Abstract
Background:
The outcomes of treatment of metastatic colorectal cancer (mCRC) is still unsatisfactory. Several trials approved that, the upfront treatment with triplet regimen included fluorouracil, leucovorin, irinotecan and oxaliplatin improved the outcomes of patients with metastatic disease as compared to standard doublet regimen. The objective of our study is evaluating the impact of upfront treatment with triplet (FOLFOXIRI) regimen on both oncological outcomes (response rate and survival) and patients’ tolerability in comparison to the standard doublet regimen.
Methods:
We randomly enrolled 64 patients with a newly diagnosed unresectable mCRC to receive either FOLFOXIRI (experimental arm) or FOLFIRI or FOLFOX4 (control arm) biweekly up to 12 cycles. The primary endpoints are overall response rate (RR) and patients’ tolerability. The secondary endpoints are the progression free and overall survival.
Result:
There was a significantly increase in RR (59% vs 37%) and complete remission rate (CR) (6.3% and 3.1%, respectively (P = 0.045) for the triplet therapy group compared to control group. Consequently, an increased rate of secondary resection of metastasis (21.9% vs 3.1% respectively; P=0.023). The FOLFOXIRI regimen was associated with higher rate of grade 3/4 toxicity but not statistically significant except febrile neutropenia (6.2%; P=0.03). There was numerical prolongation in the median PFS in the FOLFOXIRI group on compared to control group but not significantly (9 versus 8 months; P=0.11). The median OS was 20 and 22 months in FOLFOXIRI arm and control arm respectively with no statistically significant difference (P=0.57).
Conclusion:
FOLFOXIRI had a higher efficacy and higher conversion rate to secondary resection over the doublet regimen as an upfront treatment option, coupled with a manageable adverse event, but failed to improve the survival outcomes.
Key Words: Unresectable, metastatic colorectal cancer, upfront FOLFOXIRI, standard doublet, efficacy, toxicity
Introduction
Colorectal cancer (CRC) is the second cause of cancer-related death worldwide. About fifty percent of all patients with CRC will develop metastatic disease, while 25% having distant metastatic lesions at diagnosis (Sung et al., 2021). Several trials indicate that selecting robust upfront chemotherapy for patients with unresectable metastatic disease can allow a curable resection and long-term survivors in 20% to 40% of these resected patients (Adam et al., 2012). In the setting of (mCRC) cytotoxic chemotherapy (fluoropyrimidine, oxaliplatin and irinotecan) still representing the backbone of management. Doublet regimen of these drugs increased response rate (RR) up to 40% and prolonged median survival (OS) significantly up to 20 months (van der Pool et al., 2012). However, in a sequential strategy, 30% of patients are not able to receive a second-line therapy. Also the integration of a targeted agent to doublet chemotherapy has increased response rates to around 60% (Venook et al, 2017). However, due to high cost of target agents in low economic countries there is under usage of those agents in treatment regimens. So it is crucial to deliver more intensified chemotherapy regimens in an attempt to downstage the disease and improve survival outcomes. Fortunately an interesting meta-analysis study suggested that FOLFOXIRI was effective and could achieve similar response, survival outcomes and resection rates compared with target therapy added to doublet chemotherapy (Zhou et al., 2017). Moreover, several randomized trials (RCTs) provided evidence supporting the adoption of more intense chemotherapy regimens as a first line treatment showing an augmented response at the cost of higher toxicity, but still doubt remains regarding its clinical gain (Marques et al., 2017).
Thus, based on these considerations we conducted this prospective randomized phase II study to assess the efficacy and toxicity of FOLFOXIRI regimen on compared with the standard doublet regimen (FOLFIRI or FOLFOX4) as upfront treatment in patients with unresectable (mCRC). This study was registered with ClinicalTrials.gov (no. NCT05316818).
Materials and Methods
Patient selection
Patients with histologically proven colon adenocarcinoma. The metastatic disease was unresectable and measurable. Other criteria included were: age ≥ 18-60 years, performance status ≤ 2 according to Eastern Cooperative Oncology Group (ECOG); no co-morbidity disease (cardiac disease, active infections, peripheral neuropathy...), hepatic, renal, and hematologic parameters met treatment eligibility; absence of other malignancies except non-melanoma skin cancer. No previous treatment for the metastatic disease was allowed. Adjuvant fluoropyrimidine-based chemotherapy was allowed if ended more than 6 months before enrollment in the study.
Sample size was calculated using STATA 14.2 statistical software by StataCorp. 2015. for the minimum required number of patients to detect difference in overall response rate by 20% or more for the triplet regimen compared to previously reported (ORR) of 35% by Colucci (2005) for the duplet regimen. Power of 80%, confidence interval 95% and level of significance 0.05. sample size was estimated as N= 54 patients. Therefore, we planned to enroll 64 patients putting in consideration for 10% drop-out.
