Abstract
OBJECTIVE:
To summarize and evaluate the effectiveness and safety of Redcore lotion on treating vulvovaginal candidiasis (VVC) using a systematic review and Meta-analysis of randomized controlled trials.
METHODS:
A systematic literature search was performed in five English and three Chinese electronic databases up to October 2019. Randomized controlled trials in the treatment for VVC were included; only studies which compared the effectiveness and safety of Redcore lotion plus miconazole with miconazole alone were included. Relative risk (RR) and 95% confidence intervals (CI) were used in the Meta-analysis.
RESULTS:
Seven studies involving 768 patients suffering from VVC were identified; 468 of the patients were pregnant women (60.9%). Combination group (Redcore lotion plus miconazole) was more effective in reduCIng symptomatic episodes of VVC than miconazole alone, with respect to cure rate (RR, 1.31; 95% CI, 1.09-1.57; P = 0.01), fungal culture negative rate (RR, 1.21; 95% CI, 1.04-1.41; P = 0.01), and effective rate (RR, 1.18; 95% CI, 1.05-1.35; P = 0.01). Subgroup analyses for pregnant women also showed that the combination group had superior outcomes with respect to VVC cure rate (RR, 1.48; 95% CI, 1.16-1.88, P < 0.01), fungal culture negative rate (RR, 1.26; 95% CI; 1.09-1.47; P < 0.01), and effective rate (RR, 1.25; 95% CI, 1.10-1.42; P < 0.01). Additionally, the observed risk of adverse events was lower in the combination medication group (RR, 0.30; 95% CI, 0.14-0.65; P < 0.01).
CONCLUSION:
Though overall quality of individual studies was low, Redcore lotion plus miconazole can significantly improve clinical effectiveness and safety compared with miconazole alone.
Keywords: Redcore lotion; miconazole; candidiasis, vulvovaginal; systematic review; Meta-analysis
1. INTRODUCTION
Vulvovaginal candidiasis (VVC) is a common fungal infection caused by species of the genus Candida, and is one of the most common diagnoses among women seeking gynecological care.1,⇓-3 Previous studies showed that approximately 75% of child-bearing age women had at least one episode of VVC throughout their lifetimes, and the incidence of 4 VVC or more every year was about 35%.1,4,5 Hence, VVC is still a major health issue seriously affecting women’s health.6
At present, fungistatic azole drugs are recommended for patients suffering from Candida infections in VVC treatment guidelines in China, America and Canada.4,7,8 Clotrimazole and miconazole are utilized most widely for the vaginal medication treatment of vaginal candidiasis, especially for pregnant women. However, the prescription of these antifungal azoles has resulted in acquired azole resistance.9
Redcore lotion is a type of vaginal prescription that has been used for VVC treatment in China since 1998 and is dry-distillated from hawthorn seeds, and rich in chemical compounds such as phenols, aldehydes, and ketones.10 In vitro antibacterial experiments and clinical application have shown that Redcore lotion can kill or inhibit common urogenital bacteria and skin fungi.11 However, some studies did not confirm these effects.12,13 For better evaluation of the effectiveness and safety of Redcore lotion, we carried out a systematic review and Meta-analysis for clinical trials of Redcore lotion combined with miconazole, compared to miconazole alone.
2. METHODS
2.1. Systematic searching
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis.14 Eight electronic databases were used to search relevant studies up to October 2019: PubMed, EMBASE, Cochrane Library, Web of Science, ClinicalTrials.gov, China National Knowledge Infrastructure Database (CNKI), Wanfang data and China Science and Technology Journal Database (VIP). The following search terms and their synonyms were used in the literature search: (“colpitis mycotica” OR “colpomycosis” OR “vulvovaginal candidiasis” OR “monilial vaginitis” OR “candidal vaginitis” OR “candida vaginitis” or “VVC”) AND (“Honghefujiexiye” OR “Honghe Fujie lotion” OR “Honghe lotion” OR “Redcore lotion”) AND (“daktarin” OR “miconazole”). Corresponding Chinese terms with above-mentioned terms were used for searching in the following Chinese literature databases: CNKI, Wanfang, and VIP. All references were imported into Endnote X9.2 (Clarivate Analytics, Los Angeles, CA, USA) to check for eligibility, and duplicates were excluded. Possible relevance was screened and evaluated for each title and/or abstract. Then, full-texts of the retrieved records were downloaded and further reviewed to judge their eligibility. Cross-referencing was employed to supplement systematic searching and the identification process.
