Abstract
Purpose.
The aim of the present study was to assess, in healthy individuals, the impact of M1-M1 tDCS on primary motor cortex excitability using transcranial magnetic stimulation and sensorimotor metabolite concentration using 1H-MRS.
Methods.
For both experiments, each participant received the three following interventions (20 min tDCS, 1mA): left-anodal/right-cathodal, left-cathodal/right-anodal, sham. The effects of tDCS were assessed via motor evoked potentials (experiment 1) and metabolite concentrations (experiment 2) immediately after and 12 minutes following the end of stimulation and compared to baseline measurement.
Results.
No effect of M1-M1 tDCS on corticospinal excitability was found. Similarly, M1-M1 tDCS did not significantly modulate metabolite concentrations. High inter-subject variability was noted. Response rate analysis showed a tendency towards inhibition following left-anodal/right-cathodal tDCS in 50% of participants and increased GABA levels in 45% of participants.
Conclusion.
In line with recent studies showing important inter-subject variability following M1-supraorbital tDCS, the present data show that M1-M1 stimulation is also associated with large response variability. The absence of significant effects suggests that current measures may lack sensitivity to assess changes in M1 neurophysiology and metabolism associated with M1-M1 tDCS.
Keywords: magnetic resonance spectroscopy, transcranial direct current stimulation, motor cortex, GABA, glutamate
Introduction
Transcranial direct current stimulation (tDCS) is a neuromodulation technique that has gained recognition as a promising clinical tool because of its ability to modulate a large array of behaviors and cognitive functions in both healthy individuals (Jacobson et al., 2011) and clinical populations (Sandrini and Cohen, 2013; Schulz et al., 2013). It is generally assumed that when an anode is placed over the primary motor cortex (M1) and a cathode is positioned over the contralateral supraorbital area (M1-supraorbital montage; anodal tDCS, a-tDCS), corticospinal excitability is enhanced but decreased when the current flow is reversed (cathodal tDCS, c-tDCS) (Nitsche and Paulus, 2000; Lang et al., 2005). Despite a wealth of encouraging results, the apparent potential of tDCS has led to its use in clinical settings without a comprehensive understanding of optimal parameters for treatment, intra- and inter-individual variability of response, individual factors modulating response, and most importantly mechanisms of action underlying its physiological effects (Gomez Palacio Schjetnan et al., 2013; Horvath et al., 2014). For example, recent studies have shown highly variable effects in the motor cortex of healthy individuals following tDCS (López-Alonso et al., 2014; Wiethoff et al., 2014) and limited reliability to modulate cognitive function (Horvath et al., 2015; Tremblay et al., 2014a).
Pharmacological studies and animal models suggest that tDCS modulates corticospinal excitability through its effects on resting membrane potentials (Fritsch et al., 2010; Liebetanz et al., 2002; Stagg & Nitsche, 2011), and synaptic connections via long-term potentiation (LTP) and long-term depression (LTD) mechanisms (Stagg and Nitsche, 2011). In the neocortex, these mechanisms are believed to be partially mediated by GABAergic and glutamatergic neurons (Froc et al., 2000; Trepel and Racine, 2000). Using transcranial magnetic stimulation (TMS), recent studies have suggested that M1-supraorbital tDCS can modulate several indirect measures of GABA and glutamate receptor activity, such as cortical silent period duration (Tremblay et al., 2013) and intracortical facilitation (Di Lazzaro et al., 2012). However, there is currently no consensus on the effect of tDCS on neurophysiological markers of intracortical inhibition and facilitation.
Even though the effects of M1-supraorbital tDCS on M1 excitability have been widely studied using TMS, few studies have assessed the impact of M1-M1 tDCS on corticospinal excitability and current results remain unclear. As such, while some studies have shown the expected polarity-sensitive effects following M1-M1 tDCS (Mordillo-Mateos et al., 2012; Tazoe et al., 2014), one recent study reported no significant effect of M1-M1 tDCS (left anode/right cathode) over bilateral M1 excitability (O’Shea et al., 2013). Although little information is available regarding the neurophysiological mechanism underlying the cortical effects of M1-M1 tDCS, the technique has been used in several clinical studies (Di Lazzaro et al., 2014; Vines et al., 2008). For example, this protocol has shown promising results among stroke patients for which an interhemispheric imbalance, i.e. an increase of activity in the unaffected hemisphere and a reduction of activity in the affected hemisphere, is linked to poor recovery (Hummel & Cohen, 2006; Murase et al., 2004). A number of recent clinical stroke studies have shown that M1-M1 stimulation, through the combination of a-tDCS over contralesional M1 and c-tDCS over ipsilesional M1, induces greater and longer-lasting effects on motor recovery than M1-supraorbital stimulation (Lindenberg et al., 2013; Sehm et al., 2013; Vines et al., 2008). This suggests that a bi-hemispheric montage can act on the balance between both hemispheres. This montage may therefore induce additive effects on M1 excitability via interhemispheric connections, which are thought to be more focal than those associated with M1-supraorbital stimulation (Di Lazzaro et al., 2014). Despite these positive clinical results, the neurophysiology of M1-M1 stimulation remains poorly understood.
Proton magnetic resonance spectroscopy (1H-MRS), which allows direct measurement of GABA and glutamate levels, offers a powerful complementary tool to assess the mechanism of action underlying M1 tDCS. Using this method, Stagg and collaborators (2009) showed a significant reduction of GABA and glutamate + glutamine (Glx) levels in sensorimotor cortex following M1-supraorbital c-tDCS. Kim and collaborators (2014), on the other hand, found no evidence of GABA and Glx modulation with a similar stimulation protocol. Anodal M1-supraorbital tDCS has proven to be more robust in showing metabolic effects of tDCS. Indeed, GABA levels were found to be reduced after anodal tDCS in three separate studies (Kim et al., 2014; Stagg et al., 2011; Stagg et al., 2009). To our knowledge, the effect of M1-M1 tDCS on motor cortex metabolism has yet to be investigated.
The primary objective of the present study was to further assess the impact of M1-M1 tDCS on M1 corticospinal excitability using TMS and determine its effect on sensorimotor metabolism using 1H-MRS in healthy participants. Following recent studies on the effects of M1-supraorbital tDCS, we hypothesized that, when the anode was located over left M1, M1-M1 stimulation would increase corticospinal excitability and reduce GABA concentration under the anode. When the cathode was located over left M1, we predicted reduced corticospinal excitability and reduced GABA and Glx concentrations under the cathode.
Material and methods
Ethical approval
The experiments described in the current manuscript conformed to the standards set by the Declaration of Helsinki, and all of the procedures were approved by the research ethics board of the Comité Mixte d’Éthique de la Recherche du Réseau de Neuroimagerie du Québec (CMER-RNQ). All participants provided written informed consent prior to testing and all participants were aware of the aim of the experiment, including the presence of a sham condition. Both experiments were single-blinded, as only the experimenter was aware of the stimulation condition.
Experiment 1: neurophysiological effects of M1-M1 tDCS
Participants and procedure
Ten right-handed healthy volunteers (5 women; mean age: 25 ± 6 years; age range: 20–40 years) were recruited for this study. Female participants were tested irrespective of their menstrual cycle. Participants were excluded if they had any neurologic or psychiatric disorder or contraindications to tDCS or TMS, including taking drugs that can modify cortical excitability thresholds (Nitsche et al., 2008; Rossi et al., 2009). Each participant took part in the three following experimental sessions: 1) active stimulation left anodal/right cathodal (LA/RC); 2) active stimulation left cathodal/right anodal (LC/RA); 3) sham stimulation (LA/RC). The conditions were counterbalanced across groups and each session was separated by at least 72 hours.
Experimental procedures
Trancranial magnetic stimulation
TMS was delivered through an 8 cm figure-of-eight coil connected to a Magstim 2002 stimulator (Magstim Company Ltd, Spring Gardens, UK). The coil was positioned flat on the head of participants at a 45° angle from the midline, with the handle pointing backwards. A monophasic current was induced with a posterior-anterior direction. TMS was delivered over left M1. The optimal site of stimulation was defined as the coil position from which TMS produced motor evoked potentials of greatest amplitude in the FDI muscle of the contralateral hand following the method of the most recent report of the International Federation of Clinical Neurophysiology (Rossini et al., 2015). To achieve this, the coil was moved around a pre-determined spot in 1cm steps in all directions using a starting intensity of 50% maximum stimulator output. Next, the intensity of stimulation was adjusted to elicit MEPs of average amplitude of 1 mV peak-to-peak for 10 consecutive trials. Using this intensity, twenty MEPs were collected immediately before tDCS (pre), immediately after tDCS (post0) and 12 min post-tDCS (post12). TMS pulses were delivered at a frequency of 0.1 to 0.2 Hz to avoid long lasting modulation of M1 excitability (Chen et al., 1997; Rossini et al., 2015).
To ensure stable coil positioning, a stereotaxic neuronavigation system (Brainsight; NeuroConn GmbH, Ilmenau, Germany) was used. To quantify muscle contractions, two self-adhesive electrodes were positioned over the FDI muscle of the right hand and a ground electrode was positioned over the right wrist. EMG signal was filtered with a bandwith of 20–1000 Hz and digitized at a sampling rate of 4 kHz using a Powerlab 4/30 system (ADInstruments, Colorado Springs, USA). MEPs were recorded using LabChart7 software (ADInstruments, Colorado Springs, USA) and stored offline for analysis.
Transcranial direct current stimulation
Electrical current was delivered by a Magstim DC Stimulator (Magstim Company Ltd, Spring Gardens, UK) through a pair of rectangular conductive rubber electrodes (35 cm2) inserted into saline-soaked sponges. The electrode center was positioned over the left and right FDI muscle M1 representation, previously determined using TMS. The electrodes were oriented parallel to the central sulcus. For both active conditions, a constant electric current of 1 mA was applied for 20 min, and the current was gradually increased and decreased during the first and last 15 s of stimulation. For the sham condition, the current was ramped up for 15 s and then no current was delivered for 20 min.
Experiment 2: neurometabolic effects of M1-M1 tDCS
Participants and procedure
Eight right-handed healthy volunteers (4 women; mean age: 29 ± 6 years; age range: 24–40 years) participated in this study. The same exclusion criteria as in Experiment 1 were used, in addition to standard magnetic resonance (MR) contraindications. The experimental protocol consisted of three sessions of 2 h duration, separated by at least 72 h and counterbalanced across groups. Each session consisted of a first 1H-MRS acquisition, 20 min of M1-M1 tDCS that was administered inside the scanner (same conditions as for Experiment 1), and two consecutive 1H-MRS acquisitions post-stimulation.
