Abstract
Helminths or Parasitic worms of humans may cause chronic and sometimes deadly diseases, considered as neglected tropical diseases (NTDs) that infect around two billion people worldwide. Plants have been used as anthelmintics from ancient times. This review is a compilation of plants as source of anthelmintic drug. All information presented in this review article regarding the anthelmintic activities of plants from 2005 and has been acquired by approaching various electronic databases, including Scopus, Google scholar, Web of science and PubMed. Literature was surveyed for anthelmintic activity of plants which showed that secondary metabolites of plants like terpenes, glycosides, saponins, flavonoids, tannins and alkaloids were having anthelmintic activity. Since this review is a compilation of anthelmintic activity of plants from the year 2005, it will definitely be a fruitful study for researchers working in this field.
Keywords: helminths, helminthiasis, anthelmintics, biological products, anthelmintics
1. INTRODUCTION
Parasitic worms or helminths cause chronic and sometimes deadly diseases that have a major socio-economic impact worldwide.1 In humans, the disease caused by the parasitic worms is about 14 million globally, also called neglected tropical diseases (NTD).2In agricultural animals, diseases caused by parasites led to losses of about billions of dollars per year throughout the world.3,4
Gastrointestinal nematodes (GI), such as hookworms, whipworms, and roundworms affected under 15 years most.5Approximately more than 10% of the population is infected by GI nematodes worldwide.6
As of now, no vaccines are available in the market, so, control of helminths lies on the some of effective drugs, called anthelmintics, but their inadequate use causes serious drug resistance problems worldwide, so, urgent need is there for isolating, identifying new anthelmintic drugs,7 for humans, its lies on chemotherapy.8 Parasitic nematodes in human are two types : intestinal nematodes and tissue or blood nematodes.9 Intestinal nematodes includes -Ancylostoma duodenale, Trichuris trichiura, Ascaris lumbricoides, Enterobius vermicularis, and Strongyloides stercoralis, etc.10
Helminths lives in the GI tract of their hosts, and feed off living hosts, taking nutrients from host and causing infection/diseases and normally more prone to children, soil-transmitted schistosomiasis and helminthiasis are the most significant helminthiases, responsible for neglected tropical disesses.11,12 It also causes indirect disease burden through the immune system impairment, leads to malaria, tuberculosis, or human immunodeficiency virus/acquired immunodeficiency syndrome.13
Adult parasites survive for a long time in their human host and feeds directly from the blood of their hosts, thus helminths cause iron-deficiency anemia.14 Chronic helminth infections are characterized by a Type II helper T cells type response.15,⇓,⇓,⇓-19
2. HELMINTHS AND ANTHELMINTICS
Helminths cause a number of diseases in humans and animals, which are summarized in Table 1.
Table 1.
Diseases caused by helminths
| Type of Helminths | Species | Diseases |
|---|---|---|
| Cestodes (Tapeworm) |
Coenurus cerebralis - In sheep, rabbits, and rodents, - Humans become an intermediate host after ingesting food contaminated. |
Coenurosis - Involvement of CNS. -Cysts are developed into ventricles, and sometimes within the parenchyma of the brain, spinal cord.20 |
|
Diphyllobothrium latum
- The fish tapeworm, infect humans who ingest raw and pickled freshwater fish. - Compete with hosts for certain vitamins and related substances particularly for vitamin B12 and split vitamin B12 intrinsic factor complex. |
Diphyllobothriasis - Low concentration of vitamin B12 - Systemic and neurologic symptoms, including pallor, glossitis, loss of tongue papillae, numbness, paresthesias of feet and hand, depression, loss of vibratory sensation. - Optic neuropathy.21,22 |
|
|
Spirometra species - Cats and dogs (definitive host). - Humans are accidental hosts, acquire infection by drinking contaminated water. - Penetrate intestinal wall and migrate to brain and other tissues and grow to full size. |
Sparganosis - Slow-growing, tender, migratory, subcutaneous nodules develop for several weeks or years. - Symptoms like fever, chill, edema, and peripheral eosinophilia. - In rare case, helminths travel to the eye and brain.2 |
|
|
Taenia solium - Tapeworms, migrate through the mucosa and enter in the CNS, eyes, and striated muscle. |
Cysticercosis - Affects CNS, causes fever, headaches during the larval stage, progressive muscle weakness.26 |
|
| Nematodes |
Angiostrongylus cantonensis - Infect mollusks or fish. - In humans, it affects the upper respiratory tract, CNS |
Angiostrongyliasis - Characterized by eosinophilic meningitis - Symptoms are rash, pruritus, abdominal pain, headache, stiff neck, vomiting, etc.28 |
|
Gnathostoma spinigerum - Parasitizes the stomachs of cats and dogs. - Migrate through human tissue by ingestion of cooked animal flesh, drinking contaminated water |
Gnathostomiasis - Mainly CNS can be affected, the spinal cord is affected initially. - Patients experience nausea, vomiting, upper abdominal pain, urticaria, pruritus, etc.27 |
|
|
Loa loa - Group of nematodes, also called filaria. - White, threadlike worms transmitted to humans by biting tabanid flies. |
Loiasis - Infections are usually asymptomatic. - To treat loasis, one should consult an expert on tropical infectious diseases.29 |
|
|
Onchocerca volvulus - Similar to Loa loa. - Transmitted to humans by female black flies. |
Onchocerciasis - Granulomatous reaction followed by fibrosis. - Skin lesions, characterized by erythematous, pruritis, rash, etc.29 |
|
|
Strongyloides stercoralis - Small nematodes, can parasitize the small bowel of humans. - This female worm hatch in the intestinal duodenum and jejunum and pass into feces. |
Strongyloidiasis - Infection is caused in humans by skin contact with the contaminated soil. - Intestinal parasitism30 |
|
|
Toxocara canis and cati - Infects dogs and related mammals. - Eggs hatch into the small intestine of the host and larvae migrate into lungs, and trachea. |
Toxocariasis - Human toxocariasis occurs accidentally by ingesting the faeces of infected dogs. - Conditions may develop to the eye, CNS, producing nonspecific systemic manifestations.31 |
|
|
Trichinella spiralis - Human infection occurs by eating raw and undercooked pork, bear, wild boar. - After eating, the larvae migrate to the stomach, then to the small intestine. |
Trichinosis - Characterized by abdominal pain, vomiting, and diarrhea followed by fever, headache, lethargy, and severe muscle pain, weakness and tenderness.32 |
|
|
Paragonimus spices - Lung flukes, humans and other mammals are the final host, first intermediate host are snails, second intermediate host crustaceans. - Penetrating intestinal wall, entering into peritoneal cavity then migrating through diaphragm go to lungs. Schistosoma spices - Humans and other mammals are the final host, first intermediate host are snails, second intermediate host are crustaceans. - Found in intramedullary granuloma, spinal cord, CNS |
Paragonimiasis - Most cases mild and asymptomatic. - Brownish sputum with cough and intermittent hemoptysis. - Chronic bronchitis.27 Schistosomiasis - Involvement of CNS. - Chronic schistosomiasis is more common than acute. - Acute schistosomiasis is caused by an immunological response to helminths. Chronic schistosomiasis is an inflammatory response to eggs.27 |
Notes: CNS: central nervous system.
2.1. Anthelmintics
Anthelmintics, the term used for a group of drugs, used to treat several infections of humans and animals, mainly caused by parasitic worms.33
2.1.1 Secondary metabolites of plants having anthelmintic activity
Allelochemicals of plants are produced from all plants,34primary metabolites acts as precursors of different secondary metabolites.35 Secondary metabolites includeing Alkaloids, Terpenes, Flavonoids, Resins and Phenolic compounds are responsible for colour, flavour, fragrance of different plant.36 Terpinen-4-ol was having LC50 = 4.1 mM and LC90 = 20.2 mM.
Secondary metabolites having anthelmintic activity are summarized in Figure 1.
Figure 1. Secondary metabolites having anthelmintic activity.
C50: lethal concentration 50.
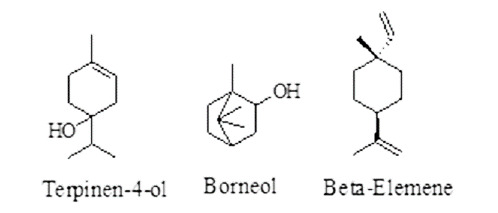
Terpenes: terpenes are the combination of different isoprene units (C5H8).36 Its shows anthelmintic activities causing intestinal damage to the parasite.37 Examples are terpinen-4-ol, borneol, and β-elemene showed activity against H. contortus by inhibiting egg hatching.38 Ter-penes having anthelmintic activity are given in Figure 2.
