Abstract
OBJECTIVE:
To evaluate the effects of moxa-burning heat stimulating acupoints Zusanli (ST36) and Shenshu (BL23) on macrophage migration inhibitory factor (MIF) and its related molecules which can provide scientific experimental basis for the clinical application of moxibustion treatment of rheumatoid arthritis (RA).
METHODS:
Thirty rabbits were randomly assigned to control group, RA model (established by injecting Freund’s Complete Adjuvant) group (RA group) and RA model with moxibustion group [Moxa group, Zusanli (ST36) and Shenshu (BL23), 5 moxa pillars/day, 6 d × 3]. The expressions of MIF mRNA were evaluated with reverse transcription polymerase chain reaction; the apoptosis rates of macrophages were detected by erminal deoxynucleotidyl transferase-mediated dTUP nick end labeling; the expressions of related signal molecules were detected with immunohistochemical S-P method and the levels of IL-2 were detected with enzyme-linked immunosorbent assay.
RESULTS:
The expressions of MIF mRNA, extracellular regulated protein kinases 2, p38 mitogen-activated protein kinase and nuclear factor-κ-gene binding p65 in synovial tissue of RA group were significantly increased when compared with control group, which were lower remarkably in moxa group than those in RA group. The apoptosis rates of macrophages in RA group were significantly down-regulated as compared with the control group, which were up-regulated in moxa group compared with the RA group. The levels of IL-2 in synovial fluid from the RA group were elevated significantly as compared with that from control group, but those of the moxa group were reduced when compared with those from RA group.
CONCLUSIONS:
Moxibustion may simultaneously regulate the expressions of MIF and its related signaling pathways molecules, the apoptosis rate of macrophages in synovial tissue, as well as the level of inflammatory factors in synovial fluid. The results suggest that the anti-inflammatory effect of moxibustion on RA may be related to inhibit the expression of MIF in synovial tissue, the molecules of some related signaling pathways and promote the apoptosis of macrophage.
Keywords: rheumatoid arthritis, moxibustion, macrophage migration-inhibitory factors, macrophages
1. INTRODUCTION
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by chronic inflammation, leading to the hyperplasia of synovium, pannus formation, the destruction of cartilage and bone, disability, and decreasing the quality of life with a worldwide prevalence of about 5 per 1000 adults.1,2 Macrophage migration inhibitory factor (MIF), a multipotent cytokine secreted by activated T lymphocytes and macrophages, is an important regulator of the innate and adaptive immune responses.3 MIF is a key cytokine of immuno-inflammatory in rheumatoid arthritis.4 MIF regulates the immunue inflammatory response by inducing pro-inflammatory cytokines and tissue-degrading molecules, promoting the proliferation and survival of synovial fibroblasts, stimulating neutrophil chemotaxis, and regulating angiogenesis and osteoclast differentiation in RA.5 MIF triggers signal pathways via mainly binding and activating the receptors CD74/CD44. Upon receptor binding, several downstream signaling pathways are shown to be activated in vivo, including nuclear factor-κ-gene binding (NF-κB), extracellular regulated protein kinases (ERK) 1/2, mitogen-activated protein kinase (MAPK) and so on.6,7 The expression of MIF in synovial tissue and synovial fluid were elevated in RA patients. The polymorphisms and serum levels of MIF play an important role in disease activity of RA.8,9
Moxibustion, a kind of Traditional Chinese Medicine therapy, mainly uses fire to ignite moxa herb and fumigates acupoints to treat diseases. It has been used to treat RA with a long history in China and has achieved good curative effect.10,⇓-12 The previous studies on the mechanism of Moxibustion in the treatment of RA have affirmed that moxibustion increases the secretion of glucocorticoid, promotes the secretion of anti-inflammatory cytokines and reduces the secretion of pro-inflammatory cytokines which relate to immunue inflammatory responses of RA.13,⇓-15
However, the question whether moxibustion regulates the expression of MIF and its related signal molecules and the apoptosis of macrophage or not remains unknown. Therefore, the aim of this study was to evaluate the effects of moxibustion at acupoints Zusanli (ST36) and Shenshu (BL23) on MIF and its related molecules in RA rabbits, which can provide scientific experimental basis for the clinical application of moxibustion for the treatment of RA.
2. MATERIALS AND METHODS
2.1. Animals
Thirty Japanese white rabbits with the same number of male and female, with weight of (2.50 ± 0.25) kg were included in the study which were purchased from Chengdu Dashuo Laboratory Animal Co., Ltd. (Certificate No. SCXK 2015-030). All animals were dwelled in a pathogen-free environment and allowed free access to food and water in the controlled room with 12 h light-dark cycles. All animal procedures were with the approval of the Institution Ethics Committee of Chengdu University of Chinese Traditional Medicine (CDUTCM 2018-10).
