Abstract
OBJECTIVE
To investigate pharmacodynamic effects of modified Gexiazhuyu decoction (MGXZYD) and explore the underlying mechanism in the treatment of chronic salpingitis
METHODS
Chronic salpingitis model rats were firstly constructed and the blood was collected to detect the whole blood viscosity and plasma viscosity. Rat oviduct were collected to evaluate the macroscopic damage and the pathological injury and fibrosis of oviduct by hematoxylin-eosin (HE) and Masson staining. Elisa assay was to detect the production interleukin-1 β (IL-1β) in serum and collagen I (COL-1), matrix metalloprotein 9 (MMP-9), tissue inhibitor of metalloproteinases 1 (TIMP-1) in oviduct tissue. And immunohistochemical staining with MMP-9 and TIMP-1 in oviduct tissue were examined. Western blot was used to detect the expressions of p38 mitogen-activated protein kinases (p38MAPK), phospho-p38MPAK (p-p38MPAK), transforming growth factor-β1 (TGF-β1) in oviduct. The expression of α-smooth muscle actin (α-SMA), p-p38MPAK, in oviduct tissue were detected by immunofluorescence method. The mRNA of p-p38MAPK, α -SMA, COL-1, MMP-9, TIMP-1 was measured by reverse transcription-polymerase chain reaction.
RESULTS
Rats administrated with MGXZYD demonstrated decreased the whole blood viscosity and plasma viscosity. MGXZYD obviously improved the tubal wall thickening, swelling and pelvic adhesion. And HE and Masson staining showed MGXZYD improved the pathological injury and fibrosis of oviduct. The results of MTT assay and flow cytometry indicated that MGXZYD could decreased the NIN-3T3 cells viability and improved the apoptosis. Besides, MGXZYD inhibited the protein and / or mRNA of TGF-β1, IL-1β, COL-1, α-SMA, p-p38MAPK expressions and increased the production of MMP-9/TIMP-1.
CONCLUSION
MGXZYD could prevent the progression of chronic salpingitis by inhibited the fibrocyte and inflammation which via inhibited the p38 MAPK signaling pathway.
Keywords: salpingitis, fibrosis, p38 mitogen-activated protein kinases, inflammation, modified Gexiazhuyu decoction
1. INTRODUCTION
Chronic salpingitis had been the usual manifestations of chronic pelvic inflammatory disease, which may damage the structure of the oviduct and even result in fallopian tube cavity conglutination, distal tube obstruction and so on.1 Pathogen infection by genital uplink and spread to tubal and pelvic operation were always the major factors that caused chronic salpingitis occurs and it had an incidence of 0.6% to 11% in healthy fertile women.2,3 According to the research of histopathology, tubal fibrosis was the common pathological characteristics of chronic salpingitis patients manifesting the excessive synthesis and abnormal deposition of collagenic extracellular stroma in and around the fallopian tube wall.4 Fibroblasts was a kind of stromal cells, characterized by proliferation and myofibroblastic activation and the latter was involving with the generation of extracellular matrix (ECM).5 Broad agreement exists that the ECM could be accumulated largely under the bombardment of various profibrotic cytokines, particularly transforming growth factor-β1 (TGF-β1).6 Except the production of ECM, the expression of alpha-smooth muscle actin (α-SMA) also demonstrated the myofibroblastic activation and the conversion degree.7 Besides, evidences showed inflammation the extent of fibrosis had long been established close relationship.8,9 Inflammatory cytokines, increased by external stimulus and bacteria et al, leaded the over proliferation of fibrocyte and activation of matrix-producing cells which produced collagen deposited in tube wall of oviduct.10 Therefore, anti-inflammation therapies also could be the strategic treatment for chronic salpingitis. So far, the primary treatment for chronic salpingitis were mainly including antibiotic therapy, physical therapy, enzyme drug, operative treatment.11 Besides, Traditional Chinese Medicine has unique advantages in the treatment of the disease. However, there was still no best treatment for chronic salpingitis patients and optimal choice needed to be developed urgently.