Patient randomization
This randomized, phase II prospective controlled study was conducted between January 2018 and April 2021. The enrolled patients were randomized in a 1:1 ratio, randomization was done using closed envelope method. Only the patients were blinded to the study treatments. The protocol was approved by the local ethical committee. All participants were asked for written informed consent.
Treatment
All staging work up had to be done within 2 weeks before the start of treatment including computing tomography (CT) scan of the thorax, abdomen and pelvis and colonoscopy if not done before. Serum tumor markers level (CEA and CA19-9) measurement as a base line. A negative pregnancy test for females of childbearing potential was required.
Patients received either FOLFOXIRI (experimental arm) regimen consisted of irinotecan 160 mg/m² in 250 ml of NaCl 0.9% over 1 hr, followed by 85 mg/m² oxaliplatin in 250 ml dextrose 5% given concurrently with a 400 mg/m² leucovorin intra venous infusion in 250 ml dextrose 5% for 120 min, followed by 2,400 mg/m² fluorouracil for 44-hr continuous infusion, or FOLFIRI or FOLFOX4 (control arm) where it consisted of 180 mg/m² intravenous infusion of irinotecan for 60 min OR 85 mg/m² oxaliplatin day 1 only followed by a 200 mg/m² intra venous infusion of leucovorin for 120 min, 400 mg/m² intravenous bolus of fluorouracil, and 600 mg/m² continuous infusion of fluorouracil for 22 hr to be repeated on day 2. Treatment was given every 2 weeks till evidence of disease progression, intolerable toxicity, patient denial, or for a maximum of 12 cycles.
Standard antiemetic prophylaxis with 5-HT3 antagonist and I.V dexamethasone 1 hour before cycle administration. G-CSF wasn’t routine prescribed as primary prophylaxis of neutropenia. Only administrated to patients suffer from febrile neutropenia, grade 4 neutropenia, or neutropenia which did not recover spontaneously on day 15.
A 20% dose reduction of 5-FU, irinotecan and oxaliplatin was done for patients who developed recurrent grade 3–4 of diarrhea, thrombocytopenia, along-lasting grade 3-4 neutropenia (>5 days) or febrile neutropenia. Also, a 20% dose reduction of oxaliplatin was implemented after the occurrence of a persistent grade 3–4 peripheral neuropathy.
Outcomes and evaluation
The primary end points included the overall response rate (RR) and safety profile.
During the trial treatment period, the response rate was defined as the percentage of patients who achieved a partial (PR) or complete response (CR). It was calculated after the first six and twelve cycles of chemotherapy according to the Response Evaluation Criteria in Solid Tumors version (RECIST) v 1.1 guidelines
The secondary end points included post-chemotherapy secondary resection of metastasis, the progression free survival (PFS) and overall survival time (OS). (PFS) was defined as the length of time from randomization to the evidence of disease progression according to (RECIST) v 1.1 or death from any cause. (OS) was defined as the time from date of random assignment to the date of death due to any cause or due to lost follow up.
Assessments during treatment had to be performed before each treatment cycle and include patients’ medical history, physical examination, complete blood, liver, and kidney tests. Cycle delay period, dose modifications, and cumulative dose intensity (CDI) were recorded for each chemotherapy agent in the both groups. Chemotherapy CDI was defined as the ratio of the given dose intensity to the standard dose intensity across the treatment period.
Adverse events were recorded and assessed before each cycle using the Common Terminology Criteria for Adverse Events version (CTCAE) 5.0, 2017.
Adverse events were recorded during the study treatment period till 30 days following the end of therapy.
Statistical analyses
The distribution of baseline clinical characteristics of patients in both treatment groups, the overall response rate (RR) and the incidence of treatment toxicity were compared by means of the Chi-square test. The hazard ratios (HR) and confidence intervals were calculated using Cox’s proportional hazards modeling (CIs;). The Kaplan-Meier method was used to calculate the median follow-up time for the complete study cohort and to perform survival analysis.
Patients were stratified according to: Patients’ related factors: Age, gender and PS. Disease related: Tumor markers (CEA and CA19-9), Primary tumor location (right or left colon), time to metastasis (synchronous vs metachronous), the number of organs affected (single or multiple), sites (liver or other sites) or treatment related factors: Primary tumor resection (yes or no), previous adjuvant therapy and Surgical resection or local ablation of metastasis.
The Kaplan– Meier analysis model was used to perform a univariate analysis on all of the preceding factors, and the differences between groups were evaluated using the log-rank test. In the univariate analysis, predictive factors with significance levels of P <0.05 were subjected to a multivariate analysis using the Cox proportional hazards model. All data were analyzed and measured by SPSS 22.0 software. The statistical tests were two-sided, and P values of 0.05 or less were considered as statistical significance.
Results
Patients’ population
Between January 2018 and December 2019, 64 patients (32 in each arm) were enrolled in the study in Kasr Alainy hospital, Cairo university, Egypt. The baseline clinical characteristics were nearly balanced among the both treatment groups except the FOLFOXIRI group had more synchronous disease (81.2% versus 56.2 %, P = 0.03) and multiple metastatic sites (65.6% versus 31.2%, P = 0.006) on compared to control group as shown in Table 1.
