Abstract
OBJECTIVE:
To compare the phenotype and adipogenic and osteogenic differentiation capacities of adipose-derived mesenchymal stem cells (AMSCs) isolated from patients with psoriasis vulgaris and healthy donors, and to explore the effects of astragaloside IV, a Traditional Chinese Medicine, on the immunoregulatory function of AMSCs.
METHODS:
AMSCs were isolated from human adipose tissue and cultured for three generations in vitro. Cell phenotype and cell cycle analysis were performed by flow cytometry. Adipogenic and osteogenic differentiation of AMSCs was examined by lipid (oil red O) and alkaline phosphatase staining, respectively. Expression of inflammatory mediators was examined by real-time quantitative polymerase chain reaction analysis, and proliferation was quantified using the cell counting kit-8 assay.
RESULTS:
Expression of CD29, CD44, and CD73 was higher in AMSCs from healthy donors than psoriasis patients, while the reverse was true for expression of CD45, CD31, and HLA-DR. AMSCs from psoriasis patients had a greater ability to undergo adipogenic differentiation than cells from healthy donors, whereas there was no significant difference in osteogenic differentiation between AMSCs from the two sources. Compared with AMSCs from healthy donors, psoriasis patient-derived AMSCs expressed lower levels of the anti-inflammatory cytokines interleukin-10 and trans-forming growth factor-β (TGF-β) and the immune checkpoint ligand programmed cell death 1 ligand 1 (PD-L1) (P < 0.05) and higher levels of the pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ). Incubation of AMSCs from psoriasis patients with astragaloside IV had no significant effect on pro-liferation but increased the expression of TGF-β and PD-L1 and decreased the expression of IFN-γ and TNF-α.
CONCLUSION:
AMSCs from patients with psoriasis vulgaris display abnormal proliferation and adipogenesis and an enhanced pro-inflammatory phenotype. These defects were normalized by treatment with astragaloside IV, suggesting that this Traditional Chinese Medicine may be useful for restoring the immunoregulatory function of AMSCs and immune homeostasis in patients with psoriasis vulgaris.
Keywords: psoriasis, mesenchymal stem cells, cell proliferation, astragaloside IV, adipogenesis, osteogenesis, immune regulation
1. INTRODUCTION
Psoriasis is a common chronic inflammatory skin disease and affects about 2% of the population worldwide. The main pathological changes in psoriasis are abnormal proliferation of keratinocytes, hyperkeratosis and hypokeratosis, and infiltration of inflammatory cells, such as monocytes and lymphocytes, into the skin.1 The etiology and pathogenesis of psoriasis have not yet been fully elucidated, but immune dysfunction mediated by T lymphocytes appears to play a key role. Mesenchymal stem cells (MSC) are present in bone marrow, fat, peripheral blood, skin, placenta, and other tissues and, in addition to their ability to self-renew, have the potential to differentiate into a variety of cell types, including adipocytes and osteocytes. MSCs also exert strong immunoregulatory effects on T and B lymphocytes, macrophages, and other immune cells2,3 by direct interaction via cell surface proteins and by the secretion of diverse soluble immunoregulatory factors.4
The use of Traditional Chinese Medicine to treat psoriasis mostly focuses on the blood theory and uses the methods of clearing away heat, cooling blood, detoxifying, eliminating wind, and activating blood.5 In recent years, a few studies have taken into account the methods of supplementing Qi, invigorating spleen, warming Yang, and nourishing Yin during clinical treatment, and have obtained good results.
From a certain viewpoint, the theory of strengthening the body and eliminating pathogenic factors in Traditional Chinese Medicine is consistent with the focus on immunoregulation mechanisms in Western Medicine. Dysregulation of stem cells and deleterious effects on immune function is equivalent to the state of the body's vital energy in Traditional Chinese Medicine. This may be one reason for the relatively poor effects of classical psoriasis treatments, especially the inability to shorten the time course of the disease or prevent recurrence. Previous work has demonstrated that bone marrow-derived MSCs from patients with psoriasis display abnormal cytokine secretion patterns,6 suggesting that this may be one mechanism underlying immune dysfunction in psoriasis patients.
