cells treated with HU (4 mM).
(g) DDX6 knockdown decreases pSTAT1 levels in Abro1 KO cells treated with HU.
We then tested whether P-bodies play a role in the innate immune response in Abro1-deficient cells. Depletion of DCP1a in WT or KO cells elevated pSTAT1 levels without HU treatment (Fig. 4f), possibly due to the role of P-body in mRNA decapping and translational repression of immune related genes 30, 40. In response to HU, depletion of DCP1 had a mild effect on pSTAT1 level at 4 h, but at 16 h, significantly downregulated pSTAT1 levels (Fig. 4f and Extended Data Fig. 5e, f). Knocking down DDX6 also greatly reduced pSTAT1 levels in HU-treated Abro1 KO cells (Fig. 4g). These suggest that P-bodies are involved in promoting the replication stress-induced innate immune response. By comparing DCP1 depletion and cGAS depletion, as well as double depletion of DCP1 and cGAS, it showed that at 16 h HU treatment, knockdown of DCP1 reduced pSTAT1 level to a similar level as knockdown of cGAS or DCP1/cGAS did in Abro1 KO cells (Fig. 4f). Comparing the effect of DCP1 depletion with that of STING knockdown or DCP1/STING double knockdown showed similar results (Extended Data Fig. 5f). Together, these data indicate that, in addition to cGAS-STING, P-bodies also contribute to the induction of innate immune response in Abro1 KO cells.
FANCD2 limits fork degradation-induced innate immune signaling
BRCA2 and FANCD2 have been implicated playing critical roles in protecting stalled replication fork integrity by inhibiting Mre11-dependent degradation 21, 22. We found that BRCA2 depletion led to increased pSTAT1 level in untreated cells (Extended Data Fig. 6a), consistent with the previous findings that induced BRCA2 inactivation triggers immune signaling 41, 42. However, HU treatment did not appear to further elicit increased pSTAT1 levels or accumulation of cytosolic ssDNA in BRCA2-depleted cells (Extended Data Fig. 6a–d). These suggest that, unlike Abro1-deficiency, BRCA2-deficiency does not induce innate immune response through cytosolic ssDNA accumulation in response to replication stress.
FANCD2 knockdown, however, led to accumulation of cytosolic ssDNA upon HU treatment (Fig. 5a, Extended Data Fig. 6b). Knockdown of FANCD2 in U2OS or MEFs also led to increased pSTAT1 (Fig. 5b and Extended Data Fig. 6e). Moreover, FANCD2-deficient cells showed increased formation of P-bodies (Fig. 5c). In comparison, knockdown of BRCA2 showed little effect on the number of P-bodies formed in the absence or presence of HU (Extended Data Fig. 6f). Furthermore, the increased pSTAT1 or pTBK1, another marker of innate immune response activation, induced by FANCD2-deficiency is dependent on cGAS (Figure 5d).
Figure 5. Stalled replication fork degradation due to FANCD2-deficiency depends on DNA2 and is linked with induction of innate immune response.
(a) FANCD2 knockdown leads to cytosolic ssDNA accumulation. Mean fluorescence intensity (MFI) of cytosolic ssDNA per cell was quantified and shown with mean ± SD.
(b) FANCD2 knockdown leads to increased pSTAT1. U2OS cells were treated with control or FANCD2 siRNAs before treatment with 4 mM HU.
(c) FANCD2 knockdown leads to increased number of P bodies in response to HU. The number of P-bodies per cell was quantified with mean ± SD.
(d) Increased pSTAT1 and pTBK1 level in FANCD2 knockdown cells is dependent on cGAS. Cells were treated HU (4 mM).
(e) FANCD2 deficiency in replication fork end protection is rescued by DNA2 knockdown. FANCD2-deficient PD20 cells expressing empty vector or FANCD2 gene were used and pulselabeled with CIdU followed by IdU and treated with HU as illustrated. IdU/CIdU ratio was quantified with mean ± SD.
(f) Knockdown of DNA2 or treatment with mirin reduces pSTAT1 or pTBK1 level in FANCD2 depleted cells. Cells were transfected with indicated siRNAs before treatment with HU.
(g) Innate immune genes IL6 and CXCL10 expression is induced in FANCD2-deficient cells and the induction is reduced by DNA2 depletion or mirin treatment. Cells were untreated or treated with HU (4 mM, 16 h). Levels of IL6 and CXCL10 mRNAs were detected by real-time qPCR and relative fold change was quantified (n=2 independent experiments of mean from triplicates of each experiment).
(h) Inhibition of DNA2 or MRE11 decreases cytosolic ssDNA in FANCD2-deficient cells. Cells were untreated or treated with HU (4 mM, 4h). Immunofluorescence was carried out with ssDNA antibody. Mean fluorescence intensity (MFI) of cytosolic ssDNA per cell was quantified mean ± SD.
(i) Cytosolic rDNA fragment accumulation in FANCD2-deficient cells detected by FISH with biotin-labelled rDNA 28S probe. Scale bars, 10 μm. Cytosolic FISH intensity (MFI, mean fluorescence intensity) was quantified with mean ± SD.
One-way Anova was used for statistics in a-i. ****p<0.0001
We then examined whether restoring fork stability in FANCD2-deficient cells eliminates the activation of innate immune response. Knocking down DNA2 or inhibition of Mre11 with mirin restores fork integrity in FANCD2-deficient cells as shown in the DNA fiber assay (Fig. 5e and Extended Data Fig. 6g) 22. We found that depletion of DNA2 or treatment with mirin reduced the increased pSTAT1/pTBK1 levels, IL6 or CXCL10 expression as well as cytosolic ssDNA accumulation in FANCD2-deficient cells (Fig. 5f–h). These data indicate that restoring fork integrity in FANCD2-deficient cells prevents cytosolic ssDNA accumulation and HU-induced activation of innate immune response.
By FISH, we found that rDNA fragments also accumulate in the cytoplasm in FANCD2 knockdown cells upon HU treatment (Fig. 5i and Extended Data Fig. 6i). Additionally, cytosolic DNA isolated from HU-treated FANCD2-deficient cells stimulated cGAS for cGAMP synthesis in the in vitro assay (Fig. 5j). Thus, FANCD2 also plays a role in protecting fork stability at rDNA loci and prevents activation of innate immune response as Abro1 does in response to replication stress.