Table 1.
Patients’ Characteristics
| Characteristics | FOLFOXIRI | Control | TOTAL | P value | |||
| (N = 32) | (N=32) | (N = 64) | |||||
| N | % | N | % | N | % | ||
| Median age (years) | 42.5 | 50 | 46 (19-60) | 0.348 | |||
| Gender | |||||||
| Male | 14 | 43.7 | 15 | 46.8 | 29 | 45.3 | 0.8 |
| Female | 18 | 56.3 | 17 | 53.2 | 35 | 54.7 | |
| PS (ECOG) | |||||||
| 0 | 7 | 21.9 | 5 | 15.6 | 12 | 18.8 | 0.8 |
| 1 | 19 | 59.4 | 20 | 62.5 | 39 | 60.9 | |
| 2 | 6 | 18.7 | 7 | 21.9 | 13 | 20.3 | |
| Site of Primary tumor | |||||||
| Right colon | 10 | 31.2 | 9 | 28.0 | 19 | 29.7 | 0.9 |
| Left colon | 22 | 68.8 | 23 | 71.9 | 31 | 48.4 | |
| Morphology | |||||||
| Mucinous carcinoma | 10 | 31.3 | 9 | 28.1 | 19 | 29.7 | 0.78 |
| Pathological grade | |||||||
| Well/moderate | 27 | 84.4 | 29 | 90.6 | 56 | 87.5 | 0.7 |
| Poor/undifferentiated | 5 | 15.6 | 3 | 9.4 | 8 | 12.5 | |
| Elevated Serum Tumor markers | |||||||
| CEA: | 19 | 59.4 | 17 | 53.0 | 36 | 56.3 | 0.8 |
| CA19-9: | 13 | 40.6 | 8 | 25.0 | 21 | 32.8 | 0.4 |
| Prior adjuvant treatment | |||||||
| Chemotherapy | 6 | 18.7 | 11 | 34.5 | 17 | 26.6 | 0.16 |
| Resection of primary | |||||||
| Yes | 28 | 87.5 | 24 | 75.0 | 52 | 81.25 | 0.2 |
| Time to metastases | |||||||
| Synchronous | 26 | 81.2 | 18 | 56.2 | 44 | 68.7 | 0.03 |
| Metachronous | 6 | 18.7 | 14 | 43.7 | 20 | 31.3 | |
| Number of Metastases | |||||||
| Single | 11 | 34.4 | 22 | 68.8 | 31 | 51.6 | 0.006 |
| Multiple | 21 | 65.6 | 10 | 31.2 | 31 | 48.4 | |
| Site of Metastases | |||||||
| Liver Frequency | 20 | 62.4 | 17 | 53.1 | 37 | 57.7 | 0.25 |
| Number > 5 | 13 | 40.6 | 8 | 25 | 21 | 32.8 | 0.2 |
| Size >5 cm | 14 | 43.7 | 11 | 34.3 | 25 | 39.0 | 0.9 |
| Peritoneum | 15 | 46.8 | 13 | 40.6 | 28 | 43.8 | 0.6 |
| Lung | 10 | 31.2 | 7 | 21.9 | 17 | 26.6 | 0.3 |
| LNs | 9 | 28.1 | 5 | 15.6 | 14 | 21.8 | 0.6 |
| Bone | 1 | 3.1 | 1 | 3.1 | 2 | 3.1 | 1 |
PS, performance status; CEA, carcino embryonic antigen; CA19:9,Carbohydrate antigen.
Treatment Administration and Safety
All patients received at least four cycles of the study treatment regimens and were evaluated for safety profile. The patients in FOLFOXIRI arm received 288 treatment cycles, with a median of 9, and the control arm received 308 cycles, with a median of 12. During the therapy period, seventy (11.7%) cycles were postponed throughout the treatment period. The incidence of cycle delay in the FOLFOXIRI group was significantly higher on compared to control group (16.6% vs 7.1% respectively, P=0.011) but not significant for dose reduction (12.1 vs 4.2 %, (P<0.27). Consequently, the average CDI was 87.9±14%, 94.2±11 %, and 93.5±12% for the FOLFOXIRI, FOLFOX4 and FOLFIRI regimens, respectively. In the FOLFOXIRI and control groups, treatment cessation due to toxicity was 15.6 % vs 12.5 %, and treatment discontinuation due to disease progression was 21.9 % vs 31.3 %, respectively with no statistically significant difference (P= 0.17). Table 2 shows the feasibility results of the study.
Table 2.