2.2. Animal grouping and models establishment
After adaptive domestication for one week prior to the experiment, 30 rabbits were randomly divided into three groups with 10 rabbits in each group: control group, RA group and RA with moxibustion group (Moxa group). RA rabbit model was established by injecting Freund’s Complete Adjuvant (FCA; Sigma-Aldrich, St. Louis, MO, USA) into the bilateral posterior knee joints as the previous study.16 The skin around the bilateral posterior knee joints of the rabbits in RA group and Moxa group was shaved and sterilized with 10% isopropanol in the slightly flexion position of knee joint, FCA of 0.5 mL/kg was slowly injected into the bilateral posterior knee joint cavities of rabbits along the lower edge of patella and lateral depression of patellar ligament. The rabbits in the control group were injected with the same volume sterile normal saline in the same way. After one week, RA models emerged the symptoms of arthritis such as redness, swelling and restricted movement of the knee while the control group did not.
2.3. Moxibustion treatment
Moxibustion treatment was performed one week after FCA injection as described previously.16 Acupoints localization was refered to the animal acupoints atlas in experimental acupuncture and moxibustion.17 Acupoint Zusanli (ST36) is located at 0.3cm outside the lower part of tibial tuberosity of dorsolateral hind limbs. Acupoint Shenshu (BL23) is located at inferior spinous process of the second lumbar spine adjacent to open 1.5 cm. The rabbits of Moxa group were fixed to the experimental table and fur on the skin was shaved to expose the acupoints. Moxa pillar (Ф 6.8 × 26 mm, Nanyang Dongsheng Moxibustion Technology Development Co., Ltd., China) was put on the acupoint and ignited. When one moxa pillar was burnt to about two-thirds, it would be withdrawn immediately and replaced by the experimenter with a new one to avoid the burn. Each acupoint was treated with 5 moxa pillars once a day. Six days was a course of treatment. There was a 1-day rest between the treatments for 3 courses. Other two groups were fixed with the same method for the same time without moxibustion.
2.4. Synovia l fluid extraction
After 3 courses of treatment, all animals were sacrificed and their synovial fluid and synovial tissue were extracted. Rabbits were anesthetized with 25% urethane (4 mL/kg; Shanghai-Rui Biological Technology Co., Ltd., Shanghai, China) and sacrifced by air injection (10 mL) into ear marginal vein.13 The extraction of synovial fluid was performed according to the previous study.11 0.3 mL sterile normal saline was injected into bilateral knee joint cavities at first, then 0.3 mL synovial fluid in joint cavity was aspirated under aseptic condition after local kneading. Finally, synovial fluid was put into Eppendorf (EP) tube which was stored at - 20 ℃ for detection. The level of IL-2 in synovial fluid was detected with enzyme-linked immunosorbent assay (ELISA).
2.5. Synovial tissue extraction
Synovial tissue was collected after the synovial fluid was extracted. The skin was cut longitudinally along the median line of the bilateral posterior knee joints until the exposed area with the knee joint as the center was about 3 × 3 cm2. The patella was lifted with tooth forceps, and cut down to the femur along the upper edge of patella about 0.3-0.4 cm and then separated down to the tibia along both sides of the patella. Naked eyes could see a layer of light yellow synovial tissue extending upward from the lower edge of the patella. The free end of the synovial tissue was gently clipped with ophthalmic forceps and cut off completely with a blade. The synovial tissue was stored at - 70 ℃ for detection. The expression of MIFmRNA in synovial tissue was measured by reverse transcription polymerase chain reaction, the apoptosis rate of macrophages in synovial tissue was detected by terminal deoxynucleotidyl transferase-mediated dTUP nick end labeling (TUNEL)-peroxidase(POD) and the expression level of ERK2, p38 MAPK and NF-κB p65 in synovial tissue were detected with immunohistochemical S-P method.
2.6. Real-Time Quantitative PCR
Total RNA from synovial tissue was extracted using the RNA extraction kit (15596-026, Invitrogen company, Carlsbad, CA, USA) according to the manufacturer’s instructions. cDNA synthesis from RNA reverse transcription was performed with a Primescript RT reagent kit (bk501, baobio Engineering Co. Ltd., Dalian, China). Real-time quantitative PCR for MIF was performed with a SYBR Premix Ex TaqⅡKit (BK402, baobio Engineering Co. Ltd., Dalian, China) on a Sequence Detection software (Applied Biosystems, Foster City, CA, USA). The amplification reaction conditions were as follows: pre-denaturation at 95 °C for 30 s repeated for 40 cycles, denaturation at 95 °C for 5 s, annealing at 55 °C for 30 s, fully extended and collecting fluorescence at 72 °C for 30 s. Relative mRNA expression was calculated by the comparative CT (2-ΔΔCt) method. National Center for Biotechnology Information was used as the reference gene. The primer sequences were listed as follows: β-action forward, 5′-GAAGATCAAGATCATTGCTCCT-3′ and reverse, 5′-TACTC CTGCTTGCTGA TCCA-3′; MIF forward, 5′-CCACCATGCCGATGTTC GT-3′ and reverse, 5′-GCTTGCTGTAGGTGCGGTTCT-3′. Relative mRNA expression was calculated by the comparative CT (2-ΔΔct) method.
