ABSTRACT
Purpose
The aim of this audit was to assess the representation of female athletes within the literature that has led to current guidelines for carbohydrate (CHO) intake in the acute periods surrounding exercise and the quality of this research.
Methods
We conducted a standardized audit of research assessing CHO loading protocols, CHO mouth rinse, and CHO intake before, during, and after exercise.
Results
A total of 937 studies were identified in this audit. There were a total of 11,202 participants across these studies, with only ~11% being women. Most studies involved male-only cohorts (~79%), with a mere 38 studies (~4%) involving female-only cohorts and 14 studies (~2%) including a methodological design for comparison of sex-based responses. The frequent use of incorrect terminology surrounding menstrual status and the failure of most studies (~69%) to provide sufficient information on the menstrual status of participants suggests incomplete understanding and concern for female-specific considerations among researchers. Of the 197 studies that included women, only 13 (~7%) provided evidence of acceptable methodological control of ovarian hormones, and no study met all best-practice recommendations. Of these 13 studies, only half also provided sufficient information regarding the athletic caliber of participants. The topics that received such scrutiny were CHO loading protocols and CHO intake during exercise.
Conclusions
The literature that underpins the current guidelines for CHO intake in the acute periods around exercise is lacking in high-quality research that can contribute knowledge specific to the female athlete and sex-based differences. New research that considers ovarian hormones and sex-based differences is needed to ensure that the recommendations for acute CHO fueling provided to female athletes are evidence based.
Key Words: WOMEN, ORAL CONTRACEPTIVE, MENSTRUAL STATUS, SPORT, PHYSICAL ACTIVITY, NUTRITION
Although women were first included in the Olympic Games in 1900, it took over a century for an almost even gender split to be achieved at the Tokyo 2020 Games (1). Despite this “equality,” female athletes continue to face barriers, such as limited resources, funding, and coverage compared with their male counterparts (2). Recent interest in the applied sport science field has focused on whether evidence-based sports science guidelines, most of them developed in male athletes, are indeed suitable and specific to female athletes; and recent data suggest that this is open for scrutiny (3–5). This is of particular interest for scientifically well-developed applied sport science themes/interventions, in which the apparent depth and sophistication of the current literature suggest that they are robust in their application, but in which most of the evidence has been based in male athletes.
Sport performance is dependent on a myriad of physiological, biomechanical, and psychological determinants, in which the availability of carbohydrate (CHO) as a substrate for the muscle and central nervous system is an important one, especially for endurance sports (6–8). Because of the finite nature of body CHO stores relative to the fuel demands of common training sessions and competitive events, there are a variety of acute nutrition strategies aimed at promoting high CHO availability for performance enhancement (6). Recommendations for the timing, amount, and type of CHO that should be consumed before, during, and between such exercise sessions are arguably the most visible and explicit guidelines within historical and contemporary positions stands, and expert statements defining sports nutrition (9). Current guidelines for acute intake of CHO around exercise are summarized in Table 1.
TABLE 1.
Acute CHO fueling strategies aimed at promoting high CHO availability with current recommendations, and study features used to classify studies in this audit.
| Strategy | Current Recommendations (6,9) | Study Features for Audit Inclusion |
|---|---|---|
| CHO loading | 10–12 g·kg−1 BM per 24 h−1 for 36–48 h | Intervention aimed at maximizing muscle glycogen stores preexercise Duration of protocol: 24 h–7 d |
| Preexercise | 1–4 g·kg−1 BM 1–4 h before exercise | Manipulation of the type, amount, and/or timing of CHO intake within the 4 h period before exercise |
| Mouth rinse | Rinse CHO-containing solution in mouth for 5–10 s during high-intensity exercise | Intervention involving the effect of CHO mouth rinse without the ingestion of CHO |
| During exercise | 30–60 g·h−1 for exercise 1–2.5 h Up to 90 g·h−1 with products providing multiple transportable CHO for exercise >2.5 h |
Manipulation of the type, amount, and/or timing of CHO ingestion during exercise Involves CHO intake <15 min before exercise, and during or in-between successive bouts of exercise |
| Postexercise | When <8 h between 2 bouts of exercise, 1.0–1.2 g·kg−1 BM·h−1 for the first 4 h | Manipulation of the type, amount, and/or timing of CHO intake in the postexercise period Duration = 0–24 h after exercise |
As with all guidelines where sex-based differences could exist, it is important to investigate the population from which these guidelines were derived, to ensure that outcomes from male populations are not being erroneously generalized to female populations. The first practical attempt to account for sex within current CHO recommendations involved the change to report and prescribe CHO intake relative to body mass, rather than absolute amounts in recognition of the often-smaller body size of women (7). However, there are also biological reasons to postulate that sex-based differences should exist for these guidelines. This includes differences in muscle fiber composition (10) and/or hormonal differences that contribute to sex-based differences in substrate utilization during exercise (11). Various reviews have highlighted the need for female athlete–specific considerations for CHO guidelines (12,13). This includes differences that might occur within and between women, to determine if different strategies are required based on menstrual cycle phase and/or situations that alter menstrual cycle hormonal fluctuations, such as hormonal contraceptive (HC) usage (14). Such insights would not only inform athlete practice but also assist future research protocols to be free of such bias or error. As such, the primary aim of this study was to audit the research that has led to the current CHO guidelines in the acute period before, during, and after exercise (9) to determine if there is evidence to support the use of these strategies in female athletes and if differences across menstrual cycle phase or with HC usage have been considered.
METHODS
The methods of this audit are in accordance with the standardized protocol previously established by Smith et al. (15), with methods specific to this audit further outlined hereinafter.