Number of Cycles, Causes of Cycle Delay and Reasons for Treatment Discontinuation
| Variable | FOLFOXIRI arm | Control arm | TOTAL | P value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| No. of cycles | |||||||
| Total | 288 | 48.3 | 308 | 51.7 | 596 | 100 | |
| Median | 9 | 12 | 10 | 0.87 | |||
| Range | 4_12 | 4_12 | 4_12 | ||||
| No. of cycles delay | 48 | 16.6 | 22 | 7.1 | 70 | 11.7 | 0.011 |
| Median duration of delay ( days) | 10 | 7 | 7 | 0.02 | |||
| Causes of cycle delay | |||||||
| Treatment toxicity: | |||||||
| Hematological | 32 | 66.6 | 11 | 50 | 43 /70 | 61.4 | 0.31 |
| Non hematological | 3 | 36.3 | 7 | 31.8 | 10 /70 | 14.3 | |
| Both hematological and non-hematological | 10 | 20.8 | 4 | 18.2 | 14/70 | 20 | |
| Non-treatment-related reasons | 3 | 6.3 | 0 | 0 | 3/70 | 4.3 | |
| Treatment Termination due to toxicity | 5 | 15.6 | 4 | 12.5 | 9 | 14 | 0.17 |
Toxicities
The treatment-related adverse events are listed in Table 3. The patients in the FOLFOXIRI arm, had a significantly higher incidence of grade 3 or 4 neutropenia on compared with patients treated in control arm (31.3% vs 6.3%; P= 0.01). Febrile neutropenia was observed in 4 patients of FOLFOXIRI group (12.5 vs 0%; P= 0.039). However, there was no significant difference between the both groups as regard to other hematological and non- hematological toxicities. G-CSF was significantly used in 13% of cycles in FOLFOXIRI arm in comparison to 2.2% of cycles in control arm (P= 0.02).
Table 3.
Adverse Events According to Treatment Group
| Adverse events | FOLFOXIRI | Control | Total | P value | |||
|---|---|---|---|---|---|---|---|
| (N = 32) | (N = 32) | (N = 64) | |||||
| N | % | N | % | N | % | ||
| Non hematological toxicity | |||||||
| Nausea | |||||||
| Grade 1 -2 | 90.6 | 29 | 100 | 32 | 61 | 95.3 | 0.3 |
| Grade 3-4 | 1.0 | 3.1 | 0 | 0 | 1 | 1.5 | |
| Vomiting | |||||||
| Grade 1 -2 | 62.5 | 20 | 71 | 22 | 42 | 65.6 | 0.5 |
| Grade 3-4 | 18.7 | 6 | 6.25 | 2 | 8 | 12.5 | |
| Diarrhea | |||||||
| Grade 1 -2 | 70.0 | 20 | 87 | 26 | 71.8 | 46 | 0.16 |
| Grade 3-4 | 22.6 | 7 | 9.4 | 3 | 15.6 | 10 | |
| Neurotoxicity | |||||||
| Grade 1 -2 | 20.0 | 62.5 | 58 | 18 | 59.3 | 38 | 0.23 |
| Grade 3-4 | 15.6 | 5 | 6.25 | 2 | 10.7 | 7 | |
| Fatigue | |||||||
| Grade 1 -2 | 90.6 | 29 | 96.6 | 30 | 92.1 | 59 | 0.7 |
| Grade 3-4 | 6.2 | 2 | 3.1 | 1 | 4.6 | 3 | |
| Hepatotoxicity | |||||||
| Grade 1-2 | 47.0 | 13 | 5 | 16 | 28.1 | 18 | 0.08 |
| Grade 3-4 | 0.0 | 0 | 3.1 | 1 | 1 | 1.56 | |
| Nephrotoxicity | |||||||
| Grade 1-2 | 4.0 | 12.4 | 3.1 | 1 | 7.8 | 5 | 0.2 |
| Grade 3-4 | 1.0 | 3.1 | 0 | 0 | 1 | 1.56 | |
| Hematological toxicity | |||||||
| Neutropenia | |||||||
| Grade 1 -2 | 59.0 | 19 | 45 | 14 | 33 | 51.6 | 0.01 |
| Grade 3-4 | 31.3 | 10 | 2 | 6.25 | 12 | 18.7 | |
| Anemia | |||||||
| Grade 1 -2 | 78.0 | 25 | 21 | 75 | 46 | 71.8 | 0.09 |
| Grade 3-4 | 15.6 | 5 | 2 | 6.25 | 7 | 10.9 | |
| Thrombocytopenia | |||||||
| Grade 1 -2 | 40.6 | 13 | 12 | 40 | 25 | 39 | 0.3 |
| Grade 3-4 | 6.2 | 2 | 0 | 0 | 2 | 3.1 | |
| Febrile neutropenia | 12.5 | 4 | 0 | 0 | 4 | 6.2 | 0.039 |
Objective Tumor Response
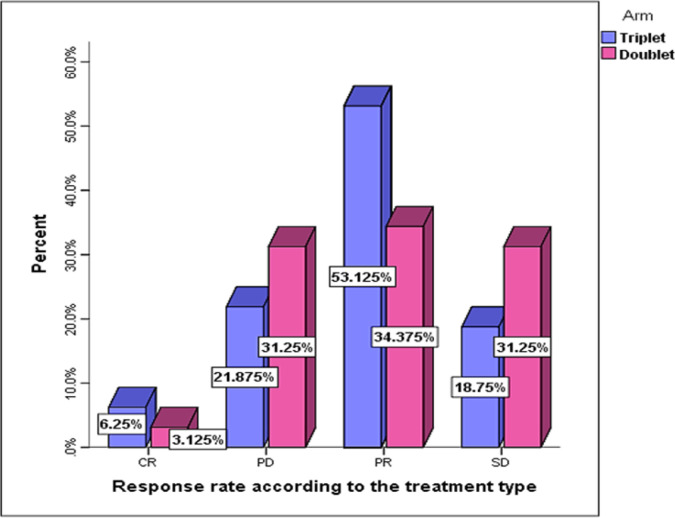
According to an intention-to-treat analysis, all patients were considered assessable for response. The RR was statistically significantly higher in FOLFOXIRI arm on compared to control group (59.4% vs 37.5%, respectively; P =0.045), Figure 1. However, there was no statistically significant difference in disease control rates between the both arms. (P=0.39). Previous un-exposure to adjuvant chemotherapy (60.9% vs 11.8%; P=0.001) and synchronous metastasis (59.1% vs 20%; P=0.004) were significantly correlated with good tumor response
Figure 1.