2.7. TUNEL-POD
Apoptosis rate of macrophages in synovial tissue was detected by the TUNEL kit (10279600, Roche Group, Basel, Switzerland) according to the manufacturer’s instructions.
2.8. Immunohistochemical (S-P) assay
Immunohistochemistry (S-P) analysis was performed to detect the expression of ERK2, p38MAPK and NF-κBp65 in synovial tissues. The following reagents were used in this assay: NF-κBp65 rabbit polyclonal antibody (1:500, bs-3485R, Bioss Biotechnology Co. Ltd., Beijing, China), ERK1/2 rabbit polyclonal antibody (1:100, bs-0022R, Bioss Biotechnology Co., Ltd., Beijing, China), p38MAPK rabbit polyclonal antibody (1:200, bs-0636R, Bioss Biotechnology Co., Ltd., Beijing, China), biotinylated Goat anti rabbit IgG (H+L) SP-9001 (13152a11, Zhongshan Jinqiao biological Co., Ltd., Beijing, China), Horseradish enzyme labeled streptomycin ovalbumin (HRP/A-V) (13152a11, Zhongshan Jinqiao biological Co., Ltd., Beijing, China), normal goat serum for blocking (13152a11,Zhongshan Jinqiao biological Co., Ltd., Beijing, China), concentrated DAB kit of color reagent (k135925c, Beijing Zhongshan Jinqiao biology Co., Ltd., Beijing, China). The BA200 digital digital micro camera system (MAC Audi Industrial Group Co., Ltd., Xiamen, China) was used to collect the images of the slices. The Image Pro plus 6.0 analysis system produced (Media Cybernetics Company, Rockville, MD, USA) was used to measure the average optical density of all the images collected.
2.9. ELISA
The levels of IL-2 in synovial fluid were measured by the ELISA double antibodies sandwich method (Rabbit IL-2 ELISA kit, dre95094, Abcam company, Cambridge, UK). The test process was performed according to the manufacturer’s instructions.
2.10. Statistical Analysis
All statistical analyses were performed by IBM SPSS Statistics for Windows, Version 22.0. (IBM Corp., Armonk, NY, USA) statistical software. The data were expressed as mean ± standard deviation. Difference between groups was evaluated by one-way analysis of variance test after the homogeneity and normal test of variance. Least significant difference was used if the variance was homogeneous, while tamhane's T2 test was used if the variance was not uniform. P < 0.05 was considered to be statistically significant.
3. RESULTS
3.1. Effect of moxibustion on macrophage migration inhibitory factor mRNA expression in RA synovium tissue
The results indicated that the expression of macrophage migration inhibitory factor (MIF) mRNA in synovial tissue of RA group significantly increased compared with control group (P < 0.01). After moxibustion therapy the expression of MIF mRNA in moxa group was lower than that of RA group (P < 0.01). The results showed that moxibustion significantly reduced the expression of MIF mRNA in synovial tissue of RA rabbits. Results statistics of macrophage migration inhibitory factor mRNA in synovial tissue are in Figure S1.
3.2. Effect of moxibustion on Apoptosis rate of macrophages in RA synovium tissue
Induction of macrophage apoptosis has a good anti-inflammatory effect on RA, so macrophage apoptosis is an effective way to limit the harmful effects of macrophages in RA.18,19 In this study, the results of apoptosis rate of macrophages was showed in Figure 1. Compared with control group, the apoptosis rate of macrophages in the synovial tissue of RA group was significantly decreased (P < 0.01). Compared with RA group, the apoptosis rate of macrophages in moxa group was significantly increased (P < 0.01). It suggested that moxibustion significantly promoted the apoptosis of macrophages in synovial tissue of RA models.
Figure 1. Apoptosis rate of macrophages in control, RA and Moxibustion group .
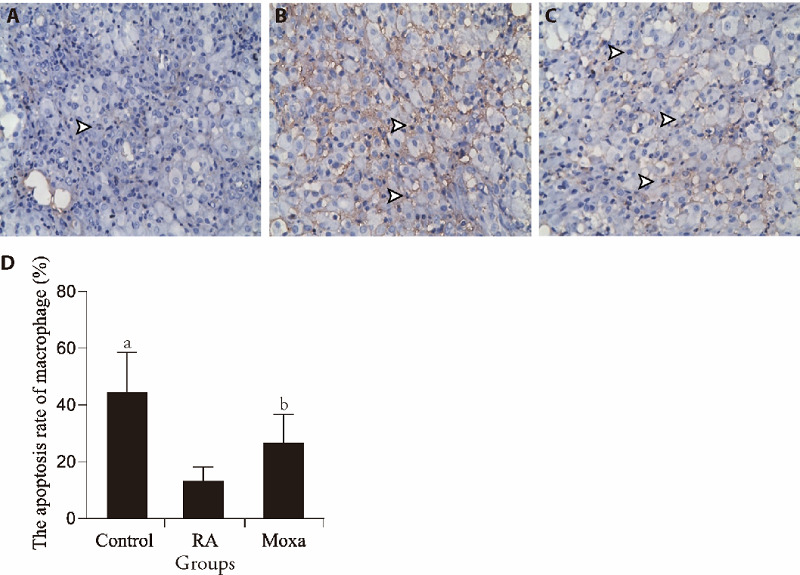
A-C: terminal deoxynucleotide transferase-mediated dUTP nick end labeling staining (× 400). A to C representative images show the apoptosis of macrophages in control (A), RA (B) and moxibustion (C) group. Black arrow indicates the apoptosis of macrophages. D showed the statistical analysis of the apoptosis of macrophages. n =10 for each group. RA group and Moxa group were established by injecting FCA (0.5 mL/kg) into the bilateral posterior knee joints. Control group was injected with the same volume sterile normal saline in the same way. Moxa group was treated by moxa-burning heat on Zusanli (ST36) and Shenshu (BL23) once 1 d for 3 courses after FCA injection. Other two groups were fixed with the same method for the same time without moxibustion. Data were expressed as the mean ± standard deviation. Compared with the Control group, aP < 0.01; compared with the RA group, bP < 0.05. RA: rheumatoid arthritis; FCA: Freund’s complete adjuvant.