Figure 2. Terpenes having anthelmintic activity.
ED50: median effective dose; EC50: half maximal effective concentration.
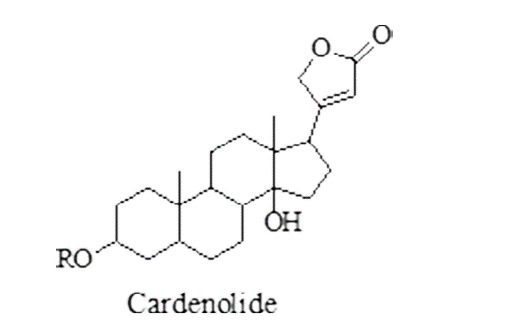
Glycosides: glycosides, have potent activity against different helminths.39,40 Cardenolide causes disturbance of sodium and potassium ions transportation into helminths, thus, causing death of helminths.41 Glycosides having anthelmintic activity are given in Figure 3.
Figure 3. Glycosides having anthelmintic activity (LC50 = 80.4 µM).
LC50: lethal concentration 50.
Saponins: saponins contains triterpene or sometimes steroidal-aglycone with sugar chains.42 Saponins show their anthelmintic activity by inhibiting acety-lcho-linesterase and thus cause worm paralysis leading to death.43 They are reported to have inhibitory activity against animal parasitic nematodes, like-Haemonchus contortus.44 β-Sitosterol was having IC50 value 58 µM. Saponins having anthelmintic activity are given in Figure 4.
Figure 4. Saponins having anthelmintic activity.
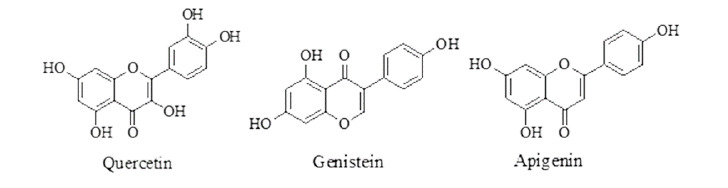
Flavonoids: flavonoids, helps in UV protection, flower coloring, allelopathy, and auxin transport inhibition.45 Flavonoidal plant, showing activity by blocking the phosphorylation reaction, thus inhibit the energy production within the parasitic worms, leading to death.46 Quercetin was having paralysis time at 10 mg/mL = 2.23 ± 4.51 min. Flavonoids having anthelmintic activity are given in Figure 5.
Figure 5. Flavonoids having anthelmintic activity.
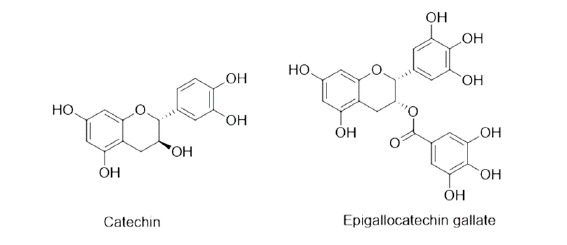
Tannins: tannins, water-soluble, polyphenolic group of compounds, help in the killing of nematodes, by interfering with nutrients absorption of worms from the host cell46 or when the condensed tannins are ingested by larvae, the tannin binds to the intestinal mucosa of the parasitic worms and thus causes autolysis.47 Epigallocatechin was having IC50 value 49 µM. Tannins having anthelmintic activity are given in Figure 6.
Figure 6. Tannins having anthelmintic activity.
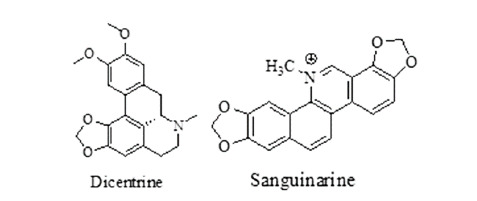
Alkaloids: alkaloids have shown anthelmintic activity by targeting acetylcholine receptor and suppressing glucose uptake, thus helminths died due to starvation.48Dicentrine was having EC90 = 6.3 µg/mL and san-guinarine was having IC50 = 58 µM. Alkaloids having anthelmintic activity are given in Figure 7.
Figure 7. Alkaloids having anthelmintic activity.
Non-protein amino acids: non-protein amino acid are the nitrogen-containing compounds having ammonia derivatives compounds with hydrogen atoms. They damage the parasitic worms by affecting the CNS of parasitic worms, leading to paralysis and followed to death.49 Mimosine was having IC50 = 16.8 µM Non-protein amino acids having anthelmintic activity are given in Figure 8. Target Sites of anthelmintics Target sites for anthelmintic are summarized in Tables 2 and 3.
Figure 8. Non-protein amino acids having anthelmintic activity.

Table 2.
Ion-channels as target sites for anthelmintics
| Parasite group | Target site | Example |
|---|---|---|
| Nematodes | Nicotinic acetylcholine receptor agonists | Levamisole |
| Large intestinal nematodes | Gamma-Aminobutyric acid receptor agonists | Piperazine |
| Nematodes and insect parasites | Glutamate-gated chloride channel receptor potentiators | Ivermectin |
| Cestodes and trematodes | Membrane calcium permeability enhancers | Praziquantel |
Table 3.
Target sites for anthelmintics (other than ion-channels)
| Parasite group | Target site | Example |
|---|---|---|
| Nematodes, cestodes and trematodes | β-tubulin binders | Thiabendazole |
| Blood feeders: flukes, Haemonchus contortus, Oestrus ovis | Proton ionophores | Closantel |
| Immature Fasciola | Malate metabolism inhibitors | Diamphenethide |
| Fasciola | Phosphoglycerate kinase and mutase inhibitors | Clorsulon |
| Filaria | Arachidonic acid metabolism inhibitors | Biethylcarbamazine |
2.1.3 Classes of natural products having anthelmintic activity
Different classes of natural products are reported to have anthelmintic activities.
Phenols: phenolic compounds contain a functional heterogenous group attached with its aromatic ring. Groups like-flavonoids, isoflavonoids and tannins are included in the phenolic compounds,45 showing activity by changing the phosphatase enzyme in the helminths tegument.50
Phenols having anthelmintic activity include:
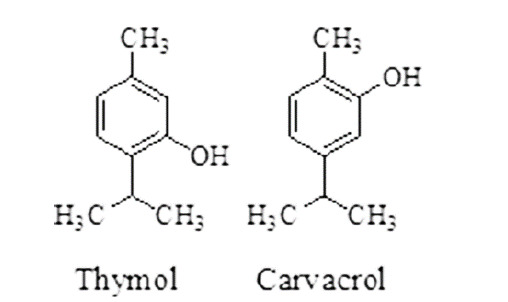
Monophenols (cresols, thymols, and carvacrol): carvacrol and thymol showed activity against C. elegans and Ascaris suum.51 Monophenols having anthelmintic activity are given in Figure 9.
Figure 9. Monophenols having anthelmintic activity.
IC50: half maximal inhibitory concentration.
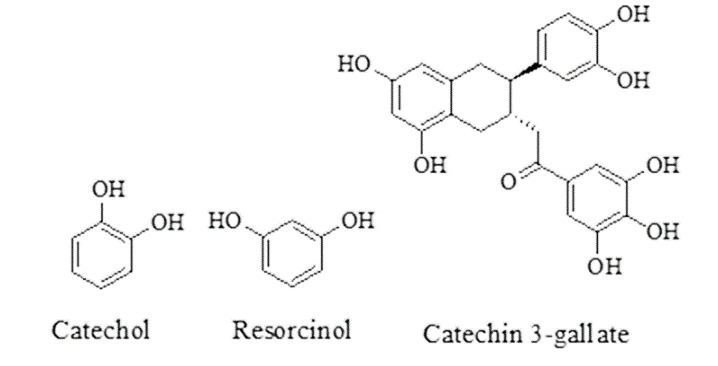
Benzene diols (resorcinols and catechols): gallic acid analogues, catechin-3-O-gallate and four related proanthocyanidins showed activity against C. elegans and O. ochengi.52 Benzene diols having anthelmintic activity are given in Figure 10.
