Abstract
Background
Although an association between COVID-19 vaccination and Bell’s palsy (BP) has been reported, a clear causal relationship has not been elucidated. We investigated the risk and clinical characteristics of BP after COVID-19 vaccination.
Methods
This retrospective chart review evaluated the association between COVID-19 vaccination and BP by comparing the number of patients diagnosed with BP during the pre-COVID-19 vaccination period (March 2018–February 2021) and the COVID-19 mass vaccination period (March 2021–February 2022). We then compared vaccine-related (time between vaccination and BP onset < 42 days) and -unrelated (time interval ≥ 42 days or non-vaccination) clinical characteristics in newly diagnosed patients with BP.
Results
BP occurred more during the COVID-19 vaccination period than in the previous three pre-vaccination years. Thirteen patients developed BP within 42 days of vaccination. All patients, except one, developed BP after mRNA-based vaccination, with most cases (9/13, 69.2%) occurring after the second or third dose. Thirteen patients with vaccine-related BP were younger (age 43.92 ± 13.14 vs. 54.32 ± 16.01 years; p = 0.033) and more frequently experienced taste changes (58.8% vs. 10.9%; p = 0.002) than 52 patients with vaccine-unrelated BP. Patients with vaccine-related BP had a greater likelihood of good and faster (p = 0.042) facial nerve function recovery than those with vaccine-unrelated BP (100% vs. 78%).
Conclusion
COVID-19 vaccines, especially mRNA-based vaccines, may be associated with BP cases with distinctive clinical characteristics, which occur more frequently in young individuals, are frequently accompanied by taste changes, and have fast and good recovery.
Keywords: Bell’s palsy, COVID-19, COVID-19 vaccines, SARS-CoV-2, Vaccination
Introduction
Bell's palsy (BP), characterized by sudden unilateral facial muscle weakness, is an idiopathic form of peripheral facial nerve palsy. Most patients recover fully within 1 year, but some experience residual facial weakness or complications, such as synkinesia and hemifacial spasm. The exact etiology is still unknown, but it is presumed that viral infection and the associated autoimmune response are involved [1, 2].
Although the health and socioeconomic burden of the coronavirus disease 2019 (COVID-19) outbreak continues [3], the development of vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has brought the hope of ending the pandemic [4]. With the increase in global vaccination rates after the approval of COVID-19 vaccines, the safety profiles of vaccines and their preventive effects have emerged as key issues.
Many previous studies have shown the risk of developing BP following influenza vaccination. Although the results are controversial, BP has been reported to have a significant association with the intranasal inactivated influenza vaccine, parenteral seasonal influenza vaccine, and pandemic influenza H1N1 vaccine [5–8]. Similarly, several case reports and case–control studies worldwide have indicated an association of BP with COVID-19 vaccines such as BNT162b2, mRNA-1273, CoronaVac, and Ad26.COV2.S [9–14], although a clear causal relationship has not yet been fully established.
Despite these growing reports of an increased risk of BP following COVID-19 vaccination, to the best of our knowledge, no studies have been published on their clinical characteristics. In the present study, we aimed to investigate the risk and clinical characteristics of BP after COVID-19 vaccination.
Materials and methods
Study design
This study included two parts: Part 1 to assess whether COVID-19 vaccination was associated with an increased risk of BP and Part 2 to analyze the clinical characteristics of BP after COVID-19 vaccination. The study protocol was approved by the Institutional Review Board of Keimyung University Dongsan Hospital (IRB No. 2022–04-044).
Part 1: COVID-19 vaccination and risk of BP
This retrospective cohort study was conducted to investigate the potential association between COVID-19 vaccination and BP. We retrieved medical record data of adult patients (older than 18 years) first diagnosed with BP at Keimyung University Dongsan Hospital during 1) the pre-COVID-19 vaccination period (March 2018 to February 2019, March 2019 to February 2020, and March 2020 to February 2021) and 2) the COVID-19 mass vaccination period (March 2021 to February 2022). As the COVID-19 mass vaccination began on February 25, 2021, in South Korea, the study period was divided into 1-year intervals from the March of each year to the February of the following year. The diagnosis of BP was determined based on the clinical practice guidelines suggested by the American Academy of Otolaryngology-Head and Neck Surgery Foundation in 2013 [15]. We compared the overall number of patients who were first diagnosed with BP during the COVID-19 vaccination period from March 2021 to February 2022 with those who were diagnosed during the same calendar months in the three preceding years before the introduction of the COVID-19 vaccine.