3.3. Effect of moxibustion on the levels of IL-2 in RA synovium fluid
IL-2 is a positive regulator of T cell proliferation and the administration of IL-2 can mediate a pro-inflammatory effect on CIA mice, increasing the animals’ arthritic scores.20,21 It is present in the RA synovial membrane, where it drives release of IFN-γ, IL-17, TNF-α and various chemokines.22 The results showed that the level of IL-2 in synovial fluid from the RA group elevated significantly as compared with that from control group (P < 0.01); the level of IL-2 from the moxa group was reduced when compared with that from RA group (P < 0.05). Results statistics of the levels of IL-2 in RA synovium fluid are in Figure S2.
3.4. Effect of moxibustion on the expression of ERK2 in RA synovium tissue
ERK2, p38MAPK and NF-κBp65 are the important molecules in the signaling pathway of MIF cascade reaction. The expression of those in synovial tissues were detected by Immunohistochemistry analysis.7,23 Figure 2 showed that the expressions of ERK2 in synovial tissue of RA models significantly increased in comparison with the control group (P < 0.01). The expressions of ERK2 in moxa group was lower remarkably than that in RA group (P < 0.05).
Figure 2. Immunohistochemical assessment the expression of ERK2 in control, RA, and moxibustion group.
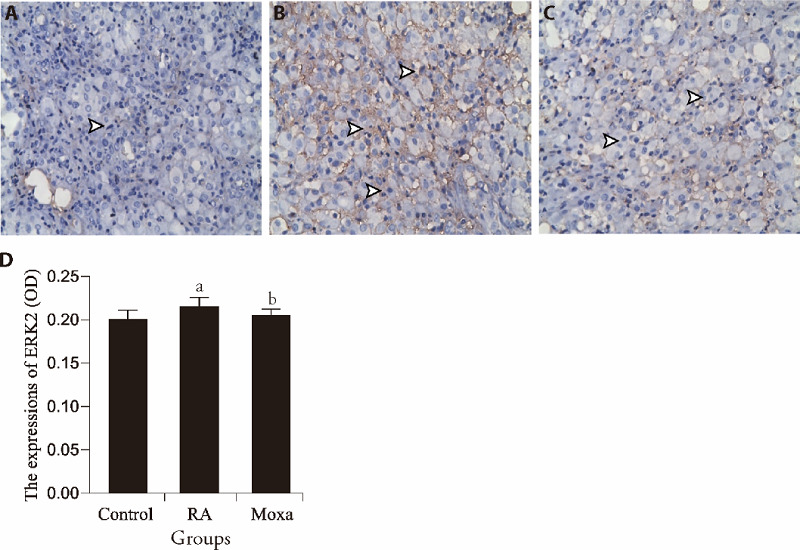
A-C: immunohistochemistry (S-P) analysis (×400). Representative images show the expression of ERK2 in synovial tissues of control (A), RA (B) and moxibustion (C) group. Black arrow indicates the position expression of ERK2. D represented the statistical analysis of the expression of ERK2. n =10 for each group. RA group and Moxa group were established by injecting FCA (0.5 mL/kg) into the bilateral posterior knee joints. Control group was injected with the same volume sterile normal saline in the same way. Moxa group was treated by moxa-burning heat on Zusanli (ST36) and Shenshu (BL23) once 1day for 3 courses after FCA injection. Other two groups were fixed with the same method for the same time without moxibustion. Data were expressed as the mean ± standard deviation. Compared with the Control group, aP < 0.01; compared with the RA group, bP < 0.05. ERK: extracellular regulated protein kinases; RA: rheumatoid arthritis; FCA: Freund’s complete adjuvant.
3.5. Effect of moxibustion on the expression of p38MAPK in RA synovium tissue
The results showed that the expression of p38MAPK in synovial tissue of RA models was significantly increased in the control group (P < 0.01). After the moxibustion treatment, the expression of p38MAPK was lower compared with the RA group (P < 0.05) (Figure 3).
