Abstract
Background:
The outcomes of relapsed childhood acute lymphoblastic leukemia (ALL) in developed countries have improved over time as a result of risk-adapted, minimal residual disease-directed therapy, hematopoietic stem cell transplantation, and immunotherapy. There are few studies that have examined survival in relapsed childhood ALL in resource-limited countries. Therefore, this study aimed to assess the prognostic factors and survival outcome of relapsed childhood ALL in a major tertiary center in Southern Thailand.
Methods:
The medical records of patients with ALL aged <15 years between January 2000 and December 2019 were retrospectively reviewed. The Kaplan-Meier method was used to depict the overall survival (OS).
Results:
A total of 472 patients with ALL were enrolled and relapsed ALL was found in 155 (32.8%) patients. Of these, 131 (84.5%) and 24 (15.5%) had B-cell and T-cell phenotypes, respectively. One hundred thirteen (72.9%) and 42 (27.1%) patients had early and late relapses, respectively. The most common site of relapse was bone marrow in 102 patients (65.8%). One hundred twenty-eight (82.6%) patients received treatment while 27 (17.4%) patients refused treatment. The 5-year OS of all relapsed patients was 11.9%. The 5-year OS among the patients with early relapse was significantly lower than in the patients with late relapse (5.3% vs. 29.1%, respectively, p <0.0001). Site and immunophenotype were not associated with survival of relapsed ALL. The median survival times among the patients who received and refused relapse chemotherapy were 11.8 and 3.1 months, respectively (p <0.0001).
Conclusion:
The relapse rate accounted for one third of patients with ALL with the 5-year OS of 12%. Early relapse and those who refused treatment were associated with poor survival outcome.
Key Words: relapsed childhood acute lymphoblastic leukemia, survival outcome, resource, limited countries
Introduction
Recent advances in the treatment of childhood acute lymphoblastic leukemia (ALL) has significantly improved outcomes with long-term survival of greater than 90% in high-income countries (Hunger et al., 2012; Jeha et al., 2019). However, the survival in resource-limited countries is significantly lower compared to high-income countries with long-term survival of around 70% (Abdelmabood et al., 2020; Al-Hadad et al., 2021; Jaime-Pérez et al., 2016, 2018; Viana et al., 2015). Various studies have reported relapse rates of 10-40% in patients with childhood ALL, which remains the most common cause of treatment failure, and these patients are more likely to die (Abdelmabood et al., 2020; Al-Hadad et al., 2021; Jaime-Pérez et al., 2016, 2018; Locatelli et al., 2012; Olbara et al., 2021; Oskarsson et al., 2016; Tuong et al., 2020; Viana et al., 2015; Zapata-Tarrés et al., 2021). Patients with relapsed ALL have inferior outcomes with long-term survival of 58-72% in high-income countries (Lew et al., 2021; Oskarsson et al., 2016; Parker et al., 2010), which is higher than the survival in resource-limited countries with long-term survival of only 27-42% (Hu et al., 2018; Jaime-Pérez et al., 2016, 2018; Tuong et al., 2020; Zapata-Tarrés et al., 2021). The factors influencing survival in relapsed ALL include immunophenotype, site and timing of relapse, and treatment. Early relapse, isolated bone marrow relapse, and T-cell phenotype are associated with poor prognosis (Hunger and Raetz, 2020; Locatelli et al., 2012), while patients who have low minimal residual disease (MRD) after re-induction and who receive hematopoietic stem cell transplantation (HSCT) have improved survival (Eckert et al., 2013; Hu et al., 2018; Lew et al., 2021; Oskarsson et al., 2016). Another important prognostic factor found in resource-limited countries which is not so common in high-income countries is high rates of chemotherapy refusal (Bhise et al., 2021, Chotsampancharoen et al., 2018). There are few studies which have evaluated survival among patients with relapsed ALL in resource-limited countries, with only one of these evaluating the consequence on survival (Hu et al., 2018). Thus, studies including patients with relapsed childhood ALL in resource-limited countries are needed to further elucidate the poor outcomes and evaluate the survival among patients with relapsed ALL who refuse chemotherapy. This study aimed to examine the incidence, prognostic factors, and survival of relapsed childhood ALL in Thailand.
