Abstract
OBJECTIVE:
To investigate the antitumour efficacy of luteolin on gastric cancer (GC) and study the mechanism underpinning the action.
METHODS:
Effects of luteolin on cell growth inhibition, apoptosis, and cell cycle arrest in MKN45 cells were investigated using the cell counting kit-8 assay. Changes in the mitochondrial membrane potential after luteolin treatment were assessed using 5,5′,6,6’-tetra-chloro-1,1’,3,3’-tetraethylbenzimidazolcarbocyanineiodi-de (JC-1) staining. To investigate whether apoptotic effect by luteolin is related to the phosphoinositide 3-kinase/v-akt murine thymoma viral oncogene (PI3K/Akt) pathway, cells were additionally treated with LY294002, a PI3K/Akt pathway inhibitor. Moreover, the expressions of apoptosis-related proteins, namely B-cell lymphoma 2(Bcl-2), Bcl-2 associated X protein (Bax), Akt, p-Akt, caspase-3, and cytochrome C, were detected after luteolin treatment.
RESULTS:
The study revealed that in MKN45 cells, luteolin could inhibit the cell proliferation in a time- and dose-dependent manner; block the cell cycle in the S-phase; induce apoptosis; reduce the mitochondrial membrane potential; increase the expression of Bax, caspase-3, and cytochrome C; and decrease the expression of Bcl-2 and p-Akt. Luteolin might be involved in the PI3K/Akt signalling pathway, indicating that this pathway could be a therapeutic target for GC treatment.
CONCLUSIONS:
Luteolin could inhibit the proliferation of GC cells and block the cell cycle in the S-phase. The mechanism of inducing apoptosis in these cells was related to the PI3K/Akt signalling pathway.
Keywords: stomach neoplasms, luteolin, apoptosis, phosphoinositide 3-kinase, Akt, signal transduction
1. INTRODUCTION
Gastric cancer (GC) is one of three leading causes of death worldwide and is an important health issue.1 Although the incidence and mortality rates of GC are declining in several countries, it cannot be effectively treated currently because of its complex aetiology.2 Surgery is the optimal treatment approach for GC at present; however, the 5-year survival rate remains low because of relapse following surgery.3 Although advanced chemotherapy can improve the survival rate, studies have shown that many chemotherapeutic drugs have potentially cytotoxic effects on healthy cells and the cancerous cells have high drug resistance.4,5 Therefore, it is necessary to explore new, effective anti-GC drugs.
Luteolin (3′, 4′, 5, 7-tetrahydroxyflavone) is a type of flavonoid. It is a pure yellow crystalline powder (chemical formula: C15H10O6). Recent studies have shown that luteolin has considerable antitumour properties. However, the underlying mechanism of its action remains largely unclear.
Luteolin is reported to have a strong anti-proliferative activity and induce apoptosis in cancerous cells, including those in gastric, prostate, and breast cancer. For example, luteolin could induce apoptosis in GC AGS cells by significantly increasing the levels of pro-apoptotic proteins, including Bax and caspases-3, -6, and -9. Moreover, it could decrease the levels of an anti-apoptotic protein, Bcl-2, by increasing the Bax/Bcl ratio, thereby facilitating apoptosis.6 Luteolin could induce apoptosis in a GC cell line through decreased Bcl-2 levels by upregulating the miR-34a expression.7 A study reported that luteolin acts by regulating proteins related to Notch1, PI3K, Akt, mTOR, ERK, STAT3, and p38 signalling pathways and modulating a series of miRNAs expression to prevent tumour growth in GC cells in vitro and in vivo.8 However, it was impossible to prove the most important antitumour pathway. A study showed that luteolin inhibits the proliferation and induces apoptosis in A375 human melanoma cells by reducing the expressions of MMP-2 and MMP-9 through the PI3K/AKT pathway.1,9 A study demonstrated that luteolin downregulates the activation of the PI3K/Akt and ERK1/2 pathways via reduction in IGF-IR signalling in HT-29 cells, which may be one of the important mechanisms for luteolin-induced cell cycle arrest and apoptosis.10 Therefore, investigating the antitumour effect of luteolin in GC cells and underlying modulated signalling pathways is essential.
In the present study, we aimed to investigate the antitumour activity of luteolin and the underlying mechanism of action.