Search strategy and data selection/extraction
An electronic search from inception to January 18, 2022, was conducted in PubMed. The full search strategy can be found in Supplemental Digital Content 1, http://links.lww.com/MSS/C724). After removal of duplicates, articles were screened using Rayyan online software (16) to identify articles that met the inclusion criteria. Studies were included if the primary aim was investigating the effects of an acute CHO fueling strategy on a performance outcome, health outcome, or underlying mechanisms associated with performance and health. Depending on study features (Table 1), acute CHO fueling strategies were categorized into one of the following: 1) CHO loading protocols of 24-h to 7-d duration, in which studies were aimed at supercompensating muscle glycogen stores; 2) studies examining the effect of CHO mouth rinsing without ingestion; and studies examining the type, amount, or timing of CHO intake either 3) before, 4) during, or 5) after exercise. Two authors independently classified each article into an acute CHO fueling strategy, and a third author resolved any conflicts. Articles were excluded using the following criteria: participants were older than 50 yr and untrained, involved populations with lifestyle disease, participants were children, the study involved an occupational task (i.e., studies involving soldiers, firefighters etc.), the CHO-based intervention was used as a control condition only, the study outcome was irrelevant to the area of interest, and/or failure to explicitly state the sex of participants. This article examined the acute effects of CHO around exercise, whereas articles that have led to guidelines for total daily CHO intake have been included in a sister analysis. For articles that met the inclusion criteria, data were extracted into a Microsoft Excel template as per the protocol by Smith et al. (15). Briefly, this allowed each article to be classified based on the following metrics:
A. Study population: females only, males only, mixed-sex cohort, male versus female design features (MvFdes), and male versus female subanalysis (MvFsub)
B. Athletic caliber as previously established by McKay et al. (17): Tier 0 (sedentary), Tier 1 (recreationally active), Tier 2 (trained/developmental), Tier 3 (highly trained/national), Tier 4 (elite/international), and Tier 5 (world-class)
C. Menstrual status and grade as previously established by Elliott-Sale et al. (14): naturally menstruating or eumenorrheic women, HC users, women with menstrual irregularities, and mixture of one of the aforementioned categories. If insufficient information was provided to determine menstrual status, then studies were defined as unclassified and were not further graded as based on methodological control of ovarian hormones as per the protocol by Smith et al. (15). Studies were awarded a Gold standard if all best-practice guidelines were implemented. Specifically, naturally menstruating women met the criteria for eumenorrhea via detailed tracking for ≥2 months with no HC usage >3 months before study commencement; HC users involved the use of a single HC type for >3 months with detailed information on the type, formulation, and active usage of HC; and women with menstrual irregularities received a diagnosis by a medical professional as part of the study, and the length of condition was provided. Studies were awarded a Silver standard if they met some but not all of these criteria, and studies were awarded a Bronze standard if even fewer criteria were met. If insufficient information to award a Gold, Silver, or Bronze standard was provided, then it remained “ungraded.” If a study involved a mixture of categories (i.e., both naturally menstruating and HC users), then each group was individually graded, with 0.5 of a study count assigned to each.
D. Research theme: performance focus/outcome, clinically established health focus/outcome, or indirect or emerging associations with performance or health
E. Study impact: Altmetric score and most recent 4-yr impact factor; Altmetric scores were retrieved for studies published after 2012 and obtained between December 23, 2021, and January 18, 2022.
F. Sample size: male and female participant number
To further assess the high-quality research specific to the female athlete, studies with sufficient methodological control of ovarian hormonal profiles (Gold, Silver, or Bronze standard) and female-specific designs (female-only cohorts, MvFdes, or MvFsub) were identified. Only studies with a female-specific design were include because these contribute knowledge to sex-based differences or directly inform female-specific guidelines. Studies with cohorts of unclassified athlete caliber were also excluded given the lack of information regarding athletic ability and competition level that prevents direct application of study findings. A spreadsheet with all included studies and their classified metrics has been provided in Supplemental Digital Content 2, List of included studies, http://links.lww.com/MSS/C725.
Statistics
Statistical analyses were performed using R Studio (version 3.5.2) with statistical significance accepted at an α level of P ≤ 0.05. Frequency-based metrics were reported as a percentage of the total studies or participants. Histogram inspection revealed nonnormally distributed data for journal impact score, Altmetric score, and male/female-specific sample sizes. As such, a Mann–Whitney U test was used to compare median numbers of male and female participants, and a Kruskal–Wallis test with a post hoc Dunn test was used to assess differences in impact factor and Altmetric scores across study population. This data are reported as median ± interquartile range.
RESULTS
Across studies of the five different acute CHO fueling strategies, a total of 937 were identified for inclusion in this audit (Fig. 1). There were a total of 11,202 participants, with ~11% being female and ~89% being male (Fig. 2A). Of the 937 studies, ~96% (n = 899) of studies included at least one male participant, whereas only ~21% (n = 197) of studies included at least one female participant (Fig. 2B).
FIGURE 1.
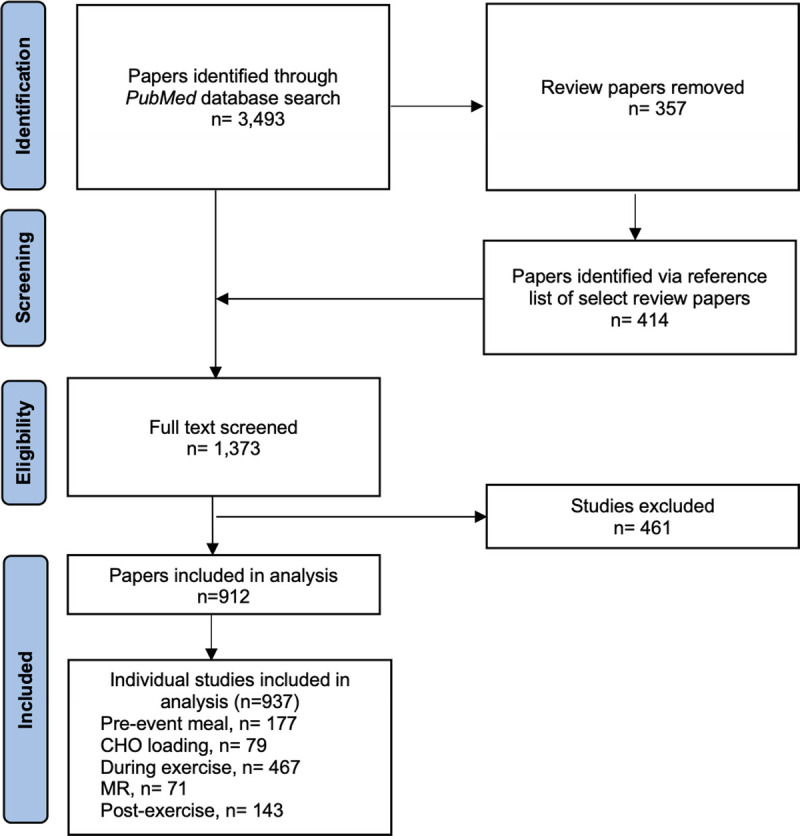
Flowchart demonstrating the screening process to identify articles examining acute CHO fueling strategies, and the number of individual studies included for each of the five acute CHO fueling strategies. MR, mouth rinse.