Experimental procedures
Transcranial direct current stimulation
Electrical current was delivered using a MR-compatible NeuroConn DC-stimulator Plus (NeuroConn GmbH, Ilmenau, Germany) through a pair of rectangular conductive rubber electrodes (35 cm2). Electrodes were entirely covered with an EEG-type conductive paste. They were positioned over C3 and C4, according to the 10/20 international system, which corresponds to the left and right primary motor regions, respectively. The electrodes were oriented parallel to the central sulcus and eyebrows. Once the electrodes were properly positioned, the impedance level was tested outside the scanning room prior to testing. If an adequate impedance level was reached (< 20 kΩ), participants were positioned comfortably in the scanner and were instructed to lie at rest for the entire scanning session. The electrodes were plugged into the MR-compatible tDCS box, which was positioned inside the scanner. Active and sham condition parameters were identical to Experiment 1. No MR data was acquired during tDCS. See Tremblay et al. (2014b) for a comprehensive description of the protocol.
Proton magnetic resonance spectroscopy
MR acquisitions were performed using a 3 T whole-body system (MAGNETOM Trio, Siemens, Erlangen, Germany) at the Unité de Neuroimagerie Fonctionnelle, Centre de recherche de l’Institut Universitaire de Gériatrie de Montréal. Radiofrequency transmission was performed with the built-in body coil, and signal was received with at 32-channel receive-only head coil. The prescription of sensorimotor voxel and detection of potential structural abnormalities were performed using anatomical images of the brain obtained with a T1-weighted MPRAGE sequence (TR = 2300 ms; TE = 2.91 ms; FA: 9°; FOV = 256 × 256 mm; 256 × 256 matrix; TI: 900 ms; 176 slices; orientation: sagittal; acquisition time: 4 min 12 s). The voxel of interest (30 × 30 × 30 mm3) was manually positioned over the left precentral knob without any angulation relative to the scanner reference space (Figure 1). The positioning of the voxel was based on the anatomical landmarks visible on the three orientation slices described by Yoursy and collaborators (1997). First and second order shims were adjusted using FAST(EST)MAP (Gruetter and Tkác, 2000). 1H-MRS data were then acquired using a MEGA-PRESS sequence (Mescher et al., 1998) as previously described (Tremblay et al., 2014b). The MEGA-PRESS acquisition consisted of four blocks of 64 metabolite scans (32 editOff and 32 editOn, interleaved) each with frequency update between blocks (TR = 3 s, TE = 68 ms, total acquisition time: 12 min), as well as single blocks of unsuppressed-water reference scans (4 editOff and 4 editOn, interleaved; acquisition time: 42 s; same parameters as for metabolite scans, but MEGA and VAPOR water suppression off). Free induction decays were stored separately in memory for individual frequency and phase correction using tCr and choline signals between 2.85 and 3.40 ppm. The final spectra were obtained by subtracting (for metabolite scans) or averaging (for unsuppressed-water reference scans) the signal from editOff and editOn scans as described previously (Tremblay et al., 2014b) (Figure 1). The 1H-MRS metabolite acquisition was performed prior to tDCS (1H-MRS pre) and repeated twice following tDCS (1H-MRS post1, 1H-MRS post2), and the 1H-MRS unsuppressed-water reference acquisition followed the acquisition of the pre and post 2 metabolite spectra. A localizer scan was performed prior to the 1H-MRS session and following the last metabolite acquisition to visually compare both scans for head movement.
Figure 1. Representative 1H-MRS spectrum.
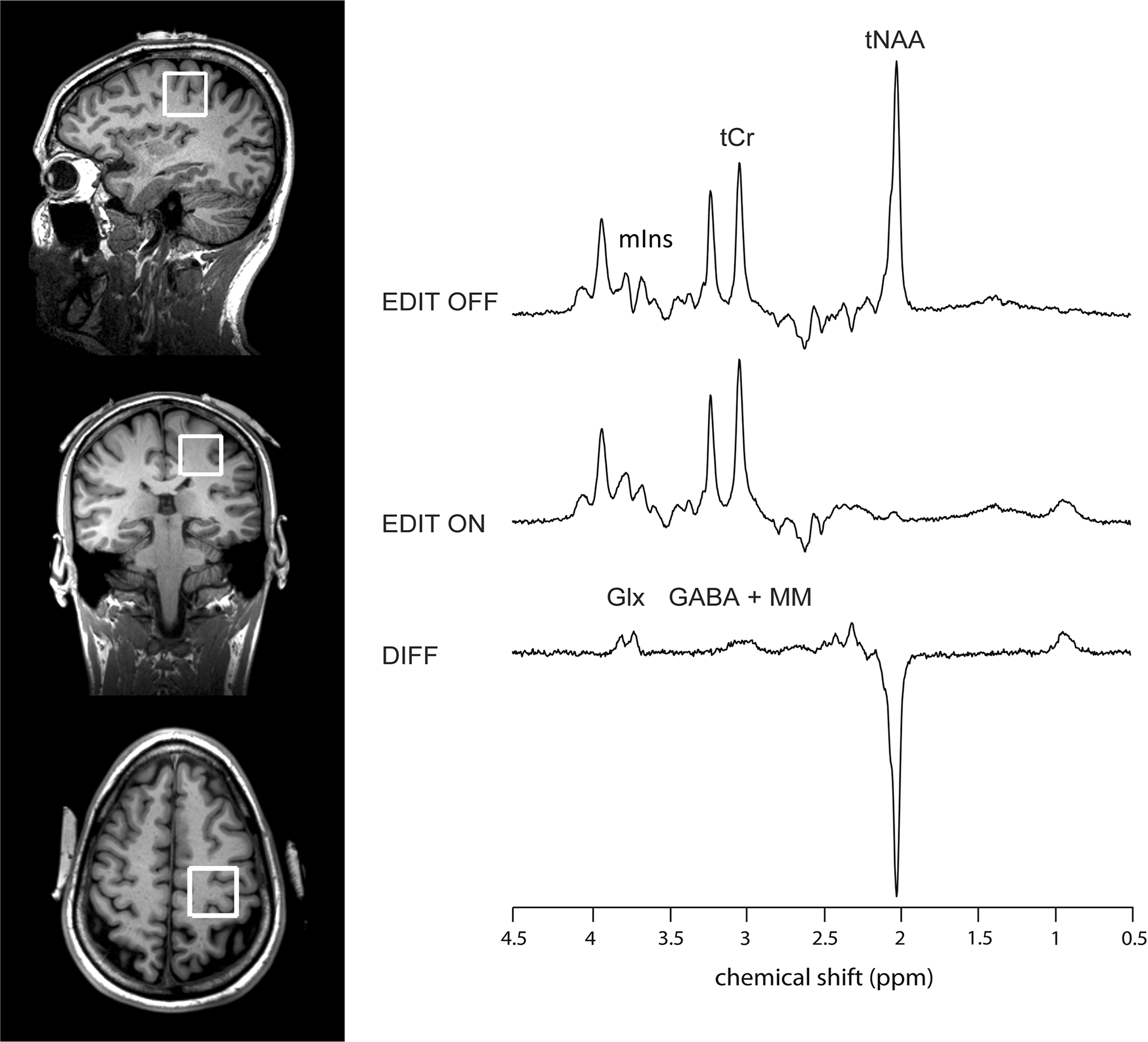
Shows placement of the 1H-MRS voxel over the left sensorimotor region in a single subject, with a representative spectrum obtained with the MEGA-PRESS sequence. tCr = total creatine; tNAA: N-acetyl-aspartate + NAAG; Glx: glutamate + glutamine; GABA + MM: γ-aminobutyric acid + macromolecules; mIns: myo-inositol.
Analysis of 1H-MRS data
Both editOff and difference spectra were analyzed using LCModel 6.3–1 (Provencher, 1993, 2001), which estimated the best fit of the experimental spectrum as a linear combination of model spectra. The basis set for editOff spectra included an experimentally measured metabolite-nulled macromolecular spectrum from the occipital region (average from 11 subjects) and metabolite spectra simulated with home-written software based on density matrix formalism (Henry et al., 2006) in MATLAB, using known chemical shifts and J couplings (Govindaraju et al., 2000) as described previously (Tremblay et al., 2014b). From LCModel’s default simulations of lipid and macromolecular resonances, only “Lip13a” (modeling a broad peak at 1.28 ppm) was allowed during the LCModel fitting that was performed over the spectral range from 0.2 to 4.0 ppm, and LCModel spline model of the baseline was deactivated using the NOBASE = T input parameter. The basis set for difference spectra included an experimentally measured metabolite-nulled macromolecular spectrum from the occipital region (average from 11 subjects) and the experimentally measured spectra from 100 mM phantoms of NAA, GABA, Glu and Gln at 37°C and with pH adjusted to 7.2. No LCModel default simulations of lipid and macromolecular resonances were allowed during the LCModel fitting that was performed over the spectral range from 0.5 to 4.0 ppm, and LCModel spline model of the baseline was also deactivated using the NOBASE = T input parameter. No baseline correction, zero-filling, or apodization functions were applied to the in vivo data prior to LCModel analysis. tCr (Cr-CH3 + PCr-CH3), mIns, and tNAA (sNAA+NAAG) concentrations were obtained from editOff spectra, and GABA and Glx concentrations were obtained from difference spectra. A scaling factor between the simulated and measured basis sets was calculated using the group average of tNAA measured from editOff spectra and the group average tNAA from difference spectra. The spectra were visually inspected for contamination from subscapular lipid signals and Cramér-Rao lower bounds (CRLB) and linewidth of water spectra were examined for outliers. Given the current methodological issues related to the use of CRLB for quality control of data when decreases in concentration are expected (see Kreis et al. 2015), the CRLB values were also examined for outliers (M ± 3 SD). This led to rescanning of 8 1H-MRS sessions. For each time point, the final CRLB were < 30% for Glx, tNAA, and tCr. For each GABA time point, CRLB were generally 40% (M = 18.13 ± 8.04). However, the CRLB value of the “post2” time point of the cathodal condition was 46% (slightly over 3 SD) for participant 1, and the three time points were > 52% (over 3 SD) for participant 4 (sham condition). After a visual inspection of the 1H-MRS data and localizer images for head motion, data from participant 1 were not excluded, but GABA concentrations from participant 4 (sham condition) were excluded from further analyses. The linewidth of each water spectra was < 10 Hz. Metabolite concentrations were quantified using the water reference and in secondary analysis using tCr.
Quantification using water reference
Quantification was performed using an unsuppressed water signal obtained from the same voxel after eddy current correction (Kolse, 1990) and after averaging editOff and editOn scans. The pre-tDCS water reference scan was used for the quantification of the pre-tDCS metabolite scan, and the post-tDCS water reference scan was used for the quantification of both post-tDCS metabolite scans. Concentrations were corrected for cerebrospinal fluid (CSF) content. The tissue composition was obtained from the high-resolution anatomical MR images of the sham session of each subject, which were segmented to gray matter (GM), white matter (WM), and CSF content using the automated FreeSurfer pipeline (V 5.3.0, http://freesurfer.net). The fractional volumes of GM, WM, and CSF were obtained for the 1H MRS voxels. The relative densities of MR-visible water for GM, WM, and CSF were assumed to be 0.78, 0.65, and 0.97 (Gasparovic et al., 2006), respectively. The T1 and T2 relaxation times of water used in the calculation of attenuation factors were taken from published reports [T1(GM) = 1.29 s, T1(WM) = 0.87 s, T1(CSF) = 4 s, T2(GM) = 110 ms, T2(WM) = 80 ms, and T2(CSF) = 400 ms] (Rooney et al., 2007; Wansapura et al., 1999). The water attenuation was computed using the fractional volume of each compartment (Gasparovic et al., 2006).