Figure 3. Immunohistochemical assessment the expression of p38MAPK in control, RA and Moxa group.
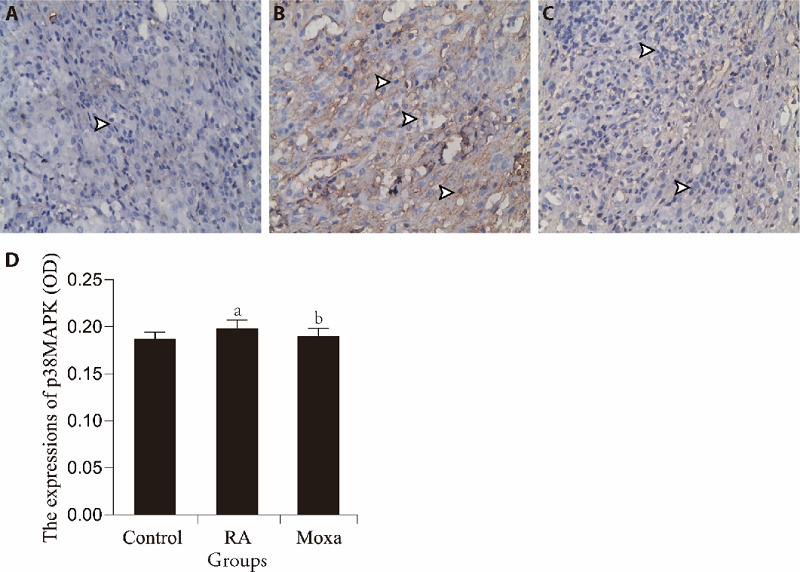
A-C: immunohistochemistry (S-P) analysis (× 400). A to C representative images show the expression of p38MAPK in synovial tissues of control (A), RA (B) and moxibustion (C) group. D represented the statistical analysis of the expression of p38MAPK. n =10 for each group. RA group and moxa group were established by injecting FCA (0.5 mL/kg) into the bilateral posterior knee joints. Control group was injected with the same volume sterile normal saline in the same way. Moxa group was treated by moxa-burning heat on Zusanli (ST36) and Shenshu (BL23) once 1 d for 3 courses after FCA injection. Other two groups were fixed with the same method for the same time without moxibustion. Data were expressed as the mean ± standard deviation. Compared with the control group, aP < 0.01; compared with RA group, bP < 0.05. MAPK: mitogen-activated protein kinase; RA: rheumatoid arthritis; FCA: Freund’s complete adjuvant.
3.6. Effect of moxibustion on the expression of NF-κBp65 in RA synovium tissue
The results showed that the expression of NF-κBp65 in synovial tissue of RA models was significantly increased in the control group (P < 0.05). However, the expression of NF-κBp65 was down-regulated compared with the RA group (P < 0.05) (Figure 4).
Figure 4. Immunohistochemical assessment the expression of NF-κBp65 in control, RA, and moxibustion group.
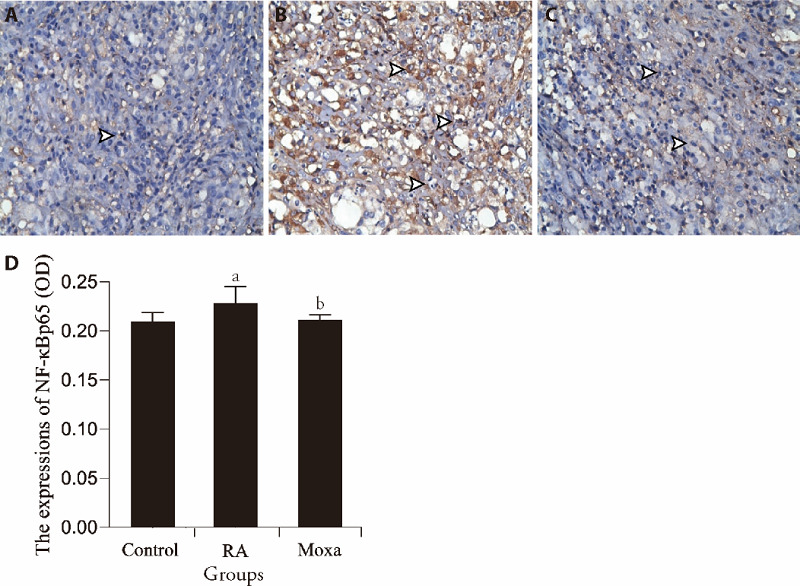
A-C: Immunohistochemistry (S-P) analysis (× 400). A to C representative images show the expression of NF-κBp65 in synovial tissues of control (A), RA (B) and moxibustion (C) group. Black arrow indicates the position expression of NF-κBp65. D represented the statistical analysis of the expression of NF-κBp65. n =10 for each group. RA group and Moxa group were established by injecting FCA (0.5 mL/kg) into the bilateral posterior knee joints. Control group was injected with the same volume sterile normal saline in the same way. Moxa group was treated by moxa-burning heat on Zusanli (ST36) and Shenshu (BL23) once 1day for 3 courses after FCA injection. Other two groups were fixed with the same method for the same time without moxibustion. Data were expressed as the mean ± standard deviation. Compared with the Control group, aP < 0.05; compared with the RA group, bP < 0.05. NF-κB: nuclear factor-κ-gene binding; RA: rheumatoid arthritis; FCA: Freund’s complete adjuvant.
