Abstract
Purpose:
This phase II clinical trial evaluated whether the addition of stereotactic radiotherapy (SAbR), which may promote tumor antigen presentation, improves the overall response rate (ORR) to high-dose IL-2 (HD-IL-2), in metastatic renal cell carcinoma (mRCC).
Patients and Methods:
Patients with pathologic evidence of clear cell RCC and radiographic evidence of metastasis were enrolled in this single arm trial and were treated with SAbR, followed by HD-IL-2. ORR was assessed based on non-irradiated metastases. Secondary endpoints included overall survival (OS), progression-free survival (PFS), toxicity, and treatment-related tumor-specific immune response. Correlative studies involved whole exome and transcriptome sequencing, T-cell receptor sequencing, cytokine analysis, and mass cytometry on patient samples.
Results:
Thirty ethnically-diverse mRCC patients were enrolled. A median of 2 metastases were treated with SAbR. Among 25 patients evaluable by RECIST-v1.1, ORR was 16% with 8% complete responses. Median OS was 16 months. Treatment-related adverse events (AEs) included 22 grade ≥3 events that were not dissimilar from HD-IL-2 alone. There were no grade 5 AEs. A correlation was observed between SAbR to lung metastases and improved PFS (p=0.0165). Clinical benefit correlated with frameshift mutational load, mast cell tumor infiltration, decreased circulating tumor-associated T-cell clones and T-cell clonal expansion. Higher regulatory/CD8+ T-cell ratios at baseline in the tumor and periphery correlated with no clinical benefit.
Conclusions:
Adding SAbR did not improve the response rate to HD-IL-2 in patients with mRCC in this study. Tissue analyses suggest a possible correlation between frameshift mutation load as well as tumor immune infiltrates and clinical outcomes.
Keywords: Renal cell carcinoma, high-dose interleukin-2, stereotactic ablative body radiotherapy
TRANSLATIONAL RELEVANCE
The benefit of IL-2 remains limited to a minority of patients due to a lack of its mechanistic understanding. Multiple strategies have been employed to increase IL-2’s response rate including selection of patients, modification of IL-2 itself, and combination with newer therapies such as stereotactic radiation (SAbR) or check-point inhibitors. We report the clinical and translational findings from a trial that focused on SAbR and IL-2 for patients with metastatic kidney cancer. Although the trial failed to reach its proposed clinical endpoint, two patients had complete response and patients who had received lung SAbR benefited from it. Patients who benefited had increased frameshift mutational loads, increased intra-tumoral mast cells, and decreased circulating tumor-associated T-cell clones and T-cell clonal expansion. The patients who did not benefit exhibited higher regulatory T-cell (Tregs)/CD8+T-cell ratios in tumor and periphery, suggesting selective expansion of Tregs in non-responders. Therefore, future studies directed toward improving IL-2 response should take these findings into account.
INTRODUCTION
Up to 30% of patients diagnosed with renal cell carcinoma (RCC) have metastatic disease at initial presentation, and about 20% of patients with localized RCC will develop metastasis during the course of their illness (1). Immune-based treatments have been exploited for metastatic RCC (mRCC) because of its high immunogenicity, but despite recent developments, prognosis remains guarded (2).
IL-2 is a pleiotropic cytokine that regulates T cell–mediated cell killing, as well as the differentiation of regulatory T (Treg) cells and the cytolytic activity of natural killer (NK) cells (3). Multiple studies have reported overall response rates (ORRs) to high-dose interleukin-2 (HD IL-2) ~20%, with complete responses (CRs) in ~7% (4). About 50% of the CRs are durable. Despite a long-standing track record of durable complete responses, HD IL-2 has been largely replaced by other therapies because of its requiring inpatient administration, its toxicity profile, and its low response rates. Recently, immune checkpoint inhibitors (ICIs) and combination therapies have changed the landscape of mRCC therapy (2). Despite an improved ORR, durable complete responses remain infrequent. As an example, the CR rate for ipilimumab/nivolumab is 9%.
Multiple clinical trials are evaluating radiation therapy (RT) as an immune adjuvant. Focused RT enhances anti-tumor immunity by promoting tumor cell death, antigen presentation, and T cell function. RT has a wide range of immune modulating effects that may synergize with immune therapies (5). The combination of IL-2 and RT improved irradiated and distant non-irradiated tumor control rates in pre-clinical models (6). The first phase II clinical trial of HD IL-2 and focal conventional RT in the mid-90s evaluated 16 patients with mRCC (7) and reported neither additional toxicity nor increased efficacy, with a partial response (PR) rate of 12.5%. Although the small cohort size precluded firm conclusions, the authors postulated that the lack of response may have been due to 1) inadequate radiation dose (immunogenicity is related to eradicating enough tumor cells to initiate antigen presentation, and 16 Gy in two sessions may have not been sufficient) (8); 2) non-conformal radiation technology, which may have ablated radiosensitive nearby lymph nodes critical for inducing an adaptive immune response; and 3) inclusion of patients with less responsive non-clear cell histologies (9).
Subsequently, a phase I trial of HD IL-2 in melanoma (N=7) and RCC (N=5) that used stereotactic ablative radiotherapy (SAbR), a focused and highly ablative form of RT, instead of conventional RT reported an ORR of 66.6% (8/12) (10). Here, we evaluated the efficacy of this regimen in a larger cohort of 30 patients with mRCC in a phase II single-arm clinical trial in which ORR was the primary endpoint. We also performed correlative studies on patient-derived tissues and blood samples to correlate treatment response with genetic, transcriptomic, and other features.
MATERIALS AND METHODS
Trial design and ethical aspects
This was a multi-center, single-arm, open-label, prospective phase II trial of SAbR to multiple sites immediately followed by HD IL-2 for patients with clear cell mRCC. The primary endpoint was objective response rate (ORR), and secondary endpoints included overall survival (OS), progression-free survival (PFS), treatment-related adverse events (AEs), and duration of response. The study was approved by the Institutional Review Board at the University of Texas Southwestern Medical Center (UTSW, protocol # STU-012013-041), was conducted in accordance with the principles of the Helsinki Declaration and the subsequent Tokyo and Venice amendments, and was registered at www.clinicaltrials.gov (NCT01896271). All patients signed written informed consent before starting treatment.
Patient selection
Patients were eligible if they were ≥18 years old, could give informed consent, had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and showed pathologic evidence of clear cell RCC and radiographic evidence of metastatic disease with at least two lesions with a combined diameter of >1.5 cm that were amenable to SAbR (minimum gross target volume ≥2 cm3). Whenever possible >1 site was treated with SAbR. Patients who had received prior oncologic treatments had to be >14 days from their most recent intervention (surgery, radiation, immunotherapy, or any targeted agent). Adequate cardiac, pulmonary, renal, and hematologic organ function were required. Patients were excluded if they had any form of immunosuppression including immunosuppressive disorders or were on steroids or other immunosuppressive therapies. Patients with brain metastases were allowed if the metastases had all been adequately treated with surgery or radiation.
Treatments
Up to six sites were treated with SAbR at the discretion of the treating radiation oncologist. One fraction (total dose 21–27 Gy) or three fractions (total dose 26.5–33 Gy) were allowed. Preference was given to treating the largest feasible disease site as well as symptomatic sites where palliative and preventative radiation is often indicated. Seeking for sufficient tumor cell killing and antigen presentation, a minimum metastasis diameter of 1.5 cm was recommended. At least one site remained un-irradiated to measure the radiographic response for the primary endpoint. The last SAbR fraction was given <84 hours before the start of the first cycle of HD IL-2. All SAbR plans were optimized to ensure adequate target volume coverage by the prescription dose. Normal tissue constraints were used as previously described (11). Breath-hold, respiratory gating, or abdominal compression techniques and image-guided radiation were used at the discretion of the treating radiation oncologist as needed.