2. MATERIALS AND METHODS
2.1. Experimental drugs
Luteolin (Tongtian biology, Shanghai, China; molecular formula C15H10O6; molecular weight 286.24); was diluted with DMSO to prepare a stock solution of 20 mmol/L, and the solution was stored at -20 ℃. LY294002 (Monmouth Junction, NJ, USA, MCE), a phosphoinositide 3-kinase/v-akt murine thymoma viral oncogene (PI3K/Akt) pathway inhibitor, was diluted with DMSO to prepare a stock solution of 4.89 mmol/L, and the solution was stored at -20 ℃.
2.2. Cell culture
MKN45 cells were purchased from the Shanghai Institute of Life Science, Chinese Academy of Sciences (Shanghai, China). The cells were grown in RPMI 1640 (HyClone, Waltham, USA) containing 10% foetal bovine serum (HyClone, Waltham, USA), 1% penicillin, and streptomycin (HyClone, Waltham, USA) at 37 ℃ with 5% CO2.
2.3. Cell viability assay
The effects of luteolin on the proliferation and viability of MKN45 cells were assessed using the CCK-8 assay. MKN45 cells were seeded into a 96-well plate (Sterilin, Caerphilly, UK) at a density of 2 × 104 cells per well. After 24 h, the cells were treated with various concentrations of luteolin for 24 h, 48 h, or 72 h. Further, the medium was replaced, and the cells were incubated with 10 μL CCK-8 for 2 h. The absorbance was detected using an instrument at nm. The survival rate of tumour cells was calculated using the following formula:
Cell survival rate = absorbance/control group luminosity value × 100.
2.4. Effect of luteolin on cell cycle
Propidium iodide (PI), a fluorescent dye, was used for flow cytometry-based determination of cell cycle distribution. MKN45 cells were seeded in 6-well plates and incubated for 48 h. Further, the cells were treated with luteolin at 0, 10, 30, and 50 μmol/L for 48 h. The cells were collected, centrifuged, washed twice with cold PBS, and fixed overnight at 4 ℃ with 70% ethanol. Further, the cells were washed once with cold PBS and resuspended in 400 μL of PI solution. The suspension was incubated for 30 min at 37 ℃ in dark. Cell cycle progression was assessed by flow cytometry and analysed using FlowJo v7.6 software (BD, Franklin Lakes, USA).
2.5. Apoptosis assessment and DAPI staining
Apoptosis rates were measured using flow cytometry following annexin V-FITC/PI double staining. MKN45 cells were seeded in 6-well plates at a density of 1 × 105 cells per well and were incubated for 48 h. Further, the cells were treated with various concentrations of luteolin for 48 h. Before harvesting, the cells were washed twice with cold PBS. The cells were resuspended in 195 μL of 1× binding buffer, and 5 μL annexin V-FITC and 5 μL PI were added. The suspension was incubated for 15 min at room temperature in dark. The apoptotic cells were analysed through flow cytometry by using FlowJo v7.6 software.
MKN45 cells were seeded in a 6-well plate at a density of 2 × 105. After 48 h, the cells were incubated with various concentrations of luteolin. The cells were fixed with 4% paraformaldehyde at room temperature for 20 min. The supernatants were discarded, and the cells were washed with PBS. Further, the cells were incubated with DAPI dye solution for 10 min. The cell morphology was observed and photographed under a fluorescence microscope (Leica Microsystems, Wetzlar, Germany). Two multiple holes were observed in each group, and three photographs were captured for each complex hole according to different areas.
2.6. JC-l staining
Mitochondrial membrane potential was determined through flow cytometry. 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-l) staining probe can detect changes in the mitochondrial transmembrane potential in the early stage of apoptosis. When the mitochondrial membrane potential decreases, JC-1 cannot accumulate in the mitochondrial matrix and exists as monomers producing green fluorescence. When the mitochondrial membrane potential is high, JC-1 accumulates in the mitochondrial matrix and exists in a polymeric form producing red fluorescence.
The cells were digested with trypsin (Sigma Aldrich, St. Louis, MO, USA) and harvested after incubation with luteolin for 48 h. Further, 1 mL of JC-1 dye solution (Sigma Aldrich, St. Louis, MO, USA) was added in the test tube, and the suspension was mixed well. The cells were incubated at 37 ℃ for 20 min. The supernatant was removed and washed twice with JC-1 dye buffer (1×; prepared by adding 4 mL distilled water to 1 mL 5× JC-1 dye buffer and further placing in an ice bath). Further, 2 mL RPMI 1640 medium was added, and the samples were subjected to flow cytometry. The experiment was performed in triplicates.