Mitogen-activated protein kinase (MAPK) was involved with cell formation, proliferation, apoptosis and so on which including p38 MAPK signaling pathway.12 It was generally agreed that p38 MAPK played an important role in the TGF-β1 induced organ fibrosis.13 Jin et al found that TGF-β1 could activated the p38 MAPK signaling pathway and then promote fibroblasts differentiation, proliferation.14 In another way, p38 MAPK signaling pathway was also a critical inflammatory pathway which could accelerate the generation of inflammatory factor to aggravate the chronic salpingitis.15
Gexiazhuyu decoction (膈下逐瘀汤加减方, GXZYD) was a Traditional Chinese Medicine and had potential use in the treatment of chronic disease.16 Deng et al 17 found GXZTD treatment could inhibit the process of ECM deposition and contribute to the resolution of liver fibrosis. However, there was no report about GXZYD in the treatment of chronic salpingitis. In this study, Gexia-zhuyu decoction was modified to obtain the modified Gexiazhuyu decoction (MGXZYD) which was com-posed with Mudanpi (Cortex Moutan Radicis), Xiangfu (Rhizoma Cyperi), Chishao (Radix Paeoniae Rubra), Zhiqiao (Fructus Aurantii Submaturus), Wuyao (Radix Linderae Aggregatae), Chuanxiong (Rhizoma Chuan-xiong), Yanhusuo (Rhizoma Corydalis Yanhusuo), Biejia (Carapax Trionycis), Taoren (Semen Persicae), Dilong (Pheretima Aspergillum). Actually, MGXZYD had been proved the good pharmacological activity either in our previous laboratory data or clinical efficacy in the tre-atment of chronic salpingitis. However, the potential me-chanism of MGXZYD was still needed to be examined.
Therefore, the present study was to evaluate the therapeutic effects of MGXZYD and explore the underlying mechanism in the treatment of chronic salpingitis in vivo and vitro experiments (the vitro experiments are in the supplemental materials).
2. MATERIALS AND METHODS
2.1. Protective effect of MGXZY on chronic salpingitis rat models
Animals and treatment: female Sprague-Dawley rats [(200 ± 10) g] were obtained from Animal Center of Heilongjiang University of Traditional Chinese Medicine. They were raised in Animal Center of Heilongjiang University of Traditional Chinese Medicine and given free way to get food and water with humidity of 40% at 22 ℃ for a 12 h light / dark cycle. All experiments were performed in accordance with the Regulations of Experimental Animal Administration issued by the Ministry of Science and Technology of the People's Republic of China. This experiment was approved by the ethics committee of Heilongjiang University of Traditional Chinese Medicine [SCXK (Hei) 2016-004].
The rats were randomly divided into 5 groups (n = 20): (a): control group; (b) model group; (c): MGXZY-high group (MGXZY-H, 1.50 g/mL); (d) MGXZY-medium group (MGXZY-M, 0.75 g/mL); (e): MGXZY-low group (MGXZY-L, 0.38 g/mL). The all of groups except control group accepted the duplication methods including injection of 2 mL staphylococcus aureus suspension (4 × 109 /mL) at the oviduct-ovarian direction at the first day, subcutaneous injection of norepinephrine at the concentration of 0.08 mL/100 g once a day from the 8th day to 14th d, stimulation of sound and light and electricity for 3 weeks. And at the 29th day during the modeling process, the rats from control group and model group were given distilled water 2 mL/100 g weight, the left given the same volume/weight medicine, respectively, lasted for 4 weeks. At the 57th day, oviduct tissues were collected followed by washing the cold saline and blood plasma were collected by abdominal aortic method, after all rats were sacrificed, stored in - 80℃ bridge.
2.2. Evaluation of rat's oviduct injury
Oviduct were exposure adequately to observe the pathological changes after rats dissected. And the pathological scoring standard was showed in Supplementary Table 1.
Table 1.
Macroscopic observation of oviduct (n)
| Group | n | Swollen veins | Tubal wall thickness | Extent of adhesions to pelvic | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | + | ++ | - | + | ++ | - | + | ++ | |||||||||||||
| Control | 20 | 16 | 3 | 1 | 16 | 2 | 2 | 14 | 4 | 2 | |||||||||||
| Model | 20 | 0 | 6 | 14 | 2 | 6 | 12 | 0 | 4 | 16 | |||||||||||
| MGXY-H | 20 | 8 | 6 | 6 | 12 | 4 | 4 | 10 | 4 | 8 | |||||||||||
| MGXY-M | 20 | 6 | 8 | 4 | 10 | 8 | 2 | 6 | 8 | 6 | |||||||||||
| MGXY-L | 20 | 8 | 6 | 6 | 6 | 6 | 6 | 3 | 8 | 9 | |||||||||||
Notes: control group and model group were given distilled water 2 mL/100 g weight. MGXZY-H, M, L groups given the MGXZY (1.5, 0.75, 0.38 g/mL), respectively, lasted for 4 weeks. MGXZY: modified Gexiazhuyu decoction.