HD IL-2 was administered in the intensive care unit (ICU) at Clements University Hospital using previously developed standard guidelines (12). Each HD IL-2 course consisted of two cycles typically separated by a one-week break. Each cycle consisted of up to 14 doses of 600,000 IU/kg (weight adjusted for obese patients) of HD IL-2 every 8 hours. A total of three courses at the discretion of the treating physician were allowed unless the patient progressed or had any unacceptable toxicity, illness, or condition that prevented further treatment.
Adverse events, outcomes, and follow-up after treatment
Participants were evaluated clinically during their SAbR and HD IL-2 treatments. After treatment, physical exams and imaging studies were performed every ~8 weeks for the first eight months, then every ~12 weeks for two years. Monitoring for AEs started with SAbR. AEs were reported based on the Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE 4.0) (13). Toxicity attribution was reviewed monthly by a panel of physicians. ORR included patients with complete response (CR) or partial response (PR). Best overall response was defined based on the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 as best tumor response until disease progression or last follow-up, and was required for all enrolled patients. Comparisons were made between RECIST, World Health Organization (WHO) and immune-related RECIST (iRECIST) criteria (14,15). Two licensed radiologists independently scored responses by applying the above response criteria and agreed on the final measurements and response designations. Clinical response was defined as a CR/PR or stable disease (SD) for at least six months. OS was calculated from the date of the first SAbR fraction until death. Progression-free survival (PFS) was calculated from the date of initial SAbR fraction until death or disease progression as defined radiographically. Patients lost to follow-up were censored at last follow-up. Irradiated tumors were considered to be locally controlled unless they progressed per RECIST.
Sample size and statistical considerations
The sample size was determined seeking to achieve a 60% improvement over the historical 23% ORR to HD IL-2 with the addition of SAbR, assuming a type I and type II error risk of 0.20, based on the limited available data from a phase I trial of HD IL-2 and SAbR (4,10). A 60% increase would mean at least a 36.8% response rate. Our null hypothesis for the binomial text was an ORR equal to 23%, and our alternative hypothesis was a response rate different from 23% (4). We used descriptive statistics to summarize patient characteristics and the Kaplan-Meier method to estimate OS and PFS and their 95% confidence intervals (CI). The exact binomial method was used to calculate the response rate and toxicity with the corresponding 95% CIs. We used univariate Cox regression to test for the associations with overall survival and progression-free survival. A one-sample log-rank test was used to test whether the survival endpoints (OS and PFS) differed significantly from the historical controls reported in McDermott et al. (4). All statistical tests were two-sided, and a p-value of ≤0.05 was considered statistically significant for all comparisons. We used SAS 9.4 (SAS Institute Inc., Cary NC) and R (version 3.6) for analysis.
Methods for translational studies, including whole exome and transcriptome sequencing, TCR sequencing, cytokine investigation and mass cytometry using different types of patient samples, are detailed in the supplementary materials and methods section.
RESULTS
Patient and treatment characteristics
The study opened for accrual in August 2013 and closed in August 2017. Thirty patients were enrolled. Baseline patient and treatment characteristics are summarized in Table 1. White patients accounted for 83% (25 patients) while non-white for 17% (5 patients). Most patients were male (19 patients, 63%) and had localized disease at initial presentation (18 patients, 60%). Six patients had progressed on systemic therapy prior to enrollment (Table S12) and the remaining were treatment naïve. Most patients were favorable to intermediate risk per Heng and Motzer criteria (16). All patients had a prior nephrectomy. Sarcomatoid component was present in four patients. Twenty-five patients (83%) had more than four metastatic lesions. A median of 2 (range 1–3) sites were treated with SAbR with a median dose of 25 Gy (21–30 Gy) for single-fraction and 30 Gy (25–36 Gy) for three-fraction treatments. Overall, 18, 9 and 3 patients received 1, 2 and 3 IL-2 courses respectively.
Table 1.
Baseline patient and treatment characteristics
| Characteristics | n=30 |
|---|---|
| Median age, years (IQR) | 51.5 (47–55) |
| Gender | |
| Female | 11 |
| Male | 19 |
| Race | |
| White | 25 |
| Non-white | 5 |
| T stage* | |
| 2 | 3 |
| 3 | 22 |
| 4 | 4 |
| Unknown^ | 1 |
| N stage* | |
| 0 | 18 |
| 1 | 5 |
| X | 6 |
| Unknown^ | 1 |
| M stage* | |
| 0 | 17 |
| 1 | 12 |
| Unknown^ | 1 |
| Grade | |
| 1 | 2 |
| 2 | 2 |
| 3 | 12 |
| 4 | 15 |
| Prior systemic therapy | 6 |
| Number of lesions | |
| <4 | 5 |
| 4–10 | 17 |
| >10 | 8 |
| Heng score | |
| 0 | 12 |
| 1 | 9 |
| 2 | 8 |
| 3 | 1 |
| Motzer score | |
| 0 | 11 |
| 1 | 8 |
| 2 | 7 |
| 3 | 3 |
| unknown | 1 |
| Median β2 microglobulin (IQR) | 2.89 (2.60–3.33) |
| Number of sites treated | |
| 1 | 14 |
| 2 | 15 |
| 3 | 1 |
| Sites treated with SAbR | |
| Lung | 21 |
| Bone | 8 |
| Liver | 2 |
| IL-2 Courses | |
| 1 | 18 |
| 2 | 9 |
| 3 | 3 |
| IL-2 Cycles | |
| 1 | 3 |
| 2 | 15 |
| 3 | 1 |
| 4+ | 11 |
| Median IL-2 doses (IQR) | 17.5 (13.25–28.75) |
IQR, interquartile range; SAbR, stereotactic ablative body radiotherapy;
IL-2, interleukin-2
Staging at initial diagnosis of RCC
Record was not available
Outcomes
According to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1, five patients were deemed ineligible due to the absence of non-irradiated measurable disease at baseline. All patients were evaluable according to the World Health Organization (WHO) response criteria. Only 20 patients were evaluable by immune-related RECIST (iRECIST) because 5 additional patients lacked second confirmatory scans prior to starting a new therapy.
By RECIST, ORR was 16% (4/25 patients). Complete responses (CRs) were observed in two patients (8%), and overall disease control rates (PR+CR+stable disease [SD]>6 months per RECIST) were 28% (7/25 patients) (Fig. 1A). These results were similar to the 30-patient cohort, which was evaluable by WHO criteria. By WHO criteria, ORR was 13% (4/30 patients), and overall disease control rates were 23% (7/30 patients) (Table 2). By iRECIST, ORR was 20% (4/20 patients). CR was seen in two patients (10%), and clinical response rates were 35% (7/20 patients).
Fig. 1. Clinical response in patients that received SAbR plus high-dose IL-2 treatment.