To probe this further, we considered that MSCs derived from subcutaneous adipose tissue (AMSCs) may play a potentially important role in regulating the inflammatory microenvironment in the skin. Relatively little is known about the immunoregulatory function of AMSCs in psoriasis patients. Based on our interest in the treatment of psoriasis using Chinese traditional medicine, we focused on investigating the effects on AMSC function by astragaloside IV, an isomer of astragaloside A, a major component of the flowering plant Huangqi (Radix Astragali Mongolici).7 This compound has been shown to have pharmacological activity in enhancing immunity and improving disease resistance, and it is thought to have a regulatory effect on the immune function of MSCs. Therefore, in the current study, we isolated AMSCs from psoriasis patients and healthy donors and examined the effects of astragaloside IV treatment in vitro on various immunomodulatory properties of AMSCs. Our results provide a theoretical basis for the use of Traditional Chinese Medicine in the clinical treatment of psoriasis.
2. MATERIALS AND METHODS
2.1. Ethical approval
All patients provided informed consent before tissue samples were obtained. The study was approved by the Hospital Ethics Committee.
2.2. Patients and tissue specimens
We enrolled three patients (2 men, 1 woman) who were diagnosed with psoriasis vulgaris at Guang'anmen Hospital, Chinese Academy of Traditional Chinese Medicine. They were aged 25-40 years old and had 5-10-year histories of psoriasis. As controls, we also enrolled three healthy volunteers; 2 men and 1 woman, aged 25-40 years old. Among the inclusion and exclusion criteria were: no treatment with biological agents or immunosuppressive agents, including retinoic acid, within 1 month before consultation; not suffering from other autoimmune diseases or other major diseases; not suffering from other medical or surgical issues. Fat specimens were obtained from abdominal fat by liposuction during plastic surgery at Peking Union Medical College Hospital.
2.3. Reagents
The major reagents and sources were as follows: fetal bovine serum (FBS) was purchased from Hyclone (South Logan, UT, USA); DF12 medium was purchased from STEMCELL Technologies Inc. (Vancouver, Canada); alkaline phosphatase reagent kit was purchased from Institute of Hematology, Chinese Academy of Medical Sciences (Beijing, China); oil red O reagent kit was purchased from Shanghai Luwen Biotechnology Co., Ltd. (Shanghai, China); fluorescein isothiocyanate labeled anti-CD29, -CD73, -CD45, -HLA-DR, -CD31, and -CD44 antibodies was purchased from Becton, Dickinson and Company (BD) (Franklin Lakes, NJ, USA); TRIzol reagent was purchased from Invitrogen/Life Technologies (Carlsbad, CA, USA); flow cytometry reagents was purchased from Dakewe Biotech Co., Ltd. (Shenzhen, China); SYBR Premix EX Taq kit was purchased from Takara Bio Inc. (Shiga, Japan); astragaloside IV was purchased from China Institute of Food and Drug Identification (Beijing, China).
2.4. Isolation, culture, and characterization of AMSCs
AMSCs were isolated from 50 mL adipose tissue, cultured in DF12 medium supplemented with 10% FBS, and passaged to the third generation. The AMSCs were then harvested, placed in 24-well plates at 1 × 105/well and allowed to proliferate to 90% confluence before analysis.
For determination of cell surface phenotype, the cells were incubated with fluorescein isothiocyanate-conjugated anti-human antibodies and analyzed using a flow cytometer. For analysis of the cell cycle, cells were incubated with RNaseA and propidium iodide and analyzed by flow cytometry with ModiFIT software.