2.2. Inclusion and exclusion criteria
The following inclusion criteria were employed to evaluate the eligibility of each study: (a) age over 18 years old; (b) all patients diagnosed with vulvovaginal candidiasis using mycological tests but were not experiencing recurrent VVC due to complexity of medication compared with primary VVC; (c) randomized controlled trials; (d) intervention group treated using combination medication of Redcore lotion and miconazole; and control group treated with miconazole alone; (e) therapeutic strategies of antifungal drugs conformed to the treatment guideline of VVC in China;7 (f) at least one effectiveness or safety outcome was reported, such as cure rate, fungal culture negative rate, effective rate, recurrence rate, and occurrence of adverse events.
2.3. Data extraction
At least two authors independently extracted the following data in a standardized manner for each eligible study: first author’s name, the year of publication, study period and cities, age of patients, drug dosages and frequency, medication duration, sample size, cure rate, fungal culture negative rate, effective rate, recurrence rate, and occurrence of adverse events between two study arms. Any disagreement was resolved in a study team after additional review of the articles or further discussion with a senior researcher (Yin Lu), if necessary.
2.4. Quality assessment
The Jadad score scale was used to evaluate the study quality of all included studies, regarding randomization (2 points), double-blinding (2 points), and withdrawals and dropouts (1 point). The sum of the above-mentioned items was computed for each study, and ranged from 0 to 5 points. Low quality was defined as 2 points or less, and high quality as 3 points or more.15
2.5. Meta-analysis
All analyses were performed by using Stata 12.0 (Stata Corp., College Station, TX, USA). Multiple outcomes were used to assess whether the effect of combination medication with Redcore lotion is superior over monotherapy. The multiple outcomes were cure rate (defined as symptoms and/or signs disappearing completely and a negative fungal culture),16 fungal culture negative rate (defined as fungal culture negative even if symptoms and/or signs partly disappeared), effective rate (defined as symptoms and sign improving, regardless of the fungal culture result), recurrence rate, and occurrence of adverse events between two arms. Relative ratio (RR) with 95% confidence interval (CI) was calculated as effect sizes (ES) for the Meta-analysis. Cochran's Q test was conducted to test the heterogeneity across included studies by summarizing variation around the estimated ES. Typically, heterogeneity is considered present if the P value is < 0.10. I 2 statistic was also calculated to estimate the degree of heterogeneity; 25%-49% was defined as low, 50%-74% as moderate, and 75% or higher as high degree. The random effect model using DerSimonian and Laird method was use if the P value for Cochran's Q test is less than 0.10, otherwise the fixed effect model based on the Mantel-Haenszel method was used.17,⇓-19 The publication bias was assessed using Egger’s linear regression tests at 0.05 level for significance.20 In order to evaluate the robustness of Meta-analysis results, sensitivity analyses were performed by deleting one study each time.
3. RESULTS
3.1. Study Selection
Though systematically searching was conducted in eight literature databases, 119 articles were identified in three Chinese databases. After removing 26 duplicates, 93 titles and/or abstracts were reviewed for eligibility, and 71 were deleted due to no eligible treatment strategy (n = 40), irrelevant articles (n = 21), not being relevant to VVC (n = 6), and not being an original study (n = 4). The full-texts of the remaining 22 articles were reviewed further for potential eligibility, among which 7 were exc-luded due to no combination group, 2 articles because of a lack of recommended dosage for miconazole in revised VVC Diagnosis and Treatment Guideline7 in China, and 6 because patients were suffering from recurrent VVC or diabetes as indicated by treatment. Finally, 7 studies were included in final Meta-analyses.21,⇓,⇓,⇓,⇓,⇓-27
3.2. Study characteristics
A total of seven randomized controlled trials were included, wherein 384 individuals received Redcore lotion plus miconazole treatments, and 384 received miconazole alone (Table 1). Four studies exclusively recruited pregnant women.21,⇓,⇓-24 The earliest two studies prescribed 10 mL Redcore lotion, diluted into 100 mL warm water, to flush vulva and vagina using vaginal irrigation tools, and 0.4 g miconazole were inserted into posterior fornix of vagina, both of which lasted 3 d.21,25 The remaining five studies had a 7-d treatment regimen, but 0.2 g miconazole was prescribed.22,⇓-24,26,27 As a whole, seven included studies were marked as low-quality; six studies had a Jadad scores of 2 and one study had a Jadad score of 1 because it failed to report “randomization” (Table 1).26
Table 1.