2.7. Western blot
The cells were lysed in lysis buffer (Sigma Aldrich, St. Louis, MO, USA) to extract whole proteins. Using a BCA kit (Beyotime, Shanghai, China) the protein content was determined as per the manufacturer’s protocol. Further, the proteins in the samples were separated using 8%-12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and were transferred to a (polyvinylidene fluoride, PVDF) membrane (Applygen, Beijing, China). Further, the PVDF membrane was blocked with 5% non-fat milk for 1h. The membrane was washed and incubated overnight with the indicated antibodies [anti-Bcl-2 (Abcam, Cambridge, England), anti-Bax (Abcam, Cambridge, England), anti-Akt (Abcam, Cam bridge, England), anti-p-Akt (Thermo, Massachusetts, USA), anti-caspase-3 (Abcam, Cam bridge, England), anti-cytochrome c (Abcam, Cam bridge, England)] at 4 ℃. The membrane was washed thrice (for more than 10 min) with PBST and was incubated with the HRP-conjugated secondary antibodies for 1 h at room temperature. The chemiluminescence was measured using the Ec3410 chemiluminescence imaging system (UVP, USA).
2.8. Statistical analysis
Quantitative data were expressed as the mean ± standard deviation ($\bar{x}\pm s$). SPSS 22.0 software (IBM Corp., Armonk, NY, USA) was used to perform one-way analysis of variance and least significant difference-t for inves-tigating differences among the groups. A P value of < 0.05 was considered statistically significant.
3. RESULTS
3.1. Luteolin inhibited the cell viability of MKN45 cells
The viability of MKN45 cells treated with luteolin was assessed using the CCK-8 assay. Luteolin inhibited the viability of MKN45 cells in both dose- and time-dependent manners. The IC50 of luteolin at 24, 48, and 72 h was found to be 59, 42.57, and 21.45 μmol/L, respectively. As the further cell exp-eriments generally involves 48-h incubation with the drug, the IC50 of luteolin value after 48-h incubation was considered for subsequent experiments. Therefore, we considered cells treated with 10, 30, and 50 μmol/L luteolin as the low, medium, and high concentration groups, respectively, and the incubation time was 48 h.
3.2. Luteolin blocked cell cycle in S-phase
MKN45 cells were treated with luteolin at various concentrations (0, 10, 30, and 50 μmol/L). With increase in the luteolin concentration, the percentage of S-phase cells increased. Compared with the control, the proportion of S-phase cells in GC cells significantly increased after luteolin treatment (P < 0.001), indicating that luteolin can block the GC cell cycle in the S-phase of DNA synthesis (Table 1 and Figure 1).
Table1.
Effect of luteolin on cell cycle of gastric cancer MKN45 ($\bar{x}\pm s$)
| Group | n | G0/G1 | S | G2/M |
|---|---|---|---|---|
| 0 | 3 | 76.12±1.74 | 7.12±0.51 | 14.12±0.07 |
| 10 μmol /L | 3 | 41.12±0.96 | 20.48±1.58a | 16.09±0.66 |
| 30 μmol/L | 3 | 43.55±2.53 | 44.15±2.00a | 12.30±0.03 |
| 50 μmol/L | 3 | 54.44±0.97 | 55.44±0.97a | 4.29±0.48 |
Notes: MKN45 cells were treated with luteolin at various concentrations (0, 10, 30, and 50 μmol/L) and the incubation time was 48 h. Compared with the control, aP < 0.001. G: Gap phase. S: Synthesis phase. M: mitotic.
Figure 1. Effect of luteolin on cell cycle of gastric cancer MKN45.

A: evaluation of cell cycle, using flow cytometry and PI staining, in MKN45 cells treated with varying concentrations of luteolin (0, 10, 30, and 50 μmol/L) for 48 h. B: the ratio of cells per cell cycle phase of MKN45 cells treated with varying concentrations of luteolin (0, 10, 30, and 50 μmol/L). Compared with the control, aP < 0.001. G: Gap phase. S: Synthesis phase. M: mitotic. Con: control.