2.3. Histological evaluations and Immunohistochemistry
The tissue of oviduct was fixed in 4% solution for 24 h, dehydrated with 95% ethanol and embedded in paraffin. Embedded sections were cut using the microtome at a thickness of 4 μm, staining with hematoxylin-eosin (HE) and Masson staining. Immunohistochemical staining with matrix metalloprotein 9 (MMP-9) was examined under the light microscope to obtain the mean optical density (MOD).
2.4. Enzyme-linked immunosorbent assay (ELISA)
IL-1β levels in serum and COL-1, MMP-9, TIMP-1 levels in oviduct tissue were detected by ELISA kit according to the protocol provided by the manufacturer.
2.5. Immunofluorescence method
The expression of α-SMA, p38MAPK, p-p38MPAK in oviduct tissue were detected by immunofluorescence method. The oviduct tissue biopsies were incubated with primary antibodies that identified α-SMA, p38MAPK, p-p38MPAK for night at 4 ℃. And then primary antibodies staining was revealed by incubated goat anti-mouse antibody or donkey anti-rabbit (1:200) for 1 h at room temperature away from light. Then they were incubated with DAPI for 15 min, using microscopy (Olympus Corporation, Tokyo, Japan) to photography.
2.6. Western blot
Proteins of oviduct tissue were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a PVDF membrane. The membrane was blocked by 5% skim milk for 2 h and then incubated with anti-body of α-SMA, p38MAPK, p-p38MPAK, TGF-β1 for night at 4 ℃. Next, membrane was incubated with horseradish peroxidase-conjugated secondary antibody after washing with TBST for 3 times. Signal were detected with enhanced chemiluminescence (ECL) detection system by Gel imaging analyzer (Nikon, Tokyo, Japan). And the density of band was analyzed by Image Lab software. All target proteins were normalized against the β-actin.
2.7. Real-time reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from oviduct tissue by TRIzol Regent according to the manufacturer's instructions. Complementary DNA (cDNA) was synthesized and the amplification of p-p38MAPK, α -SMA, COL-1, MMP-9, TIMP-1 were carried our as the following program: 95 ℃, 1 min; 95 ℃, 15 s, 55 ℃, 30 s, 72 ℃ 45 s. And the Primers were showed in Supplementary Table 2. β-acting was used as a reference gene and comparison in expression between groups was made using the 2–ΔΔCt method.
2.8. Statistical analysis
All experiments were performed for 3 time. SPSS (version 22.0, Armonk, NY: IBM Corp.) and GraphPad Prism software (Version 6.0) were used to conduct the statistical analyses, and the results were presented as mean ± standard deviation. One-way analysis of variance test with post hoc Dunnett's multiple comparison test or Tukey honestly significant difference were used for the evaluation of significance between different groups. P < 0.05 was the significant level.
3. RESULTS
3.1. Pathological evaluation of oviduct
As excepted, MGXZY improved the tubal wall thickening, swelling and pelvic adhesion (Table 1). And then HE and Masson staining were used to observe the pathological injury of oviduct. As is showed in Figure 1 A, tubal lumen of model group rats could be seen inflammatory infiltration, exfoliated epithelial cell and irregular arrangement of oviduct tissue. And MGXZY at the high and medium dose could obviously ameliorated the pathologic damage. The results of Masson staining showed that oviduct of model group rats had more collagen deposition compared with control group which indicated the sever fibrosis. And compared with model group, MGXZY group had less blue area and lighten fibrosis (Figure 1B).
Figure 1. Histopathological examination of oviduct tissue (×100) .