Abro1- or FANCD2-deficiency promotes immune cell migration
The increased innate immune response often leads to secretion of cytokines or chemokines that coordinate cell to cell communication or cell and environment interaction. Using a cytokine array, we detected multiple proinflammatory cytokines and chemokines in cultured media collected from Abro1 KO cells and found their levels were further elevated when cells were treated with HU (Fig. 6a, b). The qPCR analysis also showed increased mRNA levels for these genes (Extended Data Fig. 7). Thus, Abro1-deficiency upon replication stress leads to increased production and secretion of proinflammatory cytokines/chemokines. Chemokines direct the trafficking of immune cells. We examined whether media collected from cultured cells undergoing HU-induced replication stress promote immune cell migration using an in vitro chemotaxis essay. We isolated mouse peritoneal cavity cells which harbor a number of immune cells including macrophages, B cells and T cells and incubated these cells with collected media (Fig. 6c). We found that media collected from HU-treated Abro1−/− or FANCD2 shRNA treated MEFs promoted peritoneal cavity cells migration (Fig. 6d). As a control, growth media or media containing HU had little effect on cell migration (Fig. 6d). Importantly, knockdown of cGAS or DNA2 eliminated the capability of the media from cultured Abro1−/− cells to promote cell migration, indicating that the secretion of proinfammatory cytokines/chemokines is dependent on cGAS and fork stability (Fig. 6e).
Figure 6. Abro1-deficiency upon replication stress results in increased cytokines secretion and Abro1- or FANCD2-deficiency promotes immune cell migration.
(a) Cytokine-array analyses of secreted factors in cells untreated or treated with HU. Media from cultured Abro1 WT or KO U2OS cells untreated or treated with HU (4 mM, 16h) were collected and incubated with cytokine array. Reference spots are on the corners of the membrane (circled).
(b) Quantification of secreted cytokines spots (a.u.) using Image J.
(c) A schematic of chemotaxis assay to measure immune cell migration attracted by secreted factors from Abro1 +/+ and −/− MEFs upon HU treatment.
(d) Abro1-deficiency promotes immune cell migration and knockdown of cGAS or DNA2 abolishes the migration of immune cells attracted by media from Abro1−/− MEFs. Relative fluorescence unit (RFU) was shown (mean from triplicates of each experiment of, n=3 independent experiments). Two way Anova was used for statistics. ****p<0.0001
Discussion
Our studies have demonstrated that Abro1- or FANCD2-dependent protection of stalled replication fork in response to replications stress is critical in restricting activation of innate immune signaling in non-immune cells. In the absence of Abro1 or FANCD2, cytosolic DNA generated from self-DNA due to DNA2-mediated degradation of stalled replication forks, especially from the rDNA regions, triggers cGAS-STING-dependent and P bodies-involved innate immune signaling leading to secretion of cytokine/chemokines that promote immune cell migration.
In our studies, the accumulation of cytosolic ssDNA is directly linked to DNA2-mediated fork degradation and cGAS activation in Abro1- or FANCD2-deficient cells. However, our study does not exclude the possibility that nuclear cGAS is activated upon replication fork degradation and plays a role in the activation of innate immune signaling. It has been shown that nuclear cGAS interacts with replication fork proteins and can act as a replication fork decelerator 43. In addition, altered chromatin structure without linker histone stimulates activation of cGAS and cGAMP production 44. Although it has been indicated that nuclear cGAS is kept in an inactive conformation through its binding to chromatin 45–49, fork degradation may induce chromatin structure change that activates nuclear cGAS. In our analyses, we did not observe aberrant cGAS distribution in the nucleus as seen in cells lacking linker histones 44 or a significant change of cGAS localization in the nucleus or the cytoplasm in Abro1- or FANCD2-deficient cells upon HU treatment (Extended Data Fig. 8). It remains to be investigated whether nuclear cGAS plays a role in the activation of innate immune signaling upon stalled replication fork degradation.
How does cytosolic ssDNA contribute to the activation of cGAS-STING pathway considering that cGAS preferentially binds to dsDNA 50, 51? In our studies, it is particularly interesting that rDNA fragments are detected accumulating as cytosolic ssDNA. Since rDNA region contains highly repetitive sequence, it is possible that ssDNA fragments can anneal in the cytosol to form partially duplex DNA that are long enough to activate cGAS, or form secondary structures with ssDNA exists in the context of dsDNA as part of the region that forms flaps or loops. In fact, it has been shown that HIV-1 ssDNA can activate cGAS through cGAS stimulation by Y-form DNA structures as those presented in stem-loop structures of HIV-1 ssDNA 52.
Our studies show that P-bodies play a role in the fork degradation-induced innate immune response. Previous studies have indicated a role of several P-body components in regulating mRNA stability of inflammatory response genes 30, 40. It is possible that P-bodies are involved in regulating mRNA levels of those immune signaling genes induced by cGAS-dependent pathway, further enhancing the activation of the innate immune response. Co-staining of cGAS and DCP1a reveals that although cGAS can be detected in a few P-bodies regardless of HU treatment, it largely does not colocalizes with P-bodies (Extended Data Fig. 8). It is also possible that P-bodies and cGAS function independently in mediating the activation of innate immune signaling in Abro1 KO cells.
The results that Abro1-deficiency in replication fork protection leads to production of proinflammatory cytokines and promotes peritoneal cavity cells migration suggest that in vivo, proinflammatory cytokines/chemokines secreted from replication fork protection-deficient cells undergoing replication stress may recruit immune cells, modulate their activity and alter the tissue microenvironment. This is reminiscent of the senescence-associated secretory phenotype (SASP), a key feature of senescence which senescent cells secret proinflammatory cytokines and other secretory factors that can affect surrounding cells 53. It has been shown that the mechanisms that activate SASP involves DNA damage response and are dependent on the cGAS-STING pathway 54. We propose that the fork-protection-deficiency-induced production of proinflammatory cytokines/chemokines may be referred as Fork Degradation Associated Secretory Phenotype (FDASP), and speculate that, similar to SASP, FDASP may provide autocrine or paracrine signals that impact on the tissue or tumor environment.
Overall, our findings demonstrate that Abro1- or FANCD2-dependent protection of stalled replication fork is critical in restraining activation of cGAS-STING and P-bodies-dependent pro-inflammatory pathway in response to replication stress. Targeting this pathway may hold promise in treating inflammation-related disorders and cancer.
Methods
Cell line and cell culture
U2OS cells (ATCC, HTB-96) were grown in McCoy’s 5A Medium (Sigma Aldrich) supplemented with 10% FBS. Immortalized Abro1+/+, −/− MEF cells (generated in the lab as described 25) were grown in DMEM (Sigma Aldrich) supplemented with 10% Fetal Bovine Serum (FBS, Gibco). Abro1 KO U2OS cells were generated by CRISPR-Cas9 as described 25.FANCD2-deficient PD20 cells complement with vector or FANCD2 (a gift from Dr. Alan D’Andrea) were grown in RMPI 1640 with L-glutamine, 15% FBS. Cells were maintained at 37°C with 5% CO2. For HU treatment, cells were treated with 4 mM HU. For mirin treatment, 100 ~μM mirin was added to cells in the presence or absence of HU. For LMB treatment, cells were incubated with addition of 10 ng/ml LMB for 12 hr.