Materials and Methods
Patients
We retrospectively reviewed the medical records of all patients aged 0-15 years diagnosed with ALL between January 2000 and December 2019 at Songklanagarind Hospital, the major tertiary health care institution and a university hospital in Southern Thailand. Ethical approval was obtained from the Ethics Committee of the Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand.
The clinical characteristics and survival outcomes of patients with relapsed ALL were collected including age, gender, timing of initial diagnosis, immunophenotype, time from initial diagnosis to relapse, site of relapse, treatment for relapse, second complete remission (CR2) and second relapse if any, and survival status at the last follow up or death.
Initial diagnosis of ALL
The initial diagnoses of ALL were made based on pathological and immunochemical evaluations of the bone marrow material from bone marrow aspiration and/or biopsy, with the criterion of ≥20% lymphoblasts for the definitive diagnosis (Brown et al., 2020). Immunophenotypic classification was divided into two distinct subtypes based on the expression of cell surface markers, B-cell and T-cell.
Chemotherapy protocols for newly diagnosed ALL
The chemotherapy protocols used to treat patients with newly diagnosed ALL were divided into 3 periods as follows. Between 2000 and 2005, the patients treated according to the Children’s Cancer Group (CCG)-104, CCG-105 and CCG-106 regimens for standard, high and very high risks, respectively.(Gaynon, Bleyer, et al., 1988; Gaynon, Steinherz, et al., 1988) During the period 2006-2013, the patients treated according to the CCG-104, CCG-105 and Pediatric Oncology Group (POG) 9006 regimens for standard, high and very high risks, respectively (Lauer et al., 2001; Tubergen et al., 1993). From 2014-2019, the patients treated according to the Children’s Oncology Group (COG)-AALL00P2 regimen for standard risk and the COG-AALL0232 regimen for high and very high risks (Hunger et al., 2012). Patients were defined as having achieved a first complete remission (CR1) if they had <5% blast cells in the bone marrow and no blast cells in the cerebrospinal fluid (CSF) at the end of the induction phase.
Diagnosis of relapsed ALL
The diagnosis of relapse could be made at any time point after achieving CR1. Isolated bone marrow relapse was defined as the presence of ≥20% blast cells in the bone marrow. Isolated central nervous system (CNS) relapse was defined as the presence of ≥5/µL leukocytes with detectable blast cells in a cytocentrifuged preparation of CSF, or the presence of cranial nerve palsies. An isolated testicular relapse was defined as the presence of unilateral or bilateral testicular enlargement, with biopsy-proven testicular involvement in the absence of bone marrow involvement (<5% blast cells). Combined relapse was defined as the presence of ≥5% blast cells in the bone marrow and extramedullary disease relapse (Locatelli et al., 2012).
The timing of relapse was classified according to the COG as early (within 36 months after initial diagnosis) or late bone marrow relapse (after 36 months from initial diagnosis). Isolated extramedullary relapse was defined as early (within 18 months after initial diagnosis) or late relapse (after 18 months from initial diagnosis) (Malempati et al., 2007).
Chemotherapy protocols for relapsed ALL
The chemotherapy protocols used to treat patients with relapsed ALL were divided into 2 periods as follows. Between 2000 and 2013, relapsed patients treated according to the ALL-REZ Berlin-Frankfurt-Münster (BFM) 87 regimen (Bührer et al., 1994). In the period from 2014-2019, they treated according to UKALL R3 chemotherapy regimen (Parker et al., 2010). Patients were defined as having achieved a CR2 if they had <5% blast cells in the bone marrow and no blast cells in the CSF at the end of the re-induction phase.
Statistical analysis
Descriptive statistics are presented using mean and standard deviation or median and interquartile range (IQR) for continuous variables as appropriate, and frequency with percentage for categorical variables. The Kaplan–Meier method was used to depict the overall survival (OS) from the time of diagnosis of relapse to various time points. The log rank test was used for comparing survival between groups. A p-value less than 0.05 was considered significant.