Characteristics of the included studies
| Study | Study cities (study period) |
Pregnant (%) | Redcore lotion + Miconazole | Miconazole alone | Drug dosage, MOAa | Treatment (days) | Jadad scores15 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age (years) | ORR (%) | AE (%) | No. | Age (years) | ORR (%) |
AE (%) | Honghe | Miconazole | |||||||||
| Liu HN et al 200921 | Zhengzhou (2005-2008) |
100 | 50 | 24-34 | 98.0 | NA | 50 | 22-35 | 86.0 | NA | 10 mL, bid | 0.4 g, qd | 3 | 2 | ||||
| Jia XW et al 2010 25 | Xi’an (2009-2010) |
0 | 43 | 36.6±7.3 | 100.0 | NA | 43 | 35.8±7.6 | 97.7 | NA | 10 mL, qd | 0.4 g, qd | 3 | 2 | ||||
| Fan KL 201426 | Xi’an (NA) |
0 | 100 | 20-67 | 97.0 | 2.0 | 100 | 20-67 | 88.0 | 7.0 | 10 mL, qd | 0.2 g, qd | 7 | 1 | ||||
| Sun YF et al 201522 | Hangzhou (2012-2014) |
100 | 77 | 26.7±2.7 | 98.7 | 3.9 | 77 | 25.8±2.8 | 71.4 | 6.5 | 10 mL, tid | 0.2 g, qd | 7 | 2 | ||||
| Li YL 201723 | Lingyuan (2015-2016) |
100 | 46 | 26.9±3.2 | 97.8 | NA | 46 | 26.2±3.4 | 76.1 | NA | 10 mL, tid | 0.2 g, qd | 7 | 2 | ||||
| Yu YH 201727 | Jingdezhen (2016-2017) |
66.7 | 21 | 29.0±1.2 | 95.2 | 4.8 | 21 | 31.0±1.5 | 71.4 | 38.1 | 10 mL, bid | 0.2 g, qd | 7 | 2 | ||||
| Wang JM 201824 | Bayan Nur \(2016-2017) | 100 | 47 | 25.9±3.4 | 98.0 | 4.0 | 47 | 25.9±3.5 | 72.0 | 18.0 | 10 mL, bid | 0.2 g, qd | 7 | 2 | ||||
Notes: MOA: mode of administration; ORR: overall response rate; AE: adverse events; VVC: vulvovaginal candidiasis; NA: not available; aMode of administration for Redcore lotion and miconazole include: qd = once per day; bid = twice per day.
3.3. Syntheses results from Meta-analysis
Figure 1 forest plots illustrate the Meta-analysis results of Redcore lotion plus miconazole compared to miconazole alone. Combination medication was more effective in the treatment of VVC than single medication for the following outcomes; VVC cure rate (RR, 1.31; 95% CI, 1.09-1.57; P = 0.01; I 2 = 56.8%; P for heterogeneity = 0.04; Figure 1A), fungal culture negative rate (RR, 1.21; 95% CI, 1.04-1.41; P = 0.01; I 2 = 80.1%; P for heterogeneity < 0.01; See Figure 1B), and VVC effective rate (RR, 1.18; 95% CI, 1.05-1.35; P = 0.01; I 2 = 82.6%; P for heterogeneity < 0.01; Figure 1C). No publication bias was observed for VVC cure rate (Egger’s test P = 0.28) or fungal culture negative rate (Egger’s test P = 0.22), but obvious publication bias was found for VVC effective rate (Egger’s test P = 0.04). Further trim and fill analyses was conducted to obtain “filled” RR for effective rate by filling two studies (RR, 1.12; 95% CI, 1.00-1.23; P = 0.02), which still reached statistical significance.