A-B: oviduct tissue with hematoxylin-eosin staining (A) and Masson staining (B). Control group and model group were given distilled water 2 mL/100 g weight. MGXZY-H, M, L groups given the MGXZY (1.5, 0.75, 0.38 g/mL), respectively, lasted for 4 weeks. MGXZY: modified Gexiazhuyu decoction.
3.2. MGXZYD suppressed the levels of prion-flammatory cytokine
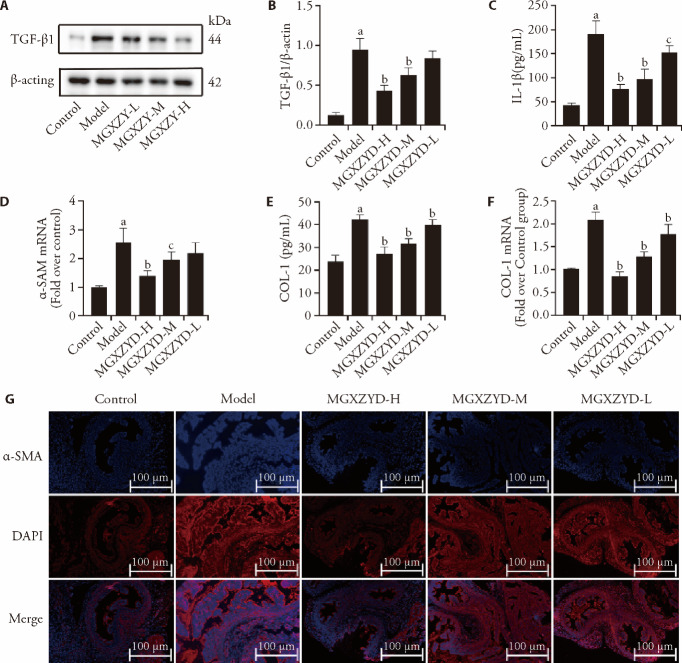
And the expression of TGF-β1 protein were also by WB, which had an identified result described above (P < 0.01, Figure 2A, 2B). Compared with control group, the generation of IL-1βwere obviously increased in model group (P < 0.01). While MGXZY significantly decreased the productions of IL-1βcompared to model group (P < 0.01, < 0.05, Figure 2C). It was found that the expression of α-SMA mRNA was increased in model group compared to control group (P < 0.01). While MGXZYD alleviated the change of α-SMA mRNA (P < 0.01, < 0.05, Figure 2D). As is shown in Figure 2G, α-SMA was retained in oviduct of model group, as evidenced by immunofluorescence staining under fluorescence micro-scope for bright reddish-blue fluorescence. COL-1 pro-duction in oviduct was detected by WB and RT-PCR. The results showed that COL-1 in model group was obviously increased compared with control group (P < 0.01) and MGXYZ decreased the COL-1 level compared to model group (P < 0.01, Figure 2E, 2F).
Figure 2. MGXZYD inhibited the production of TGF-β1, IL-1β, COL-1 and α-SMA.
A, B: expression of TGF-β1 were detected by Western blot (A) and statistical analysis on it (B). C, E: ELISA kit was used to determine the generations of IL-1β and COL-1. D, F: expression of α-SMA, COL-1 mRNA was measured by RT-PCR. G: immunofluorescence was used to measure the expression of α-SMA. Control group and model group were given distilled water 2 mL/100 g weight. MGXZY-H, M, L groups given the MGXZY (1.5, 0.75, 0.38 g/mL), respectively, lasted for 4 weeks. MGXZYD: modified Gexiazhuyu decoction; TGF-β1: transforming growth factor-β1; ELISA: enzyme-linked immunosorbent assay; IL-1β: interleukin-1β; α-SMA: alpha-smooth muscle actin; COL-1: recombinant collagen typeⅠ; RT-PCR: reverse transcription-polymerase chain reaction. Data are expressed as mean ± standard deviation of three independent experiments. aP < 0.01, compared with control group; bP < 0.01, cP < 0.05, compared with model group.