3.3. Luteolin induced apoptosis in MKN45 cells
The apoptotic effects induced by luteolin in MKN45 cells were evaluated by annexin V-FITC/PI double staining and DAPI staining (Figure 2A, 2B). Hoechst staining results showed that the GC cells in the control were stained uniformly with blue fluorescence after luteolin treatment, and their nuclei were oval in shape. The obvious characteristics of apoptosis, such as nuclear shrinkage, fragmentation, chromatin concentration, and production of apoptotic bodies, were observed in luteolin-treated cells (Figure 3).
Figure 2. Luteolin induces apoptosis of gastric cancer MKN45 cells.

A: Evaluation of cell apoptosis, using flow cytometry and DAPI staining, in MKN45 cells treated with varying concentrations of luteolin (0, 10, 30, and 50 μmol/L) for 48 h. B: The percentage of total apoptotic MKN45 cells for varying concentrations of luteolin (0, 10, 30, and 50 μmol/L). Compared with the control, aP < 0.001. Con: control.
Figure 3. Effect of luteolin on apoptotic morphology of MKN45 gastric cancer cells.
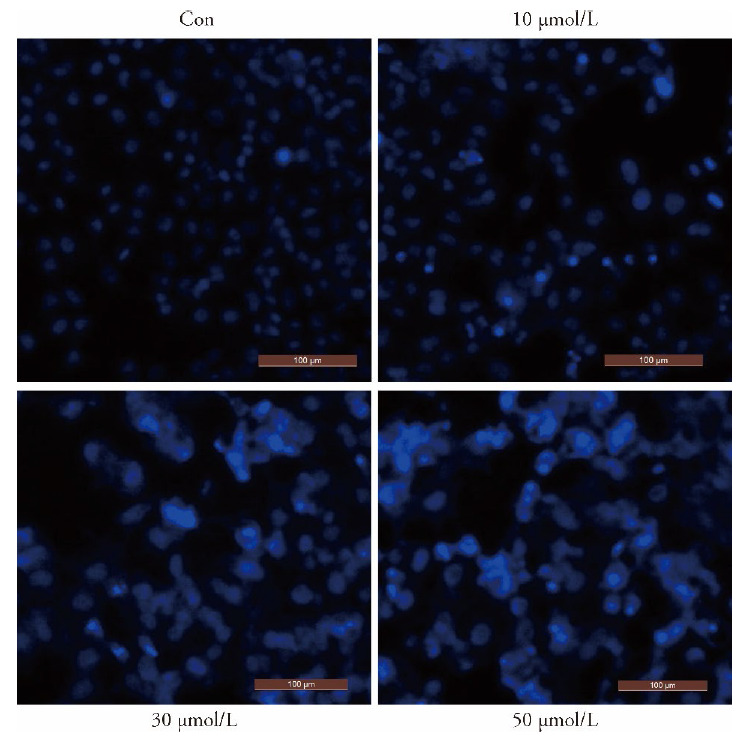
MKN45 cells treated with varying concentrations of luteolin (0, 10, 30, and 50 μmol/L) for 48 h and stained by DAPI, and observed the changes of cell morphology under a fluorescence microscope (× 200).
3.4. Luteolin decreased the mitochondrial membrane potential in MKN45 cells
The proportion of cells with decreased mitochondrial membrane potential significantly increased with an increase in the luteolin concentration compared with that in the control (P < 0.001), which indicated that luteolin could reduce the mitochondrial membrane potential in MKN45 cells and induce apoptosis. The apoptosis of mitochondrial pathway is shown in Figure 4A and 4B.
Figure 4. Effect of luteolin on mitochondrial membrane potential of MKN45 gastric cancer.

A: evaluation of mitochondrial membrane potential, using flow cytometry and JC-l staining, in MKN45 cells treated with varying concentrations of luteolin (0, 10, 30, and 50 μmol/L) for 48 h. B: The percentage of JC-l positive MKN45 cells for varying concentrations of luteolin (0, 10, 30, and 50 μmol/L). Compared with the control, aP < 0.001. Con: control.
3.5. Luteolin induced apoptosis in MKN45 cells through the PI3K/Akt signalling pathway
Luteolin (20 μmol/L), LY294002 inhibitor (20 μmol/L), and luteolin (20 μmol/L) + LY294002 inhibitor (20 μmol/L) were used for this experiment. After 48 h of incubation, the apoptotic cells were assessed using flow cytometry. Compared with the control, the proportion of apoptotic MKN45 cells significantly increased after luteolin treatment (P < 0.001). The cells treated with LY294002 and luteolin + LY294002 increased the proportion of apoptotic MKN45 cells, with the effect of luteolin + LY294002 being the most obvious. Compared with the control, luteolin, LY294002, and luteolin + LY294002 could significantly increase the incidence of apoptosis (P < 0.001; Figure 5A and 5B).