Figure 10. Benzene diols having anthelmintic activity.
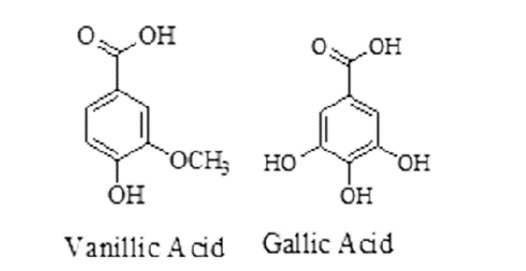
Substituted benzoic acids (vanillic acids and gallic acids): showed activity against nematode C. elegans and Onchocerca ochengi.53 Substituted benzoic acids having anthelmintic activity are given in Figure 11.
Figure 11. Substituted benzoic acids having anthelmintic activity.
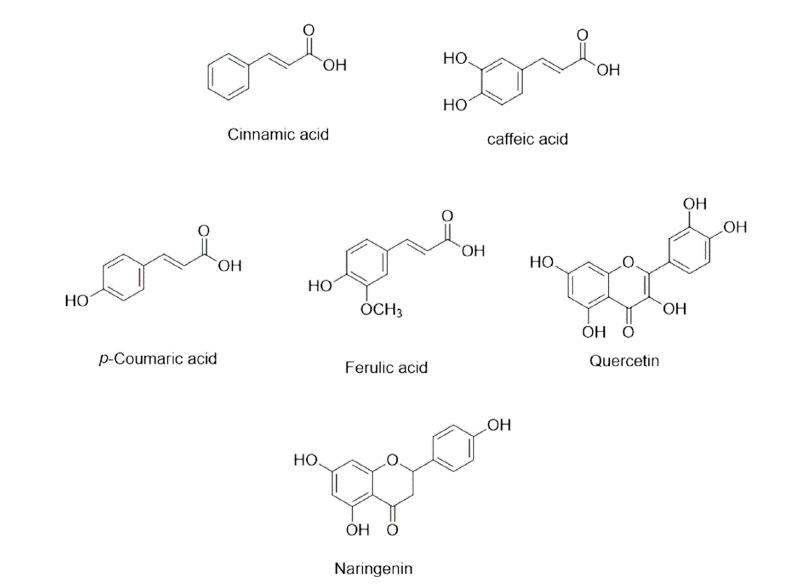
Cinnamic acid: cinnamic acid exhibited action by inhibiting egg hatching, example of some are p-coumaric, caffeic acid, ferulic acid, naringenin, quercetin and luteolin, procyanidins are having anthelmintic activity.53 Cinnamic acid derivatives having anthelmintic activity are given in Figure 12.
Figure 12. Cinnamic acid derivatives having anthelmintic activity.
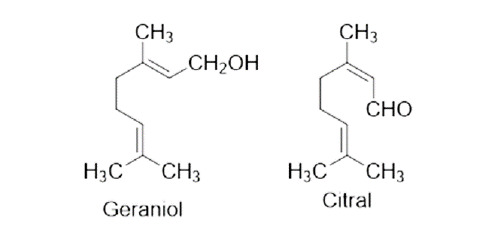
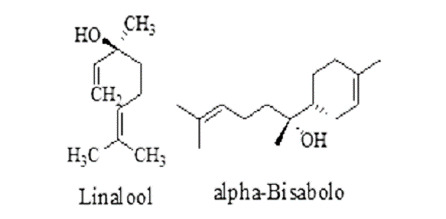
Prenyl derivatives: farnesyl pyrophosphate and geranyl pyrophosphate, precursors of prenyl groups, linalool, active against Brugia malayi and Dirofilaria immitis.54 Prenyl derivatives having anthelmintic activity are given in Figure 13.
Figure 13. Prenyl derivatives having anthelmintic activity.
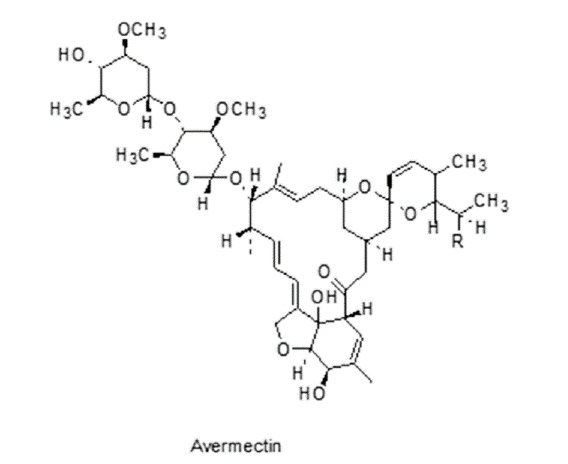
Macrocyclic Lactones: macrocycles includes macrolide and glyco lipopeptide, having anti-nematocidal activities, avermectins (Figure 14) and their aglycons are produced by soil microorganism Streptomyces species.55
Figure 14. Macrocyclic lactone having anthelmintic activity.
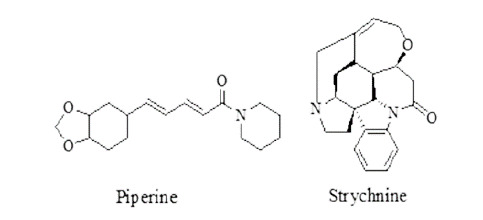
Miscellaneous compounds: piperine from Piper nigrum and strychnine from Strychnos nux-vomica showed activity against several helminths. Structures of piperine and strychnine are given in Figure 15.
Figure 15. Piperine and strychinine.
2.1.4 Plants having anthelmintic activity
A number of plants are reported to have anthelmintic activities. Some of them are as follows:
Commiphora molmol: there are a number of reports indicating that over 90% of humans are suffering from schistosomiasis, fascioliasis.56Myrrh was effective against Trichinella spiralis at a dose of 0.01 mL per infective mouse (Trichinella spiralis infective mouse), half maximal effective concentration (EC50) value for Commiphora molmol was 0.20 mg/mL.57
Ocimum sanctum: Occimum sanctum showed its activity at a dose of 62.15 mg/mL against Caenorhabditis elegans.58Crude hydro-alcoholic extract was tested at dose concentration of 20 and 40 mg/mL against Pheritima posthuma. For 20 mg/mL paralysis at (6.0 ± 0.5) min and death at (13.5 ± 1.2) min.59
Melia azedarach: hexane extract of M. azedarach fruits, effective against parasites, showed a significant lethal concentration 50 (LC50) value at 572.2 μg/mL and lethal concentration 99 (LC99) value at 1137.8 μg/mL.60
Artemisia annua: the plant crude leaves extract was tested against H. contortus, showed lowest LC99 at 1.27 μg/mL in egg hatch test assay, and in the larval development test assay, showed LC99 at 23.8 μg/mL.61
Carica papaya: the latex of the plant Carica papaya tested against Pheretima posthuma, with different concentrations (20%, 50% and 100%), at 100% concentration, it shows paralysis time, P = 24.5 min and death time, D = 56 min.62
Nigella sativa: ethanolic extract, tested against Cotylophoron cotylophorum at 5% concentration, it showed 81.02 % inhibition of the motility after the exposure of 8 h.63
Flemingia vestita: the isoflavones of the plant Flemingia vestita were tested against Rallietina echinobothrida with paralysis time for glucose 6-phosphate dehydrogenase is 0.880 ± 0.006 and for pyruvate carboxylase is 9.2 ± 0.2 and for fructose 1, 6-bisphosphatase is 0.98 ± 0.15.64
Juglans regia: crude acetone extract showed anthelmintic activity at 10 mg/mL, with paralysis time (52.00 ± 0.20) min and death at (114.00 ± 0.14) min. Crude methanolic extract showed its anthelmintic activity at 10 mg/mL, with paralysis time (100.00 ± 0.14) min and death at (133.00 ± 0.18) min when tested against Eicinia feotid.65
Mimusops elengi: the methanolic extract was tested against Pheretima posthuma, showing anthelmintic activity at 5 mg/mL, causing paralysis and death of the helminths at 163.3, 223.2 min respectively.66
Punica granatum: the crude methanolic tested against Haemonchus contortus, at 10 mg/mL it produced mortality at P < 0.05, hatching inhibition at 0.1 mg/mL upto 49.33% and 46.33%.67
Thymus vulgaris: essential oil of Thymus vulgaris was tested against H. contortus, 90% inhibition was observed at concentrations 50 to 0.781 mg/mL, and half maximal inhibitory concentration (IC50) value was 0.436 mg/mL. 97.0% larval motility inhibition observed at concen-trations 50 to 3.125 mg/mL and IC50 value 0.338 mg/mL.68
Ferula foetida: aqueous extracts were treated against Pheretima posthuma, exhibited activity at 100 mg/mL in 6 min (caused paralysis of the worms). Aqueous extract of the plant at 25 mg/mL caused helminths paralysis in (24.00 ± 0.14) min and death in (56.00 ± 0.17) min.69
Embelia ribes: methanolic extracts was tested against Ascaridia galli, at a dose of 60 mg/mL it showed activity 38.67% ± 4.10% and 38.67% ± 1.86%, after 48 h of incubation.70
Vernonia anthelmintica: ethanoic extracts were tested against Haemonchus contortus, survival rate reduced significantly at a dose conc. of 80 μg/mL.71
Chenopodium ambrosioides: the ethanolic extract was tested against Haemonchus contortus, showed lethal effect (about 96.3%) at 40 mg/mL, after 72 h incubation72 and the EC50 value of Chenopodium ambrosioides was (0.26 ± 0.02) mg/mL.73Hydro-alcoholic extract tested against Schistosoma mansoni, larvae died at different doses after 180 min.74
Piliostigma thonningii: the ethanolic extract was tested against Ascardia galli, showing anthelmintic activity, about 60% of larval paralysis at a dose conc. of 4.4 mg/mL within 24 h of exposure.75
Ginkgo biloba: aqueous extract of plant was tested against S. papillosus, 3.0% of plant extract solution, mortality rate of nematode larvae was 92.3% ± 2.9%, in 0.75% of plant extract solution.76 Petroleum-ether extract tested against Pseudodactylogyrus, at 6.0 and 2.5 mg/L, its having median effective dose (ED50) value 2.88 and 0.72 mg/L, respectively.77
Asparagus racemosus: rhizome extract at a dose of 5 mg/ ml showed mortality time (2.30 ± 0.29) h. At a dose of 10 mg/mL, plant extract showed mortality time (2.09 ± 0.05) h, etc.78
Trifolium repens: aerial shoot extract tested against the tapeworm Hymenolepis diminuta with different compounds i.e. 1 mg/mL betulinic acid, 0.50 mg/mL ursolic acid and 0.25 mg/mL biochanin A. Among them betulinic acid showed the best anthelmintic effect with a mortality time of (3.40 ± 0.66) h.79Aerial shoot extract tested against Hymenolepis diminuta, at a dose concentration of 200 and 500 mg/kg, decreased the faecal egg 47.72% and 54.59% respectively.80