Results
A total of 501 patients were diagnosed with ALL in our center during the 20-year study period. We excluded 29 patients who had incomplete data, leaving a total of 472 patients for analysis. Of these, 155 (32.8%) had relapsed ALL at a median age of 7.8 years (IQR 5.2-10.8). The percentages of males and females were 60% and 40%, respectively. Of the 155 relapsed patients, 131 (84.5%) had the B-cell phenotype and 24 (15.5%) had the T-cell phenotype. Of these, 113 (72.9%) had early relapse with a mean time from initial diagnosis of 1.3 ± 0.8 years and 42 (27.1%) had late relapse with a mean time from initial diagnosis of 4.9 ± 2.1 years. The most common site of relapse was bone marrow in 102 patients (65.8%) followed by combined sites in 28 patients (18.1%), CNS in 21 patients (13.5%) and testis/es in 4 patients (2.6%). The mean times from initial diagnosis to bone marrow and extramedullary relapse were and 2.3 ± 2.2 and 2.0 ± 2.0 years, respectively.
Of the 155 relapsed patients, 128 (82.6%) received relapse chemotherapy while 27 (17.4%) refused treatment and chose alternative medicine. Of the 128 patients who received the relapse chemotherapy, 71 (55.5%) achieved CR2, 44 (34.3%) did not, and 13 (10.2%) died during re-induction. Of the 128 patients who received the relapse chemotherapy, 47 (36.7%) had a second relapse (Table 1).
Table 1.
Characteristics of Patients with Relapsed ALL
| Characteristic | Value |
|---|---|
| Age (years) | |
| Median (IQR) | 7.8 (5.2-10.8) |
| Sex, n (%) | |
| Male | 93 (60.0) |
| Female | 62 (40.0) |
| Timing of relapse, n (%) | |
| Early | 113 (72.9) |
| Late | 42 (27.1) |
| Immunophenotype, n (%) | |
| B-cell | 131 (84.5) |
| T-cell | 24 (15.5) |
| Site of relapse, n (%) | |
| Bone marrow | 102 (65.8) |
| CNS | 21 (13.5) |
| Testes | 4 (2.6) |
| Combined (bone marrow with other site(s)) | 28 (18.1) |
| Treatment of relapse, n (%) | |
| Chemotherapy | 128 (82.6) |
| Achieved CR2 | 71 (55.5) |
| Failed CR2 | 44 (34.3) |
| Died during re-induction | 13 (10.2) |
| Alternative medicine | 27 (17.4) |
| Second relapsea, n (%) | 47 (36.7) |
| Current status, n (%) | |
| Alive | 22 (14.2) |
| Dead | 133 (85.8) |