Figure 5. Effect of luteolin on apoptosis of MKN45 cells.

A: Evaluation of cell apoptosis, using flow cytometry and DAPI staining, in MKN45 cells treated with varying concentrations of Luteolin (20 μmol/L), LY294002 inhibitor (20 μmol/L), and luteolin (20 μmol/L) + LY294002 inhibitor (20 μ mol/L) for 48 h. B: The percentage of total apoptotic MKN45 cells for varying concentrations of Luteolin (20 μmol/L), LY294002 inhibitor (20 μmol/L), and luteolin (20 μmol/L) + LY294002 inhibitor (20 μ mol/L). Compared with the control, aP < 0.001. Con: control.
3.6. Luteolin participated in the PI3K/Akt signalling pathway and affected the expression of apoptosis-related proteins
The expression of PI3K/Akt signalling pathway-related and apoptosis-related proteins was determined using western blot. The expression of caspase-3 and cytochrome C significantly increased in luteolin-treated cells (P < 0.05 and 0.001, respectively) and luteolin + LY294002-treated cells (P < 0.01 and 0.001, respectively) compared with that in control. Furthermore, the expression of caspase-3 and cytochrome c in luteolin + LY294002-treated cells significantly increased (P < 0.05 and 0.001, respectively) compared with that in luteolin-treated cells, suggesting that luteolin and LY294002 synergistically induce apoptosis in GC cells (Figure 6 and Table2).
Figure 6. Effect of luteolin on apoptotic protein of MKN45 gastric cancer cells.

1: Con; 2: Luteolin; 3: LY294002; 4: LY294002 + Luteolin. Expression of PI3K/Akt signalling pathway-related and apoptosis-related proteins was determined using western blot, in MKN45 cells treated with varying concentrations of Luteolin (20 μmol/L), LY294002 inhibitor (20 μmol/L), and luteolin (20 μmol/L) + LY294002 inhibitor (20 μmol/L) for 48 h. Con: control. Bcl: B-cell lymphoma. Bax: Bcl-2 associated X protein.
Table 2.
Luteolin quantitative analysis of the detection of apoptotic protein of mkn45 gastric cancer cells by Western blotting ($\bar{x}\pm s$)
| Group | n | Con | Luteolin | LY294002 | LY294002+ Luteolin |
|---|---|---|---|---|---|
| Akt | 3 | 1.023±0.021 | 1.044±0.027 | 1.033±0.012 | 1.013±0.022 |
| p-Akt | 3 | 1.152±0.018 | 1.033±0.031a | 0.932±0.014a | 0.872±0.011ad |
| Bax | 3 | 0.824±0.010 | 1.020±0.013a | 1.031±0.010a | 1.142±0.010ad |
| Bcl-2 | 3 | 1.140±0.009 | 0.980±0.018c | 0.871±0.038c | 0.821±0.027be |
| Caspase3 | 3 | 0.843±0.067 | 1.012±0.08c | 1.060±0.046c | 1.243±0.014be |
| Cycs | 3 | 0.612±0.053 | 1.021±0.002a | 1.043±0.014a | 1.342±0.012ad |
Notes: quantitative Analysis of PI3K/Akt signalling pathway-related and apoptosis-related proteins was determined using western blot, in MKN45 cells treated with varying concentrations of Luteolin (20 μmol/L), LY294002 inhibitor (20 μmol/L), and luteolin (20 μmol/L) + LY294002 inhibitor (20 μ mol/L) for 48 h. Compared with the control, cP < 0.05,bP < 0.01,aP < 0.001; Compared with the luteolin, eP < 0.05,dP < 0.001. Con: control. Bcl: B-cell lymphoma. Bax: Bcl-2 associated X protein.
Compared with the control, the expression of Bax in luteolin- and luteolin + LY294002-treated cells significantly increased (P < 0.001), whereas that of Bcl-2 significantly decreased in luteolin- and luteolin + LY294002-treated cells (P < 0.05).
The expression of Akt in the treated cells did not change significantly compared with that in the control. The expression of p-Akt significantly decreased in luteolin-treated cells (P < 0.001) and luteolin + LY294002-treated cells (P < 0.001) compared with that in the control. The results suggested that luteolin and LY294002 synergistically induce apoptosis in GC cells through the PI3K/Akt pathway.