Distance between M1 and the scalp
Given that previous studies have shown an influence of the scalp-to-cortex distance on TMS measures such as motor threshold (McConnell et al., 2001), scalp-to-cortex distances were assessed in all participants taking part in the 1H-MRS study to determine their impact on individual responses to stimulation. From the sagittal view of the MPRAGE scan (sham condition) of each individual participant, the distance between M1 and the scalp was measured using a previously described procedure (McConnell et al., 2001) (Figure 2).
Figure 2. Scalp-to-M1 measurements.
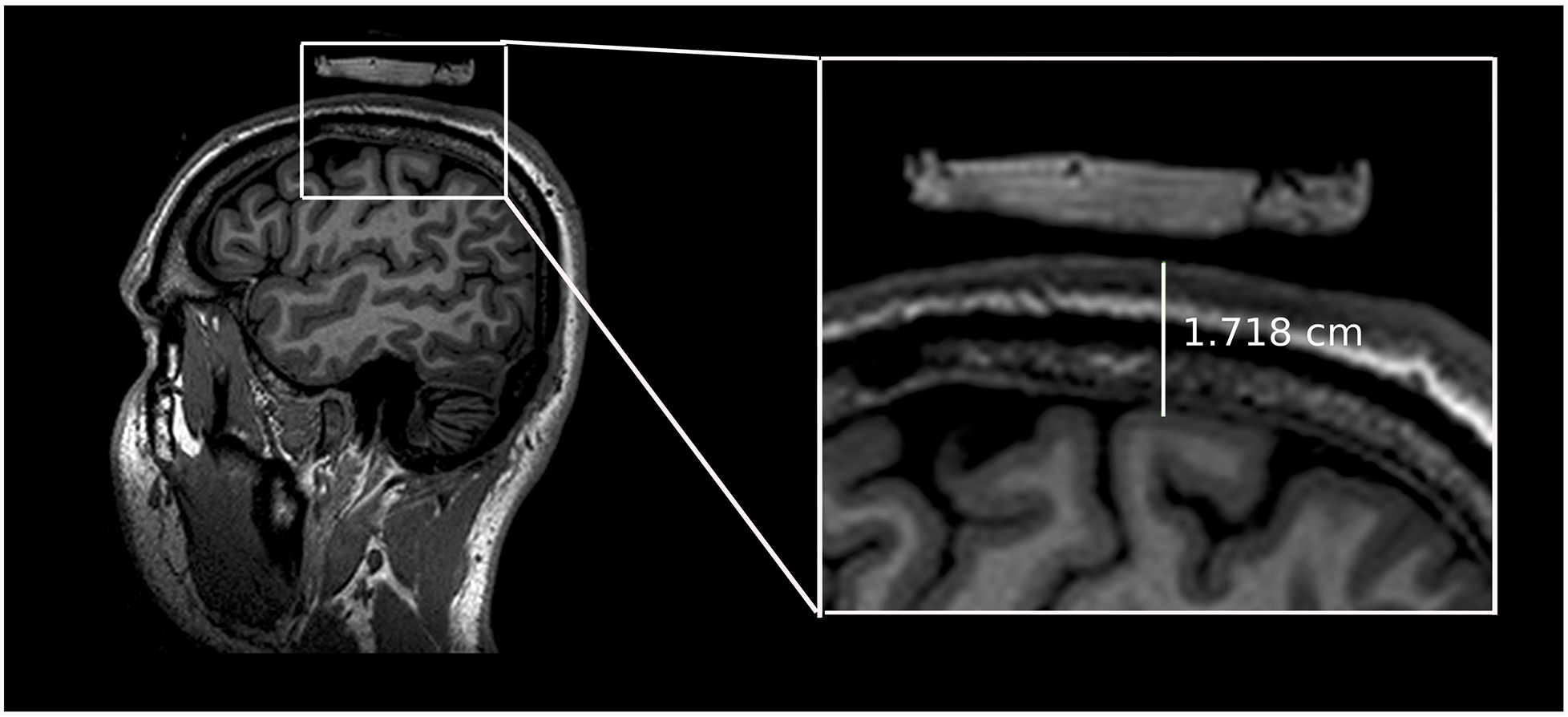
Shows an example of a measurement of the scalp-to-M1 distance for a single participant.
Statistics
Data were analyzed separately using a standard statistical software package (version 21.0, SPSS inc, Chicago, IL, USA). A p value of < .05 was considered statistically significant. When significant effects were observed, post-hoc analyses were computed and the p value was adjusted for multiple comparisons (Bonferonni correction). For the main analyses, effect size was calculated and expressed as partial eta-squared (η2partial). When necessary, non-sphericity was adjusted using Greenhouse-Weisser correction. Values that exceeded 3 standard deviations from the mean were excluded from further analyses, which led to the exclusion of one TMS data point (subject 2, post12). For both experiment, power-analyses were conducted using G*Power 3.1 (Faul et al., 2007) to determine the required sample size to obtain a significant main effect or interactions, when results were close to significance.
For Experiment 1, individual peak-to-peak MEP size was calculated offline and averaged for each subject and each condition. The mean latencies of the MEP were also calculated to ensure proper recruitment of cortical excitability. Paired-sample t-Tests were used to compare raw baseline MEPs between conditions. Then, normalized (ratios of change from baseline) MEP data were compared using a general linear model repeated-measure analysis of variance (RM-ANOVA), with factors Polarity (LA/RC, LC/RA, sham) and Time (post0, post12). To account for differences in time-dependent arousal, the root mean square (RMS) of the EMG signal recorded 50ms prior to the TMS pulse was calculated and data were compared using a repeated-measure ANOVA, with factors Polarity (LA/RC, LC/RA, sham) and Time (pre, post0, post12). To account for within-subject variability in MEP amplitudes within a time-point and therefore use all information available, individual MEPs were included in a generalized estimation equations model (GEE: Hanley et al. 2003). The model included “subjects” as cluster variable, and “time” and “condition” as within-subjects factors. Following a recent study (Feurra et al., 2013), we used an autoregressive-lag1 (AR1) with an M-dependent working correlation (value of 4). MEP values were log-transformed and the Wald test was used to assess significance. Because of the use of all time points (including the baseline) in the analysis, only the interaction time X condition was assessed. Finally, to assess inter-individual variability, classification of response rate was conducted for both active conditions. The standard error of the mean (SEM) was used as an objective criterion (Simeoni et al. 2015). The average of normalized MEPs (post0 and post12) was considered a significant change following stimulation when it exceeded ± 95% confidence interval of the grand-average of the individual baseline SEM value. Using this criterion, individuals were divided in three clusters as follows: Responders Excitation (ratio > 1.26), Non Responders (1.26> ratio < .74) and Responders Inhibition (ratio < .74). Finally, two-tailed Pearson correlation coefficients were computed between the baseline raw MEP amplitudes and the average of normalized MEP amplitudes for each active condition to assess prediction of response (see Wiethoff et al., 2014).
For Experiment 2, metabolite concentrations quantified using tCr and water (normalized as ratios of change from baseline) were compared using a repeated-measure ANOVA, with factors Polarity (LA/RC, LC/RA, sham) and Time (post1, post2). Two-tailed Pearson correlation coefficients were computed between scalp-to-cortex measures and metabolite concentrations. As for experiment 1, assessment of inter-individual variability was conducted according to a 95% of confidence interval of the SEM for GABA concentrations. Using this criterion, individuals were divided in three clusters as follows: Responders Excitation (ratio > 1.11), Non Responders (1.11> ratio < .89) and Responders Inhibition (ratio < .89).
Results
Experiment 1
Mean latencies of the MEPs as well as raw MEP amplitudes for each condition are presented in Table 1. Baseline raw MEP amplitudes were not different between conditions, as assessed with paired-sample t-tests (Bonferonni corrected). A repeated-measures ANOVA was computed on normalized MEP amplitudes (post0/pre; post12/pre; see Figure 3a). No significant main effect of Polarity (F(2,9) = 1.72; p = .22; η2partial = .22) or Time (F(2,9) = .79; p = .41 η2partial = .12) was observed. The interaction was also not significant (F(2,9) = .15; p = .86; η2partial = .03). Power analyses revealed a required sample size of 20, 36 and 143 to obtain a significant effect of polarity, time and interaction, respectively. For pre-trigger muscle activity, a repeated-measures ANOVA of RMS data revealed no significant main effect of Time (F(3,9) = 1.00; p = .39) or Polarity (F(3,9) = 0.22; p = .98) and the interaction was not significant (F(3,9) = 1.32; p = .28). The GEE analysis revealed a significant interaction between Time and Condition (Wald = 33.08, p = .0001). Post-hoc pairwise comparisons (Figure 3b) showed a significant difference between the LA/RC Post0 compared to LA/RC baseline (Mean difference = .28, p = .007), indicating a reduction of MEP size. The LA/RC Post0 time point was also significantly different from the baselines measures of the two other conditions (LC/RA: Mean difference = .23, p = .047; Sham: Mean difference = .27, p = .038).
Table 1.
Mean latency and amplitude of motor evoked potentials for each condition and time point
| tDCS condition | Pre | Post0 | Post12 | |||
|---|---|---|---|---|---|---|
| Latency (ms) | Amplitude (mV) | Latency (ms) | Amplitude (mV) | Latency (ms) | Amplitude (mV) | |
| LA/RC | 21.78 (1.61) | .91 (.26) | 22.20 (1.29) | .64 (.37) | 22.25 (1.70) | .62 (.30) |
| LC/RA | 22.10 (1.35 | .87 (.31) | 22.67 (1.50) | .82 (.33) | 22.62 (1.78) | 1.09 (.60) |
| Sham | 21.90 (1.33) | .91 (.18) | 22.00 (1.53) | .95 (.56) | 22.40 (1.52) | .92 (.48) |
Data presented as: Mean (SD).
Figure 3. Effects of M1-M1 tDCS on MEPs.