FIGURE 2.

The total number of male and female participants (A) and total number of studies with at least one male or one female participant (B) for each of the acute CHO fueling strategies. MR, mouth rinse.
Study population and sample size
Figure 3A identifies the different populations used in studies of the five acute CHO fueling strategies. Across all strategies, studies that involved female participants were most likely to recruit a mixed-sex cohort (n = 126 studies; ~64%). However, within these studies, the median number of female participants (3 ± 4) was less than that of male participants (8 ± 6; Fig. 3B; P < 0.0001). Although 740 studies (~79%) involved an exclusively male cohort, only 38 studies (~4%) involved a female-only cohort. Only 14 studies (~2%) included methodological design feature, which specifically compared sex-based responses (MvFdes), and 19 studies (~2%) conducted a sex-based comparison within the statistical procedures (MvFsub). Within MvFsub studies, the median number of female participants per study was significantly less than the number of male participants per study (8 ± 9 vs 12 ± 16; P = 0.02).
FIGURE 3.
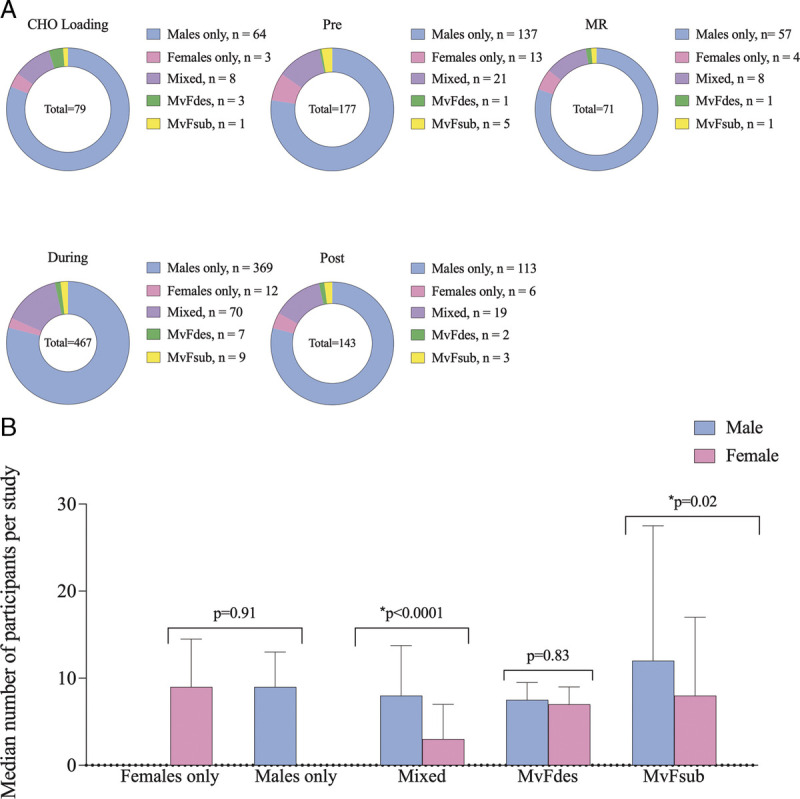
Frequency of study population for each acute CHO fueling strategy, with the number of studies indicated within the legend (A) and the median number of male and female participants within each study population (B). *Significantly different median number of male vs female participants. MR, mouth rinse.
Theme and athlete caliber
Characteristics of the study theme and the athletic caliber of participants across the five acute CHO fueling strategies are demonstrated in Figure 4. Performance themes were the dominant interest across the strategies (~54%; n = 506 studies), with the exception of studies of postexercise CHO intake, where indirect themes underpinning either performance or health (e.g., rates of glycogen synthesis or effects on exercise myokines) were the most common target (~65%; n = 93 studies). Across the 937 studies of acute CHO fueling strategies, 496 studies (~53%) failed to provide sufficient information to allow the athletic caliber of participants to be classified (17). Of the remaining 441 studies where classification was possible, most participants (~83%) were determined to be within Tier 0 (sedentary) to Tier 2 (trained/developmental), with the majority of studies involving participants within Tier 2 (~58%). Notably, no study included Tier 5 (world-class) participants.
FIGURE 4.
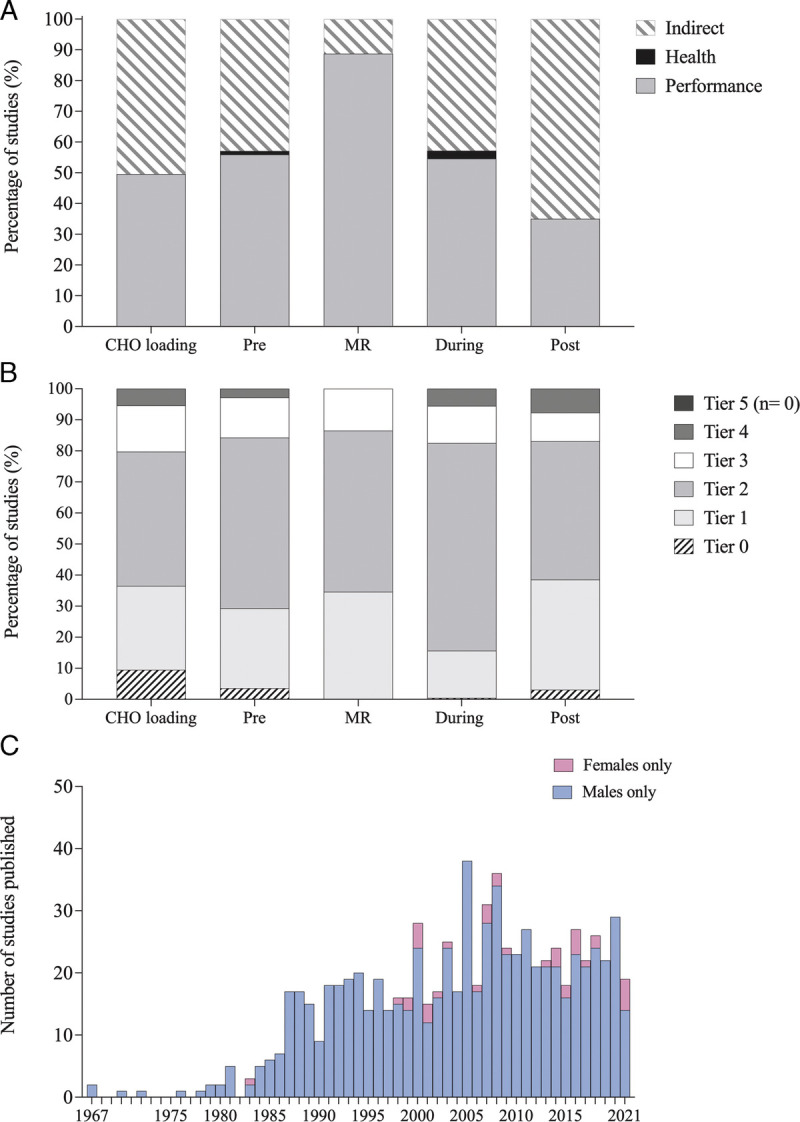
Proportion of studies examining performance, health or indirect themes (A) and proportion of participants within each athlete tier: Tier 0 (sedentary), Tier 1 (recreationally active), Tier 2 (trained/developmental), Tier 3 (highly trained/national), Tier 4 (elite/international), and Tier 5 (world-class) for each of the acute CHO fueling strategies (B). C, Histogram of yearly publication rate for male-only studies and female-only studies for all acute CHO fueling strategies combined. MR, mouth rinse.