4. DISCUSSION
GC is a great threat to human health globally that lacks effective therapeutic regimens. Luteolin, a novel dietary flavonoid from plants and foods, has been reported to inhibit tumour growth in various cancers.11,12 Understanding the mechanisms underlying the antitumour activity of luteolin may provide possible clinical applications for the treatment of GC. This study demonstrated that luteolin induces apoptosis by inhibiting the PI3K/Akt signalling pathway.
Cell apoptosis is closely associated with tumourigenicity and tumour development and could be regarded as a useful strategy in antitumour therapy.13 This study demonstrated that luteolin can inhibit cell proliferation in a time- and dose-dependent manner in MKN45 cells. Moreover, luteolin induced apoptosis in MKN45 cells. The Bcl-2 family is a crucial mediator that plays a vital role in cell apoptosis.14 Bax and Bcl‐2 are the major factors that control apoptosis.15 The present study revealed that luteolin induces apoptosis by increasing the expression of Bax and decreasing the expression of Bcl-2. Luteolin could block the cell cycle in MKN45 cells in the S-phase. A previous study reported that luteolin arrests cell cycle in the S-phase in MDA-MB-231 cells.16
Furthermore, we investigated other mechanisms involved in the antitumour activity of luteolin. The PI3K/Akt pathway is related to tumour progression, which involves mammalian cell proliferation, apoptosis, autophagy, differentiation, and survival.17,18 The PI3K signalling pathway may influence apoptosis regulation, which includes the regulation of the Bcl-2 family proteins.19,20 Activated PI3K promotes the activation of Akt, a downstream kinase of PI3K, which can enhance the expression of anti-apoptotic proteins. 21In this study, luteolin dramatically increased the expression of Bax in a dose‐dependent manner and reduced the expression of Bcl-2 in MKN45 cells. These data showed that luteolin can induce apoptosis in MKN45 cells. Luteolin remarkably reduced the expression of p-Akt. Thus, the PI3K/Akt signalling pathway was inactivated by luteolin; these results are consistent with those of previous studies conducted on several cancer cell lines.22,⇓- 24 Furthermore, we used LY294002, an inhibitor of PI3K; luteolin + LY294002 treatment induced an increase in the expression of pro-apoptotic protein Bax and a decrease in the expression of anti-apoptotic proteins Bcl-2 and p-Akt. The expression of Akt remained unchanged in each treated group. We speculated that the apoptosis in GC cells induced by luteolin is related to the PI3K/Akt pathway; however, its detailed mechanism remains to be further explored. In line with the findings of a previous study using BGC-823, 22LY294002 significantly enhanced the apoptotic effect of luteolin and further reduced cell viability, suggesting that luteolin-mediated apoptotic cell death can be achieved by at least blocking the PI3K/Akt signalling pathway. Although our findings confirmed that luteolin regulates the PI3K/Akt signalling pathway, the detailed mechanism should be explored by investigating the activators of related pathways.
5. CONCLUSIONS
This study demonstrated that luteolin exerts antitumour effects by inducing apoptotic cell death associated with cell cycle arrest in the S-phase in MKN45 cells. Moreover, luteolin could reduce the mitochondrial membrane potential of MKN45 cells, which was associated with induced apoptosis with increased expression of Bax and decreased expression of Bcl-2. Furthermore, luteolin inhibited the activity of the PI3K/Akt signalling pathway.