For the differentiation assays, AMSCs were harvested, resuspended in DF12 medium, placed in 24-well plates at a density of 1 × 105,placed in 24-well plates at a density of 1 × 105For the differentiation assays, AMSCs were harvested, resuspended in DF12 medium, placed in 24-well plates at a density of 1 × 105, and incubated for 24 h in the presence of 37 ℃, 5% CO2 and added 0.5 mL/hole osteoinductive fluid for induction of osteogenesis, or added 0.5 mL/well lipogenic induction solution for induction of adipogenesis. Change the fluid every 3 d, after 6 or 13 d in culture, the differentiated cells were harvested and examined for alkaline phosphatase or lipid content, respectively.
For experiments with astragaloside IV, the compound was resuspended in DMSO at a concentration of 20 mg/mL to make a stock solution. Astragaloside IV was diluted before addition to cells at final concentrations of 15, 30, or 60 μg/mL.
2.5. Real-time quantitative polymerase chain reaction (RT-qPCR)
AMSCs were harvested from culture and RNA was extracted using TRIzol reagent (Invitrogen/Life Technologies, Carlsbad, CA, USA). Aliquots of RNA were reverse-transcribed, and the resulting cDNA was subjected to qPCR using a PCR instrument with SYBR Green Premix EX Taq kit (Takara Bio Inc., Shiga, Japan). The qPCR reaction conditions were 95℃ pre-denaturation 60 s, 95℃ denaturation 10 s, 60℃ annealing, extension 40 s, running 40 cycles in total. The expression of mRNA was calculated by the 2-ΔΔCt method.
2.6. Cell counting kit-8 (CCK-8) proliferation assay
AMSCs were placed in 96-well culture plates at a density of 104 cells/well in triplicate. After the cells had adhered to the wells, various concentrations of astragaloside IV were added, and the plates were incubated at 37 ℃ for 24 h. CCK-8 reagent was then added at 10 μL/well and the plates were incubated at 37 ℃ for 1 h. Absorbance at 450 nm was measured using a biotek microplate reader.
2.7. Statistical analysis
All experiments were repeated three times. Data were analyzed using SPSS 16.0 software. Data are expressed as the mean ± standard deviation ($\bar{x}±s$). Group differences were analyzed using the t test, and P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Morphology and expression of surface markers on AMSCs
After isolation from psoriasis patients or healthy donors, AMSCs were passaged until the third generation and then analyzed. Under light microscopy, AMSCs from both psoriasis patients and healthy donors were fusiform or spindle-shaped, similar to a fibroblast-like morphology, with a spiral or parallel arrangement. There was no detectable difference in morphology between AMSCs from psoriasis patients and healthy donors.
Analysis of the cell surface phenotype by flow cytometry showed that a higher percentage of psoriasis patient-derived AMSCs expressed CD44, CD73, and CD29 compared with healthy donor-derived cells (mean of samples: 98.83% vs 97.86%, 98.3% vs 98.2%, and 99.1% vs 97.93%, respectively). In contrast, the percentage of AMSCs expressing CD31, CD45, and HLA-DR was lower for AMSCs from psoriasis patients compared with healthy donors (mean of samples: 0.7% vs 1.27%, 0.56% vs 0.9%, and 0.26% vs 0.43%, respectively).
3.2. Cell cycle analysis of AMSCs
To examine the cell cycle status, third generation AMSCs were stained with propidium iodide and analyzed by flow cytometry. The proportion of cells in G1 stage of the cell cycle was significantly higher for AMSCs from psoriasis patients than from healthy donors (84.3% ± 2.5% and 77.4% ± 1.1%, respectively, P < 0.05; Figure 1). Accordingly, only 6.5% ± 2.2% of psoriasis patient-derived AMSCs were in S stage compared with 17.1% ± 3.1% of AMSCs from healthy donors (P < 0.05, Figure 1). This result identified a significant decrease in cell cycle progression of AMSCs from psoriasis patients compared with normal AMSCs.
Figure 1. Cell cycle analysis of AMSCs from psoriasis patients and healthy donors.