Part 2: clinical characteristics of BP after COVID-19 vaccination
Of all patients with BP identified in Part 1, Part 2 included only patients who were newly diagnosed during the COVID-19 vaccination period (March 2021 to February 2022). We excluded patients with incomplete clinical data from the analysis. The patients were divided into two groups according to the temporal relationship between the onset of facial weakness and COVID-19 vaccination: vaccine related (time between vaccination and BP onset < 42 days) and vaccine unrelated (time interval ≥ 42 days or non-vaccination). We chose 42 days as the criterion for dividing the two groups because this period is considered a plausible time window in which adverse events may reasonably be associated with vaccination [16–18].
We collected clinical information on age, sex, medical history, vaccination status, date of vaccination, and type of vaccine administered. Clinical data of BP, including symptom onset, associated symptoms, severity of facial weakness, degree and length of recovery, magnetic resonance imaging (MRI) findings of facial nerves, facial nerve conduction study results, and prescription medications, were also recorded. The associated symptoms included pain around the ear or at the back of the head, hyperacusis, and changes in taste or lacrimation. The severity of facial weakness was graded on a scale of 1 to 6 using the House–Brackmann (H–B) facial nerve grading system [19] and then classified into normal (H–B grade 1), mild (H–B grade 2), moderate (H–B grade 3), severe (H–B grades 4–5), or very severe (H–B grade 6). To evaluate MRI enhancement patterns, the facial nerve was divided into intrameatal, labyrinthine, geniculate ganglion, tympanic, and mastoid segments [20]. Enhancement of the facial nerve was evaluated by three blinded radiologists. The degree of functional recovery was evaluated based on the H–B grade at every follow-up visit. Good recovery was defined as H–B grades 1–2 and poor recovery as H–B grades 3–6 [21]. In addition, to characterize the recovery time after BP, we assessed the proportion of patients who achieved good recovery at each of the following time points over the follow-up period: < 1 month, 1–2 months, 2–3 months, 3–4 months, and > 4 months.
Statistical analysis
The data were analyzed using SPSS Statistics (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp). The results are expressed as mean ± standard deviation or median [first interquartile—third interquartile] for continuous variables and numbers with proportions for categorical variables. Student’s t test, Mann–Whitney U test, and Chi-square or Fisher’s exact test were performed as appropriate to compare the clinical variables between the vaccine-related and vaccine-unrelated groups. Statistical significance was set at p < 0.05.
Results
Comparison of BP incidence before and after COVID-19 vaccination
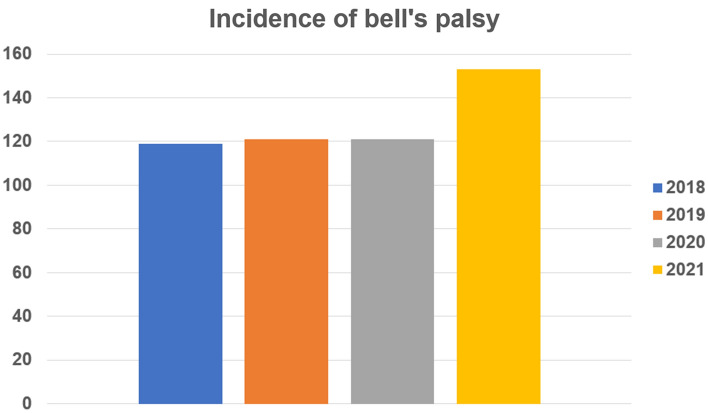
In Part 1, 153 patients were first diagnosed with BP during the COVID-19 mass vaccination period (March 2021 to February 2022) and 119, 121, and 121 during the pre-COVID-19 vaccination period (March 2018 to February 2019, March 2019 to February 2020, and March 2020 to February 2021, respectively). This represented an increase in the incidence of BP by 28.6%, 26.4%, and 26.4%, respectively, during the COVID-19 mass vaccination period compared with the previous three pre-vaccination years. Figure 1 shows the number of patients with BP during the March to February of each yearly period. The number of patients with BP was higher from March 2021 to February 2022 than previous 3 pre-vaccination years.
Fig. 1.