Journal impact and publication statistics
There was no significant difference in Altmetric score (P = 0.85) or impact factor (P = 0.60) across study population categories. Differences remained nonsignificant when each acute CHO fueling strategy was examined in isolation. Finally, over the full audit period of 1967–2021, the average publication rate for female-only studies was ~0.7 versus ~13 studies per year for male-only studies. The publication rate of female-only studies increased from ~0.8 studies per year during 2001–2010 to ~2 studies per year from 2011 to 2021, in parallel with a decreased publication rate in male-only studies during this time frame. However, the publication rate continued to be ~10–31 times greater in male-only studies, with 25 studies per year from 2001 to 2010 and 21 studies per year from 2011 to 2021 (Fig. 4C).
Menstrual status and grade
Of the 197 studies that included women, 61 studies (~31%) provided sufficient information to define menstrual status. These 61 studies included cohorts of naturally menstruating women (n = 44), a mixture of naturally menstruating and HC users (n = 11), HC users (n = 5), and women with menstrual irregularities (n = 1). Regarding the quality of the classification protocols used to identify menstrual state (14), 10 studies were awarded Bronze standard, 3 studies achieved Silver standard, and the remaining 48 studies were ungraded (Fig. 5). No studies were awarded Gold standard for their methodological control of ovarian hormones. Nineteen studies described female participants as eumenorrheic, although their characteristics failed to meet the criteria (14), and three studies provided a confusing description of their female participants as being eumenorrheic despite also being oral contraceptive pill (OCP) users.
FIGURE 5.

Proportion of studies of female participants classified according to methodology around menstrual status: Gold standard (best-practice methodologies followed), Silver/Bronze standard (some best-practice methodologies followed), ungraded (menstrual status defined but insufficient information on methodological control of ovarian hormonal profiles), and unclassified (insufficient information to define menstrual status) for each acute CHO fueling strategy. Number of studies within each menstrual classification indicated on legend. MR, mouth rinse.
Menstrual and hormonal cycle phase
Of the studies that included cohorts of naturally menstruating women (n = 55), 32 described their intent to investigate outcomes in a standardized phase of the menstrual cycle. The majority of these studies (n = 31) targeted the follicular phase, whereas 1 study examined the luteal phase. Of the remaining, 14 studies did not control or provide information on menstrual cycle phase, and 4 studies indicated that investigated outcomes were always examined in the same phase of the menstrual cycle but did not provide any information or verification of the menstrual cycle phase. The five remaining studies involving naturally menstruating women compared the effects of an acute CHO fueling strategy at different phases of the menstrual cycle, with the strategy of interest being CHO during exercise (n = 4) and CHO loading (n = 1). Although all of these studies provided a valuable comparison of outcomes in the early to midfollicular versus luteal phase, none provide enough information that allowed a classification of the athletic caliber of participants. Furthermore, only two of these studies used a design to characterize menstrual phase that was awarded Silver standard, whereas the remaining three studies were ungraded. Among studies of HC users (n = 16), 2 studies indicated that outcomes were investigated during the active phase of the OCP cycle. Of the remaining 14 studies, 8 did not provide information on the OCP cycle phase, or provided confusing terminology indicating that testing was taken during the follicular phase (n = 4), or premenstruation or postmenstruation (n = 2). One study of OCP users investigated indirect outcomes of an acute fueling strategy (CHO loading) over different phases of the oral triphasic pill (premenstruation and postmenstruation); this was undertaken in participants classified as Tier 1 (recreationally active), with characterization of the method of classifying menstrual status as Bronze standard.
Performance and indirect outcomes in female participants
Figure 6 demonstrates the studies with female-specific design with sufficient methodological control of ovarian hormones. Only two strategies involving acute CHO fueling (CHO intake during exercise and CHO loading) have investigated outcomes using an acceptable protocol of ovarian hormone control. Studies examining CHO loading protocols only involved Tier 2 (trained/development) athletes and below. Although 1 study examining CHO during exercise included Tier 4 (elite/international) athletes, only 0.5 the study was included as participants were a mixture of HC users and naturally menstruating women, with only Bronze standard being awarded to the group of OCP users (n = 3). For the remainder of studies, participants were Tier 2 and below. This databank is further limited because few studies (n = 3.5) adequately defined menstrual cycle/HC phase of study participants, and none compared outcomes across menstrual cycle phases. The characteristics and results of studies providing female-specific evidence are summarized in Table 2.
FIGURE 6.

Flowchart of studies that provided adequate methodological control of ovarian hormonal profiles (Bronze standard or above) that assessed a performance outcome (A) or an indirect theme (B) with corresponding acute CHO fueling strategy, menstrual status, menstrual cycle/HC phase, and caliber of female participants in each study. MC, menstrual cycle.
TABLE 2.