Ficus insipida: The latex was tested against Colossoma macropomum gills, immobilization of the parasite occurred after 4h on treatment with 250 µL/L of latex and with 500 µL/L, immobilization occurred after 2 h.81
Cucurbita maxima: the peel extract was tested against Pheritima posthuma, at a concentration of 50 mg/mL, the paralysis time of the earthworm took place in (90 ± 2) min and the death time of the earthworm was (11 ± 2) min.82
Trachyspermum ammi: for aqueous extract, LC50 value was found at 0.1698 mg/mL and for methanolic extract, LC50 values was found at 0.1828 mg/mL.75The seeds extract of the plant was also tested against GIT nematodes, showed activity at a dose of 3 g/kg, the maximum reduction in egg count occurred.83
Syzygium aromaticum: the ethanolic extract, tested against Pheritma posthuma, at a dose 2.5 mg/mL, causing paralysis of the worm in (4.27 ± 0.25) min and death within (45.00 ± 2.00) min. At 5mg/mL, the paralysis in (2.43 ± 0.31) min and death occurred within (35.00 ± 4.35) min.84 When tested against Cotylophoron cotylophorum, ethanolic extract at 0.5 mg/mL, showing motility (86.27%) after 8 h of treatment.85
Trichilia claussenii: anthelmintic activity of the plant Trichilia claussenii was studied against gastrointestinal nematodes, methanol extract of leaves showed a significant LC50 value at 263.8 μg/mL and LC99 value at 522.5 μg/mL.86
Withania somnifera: crude extracted hydro-alcoholic solution tested against Pheritima posthuma at dose concentration of 20 and 40 mg/mL, paralysis was shown in (6.5 ± 0.5) min and (2.8 ± 0.8) min and death occurred (13.9 ± 1.2) min and (7.1 ± 0.9) min respectively.87
Pleurospermum amabile: the crude methanolic extract when tested against whipworms and blood flukes, bergapten showed an IC50 value 8.6 μg/mL against S. mansoni and 10.6 μg/mL against T. muris.88
Macleaya cordata: the crude extract when treated against Toxocara canis, Sanguinarine was showed IC50 value 58 μΜ after 24 h.89
Ajania nubigena: the crude extract was tested against Trichuris muris. Linalool and Luteolin were showed IC50 value 20.4 μg/mL and 9.7 μg/mL, respectively after 12 h.89
Leucaena leucocephala: the crude extract was tested against C. elegans. Mimosine was showed an IC50 value 16.8 μΜ after 24 h.89 The EC50 value cotyledon extract was 0.48 mg mL-1 and seed extracts showed EC50 value 0.33 mg/mL. 90
Warburgia ugandensis: the crude extract was tested against C. elegans, Mimosine showed mortality after 24 h with IC50 value (70.1 ± 17.5) μΜ against.89
Plants having anthelmintic activity are summarized in Table 4.
Table 4.
Plants having anthelmintic activity
| Name | Biological sources | Mechanism of action | Chemical constituents |
|---|---|---|---|
| Myrrh |
Commiphora myrrha or Commiphora molmol,
Family-Burseraceae |
Reduces activities of Alanine transminase (ALT) and Aspartate transminase (AST) | Limonene, Eugenol, a-Pinene, Cadinene, Acetic acid, Formic acid etc.56 |
| Tulsi |
Ocimum sanctum Linn. Family-Lamiaceae |
Causing paralysis of infected parasitic worms or death. | Carvacrol, Caryophyllene, Eugenol, Linalool, Urosolic acid, etc.91 |
| Chinaberry tree | Melia azedarach, Family- Meliaceae | Reacting with free proteins reduces the nutrients availability, thus larval death occurs due to starvation, or React with glycoproteins in the larval cuticle, causing death. | Spathulenol, Quercetin, Astragalin, 1,7,8-Trihydroxy-2-naphtaldehyde etc.92 |
| Papaya |
Carica papaya, Family-Caricaceae |
Killing the parasite worms by eosinophils, attack on structural protein of parasite nematodes. | Papain, Cystatin, Chymopapain, Ascorbic acid, Tocopherol.93 |
| Black caraway | Nigella sativa, Family-Ranunculaceae | Inhibiting the antioxidant enzymes thus produces a defense mechanism towards the oxidants generated by the parasitic nematode. | Linoleic acid, Oleic acid, Palmitic acid, p-cymene, Carvacrol, Thymol, α-Pinene.94 |
| Sohphlang | Flemingia vestita, Family-Leguminosae | Causing paralysis of infected parasitic worms or death. | Formononetin, Genistein, Daidzein, Pseudobaptigenin.95 |
| Walnut |
Juglans regia, Family-Juglandaceae |
It binds with the free protein of GIT of the host or interferes in energy generation of helminths, causing death of parasites. | Stearic acid, Palmitic acid, alpha Linolenic acid, Oleic acid, Catechin, Tannins.96 |
| Mimusops | Mimusops elengi, Family-Sapotaceae | Denaturation of proteins, produce defense mechanism, damages reactive oxygen species (ROS) properties. | Ursolic acid, Spinasterol, Taraxerol etc.97 |
| Pomegranate |
Punica granatum Family-Punicaceae. |
Inhibit transformation of larvae from egg, produce inflammation of epithelial cells by peroxisome proliferator-activated receptors-γ and δ-dependent mechanisms | Ellagic acid, Cyanidin-3-glucose, Pelargonidin-3-glucose etc.98 |
| Embelia |
Embelia ribes, Family-Primulaceae |
Paralysis of worms and reduces fecal eggs per gram (EPG). | Vanillic acid, Christembine, Cinnamic acid, O-cumaric acid, Embelin.99 |
| Epazote | Chenopodium ambrosioides, Family-Amaranthaceae. |
Paralysis of the parasitic worms. | Limonene, α-Terpinene p-Cymene, Camphor Thymol.100 |
| Piliostigma |
Piliostigma thonningii, Family-Fabaceae. |
It stimulates the neuromuscular junction of the parasite mostly and sometimes its effects to the ganglion and cause larval paralysis. | Alepterolic acid, Anticopalic acid, Clovane-2β,9α-diol etc.101 |
| Asparagus | Asparagus officinalis, Asparagus racemosus, Family-Asparagaceae. | Causing paralysis of infected parasitic worms or death. | Racemosol, Asparagamine, Folic acid.102 |
| White clover | Trifolium repens, Family-Fabaceae. | Paralysis of worms and reduces EPG. | Rutin, Quercetin, Myricetin Kaempferol.103 |
| Fig |
Ficus insipida,
Family-Moraceae. |
Causing paralysis of infected parasitic worms or death. | Vomifoliol, Dihydrophaseic acid, Dehydrovomifoliol etc.104 |
| Squash | Cucurbita maxima, Family-Cucurbitaceae. | Inhibit transformation of larvae from egg, reduces EPG. | Palmitic acid, Oleic acid, Linoleic acid, β-sitosterol.105 |
| Ajwain |
Trachyspermum ammi, Family-Apiaceae or Umbelliferae. |
Causing paralysis of infected parasitic worms or death. | Thymol, α-Pinene, α-Terpinene, β-Pinene, γ-terpinene, p-cymene.106 |
| Cinnamon |
Cinnamomum zylanicum, Family - Lauraceae. |
Inhibition of the parasitic egg hatching inhibits the fourth stage of larvae motility. | Eugenol, Cinnamic acid Cymene, Cinnamate.107 |
| Nutmeg | Myristica fragrans, Family -Myristicaceae. | Causing paralysis by inhibiting acetyl cholinesterase. | Myristicin, Eugenol, Safrole, Terpinene, Myristic acid.108 |