3.3. MGXZYD increased the expression of MMP-9/ TIMP-1
MMP-9 and TIMP played an important in balance of the synthesis and degradation in extracellular matrix. The data showed that decreased MMP-9 level as well as the ratio of MMP-9/TIMP-1 and increased TIMP-1 in the model group, compared with control group (P < 0.05). Nonetheless treated with MGXZYD obviously reduced the production of MMP-9 and increased the production of TIMP-1 (P < 0.05, < 0.01, Figure 3A-3C). And the results of RT-PCR which detected the expression of MMP-9 and TIMP also demonstrated the similar tendency (Figure 3D, 3E). Immunohistochemical staining in the oviduct also showed that MGXZYD up-regulated the expression MMP-9 and down-regulated the expression of TIMP which was induced in model group (Figure 3F).
Figure 3. MGXZYD regulated the expression of MMP-9 and TIPM-1.

A-C: the productions of MMP-9 and TIMP-1 were detected by ELISA Kit, and statistics on ratio of MMP-9/TIPM-1; D, E: expression of MMP-9 and TIMP-1 mRNA by using RT-PCR; F: expression of MMP-9 and TIMP-1 in oviduct tissues was examined by immunohistochemical analysis ( × 400, brown yellow granules indicate positive reaction). Control group and model group were given distilled water 2 mL/100 g weight. MGXZY-H, M, L groups given the MGXZY (1.5, 0.75, 0.38 g/mL), respectively, lasted for 4 weeks. MGXZYD: modified Gexiazhuyu decoction; MMP: matrix metalloproteinase; TIMP: tissue inhibitor of metalloproteinase; ELISA: enzyme-linked immunosorbent assay; RT-PCR: reverse transcription-polymerase chain reaction. Data are expressed as mean ± standard deviation of three independent experiments. aP < 0.01, compared with control group; bP < 0.01, compared with model group.
3.4. Effect of MGXZYD on the expression of p-p38 in chronic salpingitis model rats
MAPK signaling pathway, was of great significance in the cell proliferation, apoptosis, differentiation and so on, which including p38 MPAK signaling pathway. As is shown in Figure 4A, the content of p-p38 and p38 was detected by Western blot. And statistics data indicated that the production of p-p38/p38 was obviously increased in the model group (P < 0.01), but MGXZYD retarded the change of p-p38/p38 (P < 0.05, Figure 4B-4D). Besides, p-p38 mRNA detected by RT-PCR also could be reduced by MGXZYD contrast to model group (Figure 4E). Immunofluorescence image of p-p38 was exhibited in Figure 4F, which indicated model group had brighter reddish-blue than control group and MGXZYD restored the restored the reddish-blue fluorescence.
Figure 4. MGXZYD inhibited the expression of p-p38.
Control group and model group were given distilled water 2 mL / 100 g weight. MGXZY-H, M, L groups given the MGXZY (1.5, 0.75, 0.38 g/mL), respectively, lasted for 4 weeks. A: Western bot was used to detect the expression of p-38 and p-p38; B-D: statistical data on the p38/β-actin, p-p38/β-actin, p-p38/p38; E: p-p38 mRNA was measured by RT-PCR; F: immunohistochemical image of p-p38 measured by immunofluorescence technique. MGXZYD: modified Gexiazhuyu decoction; RT-PCR: reverse transcription-polymerase chain reaction; p38 MAPK: p38 mitogen-activated protein kinase. Data are expressed as mean ± standard deviation of three independent experiments. aP < 0.01, compared with control group; bP < 0.01, cP < 0.05, compared with model group.
4. DISCUSSION
GXZYD, as the Traditional Chinese Medicine decoction remedy had been long used in the fibrosis and chronic.16 And in this study, MGXZYD was composed for the treatment of chronic salpingitis. Chronic salpingitis is a common gynecological disease and had been considered the major cause of female infertility.18 Histopathological study demonstrated that swollen veins, thickening of the oviducal wall and adhesions to pelvic were specific the pathologic manifestation of chronic salpingitis.19 Besides, blood stasis was also the manifestation and fundamental pathogenesis of chronic salpingitis.20 The chronic salpingitis rat model in this study exhibited the obviously severity of pelvic adhesions, uneven thickness and tiff and broken lumen with the observation of macroscopic oviduct. And the HE staining results also showed the exfoliated epithelial cell and irregular arrangement of oviduct tissue in model rats. And whole blood viscosity and plasma viscosity in model rat was obviously increased. According to modern pharmacological research showed that GXZYD could promote blood circulation to lessen pathological damage.21 The results in this study also indicated MGXZYD restore the pathological change of oviduct and improve the blood viscosity.