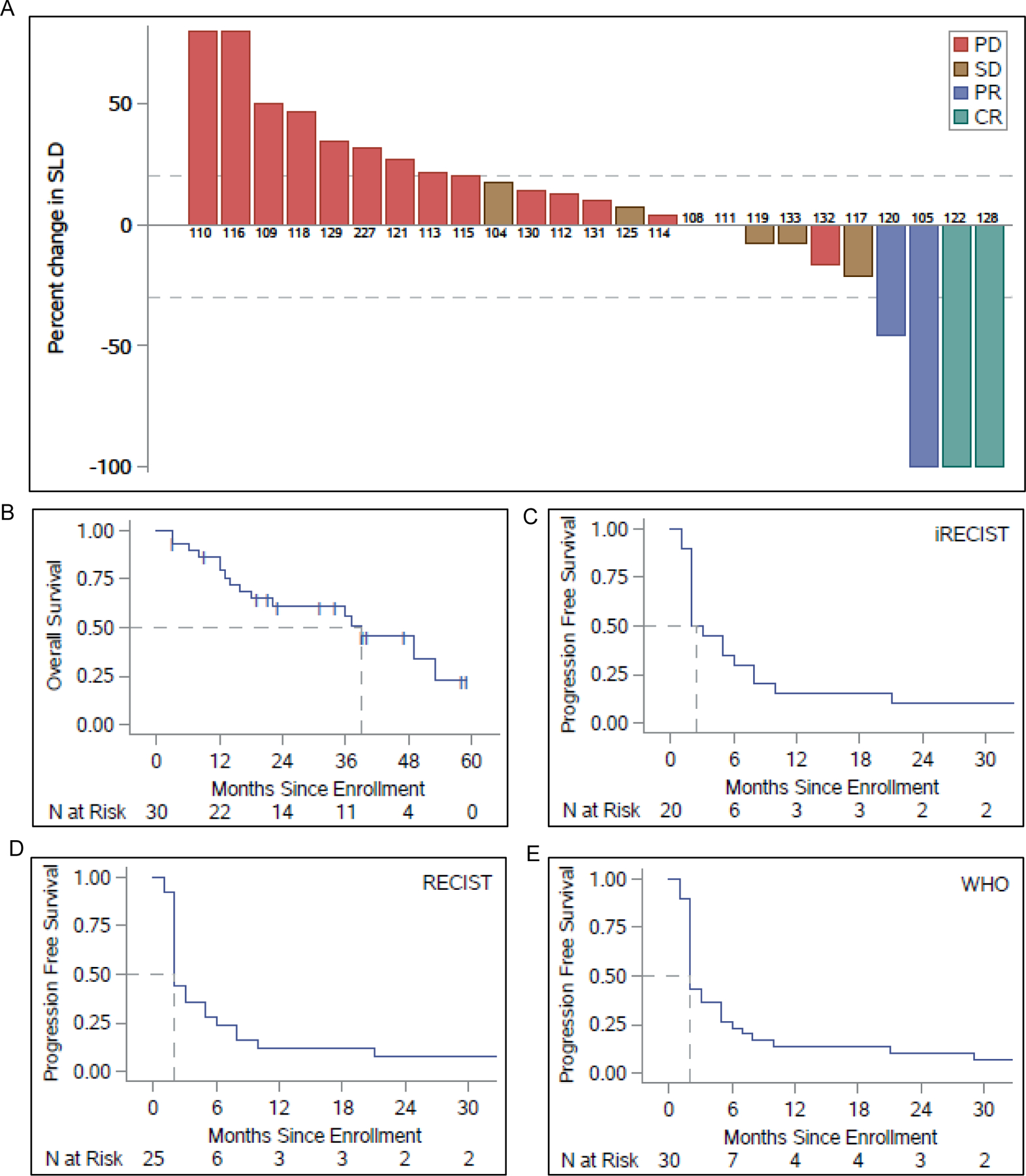
(A) Waterfall plot of maximum RECIST response shown as sum of the longest diameter (SLD) of the tumors. Patients with progressive disease (PD), stable disease (SD), partial response (PR) and complete response (CR) are denoted by red, brown, blue and green bars, respectively. (B) Kaplan-Meier (KM) plots showing overall survival (OS) following enrollment of 30 patients (N represents number of patients surviving at different time points in months following enrollment) for the trial. The hashed lines intersecting the KM plot represent the median OS. (C-E) Progression-free survival (PFS) of patients at different time points since enrollment by iRECIST, RECIST and WHO, respectively. The hashed lines intersecting the KM plot represent the median PFS.
Table 2.
Response rates
| Responder/Total (%) | Exact Binomial 95% CI |
|
|---|---|---|
| CR/PR | ||
| WHO | 4/30 (13.3%) | 3.8%−30.7% |
| RECIST 1.1* | 4/25 (16.0%) | 4.5%−36.1% |
| iRECIST*^ | 4/20 (20.0%) | 5.7%−43.7% |
| CR/PR/SD>6mo. | ||
| WHO | 7/30 (23.3%) | 9.9%−42.3% |
| RECIST 1.1* | 7/25 (28.0%) | 12.1%−49.4% |
| iRECIST*^ | 7/20 (35.0%) | 15.4%−59.2% |
5 patients without measurable disease at baseline.
5 additional patients without 2nd scan to confirm progression
CI, confidence interval; CR, complete response; PR, partial response; SD, stable disease
WHO, world health organization; RECIST, response evaluation criteria in solid tumors; iRECIST, immune-related RECIST
At the time of the last analysis, 19 patients had died (none of the deaths were related to the treatment). Median overall survival (OS) was 37 months, and median progression-free survival (PFS) was two months (Fig. 1B–E). The local control rate for SAbR-treated lesions was 96.7% (30/31).
Clinical benefit (CB) (PR+CR+stable disease [SD]>6 months per RECIST) was associated with fewer metastatic lesions at enrollment (p=0.013) and a higher number of IL-2 courses (p = 0.0033) and cycles (p = 0.0016) (Table S1). OS was associated with no prior systemic therapy (p = 0.0087), lower metastatic burden at enrollment (<4 vs. >10 lesions; p = 0.0193), lower Heng score (0 vs. 3; p = 0.0305), and lower lactate dehydrogenase (p = 0.0132) (Table S2). PFS was associated with lung SAbR (lung vs. non-lung SAbR; p = 0.0165), non-bone SAbR (bone vs. non-bone; p = 0.0385), higher number of IL-2 courses (2 vs. 1; p = 0.0482), and higher number of IL-2 injections (p = 0.0414) (Table S3).
Toxicity
Grade ≥3 AEs were observed in 22 patients (73%) (Table 3). The most common grade ≥3 AEs were renal/ electrolyte disturbances (11 patients, 37%) and hematologic (8 patients, 27%). The overall AEs and grade ≥3 AEs were comparable to historically reported rates for HD IL-2 alone except for hypotension, which was lower (6.7% vs. 56.8%), and electrolyte abnormalities, which were higher (36.6% vs. 13.7%). Three patients (10%) developed grade 4 AEs: two cases of lymphopenia and one case of leukocytosis. There were no treatment-related mortalities. All reported toxicities were expected, transient and resolved upon discontinuing treatment.
Table 3.
Treatment-related adverse events after SAbR and High-Dose IL-2 treatment
| Grade 3–4 |
||||
|---|---|---|---|---|
| Adverse event | Any Grade | SAbR and HD IL-2 (n = 30) |
HD IL-2 Historical Control (n = 95)* |
P |
| All adverse events | 30 (100%) | 22 (73.3%) | - | - |
| Cardiac | 22 (73.3%) | 3 (10.0%) | 8 (8.4%) | 0.72 |
| Constitutional | 27 (90.0%) | 0 (0.0%) | 3 (3.2%) | 1 |
| Gastrointestinal | 22 (73.3%) | 1 (3.3%) | 9 (9.5%) | 0.45 |
| Hematology/Coagulation | 28 (93.3%) | 8 (26.7%) | 13 (13.7%) | 0.16 |
| Hepatobiliary disorders | 19 (63.3%) | 3 (10.0%) | 11 (11.6%) | 1 |
| Hypotension | 18 (60.0%) | 2 (6.7%) | 54 (56.8%) | <0.0001 |
| Infection | 5 (16.7%) | 3 (10.0%) | 3 (3.2%) | 0.15 |
| Neurologic | 12 (40.0%) | 1 (3.3%) | 14 (14.7%) | 0.12 |
| Other | 14 (46.7%) | 1 (3.3%)# | - | - |
| Psychiatric | 6 (20.0%) | 0 (0.0%) | 0 (0.0%) | - |
| Renal/electrolytes | 29 (96.7%) | 11 (36.6%) | 13 (13.7%) | 0.0083 |
| Respiratory | 13 (43.3%) | 3 (10.0%) | 13 (13.7%) | 0.76 |
| Skin | 21 (70.0%) | 0 (0.0%) | - | - |
McDermott et. al. JCO 2005 [4]
Leukocytopenia
Tumor mutational burden and treatment response
All translational studies compared patients who had clinical benefit (CB) (CR+PR+SD>6 months) with those who had no clinical benefit (NCB). We analyzed by whole exome sequencing (WES) DNA from 19 patients that had either a pre-treatment nephrectomy sample or an available biopsy. We found that the somatic frameshift mutational load was higher in patients with CB than in those with NCB, both in the set of key genes implicated in RCC tumorigenesis (17) (p = 0.04) (Fig. 2A) and in all genes implicated in various cancers (p = 0.0002) (Fig. 2B). Using our previously published tumor neoantigen prediction pipeline (18), a non-significant trend towards higher frameshift neoantigen load was observed in patients that had CB (p=0.39) (Fig. 2D).