Chemicals, Plasmids, siRNAs and antibodies
Pools of four individual siRNAs were obtained from GE Dharmacon for human cGAS( L-015607–02-0005), mouse cGAS (L-055608–01-0005), human STING (L-024333–00-0005), human WRN (L-010378–00-0005), human DCP1a (L-010378–00-0005), human FANCD2 (M-016376–02-0005), human BRCA2 (M-003462–01-0005), mouse DNA2 (M-062864–01-0005). Other siRNAs used are: control non-targeting siRNA 5’U.G.G.U.U.U.A.C.A.U.G.U.C.G.A.C.U.A.A.U.U3’; human siDDX6 5’G.G.A.A.C.U.A.U.G.A.A.G.A.C.U.U.A.A.A.U.U3’; human siDNA2 #1 5’C.A.G.U.A.U.C.U.C.C.U.C.U.A.G.C.U.A.G.U.U3’; human siDNA2 #2 5’A.U.A.G.C.C.A.G.U.A.G.U.A.U.U.C.G.A.U.U.U3’; human siRAD51 #1 5’G.A.G.C.U.U.G.A.C.A.A.A.C.T.A.C.U.U.C.3’; human siRad51 #2 5’A.G.C.A.G.U.G.G.U.A.A.U.C.A.C.U.A.A.U.C.A 3’
Mouse shFANCD2: V2LMM-205183 5’T.C.C.T.C.A.A.G.G.C.C.T.G.A.A.A.G.A.G3’
Cells were transfected with siRNAs using Lipofectamine RNAiMax (Invitrogen) for 60h before treatment of cells.
Antibodies used are: Abro1 (1:5000) 25, γ-tubulin (Sigma, T-6557 1:5000), DNA2 (Proteintech, 18727–1-AP 1:500) (Abcam, ab96488), FANCD2 (Santa Cruz Biotechnology, sc-20022 1:250), RAD51 (Abcam, ab63801), ssDNA (EMD Millipore, MAB3868), WRN (Novus, nb100–472), Stat1 (D1K9Y) (Cell Signaling, 14994S), P-Stat1 (Y701) (58D6) (Cell Signaling, 9167S), TBK1/NAK (Cell Signaling, 38066S), P-TBK1 (Ser172) (Cell Signaling, 5483S), cGAS (D1D3G) (Cell Signaling, 15102), STING (D2P2F) (Cell Signaling, 13647S) GAPDH Loading Control (Invitrogen, MA5–15738 1:5000), DDX6/RCK (Cell Signaling, 9407S), BrdU (BD Bioscience, 347580, 1;500), and DCP1a (Abcam, ab47811) (Santa Cruz Biotechnology, sc-100706 1:250)
Chemicals used in this study include Picogreen (Life Technologies), HRP-coupled streptavidin (Invitrogen), streptavidin conjugated Alexa555 (Invitrogen), Cisplatin (Sigma), APH (Sigma), HU (Sigma), Mirin (Sigma), LMB (Sigma), CX5461 (MedChemExpress) and camptothecin (CPT) (Sigma).
Immunoblotting and immunoprecipitation
Cells were lysed using NETN buffer (50 mM Tris-HCl at pH 8.0, 150 mM sodium chloride, 1 mM EDTA, 0.5% nonidet P-40, 1 mM dithiothreitol [DTT], 1 mM PMSF, 5 mM NaF, 1 mM Na3VO4, 50 mM β-glyceral, protease inhibitor cocktails) and centrifuged at 12,000 xg for 10 min at 4°C, and the supernatants were collected as total cell lysate. Lysates were denatured by boiling in SDS sample buffer (62.5mM Tris-Cl pH 6.8, 2% (w/v) SDS, 10% Glycerol, 0.1% Bromophenol Blue, 50mM DTT). Proteins were separated by SDS-PAGE and transferred onto nitrocellulose membranes (Millipore) using wet transfer (Bio-Rad). Membranes were blocked with 5% (w/v) non-fat milk in 0.1% Tween-20/TBS for 1h. Primary antibodies were used at a dilution of 1:1000 except otherwise stated. Secondary HRP-coupled antibodies were used at 1:5000. Membranes were developed using Pierce™ ECL Western Blotting Substrate or SuperSignal™ West Pico PLUS Chemiluminescent Substrate (Thermo Scientific) and imaged on a Chemidoc (Bio-Rad).
For immunoprecipitation, cells were lysed in IP Lysis Buffer (20 mM Tris, pH 7.4, 50 mM Nacl, 0.5% Nonidet P-40, 0.5% deoxycholate, 0.5% SDS, 1 mM EDTA, protease inhibitor and 20 mM N-ethylmaleimide). Samples were pre-cleared by centrifugation at 12,000 xg for 10 min at 4°C before incubation with antibodies overnight at 4 C, followed by the addition of protein A/G beads (Thermo Fisher) overnight at 4 C. Beads were washed three times with lysis buffer and bound proteins were eluted by boiling in SDS sample buffer for 10 min. A portion of each whole cell lysate was retained as input controls.
Real-time PCR
Total RNA was extracted from cells using RNasey mini kit (Qiagen), and reverse transcribed using iScript cDNA Synthesis Kit (Bio-Rad Laboratories) according to manufacturer’s instructions. Real-time PCR were performed by using SYBR green supermix (Bio-Rad), and CFX-96 real-time PCR detection system (Bio-Rad). The PCR primers used are: Human IL6 F: CAGCCCTGAGAAAGGAGACAT R: GGTTCAGGTTGTTTTCTGCCA; human CXCL10 F: AGCAGAGGAACCTCCAGTCT R: AGGTACTCCTTGAATGCCACT; human rDNA 18S-1 F: GCCCGAAGCGTTTACTTTG R: CCGCGGTCCTATTCCATTAT; 18S-2 F: GCCGCTAGAGGTGAAATTCT R: TCGGAACTACGACGGTATCT; 28S F: GTAAACGGCGGGAGTAACTATG R: GACAGTGGGAATCTCGTTCATC; mouse IL6 F: GCTACCAAACTGGATATAATCAGGA, R: CCAGGTAGCTATGGTACTCCAGAA; mouse CXCL10 F: ATGACGGGCCAGTGAGAATG, R: ATTCCGGATTCAGACATCTCT; human CCL2 F: TCAAACTGAAGCTCGCACTCT R: GGCATTGATTGCATCTGGC; human MIF F: AGCATCGGCAAGATCGGC R: GTAATAGTTGATGTAGACCCTGTCC; human GM-CSF F: AATGTTTGACCTCCAGGAGCC R: AGTGCTGCTTGTAGTGGCTG; human IL8 F: AGCTCTGTGTGAAGGTGCAG, R: TGGGGTGGAAAGGTTTGGAG; human IL18 F: TGCAGTCTACACAGCTTCGG R: GCAGCCATCTTTATTCCTGCG, internal control mouse HPRT F: CTGGTGAAAAGGACCTCTCG, R: CAAGGGCATATCCAACAACA; internal control human B2M F: TTCTGGCCTGGAGGCTATC, R: TCAGGAAATTTGACTTTCCATTC.