The yearly incidence of Bell’s palsy in Dongsan Hospital between 2018 and 2021, before and after COVID-19 vaccination. The total number of patients with new-onset Bell’s palsy was 119, 121, and 121 in 2018, 2019, and 2020 (pre-COVID-19 vaccination period). The number increased to 153 in 2021, during the COVID-19 mass vaccination period
Clinical features of patients with BP related to COVID-19 vaccination
Among 153 patients who were first diagnosed with BP during the COVID-19 vaccination period in Part 1, 81 were excluded from Part 2 study due to incomplete clinical data. Consequently, 72 patients were finally included in Part 2. Of these, we identified 13 patients with vaccine-related BP according to the temporal relationship with COVID-19 vaccination. The clinical features of the 13 patients are presented in detail in Table 1. Nine were men, and four were women. The youngest was a 19-year-old man, and the oldest was a 63-year-old woman. Of the 13 patients, 9 received BNT162b2 (Pfizer, BioNTech), 3 received mRNA-1273 (Moderna), and 1 received ChAdOx1 nCoV-19 (AstraZeneca). BP developed in four patients (30.8%) after the first vaccine dose, three patients (23.1%) after the second vaccine dose, and six patients (46.2%) after the third vaccine dose. The mean interval from vaccination to BP onset was 12.8 days (range, 1–33 days). According to the H–B grade, the degree of initial facial weakness was moderate in three patients (23.1%) and severe in ten patients (76.9%). All 13 patients showed good recovery (H–B grades 1–2). The cumulative number of patients who achieved good recovery was 6 (46.2%) by 1 month, 11 (84.6%) by 2 months, and 13 by the end of 4 months. With regard to the associated symptoms, there was a decrease in taste in seven patients (53.8%), hyperlacrimation in six patients (46.2%), hyperacusis in three patients (23.1%), and pain around the ear or at the back of the head in ten patients (76.9%). MRI enhancement of the facial nerve was noted in eight patients (8/10, 80.0%). Facial nerve conduction studies revealed a significant decrease in the amplitude of compound muscle action potential (CMAP) of more than 50% compared to that in the contralesional side in nine patients (69.2%).
Table 1.
Overview of the 13 patients with Bell’s palsy associated with COVID-19 vaccination
| Patient | Sex/age (years) | Vaccine type and dose | Vac-Sxa | H–B grade (initial) | H–B grade (final) | H–B change | Duration of follow-up (days) | Associated symptomsb | MRI enhancement | Facial CMAP decrementc | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pain | Hypogeusia | Hyperacusis | Lacrimation | ||||||||||
| 1 | F/63 | BNT162b2 #3 | 3 | 5 | 2 | 3 | 85 | Yes | Yes | No | No | Yes | Yes |
| 2 | M/32 | mRNA-1273 #2 | 3 | 3 | 1 | 2 | 26 | No | No | No | No | Yes | No |
| 3 | F/56 | BNT162b2 #3 | 9 | 4 | 1 | 3 | 22 | Yes | Yes | No | Yes | Yes | No |
| 4 | M/29 | BNT162b2 #3 | 17 | 5 | 2 | 3 | 35 | Yes | Yes | No | Yes | No | Yes |
| 5 | F/51 | mRNA-1273 #3 | 1 | 4 | 2 | 2 | 41 | Yes | Yes | No | Yes | Yes | Yes |
| 6 | M/19 | BNT162b2 #1 | 1 | 4 | 2 | 2 | 15 | Yes | No | No | No | No | Yes |
| 7 | M/37 | ChAdOx1 #2 | 18 | 3 | 1 | 2 | 24 | Yes | No | Yes | No | Yes | Yes |
| 8 | M/38 | mRNA-1273 #1 | 10 | 5 | 1 | 4 | 57 | No | Yes | Yes | Yes | Yes | Yes |
| 9 | M/46 | BNT162b2 #2 | 4 | 4 | 1 | 3 | 102 | Yes | Yes | No | No | No | Yes |
| 10 | M/39 | BNT162b2 #3 | 26 | 4 | 1.5 | 2.5 | 21 | Yes | Yes | Yes | Yes | Yes | Yes |
| 11 | M/61 | BNT162b2 #1 | 13 | 4 | 1 | 3 | 22 | No | No | No | No | Yes | Yes |
| 12 | F/45 | BNT162b2 #1 | 33 | 4 | 1 | 3 | 36 | Yes | No | No | Yes | No | No |
| 13 | M/55 | BNT162b2 #3 | 30 | 3 | 1 | 2 | 33 | Yes | No | No | No | No | No |
aVac-Sx: duration between the day of vaccination and facial weakness
bAssociated symptoms: marked as “Yes”, if the symptom ever presented during the entire follow-up period, “No” if it never presented
cFacial CMAP decrement: defined as < 50% CMAP amplitude compared to that of the contralesional side
H–B House–Brackmann, MRI magnetic resonance imaging, CMAP compound muscle action potential, F female, M male