a(**superscript**) Among patients who received chemotherapy only (n = 128); ALL, acute lymphoblastic leukemia; CNS, central nervous system; CR2, second complete remission; IQR, interquartile range
Survival outcomes
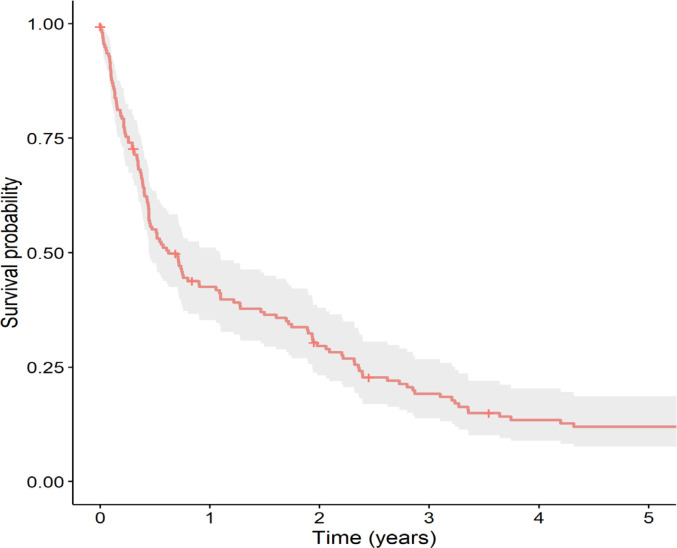
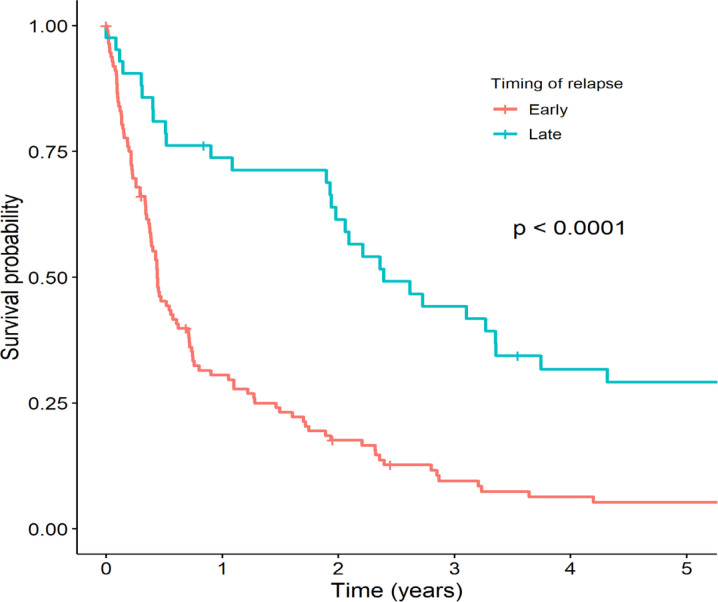
Of the 155 relapsed patients, 7 (4.5%) were lost to follow-up. The 3-year and 5-year OS of all relapsed patients were 19.2% and 11.9%, respectively (Figure 1), with a median survival time of 7.4 months (IQR 5.3-13.1). As shown in Figure 2, the timing of relapse was associated with survival of relapsed ALL. The 5-year OS among patients with early relapse was significantly lower than in patients with late relapse (5.3% vs. 29.1%, respectively, p <0.0001). Site and immunophenotype were not associated with survival of relapsed ALL.
Figure 1.
Kaplan-Meier Survival Curve of Total Patients with Relapsed Acute Lymphoblastic Leukemia
Figure 2.
Kaplan-Meier Survival Curves Stratified by Timing of Relapse between Patients with Early and Late Relapsed Acute Lymphoblastic Leukemia
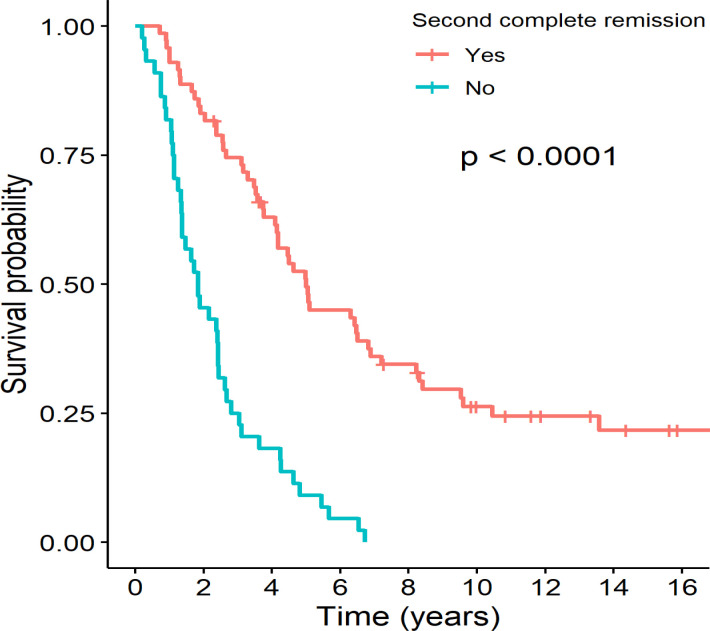
The difference in survival between the relapsed B-cell and T-cell ALL groups was not significant (12.6% vs. 8.7%, respectively, p = 0.43), nor between isolated bone marrow and other site relapses (11.3% vs. 12.8%, respectively, p = 0.29). The survival was significantly different between subgroups of relapsed patients categorized by combined timing of relapse and immunophenotype. The 5-year OS in early B- or T-cell and late B- or T-cell relapses were 4.0%, 10.5%, 32.3% and 25%, respectively (p <0.0001). Among the patients who received the relapse chemotherapy, the median survival time was 11.8 months. The median survival times were 5.0 and 1.8 years for the patients who achieved CR2 and who did not, respectively. As shown in Figure 3, the 5-year OS among the patients who achieved CR2 was significantly higher than in the patients who did not (49.4% vs. 9.1%, respectively, p <0.0001). Among the patients who did not achieve CR2, all died. Of the 27 patients who refused chemotherapy and chose alternative medicine, the median survival time was 3.1 months.