Oviducal fibrosis was the common pathology for chronic salpingitis patients.22 TGF-β1 played an important role during the process of tissue fibrosis, which could break the balance in the decomposition and synthetize of fibrous protein and then produced ECM to aggravate the oviduct adhesion.23 What's more, the enhanced expression of α-SMA was the sign that fibroblasts were translated to myofibroblasts (MFB).24 MFB had the similarly characteristic with fibrocyte and smooth muscle cell that generate a large amount of interstitial matrix components such as the type I collagens.25 Actually, MMP and TIMPs were the key to keep the normal metabolism of ECM.26 Specifically, MMPs was involved in the degradation of various proteins in the ECM and that was represented by MMP-9 which primarily degraded the denatured collagen.27 And TIMPS, as the nature inhibitor, could form stable complex with MMPs to suppress the self-activation of zymogen during the activation stage of zymogen, but it also could inhibit the activity of MMPs by forming the tight complex during the activation stage of MMPs.28 Therefore, MMPs/TIMPs exhibited the remarkably correction with the develop of chronic salpingitis.29 Our study also demonstrated that MGXZYD obviously inhibit the productions of TGF-β1, COL-1, α-SMA and increased the expression of MMP-9/TIMP-1 to alleviate the oviducal fibrosis.
Evidence is emerging that inflammatory signals and fibrosis had an intrinsic connection.30 Chronic inflammation microenvironment enhanced the activity of TGF-β1 to promote fibrosis in patients with chronic salpingitis.31,32 Especially interleukin-1β (IL-1β) could induce the TGF-β1 activation and myofibroblast accumulation which further promote cell proliferation.33 Numerous studies also showed that administration of IL-1β can stimulate collagen synthesis and proliferation in fibroblasts and drive a fibrotic response.34 And the results in the present study were consistent with the above researches that MGXZYD significantly inhibited the generation of IL-1β and TGF-β1 in chronic salpingitis model rats. Besides, the HE staining also indicated MGXZYD obviously lighted the inflammatory infiltration.
TGF-β activation was involved with cell proliferation, cell-cycle arrest, senescence, or apoptosis which could signaling by the p38MPAK.35 And the role of p38MPAK in inflammation and fibrosis had been investigated in cardiac and peritoneal fibrosis.36 Phosphorylation of p38 leaded its nucleus and the activation of transcription factors involved in the production of proinflammatory mediators and extracellular matrix proteins.37 Moreover, p38 signaling pathway also played an important in the cell apoptsis which could prevent fibrous tissue hyperplasia and scar formation after inflammation by clearing a variety of retained cells in inflammatory lesions.38 In addition, Disel et al 39 found p38 inhibitor successfully the prevented the TGF-β-induced fibrosis. In vitro experiments showed MGXZYD incubated with NIH-3T3 cells, could decreased the cells viability and improved the NIH-3T3 cells apoptosis, the same result as p38 inhibitor SB203580 treated. In the Western blot and immunofluorescence analysis, MGXZYD suppressed the p-p38 protein expression both in vitro and vivo experiments. And after treated with SB203580, NIH-3T3 also had lower expression of p-p38 than TGF-β1 incubation alone. It suggested p38 MAPK signaling pathway also contributed to the inflammation and fibrosis of chronic salpingitis.
In conclusion, this study showed that MGXZYD could decrease the accumulation of ECM (MMP-9/TIMP-1 ↑, COL-1 ↓) to alleviate the oviducal fibrosis (TGF-β1 ↓, α-SMA ↓), and inhibited inflammation response (IL-1β ↓) in the treatment of chronic salpingitis which might mediated via the p38 MAPK signaling pathways.
Contributor Information
Li LIU, Email: Liuliyouxiang2008@163.com.
Yiming SUN, Email: sym19660104@163.com.