Comparison of clinical characteristics and outcomes between COVID-19 vaccine-related and -unrelated groups
As previously mentioned, the final analysis of Part 2 study included 72 patients, of whom 13 were in the vaccine-related group and 59 were in the vaccine-unrelated group. A comparison of clinical variables between the vaccine-related and vaccine-unrelated groups is summarized in Table 2. The mean age was significantly lower in the patients in the vaccine-related group than in those in the vaccine-unrelated group (43.92 ± 13.14 vs 54.32 ± 16.01 years; p = 0.033). Sex and the comorbidity status regarding hypertension, diabetes, rheumatic disease and active cancer did not differ between the two groups. There was no significant difference in the severity of the initial facial weakness between the groups. Both groups of patients were treated identically with a combination of oral corticosteroids (prednisolone 60 mg/day for 7 days) and antiviral agents (famciclovir 750 mg/day for 7 days). In the final evaluation, only 78% of patients in the vaccine-unrelated group had good recovery, whereas all patients in the vaccine-related group achieved good recovery (difference not statistically significant). The stacked bar graphs of patients who achieved good recovery at each defined follow-up time point in both groups are shown in Fig. 2. The recovery time was significantly faster in the vaccine-related group than in the vaccine-unrelated group (p = 0.042). Regarding the associated symptoms, taste changes were significantly more frequent in the vaccine-related group than in the vaccine-unrelated group (58.8% vs. 10.9%; p = 0.002). The proportion of patients with pain around the ear or at the back of the head, hyperacusis, and changes in lacrimation did not differ between groups. There was no significant difference in the presence and segmental distribution of facial nerve enhancement between the groups based on MRI findings. No difference was found between the groups in the facial nerve conduction study results.
Table 2.
Comparison of clinical parameters of patients with Bell’s palsy with and without relation to COVID-19 vaccination
| Vaccine related (n = 13) |
Vaccine unrelated (n = 59) |
P-value | ||
|---|---|---|---|---|
| Patient information | ||||
| Age, years | 43.92 ± 13.14 | 54.32 ± 16.01 | 0.033* | |
| Sex (male) | 9 (69.2) | 34 (57.6) | 0.44 | |
| Clinical assessment | ||||
| Initial H–B grade | 0.567 | |||
| Normal or mild (1–2) | 0 (0) | 2 (3.4) | ||
| Moderate (3–4) | 10 (76.9) | 35 (59.3) | ||
| Severe (5–6) | 3 (23.1) | 22 (37.3) | ||
| Final H–B grade | 0.209 | |||
| Normal or mild (1–2) | 13 (100) | 46 (78.0) | ||
| Moderate (3–4) | 0 (0) | 12 (20.3) | ||
| Severe (5–6) | 0 (0) | 1 (1.7) | ||
| H–B grade change | 2.65 ± 0.63 | 2.25 ± 1.06 | 0.194 | |
| Duration from initial to final assessment, days (median [Q1–Q3]) | 33.0 [22.0–49.0] | 56.0 [27.0–76.0] | 0.078 | |
| Associated symptoms (n = 68) | (n = 13) | (n = 55) | ||
| Pain | 10 (76.9) | 30 (54.5) | 0.14 | |
| Hypogeusia | 7 (53.8) | 6 (10.9) | 0.002* | |
| Hyperacusis | 3 (23.1) | 12 (21.8) | 1 | |
| Lacrimation | 6 (46.2) | 33 (60.0) | 0.364 | |
| Presence of MRI enhancement of facial nerve (n = 46) | (n = 10) | (n = 36) | ||
| Overall | 8 (80.0) | 29 (80.6) | 1 | |
| Segmental | ||||
| Intrameatal | 8 (80.0) | 24 (66.7) | 0.699 | |
| Labyrinthine | 2 (20.0) | 7 (19.4) | 1 | |
| Geniculate ganglion | 3 (30.0) | 9 (25.0) | 0.706 | |
| Tympanic | 1 (10.0) | 4 (11.1) | 1 | |
| Mastoid | 0 (0) | 0 (0) | 1 | |
| Facial NCS findings (n = 70) | (n = 13) | (n = 57) | ||
| > 50% CMAP decrement | 9 (69.2) | 44 (77.2) | 0.721 | |
| CMAP ratio (ipsilesional/contralesional) | 0.46 ± 0.32 | 0.32 ± 0.25 | 0.172 | |
| Time required for good recoverya | 0.042* | |||
| < 1 month | 6 (46.2) | 10 (16.9) | ||
| 1–2 months | 5 (38.5) | 16 (27.1) | ||
| 2–3 months | 1 (7.7) | 12 (20.3) | ||
| 3–4 months | 1 (7.7) | 5 (8.5) | ||
| > 4 months or poor recovery | 0 (0) | 16 (27.1) | ||
Results are shown as mean ± SD or n (%)
H–B House–Brackmann, MRI magnetic resonance imaging, NCS nerve conduction study, CMAP compound muscle action potential
aGood recovery: H–B grade 1 or 2
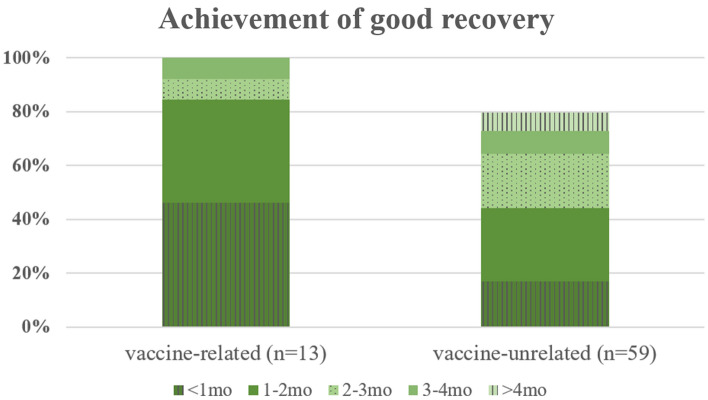
Fig. 2.