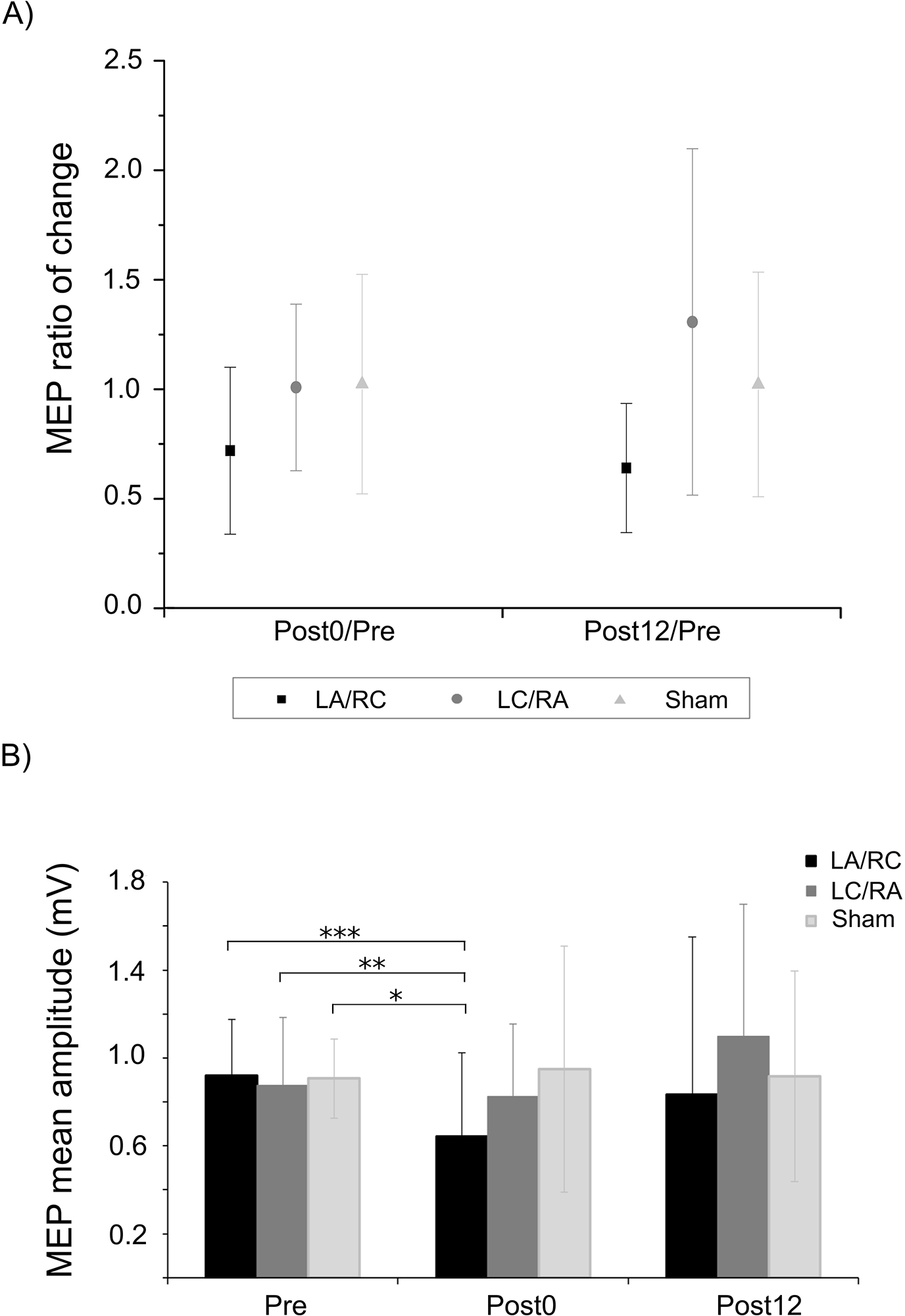
A) Shows average change ratio (± SD) at the two time-points following the three M1-M1 tDCS conditions. No modulation is observed for both active M1-M1 conditions when compared to sham. B) Shows average raw MEP amplitudes (± SD) at the three time points. A significant reduction of MEP amplitude is observed at Post0 following the LA/RC condition when compared to all baseline values. Note that the analysis was conducted on non-averaged MEPs. *** p < .001; ** p < .01; * p < .05
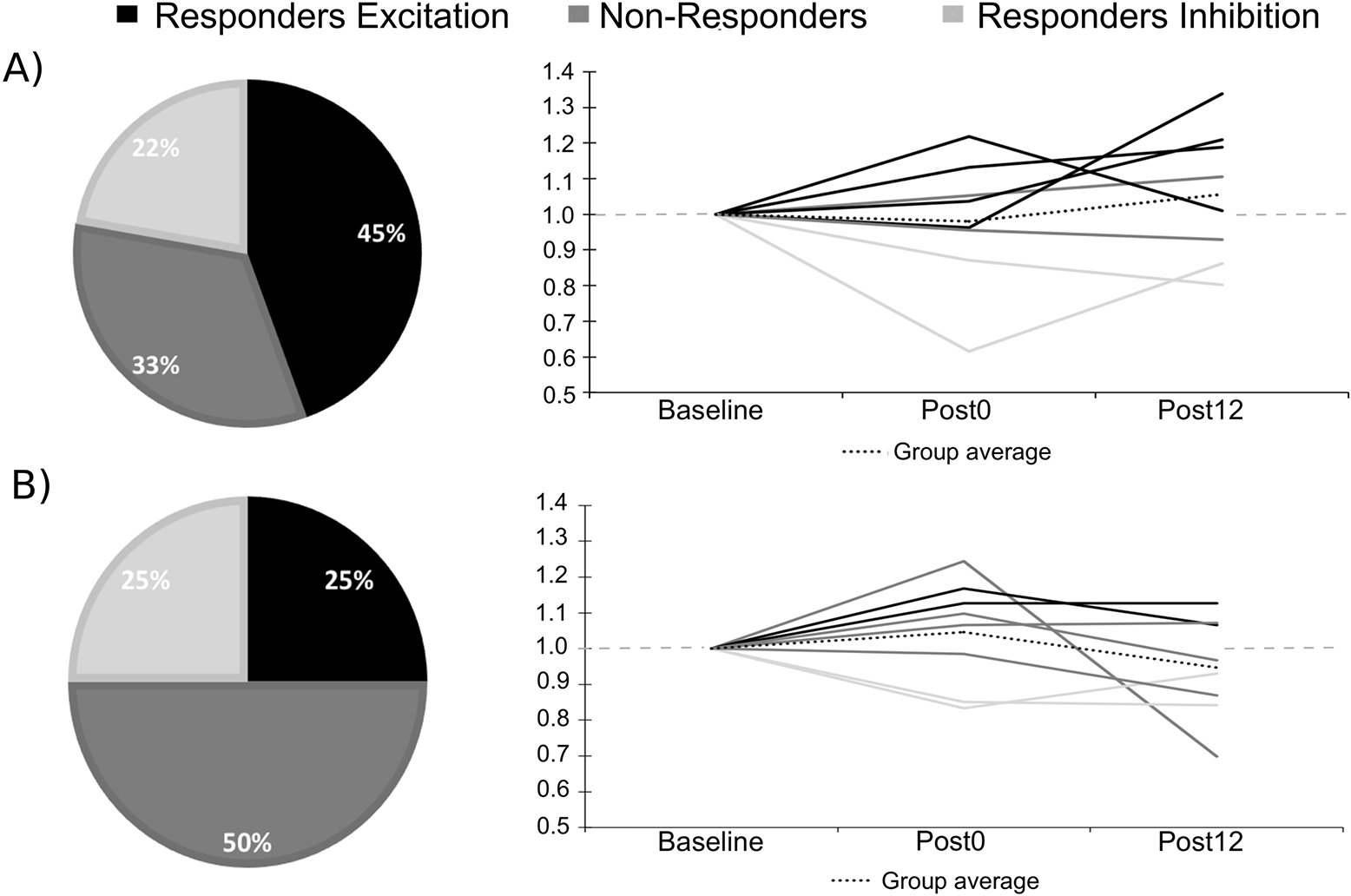
Response rate for both active conditions, individual changes in normalized MEP amplitudes and rates of response are shown in Figure 4. Pearson correlations showed a significant negative correlation between baseline MEP amplitudes and the mean ratio of change following LC/RA tDCS (r = −.66; p = .04; see Figure 5) whereas the correlation was not significant for the LA/RC condition (r = −.33; p = .35).
Figure 4. Response rate following the two active tDCS conditions and correlations with baseline MEP values.
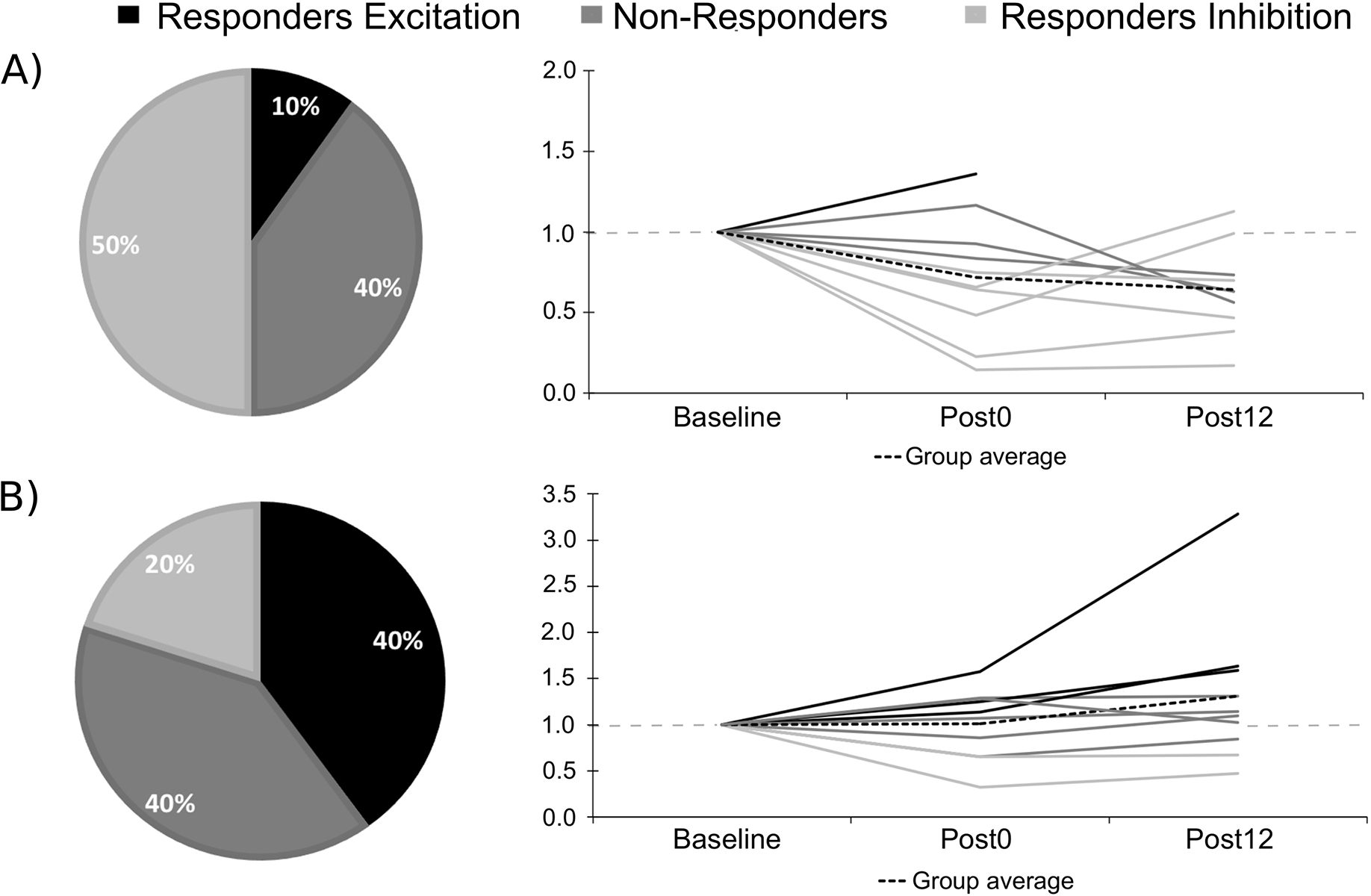
Shows the response rate for the three clusters and the individual MEP ratios of change for A) LA/RC; B) LC/RA. Note that the doted grey line indicates a ratio of 1.00 (no change).