Figure 1. Forest plots for r treatment effect of redcore lotion plus miconazole vs miconazole alone.
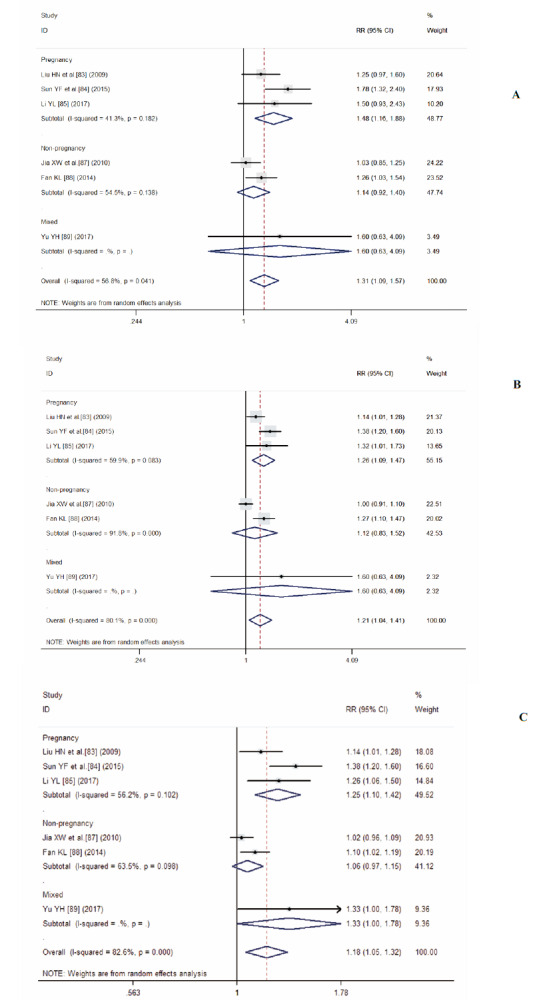
A: vulvovaginal candidiasis cure rate; B: fungal culture negative rate; C: effective rate. Redcore lotion plus miconazole vs miconazole alone.
Figure 1 also illustrates the results of subgroup analyses for pregnant women. Combination medication appears to be even more efficacious among pregnant women: VVC cure rate (RR, 1.48; 95% CI, 1.16-1.88, P < 0.01, I 2 = 41.3%; P for heterogeneity = 0.18), fungal culture negative rate (RR, 1.26; 95% CI; 1.09-1.47; P < 0.01; I 2 = 59.9%; P for heterogeneity = 0.08), effective rate (RR, 1.25; 95% CI, 1.10-1.42; P < 0.01; I 2 = 56.2%; P for heterogeneity = 0.10). However, no statistically significant differences were detected found for these three outcomes among non-pregnant women. Sensitivity analyses indicated that results pooled effect sizes remained stable, regardless of which study was deleted.
Only two individual studies reported VVC recurrence rate among pregnant women.22,23 One observed VVC recurrence in six months after trial treatment (combination vs. single medication: 6.5% vs 16.9%),22 another did not describe observation time for VVC recurrence after medication (4.4% vs 17.4%).23 After combining these two studies, VCC recurrence rate was much lower in combination medication than single medication (RR, 0.34; 95% CI, 0.15-0.77; P = 0.01; I 2 = 0.0%; P for heterogeneity = 0.64).
Four included studies reported adverse events during trials,22,24,26,27 including eight AE in combination medication and twenty-nine in single medication, such as papule, urticaria, local irritation, burning sensation, and anaphylactic reaction. Meta-analysis presented a significantly reduced risk of adverse events in combination medication (RR, 0.30; 95% CI, 0.14-0.65; P < 0.01; I 2 = 0.0%, P for heterogeneity = 0.60).
4. DISCUSSION
This systematic review is the first to attempt to summarize available evidence so far and evaluate the effectiveness of combination Redcore lotion and topical azoles treatment for VVC. Our Meta-analysis included 768 individuals from 7 studies. Results suggest that miconazole combined with Redcore lotion is superior to miconazole alone, including among pregnant women, in terms of VVC cure rate, fungal negative-inverted rate, effective rate, and recurrence rate. In addition, combination therapy also appears to have fewer adverse events. However, the quality of included studies was low, and only 7 studies were included.