Response Rate According to Treatment Arm
Local therapy
Secondary Surgery
There was a statistically significantly increase of post-chemotherapy secondary resection of hepatic and peritoneal metastasis in patients treated with FOLFOXIRI regimen on compared to standard regimen (21.9% vs 3.1%, respectively; P=0.023).
Local ablative therapy
A total of 8 patients (12.5%), 4 in each group, received further local ablation therapy for hepatic metastasis after the first line systemic treatment.
Second-line chemotherapy
Forty-three (67%) patients who progressed during or after first line therapy received second line chemotherapy. Most of patients in control arm received second line chemotherapy (81.3% vs 53.1%; P= 0.01). Irinotecan-based therapy was most commonly used 25/43 (58.1%), followed by oxaliplatin -based therapy in 6/43 (14%) and single agent capecitabine in 9/43 (20.9%). While 3 (6.9%) patients re-challenged by FOLFOXIRI after durable response (3- 5.5 months). RR was sluggish with second line treatment, stationary disease was observed in 13 (30.2%) and disease control rate in 39.6 % of patients.
Survival
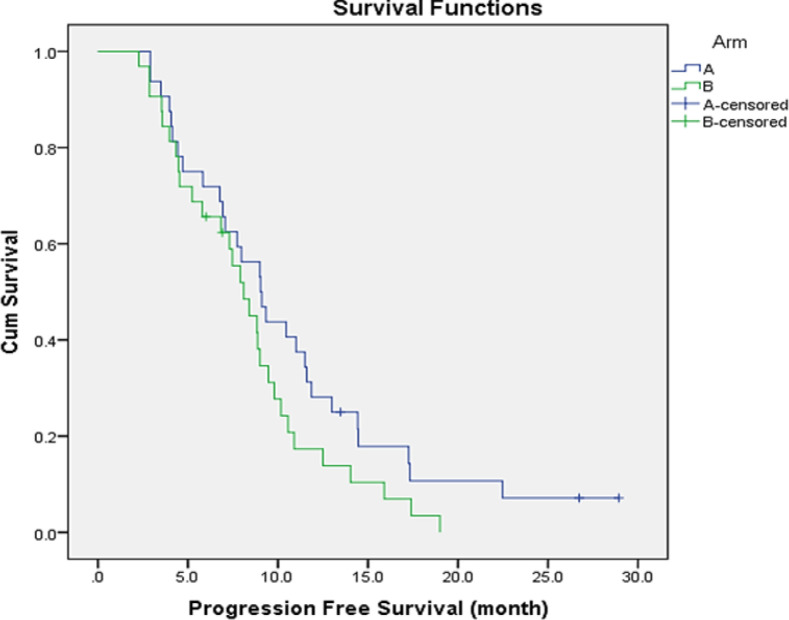
After the median follow-up of 18.4 months (4-31 months), 37 (57.8%) patients died, 18 (56.3%) in the FOLFOXIRI arm and 19 (59.4%) in the control arm. Disease progression was the leading cause of death among 94.6% of patients. The median PFS for all study group was 8.8 months (95% CI, 7.06- 10.16 months). According to the treatment type, the median PFS was 9 months (95% CI: 7.13- 10.92 months;) in the FOLFOXIRI group compared to 8 months (95% CI: 6.34- 9.85 months;) in the control group (P=0.11), Figure 2. The PFS rate at 6 and 12 months was 69% vs 62% and 28% vs 14% for FOLFOXIRI and control group respectively. By univariate analysis, prolonged PFS was significantly related to treatment response (10.4 vs 5.8 months, P=0.003), good PS (0-1 vs 2), 8.9 vs 4.5 months; P=0.02) and normal CEA level (9.3 vs 7.3 months; P=0.02). Additionally, patients who underwent local treatment in combination to systemic chemotherapy had a longer PFS. (11.9 vs 7.4; p=0.011).