3.7. Effect of moxibustion on the Pathological changes of joint
In the control group, the synovial tissue was smooth and arranged regularly without inflammatory cell infiltration. In the RA group, joint space was narrowed, synovial tissue was congested and edematous, and a large number of inflammatory cells could be seen. The degree of joint space narrowing and inflammatory cell infiltration in moxibustion group was less than that of the RA group. Figures of the pathological changes of joint are in Figure S3.
4. DISCUSSION
Moxibustion is a kind of Traditional Chinese Medicine therapy, which has the functions of warming the meridians, promoting blood circulation and dispersing stasis, relieving inflammation and pain, improving immunity, and so on.24,⇓,⇓-27 Therefore, moxibustion is widely used in the treatment of diseases. The effect of moxibustion on rheumatoid arthritis is accurate,28 but the mechanism of moxibustion has not been fully elucidated. It has been found that moxibustion treatment of rheumatoid arthritis is a multi-pathway and multi-target effect through neuroendocrine immune network.29,⇓-31 Previous studies revealed that moxibustion regulated NF-κB, JAK-STAT and MAPK signaling pathways moleculars expression and inflammatory factor levels in rheumatoid arthritis.32,⇓,⇓-35 ERK2, p38MAPK and NF-κBp65 are important signal molecules involved in the above signal pathways.
MIF is a pleiotropic inflammatory cytokine with upstream immunoregulatory effects that is induced as part of the innate and adaptive immune responses. MIF is constitutively expressed among a broad distribution of cell types, and in the setting of an appropriate stimulus is released from intracellular pools as well as synthesized de novo. MIF signals through binding with the CD74/CD44 receptor complex to activate such as NF-κB, MAPK, ERK, PI3K-AKT and other transcriptional pathways so as to stimulate synthesis of downstream inflammatory cytokines, promote cell proliferation, and arrest apoptotic pathways.6,7,16,36 Therefore, MIF may play an important role in regulating signaling pathway molecules, macrophage apoptosis and inflammatory factors.
It was hypothesized that moxibustion might modulate the immunity via affecting MIF, signaling pathway molecules and macrophage apoptosis of RA animals. To prove this hypothesis,MIF was used as a breakthrough point, RA model was applied and moxibustion was treated at acupoints Zusanli (ST36) and Shenshu (BL23). ST36 is located near the knee joint, which can treat the diseases of knee and leg according to acupuncture theory. It is also the He points and Xiahe point of stomach meridian, so it can regulate gastrointestinal function. In addition, it has the function of systemic regulation, which can improve the disease resistance. Shenshu (BL23) is located in the waist which is the Back Shu point of kidney. On the basis of acupuncture theory,it can treat kidney-related diseases, enhance physical fitness and improve the disease resistance. According to ancient and modern clinical literature records, these two acupoints are the most commonly used acupoints for the treatment of RA. In the experimental study of acupuncture and moxibustion treatment of RA, these two acupoints were also the most commonly used,16,37,⇓ -39 so the acupoints of moxibustion in this study were Zusanli (ST36) and Shenshu (BL23).
In conclusion, this study results showed that moxibustion reduced the high expression of MIF mRNA, promoted the apoptosis of macrophages and decreased the expressions of ERK2, p38MAPK and NF-κBp65 in synovium tissue, reduced the content of IL-2 in synovial fluid. The results of p38MAPK, NF-κBp65 and IL-2 consistented with previous literatures.40,⇓-42 This study found that moxibustion simultaneously regulated the MIF expression of synovial tissue, the apoptosis rate of macrophages and the related molecules of several signaling pathways, as well as regulated the level of inflammatory factors in synovial fluid. There may be a close relationship between them. MIF may play a key role in several signaling pathways in moxibustion regulating RA inflammatory response. However, further research is required for verification.
Contributor Information
Min JIA, Email: 401877133@qq.com.
Xin YANG, Email: yangxin@cdutcm.edu.cn.