Summary of female-specific study with sufficient methodological control of ovarian hormones.
| Study | CHO Strategy and Protocol | Study Population | Athletic Caliber | Menstrual Status and Grade | Key Findings |
|---|---|---|---|---|---|
| James et al. (18) | CHO loading 10 g·kg−1 BM·d−1 × 3 d |
MvFdes: n = 6 males, n = 6 females | Tier 1 | HC users HC type: triphasic OCP Bronze standard |
No sex differences in ability to increase muscle glycogen |
| Tarnopolsky et al. (19) | CHO loading Male: 8.4 g·kg−1 BM·d−1 × 4 d; female: 5.4 g·kg−1 BM·d−1 × 4 d |
MvFdes: n = 7 males, n = 8 females | Tier 1 | HC users (n = 4) HC type: triphasic OCP for >1 month Silver standard (HC users only) |
Males only able to increase muscle glycogen and improvements in performance |
| Tarnopolsky et al. (20) | CHO loading Male: 10.5 g·kg−1 BM·d−1 × 5 d; female: 8.8 g·kg−1 BM·d−1 × 5 d |
MvFdes: n = 6 males, n = 7 females | Tier 1 | HC users (n = 4) HC type: triphasic OCP Bronze standard (HC users only) |
No gender differences in ability to increase muscle glycogen |
| Walker et al. (21) | CHO loading 8.2 g·kg−1 BM·d−1 × 4 d |
Females only: n = 6 | Tier 2 | Naturally menstruating Tested during luteal phase Bronze standard |
Improved performance and increased muscle glycogen stores |
| Pettersson et al. (22) | CHO during exercise 132 g·h−1 during 120-min roller-skiing |
MvFdes: n = 6 males, n = 6 females | Tier 4 | HC users (n = 3) HC type: monophasic OCP (n = 2); hormonal spiral (n = 1) Bronze standard (HC users only) |
No improvements in performance for either males or females or sex differences in substrate utilization |
| Wallis et al. (23) | CHO during exercise 10, 60, and 90 g·h−1 during 2-h cycling at 60% of V̇O2max |
Female only: n = 8 | Tier 2 | Naturally menstruating Tested during follicular phase Bronze standard |
Highest rate of CHO oxidation with 60 g·h−1 with no further increase at 90 g·h−1 |
| Tremblay et al. (24) | CHO during exercise Male: ~63 g·h−1; naturally menstruating ~58 g·h−1; HC users ~56 g·h−1 during 120-min cycling at 50% of maximum wattage Performed once following a normal diet and once following a high-CHO diet |
MvFdes: n = 6 males, n = 12 females | Tier 1 | HC users (n = 6) HC type: triphasic OCP Silver standard Naturally menstruating (n = 6) Tested 6–10 d after menses Bronze standard |
No difference in fuel selection between HC users and naturally menstruating women Sex differences in CHO oxidation seen during control trial disappeared with CHO supplementation |
| Wallis et al. (25) | CHO during exercise 90 g·h−1 during 120 min of cycling at 55% of maximum wattage |
MvFdes: n = 8 males, n = 8 females | Tier 1 | Naturally menstruating Tested during follicular phase Bronze standard |
Similar metabolic responses seen in men and women |
V̇O2max, maximal aerobic capacity.
DISCUSSION
We assessed the quality and quantity of representation of female athletes within the research that underpins current sports nutrition guidelines for the achievement of acute high CHO availability around key training sessions and competition. We used a standardized auditing tool (15) that allows us to make direct comparison to other projects of this type (5) as well as the wider survey literature (3,4). Our audit found that only ~11% of the participants in studies of acute CHO fueling strategies were women; this is significantly lower than the findings of ~23% representation in studies examining performance supplements using the same audit tool (5). In addition to the lack of general participation in the studies included in our audit, we found that female athletes were not well served with investigations of the efficacy of acute CHO fueling strategies for their specific needs (e.g., biological differences or event characteristics). Indeed, there were nearly 20 times more studies involving male-only cohorts compared with female-only cohorts, and the smaller median number of women in mixed cohort studies suggests that they may have been included to increase the sample size of the total group rather than allow an investigation of between-sex differences. It may also reflect volunteer bias and sex differences in willingness to participate in certain types of research studies that may make it more difficult to recruit female participants (26). Despite the potential effect of ovarian hormones on CHO metabolism (11), the current literature provides minimal attention to differences in outcomes of the various acute CHO fueling strategies between male and female athletes or within females according to menstrual status or phase, particularly when the robustness of the research design is considered. Our audit must therefore conclude that, despite the wealth (>900) of studies on acute strategies for CHO fueling for exercise, the lack of high-quality studies with a female-specific design represents a large gap in the evidence base supporting their implementation by female athletes. Although this does not demonstrate the lack of efficacy of the sports nutrition guidelines regarding the achievement of high CHO availability for training and competition by a female athlete, it calls for new literature to consider any potential adjustments associated with her special needs.
There is growing awareness of the underrepresentation of female participants in sports science research (3,4). Because the paucity of female-specific sports science research is likely to be widespread, it is important that the emerging interest in tackling female-focused research is directed to areas that are deemed high priority. We have previously identified these as being themes that have received poor (and/or poor quality) attention to date, those in which sex-specific differences are suspected, and those in which successful strategies are likely to achieve detectable changes in health or performance (15). Acute strategies to achieve high CHO availability for a key exercise session seem to meet all three criteria given the clear underrepresentation of women noted, sex differences being plausible (11), and strategies that match body CHO stores to the fuel requirements of a performance test achieve enhancements that are both statistically significant and substantial in terms of the outcomes of real-life sporting events. For example, prolonged time trial protocols are improved by ~2%–3% by glycogen supercompensation (27) and ~2%–6% by consuming CHO during exercise according to meta-analyses of the general literature (28).