Figure 5. Relationship between baseline MEP values and mean MEP ratio of change.
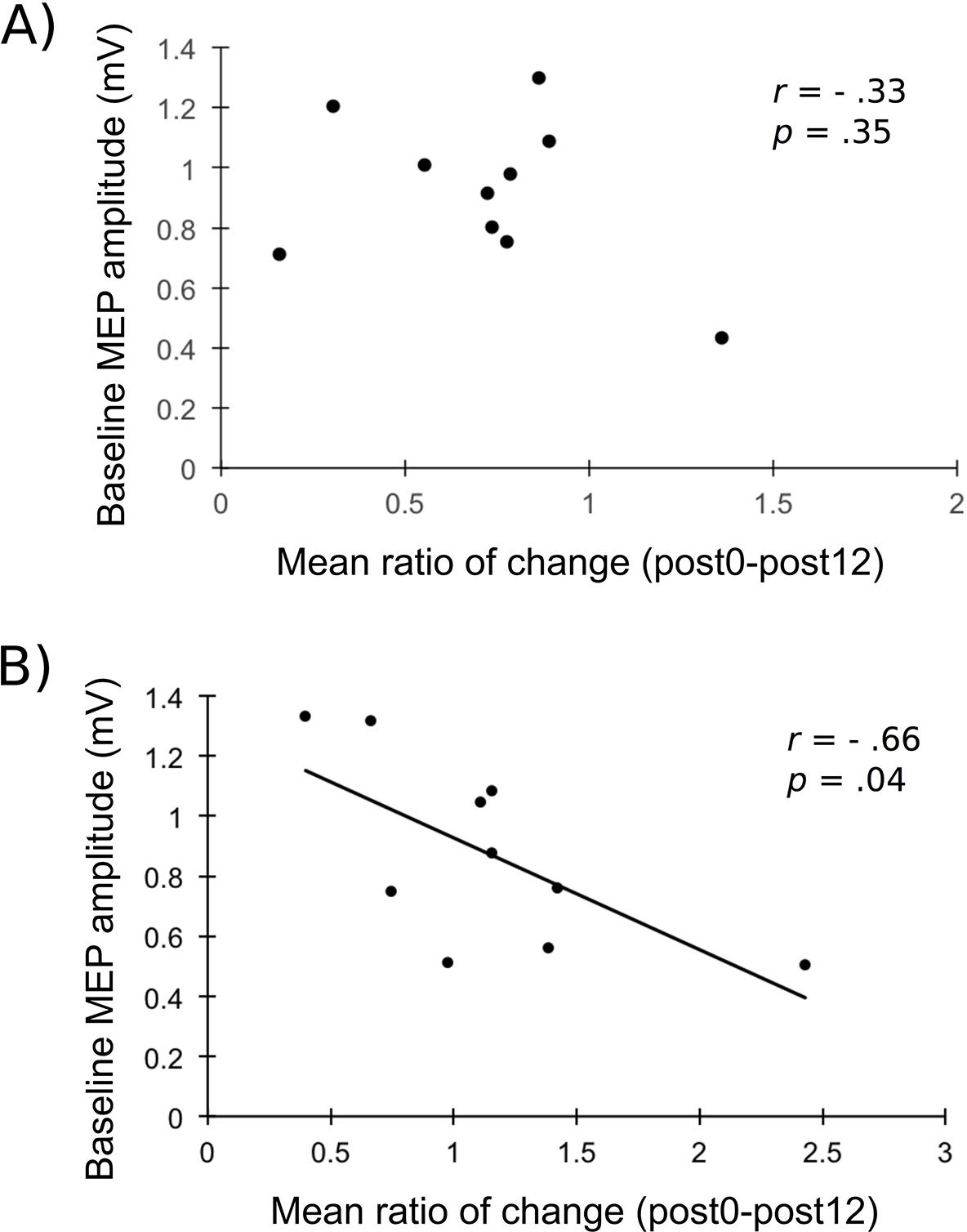
Shows the correlation between the raw baseline average MEP amplitudes and the mean ratio of change (Post0 and Post12 averaged) for A) LA/RC; B) LC/RA. A significant relationship is observed for the LA/RC condition.
Experiment 2
Water-quantified metabolite concentrations
Average percent change in concentration of metabolites following M1-M1 tDCS are shown in Table 2. Change ratios between pre-tDCS and post-tDCS measures (Post1/Pre; Post2/Pre) were calculated for each metabolite of interest and were used for analysis. To assess the effects of tDCS on metabolite concentration, a 2 X 3 repeated measures ANOVA with Time and Polarity as factors was computed for each metabolite of interest. Figure 6 shows changes in concentration ratios. For GABA, no significant main effect of Time (F(2,6) = .16; p = .70; η2partial = .02) or Polarity (F(2,6) = .03; p = .97; η2partial = . 005) was found. A trend towards significance was shown for the interaction between factors (F(2,6) = 3.51; p = .063; η2partial = .37). Power analyses revealed that sample sizes of 212, 859 and 12 would be required to obtain a significant effect of time, polarity and interaction, respectively. Response rate for both active conditions and individual changes in GABA concentration are shown in Figure 7.
Table 2.
Percent change in water-quantified concentrations of metabolites following M1-M1 tDCS in 8 participants
| Metabolite | Sham tDCS Percent change (M ± SD) |
LA/RC tDCS Percent change (M ± SD) |
LC/RA tDCS Percent change (M ± SD) |
|||
|---|---|---|---|---|---|---|
| Post1 | Post2 | Post1 | Post2 | Post1 | Post2 | |
| GABA | −5.78 ± 16.04 | 4.71 ± 12.44 | −1.96 ± 18.27 | 5.53 ± 18.67 | 4.65 ± 14.65 | −5.36 ± 14.25 |
| Glx | −1.53 ± 8.32 | −3.07 ± 2.17 | −1.56 ± 2.85 | −2.03 ± 4.58 | −1.84 ± 3.26 | −2.64 ± 2.81 |
| tNAA | 0.85 ± 1.01 | 1.82 ± 1.73 | −0.82 ± 1.28 | −0.19 ± 1.65 | 0.14 ± 1.68 | 0.12 ± 1.44 |
| mIns | 2.51 ± 6.84 | 5.40 ± 9.39 | −1.30± 3.44 | −0.50 ± 2.42 | 1.12 ± 6.24 | 2.11 ± 8.44 |
Figure 6. Effects of M1-M1 tDCS on water-quantified concentrations of metabolites.
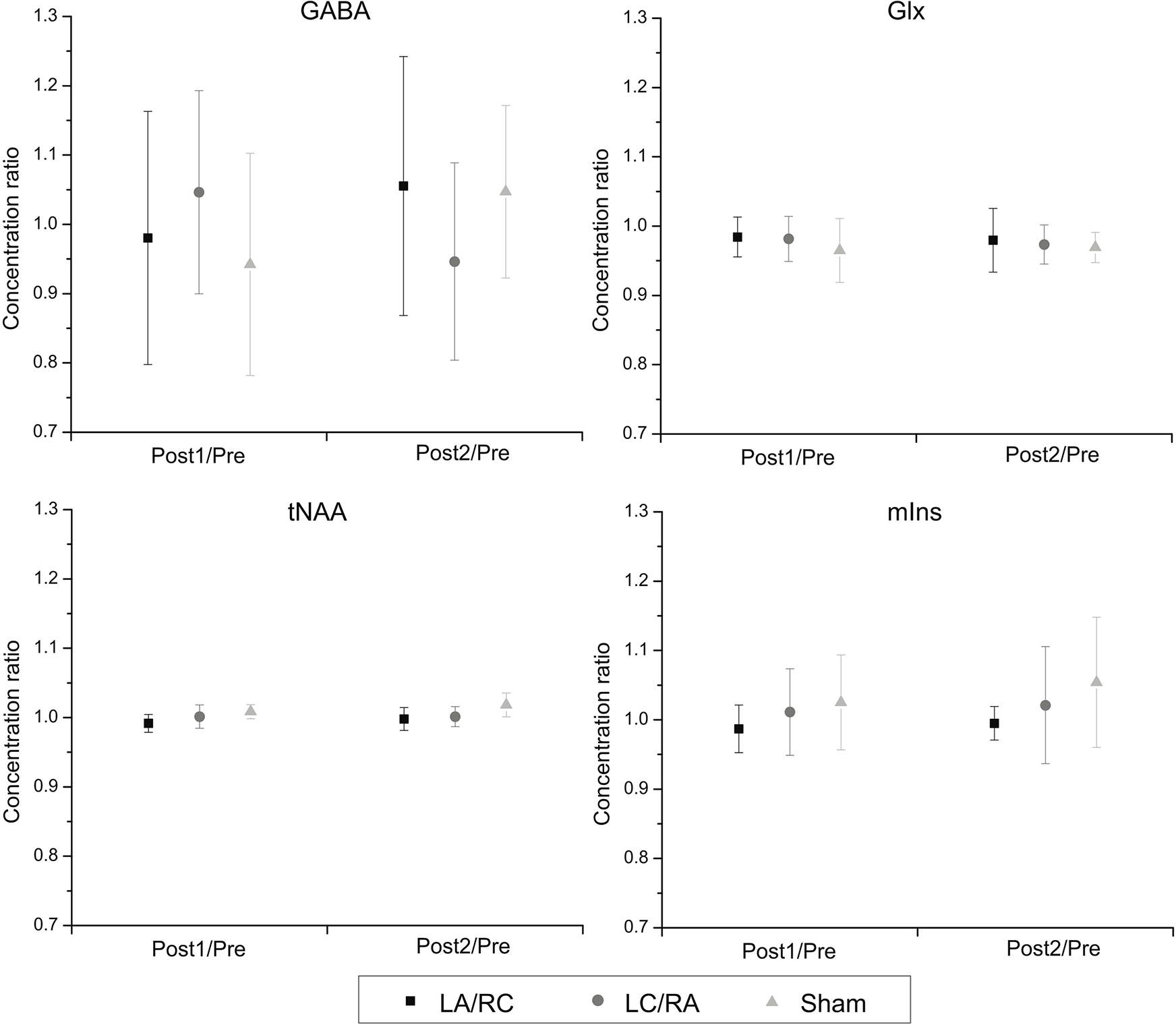
Shows average change ratios (± SD) of water-quantified metabolite concentrations between pre-tDCS and post-tDCS measures. No significant modulation is observed for any metabolite concentration ratios at all time points.