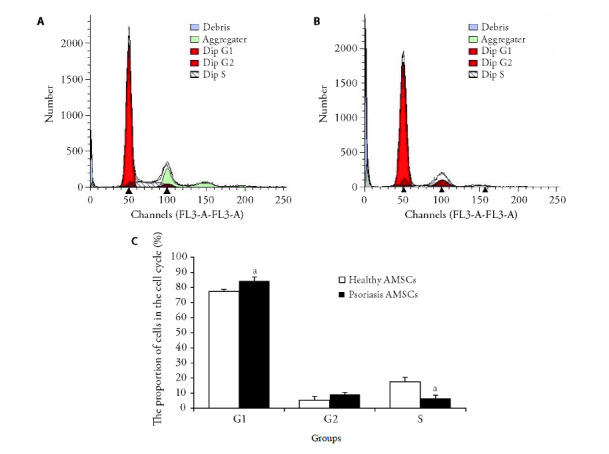
A: flow cytometric analysis of propidium iodide-stained third-generation AMSCs (adipose-derived mesenchymal stem cells) derived from healthy donors; B: flow cytometric analysis of propidium iodide-stained third-generation AMSCs derived from psoriasis patients; C: quantification of AMSCs of healthy donors and psoriasis patients in the indicated cell cycle stages. AMSCs: adipose-derived mesenchymal stem cells. Compared with the healthy donors AMSCs, aP < 0.05.
3.3. Comparison of the adipogenic and osteogenic differentiation potential of AMSCs
To investigate the differentiation potential, isolated AMSCs were induced to undergo either adipogenic or osteogenic differentiation by culture with Osteogenesis or adipogenesis induction fluid. On day 13, AMSCs from psoriasis patients (Figure 2A) displayed greater adipogenic differentiation than cells from healthy donors (Figure 2B), as indicated by the greater lipid content (oil red O staining). In contrast, staining for alkaline phosphatase, a marker of osteoclasts, in day 6 osteogenic cultures showed no significant difference between cells derived from psoriasis patients (Figure 2C) and healthy donors (Figure 2D). These results indicate that adipogenic differentiation, but not osteogenic differentiation, of AMSCs is abnormal in psoriasis patients.
Figure 2. Induction of adipogenic and osteogenic differentiation in cultured AMSCs.
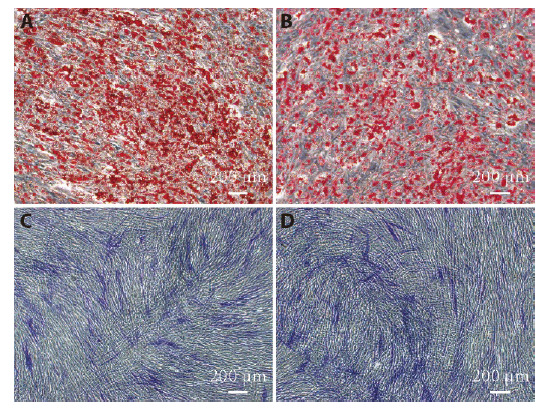
A, C: third generation AMSCs from psoriasis patients; B, D: third generation AMSCs from healthy donors. A, B: induction of adipogenic differentiation of AMSCs by Oil Red O staining on day 13 (× 20); C, D: induction of osteogenic differentiation of AMSCs by alkaline phosphatase staining on day 6 (× 20). AMSCs: adipose-derived mesenchymal stem cells.
3.4. Expression of inflammatory factors by AMSCs
To assess the inflammatory status of AMSCs from psoriasis patients compared with healthy donors, we performed RT-qPCR analysis of third generations cells. Cells from psoriasis patients showed trends, albeit not significant, towards lower expression of the anti-inflammatory cytokines TGF-β and IL-10, and higher expression of the pro-inflammatory cytokines IFN-γ and TNF-α, compared with cells from healthy donors (Table 1). However, expression of programmed cell death 1 ligand 1 (PD-L1) mRNA was significantly lower in AMSCs from psoriasis patients than from healthy donors (P < 0.01, Table 1). These data suggest that AMSCs from psoriasis patients display an enhanced infla-mmatory phenotype compared with normal AMSCs.
Table 1.