Stacked bar chart displaying proportion of patients who achieved H–B grade ≤ 2 in each monthly period during follow-up in vaccine-related and -unrelated groups. The recovery from facial palsy seemed faster in the vaccine-related group. In the first month of follow-up, 46.2% and 16.9% of the patients showed good recovery in the vaccine-related and -unrelated groups, respectively. The cumulative proportion of good recovery was 84.7% vs. 44.0% and 92.4% vs. 64.3% in the second and third months, respectively
Discussion
Part 1 of this study showed a higher occurrence of BP during the COVID-19 mass vaccination period than during the previous three pre-vaccination years, which may reflect an association between the COVID-19 vaccine and an increased risk of BP. In Part 2 of the study, we identified 13 patients who developed BP within 42 days of COVID-19 vaccination during the study period (March 2021 to February 2022). All patients, except one, developed BP after receiving the mRNA-based COVID-19 vaccines. Most cases (9/13, 69.2%) occurred after the second or third dose. To our knowledge, this is the first report to compare the clinical characteristics of patients with COVID-19 vaccine-related and vaccine-unrelated BP. Patients with COVID-19 vaccine-related BP were younger and more frequently experienced taste changes than those with vaccine-unrelated BP. The patients with vaccine-related BP showed favorable recovery of facial nerve function with a faster time to recovery than that of the patients with vaccine-unrelated BP.
In phase-3 clinical trials of mRNA COVID-19 vaccines, a discrepancy in the occurrence of BP was observed in the vaccine groups (four in the BNT162b2 trial and three in the mRNA-1273 trial) compared to the placebo groups (none in the BNT162b2 trial and one in the mRNA-1273 trial), raising concerns about the association between mRNA COVID-19 vaccines and the risk of BP [22, 23]. Although the incidence of BP observed in the vaccine group of these clinical trials is interpreted differently among researchers, it is considered to be 1.5 to 7 times higher than that of the general population [24, 25]. Two recent studies have also shown that mRNA COVID-19 vaccines are associated with an increased risk of BP. A study using a large self-reporting database in the United States showed significantly high reporting of BP after receiving BNT162b2 and mRNA-1273 (reporting odds ratio 1.84 and 1.54, respectively) [26]. Another study conducted using the largest healthcare database in Israel found an association between BNT162b2 and an increased incidence of BP [27]. In our study, we observed an approximately 26% increase in the number of patients first diagnosed with BP during the COVID-19 mass vaccination period compared to the previous three pre-vaccination years. Given that by the end of February 2022, 87.5% of the population in South Korea had received at least one dose of the COVID-19 vaccine [28], this increase in the number of patients with BP may be attributable to the impact of COVID-19 vaccination. In addition, all but one of the 13 vaccine-related BP patients received mRNA COVID-19 vaccines (nine for BNT162b2 and three for mRNA-1273). Our results support previous findings of a possible relationship between COVID-19 vaccines, particularly mRNA-based vaccines, and BP.
In Part 2 of this study, most of our patients with post-vaccination BP (9/13; 69.2%) developed BP after the second or third vaccination dose. This finding is similar to findings from recent clinical trials of mRNA COVID-19 vaccines in which BP was reported more frequently after the second vaccination dose (two out of four in the BNT162b2 trial and all three in the mRNA-1273 trial) [22, 23, 27]. In addition, previous studies have shown that many types of adverse reactions, including myocarditis, from the mRNA COVID-19 vaccines are more frequent and severe after the second dose than after the first [29–32]. Although the reason for this remains unclear, boosting with another dose of the vaccine can induce a stronger immune response than the first vaccination dose [33], which may cause more frequent and severe adverse reactions.