Fig. 2. Next generation sequencing analysis (NGS) of tumor tissue in clinically benefiting (CB) vs. non-clinically benefiting (NCB) patients.
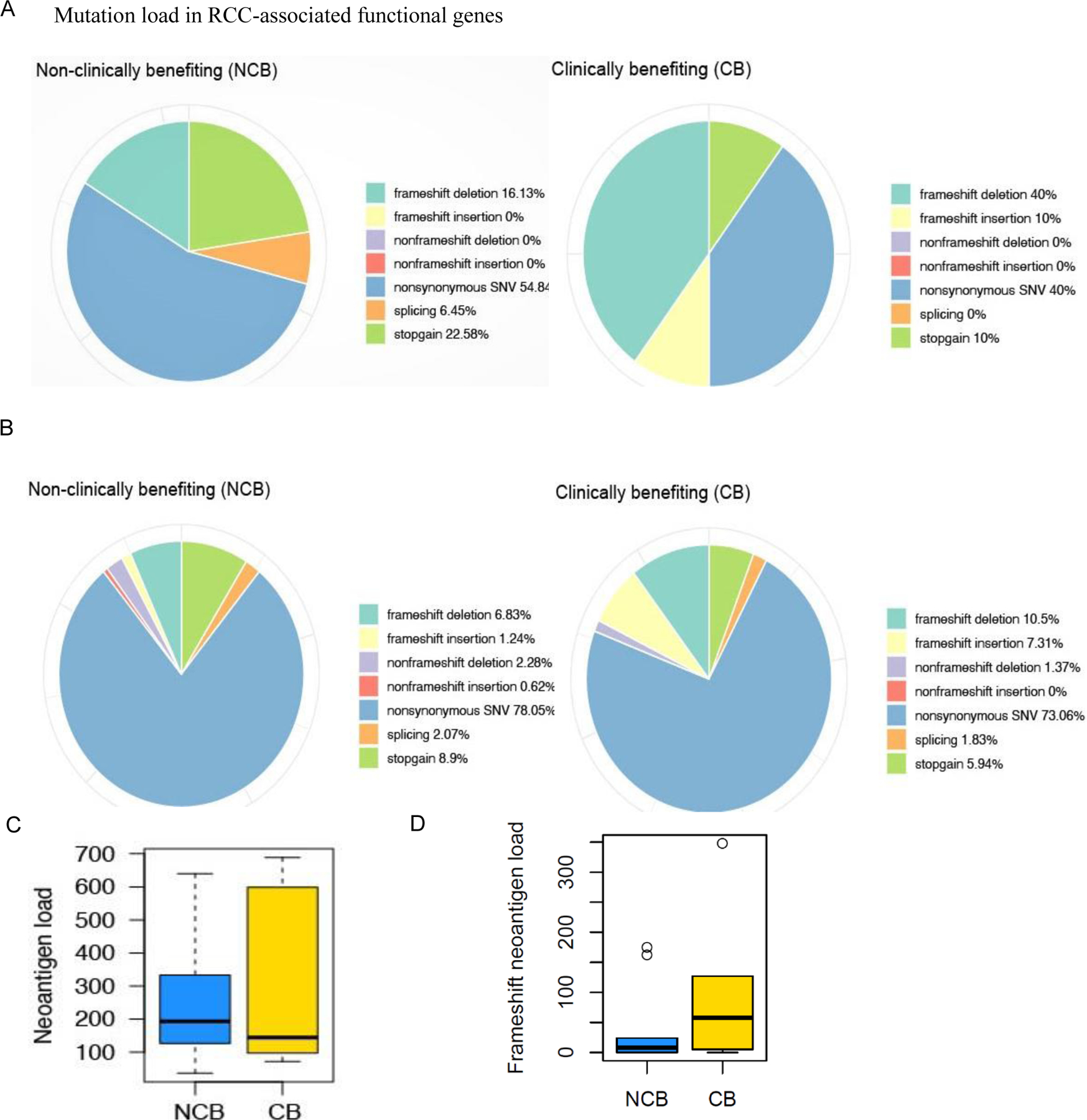
(A-B) Pie charts showing the distribution of mutation load in CB vs. NCB patients enrolled in the SAbR and high-dose IL-2 trial: (A) different mutations in RCC associated functional genes and (B) genome-wide mutation load in RCC. Mutations including single-nucleotide variants (SNVs) were called by the Quantitative Biomedical Research Center (QBRC) mutation calling pipeline (Supplementary Materials and Methods) from whole exome sequencing data. The sequencing libraries from CB and NCB patients were generated from the DNA samples derived from formalin-fixed paraffin-embedded (FFPE) tissue blocks collected as a baseline. (C) Total predicted neoantigen load for patients enrolled in the SAbR + IL-2 trial comparing CB and NCB patients. Bold lines represent median neoantigen count. No difference in the predicted neoantigen load was identified between CB and NCB patients (p = 0.569). (D) Total frameshift neoantigen load for patients enrolled in the SAbR + IL-2 trial comparing CB and NCB patients. No difference in the predicted frameshift neoantigen load was identified between CB and NCB patients (p = 0.39).
Pathways that correlate with treatment response
Next, we analyzed gene expression by using RNA-Seq in 14 patients for whom suitable data were available (4 CB and 10 NCB). In patients with NCB several significantly upregulated genes were identified (e.g., SLAMF9, RAB37, LTB4R2, CARD17) previously associated with tumor growth, progression, immune regulation, and prognosis in patients with many cancers, including mRCC (Fig. 3A, Table S6) (19–22). Deregulated pathways associated with NCB included those implicated in vasculature development, angiogenesis, and endothelial cell migration, which may lead to resistance to immunotherapy, including SAbR/ IL-2 (Fig. 3B). Results for genes with the largest fold changes and the corresponding pathway are shown in Fig. 3C.
Fig. 3. RNA-seq analysis and proteomics/mass spectrometry investigation of tumor tissue in clinically benefiting (CB) vs. non-clinically benefiting (NCB) patients.