Figure 7. Individual change in GABA concentrations and response rate following the two active tDCS conditions.
Shows the response rate for the three clusters and the individual GABA ratios of change for A) LA/RC; B) LC/RA. Note that the doted grey line indicates a ratio of 1.00 (no change).
For Glx, no significant main effect of Time (F(2,7) = .10; p = .78; η2partial = .02) or Polarity (F(2,7) = .50; p = .61 η2partial = .07) was found. The interaction was also not significant (F(2,7) = .30; p = .75; η2partial = .04). For mIns, no significant main effect of Time (F(2,7) = 3.31; p = .11 η2partial = .32) or Polarity (F(2,7) = 1.32; p = .30 η2partial = .16) was found. The interaction was also not significant (F(2,7) = .38; p = .69; η2partial = .05). For tNAA, no main effect of Time (F(2,7) = 3.63; p = .10; η2partial = .34) or Polarity (F(2,7) = 3.08; p = .08; η2partial = .31) was found. The interaction was also not significant (F(2,7) = 2.26; p = .14; η2partial = .24). Ratios of Glx over GABA were also computed to measure the interaction between the two metabolites (Figure 8). Change ratios were computed as for previous metabolite measurements. No significant main effect of Time (F(2,7) = 3.90; p = .10; η2partial = .36) or Polarity (F(2,7) = .004; p = .99; η2partial = .001) was found. The interaction was also not significant (F(2,7) = 2.44; p = .13; η2partial = .26).
Figure 8. Effects of M1-M1 tDCS on Glx/GABA ratios.
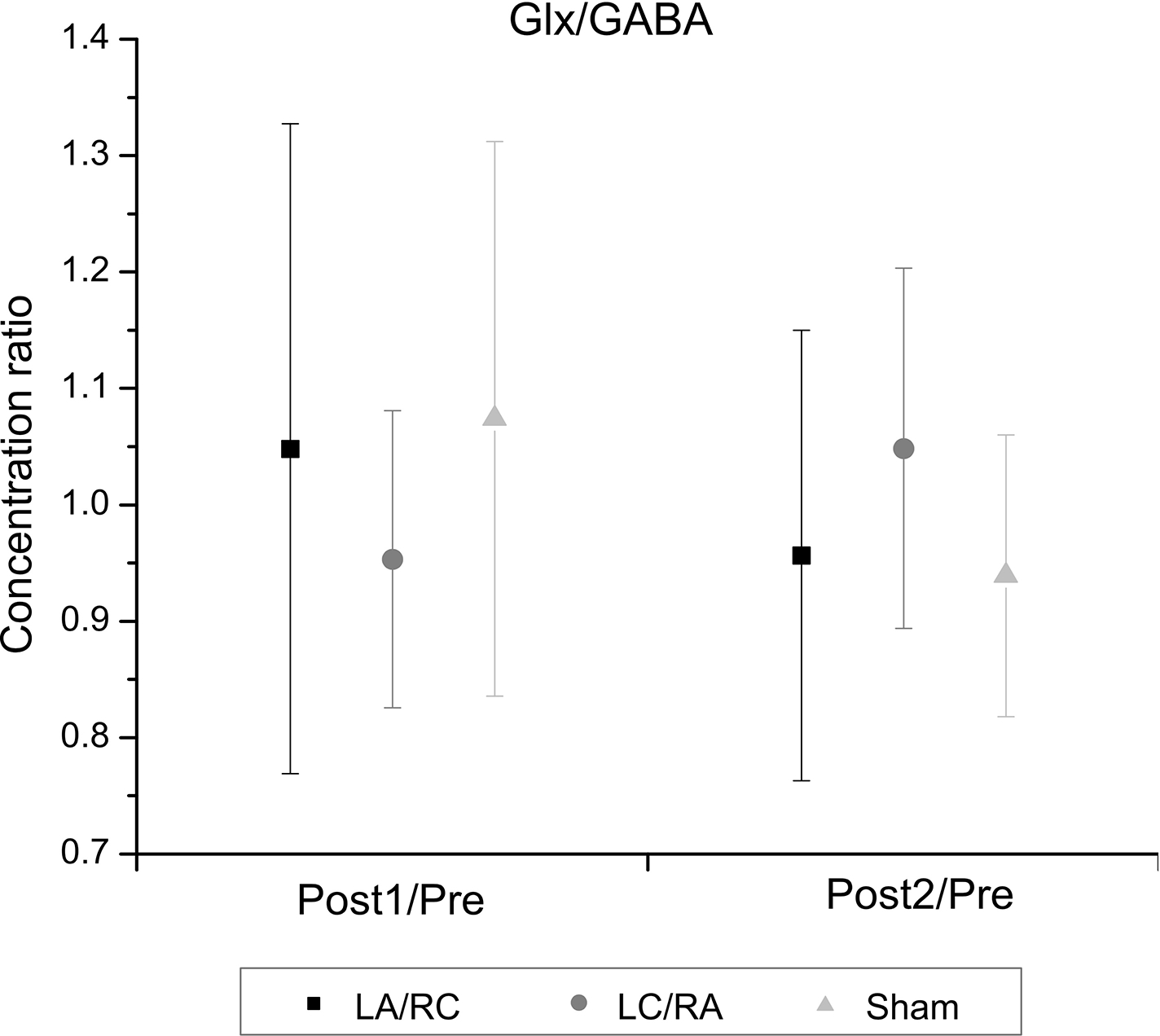
Average change ratios (± SD) following M1-M1 tDCS for the ratio of water-quantified Glx over GABA levels are shown. No significant change is observed.
tCr quantified metabolite concentrations
Prior to tCr scaling, a 3 × 3 repeated measures ANOVA with time and polarity as factors was computed on raw tCr concentrations to confirm the stability of the reference metabolite. No significant main effect of Time (F(2,7) = .49; p = .63) or Polarity (F(2,7) = .74; p = .49) was found. The interaction was also not significant (F(2,7) = .90; p = .48). Metabolite concentrations were then computed as ratios over tCr for secondary analyses. Results were highly similar to those of water-quantified metabolites. Statistical analyses are presented in Table 3.
Table 3.
Repeated measures ANOVA for concentration of metabolites quantified using tCr.
| Metabolite | Main effect: Time | Main effect: Polarity | Interaction | |||
|---|---|---|---|---|---|---|
| F value | p value | F value | p value | F value | p value | |
| GABA | .16 | .70 | .02 | .98 | 2.89 | .10 |
| Glx | .57 | .47 | .67 | .53 | .07 | .93 |
| tNAA | .20 | .67 | 2.89 | .09 | .05 | .95 |
| mIns | 1.58 | .25 | 1.16 | .34 | .31 | .74 |
| GABA/Glx | 2.85 | .14 | .04 | .96 | 2.35 | .14 |
Scalp-to-cortex measures
The average scalp-to-cortex distance was 17.36 mm (SD = 2.73 mm), which is comparable to previous studies using the same protocol (Lepage et al., 2011; McConnell et al., 2001). Bivariate Pearson’s correlations were performed between scalp-to-M1 distance and absolute percent change (water-scaled) following both active conditions. Bonferonni corrections were applied for multiple comparisons. No significant correlation was found for any metabolite of interest. Given the exploratory nature of this analysis, absolute percent change for all metabolites and all conditions was also computed in a single correlation (N = 128) to increase statistical power. The correlation was not significant (r = .10, p = .25).
Discussion
The present set of experiments investigated the effects of M1-M1 tDCS on corticospinal excitability, as well as the effects of M1-M1 tDCS on sensorimotor cortex metabolism. The two active tDCS interventions (LA/RC and LC/RA) did not significantly modulate left M1 corticospinal excitability compared to sham stimulation. Similar results were obtained with 1H-MRS, where M1-M1 tDCS failed to modulate GABA, Glx, tNAA or mIns concentrations.
Neurophysiological effects of M1-M1 tDCS
The failure of M1-M1 tDCS to modulate corticospinal excitability in the present study is in line with a previous report where identical stimulation parameters were used (O’Shea et al., 2013). In that study, M1-M1 tDCS (1 mA, 20 min, 35 cm2 electrode size) resulted in no MEP size difference between LA/RC stimulation and sham tDCS. These results contrast with previous studies that reported “classical” effects of M1-M1 tDCS (increased excitability under the anode and decreased excitability under the cathode) (Kidgell et al., 2013; Mordillo-Mateos et al., 2012; Tazoe et al., 2014). In fact, examination of individual responses of the present sample shows that corticospinal excitability was reduced in 50% of the sample following LA/RC stimulation and increased in 40% of the sample after LC/RA stimulation. The reduction in MEP amplitude was significant immediately after LA/RC stimulation, as revealed by the GEE analysis, but not different from sham. Although caution should be taken in the interpretation of the present data given small sample sizes and non-significant group effects, they suggest that our sample showed a tendency towards opposite polarity-dependent effects.
A possible explanation for this discrepancy may reside in recently reported non-linear effects of duration and intensity of M1-supraorbital tDCS on corticospinal excitability (Batsikadze et al., 2013; Fricke et al., 2011). A systematic evaluation of M1-M1 stimulation parameter efficacy is needed to determine whether changing stimulation duration or intensity, for example, can reverse polarity-dependent effects or increase efficacy. This has significant importance considering the use of M1-M1 tDCS in clinical populations (e.g. Lindenberg et al. 2013; Sehm et al. 2013; Vines et al. 2008).
In addition, fine changes in the balance between excitation and inhibition within the motor system could account for our contrasting results. It was recently suggested that the excitatory/inhibitory balance differs between individuals and may contribute to inter-individual variability following supraorbital-M1 stimulation (Krause, 2013). Due to strong interactions between primary motor cortices, the effects of M1-M1 tDCS on corticospinal excitability may be more complex than those of M1-supraorbital tDCS. In fact, by stimulating both M1 simultaneously, it is likely that the effects of tDCS not only occur in each M1 separately, but also in the balance of inhibitory/excitatory interactions within and between both areas. For example, Tazoe et al. (2014) found reduced interhemispheric inhibition (IHI) from the left M1 to the right M1 and increased IHI from the right to the left M1 in a LC/RA stimulation protocol. In another study, short-interval intracortical inhibition (SICI) was found to be significantly reduced under the anode following M1-M1 stimulation (Kidgell et al., 2013). Consequently, the absence of MEP modulation following M1-M1 tDCS does not necessarily imply a lack of stimulation effects. In fact, MEP amplitudes may not be the most reliable measure of bi-hemispheric stimulation because of these interactions. Other measures of M1 excitability, such as IHI and SICI, may be more sensitive to M1-M1 stimulation effects than MEPs..