| Elecampane | Inula helenium, Family-Asteraceae. | Inhibitory effects on process of embryo development, paralysis by inhibiting acetylcholinesterase. | Alantolactone, Inulin, Helenin Stearoptene.109 |
| Clausena anisata | Clausena anisata, Family-Rutaceae. | Causing paralysis by inhibiting acetylcholinesterase. | Coumarins, Linalool, Myrcene, Anethole, Lomonene etc.110 |
| Zanthoxylum | Zanthoxylum zanthoxyloides, Family-Rutaceae. | Inhibition of the parasitic egg hatching prevents larvae from migrating. | Lomonine, Citronellal, Myrcene, α-pinene.111 |
| Annona | Annona squamosa, Family-Annonaceae. | Inhibition of the parasitic egg hatching inhibits cell division. | Anonain, OxophoebineIsocorydine, Reticulin.112 |
| False daisy | Eclipta prostrata, Family-Asteraceae. | Causing paralysis of infected parasitic worms or death. | Quercetin, β-Sitosterol, Luteoloside, Apigenin, Luteolin.113 |
| Name | Biological sources | Mechanism of action | Chemical constituents |
| Turkey berry | Solanum torvum, Family-Solanaceae. | Causing paralysis of infected parasitic worms, or reduces EPG. | Quercetin, Isoquarecetin Kaempferol, Rutin etc.114 |
| Myrobalan |
Terminalia chebula, Family -Combretaceae. |
Interrupts in energy production by binds free protein from GI tract or oxidative phosphorylation. | Arjungenin, Chebulin, Ellagic acid, Chebulic acid, Gallic acid.115 |
| Vinca | Catharanthus roseus, Family-Apocynaceae | Prevents polymerization of tubulin into microtubules. | Vincristine, Vinblastine Catharanthine etc.116 |
| Celandine |
Chelidonium majus, Family-Papaveraceae |
Reduce ROS generation, paralysis the parasitic worms. | Chelidonine, Sanguinarine, Caffeic acid, Protopine.117 |
| Mentha | Mentha cordifolia, Family-Lamiaceae | Causing paralysis of infected parasitic worms or death. | Carvone, Limonene, Menthol.118,8 |
| Sainfoin | Onobrychis viciifolia, Family-Fabaceae. | Reduce nematode excretion from GI tract, delay in egg maturation. | Tannin, Rutin, Nicotiflorin.119 |
| Ashwagandha | Withania somnifera, Family -Solanaceae | Causing paralysis of infected parasitic worms or death. | Withanolides, Anaferine, Sitoindoside.120 |
| Coriander | Coriandrum sativum, Family - Apiaceae | Reduce faecal egg count of worm and also inhibit the egg hatching process. | Linalool, Camphor, Geraniol, Coumarins, Linoleic acid.121 |
3. CONCLUSIONS
A number of plants/extracts are reported to have anthelmintic activity. Most of the studies reported in vitro activity and only a few studies report in vivo activity. So, more research is needed to investigate the molecular mechanism of action of the reported anthelmintic plants/extracts as well as there is a need to either modify existing anthelmintic agents or explore new molecular targets to get next generation anthelmintic agents.
3.1. Future perspectives
Now a days novel anthelmintic targets like lysine deacetylases, lysine deacetylases, KDAC inhibitors, kinase inhibitors are explored. Modification of chemical structure and combination of known anthelmintics is also one of the ways to combat this challenge. Drug repurposing is also an emerging trend in anthelmintic drug discovery e.g. trichlorfon, a broad-spectrum organophosphorus insecticide has recently proved as anthelmintic agent.
REFERENCES
- 1. Fenwick A. The global burden of neglected tropical diseases. J Public Health 2012; 126: 233-6. [DOI] [PubMed] [Google Scholar]
- 2. Hotez PJ, Bottazzi ME, Strych U. New vaccines for the world's poorest people. Annu Rev Med 2016; 67: 405-17. [DOI] [PubMed] [Google Scholar]
- 3. Knox M, Besier R, Le LJ. Novel approaches to the control of helminth parasites of livestock. Foreword. Vet Parasitol 2012; 186: 1-1. [DOI] [PubMed] [Google Scholar]
- 4. Stepek G, Behnke JM, Buttle DJ, et al. Natural plant cysteine proteinases as anthelmintics? Trends Parasitol 2004; 20: 322-7. [DOI] [PubMed] [Google Scholar]
- 5. Kappus KD, Lundgren Jr RG, Juranek DD, et al. Intestinal parasitism in the United States: Update on a continuing problem. Am J Trop Med Hyg 1994; 50: 705-13. [DOI] [PubMed] [Google Scholar]
- 6. Sharpe C, Thornton DJ, Grencis RK. A sticky end for gastrointestinal helminths; the role of the mucus barrier. Parasite Immunol 2018; 40: e12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Preston S, Jiao Y, Baell JB, et al. Screening of the ‘Open Scaffolds’ collection from compounds Australia identifies a new chemical entity with anthelmintic activities against different developmental stages of the barber's pole worm and other parasitic nematodes. Int J Parasitol 2017; 7: 286-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu M, Panda SK, Luyten W. Plant-based natural products for the discovery and development of novel anthelmintics against nematodes. Biomolecules 2020; 10: 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu Y, Georghiou SB, Kelleher AJ, et al. Bacillus thuringiensis Cry5B protein is highly efficacious as a single-dose therapy against an intestinal roundworm infection in mice. PLoS Negl Trop Dis 2010; 4: e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bogitsh BJ, Carter CE, Oeltmann TN. Intestinal Nematodes. In: Human parasitology. 5th ed. Massachusetts: Academic Press, 2019: 277- 311. [Google Scholar]
- 11. Hotez PJ. Neglected parasitic infections and poverty in the United States. PLoS Negl Trop Dis 2014; 8: e3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brooker S, Kabatereine N, Gyapong J, et al. Rapid mapping of schistosomiasis and other neglected tropical diseases in the context of integrated control programmes in Africa. Parasitol 2009; 136: 1707-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geary TG, Chibale K, Abegaz B, et al. A new approach for anthelmintic discovery for humans. Trends Parasitol 2012; 28: 176-81. [DOI] [PubMed] [Google Scholar]
- 14. Minciullo P, Cascio A, David A, et al. Anaphylaxis caused by helminths: review of the literature. Eur Rev Med Pharmacol Sci 2012; 16: 1513-8. [PubMed] [Google Scholar]
- 15. van Riet E, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections induce immunomodulation: Consequences and mechanisms. J Immunobiol 2007; 212: 475-90. [DOI] [PubMed] [Google Scholar]
- 16. Kaewkes S. Taxonomy and biology of liver flukes. Acta Tropica 2003; 88: 177-86. [DOI] [PubMed] [Google Scholar]
- 17. Tsai IJ, Zarowiecki M, Holroyd N, et al. The genomes of four tapeworm species reveal adaptations to parasitism. Nature 2013; 496: 57-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Traversa D. Pet roundworms and hookworms: a continuing need for global worming. Parasit Vectors 2012; 5: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bethony J, Brooker S, Albonico M, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 2006; 367: 1521-32. [DOI] [PubMed] [Google Scholar]