In the analysis of the COVID-19 vaccine-related and unrelated BP patient groups, we found that the patients with vaccine-related BP tended to be younger than those with vaccine-unrelated BP. This finding is in line with the results of previous studies in which adverse reactions ranging from minor skin irritation to serious conditions such as myocarditis or pericarditis after mRNA COVID-19 vaccination were more frequent in younger individuals [29, 31, 32, 34]. Interestingly, although the frequency of the majority of clinical features did not differ between the two groups, accompanying hypogeusia was more frequent in the vaccine-related group. Hypogeusia or ageusia is well known as one of the chief symptoms of COVID-19. SARS-CoV-2 is thought to enter target cells by binding its spike protein to the angiotensin-converting enzyme 2 (ACE2) receptor on the cell surface. Because ACE2 is abundant in the epithelium of taste buds on the tongue [35], SARS-CoV-2 is thought to cause taste changes through direct damage or inflammatory reactions in the taste cells and chorda tympani nerve endings in taste buds [36, 37]. It is not clear why taste changes were more frequent in patients with vaccine-related BP in our study. However, as the mRNA of COVID-19 vaccines encodes the spike protein of SARS-CoV-2, it may bind to ACE2 in taste buds and cause taste changes through a series of processes similar to those of SARS-CoV-2 infection. In this study, there was no difference in the segmental distribution of facial nerve MRI enhancement, even in the mastoid segment, despite the difference in the accompanying frequency of hypogeusia between the two groups. This may be because the lesion causing hypogeusia in the vaccine-related group was in the chorda tympani nerve endings near the taste buds, which are more distal than the mastoid segment of the facial nerve.
Patients with vaccine-related BP had a greater likelihood of good recovery of facial nerve function, with a faster time to recovery, than those with vaccine-unrelated BP. In the final evaluation, only 78% of the patients in the vaccine-unrelated group had good recovery, whereas all patients in the vaccine-related group achieved good recovery. In terms of recovery time, 46.2% of BP patients with vaccine-related BP recovered within 1 month and 84.6% within 2 months, whereas only 16.9% of those with vaccine-unrelated BP recovered within 1 month and 44.0% within 2 months. In our study, the patients with vaccine-related BP were younger than those with vaccine-unrelated BP, which may have affected the difference in recovery between the two groups. Age has been considered an important prognostic factor affecting the final outcome and recovery time of BP in several previous studies that showed a higher final recovery rate and shorter recovery time in younger patients [38–40]. It has been suggested that the better recovery outcome in younger patients may be due to the greater ability of damaged facial nerve axons to regenerate faster in younger patients than in older patients.
In conclusion, this study suggests that COVID-19 vaccines, especially mRNA-based vaccines, may be associated with BP with distinctive clinical characteristics that occur more frequently in young individuals, are frequently accompanied by taste changes, and have a fast and good recovery. The present study has limitations in terms of the small sample size from a single center and its retrospective study design. Also, the baseline medical condition including the immunological status of patients were not strictly controlled and this heterogeneity might potentially influence the result. It is impossible to define a causal relationship between vaccination and the disease with the observations of the study. Despite these shortcomings, it would be informative for neurologists and physicians to investigate COVID-19 vaccination-related BP in this pandemic era. Future studies with larger sample sizes and a prospective design are needed to demonstrate causality.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (NRF-2022R1C1C1009723).
Data availability
Data can be obtained by contacting the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. the Institutional Review Board of Keimyung University Dongsan Hospital approved this study.
Informed consent
Informed consent was waived by our institutional ethical board since the research involves no more than minimal risk to participants and does not adversely affect the rights and welfare of them.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sohyeon Kim and Minsung Kang have contributed equally to this work as the first authors.
Contributor Information
Jin-Sung Park, Email: neurojspark@gmail.com.
Hung Youl Seok, Email: shy2354@gmail.com.