6. ACKNOWLEDGMENTS
We thank Prof. Yang Ming and Chen Qi-long, for their kind and professional assistance in experimental guidance and manuscript writing during our revision.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69: 7-34. [DOI] [PubMed] [Google Scholar]
- 2. Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell 2011; 147: 275-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allemani C, Weir HK, . Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25, 676, 887 patients from 279 population-based registries in 67 countries ( CONCORD-2). Lancet 2015;385: 977-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dai ZJ, Gao J, . Ji ZZ, et al. Matrine induces apoptosis in gastric carcinoma cells via alteration of Fas/FasL and activation of caspase-3. J Ethnopharmacol 2009; 123: 91-6. [DOI] [PubMed] [Google Scholar]
- 5. Davern M, Lysaght J. Cooperation between chemotherapy and immunotherapy in gastroesophageal cancers-ScienceDirect. Cancer Lett 2020; 495: 89-99. [DOI] [PubMed] [Google Scholar]
- 6. Wu B, Zhang Q, Shen W, et al. Anti-proliferative and chemo-sensitizing effects of luteolin on human gastric cancer AGS cell line. Mol Cell Biochem 2008; 313: 125-32. [DOI] [PubMed] [Google Scholar]
- 7. Wu H, Huang M, Liu Y, et al. Luteolin induces apoptosis by up-regulating mir-34a in human gastric cancer cells. Technol Cancer Res Treat 2015; 14: 747-55. [DOI] [PubMed] [Google Scholar]
- 8. Tan J, Tian Y, Cai R, et al. Antiproliferative and proapoptotic effects of a protein component purified from Aspongopus chinensis Dallas on cancer cells in vitro and in vivo. Evid Based Complement Alternat Med 2019; 2019: 8934794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yao X, Jiang W, Yu D, et al. Luteolin inhibits proliferation and induces apoptosis of human melanoma cells in vivo and in vitro by suppressing MMP-2 and MMP-9 through the PI3K/AKT pathway. Food Funct 2019; 10: 703-12. [DOI] [PubMed] [Google Scholar]
- 10. Lim D, Cho H, Kim J, et al. Luteolin decreases IGF-II production and downregulates insulin-like growth factor-I receptor signaling in HT-29 human colon cancer cells. BMC Gastroenterol 2012; 12: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen KC, Hsu WH, Ho JY, et al. Flavonoids luteolin and quercetin inhibit RPS19 and contributes to metastasis of cancer cells through c-Myc reduction. J Food Drug Anal 2018; 26: 1180-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Imran M, Rauf A, Abu-Izneid T, et al. Luteolin, a flavonoid, as an anticancer agent: a review. Biomed Pharmacother 2019; 112: 108612. [DOI] [PubMed] [Google Scholar]
- 13. Kou Y, Tong B, Wu W, et al. Berberine improves chemo-sensitivity to cisplatin by enhancing cell apoptosis and repressing PI3K/AKT/mTOR signaling pathway in gastric cancer. Front Pharmacol 2020; 11: 616251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hassan M, Watari H, AbuAlmaaty A, et al. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int 2014; 2014: 150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. Du L, Fei Z, Song S, et al. Antitumor activity of Lobaplatin against esophageal squamous cell carcinoma through caspase-dependent apoptosis and increasing the Bax/Bcl-2 ratio. Biomed Pharma-cother 2017; 95: 447-52. [DOI] [PubMed] [Google Scholar]
- 16. Huang L, Jin K, Lan H. Luteolin inhibits cell cycle progression and induces apoptosis of breast cancer cells through downregulation of human telomerase reverse transcriptase. Oncol Lett 2019; 17: 3842-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer 2020; 20: 74-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Faes S, Dormond O. PI3K and AKT: unfaithful partners in cancer. Int J Mol Sci 2015; 16: 21138-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Q, Dong H, . Sun W, et al. Apoptosis initiation of β-ionone in SGC-7901 gastric carcinoma cancer cells via a PI3K-AKT pathway . Arch Toxicol 2013; 87: 481-90. [DOI] [PubMed] [Google Scholar]
- 20. Yin J, Ji Z, Hong Y, et al. Sh-MARCH. 8 inhibits tumorigenesis via PI3K pathway in gastric cancer. Cell Physiol Biochem 2018; 49: 306-21. [DOI] [PubMed] [Google Scholar]
- 21. Sathe A, Nawroth R. Targeting the PI3K/AKT/mTOR pathway in bladder cancer. Methods Mol Biol 2018; 1655: 335-50. [DOI] [PubMed] [Google Scholar]
- 22. Lu X, Li Y, Li X, et al. Luteolin induces apoptosis in vitro through suppressing the MAPK and PI3K signaling pathways in gastric cancer. Oncol Lett 2017; 14: 1993-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hasibuan PAZ, Harahap U, Sitorus P, et al. The anticancer activities of Vernonia amygdalina Delile. leaves on 4T1 breast cancer cells through phosphoinositide 3-kinase (PI3K) pathway. Heliyon 2020; 6: e04449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yan W, Ma X, Zhao X, et al. Baicalein induces apoptosis and autophagy of breast cancer cells via inhibiting PI3K/AKT pathway in vivo and vitro. Drug Des Devel Ther 2018; 12: 3961-72. [DOI] [PMC free article] [PubMed] [Google Scholar]