- 20. Sabattani S, Marliani AF, Roncaroli F, et al. Cerebral coenurosis: case illustration. J Neurosurg 2004; 100: 964. [DOI] [PubMed] [Google Scholar]
- 21. Marcial-Rojas RA. Pathology of protozoal and helminthic diseases, with clinical correlation. Baltimore: Williams & Wilkins Co. Edinburgh: Churchill Livingstone; 1971: 698-710. [Google Scholar]
- 22. Ohnishi K, Murata M. Single dose treatment with praziquantel for human diphyllobothrium nihonkaiense infections. Trans R Soc Trop Med Hyg 1993; 87: 482-3. [DOI] [PubMed] [Google Scholar]
- 23. Gökçek C, Bayar N, Buharal Z. Total removal of an unruptured orbital hydatid cyst. Can J Ophthalmol 2001; 36: 218-20. [DOI] [PubMed] [Google Scholar]
- 24. Demir K, Karsli A, Kaya T, et al. Cerebral hydatid cysts: CT findings. Neuroradiol J 1991; 33: 22-4. [DOI] [PubMed] [Google Scholar]
- 25. Botterel F, Bourée P. Ocular sparganosis:a case report. J Travel Med 2003; 10: 245-6. [DOI] [PubMed] [Google Scholar]
- 26. Pushker N, Bajaj MS, Betharia SM. Orbital and adnexal cysticercosis. Clin Exp Ophthalmol 2002; 30: 322-33. [DOI] [PubMed] [Google Scholar]
- 27. Hughes A, Biggs B. Parasitic worms of the central nervous system: An Australian perspective. Intern Med J 2002; 32: 541-53. [DOI] [PubMed] [Google Scholar]
- 28. Niu M, Duma R. Meningitis due to protozoa and helminths. Infect Dis Clin North Am 1990; 4: 809-41. [PubMed] [Google Scholar]
- 29. Remme J, Boatin B, Boussinesq M. Helminthic diseases:onchocerciasis and loiasis. In: Quah SR, Cockerham WC, editors. International encyclopedia of public health. 2nd ed. DC: Academic Press; 2017: 576- 87. [Google Scholar]
- 30. Marti H, Haji HJ, Savioli L, et al. A comparative trial of a single-dose ivermectin versus three days of albendazole for treatment of Strongyloides stercoralis and other soil-transmitted helminth infections in children. Am J Trop Med Hyg 1996; 55: 477-81. [DOI] [PubMed] [Google Scholar]
- 31. Ament CS, Young LH. Ocular manifestations of helminthic infections: Onchocersiasis, cysticercosis, toxocariasis, and diffuse unilateral subacute neuroretinitis. Int Ophthalmol Clin 2006; 46: 1-10. [DOI] [PubMed] [Google Scholar]
- 32. Grove DI, Mahmoud A, Warren KS. Eosinophils and resistance to Trichinella spiralis. J Exp Med 1977; 145: 755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Katz M. Anthelmintics. Drugs 1977; 13: 124-36. [DOI] [PubMed] [Google Scholar]
- 34. De los Reyes-Gavilán CG, Fernández M, Hudson JA, et al. Role of microorganisms present in dairy fermented products in health and disease. BioMed Res Int 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saltveit ME. Fruit and vegetable phytochemicals: chemistry and human health, synthesis and metabolism of phenolic compounds. 2nd ed. Hoboken: John Wiley & Sons, 2017: 115-24. [Google Scholar]
- 36. Chanda S, Ramachandra T. A review on some Therapeutic aspects of phytochemicals present in medicinal plants. Int J Pharm Life Sci 2019; 10: 6052-8. [Google Scholar]
- 37. Mukherjee N, Mukherjee S, Saini P, et al. Phenolics and Terpenoids; The promising new search for anthelmintics: A critical review. Mini Rev Med Chem 2016; 16: 1415-41. [DOI] [PubMed] [Google Scholar]
- 38. Qi H, Wang W, Dai J, et al. In vitro anthelmintic activity of Zanthoxylum simulans essential oil against Haemonchus contortus. Vet Parasitol 2015; 211: 223-7. [DOI] [PubMed] [Google Scholar]
- 39. Overend W. 9 Glycosides. Carbohydr 2012; 1: 279. [Google Scholar]
- 40. Kaingu F, Kibor A, Waihenya R, et al. Efficacy of Aloe secundiflora crude extracts on Ascaridia galli in vitro. Sustain Agric Res 2013; 2: 49-53. [Google Scholar]
- 41. Hussein RA, El-Anssary AA. Herbal medicine, plants secondary metabolites. In: the key drivers of the pharmacological actions of medicinal plants. 2018; 11- 30. [Google Scholar]
- 42. Güçlü-Üstündağ Ö, Mazza G. Saponins: properties, applications and processing. Crit Rev Food Sci Nutr 2007; 47: 231-58. [DOI] [PubMed] [Google Scholar]
- 43. Ali N, Shah SWA, Shah I, et al. Cytotoxic and anthelmintic potential of crude saponins isolated from Achillea Wilhelmsii C. Koch and Teucrium Stocksianum boiss. BMC Compl Alternative Med 2011; 11: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cavalcante GS, de Morais SM, Andre WP, et al. Chemical composition and in vitro activity of Calotropis procera (Ait.) latex on Haemonchus contortus. Vet Parasitol 2016; 226: 22-5. [DOI] [PubMed] [Google Scholar]
- 45. Ohri P, Pannu SK. Effect of phenolic compounds on nematodes. J Nat Appl Sci 2010; 2: 344-50. [Google Scholar]
- 46. Symeonidou I, Bonos E, Moustakidis K, et al. Botanicals: a natural approach to control ascaridiosis in poultry. J Hellenic Vet Med Soc 2018; 69: 711-22. [Google Scholar]
- 47. Del Carmen Acevedo-Ramírez PM, Hallal-Calleros C, Flores-Pérez I, et al. Anthelmintic effect and tissue alterations induced in vitro by hydrolysable tannins on the adult stage of the gastrointestinal nematode Haemonchus contortus. Vet Parasitol 2019; 266: 1-6. [DOI] [PubMed] [Google Scholar]
- 48. Badarina I, Putranto HD, Sulistyowati E. In vitro anthelmintic activity of the extract of coffee husk fermented with Pleurotus ostreatus for Ascaridia galli. Anim Prod Sci 2017; 19: 55-60. [Google Scholar]
- 49. Huang T, Jander G, de Vos M. Non-protein amino acids in plant defense against insect herbivores: representative cases and opportunities for further functional analysis. Phytochemistry 2011; 72: 1531-7. [DOI] [PubMed] [Google Scholar]
- 50. Swargiary A, Roy B. In vitro anthelmintic efficacy of Alpinia nigra and its bioactive compound, astragalin against Fasciolopsis buski. Int J Pharm Pharm Sci 2015; 7: 30-5. [Google Scholar]
- 51. Lei J, Leser M, Enan E. Nematicidal activity of two monoterpenoids and SER-2 tyramine receptor of Caenorhabditis elegans. Biochem Pharmacol 2010; 79: 1062-71. [DOI] [PubMed] [Google Scholar]
- 52. Vildina JD, Kalmobe J, Djafsia B, et al. Anti-onchocerca and anti-caenorhabditis activity of a hydro-alcoholic extract from the fruits of Acacia nilotica and some proanthocyanidin derivatives. Molecules 2017; 22: 748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ndjonka D, Abladam E, Djafsia B, et al. Anthelmintic activity of phenolic acids from the axlewood tree Anogeissus leiocarpus on the filarial nematode Onchocerca ochengi and drug-resistant strains of the free-living nematode Caenorhabditis elegans. J Helminthol 2014; 88: 481-8. [DOI] [PubMed] [Google Scholar]
- 54. Pereira I, Severino P, Santos AC, et al. Linalool bioactive properties and potential applicability in drug delivery systems. Colloids Surf B Biointerfaces 2018; 171: 566-78. [DOI] [PubMed] [Google Scholar]