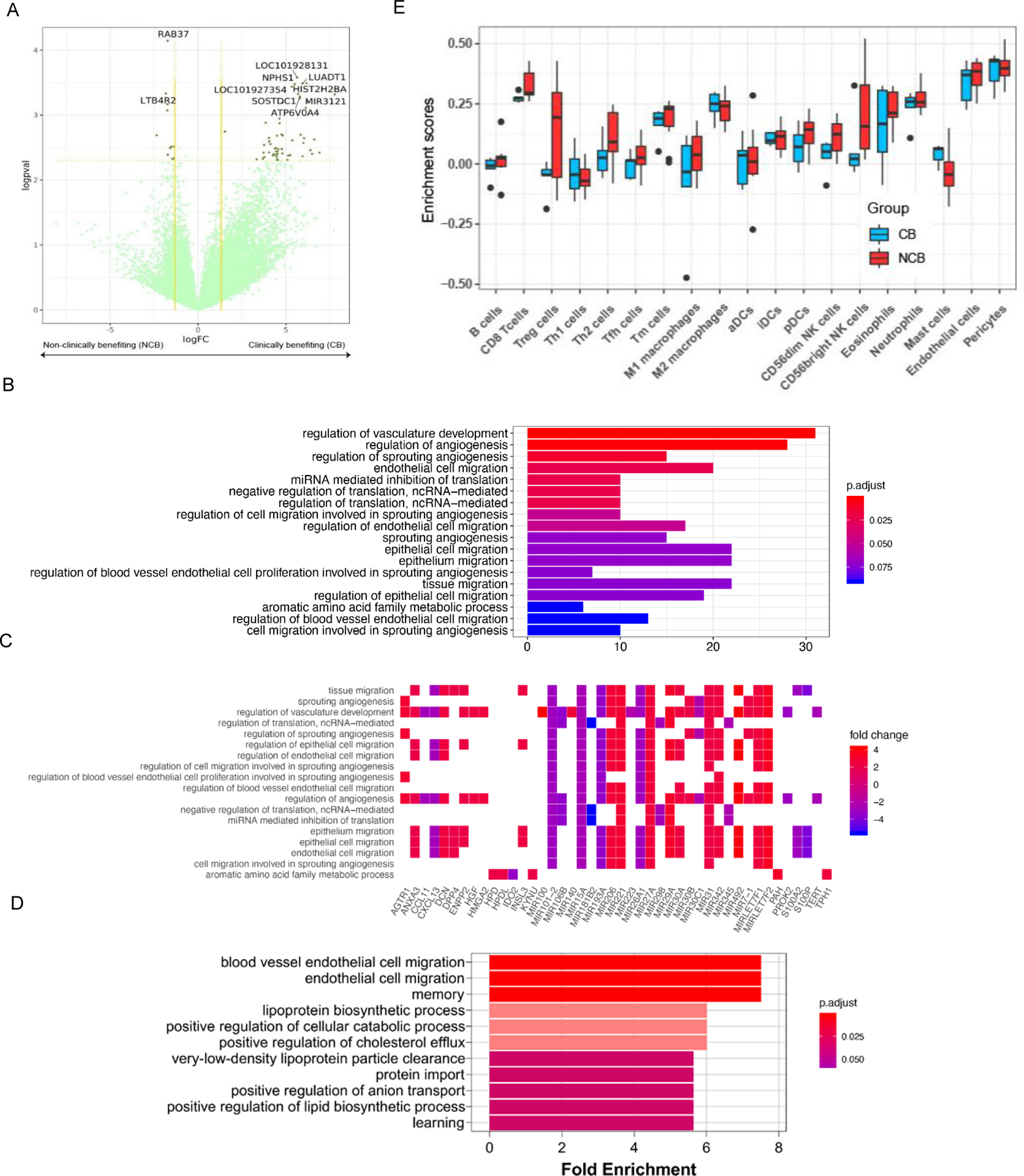
(A) Differential gene expression analyses for clinically benefiting (CB) vs. non-clinically benefiting (NCB) patients to SAbR+IL-2. Gene expression has been denoted as logarithm of the fold change (logFC) values along the x-axis. The negative logarithm of p-values (logpval) have been plotted along the y-axis. Vertical yellow lines along the logFC value 1.5 on both sides and a horizontal yellow line along the p-value=0.1 have been used as cut-offs for gene expression to be considered real gene expression. (B) Gene ontology pathway analysis, including both up- and downregulated genes, of CB compared to NCB. The 18 most significantly different pathways have been represented in the bar chart. False discovery rate (FDR) adjusted p-values were plotted as the heights and the colors of the bars. (C) A heat map showing log fold changes of the expression levels of the most overlapping genes (CB over NCB) in the pathways that were most significant. (D) Gene ontology pathway analysis of upregulated proteins in responders compared to non-responders. From the significantly enriched pathways, the 11 pathways with the highest fold enrichment are represented in the bar chart. (E) Tumor immune infiltration at baseline in patients with CB as compared to those in NCB.
We further performed mass spectrometry on sera from 8 patients (4 CB and 4 NCB) to identify protein level differences between the two groups. Consistent with our gene ontology analysis at the RNA level, blood vessels and endothelial cell migration pathways were enriched in NCB group (Fig. 3D, Table S7). Thus, our proteomics analysis extends transcriptome analyses further implicating the vasculature and endothelial cell migration as potential determinants of resistance to SAbR/ IL-2.
Using our validated algorithm for estimating the relative abundance of different immune cell types in the tumor microenvironment (TME) from whole transcriptome data (23), we analyzed the baseline tumor infiltrating immune cells of patients enrolled in the trial and compared between the CB and NCB groups (Fig. 3E). The analysis revealed that the NCB group tended to have higher levels of infiltrating Treg cells (p value = 0.032), while CB patients had higher levels of mast cells (p value = 0.033).
TCR repertoire in tumors and in circulation
We used the VDJ Server analysis portal and custom Python scripts to analyze the T-cell receptor (TCR) beta-chain complementarity determining region 3 (CDR3) sequences from patient-matched peripheral blood mononuclear cells (PBMCs) (pre- and post-treatment) and tumor compared to adjacent healthy tissue (pre-treatment) (24). Summary statistics for the data are shown in Table S8.
We first compared the pre- and post-treatment blood values between CB (N=4) and NCB (N=3) patients. We evaluated the total number of Productive Templates (Supplementary Fig. S1A), where a template corresponds to a single rearranged TCR gene before PCR amplification, and where a template corresponding to a gene expected to encode a functional TCR Beta protein is referred to as a productive template. In addition, we also evaluated the total number of Productive Rearrangements (Supplementary Fig. S1B), defined as unique nucleotide sequences generated through V(D)J recombination representing clonal lineages. The number of productive templates was expected to correlate with the number of T cells in a sample, and the number of productive rearrangements was expected to correlate with the number of T cell clones. We found that clinically benefiting patients tended to have lower numbers of productive templates and productive rearrangements in both pre- and post-treatment blood samples, and we observed a larger difference in post-treatment samples (Supplementary Figs. S1A, S2A). Assessing the interaction between treatment response group and change in Template or Rearrangement counts pre- to post-treatment via Generalized Estimating Equations (GEEs) revealed a significant interaction effect in both cases (p < 0.001 and p = 0.049, respectively).
We also compared measures expected to be associated with the extent of clonal expansion: (1) Percent Unique, which is the number of clones (Rearrangements) divided by the number of T cells (Templates) (Supplementary Fig. S2B); (2) Max Productive Frequency, defined as the relative abundance of the largest clone in a sample (Supplementary Fig. S2C); and (3) Productive Simpson Clonality, which captures the extent to which all relative clonal abundances in a sample have shifted away from a uniform distribution (Supplementary Fig. S1B). We found that CB patients tended to show more clonal expansion in their blood repertoires, particularly before treatment, with a larger Max Productive Frequency (largest clone size) and a higher Simpson Clonality. Furthermore, the level of peripheral blood clonality appeared to decrease after treatment in the CB group while remaining constant or increasing slightly in the NCB group (Supplementary Figs. S1B, S2B–C). Indeed, GEEs show a significant interaction between treatment response and the pre- to post-treatment change in the Percent Unique (p < 0.001), with a decreasing Percent Unique in the NCB group, which represents a trend towards a larger number of T cells (Templates) per clone (Rearrangement), and an increasing Percent Unique in the CB group, which represents a trend towards a smaller number of T cells per clone.
Next, we sought to determine whether any of the peripheral blood clones were tumor-associated (TA), defined as being present in the tumor but not in the adjacent healthy tissue. We identified TA CDR3 sequences for the four CB patients and two NCB patients for whom tumor and adjacent healthy samples were available. We then determined whether the TA CDR3s were also present in the blood before or after treatment. Although the percentage of TA CDR3s present in pre- or post-treatment blood did not appear to differ between the two groups of patients, CB patients appeared to have a decrease in the percentage, while NCB patients seemingly had an increase after treatment (Supplementary Fig. S1C). GEEs revealed a significant interaction effect between this change and treatment response group (p = 0.051). We observed that, of the TA CDR3s present in blood at both time points, very few changed in abundance. However, CB patients appeared to be more likely to have TA CDR3s that were present in pre-treatment blood but not in post-treatment blood than to have TA CDR3s appear for the first time in their blood after treatment (Supplementary Fig. S1B, Table S9).
Cytokines and T cell subpopulations and treatment response
Most of the cytokines/chemokines investigated were upregulated at baseline, especially in NCB group (Table S10). However, most of the molecules that were significantly different between the groups were chemokines (e.g., GRO-a, MIG, MIP-1b, and SDF-1a).