Figure 2.
Progression Free Survival According to Treatment Arm, A (Triplet) and B (Duplet)
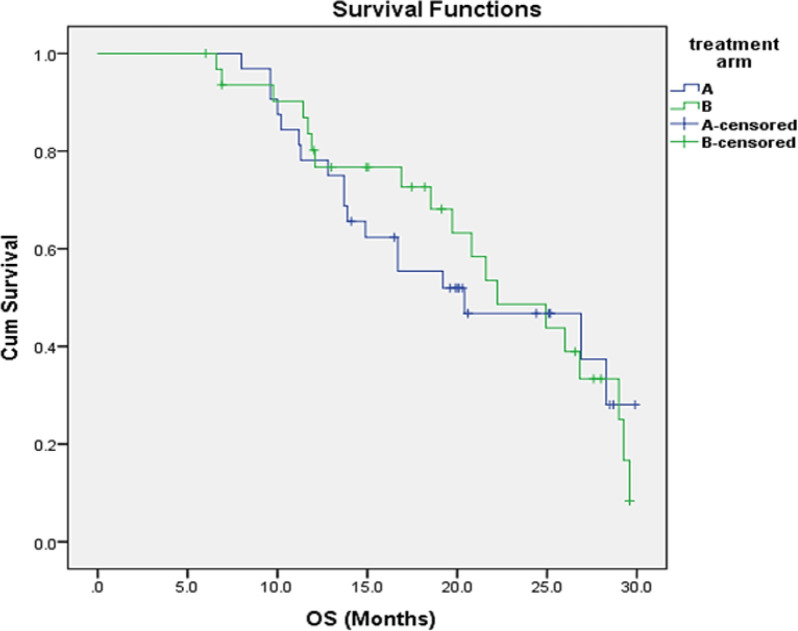
The whole trial group’s median OS was 21 months (95 percent CI: 15.67-27.32), while the FOLFOXIRI arm and control arm’s median OS was 20 and 22 months, respectively, with no statistically significant difference (p=0.57) Figure 3. By univariate analysis, the median OS is significantly longer for patients with PS 0-1 (P=0.04), single site of metastasis (P=0.03), who achieved treatment response (P=0.034), received combined local and systemic therapy (P= 0.001) and patients with CEA normal level (P=0.03), all were still significant in multivariate analysis.
Figure 3.
Overall Survival According to Treatment Arm, A (Triplet) and B (Duplet)
Discussion
Patients with (mCRC) usually exposed to the three active drugs (5-FU, irinotecan and oxaliplatin) in sequential strategy during their treatment and only 60 - 80% were able to receive second-line therapy with the risk of impairing its activity and efficacy (Cremolini et al., 2015). In attempt to downstage the disease and improve survival outcomes, several trials examined the administration of three active drugs as an upfront treatment, but results were conflicted. Thus, we conducted our study to assess the efficacy and toxicity of FOLFOXIRI regimen in mCRC patients. For the current study, the primary response outcome was consistent with several trials and pooled analysis which investigated the efficacy of FOLFOXIRI regimen as upfront treatment in mCRC. In our study, FOLFOXIRI was successful in inducing 21.9% increase in tumor response rate (59.4%; P =0.045) and doubling the rate of CR (6.3%; P < 0.033) on compared to doublet arm. In addition, it is an interesting finding that FOLFOXIRI yielded significantly higher RR among patients with heavily disease burden on compared to patients in control arm (P=0.006). Consequently, this improvement in RR in FOLFOXIRI arm allowed a significant increase in the percentage of patients (21.8%) candidates for surgical metastasectomy (P =0.023).
These findings confirmed that achieving deeper response that enables for metastasectomy was associated significantly with FOLFOXIRI as independent predictive factor (P=0.04). Despite small sample size and the difference dose schedule, our findings are in line with results of phase III GONO trial that reported 26% increase in the response rate in FOLFOXIRI arm (p < 0.0001), 2% CR and 9% secondary resection rate of metastases (P=0.033) (Falcone et al., 2007). In addition, in the METHEP trial FOLFIRINOX yielded the highest RR rate versus controls group (73% vs 60%) and provided the highest conversion rate to resectability at 67 % of patients (Ychou et al., 2013). In contrast, some trials like the HORG phase III trial, reported numerically improvement of the RR, CR and secondary metastasectomy in favor of FOLFOXIRI but with no statistical significance (Souglakos et al., 2006). Likewise, in MRC FOCUS3 trial, there was no statistically significant gain in RR and CR with the addition of oxaliplatin to FOLFIRI versus FOLFIRI alone (Maughan et al., 2014). The possible explanations for the differences of RRs between the trials attributed to differences in patients’ inclusion criteria and treatment schedules applied. Another interesting finding in our trial was a major impact of prior exposure to adjuvant chemotherapy and time of metastasis occurrence on RR. Over a quarter of our patients who had previously received oxaliplatin-containing adjuvant treatment did not benefit from FOLFOXIRI treatment. Theoretically, this may have some degree of resistance to chemotherapy. This finding is in line with a meta-analysis published by Loupakis F et al., and the theory of sequential exposure to active drugs in colon cancer yield impairing efficacy (Asmis et al., 2014; Loupakis et al., 2014). Furthermore, we observed significant RR to FOLFOXIRI in patients with synchronous metastases (65.4%) on compared to control group (50%; p= 0.03). This finding may be explained by patients with synchronous metastases have less tumor- resistant chemotherapy as result of prior un exposure to chemotherapy. This observation is consistent with the finding of Mekenkamp et al study which found that patients with synchronous metastasis had a significantly higher response rate than those with metachronous metastasis (38 vs 28%, respectively; P= 0.02) (Mekenkamp et al., 2010).