Several of antifungal drugs are used in clinical practice, but acquired azole resistance has increased, especially for frequent dosing or intermittent medication. A Bayesian network Meta-analysis study involving 41 RCTs was conducted to assess the effectiveness of various antifungal drugs for the treatment of VVC. Results from that study confirmed that antifungal drugs are effective.28 Moreover, azole resistance is very common in fluconazole treatment.9,29,30
As we know, Traditional Chinese Medicines such as decoction of medicinal ingredients and Chinese patent drugs have been used widely in clinical practice with a long history in China and some Asian countries despite the dominance of Western Medicine in modern history. Redcore lotion is distillated by hawthorn seeds and is rich in anion, which maybe adhere and infiltrate the pathological cells whose cell membrane polarity has changed.31 Six compounds of destructive distillation extracts have been determined using gas chromatography mass spectrometer, including 2,6-dimethoxyphenol, furfural, 2-methoxy-4-methylphenol, 5-tertbutyl-pyrogallol, 2-methoxyphenol, and 4-ethyl-2-metho-xyphenol, all of which have been confirmed to affect the growth of yeast cells together.32 Originated from plants, Redcore lotion has lower risk of developing drug resistance. In vitro antibacterial experiments showed that the sterilizing rate was 100% on eleven test organisms in 10-fold diluent for 4 minutes, including candida albicans.33 Vaginal secretions can be dramatically reduced and the effect of other antibiotics may be enhanced after topical treatment of Redcore lotion.23,34 Hence, their combination with azoles can either increase azole potency or delay the development of azole resistance. Our results found better improvement for effectiveness and safety of combination medication, though more high-quality clinical trials are needed to confirm the effectiveness of Redcore lotion on VVC treatment.
It is worth mentioning that pregnant women are more prone to fungal infection.35 There is some emerging evidence to suggest that fungal infection in pregnancy may increase risks to the embryo and new born children, such as chorioamnionitis, premature rupture of membranes, preterm labor, and congenital cutaneous candidiasis.35 Most gynecologists prescribe vaginal imidazole for seven days for symptomatic episodes of VVC in pregnancy to avoid the risks of oral antifungal drugs. A Meta-analysis including ten randomized clinical trials confirmed imidazole drugs were more effective than nystatin when treating vaginal candidiasis in pregnancy.36 Results of our systematic review and Meta-analysis provide new evidence for combination medication of Redcore lotion and miconazole in the treatment of VVC in pregnancy, though more studies should be conducted for in-depth analyses.
The strength of this study is that it is the first to summarize available literature using Meta-analysis and estimate the effectiveness and safety of combination medication of Redcore lotion and miconazole in treating patients with VVC, compared to those being treated only by miconazole alone. However, our study has several limitations. Firstly, the anti-inflammatory mechanisms of Redcore lotion are still unclear, despite widespread clinical use in China. Clear effects have been observed in several clinical studies,26,27,31,37,⇓,⇓ -40 but the quality of available studies is quite low. Secondly, we cannot guarantee that no relevant studies were missed, though eight literature databases were used for searching and additive checks using cross-referencing were also conducted. In particular, only Chinese articles were found in three Chinese literature databases. Finally, potential publication bias could not be avoided for VVC effective rate. Lastly, only Chinese studies were included into our Meta-analysis. Further trim and fill analyses were used to evaluate the effective rate after filling two studies, and effect sizes decreased slightly, but statistical significance was maintained.
In conclusion, despite the above-mentioned limitations, our study is the first to assess the synergistic effect of Redcore lotion and azoles drugs using comprehensive approaches of systematic review and Meta-analysis. Findings indicate improvement of effectiveness and safety of combination medication with Redcore lotion in topical treatment of VVC, especially among pregnant women. However, only a few low-quality studies were included. The low number of studies precluded in-depth subgroup analyses and Meta-regression evaluation of combination medication with other azoles drugs.
5. ACKNOWLEDGMENTS
We are grateful to Stephen W. Pan for proofreading the manuscript for language and grammar.