The cycling program used was: 95 °C for 5 min; 40 cycles of 95 °C for 15 sec and 60 °C for 30 sec. Quantification cycle (Cq) for the mRNAs of interest were normalized to reference mRNA and data was presented as fold change.
Immunofluorescence
Cells grown on coverslips were fixed with freshly made 3% paraformaldehyde solution for 20 min at room temperature followed by permeabilization with 1% Triton X-100 solution for 20 min on ice, washed with PBS, and then incubated with indicated primary antibodies overnight at 4°C followed by secondary antibodies conjugated with Alexa 488 or Alexa 555 for 1 h at room temperature. Coverslips were then washed with 0.1% Tween-20/PBS and mounted using DAPI containing antifade solution (Invitrogen). For picogreen staining, cells were incubated with 1X Quant-iT™ PicoGreen® dsDNA reagent (Invitrogen) at 37°C incubator with 5% CO2 for 1 h before fixation and permeablization as described above. For examining the ssDNA antibody specificity, cells were fixed, permeabilized and treated with S1 nuclease (200 U/ml) ( Thermo Fisher) for 1 h at 37° before they were stained with ssDNA antibody. Images were captured by a Nikon 80i upright microscope or Nikon A1-Confocal microscopy. Quantification of cytosolic ssDNA was performed using NIS Elements AR 5.31.01 64 Bit software (Nikon).
Native BrdU staining
The native BrdU staining was performed as previously described 25. Briefly, cells were pulse labelled with 10 μM BrdU for 30 min before treatment with 4 mM HU for 4 h. Cells were then fixed with 4% paraformaldehyde for 15 min, and immunostained with an anti-BrdU antibody (BD Biosciences, Franklin Lakes, NJ) without a DNA denaturation step. Images were acquired using an 80i eclipse Nikon microscope using ×60 objective. The mean cytosolic BrdU intensities were measured using NIS Elements AR 5.31.01 64 Bit software (Nikon).
Nuclear and cytosolic DNA extraction and quantification
For nuclear or cytosolic DNA, cell fraction using Mitochondria Isolation Kit for Cultured Cells (Thermo Scientific) was carried out before DNA extraction. Nuclear or cytosolic fraction of cells were then extracted by treated with DNA extraction buffer (10 mM Tris pH 8, 100 mM EDTA pH 8, 0.5% SDS, 200 μg/ml Proteinase K, 1mg/ml RNase A) and incubated at 56°C for 1 h, followed by extraction with equal volume phenol/chloroform/isoamyl alcohol (25:24:1) and chloroform/isoamyl alcohol (24:1) and sequentially. After centrifugation, supernatant was collected and equal volume of isopropanol and 1/10 volume of 3M sodium acetate (pH 5.8) was added and incubated at −20°C overnight for DNA precipitation. Nanodrop (Thermo Scientific) was used for total DNA quantification. Qubit™ 4 Fluorometer (Thermo Scientific) and Qubit™ ssDNA Assay Kit (Invitrogen) were used for ssDNA or dsDNA quantification according to manufacturer’s instructions. For nucleases treatment, 20 U/ml S1 nuclease (Thermo Fisher), 100 U/ml RNaseH (Invitrogen), 10U/μl RNaseT1 (Thermo Fisher) or 100 U/ml RNaseIII (Ambion) were incubated with cytosolic DNA for 30 min at 37°C.
cGAS IP and slot blot or qPCR for rDNA detection
Cells were fractionated using Mitochondria Isolation Kit for Cultured Cells (Thermo Scientific) for preparation of nuclear and cytosolic fraction. DNA was extracted from nuclear and half of the cytosolic fraction as described above. The rest half of the cytosolic fraction was added with equal volume of IP Lysis Buffer (20 mM Tris, pH 7.4, 50 mM Nacl, 0.5% Nonidet P-40, 0.5% deoxycholate, 0.5% SDS, 1 mM EDTA, protease inhibitor and 20 mM N-ethylmaleimide), pre-cleared by centrifugation at 12,000 xg for 10 min at 4°C before incubation with cGAS antibody overnight at 4 C, followed by the addition of protein A/G beads (Thermo Fisher) overnight at 4 °C. Beads were washed three times with lysis buffer followed by addition of DNA extraction buffer and DNA extraction was carried out using phenol/chloroform as described above. Slot blot for rDNA detection was modified from a previously described method for rDNA detection using dot blot 55. Briefly, a membrane (Biodyne B 0.45 um) was wetted with the 10хSSC solution and dried before DNA was applied. DNA was first denatured with 1/10 vol 1M sodium hydroxide at 0 °C for 10 min, then neutralized by addition of equal volume of 20 xSSC (pH 5.0). The denatured DNA samples were applied to the prepared membrane using slot apparatus and vacuum. DNA was then crosslinked to the membrane using UV (1200 J, twice, Stratalinker 1800). The membrane was pre-hybridized with hybridization buffer (10 × Denhardt solution, 0.05 M phosphate buffer, pH 7.0; 0.7 M sodium chloride, 50% formamide, 100 μg/ml salmon sperm DNA) for 5 h at 42 °C, followed by hybridization with biotinylated DNA probe (18S probe: biotin-CTGTAATGATCCTTCCGCAGGTTCACCTAC, 28S probe: biotin-TATCGGTCTCGTGCCGGTATTTAGCCTTAG) in the concentration of 5 ng/ml for 16h at 42◦C. After hybridization, the membrane was washed sequentially with wash buffer 1 (2 × SSC, 0.1% SDS) 2 × 15 min at 25° C, wash buffer 2 (0.01 × SSC, 0.1% SDS) for 20 min at 65° C, and wash buffer 3 (2 × SSC) for 10 min at 25° C. The membrane was then blocked for 30 min at 37° C with a solution of 0.1% fat-free milk, 0.1% gelatin, tris-HCL buffer, pH 7.5, 0.1 M sodium chloride. After that, the membrane was incubated with a conjugate of streptavidin with hydrogen peroxidase (1 ug/ml, Invitrogen) in the solution of 0.1 M tris-HCL buffer, pH 7.5, 0.1 M sodium chloride, 0.005 M magnesium chloride for 20 min (25 ◦ C). Finally, the membrane was washed (3 × 10 min) with the solution of Tris-HCL buffer (pH 7.5), 0.1 M sodium chloride, 0.005 M magnesium chloride. The membrane was developed using Pierce™ ECL Western Blotting Substrate (Thermo Scientific) and imaged on a Chemidoc (Bio-Rad). For qPCR detection of cGAS-bound rDNA, extracted DNA from cGAS IP was used as template with either rDNA 28S primers (F: GTAAACGGCGGGAGTAACTATG R: GACAGTGGGAATCTCGTTCATC) or 18S-1 primers (F: GCCCGAAGCGTTTACTTTG R: CCGCGGTCCTATTCCATTAT).