M1-M1 tDCS effect on sensorimotor metabolism
In line with neurophysiological results, M1-M1 tDCS did not significantly modulate metabolite concentrations in sensorimotor cortex. Although this is the first study to report 1H-MRS-derived metabolite levels following M1-M1 tDCS, previous studies investigating the effects of M1-supraorbital tDCS have reported metabolite concentration modulation following stimulation. Cathodal stimulation has been associated with reduced Glx concentration (Stagg et al., 2009), while no effect was observed in another study (Kim et al., 2014). Anodal stimulation, on the other hand, failed to modulate Glx levels (Kim et al., 2014; Stagg et al., 2009). For GABA, four studies have reported reduced concentrations following anodal stimulation (Bachtiar et al., 2015; Kim et al., 2014; Stagg et al., 2009;2011), which were associated with motor learning (Stagg et al., 2011), whereas cathodal stimulation has been associated with both a reduction (Stagg et al., 2009) and an absence of effect (Kim et al., 2014). Taken together, these studies suggest an involvement of GABA in the physiological effects of M1-supraorbital a-tDCS. In the present study, examination of response rates revealed a tendency towards increased GABA concentration in 45% of participants following LA/RC stimulation, whereas no clear pattern of responders/non-responders emerged following LC/RA tDCS. In line with results from the TMS experiment, these trends are opposite to our hypotheses based on M1-supraorbital studies and further suggest that M1-M1 stimulation may induce more complex effects on excitatory and inhibitory mechanisms within primary motor cortex.
Very few studies have directly compared the effects of uni-hemispheric and bi-hemispheric tDCS protocols. Using TMS, Mordillo-Mateos et al. (2012) found no significant difference in the induced corticospinal excitability changes between the two protocols. Interestingly, Linderberg and collaborators (2013) reported greater BOLD activity in bilateral M1 during a reaction time task following M1-M1 tDCS compared to M1-supraorbital tDCS, which may be partly explained by state-dependent effects (Silvanto et al., 2008). As a result, coupling M1-M1 tDCS with a motor task may possibly prove more effective in producing robust neurophysiological, metabolic and behavioral effects.
In non-motor regions, a recent study using a bilateral prefrontal tDCS montage reported elevations in prefrontal NAA and striatal Glx concentrations during stimulation, which were no longer present following stimulation (Hone-Blanchet et al., 2016). This suggests that modulation of GABA and Glx could have been present during stimulation in the current study. In line with this, a recent study showed a slight decrease of GABA levels during M1-supraorbital anodal tDCS (Bachtiar et al., 2015). Online effects on sensorimotor metabolism remain to be assessed using a M1-M1 montage.
Variability of tDCS effects
A series of recent studies have highlighted the fact that large inter-individual variability in the response to M1-supraorbital tDCS (López-Alonso et al., 2014; Strube et al., 2015; Wiethoff et al., 2014) is one of the main factors driving inconsistent or negative results in the response to M1-supraorbital tDCS, with an average of half of the sample showing the expected changes in M1 cortical excitability. The present study suggests that a M1-M1 stimulation montage does not reduce the inherent variability associated with tDCS.
It is unlikely, however, that variability in 1H-MRS measures per se can explain this result. Indeed, the within-session reproducibility of four MEGA-PRESS acquisitions over the dorso-lateral prefrontal region at 3 T was recently reported (O’Gorman et al., 2011). High reproducibility was found, with low coefficients of variation between the four acquisitions: 0.07 for GABA and 0.06 for Glx. Similar coefficients of variation were observed in the present study for sham tDCS (pre, post1, post2; GABA = 0.09; Glx = 0.03). Coefficients of variation, however, were much more elevated for TMS measures: in the sham condition (pre, post1, post2), the coefficient of variation was 0.25. Intra-individual variation of TMS-induced MEP amplitudes is a well-documented phenomenon (Darling et al., 2006; Kiers et al.,1993; Pitcher et al., 2003), with MEP trial-to-trial coefficients of variation reaching upwards of 0.5 depending on TMS intensity (Darling et al., 2006; Pitcher et al., 2003).
The present data suggest that the above-mentioned inter-individual variability should be taken into account in clinical studies using a bi-hemispheric M1-M1 montage, such as with a population of stroke patients. Further studies are needed to investigate predictors of response to M1-M1 tDCS, such as I-wave recruitment (Hamada et al., 2013; Wiethoff et al., 2014), synchronicity in brain oscillations as revealed by electroencephalography (Ferreri et al., 2014) and sensitivity to TMS (Labruna, 2015) that may help identify responders. Exploratory correlational analyses from the present study suggest that individuals with a lower MEP baseline show a tendency towards higher ratios of change, a result that was also reported by Wiethoff and collaborators (2014). This raises important questions regarding the intensity of TMS stimulation used to determine baseline excitability, which may contribute to inter-subject variability (Vallence et al., 2015). Indeed, the findings suggest that smaller MEPs may be associated with higher response rates. As a result, a 1mV criterion for determining stimulation intensity may be too high for some individuals and lead to ceiling effects and reducing sensitivity. It should however be noted, conversely, that a recent study showed that higher stimulation intensities at baseline (150% of the resting motor threshold) induce stronger suppression of MEPs following continuous theta burst stimulation (Vallence et al., 2015).
Finally, it is important to note that numerous approaches have been proposed to maximize the effects of tDCS on cortical excitability (e.g. Dmochowski et al. 2013). In clinical populations where interhemipheric balance is compromised, such as the case of stroke, monopolar bilateral stimulation could be a viable alternative to bi-hemispheric M1-M1 tDCS. In monopolar bilateral stimulation, M1-supraorbital stimulation is applied to each hemisphere (either anodal or cathodal). This type of stimulation has been used successfully in healthy individuals (Xu et al., 2015) and, in stroke patients, may theoretically be more effective in modulating activity independently in each M1. This is also relevant in healthy individuals, where transcallosal connections may be dynamically involved in updating the excitatory/inhibitory balance between the two hemispheres.
Also, generalization of the present data may be limited by the lack of a double-blind design, as well as the small sample sizes in both studies. Sample size was based on current literature reporting significant MEP and metabolite concentration modulation with an average of 8 and 10 participants for 1H-MRS and TMS studies, respectively. Power calculations showed that considerable increases in sample size would be required to obtain a significant effect of active vs. sham stimulation for both 1H-MRS and TMS experiments. In light of the high cost and time-intensive analyses associated with MR spectroscopy, the present data suggest that 1H-MRS may not be an effective tool to assess tDCS treatment response in clinical studies where eligible patients are limited, such as stroke survivors. As a result, the present study suggests that small exploratory studies of tDCS response and mechanism are probably underpowered to account for the large inter-subject variability associated with tDCS.
Conclusion
The present study suggests a limited impact of M1-M1 tDCS on both TMS and 1H-MRS measures of neurophysiology and metabolism. These negative results are in line with recent studies showing highly variable inter-individual responses to M1-supraorbital tDCS and therefore suggest that both techniques may lack sensitivity to reliably quantify the neurophysiological and metabolic effects of M1-M1 tDCS. It remains to be determined whether specific behavioral outcomes are more sensitive to the effects of M1-M1 tDCS than TMS and 1H-MRS alone. Importantly, these findings further highlight the importance of uncovering the factors that underlie inter-individual variability in response to non-invasive brain stimulation protocols. Multimodal studies that combine behavioral outcome with neurophysiological and metabolic measures, that systematically evaluate stimulation parameters effects, and that identify factors predicting outcome are greatly needed to support its use in clinical settings.
Acknowledgements
The authors would like to thank Edward J. Auerbach, Ph.D. (Center for Magnetic Resonance Research, University of Minnesota) for implementing MEGA-PRESS sequence on Siemens, and Romain Valabregue, Ph.D. (Centre de NeuroImagerie de Recherche, Paris, France) and Brice Tiret (Unité de neuroimagerie fonctionnelle, Montréal) for developing processing tools. This work was supported by grants from the Canadian Institutes of Health Research and the Fonds de Recherche en Santé du Québec to HT. ST was supported by a Vanier Canada Graduate scholarship of the Canadian Institutes of Health Research. MM acknowledges the support from Biotechnology Research Center (BTRC) grant P41 RR008079 and P41 EB015894 (NIBIB), and NCC P30 NS057091.