References
- 1.Heckmann JG, Urban PP, Pitz S, Guntinas-Lichius O, Gágyor I. The diagnosis and treatment of idiopathic facial Paresis (Bell's Palsy) Dtsch Arztebl Int. 2019;116:692–702. doi: 10.3238/arztebl.2019.0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reich SG. Bell's Palsy. Continuum (Minneap Minn) 2017;23:447–466. doi: 10.1212/CON.0000000000000447. [DOI] [PubMed] [Google Scholar]
- 3.Richards F, Kodjamanova P, Chen X, Li N, Atanasov P, Bennetts L, et al. Economic burden of COVID-19: a systematic review. Clinicoecon Outcomes Res. 2022;14:293–307. doi: 10.1212/CON.0000000000000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22:1293–1302. doi: 10.1016/S1473-3099(22)00320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardage C, Persson I, Ortqvist A, Bergman U, Ludvigsson JF, Granath F. Neurological and autoimmune disorders after vaccination against pandemic influenza A (H1N1) with a monovalent adjuvanted vaccine: population based cohort study in Stockholm. Sweden. Bmj. 2011;343:d5956. doi: 10.1136/bmj.d5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang WT, Huang WI, Huang YW, Hsu CW, Chuang JH. The reporting completeness of a passive safety surveillance system for pandemic (H1N1) 2009 vaccines: a capture-recapture analysis. Vaccine. 2012;30:2168–2172. doi: 10.1016/j.vaccine.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med. 2004;350:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 8.Zhou W, Pool V, DeStefano F, Iskander JK, Haber P, Chen RT. A potential signal of Bell’s palsy after parenteral inactivated influenza vaccines: reports to the Vaccine Adverse Event Reporting system (VAERS)–United States, 1991–2001. Pharmacoepidemiol Drug Saf. 2004;13:505–510. doi: 10.1002/pds.998. [DOI] [PubMed] [Google Scholar]
- 9.Colella G, Orlandi M, Cirillo N. Bell’s palsy following COVID-19 vaccination. J Neurol. 2021;268:3589–3591. doi: 10.1007/s00415-021-10462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iftikhar H, Noor SMU, Masood M, Bashir K. Bell's Palsy after 24 hours of mRNA-1273 SARS-CoV-2 vaccine. Cureus. 2021;13:e15935. doi: 10.7759/cureus.15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishizawa Y, Hoshina Y, Baker V. Bell's palsy following the Ad26.COV2.S COVID-19 vaccination. QJM. 2021;114:657–658. doi: 10.1093/qjmed/hcab143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obermann M, Krasniqi M, Ewers N, Fayad J, Haeberle U. Bell's palsy following COVID-19 vaccination with high CSF antibody response. Neurol Sci. 2021;42:4397–4399. doi: 10.1007/s10072-021-05496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shemer A, Pras E, Hecht I. Peripheral facial nerve palsy following BNT162b2 (COVID-19) vaccination. Isr Med Assoc J. 2021;23:143–144. [PubMed] [Google Scholar]
- 14.Wan EYF, Chui CSL, Lai FTT, Chan EWY, Li X, Yan VKC, et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2022;22:64–72. doi: 10.1016/S1473-3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baugh RF, Basura GJ, Ishii LE, Schwartz SR, Drumheller CM, Burkholder R, et al. Clinical practice guideline: Bell's palsy. Otolaryngol Head Neck Surg. 2013;149:S1–27. doi: 10.1177/0194599813505967. [DOI] [PubMed] [Google Scholar]
- 16.Butler M, Tamborska A, Wood GK, Ellul M, Thomas RH, Galea I, et al. Considerations for causality assessment of neurological and neuropsychiatric complications of SARS-CoV-2 vaccines: from cerebral venous sinus thrombosis to functional neurological disorder. J Neurol Neurosurg Psychiatry. 2021;92:1144–1151. doi: 10.1136/jnnp-2021-326924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Netravathi M, Dhamija K, Gupta M, Tamborska A, Nalini A, Holla VV, et al. COVID-19 vaccine associated demyelination & its association with MOG antibody. Mult Scler Relat Disord. 2022;60:103739. doi: 10.1016/j.msard.2022.103739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woo EJ, Mba-Jonas A, Dimova RB, Alimchandani M, Zinderman CE, Nair N. Association of receipt of the Ad26.COV2.S COVID-19 vaccine with presumptive Guillain-Barré syndrome, February-July 2021. JAMA. 2021;326:1606–1613. doi: 10.1001/jama.2021.16496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93:146–147. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- 20.Seok JI, Lee DK, Kim KJ. The usefulness of clinical findings in localising lesions in Bell's palsy: comparison with MRI. J Neurol Neurosurg Psychiatry. 2008;79:418–420. doi: 10.1136/jnnp.2007.118489. [DOI] [PubMed] [Google Scholar]