Fluorescence in situ hybridization (FISH) detection of rDNA
This approach is adapted from a previously described method for rDNA detection using FISH 56. Briefly, cells were grown on coverslips and fixed with fresh 3% paraformaldehyde solution for 20 min at room temperature followed by permeabilization with 0.5% Triton X-100 solution for 20 min on ice. The coverslips were then treated with 1 mg/ml RNase A (Qiagen, 1:100 dilution) in 2× SSC for 45 min at 37°C. For S1 nuclease treatment, the coverslips were incubated with S1 (200 U/ml) in 2x SSC for 1 h at 37°C before treatment with RNase A. For the effect of other nucleases, RNaseH (50 U/ml), RNaseT1 (5000 U/ml) or RNaseIII (5 U/ml) were added together with RNaseA in 2x SSC for 45 min at 37°. After that, the coverslips were pretreated with 0.1 N HCl for 15 min, washed in 2× SSC buffer, and incubated in 50% formamide/2× SSC for 30 min, followed by addition of biotinylated DNA probes (5 ng/ml) and denatured at 85°C for 7 min. Hybridization was carried out in a humidified chamber at 37°C for 16 h. After hybridization, the coverslips were washed with 50% formamide/2× SSC (3× 5min) at 45°C, 1× SSC (2× 5min) at 45°C, 1 x SSC (1× 5 min) at room temperature once. After that, the coverslips were incubated with a conjugate of streptavidin conjugated with Alexa 555 for 30 min at room temperature, followed by washes with 50% formamide/2× SSC (3× 5 min) at 45°C, 1× SSC solution at 45°C for 5 min twice and at room temperature once. Coverslips were then rinsed with double-deionized H2O, air dried, mounted to slides with addition of DAPI containing antifade solution (Invitrogen). Images were captured by a Nikon 80i upright microscope. Cytosolic rDNA FISH intensity was measured using NIS Elements AR 5.31.01 64 Bit software (Nikon).
cGAS activity assay and 2’,3’-cGAMP concentration measurements
The cGAS activity (cGAMP synthesis) assay was performed with 150 nM recombinant cGAS (Cayman Chemical) in 80 mM Tris–HCl (pH 7.5), 200 mM NaCl, and 40 mM MgCl2 in the presence of 0.5 mM ATP and 0.5 mM GTP with addition of DNA in a total volume of 100 μl at 37°C overnight, followed by heat inactivation at 95°C for 10 min. For cytosolic DNA samples, 10 ul out of 50 ul cytosolic DNA isolated from 6 × 106 cells were added. For the control, salmon sperm DNA, rDNA PCR products from genomic DNA using 28S primers targeting 28S rDNA region or 18S-1 and 18S-2 primers targeting 18S rDNA region were used. cGAMP levels were measured with the DetectX Direct 2′,3′-Cyclic GAMP Enzyme Immunoassay Kit (Arbor Assays), according to the manufacturer’s instruction. Briefly, 50 μl from the above in vitro cGAS reaction was added to the kit microplate well with addition of 50 μl Assay buffer, 25 μl conjugate, and 25 μl Antibody for incubation at room temperature for 2h. The standards were prepared with serial-dilution in Assay buffer freshly before use following the manufacturer’s instruction. Quantification of cGAMP levels was performed on BioTeck microplate reader at 450 nm.
DNA Fiber assay
DNA fiber assay was carried out following procedures published previously 25. Exponentially growing Cells were incubated consecutively with 100 μM CldU and 250 μM IdU for 30 min, followed by 4mM HU treatment for another 4 hr. Next, cells were harvested, lysed and spread on coated microscope slides. After fixation and denature, slides were incubated with primary antibodies (Abcam 6326 for detection of CIdU; BD 347580 for detection of IdU), washed with PBS, and then incubated with secondary antibodies including Alexa fluor 555 (red) and Alexa fluor 488 (green) (Invitrogen). Following mounting, slides were imaged using Nikon 80 microscope with a 60x lens and analyzed using ImageJ software. At least 200 ratios of IdU stained DNA tract to CldU stained DNA tract were quantified for each sample. Statistical analysis was performed using Prism software.
Cytokine array analysis
3 × 10^5 cells were cultured in six-well plates for 6 h prior to treatment. Cultured media were collected and cleared by centrifugation at 2000 xg for 10 min. 1 ml supernatant was used with addition of 0.5 ml buffer provided from the kit and applied to the Proteome Profiler™ Human Cytokine Array Kit (R&D Systems® ARY005B). Spot intensity was quantified with ImageJ and normalized to reference spots.
Animals and Chemotaxis assay
Mice were housed and handled in accordance with protocol 00001247-RN03, approved by the Institutional Animal Care and Use Committee (IACUC) of the MD Anderson Cancer Center. Four males and three female (C57BL/6) mice between 8–10 weeks were used to isolate peritoneal immune cells. Isolation of immune cells from mice peritoneum was carried out following a published method 57. Briefly, euthanized mice were cleaned with 70% ethanol and outer skin of the peritoneum were cut. 5 ml of 3% FBS in ice cold PBS were injected into the peritoneum. The peritoneum was massaged to detach any immune cells. Fluid containing cells were collected and centrifuged at 900 xg for 8 minutes. Chemotaxis assay were performed using a CytoSelect 96-well cell migration assay (5 μm; Fluorometric Format; Cell Biolabs, San Diego, CA, USA) according to the manufacturer’s instructions. Media from Abro1 +/+ or −/− MEFs or MEFs treated with siRNAs to DNA2 or FANCD2, untreated or treated with HU were collected and cleared with low speed centrifugation. 150 ul of supernatant after centrifugation was transferred into the bottom wells (feeder tray). Serum-free medium containing 1 × 10^6 peritoneum cells (100 μL) was placed in the migration chamber, and the chemotaxis plate was cultured at 37 °C for 12 h. After incubation, migratory cells were incubated for 20 min with 50 μL of lysis buffer/dye solution. Fluorescence was read at 480/520 nm, and values were expressed as relative fluorescence units (RFU).
Nuclear/Cytosolic Fractionation (for Extended Data Fig.8)
Cell pellet (from 2 × 106 cells) was incubated with 200 μL of cold solution A (10 mM HEPES at pH 7.9, 10 mM KCL, 1.5 mM MgCL2, 0.34 M sucrose, 10% glycerol, 5 mM NaF, 1 mM Na3VO4, 1 mM DTT, protease cocktails, 0.1% Triton X-100) for 3 min on ice. After centrifugation at 2500 xg for 5 min at 4°C, the supernatant was collected and used as cytosolic fraction. The remaining pellet was washed with cold solution A, then suspended in NETN buffer followed by sonication and centrifugation at 4 °C using 21,000 × g for 10 min. The supernatant was then collected as nuclear fraction.