We performed mass cytometry of PBMCs by using a comprehensive panel of heavy metal–conjugated antibodies specific to human T cells to investigate the baseline and dynamics of T cell subpopulations. The baseline percentages of CD4+ and CD8+ naïve (CD45+CCR7+) T cell populations were much lower in the group of patients who eventually showed CB. Within this same group, we also observed higher baseline percentage in the subset of effector (CCR7−CD45A+) and effector memory (CCR7−CD45A−) CD4+ T cells (Supplementary Table S4). Moreover, CD4+CD25+CD127− Treg populations were significantly higher at baseline in NCB patients and showed a clear incremental trend at follow-up visits. This finding correlates with the observed higher intratumoral Treg infiltration at baseline in patients with NCB (Fig. 3D). When we investigated Tregs further, we observed that only antigen-experienced “memory” (CD45RA−), but not naïve (CD45RA+), Tregs were significantly higher at baseline and increased at follow-up only in NCB patients. We further analyzed the CD161 phenotype of Tregs, a marker that defines a subpopulation of Th17-like cells producing IL-17 that are known for preserving a pro-inflammatory regulatory environment (25). We observed the same pattern of increased CD161+ Treg cells at baseline and follow-up only in patients with no clinical benefit.
DISCUSSION
In this study investigating SAbR in combination with HD IL-2 for patients with metastatic RCC, we postulated that upfront SAbR would prime the immune response with tumor-specific neoantigens so that when IL-2 is delivered, the anti-tumor immune response would be boosted leading to a measurable clinical benefit. The literature is growing with studies demonstrating the immunogenic properties of hypofractionated SAbR and showing the safety and promise of combining SAbR with immune-based therapies for advanced and metastatic cancers, including RCC (10,26). SAbR ablative capabilities create a state of local inflammation in the tumor, which facilitates antigen presentation and T cell activation. Its focused nature spares nearby lymph nodes, which are vital to mounting an effective immune response (27). Additionally, tumor ablation with SAbR may reduce distant tumor seeding and immunosuppressive effects of bulky tumors. We chose our primary endpoint, ORR, because of its correlation with overall survival for patients with mRCC treated with IL-2 (28). The SAbR dose and fractionation schemes were selected based on published reports and our institutional experience to maximize the immune response. Single- or three-fraction treatments were selected to reduce the negative impact of prolonged fractionation on tumor-infiltrating immunocytes and to take advantage of the immune dose response at higher doses per fraction (29,30). However, SAbR did not improve upon the historically reported ORR or CR rate.
Interestingly, when we compared CB (CR+PR+SD>6 months) and NCB patients within this trial cohort, those who received lung SAbR showed better ORR and PFS than those who did not. However, the data is confounded by the possible overall better outcomes of patients with lung metastases. SAbR to bone metastases proved less immunogenic than SAbR to non-bone metastasis. Such organ-specific differences in SAbR immunogenicity have been reported previously (31,32). Of the four patients with a sarcomatoid component, one had a CR and the remaining PD.
Although adding SAbR to HD IL-2 did not improve ORR, it did not increase toxicity over historically reported rates either. The decrease in hypotension in our cohort can be attributed to the aggressive management of blood pressure in the ICU and adherence to strict blood pressure criteria.
Although multiple clinical trials have shown that HD IL-2 can induce durable CR (7–9%) and PR (20–23.2%) in patients with mRCC (4,28,33), extensive searches for biomarkers and alternative regimens have failed to improve on the outcome for patients with mRCC treated with IL-2 (4,9,33,34). Because of its acute AEs and inpatient administration, HD IL-2 has not become a common choice for treating patients (4,33,34). The combination of IL-2 and radiation has been explored in animal models and has shown improved local control of irradiated tumors and regression of non-irradiated tumors (6,35). Accordingly, this combination has been tested in a phase I clinical trial (10). Additionally, a previous phase II study of patients with mRCC treated with HD IL-2 and one fraction of 8 Gy to a single lesion did not show an overall improvement in response rate (36).
Our analysis of somatic mutations showed that RCC patients with high levels of frameshift mutations may be more likely to respond to SAbR/ IL-2. Somatic mutations have been reported as key mediators of anti-tumor immunity (37,38). Frameshift mutations can generate neoantigens with greater diversity promoting the recruitment of APCs and neoantigen-specific cytotoxic CD8+ T cells (39). In addition, other cancers, such as Merkel cell carcinoma, non-small cell lung cancer, and melanoma have been reported to carry high affinity neoantigens from frameshift mutations, and have shown response to immunotherapy (38,40). Further studies are required to investigate whether frameshift mutation load can be used together with other selection criteria such as clear cell histology and favorable prognostic grouping to select patients for HD IL-2 treatment (9).
The similar baseline-predicted neoantigen load in the two response groups may suggest that quality, rather than quantity, of neoantigens, in terms of binding affinity with HLA, may be an important factor in determining response after a SAbR and HD IL-2 combination regimen (41,42). This is constant with the observation that there were a trend towards higher frameshift-induced neoantigens (Fig 2D) in the CB group. We sequenced the TCR repertoire of a small number of the patients, and, although limited by the sample size, several trends were apparent. We found that CB patients appear to have a smaller number of T cells and T cell clones in their peripheral blood both pre- and post-treatment. This raises the question whether CB patients may have more T cells in their tumors and metastatic sites. This hypothesis is supported by the opposing trends between the two response groups, with increased T cell and clone numbers after treatment for NCB patients and decreased T cell and clone numbers after treatment for CB patients. The hypothesis is further supported by the finding that CB patients are more likely to have TA CDR3s that are present in their blood before but not after treatment and fewer that appear for the first time after treatment. This hypothesis is at odds with the RNA-seq data, which show similar overall levels of CD8 and CD4 cells in the tumors of CB patients, but the interpretation is limited as tumor tissue for RNA-seq was often obtained from initial nephrectomy samples, which in some cases were acquired multiple years prior to enrollment.
TCR repertoire analysis also revealed greater clonal expansion in CB patients in both pre- and post-treatment blood samples. However, peripheral clonality appears to trend downward after treatment in responders and is accompanied by an increase in the percentage of unique clones. Consistent with the above hypothesis that CB patients may be experiencing T cell recruitment into the tumor and metastatic sites, this observation suggests that T cell emigrants from blood are members of expanded clones. This result is also consistent with the notion that treatment increased T cell recirculation in CB patients. In NCB patients, we observed the opposite pre- to post-treatment T cell dynamics with an increase in clonality following treatment. We hypothesize that this treatment-induced clonal expansion in NCB patients may be an expansion of regulatory T cells. This is supported by our mass cytometry data, which showed higher levels of CD4+ cells in NCB patients.
Bulk RNA-seq analyses of immune cells in the TME found a significant difference in Tregs at baseline in tumors of patients who did not respond (Table S5). Tregs are well known for their inhibitory immunosuppressive effects. Tregs may use their high affinity IL-2 receptors to compete with antigen-specific CD8 T cells for exogenous IL-2 (43). IL-2 is essential for the growth, proliferation and function of tumor infiltrating cytotoxic T cells. Thus, Tregs may prevent SAbR-induced antigen presentation from inducing the proliferation of short-lived tumor-associated CD8+ cytotoxic T cells. Taken together, our genomic and mass cytometry results suggest that patients with higher ratios of CD4+ to CD8+ T cell populations with functional Tregs at baseline are less likely to respond to SAbR/ IL-2, as observed in previous reports (44). Importantly, memory CD45RA− Tregs negatively correlate with clinical benefit in other types of cancer (45).
The finding of more tumor infiltrating mast cells in CB patients needs to be validated. Previous studies have shown that tumor infiltrating mast cells are involved in angiogenesis and metastasis of invasive cancers, including RCC (46,47). However, other studies have also suggested tumor infiltrating mast cells may portend a favorable prognosis (48). Finally, differences in cytokine profiles at baseline predicted which group would not benefit from SAbR/ IL-2. Higher levels translate to impaired immunity, probably mediated by the recruitment of myeloid-derived suppressor cells. Previous studies have also shown that higher plasma cytokine levels are associated with worse prognosis (49).