REFERENCES
- [1]. Sparks JA.. Rheumatoid arthritis. Ann Intern Med 2019; 170: ITC1-16. [DOI] [PubMed] [Google Scholar]
- [2]. Aletaha D, Smolen JS.. Diagnosis and management of rheumatoid arthritis: a review. JAMA 2018; 320: 1360-72. [DOI] [PubMed] [Google Scholar]
- [3]. De R, Sarkar S, Mazumder S, et al. Macrophage migration inhibitory factor regulates mitochondrial dynamics and cell growth of human cancer cell lines through CD74-NF-kappaB signaling. J Biol Chem 2018; 293: 19740-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Morand EF, Leech M, Bernhagen J.. MIF: a new cytokine link between rheumatoid arthritis and atherosclerosis. Nat Rev Drug Discov 2006; 5: 399-410. [DOI] [PubMed] [Google Scholar]
- [5]. Kim KW, Kim HR.. Macrophage migration inhibitory factor: a potential therapeutic target for rheumatoid arthritis. Korean J Intern Med 2016; 31: 634-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Bilsborrow JB, Doherty E, Tilstam PV, Bucala R.. Macrophage migration inhibitory factor (MIF) as a therapeutic target for rheumatoid arthritis and systemic lupus erythematosus. Expert Opin Ther Targets 2019; 23: 733-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Jankauskas SS, Wong DWL, Bucala R, Djudjaj S, Boor P. . Evolving complexity of MIF signaling. Cell Signal 2019; 57: 76-88. [DOI] [PubMed] [Google Scholar]
- [8]. Santoscoy-Ascencio G, Banos-Hernandez CJ, Navarro-Zarza JE, et al. Macrophage migration inhibitory factor promoter polymorphisms are associated with disease activity in rheumatoid arthritis patients from Southern Mexico. Mol Genet Genomic Med 2020; 8: e1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Gong Y, Yu Z, Wang Y, et al. Effect of moxibustion on HIF-1alpha and VEGF levels in patients with rheumatoid arthritis. Pain Res Manag 2019; 2019: 4705247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Sun ZL, Xu X, Du SZ, Jiang X.. Moxibustion for treating rheumatoid arthritis: a systematic review and meta-analysis of randomized controlled trials. Eur J Integr Med 2014; 6: 621-30. [Google Scholar]
- [11]. Seca S, Miranda D, Cardoso D, et al. Effectiveness of acupuncture on pain, physical function and health-related quality of life in patients with rheumatoid arthritis: a systematic review of quantitative evidence. Chin J Integr Med 2019; 25: 704-9. [DOI] [PubMed] [Google Scholar]
- [12]. Zhou DP, Jiang X, Ji W, Xu X, Sun ZL.. bibliometric analysis on moxibustion for the treatment of rheumatoid arthritis. Beijing Zhong Yi Yao 2015; 34: 434-7. [Google Scholar]
- [13]. Zhu TT, Zhao ZT, Zhao YK, Yan XK.. Review on modern repair mechanism of moxibustion for treating inflammatory damage of rheumatoid arthritis. Zhen Ci Yan Jiu 2017; 42: 271-4. [PubMed] [Google Scholar]
- [14]. Gao XH, Liu XG, Jin S, et al. Effect of moxibustion therapy on the balance of Th17/Treg in rabbits with rheumatoid arthritis. Journal of Basic Chinese Medicine 2019; 25: 1404-06,19. [Google Scholar]
- [15]. Ma WB, Liu XG, Zhou HY.. Effects of chronological moxibustion on circadian rhythm activities of hypothalamus-pituitary-axis in rheumatoid arthritis rats. Zhen Ci Yan Jiu 2016; 41: 100-7. [PubMed] [Google Scholar]
- [16]. Chen Y, Li H, Luo X, et al. Moxibustion of Zusanli (ST36) and Shenshu (BL23) alleviates cartilage degradation through RANKL/OPG signaling in a rabbit model of rheumatoid arthritis. Evid Based Complement Alternat Med 2019; 2019: 6436420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Li Z,. Experimental acupuncture and moxibustion. Beijing: China Press of Traditional Chinese Medicine, 2003: 314- 6. [Google Scholar]
- [18]. Liu H, Huang Q, Shi B, Eksarko P, Temkin V, Pope RM.. Regulation of Mcl-1 expression in rheumatoid arthritis synovial macrophages. Arthritis Rheum 2006; 54: 3174-81. [DOI] [PubMed] [Google Scholar]
- [19]. Yang X, Chang Y, Wei W.. Emerging role of targeting macrophages in rheumatoid arthritis: focus on polarization, metabolism and apoptosis. Cell Prolif 2020; 53: e12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Thornton S, Boivin GP, Kim KN, Finkelman FD, Hirsch R.. Heterogeneous effects of IL-2 on collagen-induced arthritis. J Immunol 2000; 165: 1557-63. [DOI] [PubMed] [Google Scholar]