Statistics and reproducibility
Statistical analysis was performed with GraphPad Prism 9. One-way or Two-way Anova was used for statistical analyses as indicated. P < 0.05 was considered statistically significant. All experiments were performed with at least three biological repeats except indicated in the figure legends. No statistical method was used to predetermine sample sizes. No data were excluded from the analyses. The experiments were not randomized. The investigators were not blinded to allocation during experiments and outcome assessment. Western blot, slot blot and microscopic imaging are representative of at least three biological repeats unless specified in the legends.
Data availability:
All data supporting the findings of this study are available within the paper and its supplementary information. Source data are provided with this study. All other data supporting the findings of this study are available from the corresponding author on reasonable request
Extended Data
Extended Data Fig. 1: Abro1 limits cytosolic ssDNA and replication stress-induced cGAS-STING-dependent innate immune response.
(a) Increased cytosolic DNA detected by picogreen in Abro1 KO cells treated with 4 mM HU. Scale bar, 10 μm. (b) Treatment with S1 nuclease eliminates ssDNA staining in immunofluorescence. Cells were fixed, untreated or treated with S1 nuclease before the staining with ssDNA antibody. Scale bar, 10 μm. (c) Isolated cytosolic ssDNA is sensitive to S1 nuclease but not to RNase H, RNase T1 or RNase III. Equal amount of isolated cytosolic ssDNA was used for each condition. After the indicated treatment, the amount of ssDNA is quantified by Qubit ssDNA assay, the amount of total DNA is measured by nanodrop (n = 3 independent experiments). One way Anova was used within each group for the statistics. (d) Detection of cytosolic ssDNA from newly synthesized DNA. Cells were treated as illustrated and BrdU staining was performed under non-denatured condition. Scale bars, 50 μm. Cytosolic BrdU intensity was measured for each cell with mean fluorescence intensity (MFI) and shown as mean ± SD. One way Anova was used for statistics. ‘n’ refers the number of cells analysed across two independent experiments. (e) Re-introduction of Abro1 expression in KO cells reduced pSTAT1 levels in response to HU. GFP-tagged Abro1 was expressed in KO cells. (f) Increased pSTAT1 in Abro1−/− MEFs treated with CPT (25 nM). (g) STING knockdown decreases the elevated pSTAT levels in Abro1 KO cells upon treatment of HU. (h) cGAS knockdown decreases the elevated pSTAT levels in Abro1 −/− MEFs upon treatment of HU. Data shown represent three independent experiments in a, b, f-h and two independent experiments in e.
Extended Data Fig. 2: Increased innate immune signaling in Abro1-deficient cells depends on DNA2 but not MRE11.
(a) Inhibition of Mre11 does not decrease pSTAT levels in Abro1 KO cells. Cells were treated with 4 mM HU in the absence or presence of Mirin. (b) Inhibition of Mre11 does not decrease cytosolic ssDNA in Abro1 KO cells. Immunofluorescence was performed with ssDNA antibody. Scale bars, 10 μm. Quantification of cytosolic ssDNA (mean fluorescence intensity, MFI) is shown with mean ± SD. One way Anova was used for statistics. ‘n’ refers the number of cells analysed across three independent experiments. (c) Knockdown of RAD51 decreases pSTAT1 levels in Abro1 KO cells. Cells were treated with 4 mM HU. Data shown represent three independent experiments in a and c.
Extended Data Fig. 3: rDNA fragments accumulate in the cytoplasm of Abro1-deficient cells.
(a) Co-staining of ssDNA and nucleolin in Abro1 KO cells treated with HU (4 mM, 4 h). Scale bars, 10 μm. (b) Leptomycin B (LMB) blocks accumulation of cytosolic ssDNA. Cells incubated with LMB for 8 h followed by 4 h continuous incubation with or without addition of 4 mM HU were stained with antibody to ssDNA. Scale bars, 10 μm. (c) pSTAT1 levels were reduced when cells were treated with LMB. (d) Detection of rDNA in the cytoplasm of Abro1 KO cells by slot blot with biotin-labelled rDNA probe. ‘Nuclear DNA’ (from 2 × 105 cells) and ‘Cytosolic DNA’ (‘1x’ from 5 × 105 cells, ‘2x’ from 1 × 106 cells) were loaded. Band intensity (a.u.) is quantified using ImageJ. (e) Knockdown DNA2 reduces cytosolic rDNA in the cytoplasm of Abro1 KO cells by slot blot with biotin-labelled rDNA 18S probe. ‘Nuclear DNA’ (from 2 × 105 cells) and ‘Cytosolic DNA’ (from 5 × 105 cells) were loaded. Band intensity (a.u.) is quantified using ImageJ. (f) Detection of rDNA by FISH with biotin-labelled rDNA 18S probe. Scale bars, 10 μm. (g) Treatment with RNAPI inhibitor, CX5461, reduces cytosolic ssDNA accumulation in Abro1 KO cells treated with HU (4 mM, 4 h). CX-5461 (1 μM) was added for 1 h before the end of the HU treatment. IF staining was carried out with ssDNA antibody. Scale bars, 10 μm. Data shown represent three independent experiments in a, d-g and two independent experiments in b, c. (h) Treatment with CX5461 reduces the upregulation of IL6 and CXCL10 in Abro1 KO cells treated with HU (4 mM, 16 h). CX-5461 (1 μM) was added for 1 h before the end of the HU treatment. Relative fold change was quantified and shown with mean ± SD (n = 3 independent experiments). Two way Anova was used for statistics.
Extended Data Fig. 4: Cytosolic rDNA fragments are detected by cGAS.
(a) Nuclear and cytosolic fraction used in slot blots were confirmed by western blot using antibodies to Lamin (nuclear) or GAPDH (cytosolic). (b) Western blot of IgG and cGAS immunoprecipitates with antibodies to cGAS and ssDNA. Immunoprecipitatation was carried out from the cytoplasmic fraction after cell fractionation. (c) Cytosolic rDNA is detected bound to cGAS by slot blot with biotin-labelled rDNA 18S probe. ‘Nuclear DNA’ (from 2 × 105 cells); ‘Cytosolic DNA’ (from 5 × 105 cells), or ‘cGAS bound DNA’ (prepared from 1.25 × 106 cells) were loaded. Band intensity (a.u.) is quantified using ImageJ. Data shown represent three independent experiments in a-c. (d) Detection of cGAS bound rDNA by qPCR using 18S-1 primers and cGAS bound DNA extracted from cGAS IP. Cells were untreated or treated with HU (4 mM, 4 h). Relative fold change was shown with mean ± SD (n = 3 biological replicates). One way Anova was used for statistics. (e) rDNA PCR fragments activates cGAS in cGAMP synthesis in vitro. Salmon sperm DNA (100 ng) or rDNA PCR products using indicated primers and genomic DNA as a template (28S (145 ng), 18S-1 (151 ng), 18S-2 (88 ng) rDNA PCR products) were used in the reaction with recombinant cGAS. The amount of cGAMP was measured by ELISA and shown with mean from 2 independent experiments.