This study has multiple limitations. The foremost is the lack of a control arm. A second limitation is the small sample size. In addition, while the study enrolled 30 patients, only 25 were evaluable. The study failed to show that SAbR (at least in its current dose, fractionation and sequencing) increased the response rate of HD IL-2. The correlative tissue and blood-based studies suffered most from the small sample size and lack of a control arm, and conclusions are primarily hypothesis generating.
In conclusion, this prospective phase II single-arm trial showed that SAbR did not improve the ORR of HD IL-2 alone in mRCC patients, but also did not appear to increase toxicities. Promising next generation IL-2 molecules with greater avidity for CD8+ T cells and less affinity for CD4+ T cells have been developed (50,51), and pegylated IL-2, which can be administered in the ambulatory setting, is being evaluated in clinical trials in combination with ICIs (52,53). Retrospective and prospective data for the combination of SAbR and ICIs are now available (31,32). Successful trials may require a better understanding of the optimal immunogenic effects of SAbR dose/fractionation/sequencing in the cancer-specific and clinical setting and a more potent combination of IL-2 and ICIs to improve the outcome for patients with mRCC.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the patients that participated in the study and their families. The authors thank Dr. Jonathan Feinberg for scientific editing of the manuscript. The authors also thank the Genomics (Dr. Edward Wakeland and Dr. Prithvi Raj), Microarray (Dr. Quan-Zhen Li and Ms. Indu Raman), Proteomics (Dr. Andrew Lemoff) and Tissue Management Shared Resource (Dr. Cheryl Lewis) Core Facilities at UT Southwestern, Dallas, TX.
Funding:
Clinigen Inc. (formerly Prometheus Laboratories) grant
National Institutes of Health (NIH) grant CCSG 5P30CA142543 (TW)
Cancer Prevention Research Institute of Texas grant CPRIT RP190208 (TW)
Simmons Cancer Center DSSR, UT Southwestern Medical Center 5P30CA142543(TW)
National Cancer Institute (NCI) SPORE grant P50CA196516 (AC, CA, PK, KC, IP, TA, VM, JB)
NIH grant 5RO1CA154475 (PK, IP)
NIH grant U01CA207091 (IP)
American Cancer Society grant RSG-16-004-01-CCE (RH)
CPRIT MIRA RP180725 (RH)
Footnotes
Conflict of interests:
Authors declare that they have no conflict of interests.
Data and materials availability:
Please see the supplementary materials and methods section.
REFERENCES
- 1.Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev 2008;34(3):193–205 doi 10.1016/j.ctrv.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. The New England journal of medicine 2018;378(14):1277–90 doi 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao W, Lin J-X, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Current Opinion in Immunology 2011;23(5):598–604 doi 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol 2005;23(1):133–41 doi 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 5.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. The Lancet Oncology 2015;16(13):e498–509 doi 10.1016/s1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 6.Everse LA, Renes IB, Jurgenliemk-Schulz IM, Rutgers DH, Bernsen MR, Dullens HF, et al. Local low-dose interleukin-2 induces systemic immunity when combined with radiotherapy of cancer. A pre-clinical study. Int J Cancer 1997;72(6):1003–7. [DOI] [PubMed] [Google Scholar]
- 7.Redman BG, Hillman GG, Flaherty L, Forman J, Dezso B, Haas GP. Phase II trial of sequential radiation and interleukin 2 in the treatment of patients with metastatic renal cell carcinoma. Clinical Cancer Research 1998;4(2):283–6. [PubMed] [Google Scholar]
- 8.Ranck MC, Golden DW, Corbin KS, Hasselle MD, Liauw SL, Stadler WM, et al. Stereotactic Body Radiotherapy for the Treatment of Oligometastatic Renal Cell Carcinoma. American journal of clinical oncology 2012. doi 10.1097/COC.0b013e31825d52b2. [DOI] [PubMed] [Google Scholar]
- 9.McDermott DF, Cheng SC, Signoretti S, Margolin KA, Clark JI, Sosman JA, et al. The high-dose aldesleukin “select” trial: a trial to prospectively validate predictive models of response to treatment in patients with metastatic renal cell carcinoma. Clin Cancer Res 2015;21(3):561–8 doi 10.1158/1078-0432.CCR-14-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seung SK, Curti BD, Crittenden M, Walker E, Coffey T, Siebert JC, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2--tumor and immunological responses. Science translational medicine 2012;4(137):137ra74 doi 10.1126/scitranslmed.3003649. [DOI] [PubMed] [Google Scholar]
- 11.Kim DWN, Medin PM, Timmerman RD. Emphasis on Repair, Not Just Avoidance of Injury, Facilitates Prudent Stereotactic Ablative Radiotherapy. Seminars in radiation oncology 2017;27(4):378–92 doi 10.1016/j.semradonc.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Schwartzentruber DJ. Guidelines for the safe administration of high-dose interleukin-2. J Immunother 2001;24(4):287–93. [DOI] [PubMed] [Google Scholar]
- 13.NIH. 2009 August 2017. Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0 <https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf>. August 2017.
- 14.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2):228–47 doi 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clinical cancer research : an official journal of the American Association for Cancer Research 2009;15(23):7412–20 doi 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 16.Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. The Lancet Oncology 2013;14(2):141–8 doi 10.1016/s1470-2045(12)70559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turajlic S, Litchfield K, Xu H, Rosenthal R, McGranahan N, Reading JL, et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. The Lancet Oncology 2017;18(8):1009–21 doi 10.1016/s1470-2045(17)30516-8. [DOI] [PubMed] [Google Scholar]
- 18.Lu T, Wang S, Xu L, Zhou Q, Singla N, Gao J, et al. Tumor neoantigenicity assessment with CSiN score incorporates clonality and immunogenicity to predict immunotherapy outcomes. Sci Immunol 2020;5(44) doi 10.1126/sciimmunol.aaz3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park J, Jang J-H, Park G-S, Chung Y, You HJ, Kim J-H. BLT2, a leukotriene B4 receptor 2, as a novel prognostic biomarker of triple-negative breast cancer. BMB Rep 2018;51(8):373–7 doi 10.5483/BMBRep.2018.51.8.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dollt C, Michel J, Kloss L, Melchers S, Schledzewski K, Becker K, et al. The novel immunoglobulin super family receptor SLAMF9 identified in TAM of murine and human melanoma influences pro-inflammatory cytokine secretion and migration. Cell Death Dis 2018;9(10):939 doi 10.1038/s41419-018-1011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tzeng H-T, Tsai C-H, Yen Y-T, Cheng H-C, Chen Y-C, Pu S-W, et al. Dysregulation of Rab37-Mediated Cross-talk between Cancer Cells and Endothelial Cells via Thrombospondin-1 Promotes Tumor Neovasculature and Metastasis. Clinical Cancer Research 2017;23(9):2335–45 doi 10.1158/1078-0432.Ccr-16-1520. [DOI] [PubMed] [Google Scholar]