REFERENCES
- 1. Dun EC, Nezhat CH. Tubal factor infertility: diagnosis and management in the era of assisted reproductive technology. Obstet Gynecol Clin North Am 2012; 39: 551-66. [DOI] [PubMed] [Google Scholar]
- 2. Barkwill D, Tobler KJ. Salpingitis isthmica nodosa. In: StatPearls. edn. Treasure Island: Statpearls Publishing, 2020: 77-80. [PubMed] [Google Scholar]
- 3. Wiesenfeld HC, Hillier SL, Krohn MA, et al. Lower genital tract infection and endometritis: insight into subclinical pelvic inflammatory disease. Obstet Gynecol 2002; 100: 456-63. [DOI] [PubMed] [Google Scholar]
- 4. Li C, Meng CX, Sun LL, et al. Reduced prevalence of chronic tubal inflammation in tubal pregnancies after levonorgestrel emergency contraception failure. Pharmacoepidemiol Drug Saf 2015; 24: 548-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Avery D, Govindaraju P, Jacob M, Todd L, Monslow J, Pure E. Extracellular matrix directs phenotypic heterogeneity of activated fibroblasts. Matrix Biol 2018; 67: 90-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Humphreys BD, Lin SL, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 2010; 176: 85-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 2011; 7: 684-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vernon MA, Mylonas KJ, Hughes J. Macrophages and renal fibrosis. Semin Nephrol 2010; 30: 302-17. [DOI] [PubMed] [Google Scholar]
- 9. Grande MT, Perez-Barriocanal F, Lopez-Novoa JM. Role of inflammation in tubulo-interstitial damage associated to obstructive nephropathy. J Inflamm (Lond) 2010; 7: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karsdal MA, Nielsen SH, Leeming DJ, et al. The good and the bad collagens of fibrosis - their role in signaling and organ function. Adv Drug Deliv Rev 2017; 121: 43-56. [DOI] [PubMed] [Google Scholar]
- 11. Terzic M, Kocijancic D. Pelvic inflammatory disease: contemporary diagnostic and therapeutic approach. Srp Arh Celok Lek 2010; 138: 658-63. [DOI] [PubMed] [Google Scholar]
- 12. Liu Q, Zhuang Y, Ouyang N, Yu H. Cytochalasin D promotes osteogenic differentiation of MC3T3-E1 cells via p38-MAPK signaling pathway. Curr Mol Med 2019; 20: 79-88. [DOI] [PubMed] [Google Scholar]
- 13. Ji L, Wang T, Tian L, Song H, Gao M. Roxatidine inhibits fibrosis by inhibiting NF kappa B and MAPK signaling in macrophages sensing breast implant surface materials. Mol Med Rep 2020; 21: 161-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jin YK, Li XH, Wang W, et al. Follistatin-Like 1 promotes bleomycin-induced pulmonary fibrosis through the transforming growth factor beta 1/Mitogen-activated protein kinase signaling pathway. Chin Med J (Engl) 2018; 131: 1917-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun H, Ding JM, Zheng HH, et al. The effects of Sidt2 on the inflammatory pathway in mouse mesangial cells. Mediators Inflamm 2020; 2020: 3560793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen JY, Chen HL, Cheng JC, et al. A Chinese herbal medicine, Gexia-Zhuyu Tang (GZT), prevents dimethylnitrosamine-induced liver fibrosis through inhibition of hepatic stellate cells proliferation. J Ethnopharmacol 2012; 142: 811-8. [DOI] [PubMed] [Google Scholar]
- 17. Deng Z, Zhang S, Ge S, Kong F, Cao S, Pan Z. Gexia-Zhuyu decoction attenuates carbon tetrachloride-induced liver fibrosis in mice partly via liver angiogenesis mediated by myeloid cells. Med Sci Monit 2019; 25: 2835-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Z, Zhang Z, Chen X, Zhou J, Xiao XM. Treatment evaluation of Wharton's jelly-derived mesenchymal stem cells using a chronic salpingitis model: an animal experiment. Stem Cell Res Ther 2017; 8: 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bonfante F, Mazzetto E, Zanardello C, et al. A G1-lineage H9N2 virus with oviduct tropism causes chronic pathological changes in the infundibulum and a long-lasting drop in egg production. Vet Res 2018; 49: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma K, Wang KL, Chen YX. Infertility caused by salpingitis treated based on theory of kidney deficiency and blood stasis. Zhong Guo Zhong Yao Za Zhi 2019; 44: 1099-103. [DOI] [PubMed] [Google Scholar]