Extended Data Fig. 5: Replication stress induced P-bodies are involved in modulating Abro1 deficiency-elicited innate immune response.
(a) GFP-Abro1 forms cytoplasmic foci in cells treated with various replication stressing agents including HU (4 mM), camptothecin (CPT, 100 nM), aphidicholin (APH, 0.1 uM), or cisplatin (5 uM) at 4 h after treatment. IF staining was carried out with anti-GFP antibody. Scale bars, 10 μm. (b) Increased P-bodies in Abro1−/− MEFs. Cells were untreated or treated with 4 mM HU. Number of P-bodies per cell was quantified and shown with mean ± SD. ‘n’ refers the number of cells analysed across three independent experiments. One way Anova was used for statistics. (c) Knockdown of DCP1a or DDX6 disrupts P-bodies formation and cytoplasmic GFP-Abro1 foci upon HU treatment (4 mM, 4 h). DCP1a antibody was used for the staining. (d) Knockdown of DCP1a or DDX6 does not reduce rDNA accumulation in the cytoplasm in Abro1 KO cells. FISH was carried out with cells transfected with indicated siRNAs, either untreated or treated with HU (4 mM, 4 h), and biotin-labelled rDNA 28S probe. Scale bars, 10 μm. (e) DCP1a knockdown decreases pSTAT1 levels in Abro1 KO cells treated with HU (4 mM). (f) DCP1a knockdown or DCP1a/STING double knockdown decreases pSTAT1 levels in Abro1 KO cells treated with HU (4 mM). Data shown represent three independent experiments in a, c-f.
Extended Data Fig. 6: Stalled replication fork degradation due to FANCD2- but not BRCA2-deficiency is linked with induction of innate immune response.
(a) pSTAT1 levels in BRCA2 knockdown cells at different times after treatment with HU (4 mM). (b) Knockdown of FANCD2 but not BRCA2 leads to cytosolic ssDNA accumulation by immunofluorescence staining with ssDNA antibody. Scale bars, 10 μm. (c) BRCA2 knockdown does not lead to HU-induced cytosolic ssDNA accumulation by quantification of Mean fluorescence intensity (MFI) with mean ± SD. One way Anova was used for statistics. (d) Picogreen staining of BRCA2 knockdown does not show HU-induced cytosolic DNA accumulation. Scale bars, 10 μm. Data shown represent three independent experiments in a, b, d, e. (e) FANCD2 knockdown leads to increased pSTAT1 in MEFs. (f) BRCA2 knockdown does not induce an increase of P-bodies. Number of P-bodies per cell was quantified and shown with mean ± SD. One way Anova was used for statistics. (g) Mirin restores fork protection in FANCD2 depleted cells. FANCD2-deficient PD20 cells expressing empty vector or FANCD2 gene were used and treated as illustrated. IdU/CIdU ratio was quantified with mean ± SD. One-way Anova was used for statistics. (h) Knockdown of DNA2 decreases P-bodies number in FANCD2-deficient cells. Number of P-bodies per cell was quantified and shown with mean ± SD. One-way Anova was used for statistics. (i) Cytosolic rDNA fragment accumulation in FANCD2-deficient cells detected by FISH with biotin-labelled rDNA 18S probe. Scale bars, 10 μm. Mean fluorescence intensity was quantified with mean ± SD. One-way Anova was used for statistics. ‘n’ refers the number of cells analysed across three independent experiments in c, f-i.
Extended Data Fig. 7: Abro1 deficiency upon replication stress results in increased cytokines secretion.
Realtime qPCR of indicated cytokine gene expression in Abro1 WT or KO U2OS cells treated with HU is quantified and shown with mean from 2 independent experiments.
Extended Data Fig. 8: cGAS localization in Abro1- or FANCD2-deficient cells.
(a) cGAS rarely colocalizes with P-bodies. Abro1 WT or KO cells untreated or treated with HU (4 mM, 4 h) were stained with antibodies to cGAS and DCP1a. Images with DAPI, cGAS and DCP1 staining were collected by Nikon A1-Confocal using ×60 objective (left panel). The scare bar=10 μm. NIH Elements AR software was used to analyse co-localization of cGAS and DCP1 (right panel). Red lines represent cGAS signal and green lines represent DCP1 signal. Occasionally, cGAS (red) signal can be detected within DCP1a foci (green) as shown in ‘1’ for each sample. In majority of the P-bodies (green), cGAS signal (red) can not be detected as shown in ‘2’. (b) Cytosolic and nuclear cGAS levels in Abro1 KO cells. Cell fractionation was carried out with cells untreated or treated with HU (4 mM, 4 h). (k) Immunofluorescence of cGAS and DCP1a in FANCD2-deficient cells. Co-staining of cGAS and DCP1a was carried out with cells treated with indicated siRNAs untreated or treated with HU (4 mM, 4 h). (d) Cytosolic and nuclear cGAS levels in FANCD2 depleted cells. Cell fractionation was carried out with cells untreated or treated with HU (4 mM, 4 h). Data shown represent three independent experiments in a-d.
Supplementary Material
Acknowledgements:
We thank Dr. Adriana Paulucci and Department of Genetics Microscope Core Facility for assistance of imaging acquisition, Sophie Lu (Williams College) for a pilot study as a summer intern, Dr. Alan D’Andrea (Dana Farber Cancer Institute) for PD20 cells. A.E., X.W., S.X., L.W., S.L. and B.W. were partially supported by NIH grant (CA155025 and CA248088 to B.W.) and Cancer Prevention Research Institute of Texas (CPRIT) grant (RP180244 to B.W.). Use of A1-Nikon microscope was made possible via NIH shared Instrumentation Grant (1S10OD024976-01 to MDACC Department of Genetics).
Footnotes
Competing interests: The authors declare no competing interests.