Figure 3.
Kaplan-Meier Survival Curves Stratified by Status of Second Complete Remission (CR2) between Patients who Achieved CR2 and who did Not
Discussion
In our study, the relapse rate of ALL was 33%, which was higher than some studies from high-income countries with reported rates ranging from 15% to 20% (Locatelli et al., 2012; Oskarsson et al., 2016). However, the relapse rate in our study was similar to some studies from resource-limited countries with reported rates ranging from 19% to 40% (Abdelmabood et al., 2020; Jaime-Pérez et al., 2016, 2018; Viana et al., 2015). The percentage of relapsed patients who refused chemotherapy in our study was 14%, which was significantly higher than a study from the Nordic countries in which none of the patients refused treatment (Oskarsson et al., 2016). However, the percentage of patients refusing chemotherapy in our study was significantly lower than a study from China in which 41% of the patients refused chemotherapy (Hu et al., 2018).
The 5-year OS of patients with relapsed ALL in our study was 12%, which was significantly lower than other studies from high-income countries with 5-year OS of 58% and 65% (Lew et al., 2021; Oskarsson et al., 2016). The 5-year OS of our study was also significantly lower compared to other studies in resource-limited countries with rates of 24% and 42% (Hu et al., 2018; Jaime-Pérez et al., 2018). The inferior survival outcome in our study was likely due to a lower rate of CR2, and limited availability of risk-adapted, minimal residual disease (MRD)-directed therapy, HSCT and immunotherapy, which can have a significant influence on survival where available. However, the survival outcomes of patients with relapsed ALL in our study were similar to the study of Tuong (2020), which also had limited access to risk-adapted, MRD-directed therapy, HSCT and immunotherapy. The median survival times of our cohort and the study of Tuong (2020) were 7.4 and 7.5 months, respectively, and the 3-year OS were 19% and 27%, respectively.
Our finding regarding the timing of relapse was concordant with other studies which found that early relapse was an adverse prognostic factor of survival (Hu et al., 2018; Hunger and Raetz, 2020; Locatelli et al., 2012; Oskarsson et al., 2016). Although immunophenotypes have been reported in the literature, the findings have been inconsistent, with other studies reporting that relapsed B-cell ALL was associated with better survival (Hunger and Raetz, 2020; Locatelli et al., 2012). More recent studies by Oskarsson (2016) and Hu (2018) reported no significant differences in survival between patients with relapsed B-cell and T-cell ALL, as in our study in which we also found that immunophenotype was not associated with survival. Site of relapse has also been reported to be associated with survival in the literature, with several studies reporting that isolated bone marrow relapse was associated with the worst prognosis (Hu et al., 2018; Locatelli et al., 2012; Oskarsson et al., 2016). However, our study did not find any survival differences between isolated bone marrow and other relapse site. We found that patients who did not achieve CR2 after re-induction had very poor survival, which was consistent with other studies (Eckert et al., 2013; Hu et al., 2018; Parker et al., 2010). Hu (2018) reported that refusal of chemotherapy was associated with very poor survival with a 5-year OS of only 5%, a rate similar to our study where refusal of chemotherapy was associated with a 5-year OS of 9%.
Our study demonstrated that the survival of patients with relapsed ALL was poor in the context of lacking risk-adapted, MRD-directed therapy, HSCT, and immunotherapy. Therefore, we suggest that these treatments would be of benefit for patients with relapsed ALL, particularly those who suffer early relapse and have poor prognoses. MRD-directed therapy, HSCT, and immunotherapy should all be included in the treatment plan. Our study also found poorer survival among patients who refused chemotherapy. Thus, another strategy to improve overall survival among patients with relapsed ALL in resource-limited countries would be devising programs to assist healthcare providers convince these patients to accept this life-lengthening treatment.