- 21. Zhao Z, Yu H, Peng Y, et al. Comparison of effect of formulas clearing away heat and promoting blood circulation on prevention and treatment of liver fibrosis in CCl4 mice. Zhong Guo Zhong Yao Za Zhi 2012; 37: 1804-8. [PubMed] [Google Scholar]
- 22. Hafner LM. Pathogenesis of fallopian tube damage caused by Chlamydia trachomatis infections. Contraception 2015,92: 108-15. [DOI] [PubMed] [Google Scholar]
- 23. Zhou F, Shi LB, Zhang SY. Ovarian fibrosis: a phenomenon of concern. Chin Med J (Engl) 2017; 130: 365-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manetti M, Romano E, Rosa I, et al. Endothelial-to-mesenchymal transition contributes to endothelial dysfunction and dermal fibrosis in systemic sclerosis. Ann Rheum Dis 2017; 76: 924-34. [DOI] [PubMed] [Google Scholar]
- 25. Rubis P, Wisniowska-Smialek S, Wypasek E, et al. Fibrosis of extracellular matrix is related to the duration of the disease but is unrelated to the dynamics of collagen metabolism in dilated cardiomyopathy. Inflamm Res 2016; 65: 941-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cui N, Hu M, Khalil RA. Biochemical and biological attributes of Matrix Metalloproteinases. Prog Mol Biol Transl Sci 2017; 147: 1-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mitrut R, Stepan AE, Margaritescu C, et al. Immunoexpression of MMP-8, MMP-9 and TIMP-2 in dilated cardiomyopathy. Rom J Morphol Embryol 2019; 60: 119-24. [PubMed] [Google Scholar]
- 28. Arpino V, Brock M, Gill SE, The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol 2015; 44-6: 247-54. [DOI] [PubMed] [Google Scholar]
- 29. Juica NE, Rodas PI, Solar P, et al. Neisseria gonorrhoeae challenge increases matrix metalloproteinase-8 expression in fallopian tube explants. Front Cell Infect Microbiol 2017; 7: 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappa B is required for inflammation-induced cell migration and invasion. Cancer Cell 2009; 15: 416-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bristol-Gould SK, Hutten CG, Sturgis C, Kilen SM, Mayo KE, Woodruff TK. The development of a mouse model of ovarian endosalpingiosis. Endocrinology 2005; 146: 5228-36. [DOI] [PubMed] [Google Scholar]
- 32. Witkin SS, Toth M, Jeremias J, Ledger WJ. Increased inducibility of inflammatory mediators from peripheral blood mononuclear cells of women with salpingitis. Am J Obstet Gynecol 1991; 165: 719-23. [DOI] [PubMed] [Google Scholar]
- 33. Kim KK, Sheppard D, Chapman HA. TGF-beta1 signaling and tissue fibrosis. Cold Spring Harb Perspect Biol 2018; 10: a022293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kahari VM, Heino J, Vuorio E. Interleukin-1 increases collagen production and mRNA levels in cultured skin fibroblasts. Biochim Biophys Acta 1987; 929: 142-7. [DOI] [PubMed] [Google Scholar]
- 35. Kulasekaran P, Scavone CA, Rogers DS, Arenberg DA, Thannickal VJ, Horowitz JC. Endothelin-1 and transforming growth factor-beta1 independently induce fibroblast resistance to apoptosis via AKT activation. Am J Respir Cell Mol Biol 2009; 41: 484-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lea S, Harbron C, Khan N, Booth G, Armstrong J, Singh D. Corticosteroid insensitive alveolar macrophages from asthma patients; synergistic interaction with a p38 mitogen-activated protein kinase (MAPK) inhibitor. Br J Clin Pharmacol 2015; 79: 756-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang Y, Kim SC, Yu T, et al. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediators Inflamm 2014; 2014: 352371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. ACOG Committee on Practice Bulletins--Gynecology. ACOG practice bulletin No. 51. Chronic pelvic pain. Obstet Gynecol 2004,103: 589-605. [PubMed] [Google Scholar]
- 39. Disel U, Paydas S, Dogan A, Gulfiliz G, Yavuz S. Effect of colchicine on cyclosporine nephrotoxicity, reduction of TGF-beta overexpression, apoptosis, and oxidative damage: an experimental animal study. Transplant Proc 2004; 36: 1372-6. [DOI] [PubMed] [Google Scholar]