References
- 1.Chen Q, Sun L. & Chen ZJ Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol 17, 1142–1149 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Li T. & Chen ZJ The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J Exp Med 215, 1287–1299 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakhoum SF & Cantley LC The Multifaceted Role of Chromosomal Instability in Cancer and Its Microenvironment. Cell 174, 1347–1360 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ablasser A. & Chen ZJ cGAS in action: Expanding roles in immunity and inflammation. Science 363 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Ablasser A. et al. cGAS produces a 2’−5’-linked cyclic dinucleotide second messenger that activates STING. Nature 498, 380–384 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li XD et al. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 341, 1390–1394 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motwani M, Pesiridis S. & Fitzgerald KA DNA sensing by the cGAS-STING pathway in health and disease. Nat Rev Genet 20, 657–674 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Sun L, Wu J, Du F, Chen X. & Chen ZJ Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J. et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339, 826–830 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X. et al. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell 51, 226–235 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackenzie KJ et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 548, 461–465 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaillard H, Garcia-Muse T. & Aguilera A. Replication stress and cancer. Nat Rev Cancer 15, 276–289 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Zeman MK & Cimprich KA Causes and consequences of replication stress. Nat Cell Biol 16, 2–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berti M, Cortez D. & Lopes M. The plasticity of DNA replication forks in response to clinically relevant genotoxic stress. Nat Rev Mol Cell Biol 21, 633–651 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Erdal E, Haider S, Rehwinkel J, Harris AL & McHugh PJ A prosurvival DNA damage-induced cytoplasmic interferon response is mediated by end resection factors and is limited by Trex1. Genes Dev 31, 353–369 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf C. et al. RPA and Rad51 constitute a cell intrinsic mechanism to protect the cytosol from self DNA. Nat Commun 7, 11752 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhattacharya S. et al. RAD51 interconnects between DNA replication, DNA repair and immunity. Nucleic Acids Res 45, 4590–4605 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coquel F, Neumayer C, Lin YL & Pasero P. SAMHD1 and the innate immune response to cytosolic DNA during DNA replication. Curr Opin Immunol 56, 24–30 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Coquel F. et al. SAMHD1 acts at stalled replication forks to prevent interferon induction. Nature 557, 57–61 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Guan J. et al. MLH1 Deficiency-Triggered DNA Hyperexcision by Exonuclease 1 Activates the cGAS-STING Pathway. Cancer Cell 39, 109–121 e105 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlacher K. et al. Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell 145, 529–542 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlacher K, Wu H. & Jasin M. A distinct replication fork protection pathway connects Fanconi anemia tumor suppressors to RAD51-BRCA1/2. Cancer Cell 22, 106–116 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kais Z. et al. FANCD2 Maintains Fork Stability in BRCA1/2-Deficient Tumors and Promotes Alternative End-Joining DNA Repair. Cell Rep 15, 2488–2499 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michl J, Zimmer J, Buffa FM, McDermott U. & Tarsounas M. FANCD2 limits replication stress and genome instability in cells lacking BRCA2. Nat Struct Mol Biol 23, 755–757 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu S. et al. Abro1 maintains genome stability and limits replication stress by protecting replication fork stability. Genes Dev 31, 1469–1482 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo Y, Na Z. & Slavoff SA P-Bodies: Composition, Properties, and Functions. Biochemistry 57, 2424–2431 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Standart N. & Weil D. P-Bodies: Cytosolic Droplets for Coordinated mRNA Storage. Trends Genet 34, 612–626 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Youn JY et al. Properties of Stress Granule and P-Body Proteomes. Mol Cell 76, 286–294 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Parker R. & Sheth U. P bodies and the control of mRNA translation and degradation. Mol Cell 25, 635–646 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Anderson P. Post-transcriptional regulons coordinate the initiation and resolution of inflammation. Nat Rev Immunol 10, 24–35 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Loll-Krippleber R. & Brown GW P-body proteins regulate transcriptional rewiring to promote DNA replication stress resistance. Nat Commun 8, 558 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tkach JM et al. Dissecting DNA damage response pathways by analysing protein localization and abundance changes during DNA replication stress. Nat Cell Biol 14, 966–976 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durkin SG & Glover TW Chromosome fragile sites. Annu Rev Genet 41, 169–192 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Boisvert FM, van Koningsbruggen S, Navascues J. & Lamond AI The multifunctional nucleolus. Nat Rev Mol Cell Biol 8, 574–585 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Weitao T, Budd M. & Campbell JL Evidence that yeast SGS1, DNA2, SRS2, and FOB1 interact to maintain rDNA stability. Mutat Res 532, 157–172 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Weitao T, Budd M, Hoopes LL & Campbell JL Dna2 helicase/nuclease causes replicative fork stalling and double-strand breaks in the ribosomal DNA of Saccharomyces cerevisiae. J Biol Chem 278, 22513–22522 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Muse T. & Aguilera A. Transcription-replication conflicts: how they occur and how they are resolved. Nat Rev Mol Cell Biol 17, 553–563 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi Y, Horiuchi T. & Kobayashi T. Transcription-dependent recombination and the role of fork collision in yeast rDNA. Genes Dev 17, 1497–1506 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ayache J. et al. P-body assembly requires DDX6 repression complexes rather than decay or Ataxin2/2L complexes. Mol Biol Cell 26, 2579–2595 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hubstenberger A. et al. P-Body Purification Reveals the Condensation of Repressed mRNA Regulons. Mol Cell 68, 144–157 e145 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Reislander T. et al. BRCA2 abrogation triggers innate immune responses potentiated by treatment with PARP inhibitors. Nat Commun 10, 3143 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heijink AM et al. BRCA2 deficiency instigates cGAS-mediated inflammatory signaling and confers sensitivity to tumor necrosis factor-alpha-mediated cytotoxicity. Nat Commun 10, 100 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen H. et al. cGAS suppresses genomic instability as a decelerator of replication forks. Sci Adv 6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uggenti C. et al. cGAS-mediated induction of type I interferon due to inborn errors of histone pre-mRNA processing. Nat Genet 52, 1364–1372 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Boyer JA et al. Structural basis of nucleosome-dependent cGAS inhibition. Science 370, 450–454 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kujirai T. et al. Structural basis for the inhibition of cGAS by nucleosomes. Science 370, 455–458 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michalski S. et al. Structural basis for sequestration and autoinhibition of cGAS by chromatin. Nature 587, 678–682 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Pathare GR et al. Structural mechanism of cGAS inhibition by the nucleosome. Nature 587, 668–672 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Zhao B. et al. The molecular basis of tight nuclear tethering and inactivation of cGAS. Nature 587, 673–677 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gasser S. et al. Sensing of dangerous DNA. Mech Ageing Dev 165, 33–46 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Dhanwani R, Takahashi M. & Sharma S. Cytosolic sensing of immuno-stimulatory DNA, the enemy within. Curr Opin Immunol 50, 82–87 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herzner AM et al. Sequence-specific activation of the DNA sensor cGAS by Y-form DNA structures as found in primary HIV-1 cDNA. Nat Immunol 16, 1025–1033 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coppe JP, Desprez PY, Krtolica A. & Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5, 99–118 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gorgoulis V. et al. Cellular Senescence: Defining a Path Forward. Cell 179, 813–827 (2019). [DOI] [PubMed] [Google Scholar]
- 55.Korzeneva IB et al. Human circulating ribosomal DNA content significantly increases while circulating satellite III (1q12) content decreases under chronic occupational exposure to low-dose gamma- neutron and tritium beta-radiation. Mutat Res 791–792, 49–60 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Potapova TA et al. Superresolution microscopy reveals linkages between ribosomal DNA on heterologous chromosomes. J Cell Biol 218, 2492–2513 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ray A. & Dittel BN Isolation of mouse peritoneal cavity cells. J Vis Exp (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper and its supplementary information. Source data are provided with this study. All other data supporting the findings of this study are available from the corresponding author on reasonable request