RT-qPCR analysis of the indicated cytokines or PD-L1 in AMSCs from healthy donors compared with those from psoriasis patients ($\bar{x}±s$)
| Group | n | IL-10 | TGF-β | PD-L1 | IFN-γ | TNF-α |
|---|---|---|---|---|---|---|
| Psoriasis AMSCs | 3 | 0.33±0.08 | 0.98±0.14 | 0.23±0.04a | 2.34±1.63 | 1.31±0.24 |
| Healthy AMSCs | 3 | 1.00±0.42 | 1.00±0.19 | 1.00±0.18 | 1.00±0.57 | 1.00±0.46 |
Notes: RT-qPCR: real-time quantitative polymerase chain reaction analysis; PD-L1: programmed cell death 1 ligand 1; AMSCs: adipose-derived mesenchymal stem cells; IL-10: interleukin-10; TGF-β: transforming growth factor-β; IFN-γ: interferon-γ; TNF-α: tumor necrosis factor-α. Psoriasis AMSCs: extracted from psoriasis patients; Healthy AMSCs: extracted from healthy donors. Compared with the healthy AMSCs, aP < 0.01.
3.5. Effect of astragaloside IV on the proliferation of AMSCs from psoriasis patients
Many drugs have toxic side effects on normal cells. Therefore, before examining the effects of astragaloside Ⅳ on expression of inflammatory mediators by AMSCs from psoriasis patients, we performed a CCK-8 proliferation assay to determine the optimal non-toxic concentration of astragaloside Ⅳ for treatment. The OD values (450 nm) of cells incubated with 0, 15, 30 and 60 μg/mL Astragaloside Ⅳ were (0.20 ± 0.02), (0.20 ± 0.03), (0.20 ± 0.01), (0.22 ± 0.01). Compared with control (cells were incubated with 0 μg/mL astragaloside Ⅳ), we detected no inhibitory effects on cell proliferation by incubation with 15, 30, or 60 μg/mL astragaloside Ⅳ for 24 h.
3.6. Effect of astragaloside Ⅳ on the expression of cytokines and PD-L1 by AMSCs from psoriasis patients
Next, we performed RT-qPCR analysis of cytokine and PD-L1 expression after culture of AMSCs from psoriasis patients with 15, 30, or 60 μg/mL for 24 h. As shown in Table 2, IL-10 mRNA levels were not significantly affected by astragaloside Ⅳ at any dose tested. However, TGF-β mRNA levels were significantly increased by treatment with 30 μg/mL astragaloside Ⅳ compared with the control cells (P < 0.05), whereas PD-L1 mRNA levels were significantly increased by 30 and 60 μg/mL astragaloside Ⅳ (P < 0.05). Expression of the pro-inflammatory cytokine IFN-γ was dramatically suppressed by astragaloside IV treatment, even at the lowest concentration tested (P < 0.05), whereas TNF-α mRNA levels were not significantly affected. Thus, astragaloside Ⅳ normalized the dysregulated expression of some, but not all, inflammatory factors in AMSCs from psoriasis patients.
Table 2.
Effect of astragaloside IV on the expression of cytokines and PD-L1 by AMSCs from psoriasis patients ($\bar{x}±s$)
| Group | n | IL-10 | TGF-β | PD-L1 | IFN-γ | TNF-α |
|---|---|---|---|---|---|---|
| Control | 3 | 1.00±0.25 | 1.00±0.14 | 1.00±0.16 | 1.00±0.33 | 1.00±0.17 |
| As1 | 3 | 0.97±0.24 | 1.17±0.13 | 1.09±0.21 | 0.01±0.34b | 0.77±0.27 |
| As2 | 3 | 1.10±0.18 | 1.57±0.20a | 1.82±0.27a | 0.42±0.36a | 1.02±0.29 |
| As3 | 3 | 1.08±0.34 | 1.36±0.35 | 1.82±0.51a | 0.41±0.17a | 0.95±0.22 |
Notes: PD-L1: programmed cell death 1 ligand 1; AMSCs: adipose-derived mesenchymal stem cells; IL-10: interleukin-10; TGF-β: transforming growth factor-β; IFN-γ: interferon-γ; TNF-α: tumor necrosis factor-α. control: AMSCs from psoriasis patients were incubated with astragaloside IV at 0 μg/mL for 24 h; As1: AMSCs from psoriasis patients were incubated with astragaloside Ⅳ at 15 μg/mL for 24 h; As2: AMSCs from psoriasis patients were incubated with astragaloside IV at 30 μg/mL for 24 h; As3: AMSCs from psoriasis patients were incubated with astragaloside IV at 60 μg/mL for 24 h. Compared with the control, aP < 0.05,bP < 0.01.