Our auditing tool captures a wealth of information about the targeted literature including the size and focus of study sample sizes, characteristics of the sex and athletic caliber of study participants, study interests, secular changes in publication rates, and impact factors and Altmetric scores (15). Although these outcomes have been presented in the Results section of this article and discussed more widely in our previous audit of evidence-based performance supplements (5), the discussion theme of greatest interest from the literature on acute fueling strategies around exercise involves the inadequate understanding and investigation of menstrual status and phase in female athletes. Of the 197 studies that included female participants, the majority (~69%) failed to provide sufficient information to classify the menstrual cycle status of participants, and even in the initial literature search, 46 studies needed to be excluded because of failure to explicitly state the sex of study participants. For studies where the menstrual status of participants could be classified, poor knowledge or mixed terminology used to describe menstrual status characteristics was noted. For example, several studies described taking measurements during the “follicular” phase or “premenstruation and postmenstruation” in participants who were OCP users (18,29) rather than indicating if measurements were taken during the active or nonactive pill phase and before or after the withdrawal bleed. Various factors may underpin the incorrect terminology and lack of information pertaining to the menstrual status of female participants in these studies. This may include the poor knowledge of the menstrual cycle and HC of those involved in the research and authorship, lack of importance placed on the control of ovarian hormones or the possibility for sex-based differences in study outcomes by these individuals and/or the oversimplification of description of study methods within a publication word count limit. Indeed, because the appreciation of physiological differences across menstrual cycle phase or the influence of HC on ovarian hormones may have preceded many early studies, information now seen as pertinent and/or appropriate terminology may not have been included. However, this can no longer be an excuse in view of guidance that is now available to address the challenges of experimental protocols and make research designs more robust (14).
Of the acute CHO fueling strategies in this audit, studies examining CHO loading protocols contained the highest proportion of studies with sufficient methodological control of ovarian hormones (n = 2.5; 27%). The popularity of this topic may reflect the longevity of interest in a protocol that emanated in the 1960s and has continued to evolve in the simplified format recommended for present day athletes (6) (Table 1). Early interest in sex-based differences was trigged by a study in which female participants were reported to fail to respond to a CHO loading protocol (i.e., achieve the same increase in glycogen stores) in comparison to the male participants (19). These results were later shown to be an artifact created by differences in energy intake, with later studies demonstrating that female athletes were able to increase muscle glycogen stores when the energy content of the habitual diet was increased to improve energy availability and support an additional increase in CHO intake (20,21). This suggests that female athletes may need to increase energy intake above their apparent (self-reported) or true habitual intake, both to achieve CHO targets and to allow an energy availability that will support an increase in muscle glycogen. Despite the continued investigation of CHO loading protocols, with topics such as the localization or structure of glycogen particles (30), these have been undertaken with male-only or mixed-sex cohorts. Indeed, no studies of CHO loading involving separate analysis of female participants have been published over the past 15 yr, despite the likelihood of sex-based differences in muscle fibers (10). Furthermore, of the currently available studies on glycogen supercompensation in female athletes, the only two that have demonstrated functional benefits used protocols involving exercise capacity rather than performance (21,31). Because female athletes are more likely to consume suboptimal amounts of CHO intake in their daily training diet (32), there is a delicate interaction between energy availability, glycogen storage, and performance that requires further investigation.
Even when sufficient control of ovarian hormones has been achieved, there are other limitations of the sparse literature on CHO loading in female athletes as outlined in Table 2. This includes the involvement of athletes of lower caliber (Tiers 1–2; recreational to trained/developed), leaving an absence of evidence on highly trained and elite female athletes. In addition, most studies have involved triphasic OCP users in an unknown phase of the OCP cycle (18–20), whereas there is only one investigation in naturally menstruating women (21). This study, which measured outcomes in the luteal phase of the menstrual cycle, is an anomaly because most research was conducted during the follicular phase of the menstrual cycle, to minimize the impact of ovarian hormones on study outcomes by choosing the phase during which they are at their lowest. Of course, from a real-world perspective, there is greater interest in understanding how higher concentrations of ovarian hormones affect glycogen storage and utilization. Here, there is evidence that resting glycogen concentrations (33) or glycogen repletion rates (34) are higher in the luteal versus follicular phases, although the findings and their implications are unclear. For example, an investigation of female participants of unclassified athletic caliber reported that, although resting muscle glycogen was lower in the follicular phase but could be increased with a CHO loading protocol, the CHO loading protocol failed to increase muscle glycogen in the luteal phase or improve performance in either phase (33). Although CHO loading increased rates of CHO oxidation during exercise, there were no differences between phases (33). More research is needed to examine the effects of CHO loading in different phases of the menstrual cycle as well as in athletes using monophasic OCP and increasingly popular HC such as intrauterine devices.
The benefits of consuming CHO during exercise on performance are well documented (28), with guidelines including hourly targets based on the duration of the event, as well as recommendations for different types of CHO sources according to the targeted amounts (6,9) (Table 1). The use of absolute (in grams per hour) rather than scaled (in grams per kilogram of body mass (BM) per hour) targets for event fueling is based on the understanding that the maximal oxidation rate of ingested CHO is limited by the intestinal absorption of CHO and is not changed by body size (35). When targets exceed 60 g·h−1, such as during events >2.5 h where dwindling endogenous CHO stores necessitate a greater contribution from exogenous CHO sources, it is recommended that this is supplied by “multiple transportable CHOs” blends of CHO sources with different intestinal transport routes such as glucose and fructose (35). Our search for studies with a female-specific design and sufficient methodological control of ovarian hormones that could have contributed to these guidelines was only able to locate one article as outlined in Table 2 (22). This involved an investigation of Tier 4 (elite) female athletes, comparing the performance effects of consuming large amounts of multiple transportable CHO in a hydrogel versus placebo treatment (22). Notably, this study involved a mixture of HC users and naturally menstruating women, with only the HC users treated according to best-practice guidelines for hormonal considerations (12) and were therefore eligible for inclusion. We also identified a further three studies involving the effects on CHO intake during exercise on indirect measures of health and performance (23–25). Although one demonstrated that rates of exogenous glucose oxidation during exercise in female athletes are similar to male athletes (23), we failed to find any studies of sex-based differences in the ability to oxidize multiple transportable CHO. Evidently, research following best-practice recommendations for methodological control of ovarian hormones in high-caliber female athletes is needed to assess this gap within the literature. There is also a need to consider whether the recommendations for doses and sources of CHO are the same for female as for male athletes. Although there may not be a relationship between body mass and maximal rates of CHO oxidation based on similar intestinal absorption capacity (35), differences in body size may affect the gastrointestinal comfort associated with consuming different amounts or forms of CHO. For example, a 6%–10% CHO beverage is typically recommended to provide the ideal balance of CHO and fluid replacement while minimizing gastrointestinal disturbances (36). However, this would require drinking 600–1000 mL·h−1 of fluid to achieve the recommended CHO target of 60 g·h−1, which may pose a risk of gastrointestinal discomfort for a small female athlete. Differences in gastrointestinal tract function, such as differences in gastric emptying (37), or premenstrual symptoms that often include gastrointestinal disturbances (38) could have further implications for best-practice guidelines for the female athlete regarding CHO intake during exercise.