- 55. Burg RW, Miller BM, Baker EE, et al. Avermectins, new family of potent anthelmintic agents: producing organism and fermentation. Antimicrob Agents Chemother 1979; 15: 361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yakoot M. A short review of the anthelmintic role of Mirazid. Arq Gastroenterol 2010; 47: 393-4. [DOI] [PubMed] [Google Scholar]
- 57. Basyoni MM, El-Sabaa AAA. Therapeutic potential of myrrh and ivermectin against experimental Trichinella spiralis infection in mice. Korean J Parasitol 2013; 51: 297-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Asha M, Prashanth D, Murali B, et al. Anthelmintic activity of essential oil of Ocimum sanctum and eugenol. Fitoterapia 2001; 72: 669-70. [DOI] [PubMed] [Google Scholar]
- 59. Kirtiman S. Comparative study of Withania somnifera and Ocimum sanctum for anthelmintic activity. ISCA J Bio Sci 2012; 1: 74-6. [Google Scholar]
- 60. Cala A, Chagas A, Oliveira M, et al. In vitro anthelmintic effect of Melia azedarach L. and Trichilia claussenii C. against sheep gastrointestinal nematodes. Exp Parasitol 2012; 130: 98-102. [DOI] [PubMed] [Google Scholar]
- 61. Cala AC, Ferreira JF, Chagas ACS, et al. Anthelmintic activity of Artemisia annua L. extracts in vitro and the effect of an aqueous extract and artemisinin in sheep naturally infected with gastrointestinal nematodes. Parasitol Res 2014; 113: 2345-53. [DOI] [PubMed] [Google Scholar]
- 62. Kanthal LK, Mondal P, De S, et al. Evaluation of anthelmintic activity of carica papaya latex using Pheritima posthuma. Int J Life Sci Pharma Res 2012; 2: 10-2. [Google Scholar]
- 63. Selvaraju A, Dhanraj S. Phytochemical analysis and anthelmintic potential of Nigella sativa against the trematode, Cotylophoron cotylophorum. J Pharmacogn Phytochem 2019; 8: 3161-6. [Google Scholar]
- 64. Tandon V, Das B. In vitro testing of anthelmintic efficacy of Flemingia vestita (Fabaceae) on carbohydrate metabolism in Rallietina echinobothrida. Methods 2007; 42: 330-8. [DOI] [PubMed] [Google Scholar]
- 65. Kale AA, Gaikwada SA, Kamble G. In vitro anthelmintic activity of stem bark of Juglans regia L. J Chem Pharm Res 2011; 3: 298-302. [Google Scholar]
- 66. Jana GK, Dhanamjayarao M, Vani M. Evaluation of anthelmintic potential of Mimusops elengi Linn (sapotaceae) leaf. J Pharm Res 2010; 3: 2514-5. [Google Scholar]
- 67. Ahmed AH, Ejo M, Feyera T, et al. In vitro anthelmintic activity of crude extracts of artemisia herba-alba and Punica granatum against Haemonchus contortus. J Parasitol 2020; 1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ferreira LE, Benincasa BI, Fachin AL, et al. Thymus vulgaris L. essential oil and its main component thymol: anthelmintic effects against Haemonchus contortus from sheep. Vet Parasitol 2016; 228: 70-6. [DOI] [PubMed] [Google Scholar]
- 69. Gundamaraju R. Evaluation of anti-helmintic activity of Ferula foetida “Hing-A natural Indian spice” aqueous extract. Asian Pac J Trop Dis 2013; 3: 189-91. [Google Scholar]
- 70. Sen D, Agnihotri RK, Sharma D, et al. In vitro assays on mangifera indica and embelia ribes against Ascaridia galli of poultry. Himachal J Agric Res 2018; 44: 117-24. [Google Scholar]
- 71. Hördegen P, Cabaret J, Hertzberg H, et al. In vitro screening of six anthelmintic plant products against larval Haemonchus contortus with a modified methyl-thiazolyl-tetrazolium reduction assay. J Ethnopharmacol 2006; 108: 85-9. [DOI] [PubMed] [Google Scholar]
- 72. Zamilpa A, García-Alanís C, López-Arellano M, et al. In vitro nematicidal effect of Chenopodium ambrosioides and Castela tortuosa n-hexane extracts against Haemonchus contortus (Nematoda) and their anthelmintic effect in gerbils. J Helminthol 2019; 93: 434-9. [DOI] [PubMed] [Google Scholar]
- 73. Ali N, Nabi M, Shoaib M, et al. GC/MS analysis, anti-leishmanial and relaxant activity of essential oil of Chenopodium ambrosioides from Malakand region. Pak J Pharm Sci 2021; 34: 577-83. [PubMed] [Google Scholar]
- 74. Rodrigues JGM, Albuquerque PSV, Nascimento JR, et al. The immunomodulatory activity of Chenopodium ambrosioides reduces the parasite burden and hepatic granulomatous inflammation in Schistosoma mansoni-infection. J Ethnopharmacol 2021; 264: 113287. [DOI] [PubMed] [Google Scholar]
- 75. Mali RG, Mehta AA. A review on anthelmintic plants. Nat Prod Rad 2008; 7: 466-75. [Google Scholar]
- 76. Boyko OO, Kabar A, Brygadyrenko V. Nematicidal activity of aqueous tinctures of medicinal plants against larvae of the nematodes Strongyloides papillosus and Haemonchus contortus. Biosyst Divers 2020; 28: 119-23. [Google Scholar]
- 77. Wang GX, Jiang Dx, Zhou Z, et al. In vivo assessment of anthelmintic efficacy of ginkgolic acids on removal of Ps-eudodactylogyrus in European eel. Aquaculture 2009; 297: 38-43. [Google Scholar]
- 78. Bhavare V, Pokharka R. Comparative in vitro anticestodal activity of some medicinal plants from western India. Pharmacologyonline 2010; 3: 142-5. [Google Scholar]
- 79. Yadav AK. In vitro anthelmintic assessment of selected phytochemicals against Hymenolepis diminuta, a zoonotic tapeworm. J Parasit Dis 2016; 40: 1082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tangpu V, Temjenmongla K, Yadav AK. Anticestodal activity of Trifolium repens extract. Pharm Biol 2005; 42: 656-8. [Google Scholar]
- 81. Gonzales APPF, Santos GG, Tavares-Dias M. Anthelminthic potential of the Ficus insipida latex on monogeneans of Colossoma macropomum (Serrasalmidae), a medicinal plant from the Amazon. Acta Parasitol 2019; 64: 927-31. [DOI] [PubMed] [Google Scholar]
- 82. Chand J, Naaz Y, Nainwal P. In vitro Anthelmintic activity of peel extracts of Cucurbita Maxima. Int Res J Pharm 2019; 10: 22-5. [Google Scholar]
- 83. Lateef M, Iqbal Z, Akhtar M, et al. Preliminary screening of Trachyspermum ammi seed for anthelmintic activity in sheep. Trop Anim Health Prod 2006; 38: 491-6. [DOI] [PubMed] [Google Scholar]
- 84. Patil R, Kadam J, Chavan J, et al. Anthelmintic activity of ethanolic bud extract of Syzygium aromaticum against Pheretima posthuma. Weekly Sci Int Res J 2013; 1-5. [Google Scholar]
- 85. Dhanraj KM, Veerakumari L. In vitro effect of Syzygium aromaticum on the motility and acetylcholinesterase of Cotylophoron cotylophorum. Ind J Vet Anim Sci Res 2014; 43: 187-94. [Google Scholar]
- 86. Cala A, Chagas A, Oliveira M, et al. In vitro anthelmintic effect of Melia azedarach L. and Trichilia claussenii C. against sheep gastrointestinal nematodes. Exp Parasitol 2012; 130: 98-102. [DOI] [PubMed] [Google Scholar]
- 87. Kirtiman S. Comparative study of Withania somnifera and Ocimum sanctum for anthelmintic activity. ISCA J Bio Sci 2012; 1: 74-6. [Google Scholar]