4. DISCUSSION
MSCs originate from the mesoderm and can differentiate into many cell lineages. MSCs are widely distributed in the bone marrow, adipose tissue, placenta, umbilical cord, epidermis, and other tissues. These cells not only participate in tissue regeneration and reconstruction but also in immunoregulation, and they are being widely explored in clinical research for their potential to treat various immune-mediated diseases. Adipose MSCs exist in the subcutaneous fat microenvironment of psoriasis lesions, raising the possibility that abnormal biological characteristics or immune functions of AMSCs may contribute to the disorder.
MSCs are easy to isolate and maintain, allowing their self-renewal ability and multi-differentiation potential to be examined in detail in vitro.8 In the present study, we exploited these properties to conduct a preliminary study of the general biological characteristics and immune-ological functions of AMSCs from psoriasis patients. We found that AMSCs isolated from psoriasis patients and healthy donors had similar cell surface phenotypes and expressed CD29, CD44, and CD73, but were negative for CD45, CD31, and HLA-DR. Similarly, AMSCs from healthy donors and psoriasis patients did not differ significantly in their proliferative capacity (data not shown) or their ability to differentiate into osteoblasts. Strikingly, however, adipogenic differentiation was more efficient for psoriasis patient-derived AMSCs compared with healthy donor-derived cells in the presence of specific inducing factors. Previous work has shown that bone marrow-derived MSCs from psoriasis patients have a reduced proliferative ability in vitro compared with cells from healthy bone marrow.9 In addition, an inflammatory environment is thought to promote the differentiation of bone marrow MSCs into adipocytes.10,11 Therefore, we speculate that the abnormal proliferation and adipogenic differentiation of AMSCs from psoriasis patients detected in the present study may be related to the more inflammatory local environment in psoriasis patients.
MSCs have multiple roles in immunoregulation. They can inhibit the activation, proliferation, differentiation, and maturation of several types of immune cells, including T and B lymphocytes, natural killer cells, and antigen-presenting cells, and they can affect the spectrum of cytokines secreted by these cells. Overall, MSCs tend to have anti-inflammatory activities and promote immune tolerance via the development of regulatory T cells (Tregs) and regulatory B cells and the differentiation of monocytes into M2 macrophages, all of which tend to inhibit inflammation.12 Thus, MSCs can help maintain immune homeostasis and promote the formation of an immune microenvironment conducive to tissue damage repair.
The immunoregulatory functions of MSCs are mediated in part by secretion of cytokines such as IL-10 and TNF-α,13 which have been shown to promote the proliferation and differentiation of Tregs.14 In addition, the immunoregulatory capacity of MSCs is modulated by binding of microbial ligands to Toll-like receptors (e.g., TLR3 and TLR4). TLR3 activation causes the expression of immunosuppressive molecules, such as IL-10, TGF-β, and indoleamine-pyrrole 2,3-dioxygenase, whereas activation of TLR4 induces secretion of pro-inflammatory factors such as TNF-α and IFN-γ.15 The plasticity of immunoregulatory MSCs can help to balance pro-inflammatory and anti-inflammatory responses and thus plays an important role in maintaining organizational homeostasis.16
At present, few studies have examined the immune function of AMSCs from psoriasis patients or their possible role in the pathogenesis of the disease. Here, we found that AMSCs from psoriasis patients expressed lower levels of anti-inflammatory molecules and higher levels of pro-inflammatory cytokines compared with AMSCs from healthy donors. Therefore, we speculate that the immunoregulatory functions of AMSCs is suppressed in cells from psoriasis patients compared with healthy donors.