Our study had some limitations. First, there is the potential bias inherent in all retrospective studies. Second, our study covered a 20-year period in which advances in diagnosis, chemotherapy regimens and supportive care had been made. Therefore, our data may be considered to be heterogenous in this respect. Third, molecular studies and MRD, which are important for determining ALL risk and treatment, were not done in our study. Finally, since our setting was a major tertiary health care institution and university hospital, which receives mostly high-risk and complicated cases with ALL, there is a possibility of referral bias.
Author Contribution Statement
T.C., N.S., S.C., P.S., and E.M. all contributed significantly to the conception and design, acquisition of data, and/or analysis and interpretation of data. T.C. and N.S. were primarily responsible for drafting and revising the manuscript for critical intellectual content. The manuscript was given final approval by all authors before it was submitted for publication.
Acknowledgments
We would like to thanks Mr. Dave Patterson of the Office of International Affairs, Faculty of Medicine, Prince of Songkla University for his English editing.
Ethical declaration
Ethical approval was obtained from the Ethics Committee of the Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand.
Data availability statement
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Conflict of interest
The authors report there are no competing interests to declare. There was no funding support for this study.
References
- 1.Abdelmabood S, Fouda AE, Boujettif F, Mansour A. Treatment outcomes of children with acute lymphoblastic leukemia in a middle-income developing country: high mortalities, early relapses, and poor survival. J Pediatr. 2020;96:108–16. doi: 10.1016/j.jped.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Hadad SA, Al-Jadiry MF, Ghali HH, et al. Treatment of childhood acute lymphoblastic leukemia in Iraq: a 17-year experience from a single center. Leuk Lymphoma. 2021;62:3430–9. doi: 10.1080/10428194.2021.1961237. [DOI] [PubMed] [Google Scholar]
- 3.Bhise R, Ahmed I, Imtiaz S. Acute promyelocytic leukemia: Simplifying treatment in a resource poor setting. Asian Pac J Cancer Care. 2021;6:383–7. [Google Scholar]
- 4.Brown P, Inaba H, Annesley C, et al. Pediatric acute lymphoblastic leukemia, version 2 2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2020;18:81–112. doi: 10.6004/jnccn.2020.0001. [DOI] [PubMed] [Google Scholar]
- 5.Bührer C, Hartmann R, Fengler R, et al. Importance of effective central nervous system therapy in isolated bone marrow relapse of childhood acute lymphoblastic leukemia. BFM (Berlin-Frankfurt-Münster) Relapse Study Group. Blood. 1994;83:3468–72. [PubMed] [Google Scholar]
- 6.Chotsampancharoen T, Sripornsawan P, Duangchu S, et al. Survival outcome of alternative medicine treatment for newly diagnosed acute leukemia in children. Acta Haematol. 2018;140:203–8. doi: 10.1159/000493417. [DOI] [PubMed] [Google Scholar]
- 7.Eckert C, von Stackelberg A, Seeger K, et al. Minimal residual disease after induction is the strongest predictor of prognosis in intermediate risk relapsed acute lymphoblastic leukaemia - long-term results of trial ALL-REZ BFM P95/96. Eur J Cancer. 2013;49:1346–55. doi: 10.1016/j.ejca.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Gaynon PS, Bleyer WA, Steinherz PG, et al. Modified BFM therapy for children with previously untreated acute lymphoblastic leukemia and unfavorable prognostic features Report of Children’s Cancer Study Group Study CCG-193P. Am J Pediatr Hematol Oncol. 1988;10:42–50. doi: 10.1097/00043426-198821000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Gaynon PS, Steinherz PG, Bleyer WA, et al. Intensive therapy for children with acute lymphoblastic leukaemia and unfavourable presenting features Early conclusions of study CCG-106 by the Children’s Cancer Study Group. Lancet. 1988;2:921–4. doi: 10.1016/s0140-6736(88)92596-2. [DOI] [PubMed] [Google Scholar]
- 10.Hu Q, Hu W, Chen X, et al. Relapsed childhood acute lymphoblastic leukemia: current situation in China; a multicenter observational study. Pediatr Hematol Oncol J. 2018;3:59–63. [Google Scholar]