REFERENCES
- 1. Liu XP, Fan SR, Peng YT, et al. Species distribution and susceptibility of Candida isolates from patient with vulvovaginal candidiasis in Southern China from 2003 to 2012. J Mycol Med 2014; 24: 106-11. [DOI] [PubMed] [Google Scholar]
- 2. Shi XY, Yang YP, Zhang Y, et al. Molecular identification and antifungal susceptibility of 186 Candida isolates from vulvovaginal candidiasis in southern China. J Med Microbiol 2015; 64: 390-3. [DOI] [PubMed] [Google Scholar]
- 3. Fakhim H, Vaezi A, Javidnia J, et al. Candida africana vulvovaginitis: prevalence and geographical distribution. J Mycol Med 2020; 30: 100966. [DOI] [PubMed] [Google Scholar]
- 4. Workowski KA. Centers for disease control and prevention sexually transmitted diseases treatment guidelines. Clin Infect Dis 2015; 61: S759-62. [DOI] [PubMed] [Google Scholar]
- 5. Yano J, Soble JD, Nyirjesy P, et al. Current patient perspectives of vulvovaginal candidiasis: incidence, symptoms, management and post-treatment outcomes. BMC Womens Health 2019; 19: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Denning DW, Kneale M, Sobel JD, Rautemaa-Richardson R. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect Dis 2018; 18: e339-47. [DOI] [PubMed] [Google Scholar]
- 7. Liu CH, Liao QP. Revised regulations for diagnosis and treatment of vulvovaginal candidiasis (VVC). Zhong Guo Shi Yong Fu Ke Yu Chan Ke Za Zhi 2012; 28: 401-2. [Google Scholar]
- 8. Schalkwyk JV, Yudin MH, infectious disease committee . Vulvovaginitis: screening for and management of trichomoniasis, vulvovaginal candidiasis, and bacterial vaginosis. J Obstet Gynaecol Can 2015; 37: 266-74. [DOI] [PubMed] [Google Scholar]
- 9. Zavrel M, White TC. Medically important fungi respond to azole drugs: an update. Future Microbiol 2015; 10: 1355-73. [DOI] [PubMed] [Google Scholar]
- 10. Lin L, Gu GQ, Cui QH, Ma ML, Lin YQ. Establishment of GC fingerprint and quantitative determination of multiple components of Honghe lotion. Zhong Cao Yao 2019; 50: 3833-9. [Google Scholar]
- 11. Wen N, Lu SH, Tang ZS, et al. Application and research of Honghe Fujie lotion. Zhong Guo Min Zu Min Jian Yi Yao 2017; 26: 54-6. [Google Scholar]
- 12. Chang LL, Xi YN, Wu J. Application effect of clotrimazole vaginal tablets combined with Honghe Fujie lotion in the treatment of mycotic vaginitis. Lin Chuang Yi Xue Yan Jiu Yu Shi Jian 2018; 3: 81-3. [Google Scholar]
- 13. Zeng XM, Yan PK, Ruan XX, Kang J, Pan W. A survey of topical medication in 800 cases of vaginitis. Xian Dai Yi Yuan 2009; 9: 78-9. [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and Meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006-12. [DOI] [PubMed] [Google Scholar]
- 15. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1-12. [DOI] [PubMed] [Google Scholar]
- 16. Sun CX. Diagnosis of clinical diseases according to the standard of cure and improvement. Beijing: People's Military Medical Press; 2002; 529- 34. [Google Scholar]
- 17. Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177-88. [DOI] [PubMed] [Google Scholar]
- 18. Lipsey MW, Wilson DB. Practical Meta-analysis. London: Sage Publications, Inc. 2001; 1- 168. [Google Scholar]
- 19. Normand SL. Meta-analysis: formulating, evaluating, combining, and reporting. Stat Med 1999; 18: 321-59. [DOI] [PubMed] [Google Scholar]
- 20. Rothstein H, Sutton AJ, Borenstein M. Publication bias in meta-analysis:prevention, assessment and adjustments. Chichester: Wiley, 2005: 1- 356. [Google Scholar]