Studies on the intake of CHO during exercise encompass the newer area of research around CHO mouth sensing. Here, the ergogenic effects of consuming (or even mouth rinsing) CHO are achieved via interaction between receptors in the oral cavity and reward areas in the brain (39). Although this may provide a separate and additional benefit to the role of exogenous CHO as a substrate for the muscle and central nervous system in longer exercise protocols, benefits are apparent in shorter events in which there is minimal need or opportunity for exercise CHO intake to play a metabolic role (40). This has led to separate recommendations for exercise CHO intake/mouth sensing within sports nutrition guidelines (6,9) (Table 1) as well as a dedicated literature in which the intervention is applied as a CHO mouth rinse rather than CHO ingestion, to divorce it from a possible metabolic role. Notwithstanding the briefer life span of this research theme, our audit identified six studies with a female-specific design examining a performance outcome, of which only two reported improvements in performance with CHO mouth rinse (41,42). However, these studies involved a broad range of female participants in terms of sports/exercise protocols and athletic caliber, making it difficult to make a general conclusion about the effectiveness of CHO mouth rinsing in female athletes. In terms of potential sex differences, it is noted that the use of a CHO mouth rinse could result in performance impairment if an improved psychological sensation leads to a misguided pacing strategies and the adoption of an unsustainable pace (39). Because female athletes are suggested to have better pacing strategies than male athletes (43), CHO mouth rinse could arguably be either less effective in female athletes if they are already adopting optimal event pacing or more effective if they are less likely to mis-pace because of increased psychological input. Furthermore, there is evidence of sex-based differences in brain activation in response to food cues (44). Unfortunately, we were unable to find any studies of CHO mouth sensing that compared this in male and female athletes. Obviously, more research is needed to examine the use of CHO mouth rinse in female athletes, including sex-based differences in the ergogenic mechanism behind this strategy, and how this interacts with differences or changes in psychological sensations, such as perceived exertion or cravings/sense of sweetness, across menstrual cycle phase or HC usage (38).
Consuming CHO in the hours before or between events (Table 1) has the potential to acutely alter muscle and liver glycogen stores for contribution to the fuel requirements of the subsequent session. Our audit included five studies with a female-specific design that have examined the effect of preexercise CHO intake within the recommended range of 1–4 g·kg−1 BM on a performance outcome, with two of these demonstrating a significant benefit (45,46). Unfortunately, none of the studies examining preexercise CHO fueling and female participants were considered to have achieved ideal control of ovarian hormones. Although one study examining postexercise CHO fueling was awarded a Bronze standard for this characteristic (47), its failure to provide sufficient information on the caliber of female participants limits the direct application of the findings. Meanwhile, in terms of immediate (0–4 h) postexercise refueling, we located three studies using a female-specific design to examine the effect of CHO intake recommendations (1.0–1.2 g of CHO·kg−1 BM·h−1; Table 1) on the performance of a subsequent exercise bout (47–49). The unanimous finding of a lack of performance improvement in these studies could be due to a type 2 error involving the failure to control ovarian hormones appropriately, or it could signal that the refueling guidelines, derived from research in men, are not optimal for the female athlete. However, the recommended CHO intakes are based on enhancing postexercise muscle glycogen synthesis, which, while only being directly measured in one study, has been found to be similar in male and female athletes (50). Although not the focus of this audit, it is noted that sports nutrition guidelines recommend the coingestion of protein intake to enhance postexercise refueling when CHO ingestion is suboptimal (<1.2 g·kg−1 BM·h−1) (51). Of the nine studies that contributed to a recent review of this topic (50), eight included male-only cohorts and one did not indicate the sex of participants. In light of the limited number of female-specific studies of preexercise and postexercise CHO intake, and the lack of adequate ovarian hormonal control within these studies, we are unable to make firm conclusions about female-focused recommendations, and sex or menstrual phase differences around these themes.
CONCLUSIONS
In conclusion, as has been seen in other areas of sports science research, women are underrepresented in the research that has led to the current guidelines for acute CHO fueling strategies. Audit results suggest a lack of understanding of female-specific considerations, and poor appreciation for the need to control for ovarian hormones when conducting research. Given the lack of high-quality research that contributes knowledge specific to the female athlete and sex-based differences, current guidelines for acute strategies to achieve optimal CHO availability for key training sessions and competition may not represent best practice for female athletes. Although this does not mean that the sports nutrition guidelines should not be used by female athletes, it calls for new literature to consider any potential adjustments associated with special needs or considerations. High-priority areas of research have been highlighted throughout this audit that will help to ensure that recommendations provided to female athletes regarding CHO fueling strategies around training are evidence based.
Supplementary Material
Acknowledgments
The authors acknowledge the support from the Wu Tsai Human Performance Alliance and the Joe and Clara Tsai Foundation for this work.
The authors declare no conflicts of interest. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
Contributor Information
MEGAN A. KUIKMAN, Email: Megan.Kuikman@acu.edu.au.
ELLA S. SMITH, Email: Ella.Smith@acu.edu.au.
KATHRYN E. ACKERMAN, Email: Kathryn.Ackerman@childrens.harvard.edu.
RACHEL HARRIS, Email: drrachharris@gmail.com.
KIRSTY J. ELLIOTT-SALE, Email: K.Elliott-Sale@mmu.ac.uk.
TRENT STELLINGWERFF, Email: tstellingwerff@csipacific.ca.
LOUISE M. BURKE, Email: Louise.Burke@acu.edu.au.
REFERENCES
- 1.Tokyo 2020 first ever gender-balanced Olympic Games in history, record number of female competitors at Paralympic Games—Olympic News [date unknown]; [cited 2021 Dec 15]. Available from: https://olympics.com/ioc/news/tokyo-2020-first-ever-gender-balanced-olympic-games-in-history-record-number-of-female-competitors-at-paralympic-games.