- 21.Flifel ME, Belal T, Abou Elmaaty AA. Bell’s palsy: clinical and neurophysiologic predictors of recovery. Egypt J Neurol Psychiatry Neurosurg. 2020;56:40. doi: 10.1186/s41983-020-00171-6. [DOI] [Google Scholar]
- 22.(2020) Vaccines and Related Biological Products Advisory Committee meeting; December 10, 2020. FDA briefing document. https://www.fda.gov/media/144245/download. Accessed 1 July 2021
- 23.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cirillo N, Doan R. Bell's palsy and SARS-CoV-2 vaccines-an unfolding story. Lancet Infect Dis. 2021;21:1210–1211. doi: 10.1016/S1473-3099(21)00273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozonoff A, Nanishi E, Levy O. Bell's palsy and SARS-CoV-2 vaccines. Lancet Infect Dis. 2021;21:450–452. doi: 10.1016/S1473-3099(21)00076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato K, Mano T, Niimi Y, Toda T, Iwata A, Iwatsubo T. Facial nerve palsy following the administration of COVID-19 mRNA vaccines: analysis of a self-reporting database. Int J Infect Dis. 2021;111:310–312. doi: 10.1016/j.ijid.2021.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibli R, Barnett O, Abu-Full Z, Gronich N, Najjar-Debbiny R, Doweck I, et al. Association between vaccination with the BNT162b2 mRNA COVID-19 vaccine and Bell's palsy: a population-based study. Lancet Reg Health Eur. 2021;11:100236. doi: 10.1016/j.lanepe.2021.100236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edouard Mathieu HR, Lucas Rodés-Guirao, Cameron Appel, Charlie Giattino, Joe Hasell, Bobbie Macdonald, Saloni Dattani, Diana Beltekian, Esteban Ortiz-Ospina and Max Roser (2020) Coronavirus Pandemic (COVID-19). OurWorldInData.org. https://ourworldindata.org/coronavirus. Accessed 8 Novemb 2022
- 29.Kitagawa H, Kaiki Y, Sugiyama A, Nagashima S, Kurisu A, Nomura T, et al. Adverse reactions to the BNT162b2 and mRNA-1273 mRNA COVID-19 vaccines in Japan. J Infect Chemother. 2022;28:576–581. doi: 10.1016/j.jiac.2021.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai FTT, Li X, Peng K, Huang L, Ip P, Tong X, et al. Carditis after COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine : a case-control study. Ann Intern Med. 2022;175:362–370. doi: 10.7326/M21-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US From December 2020 to August 2021. JAMA. 2022;327:331–340. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urakawa R, Isomura ET, Matsunaga K, Kubota K, Ike M. Impact of age, sex and medical history on adverse reactions to the first and second dose of BNT162b2 mRNA COVID-19 vaccine in Japan: a cross-sectional study. BMC Infect Dis. 2022;22:179. doi: 10.1186/s12879-022-07175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwon SR, Kim N, Park H, Minn D, Park S, Roh EY, Yoon JH, Shin S. Strong SARS-CoV-2 Antibody Response After Booster Dose of BNT162b2 mRNA Vaccines in Uninfected Healthcare Workers. J Korean Med Sci. 2022;37:e135. doi: 10.3346/jkms.2022.37.e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326:1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karuppan MKM, Devadoss D, Nair M, Chand HS, Lakshmana MK. SARS-CoV-2 infection in the central and peripheral nervous system-associated morbidities and their potential mechanism. Mol Neurobiol. 2021;58:2465–2480. doi: 10.1007/s12035-020-02245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gholami M, Safari S, Ulloa L, Motaghinejad M. Neuropathies and neurological dysfunction induced by coronaviruses. J Neurovirol. 2021;27:380–396. doi: 10.1007/s13365-021-00977-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lozada-Nur F, Chainani-Wu N, Fortuna G, Sroussi H. Dysgeusia in COVID-19: possible mechanisms and implications. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130:344–346. doi: 10.1016/j.oooo.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kafle DR, Thakur SK. Evaluation of prognostic factors in patients with Bell's palsy. Brain Behav. 2021;11:e2385. doi: 10.1002/brb3.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee Y, SooYoon H, Yeo SG, Lee EH. Factors associated with fast recovery of bell palsy in children. J Child Neurol. 2020;35:71–76. doi: 10.1177/0883073819877098. [DOI] [PubMed] [Google Scholar]
- 40.Peng CH, Chen JL, Liao MF, Hsu JL, Hsu HC, Ro LS. Reappraisal of the prognostic factors of outcome and recovery time in patients with idiopathic bell’s palsy: a retrospective single-center analysis. J Pers Med. 2021 doi: 10.3390/jpm11030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be obtained by contacting the corresponding author upon reasonable request.