- 21. Liu HN, Chen SM. Clinical observation of Honghe lotion combined with dacornin on the treatment of vulvovaginal candidiasis in pregant women. Yi Xue Xin Xi 2009; 22: 159-60. [Google Scholar]
- 22. Sun YF, Fang YJ. The observation of cure effective of Honghe lotion on vulvovaginal candiasis during pregnancy. Xin Zhong Yi 2015; 47: 148-9. [Google Scholar]
- 23. Li YL. Clinical observation of the treatment of Honghe lotion on vulvovaginal candidiasis in pregant women. Zhong Guo Yi Yao Zhi Nan 2017; 15: 175. [Google Scholar]
- 24. Wang JM. Clinical observation of the treatment of Honghe lotion in vulvovaginal candidiasis in pregnant women. Shi Yong Fu Ke Nei Fen Mi Za Zhi 2018; 5: 99-100. [Google Scholar]
- 25. Jia XW, He FJ, Zhang R, Gao XY. Effect of rinsing vagina with Honghe lotion on vaginal microecology of vulvovaginal candidiasis. Shaanxi Zhong Yi Xue Yuan Xue Bao 2010; 33: 42-4. [Google Scholar]
- 26. Fan KL. The clinical effect of Honghe lotion combined with miconazole nitrate in mycotic vaginitis. Yi Yao Qian Yan 2014; 11: 191-2. [Google Scholar]
- 27. Yu YH. Clinical effect of Honghe lotion on the uncomplicated vulvovaginal candidiasis. Dang Dai Yi Xue 2017; 23: 111-2. [Google Scholar]
- 28. Qin F, Wang Q, Zhang C, et al. Efficacy of antifungal drugs in the treatment of vulvovaginal candidiasis: a Bayesian network Meta-analysis. Infect Drug Resist 2018; 11: 1893-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marchaim D, Lemanek L, Bheemreddy S, et al. Fluconazole-resistant Candida albicans vulvovaginitis. Obstet Gynecol 2012; 120: 1407-14. [DOI] [PubMed] [Google Scholar]
- 30. Sobel JD, Sobel R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin Pharmacother 2018; 19: 971-7. [DOI] [PubMed] [Google Scholar]
- 31. Zhu CY, Cao YK, Li YY, et al. A randomised,case-control study of Honghe Fujie lotion for treating uncomplicated vulvovaginal candidiasis. Xian Dai Fu Chan Ke Jin Zhan 2017; 26: 426-30. [Google Scholar]
- 32. Rao HY, Li PB, Wu H, Liu C, Peng W, Su WW. Simultaneous determination of six compounds in destructive distillation extracts of hawthorn seed by GC-MS and evaluation of their antimicrobial activity. Molecules 2019; 24: 4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mei L, Lin R, Lu CH. Study on antibacterial activity in vitro of Honghe lotion. Zhong Guo Fu Chan Ke Lin Chuang Za Zhi 2014; 15: 145-7. [Google Scholar]
- 34. Liu PZ. Study on the combination therapy effect of Honghe lotion combined with clotrizole in the treatment of candidiasis vulvovaginalis in pregnant women. Zhong Guo Xian Dai Yao Wu Ying Yong 2010; 4: 160-1. [Google Scholar]
- 35. Aguin TJ, Sobel JD. Vulvovaginal candidiasis in pregnancy. Curr Infect Dis Rep 2015; 17: 462. [DOI] [PubMed] [Google Scholar]
- 36. Young GL, Jewell D. Topical treatment for vaginal candidiasis (thrush) in pregnancy. Cochrane Database Syst Rev 2001; 4: CD000225. [DOI] [PubMed] [Google Scholar]
- 37. Ma LH, Chan XL. Analysis on15 cases with intractable vaginal candidiasis treated by combination therapy. Zhong Wai Yi Liao 2010; 29: 114. [Google Scholar]
- 38. Zhang YL, Zhang Y, Li SY. The clinical effect analysis of 32 cases of refractory mycotic vaginitis treated with fluconazole combined with miconazole. Zhong Guo She Qu Yi Shi 2011; 13: 125. [Google Scholar]
- 39. Zhang L, Liu JH. Study on the effect of anti-candida drugs on the growth of growth of common lactobacillus vaginalis. Sheng Zhi Yu Bi Yun 2012; 32: 500-3. [Google Scholar]
- 40. Liu YL. 56 cases of mycotic vaginitis treated with miconazole nitrate combined with fuyanjie lotion. Chin J Modern Drug Application 2013; 7: 74-5. [Google Scholar]