- 22.Lu A, Li Y, Schmidt FI, Yin Q, Chen S, Fu T-M, et al. Molecular basis of caspase-1 polymerization and its inhibition by a new capping mechanism. Nat Struct Mol Biol 2016;23(5):416–25 doi 10.1038/nsmb.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Tao, Lu Rong, Kapur Payal, Jaiswal Bijay S., Hannan Raquibul, Zhang Ze, et al. An Empirical Approach Leveraging Tumorgrafts to Dissect the Tumor Microenvironment in Renal Cell Carcinoma Identifies Missing Link to Prognostic Inflammatory Factors. Cancer discovery 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christley S, Scarborough W, Salinas E, Rounds WH, Toby IT, Fonner JM, et al. VDJServer: A Cloud-Based Analysis Portal and Data Commons for Immune Repertoire Sequences and Rearrangements. Front Immunol 2018;9:976 doi 10.3389/fimmu.2018.00976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afzali B, Mitchell PJ, Edozie FC, Povoleri GA, Dowson SE, Demandt L, et al. CD161 expression characterizes a subpopulation of human regulatory T cells that produces IL-17 in a STAT3-dependent manner. European journal of immunology 2013;43(8):2043–54 doi 10.1002/eji.201243296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nature reviews Clinical oncology 2016;13(8):516–24 doi 10.1038/nrclinonc.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeshima T, Chamoto K, Wakita D, Ohkuri T, Togashi Y, Shirato H, et al. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res 2010;70(7):2697–706 doi 10.1158/0008-5472.CAN-09-2982. [DOI] [PubMed] [Google Scholar]
- 28.Klapper JA, Downey SG, Smith FO, Yang JC, Hughes MS, Kammula US, et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma : a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer 2008;113(2):293–301 doi 10.1002/cncr.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee Y, Auh SL, Wang Y, Burnette B, Meng Y, Beckett M, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 2009;114(3):589–95 doi 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys 2012;83(4):1306–10 doi 10.1016/j.ijrobp.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang C, Welsh JW, de Groot P, Massarelli E, Chang JY, Hess KR, et al. Ipilimumab with Stereotactic Ablative Radiation Therapy: Phase I Results and Immunologic Correlates from Peripheral T Cells. Clin Cancer Res 2017;23(6):1388–96 doi 10.1158/1078-0432.CCR-16-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohamad O, Diaz de Leon A, Schroeder S, Leiker A, Christie A, Zhang-Velten E, et al. Safety and efficacy of concurrent immune checkpoint inhibitors and hypofractionated body radiotherapy. Oncoimmunology 2018;7(7):e1440168 doi 10.1080/2162402X.2018.1440168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol 2003;21(16):3127–32 doi 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Negrier S, Escudier B, Lasset C, Douillard JY, Savary J, Chevreau C, et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d’Immunotherapie. The New England journal of medicine 1998;338(18):1272–8 doi 10.1056/NEJM199804303381805. [DOI] [PubMed] [Google Scholar]
- 35.Jurgenliemk-Schulz IM, Renes IB, Rutgers DH, Everse LA, Bernsen MR, Den Otter W, et al. Anti-tumor effects of local irradiation in combination with peritumoral administration of low doses of recombinant interleukin-2 (rIL-2). Radiation oncology investigations 1997;5(2):54–61 doi [DOI] [PubMed] [Google Scholar]
- 36.Redman BG, Hillman GG, Flaherty L, Forman J, Dezso B, Haas GP. Phase II trial of sequential radiation and interleukin 2 in the treatment of patients with metastatic renal cell carcinoma. Clin Cancer Res 1998;4(2):283–6. [PubMed] [Google Scholar]
- 37.Yang H, Zhong Y, Peng C, Chen J-Q, Tian D. Important role of indels in somatic mutations of human cancer genes. BMC Medical Genetics 2010;11(1):128 doi 10.1186/1471-2350-11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turajlic S, Litchfield K, Xu H, Rosenthal R, McGranahan N, Reading JL, et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. The Lancet Oncology 2017;18(8):1009–21 doi 10.1016/S1470-2045(17)30516-8. [DOI] [PubMed] [Google Scholar]
- 39.Hansen UK, Ramskov S, Bjerregaard A-M, Borch A, Andersen R, Draghi A, et al. Tumor-Infiltrating T Cells From Clear Cell Renal Cell Carcinoma Patients Recognize Neoepitopes Derived From Point and Frameshift Mutations. Frontiers in immunology 2020;11:373- doi 10.3389/fimmu.2020.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maletzki C, Schmidt F, Dirks WG, Schmitt M, Linnebacher M. Frameshift-derived neoantigens constitute immunotherapeutic targets for patients with microsatellite-instable haematological malignancies: Frameshift peptides for treating MSI+ blood cancers. European Journal of Cancer 2013;49(11):2587–95 doi 10.1016/j.ejca.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 41.Borden ES, Kang P, Natri HM, Phung TN, Wilson MA, Buetow KH, et al. Neoantigen Fitness Model Predicts Lower Immune Recognition of Cutaneous Squamous Cell Carcinomas Than Actinic Keratoses. Frontiers in Immunology 2019;10(2799) doi 10.3389/fimmu.2019.02799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu T, Wang S, Xu L, Zhou Q, Singla N, Gao J, et al. Tumor neoantigenicity assessment with CSiN score incorporates clonality and immunogenicity to predict immunotherapy outcomes. Science Immunology 2020;5(44):eaaz3199 doi 10.1126/sciimmunol.aaz3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kastenmuller W, Gasteiger G, Subramanian N, Sparwasser T, Busch DH, Belkaid Y, et al. Regulatory T Cells Selectively Control CD8+ T Cell Effector Pool Size via IL-2 Restriction. The Journal of Immunology 2011;187(6):3186–97 doi 10.4049/jimmunol.1101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cesana GC, DeRaffele G, Cohen S, Moroziewicz D, Mitcham J, Stoutenburg J, et al. Characterization of CD4+CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol 2006;24(7):1169–77 doi 10.1200/JCO.2005.03.6830. [DOI] [PubMed] [Google Scholar]
- 45.Ihara F, Sakurai D, Horinaka A, Makita Y, Fujikawa A, Sakurai T, et al. CD45RA(−)Foxp3(high) regulatory T cells have a negative impact on the clinical outcome of head and neck squamous cell carcinoma. Cancer immunology, immunotherapy : CII 2017;66(10):1275–85 doi 10.1007/s00262-017-2021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cherdantseva TM, Bobrov IP, Avdalyan AM, Klimachev VV, Kazartsev AV, Kryuchkova NG, et al. Mast Cells in Renal Cancer: Clinical Morphological Correlations and Prognosis. Bulletin of experimental biology and medicine 2017;163(6):801–4 doi 10.1007/s10517-017-3907-7. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Li C, Xie H, Fan Y, Yang Z, Ma J, et al. Infiltrating mast cells promote renal cell carcinoma angiogenesis by modulating PI3K→︀AKT→︀GSK3β→︀AM signaling. Oncogene 2017;36(20):2879–88 doi 10.1038/onc.2016.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dabiri S, Huntsman D, Makretsov N, Cheang M, Gilks B, Badjik C, et al. The presence of stromal mast cells identifies a subset of invasive breast cancers with a favorable prognosis. Modern Pathology 2004;17(6):690–5 doi 10.1038/modpathol.3800094. [DOI] [PubMed] [Google Scholar]
- 49.Chen ZY, He WZ, Peng LX, Jia WH, Guo RP, Xia LP, et al. A prognostic classifier consisting of 17 circulating cytokines is a novel predictor of overall survival for metastatic colorectal cancer patients. Int J Cancer 2015;136(3):584–92 doi 10.1002/ijc.29017. [DOI] [PubMed] [Google Scholar]
- 50.Levin AM, Bates DL, Ring AM, Krieg C, Lin JT, Su L, et al. Exploiting a natural conformational switch to engineer an interleukin-2 ‘superkine’. Nature 2012;484(7395):529–33 doi 10.1038/nature10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosalia RA, Arenas-Ramirez N, Bouchaud G, Raeber ME, Boyman O. Use of enhanced interleukin-2 formulations for improved immunotherapy against cancer. Curr Opin Chem Biol 2014;23:39–46 doi 10.1016/j.cbpa.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Yang JC, Topalian SL, Schwartzentruber DJ, Parkinson DR, Marincola FM, Weber JS, et al. The use of polyethylene glycol-modified interleukin-2 (PEG-IL-2) in the treatment of patients with metastatic renal cell carcinoma and melanoma. A phase I study and a randomized prospective study comparing IL-2 alone versus IL-2 combined with PEG-IL-2. Cancer 1995;76(4):687–94. [DOI] [PubMed] [Google Scholar]
- 53.Charych DH, Hoch U, Langowski JL, Lee SR, Addepalli MK, Kirk PB, et al. NKTR-214, an Engineered Cytokine with Biased IL2 Receptor Binding, Increased Tumor Exposure, and Marked Efficacy in Mouse Tumor Models. Clin Cancer Res 2016;22(3):680–90 doi 10.1158/1078-0432.CCR-15-1631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