- 88. Wangchuk P, Giacomin PR, Pearson MS, et al. Identification of lead chemotherapeutic agents from medicinal plants against blood flukes and whipworms. Sci Rep 2016; 6: 32101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Liu M, Panda SK, Luyten W. Plant-based natural products for the discovery and development of novel anthelmintics against nematodes. Biomolecules 2020; 10: 425-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Soares AM, Lopes SG, et al. Anthelmintic activity of Leucaena leucocephala protein extracts on Haemonchus contortus. Rev Bras Parasitol Vet 2015; 24: 396-401. [DOI] [PubMed] [Google Scholar]
- 91. Kanojiya D, Shanker D, Sudan V, et al. Anthelmintic activity of Ocimum sanctum leaf extract against ovine gastrointestinal nematodes in India. Res Vet Sci 2015; 99: 165-70. [DOI] [PubMed] [Google Scholar]
- 92. Szewczuk VD, Mongelli ER, Pomilio AB. In vitro anthelmintic activity of Melia azedarach naturalized in Argentina. Phytother Res 2006; 20: 993-6. [DOI] [PubMed] [Google Scholar]
- 93. Ameen S, Azeez O, Baba Y, et al. Anthelmintic Potency of Carica papaya seeds against Gastro-intestinal Helminths in Red Sokoto goat. Ceylon J Sci 2018; 47: 137-41. [Google Scholar]
- 94. Al-Shaibani I, Phulan M, Arijo A, et al. Anthelmintic activity of Nigella sativa L, seeds on gastrointestinal nematodes of sheep. Pak J Nematol 2008; 26: 207-18. [Google Scholar]
- 95. Pal P, Tandon V. Anthelmintic efficacy of Flemingia vestita (Fabaceae) genistein-induced alterations in the ultrastructure of the tegument in the cestode, Raillietina echinobothrida. J Parasit Dis 2010; 22: 104-9. [Google Scholar]
- 96. Hayes D, Angove MJ, Tucci J, et al. Walnuts (Juglans regia) chemical composition and research in human health. Crit Rev Food Sci Nutr 2016; 56: 1231-41. [DOI] [PubMed] [Google Scholar]
- 97. Mali RG, Mahajan SG, Mehta AA. In vitro anthelmintic activity of stem bark of Mimusops elengi Linn. PhcogMag 2007; 3: 73-6. [Google Scholar]
- 98. Aggarwal R, Kaur K, Suri M, et al. Anthelmintic potential of Calotropis procera, Azadirachta indica and Punica granatum against Gastrothylax indicus. J Parasit Dis 2016; 40: 1230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jalalpure S, Alagawadi K, Mahajanashetti C, et al. In vitro anthelmintic property of various seed oils against Pheritima posthuma. Indian J Pharm Sci 2007; 69: 158. [Google Scholar]
- 100. Jabbar A, Zaman MA, Iqbal Z, et al. Anthelmintic activity of Chenopodium album and Caesalpinia crista against trichostrongylid nematodes of sheep. J Ethnopharmacol. 2007; 114: 86-91. [DOI] [PubMed] [Google Scholar]
- 101. Afolayan M, Srivedavyasasri R, Asekun OT, et al. Phytochemical study of Piliostigma thonningii, a medicinal plant grown in Nigeria. Med Chem Res 2018; 27: 2325-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kiranmayi G, Ravishankar K, Priyabandhavi P. Phytochemical screening and in vitro comparative study of anthelmintic activity of Asparagus racemosus and Cucurbita maxima. J Pharm Res 2012; 5: 1545-7. [Google Scholar]
- 103. Tangpu V, Temjenmongla K, Yadav AK. Anticestodal activity of Trifolium repens extract. Pharm Biol 2005; 42: 656-8. [Google Scholar]
- 104. Hansson A, Zelada JC, Noriega HP. Reevaluation of risks with the use of Ficus insipida latex as a traditional anthelmintic remedy in the Amazon. J Ethnopharmacol 2005; 98: 251-7. [DOI] [PubMed] [Google Scholar]
- 105. Ayaz E, Gökbulut C, Coşkun H, et al. Evaluation of the anthelmintic activity of pumpkin seeds (Cucurbita maxima) in mice naturally infected with Aspiculuris tetraptera. J Pharmacognosy Phytother 2015; 7: 189-93. [Google Scholar]
- 106. Bairwa R, Sodha R, Rajawat B. Trachyspermum ammi. Phcog Rev 2012; 6: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Williams AR, Ramsay A, Hansen TV, et al. Anthelmintic activity of trans-cinnamaldehyde and A-and B-type proanthocyanidins derived from cinnamon (Cinnamomum verum). Sci Rep 2015; 5: 14791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dwivedi G, Bairagi M, Rawal D, et al. Anthelmintic activity of Myristica fragrans (Nutmeg) extract. Res J Pharm Bio Chem Sci 2011; 2: 315-8. [Google Scholar]
- 109. Yan H, Haiming S, Cheng G, et al. Chemical constituents of the roots of Inula helenium. Chem Nat Compd 2012; 48: 522-4. [Google Scholar]
- 110. Muthee J, Gakuya D, Mbaria J, et al. Ethnobotanical study of anthelmintic and other medicinal plants traditionally used in Loitoktok district of Kenya. J Ethnopharmacol 2011; 135: 15-21. [DOI] [PubMed] [Google Scholar]
- 111. Olounladé P, Azando E, Hounzangbé-Adoté M, et al. In vitro anthelmintic activity of the essential oils of Zanthoxylum zanthoxyloides and Newbouldia laevis against Strongyloides ratti. Parasitol Res 2012; 110: 1427-33. [DOI] [PubMed] [Google Scholar]
- 112. Souza M, Bevilaqua CM, Morais SM, et al. Anthelmintic acetogenin from Annona squamosa L. Seeds. An Acad Bras Ciênc 2008; 80: 271-7. [DOI] [PubMed] [Google Scholar]
- 113. Bhinge SD, Hogade MG, Chavan C, et al. In vitro anthelmintic activity of herb extract of Eclipta prostrate L. against Pheretima posthuma. Asian J Pharm Clin Res 2010; 3: 229-30. [Google Scholar]
- 114. Karumari RJ, Sumathi S, Vijayalakshmi K, et al. Anthelmintic efficacy of Sesbania grandiflora leaves and Solanum torvum fruits against the nematode parasite Ascaridia galli. Am J Ethno Med 2014; 1: 326-33. [Google Scholar]
- 115. Nirmal S, Gagare P, Dighe S, et al. Anthelmintic activity of some existing polyherbal Ayurvedic formulations. Pharmacologyonline 2008; 3: 76-9. [Google Scholar]
- 116. Gajalakshmi S, Vijayalakshmi S, Devi RV. Pharmacological activities of Catharanthus roseus. Int J Pharm Bio Sci 2013; 4: 431-9. [Google Scholar]
- 117. Maji AK, Banerji P. Chelidonium majus L.(Greater celandine)-A review on its phytochemical and therapeutic perspectives. Int J Herb Med 2015; 3: 10-27. [Google Scholar]
- 118. Kozan E, Küpeli E, Yesilada E. Evaluation of some plants used in Turkish folk medicine against parasitic infections for their in vivo anthelmintic activity. J Ethnopharmacol 2006; 108: 211-6. [DOI] [PubMed] [Google Scholar]
- 119. Barrau E, Fabre N, Fouraste I, et al. Effect of bioactive compounds from Sainfoin (Onobrychis viciifolia Scop.) on the in vitro larval migration of Haemonchus contortus: role of tannins and flavonol glycosides. Parasitology 2005; 131: 531-8. [DOI] [PubMed] [Google Scholar]
- 120. Saddiqe Z, Khalid S, Maimoona A. In vitro antelmintic activity of extracts of Withania Somnifera. J Nat Appl Sci Pakistan 2019; 1: 89-97. [Google Scholar]
- 121. Eguale T, Tilahun G, Debella A, et al. In vitro and in vivo anth-elmintic activity of crude extracts of Coriandrum sativum against Haemonchus contortus. J Ethnopharmacol 2007; 110: 428-33. [DOI] [PubMed] [Google Scholar]