References
- Bachtiar V, Near J, Johansen-Berg H, & Stagg CJ (2015). Modulation of GABA and resting state functional connectivity by transcranial direct current stimulation. eLife, 4, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batsikadze G, Moliadze V, Paulus W, Kuo MF, & Nitsche MA (2013). Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. Journal of Physiology, 591, 1987–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, & Cohen LG (1997). Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology, 48, 1398–1403. [DOI] [PubMed] [Google Scholar]
- Darling WG, Wolf SL, & Butler AJ (2006). Variability of motor potentials evoked by transcranial magnetic stimulation depends on muscle activation. Experimental Brain Research, 174, 376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Dileone M, Capone F, Pellegrino G, Ranieri F, Musumeci G, Florio L, Di Pino G, & Fregni F, (2014). Immediate and late modulation of interhemipheric imbalance with bilateral transcranial direct current stimulation in acute stroke. Brain Stimulation, 7, 841–848. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Manganelli F, Dileone M, Notturno F, Esposito M, Capasso M, Dubbioso R, Pace M, Ranieri F, Minicuci G, & Santoro L (2012). The effects of prolonged cathodal direct current stimulation on the excitatory and inhibitory circuits of the ipsilateral and contralateral motor cortex. Journal of Neural Transmission, 119, 1499–1506. [DOI] [PubMed] [Google Scholar]
- Dmochowski JP, Datta A, Huang Y, Richardson JD, Bikson M, Fridriksson J, & Parra LC (2013). Targeted transcranial direct current stimulation for rehabilitation after stroke. Neuroimage, 75, 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, & Buchner A (2007). G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Vecchio F, Ponzo D, Pasqualetti P, & Rossini PM (2014). Time‐varying coupling of EEG oscillations predicts excitability fluctuations in the primary motor cortex as reflected by motor evoked potentials amplitude: An EEG-TMS study. Human Brain Mapping, 35, 1969–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke K, Seeber AA, Thirugnanasambandam N, Paulus W, Nitsche MA, & Rothwell JC (2011). Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation of the human motor cortex. Journal of Neurophysiology, 105, 1141–1149. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, & Lu B (2010). Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron, 66, 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froc DJ, Chapman CA, Trepel C, & Racine RJ (2000). Long-term depression and depotentiation in the sensorimotor cortex of the freely moving rat. Journal of Neuroscience, 20, 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, & Morrison LA (2006). Use of tissue water as a concentration reference for proton spectroscopic imaging. Magnetic Resonance in Medicine, 55, 1219–1226. [DOI] [PubMed] [Google Scholar]
- Gomez Palacio Schjetnan A, Faraji J, Metz GA, Tatsuno M, & Luczak A (2013). Transcranial direct current stimulation in stroke rehabilitation: a review of recent advancements. Stroke Research and Treatment, 2013, 170256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaraju V, Young K, & Maudsley AA (2000). Proton NMR chemical shifts and coupling constants for brain metabolites. NMR in Biomedicine, 13, 129–153. [DOI] [PubMed] [Google Scholar]
- Gruetter R, & Tkác I (2000). Field mapping without reference scan using asymmetric echo-planar techniques. Magnetic Resonance in Medicine, 43, 319–323. [DOI] [PubMed] [Google Scholar]
- Hanley JA, Negassa A, & Forrester JE (2003). Statistical analysis of correlated data using generalized estimating equations: an orientation. American Journal of Epidemiology, 157, 364–375. [DOI] [PubMed] [Google Scholar]
- Hamada M, Murase N, Hasan A, Balaratnam M, & Rothwell JC (2013). The role of interneuron networks in driving human motor cortical plasticity. Cerebral cortex, 23, 1593–1605. [DOI] [PubMed] [Google Scholar]
- Henry P-G, Marjanska M, Walls JD, Valette J, Gruetter R, & Ugurbil K (2006). Proton-observed carbon-edited NMR spectroscopy in strongly coupled second-order spin systems. Magnetic Resonance in Medicine, 55, 250–257. [DOI] [PubMed] [Google Scholar]
- Hone-Blanchet A, Edden RA, & Fecteau S (2016). Online effects of transcranial direct current stimulation in real time on human prefrontal and striatal metabolites. Biological Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath JC, Carter O, & Forte JD (2014). Transcranial direct current stimulation: five important issues we aren’t discussing (but probably should be). Frontiers in Systems Neuroscience, 8, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath JC, Forte JD, & Carter O (2015). Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects. A systematic review. Neuropsychologia, 66, 213–236. [DOI] [PubMed] [Google Scholar]
- Hummel FC, & Cohen LG (2006). Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurology, 5, 708–712. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Koslowsky M, & Lavidor M (2011). tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Experimental Brain Research, 216, 1–10. [DOI] [PubMed] [Google Scholar]
- Kidgell DJ, Goodwill AM, Frazer AK, & Daly RM (2013). Induction of cortical plasticity and improved motor performance following unilateral and bilateral transcranial direct current stimulation of the primary motor cortex. BMC Neuroscience, 14, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiers L, Cros D, Chiappa KH, & Fang J (1993). Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogry and Clinical Neurophysiolology, 89, 415–423. [DOI] [PubMed] [Google Scholar]
- Kim S, Stephenson MC, Morris PG, & Jackson SR (2014). tDCS-induced alterations in GABA concentration within primary motor cortex predict motor learning and motor memory: a 7 T magnetic resonance spectroscopy study. Neuroimage, 99, 237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose U (1990). In vivo proton spectroscopy in presence of eddy currents. Magnetic Resonance in Medicine, 14, 26–30. [DOI] [PubMed] [Google Scholar]
- Krause B (2013). The effect of transcranial direct current stimulation: a role for cortical excitation/inhibition balance? Frontiers in human neuroscience, 7, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis R (2015). The trouble with quality filtering based on relative cramér-rao lower bounds. Magnetic Resonance in Medicine, 75, 15–18. [DOI] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, Rothwell JC, Lemon RN, & Frackowiak RS. (2005). How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? European Journal of Neuroscience, 22, 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage J-F, Clouchoux C, Lassonde M, Evans AC, Deal CL, & Théoret H (2011). Abnormal motor cortex excitability is associated with reduced cortical thickness in X monosomy. Human Brain Mapping, 34, 936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Tergau F, & Paulus W (2002). Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain, 125, 2238–2247. [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Nachtigall L, Meinzer M, Sieg MM, & Floel A (2013). Differential effects of dual and unihemispheric motor cortex stimulation in older adults. Journal of Neuroscience, 33, 9176–9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Alonso V, Cheeran B, Río-Rodríguez D, & Fernández-del-Olmo M (2014). Inter-individual Variability in Response to Non-invasive Brain Stimulation Paradigms. Brain Stimulation, 7, 372–380. [DOI] [PubMed] [Google Scholar]
- McConnell KA, Nahas Z, Shastri A, Lorberbaum JP, Kozel FA, Bohning DE, & George MS (2001). The transcranial magnetic stimulation motor threshold depends on the distance from coil to underlying cortex: a replication in healthy adults comparing two methods of assessing the distance to cortex. Biolological Psychiatry, 49, 454–459. [DOI] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, & Gruetter R (1998). Simultaneous in vivo spectral editing and water suppression. NMR Biomedicine, 11, 266–272. [DOI] [PubMed] [Google Scholar]
- Mordillo-Mateos L, Turpin-Fenoll L, Millán-Pascual J, Núñez-Pérez N, Panyavin I, Gómez-Argüelles JM, Botia-Paniagua E, Foffani G, Lang N, & Oliviero, A. (2012). Effects of simultaneous bilateral tDCS of the human motor cortex. Brain Stimulation, 5, 214–222. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, & Cohen LG (2004). Influence of interhemispheric interactions on motor function in chronic stroke. Annals of Neurology, 55, 400–409. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, & Pascual-Leone A (2008). Transcranial direct current stimulation: State of the art 2008. Brain Stimulation, 1, 206–223. [DOI] [PubMed] [Google Scholar]
- O’Gorman RL, Michels L, Edden RA, Murdoch JB, & Martin E (2011). In vivo detection of GABA and glutamate with MEGA-PRESS: reproducibility and gender effects. Journal of Magnetic Resonance Imaging, 33, 1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea J, Boudrias M-H, Stagg CJ, Bachtiar V, Kischka U, Blicher JU, & Johansen-Berg H (2013). Predicting behavioural response to TDCS in chronic motor stroke. Neuroimage, 15, 924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher JB, Ogston KM, & Miles TS (2003). Age and sex differences in human motor cortex input-output characteristics. Journal of Physiology, 546, 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW (1993). Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic Resonance in Medicine, 30, 672–679. [DOI] [PubMed] [Google Scholar]
- Provencher SW (2001). Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomedicine, 14, 260–264. [DOI] [PubMed] [Google Scholar]
- Rooney WD, Johnson G, Li X, Cohen ER, Kim SG, Ugurbil K, & Springer CS (2007). Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magnetic Resonance in Medicine, 57, 308–318. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, & Safety of TMS Consensus Group. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120, 2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, & Ziemann U (2015). Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an IFCN Committee. Clinical Neurophysiology, 126, 1071–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Gerloff C, & Hummel FC (2013). Non-invasive brain stimulation in neurological diseases. Neuropharmacology, 64, 579–587. [DOI] [PubMed] [Google Scholar]
- Sehm B, Kipping J, Schäfer A, Villringer A, & Ragert P (2013). A Comparison between Uni- and Bilateral tDCS Effects on Functional Connectivity of the Human Motor Cortex. Frontiers in Human Neuroscience, 7, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Muggleton N, & Walsh V (2008). State-dependency in brain stimulation studies of perception and cognition. Trends in Cognitive Science, 12, 447–454. [DOI] [PubMed] [Google Scholar]
- Simeoni S, Hannah R, Sato D, Kawakami M, Rothwell JC, & Gigli GL (2015). Effects of Quadripulse Stimulation on Human Motor Cortex Excitability: A Replication Study. Brain Stimulation. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, & Johansen-Berg H (2011). The role of GABA in human motor learning. Current Biology, 21, 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Best JG, Stephenson MC, O’Shea J, Wylezinska M, Kincses ZT, Morris PG, Matthews PM, & Johansen-Berg H (2009). Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. Journal of Neuroscience, 29, 5202–5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, & Nitsche MA (2011). Physiological basis of transcranial direct current stimulation. Neuroscientist, 17, 37–53. [DOI] [PubMed] [Google Scholar]
- Strube W, Bunse T, Malchow B, & Hasan A (2015). Efficacy and Interindividual Variability in Motor-Cortex Plasticity following Anodal tDCS and Paired-Associative Stimulation. Neural Plasticity, 2015, 530423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazoe T, Endoh T, Kitamura T, & Ogata T (2014). Polarity specific effects of transcranial direct current stimulation on interhemispheric inhibition. PloS One, 9, e114244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, Beaulé V, Lepage JF, & Théoret H (2013). Anodal transcranial direct current stimulation modulates GABAB-related intracortical inhibition in the M1 of healthy individuals. Neuroreport, 24, 46–50. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Beaulé V, Proulx S, Lafleur L-P, Doyon J, Marjańska M, & Théoret H (2014b). The Use of Magnetic Resonance Spectroscopy as a Tool for the Measurement of Bi-hemispheric Transcranial Electric Stimulation Effects on Primary Motor Cortex Metabolism. Journal of Visualized Experiment, 93, e51631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, Lepage JF, Latulipe-Loiselle A, Fregni F, Pascual-Leone A, & Théoret H (2014a). The Uncertain Outcome of Prefrontal tDCS. Brain Stimulation, 7, 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepel C, & Racine RJ (2000). GABAergic modulation of neocortical long-term potentiation in the freely moving rat. Synapse, 35, 120–128. [DOI] [PubMed] [Google Scholar]
- Vallence AM, Goldsworthy MR, Hodyl NA, Semmler JG, Pitcher JB, & Ridding MC (2015). Inter- and intra-subject variability of motor cortex plasticity following continuous theta-burst stimulation. Neuroscience, 304, 266–278. [DOI] [PubMed] [Google Scholar]
- Vines BW, Cerruti C, & Schlaug G (2008). Dual-hemisphere tDCS facilitates greater improvements for healthy subjects’ non-dominant hand compared to uni-hemisphere stimulation. BMC Neuroscience, 9, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansapura JP, Holland SK, Dunn RS, & Ball WS (1999). NMR relaxation times in the human brain at 3.0 tesla. Journal of Magnetic Resonance Imaging, 9, 531–538. [DOI] [PubMed] [Google Scholar]
- Wiethoff S, Hamada M, & Rothwell JC (2014). Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimulation, 7, 468–475. [DOI] [PubMed] [Google Scholar]
- Xu J, Healy SM, Truong DQ, Datta A, Bikson M, & Potenza MN (2015). A Feasibility Study of Bilateral Anodal Stimulation of the Prefrontal Cortex Using High-Definition Electrodes in Healthy Participants. Yale Journal of Biology and Medicine, 88, 219–225. [PMC free article] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, & Winkler P (1997). Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain, 120, 141–157. [DOI] [PubMed] [Google Scholar]