As regard to our primary safety outcome, FOLFOXIRI had a less favorable toxicity profile. Although grade 3/4 were two-fold more frequent among our patients, if compared to a doublet, but the difference was not statistically significant.
However, grade 3/4 neutropenia was 4.7-fold higher (31.3%) in FOLFOXIRI group, as was the incidence rate of febrile neutropenia (12.5%) necessitating the use of G-CSF in 13% of cycles. Consequently, treatment delay, interruptions and dose reduction were observed more frequent in the triplet arm. However, there was no difference between treatment groups in terms of overall treatment withdrawal rate. (P =0.17). The toxicity profiles among our patients were generally acceptable, manageable and no serious adverse events were recorded in both groups except in one case (3.1%) mortality due to treatment-related toxic effects in FOLFOXIRI group. Our findings were consistent with the incidence of grade 3/4 toxicity rates in several studies reviewed by Loupakis et al. where neutropenia was (33–59%), neurotoxicity (2–17%) and diarrhea (16–30%) (Loupakis et al., 2016). Because of the regimen’s tolerability, it was possible to administer 87.9% of the average FOLFOXIRI CDI. throughout the whole treatment period. In our opinion, good PS of our patients, close monitoring process, the use of G-CSF and prompt dynamic management of adverse events were the main factors for eliminating the severity of adverse events. However, health-related quality of life is necessary to evaluate whether the effects of toxicity in the experimental arm will have an influence on patients’ quality of life. As regard to survival outcomes, our trial failed to show any significant superiority for the FOLFOXIRI arm. This result was comparable to (HORG) trial that reported no significant differences in PFS (8.4 vs 6.9 months; P= 0.17) and OS (21.5 vs 19.5 months; P= 0.337) between FOLFOXIRI and FOLFIRI arms, respectively ( Souglakos et al., 2014). In contrast to GONO trial that reported a significant improvement in OS (23.4 vs 16.7 months; p=0.026) and PFS (9.5 vs 6.6 months; p<0.001) in FOLFOXIRI arm compared to FOLFIRI arm.
Furthermore, our findings show that adding local treatment, such as surgery or local ablation, to systemic treatment improves PFS (11.9 vs 7.4; p=0.011) and OS (NR vs 19.7; p=0.001) significantly when compared to systemic treatment alone. This finding was supported by (Xu et al., 2016; Ruers et al., 2017). In addition, a subgroup analysis in our study revealed that patients with a single metastatic site and a normal CEA level had the highest survival outcomes. These finding are in consistent with previous trials had proven the importance of a low CEA level and a solitary metastatic location as independent prognostic variables for prolonged survival (Eker et al., 2015; McNally et al., 2015).
Our study, however, had some limitations. First, because of the small sample size of patients, the lack effect of FOLFOXIRI on survival outcomes must be interpreted carefully, Second, the impact of the FOLFOXIRI regimen on the patients’ quality of life was not assessed.
In Conclusion, mCRC represents a critical clinical condition and the present study demonstrated the efficacy of triplet regimen (reflected in high conversion rate to secondary resection) over doublet regimen as an upfront treatment strategy, coupled with a manageable adverse events, in patients with initially unresectable mCRC. Despite, triplet regimen failed to improve the survival outcomes compared to standard doublet regimen, we recommend it as the best choice therapy for fit patients with potentially resectable tumor who cannot afford for expensive target therapy.
Author Contribution Statement
All authors designed and approved the study protocol. KK recruited patients and collected data, All the authors analyzed and interpreted the data. All except MA contributed to the manuscript writing. All the authors revised and approved the final version of the manuscript.
Acknowledgements
The authors would like to thank and appreciate our residents, colleagues, pharmacists and nursing staffs for their supports and contribution in the study.
Scientific Body/ if it is part of an approved student thesis
It was thesis study submitted for partial fulfillment of Medical Doctorate degree in clinical oncology, it was approved by scientific committee in clinical oncology department, Kasr Alainy hospital
Ethical Declaration
This study was approved by the Research Ethics Committee at Faculty of Medicine, Cairo University, (approval number: I-160317) based on the Declaration of Helsinki and Good Clinical Practice.
Availability of data
The data of the study is available upon request.