Psoriasis belongs to the category of “white cruste” in Traditional Chinese Medicine. According to the color and shape of skin lesions, three syndrome types can be identified: blood-heat, blood-dryness, and blood-stasis. Therefore, treatment methods of clearing heat, cooling blood, detoxification, dispelling wind, and promoting blood circulation are often used to eliminate evil. In recent years, a few studies have reported that methods of supplementing Qi, invigorating spleen, warming Yang, and nourishing Yin have achieved good results in diagnosis and treatment. These treatments embody the characteristics of the theory of strengthening the body and eliminating pathogenic factors in Traditional Chinese Medicine. It is believed that the destruction of immune regulation is due to the deficiency of righteous Qi, the invincibility of righteousness, and the two pathological factors of righteousness and evil toxin, which act on the human body. Therefore, strengthening the body and eliminating evil is the main principle for the treatment of immune disorders.17
Psoriasis patients often relapse or experience disease exacerbation in winter. Most patients with chronic psoriasis have clinical manifestations of abdominal distension, loose stools, and weak waist and knee. Some patients have joint pain, and all of these symptoms may be alleviated by warmth.18 This suggest that the patient's body is in a state of emptiness, and the symptoms are characterized by insufficient Yang-Qi. In previous studies, we found that AMSCs from psoriasis patients had weaker immunoregulatory function than normal AMSCs.19 It is possible that the reduced immune function of AMSCs, that is, the state of the body's vital energy, may be a reason for the poor therapeutic response in psoriasis patients, especially for prolonged and recurrent psoriasis. Therefore, based on the methods of clearing away heat, cooling blood, and activating blood circulation, combined with drugs derived from Huangqi (Radix Astragali Mongolici), Bajitian (Radix Morindae Officinalis), Yinyanghuo (Herba Epimedii Brevicornus), treatment of psoriasis with Chinese traditional medicine may achieve a better curative effect than conventional treatment. Based on the results described here, we speculate that astragaloside IV may be able to restore the immune function of AMSCs in patients with psoriasis and promote their immunoregulatory properties.
There are some limitations to this study. We examined AMSCs from only three patients, and only a few representative pro- and anti-inflammatory factors expressed by AMSCs were selected for study. In addition, how astragaloside IV affects the ability of AMSCs to express inflammatory factors and the possible underlying immunoregulatory mechanisms of AMSCs remain to be elucidated. Therefore, further in-depth and more comprehensive research using larger sample sizes will be needed to confirm and extend our findings before they can be translated into clinical applications.
In conclusion, we found that AMSCs from psoriasis vulgaris patients differ significantly in their ability to secrete inflammatory mediators compared with AMSCs from healthy donors. Astragaloside IV, a constituent of Huangqi (Radix Astragali Mongolici), reversed some of the immune defects in AMSCs from psoriasis patients, suggesting that it can promote their immunoregulatory functions. This study contributes to our understanding of AMSC function in psoriasis patients and identifies a novel potential therapeutic target for the treatment of psoriasis with Traditional Chinese Medicine. However, the specific mechanism of action by which astragaloside IV modulates the immunoregulatory properties of AMSCs remains to be fully elucidated.
5. ACKNOWLEDGEMENTS
We thank Anne M. O'Rourke, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Contributor Information
Rongjia ZHU, Email: zrjia2006@126.com.
Ping SONG, Email: songping@vip.126.com.