- [21]. Teixeira JH, Silva AM, Almeida MI, et al. The systemic immune response to collagen-induced arthritis and the impact of bone injury in inflammatory conditions. Int J Mol Sci 2019; 20: 5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Brennan FM, McInnes IB.. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest 2008; 118: 3537-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Zhang J, Zhang G, Yang S, et al. Macrophage migration inhibitory factor regulating the expression of VEGF-C through MAPK signal pathways in breast cancer MCF-7 cell. World J Surg Oncol 2016; 14: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Singh H, Chetha AS, Shalikar H. . Moxibustion-septic shock and necrotizing fasciitis. IDCases 2020; 22: e00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Wu Z, Xu G, Xiong J, Zuo Z, Yu X, Xie Q.. Moxibustion therapy on myofascial pain syndrome: an evidence-based clinical practice guideline. Medicine (Baltimore) 2020; 99: e22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Xue N, Fu X, Zhu Y, Da N, Zhang J.. Moxibustion enhances chemotherapy of breast cancer by affecting tumor microenvironment. Cancer Manag Res 2020; 12: 8015-22. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [27]. Xu J, Pan LJ, Jia CS.. Exploration on the feasibility of moxibustion in prevention and treatment of COVID-19 from the perspective of modern medical mechanism. World J Acupunct Moxibustion 2020; 30: 81-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Huang A, Pang Y, Tang Q, Xu J, Lin J, Li J.. Clinical therapeutic effects on rheumatoid arthritis treated with the assisted therapy of acupuncture at the points detected with thermosensitive moxibustion in Zhuang medicine. Zhong Guo Zhen Jiu 2018; 38: 245-50. [DOI] [PubMed] [Google Scholar]
- [29]. Liu J, Huang Z, Zhang GH.. Involvement of NF-kappa B signal pathway in acupuncture treatment of patients with rheumatoid arthritis. Zhen Ci Yan Jiu 2020; 45: 914-9. [DOI] [PubMed] [Google Scholar]
- [30]. Yu Z, Wang Y, Li Y, et al. Effect of moxibustion on the serum levels of MMP-1, MMP-3, and VEGF in patients with rheumatoid arthritis. Evid Based Complement Alternat Med 2020; 2020: 7150605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Su X, Zhang H, Wang H, Sun P.. MiR-130a/Ndrg2 axis inhibits the proliferation of fibroblast-like synoviocytes in rheumatoid arthritis. Inflammation 2020; 43: 2048-60. [DOI] [PubMed] [Google Scholar]
- [32]. Zhang CY, Hu L, Cai RL, Peng CY, Yuan J.. Toll-like receptor 4/nuclear factor-kappaB signaling in synovial tissue is involved in the anti-inflammatory effect of moxibustion in rats with rheumatoid arthritis. Zhen Ci Yan Jiu 2018; 43: 687-91. [DOI] [PubMed] [Google Scholar]
- [33]. Liu Z, Li X, Zhao C, et al. Effects of moxibustion on Treg/Th17 cell and its signal pathway in mice with rheumatoid arthritis. Zhong Guo Zhen Jiu 2017; 37: 1083-91. [DOI] [PubMed] [Google Scholar]
- [34]. Zhang CY, Shao FR, Cai RL, Yuan J, Yin G, Tang ZL.. Effects of moxibustion on expression of STAT 1, SOCS mRNA in synovium of rats with rheumatoid arthritis. Zhen Ci Yan Jiu 2015; 40: 205-9. [PubMed] [Google Scholar]
- [35]. Yang X, Liu XG, Wang Y, Yang SQ, Jin RJ.. Effects of moxibustion intervention on inflammatory reactions and expression of suppressor of cytokine signaling proteins of synovium cells in rheumatoid arthritis rabbits. Zhen Ci Yan Jiu 2013; 38: 129-33, 57. [PubMed] [Google Scholar]
- [36]. Yoo SA, Leng L, Kim BJ, et al. MIF allele-dependent regulation of the MIF coreceptor CD44 and role in rheumatoid arthritis. Proc Natl Acad Sci U S A 2016; 113: E7917-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Zhong YM, Cheng B, Zhang LL, Lu WT, Shang YN, Zhou HY.. Effect of moxibustion on inflammatory cytokines in animals with rheumatoid arthritis: a systematic review and Meta-analysis. Evid Based Complement Alternat Med 2020; 2020: 6108619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Zhao C, Li X, Yang Y, et al. An analysis of Treg/Th 17 cells imbalance associated microRNA networks regulated by moxibustion therapy on Zusanli (ST36) and Shenshu (BL23) in mice with collagen induced arthritis. Am J Transl Res 2019; 11: 4029-45. [PMC free article] [PubMed] [Google Scholar]
- [39]. He TF, Yang WJ, Zhang SH, Zhang CY, Li LB, Chen YF.. Electroacupuncture inhibits inflammation reaction by upregulating vasoactive intestinal peptide in rats with adjuvant-induced arthritis. Evid Based Complement Alternat Med 2011; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Yang X, Yang SQ, Zhou HY, et al. The influence on MAPK signal pathway in RA synovial cell of experimental rabbits by moxibustion. Zhong Hua Zhong Yi Yao Xue Kan 2007; 25: 470-4. [Google Scholar]
- [41]. Gao J, Liu XG, Huang DJ, et al. Involvement of the hypothalamus-pituitary-adrenal axis in moxibustion-induced changes of NF-kB signaling in the synovial tissue in rheumatic arthritis rats. Zhen Ci Yan Jiu 2010; 35: 198-203. [PubMed] [Google Scholar]
- [42]. Zhang CY, Cai RL, Tang ZL.. Influences of moxibustion on inflammatory factors and synoviocytes in rats with rheumatoid arthritis. Beijing Zhong Yi Yao Da Xue Xue Bao 2014; 37: 190-5. [Google Scholar]