Study Registration
This study was registered in ClinicalTrials.gov registry (no. NCT05316818).
Conflict of Interest
The authors declare no conflict of interest; no conflict exists for drugs used in the study.
References
- 1.Adam R, De Gramont A, Figueras J, et al. The oncosurgery approach to managing liver metastases from colorectal cancer: A Multidisciplinary International Consensus. Oncologist. 2012;17:1225–9. doi: 10.1634/theoncologist.2012-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asmis T, Berry S, Cosby R, et al. Strategies of sequential therapies in unresectable metastatic colorectal cancer: A meta-analysis. Curr Oncol. 2014;21:318–8. doi: 10.3747/co.21.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: A Multicenter Study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol. 2005;23:4866–5. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 4./Cremolini C, Schirripa M, Antoniotti C, et al. First-line chemotherapy for mCRC—a review and evidence-based algorithm. Nat Rev Clin Oncol. 2015;12:607–9. doi: 10.1038/nrclinonc.2015.129. [DOI] [PubMed] [Google Scholar]
- 5.Eker B, Ozaslan E, Karaca H, et al. Factors affecting prognosis in metastatic colorectal cancer patients. Asian Pac J Cancer Prev. 2015;16:3015–1. doi: 10.7314/apjcp.2015.16.7.3015. [DOI] [PubMed] [Google Scholar]
- 6.Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: The gruppo oncologico nor. J Clin Oncol. 2007;25:1670–6. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 7.Loupakis F, Cremolini C, Salvatore L, et al. FOLFOXIRI plus bevacizumab as first-line treatment in BRAF mutant metastatic colorectal cancer. Eur J Cancer. 2014;50:57–3. doi: 10.1016/j.ejca.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Loupakis F, Stein A, Ychou M, et al. A review of clinical studies and practical guide for the administration of triplet chemotherapy regimens with bevacizumab in first-line metastatic colorectal cancer. Target Oncol. 2016;11:293–8. doi: 10.1007/s11523-015-0400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marques RP, Duarte GS, Sterrantino C, et al. Triplet (FOLFOXIRI) versus doublet (FOLFOX or FOLFIRI) backbone chemotherapy as first-line treatment of metastatic colorectal cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;118:54–62. doi: 10.1016/j.critrevonc.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Maughan TS, Meade AM, Adams RA, et al. A feasibility study testing four hypotheses with phase II outcomes in advanced colorectal cancer (MRC FOCUS3): a model for randomised controlled trials in the era of personalised medicine? Br J Cancer. 2014;110:2178–6. doi: 10.1038/bjc.2014.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNally GE, Lloyd DM, Grondona JP. Carcinoembryonic antigen as a prognostic factor in colorectal cancer with liver metastases. J Cancer Ther. 2015;6:1035–4. [Google Scholar]
- 12.Mekenkamp LJ, Koopman M, Teerenstra S, et al. Clinicopathological features and outcome in advanced colorectal cancer patients with synchronous vs metachronous metastases. Br J Cancer. 2010;103:159–4. doi: 10.1038/sj.bjc.6605737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruers T, Van Coevorden F, Punt CJ, et al. Local treatment of unresectable colorectal liver metastases: Results of a Randomized Phase II Trial. J Natl Cancer Inst. 2017;109:1–10. doi: 10.1093/jnci/djx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Souglakos J, Androulakis N, Syrigos K, et al. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): A multicentre randomised phase III trial from the Hellenic Oncolog. Br J Cancer. 2006;94:798–5. doi: 10.1038/sj.bjc.6603011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–9. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 16.Van der Pool AE, Damhuis RA, IJzermans JN, et al. Trends in incidence, treatment and survival of patients with stage IV colorectal cancer: a population-based series. Color Dis. 2012;14:56–1. doi: 10.1111/j.1463-1318.2010.02539.x. [DOI] [PubMed] [Google Scholar]
- 17.Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer. JAMA. 2017;317:2392–1. doi: 10.1001/jama.2017.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu C, Huang XE, Lv PH, et al. Radiofrequency ablation in treating colorectal cancer patients with liver metastases. Asian Pac J Cancer Prev. 2016;16:8559–1. doi: 10.7314/apjcp.2015.16.18.8559. [DOI] [PubMed] [Google Scholar]
- 19.Ychou M, Rivoire M, Thezenas S, et al. A randomized phase II trial of three intensified chemotherapy regimens in first-line treatment of colorectal cancer patients with initially unresectable or not optimally resectable liver metastases the METHEP trial. Ann Surg Oncol. 2013;20:4289–7. doi: 10.1245/s10434-013-3217-x. [DOI] [PubMed] [Google Scholar]
- 20.Zhou M, Yu P, Davin DBH, et al. Is FOLFOXIRI alone or combined with targeted therapy administered as first-line treatment a reasonable choice for most patients with mCRC? Systematic review and network meta-analysis. Oncotarget. 2017;8:62339. doi: 10.18632/oncotarget.17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of the study is available upon request.