REFERENCES
- 1. Mahler, Jackson C, Ijacu H. The burden of psoriasis and barriers to satisfactory care: results from a Canadian patient survery. J Cutan Med Surg 2009; 13: 283-93. [DOI] [PubMed] [Google Scholar]
- 2. Akiyama K, Chen C, Wang DD, et al. Mesenchymalstem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem cell 2012; 10: 544-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Q, Ma Y, Li L, et al. Flagellin influences the expression of a variety of important cytokines and chemokines without affecting the immune status of umbilical cord mesenchymal stem cells. Mol Med Rep 2015; 12: 6955-61. [DOI] [PubMed] [Google Scholar]
- 4. Cao K, Zhang T. Progress in immunoregulatory plasticity of mesenchymal stem cells. Medical review 2018; 24 : 34-9. [Google Scholar]
- 5. Zhou DM, Wang P, Chen WW, et al. An analysis of the theoretical evolution from treating psoriasis from blood to Qi, blood and body fluid. Beijing Zhong Yi Yao 2019; 38: 755-9. [Google Scholar]
- 6. Zhang K, Liu R, Yin G, et al. Differential cytokine secretion of cultured bone marrow stromal cells from patiens with psoriasis and healthy volunteers. Eur J Dermatol 2010; 20, 49-53. [DOI] [PubMed] [Google Scholar]
- 7. Peng SN, Tao YX, Hong T, et al. Astragaloside Ⅳ regulating differentiation of splenic lymphocyte of IgA nephropathy rats via mTORC 1 pathway. Zhong Guo Mian Yi Xue Za Zhi 2021; 37: 421-5. [Google Scholar]
- 8. Zhou X, Liu Rf, Zhang K, et al. Detection of interleukin and vascular endothelial growth factor secreted by psoriatic bone marrow mesenchymal stem cells. Lin Chuang Pi Fu Za Zhi 2014; 5: 22-8. [Google Scholar]
- 9. Hou R, Liu R, Niu X, et al. Biological characteristics and gene expression pattern of bone marrow mesenchymal stem cells in patients with psoriasis. Exp Dermatol 2014; 23: 521-3. [DOI] [PubMed] [Google Scholar]
- 10. Munir H, Ward LSC, Sheriff L, et al. Adipogenic differentiation of MSC alters their immunomodulatory properties in a tissue-specific manner. Stem Cells 2017; 35: 1636-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jia YR, Jia XL, Hou RX, et al. Gene expression and biological characteristics of bone marrow mesenchymal stem cells in patients with psoriasis. Shanxi Yi Yao Za Zhi 2016; 45: 20-3. [Google Scholar]
- 12. Kode JA, Mukherjee S, Joglekar MV, et al. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy 2009; 11: 377-91. [DOI] [PubMed] [Google Scholar]
- 13. English K. Mechanisms of mesenchymal stromal celli-mmunomodulation. Immunol Cell Biol 2013; 91: 19-26. [DOI] [PubMed] [Google Scholar]
- 14. Qu X, Liu X, Cheng K, et al. Mesenchymal stem cells inhibit Th17 cell differentiation by IL-10 secretion. Exp Hematol 2012; 40: 761-70. [DOI] [PubMed] [Google Scholar]
- 15. Huang QL, Wei XF. Plasticity of immune regulation of mesenchymal stem cells. Zhong Guo Ke Xue: Sheng Ming Ke Xue 2016; 46: 79-808. [Google Scholar]
- 16. Waterman RS, Tomchuck SL, Henkle SL, et al. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS One 2010; 5: e10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhong QC. Discussing the pathogenesis and treatment of systemic lupus erythematosus from the connection between Traditional Chinese Medicine and modern immunology. Xin Zhong Yi 2004; 36: 3-6. [Google Scholar]
- 18. Song P, Wang XX, Yang MY, et al. Observation of 120 cases of plaque psoriasis treated by opening Xuanfu and dredging collaterals and detoxifying methods. Zhong Yi Za Zhi 2013; 54: 1476-1479. [Google Scholar]
- 19. Yin XP. Zhou RJ, Zhuang C, et al. Isolating culture of adipose mesenchymal stem cells in psoriasis vulgaris patients and differentiation into immune regulation function. Ji Chu Yi Xue Yu Lin Chuang 2017; 37: 975-80. [Google Scholar]