- 11.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30:1663–9. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunger SP, Raetz EA. How I treat relapsed acute lymphoblastic leukemia in the pediatric population. Blood. 2020;136:1803–12. doi: 10.1182/blood.2019004043. [DOI] [PubMed] [Google Scholar]
- 13.Jaime-Pérez JC, López-Razo ON, García-Arellano G, et al. Results of treating childhood acute lymphoblastic leukemia in a low-middle income country: 10 year experience in Northeast Mexico. Arch Med Res. 2016;47:668–76. doi: 10.1016/j.arcmed.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Jaime-Pérez JC, Pinzón-Uresti MA, Jiménez-Castillo RA, et al. Relapse of childhood acute lymphoblastic leukemia and outcomes at a reference center in Latin America: organomegaly at diagnosis is a significant clinical predictor. Hematology. 2018;23:1–9. doi: 10.1080/10245332.2017.1333294. [DOI] [PubMed] [Google Scholar]
- 15.Jeha S, Pei D, Choi J, et al. Improved CNS control of childhood acute lymphoblastic leukemia without cranial irradiation: St Jude Total Therapy Study 16. J Clin Oncol. 2019;37:3377–91. doi: 10.1200/JCO.19.01692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauer SJ, Shuster JJ, Mahoney DH Jr, et al. A comparison of early intensive methotrexate/mercaptopurine with early intensive alternating combination chemotherapy for high-risk B-precursor acute lymphoblastic leukemia: a Pediatric Oncology Group phase III randomized trial. Leukemia. 2001;15:1038–45. doi: 10.1038/sj.leu.2402132. [DOI] [PubMed] [Google Scholar]
- 17.Lew G, Chen Y, Lu X, et al. Outcomes after late bone marrow and very early central nervous system relapse of childhood B-acute lymphoblastic leukemia: a report from the Children’s Oncology Group phase III study AALL0433. Haematologica. 2021;106:46–55. doi: 10.3324/haematol.2019.237230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locatelli F, Schrappe M, Bernardo ME, Rutella S. How I treat relapsed childhood acute lymphoblastic leukemia. Blood. 2012;120:2807–16. doi: 10.1182/blood-2012-02-265884. [DOI] [PubMed] [Google Scholar]
- 19.Malempati S, Gaynon PS, Sather H, et al. Outcome after relapse among children with standard-risk acute lymphoblastic leukemia: Children’s Oncology Group study CCG-1952. J Clin Oncol. 2007;25:5800–7. doi: 10.1200/JCO.2007.10.7508. [DOI] [PubMed] [Google Scholar]
- 20.Olbara G, van der Wijk T, Njuguna F, et al. Childhood acute lymphoblastic leukemia treatment in an academic hospital in Kenya: treatment outcomes and health-care providers’ perspectives. Pediatr Blood Cancer. 2021;68:e29366. doi: 10.1002/pbc.29366. [DOI] [PubMed] [Google Scholar]
- 21.Oskarsson T, Söderhäll S, Arvidson J, et al. Relapsed childhood acute lymphoblastic leukemia in the Nordic countries: prognostic factors, treatment and outcome. Haematologica. 2016;101:68–76. doi: 10.3324/haematol.2015.131680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker C, Waters R, Leighton C, et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. Lancet. 2010;376:2009–17. doi: 10.1016/S0140-6736(10)62002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tubergen DG, Gilchrist GS, O’Brien RT, et al. Improved outcome with delayed intensification for children with acute lymphoblastic leukemia and intermediate presenting features: a Children’s Cancer Group phase III trial. J Clin Oncol. 1993;11:527–37. doi: 10.1200/JCO.1993.11.3.527. [DOI] [PubMed] [Google Scholar]
- 24.Tuong PN, Kiem Hao T, Kim Hoa NT. Relapsed childhood acute lymphoblastic leukemia: a single-institution experience. Cureus. 2020;12:e9238. doi: 10.7759/cureus.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viana SS, de Lima LM, do Nascimento JB, et al. Secular trends and predictors of mortality in acute lymphoblastic leukemia for children of low socioeconomic level in Northeast Brazil. Leuk Res. 2015;39:1060–5. doi: 10.1016/j.leukres.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Zapata-Tarrés M, Balandrán JC, Rivera-Luna R, Pelayo R. Childhood acute leukemias in developing nations: successes and challenges. Curr Oncol Rep. 2021;23:56. doi: 10.1007/s11912-021-01043-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.