- 2.Sainz-De-baranda C, Adá-Lameiras A, Blanco-Ruiz M. Gender differences in sports news coverage on twitter. Int J Environ Res Public Health. 2020;17(14):5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costello JT, Bieuzen F, Bleakley CM. Where are all the female participants in sports and exercise medicine research? Eur J Sport Sci. 2014;14(8):847–51. [DOI] [PubMed] [Google Scholar]
- 4.Cowley ES, Olenick AA, Mcnulty KL, Ross EZ. “Invisible sportswomen”: the sex data gap in sport and exercise science research. Women Sport Phys Act J. 2021;29(2):146–51. [Google Scholar]
- 5.Smith ES Mckay AKA Kuikman M, et al. Auditing the representation of female versus male athletes in sports science and sports medicine research: evidence-based performance supplements. Nutrients. 2022;14(5):953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke LM, Hawley JA, Wong SH, Jeukendrup AE. Carbohydrates for training and competition. J Sports Sci. 2011;29(1 Suppl):S17–27. [DOI] [PubMed] [Google Scholar]
- 7.Burke LM, Kiens B, Ivy JL. Carbohydrates and fat for training and recovery. J Sports Sci. 2004;22(1):15–30. [DOI] [PubMed] [Google Scholar]
- 8.Coyle EF. Timing and method of increased carbohydrate intake to cope with heavy training, competition and recovery. J Sports Sci. 1991;9: Spec No. 29–51; discussion 51–2. [DOI] [PubMed] [Google Scholar]
- 9.Thomas DT, Erdman KA, Burke LM. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: nutrition and athletic performance. J Acad Nutr Diet. 2016;116(3):501–28. [DOI] [PubMed] [Google Scholar]
- 10.Haizlip KM, Harrison BC, Leinwand LA. Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology (Bethesda). 2015;30(1):30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell SE, Febbraio MA. Effects of ovarian hormones on exercise metabolism. Curr Opin Clin Nutr Metab Care. 2001;4(6):515–20. [DOI] [PubMed] [Google Scholar]
- 12.Moore DR, Sygo J, Morton JP. Fuelling the female athlete: carbohydrate and protein recommendations. Eur J Sport Sci. 2022;22(5):684–96. [DOI] [PubMed] [Google Scholar]
- 13.Holtzman B, Ackerman KE. Recommendations and nutritional considerations for female athletes: health and performance. Sports Med. 2021;51(1 Suppl):43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott-Sale KJ Minahan CL de Jonge XAKJ, et al. Methodological considerations for studies in sport and exercise science with women as participants: a working guide for standards of practice for research on women. Sports Med. 2021;51(5):843–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith ES McKay AKA Ackerman KE, et al. Methodology review: a protocol to audit the representation of female athletes in sports science and sports medicine research. Int J Sport Nutr Exerc Metab. 2022;32(2):114–27. [DOI] [PubMed] [Google Scholar]
- 16.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKay AKA Stellingwerff T Smith ES, et al. Defining training and performance caliber: a participant classification framework. Int J Sports Physiol Perform. 2022;17(2):317–31. [DOI] [PubMed] [Google Scholar]
- 18.James AP Lorraine M Cullen D, et al. Muscle glycogen supercompensation: absence of a gender-related difference. Eur J Appl Physiol. 2001;85(6):533–8. [DOI] [PubMed] [Google Scholar]
- 19.Tarnopolsky MA, Atkinson SA, Phillips SM, MacDougall JD. Carbohydrate loading and metabolism during exercise in men and women. J Appl Physiol (1985). 1995;78(4):1360–8. [DOI] [PubMed] [Google Scholar]
- 20.Tarnopolsky MA Zawada C Richmond LB, et al. Gender differences in carbohydrate loading are related to energy intake. J Appl Physiol (1985). 2001;91(1):225–30. [DOI] [PubMed] [Google Scholar]
- 21.Walker JL, Heigenhauser GJ, Hultman E, Spriet LL. Dietary carbohydrate, muscle glycogen content, and endurance performance in well-trained women. J Appl Physiol (1985). 2000;88(6):2151–8. [DOI] [PubMed] [Google Scholar]
- 22.Pettersson S, Edin F, Bakkman L, McGawley K. Effects of supplementing with an 18% carbohydrate-hydrogel drink versus a placebo during whole-body exercise in −5°C with elite cross-country ski athletes: a crossover study. J Int Soc Sports Nutr. 2019;16(1):46–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallis GA, Yeo SE, Blannin AK, Jeukendrup AE. Dose–response effects of ingested carbohydrate on exercise metabolism in women. Med Sci Sports Exerc. 2007;39(1):131–8. [DOI] [PubMed] [Google Scholar]
- 24.Tremblay J, Peronnet F, Massicotte D, Lavoie C. Carbohydrate supplementation and sex differences in fuel selection during exercise. Med Sci Sports Exerc. 2010;42(7):1314–23. [DOI] [PubMed] [Google Scholar]
- 25.Wallis GA, Dawson R, Achten J, Webber J, Jeukendrup AE. Metabolic response to carbohydrate ingestion during exercise in males and females. Am J Physiol Endocrinol Metab. 2006;290(4):E708–15. [DOI] [PubMed] [Google Scholar]
- 26.Nuzzo J. Volunteer bias and female participation in exercise and sports science research. Quest. 2021;73(1):82–101. [Google Scholar]
- 27.Hawley JA, Schabort EJ, Noakes TD, Dennis SC. Carbohydrate-loading and exercise performance. Sports Med. 1997;24(2):73–81. [DOI] [PubMed] [Google Scholar]
- 28.Stellingwerff T, Cox GR. Systematic review: carbohydrate supplementation on exercise performance or capacity of varying durations. Appl Physiol Nutr Metab. 2014;39(9):998–1011. [DOI] [PubMed] [Google Scholar]
- 29.Brown MA, Green BP, James LJ, Stevenson EJ, Rumbold PLS. The effect of a dairy-based recovery beverage on post-exercise appetite and energy intake in active females. Nutrients. 2016;8(6):355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen R Skjærbæk MC Plomgaard P, et al. Glycogen supercompensationis due to increased number, not size, of glycogen particles in human skeletal muscle. Exp Physiol. 2021;106(5):1272–84. [DOI] [PubMed] [Google Scholar]
- 31.Brewer J, Williams C, Patton A. The influence of high carbohydrate diets on endurance running performance. Eur J Appl Physiol Occup Physiol. 1988;57(6):698–706. [DOI] [PubMed] [Google Scholar]
- 32.Burke LM, Cox GR, Culmmings NK, Desbrow B. Guidelines for daily carbohydrate intake: do athletes achieve them? Sports Med. 2001;31(4):267–99. [DOI] [PubMed] [Google Scholar]
- 33.McLay RT, Thomson CD, Williams SM, Rehrer NJ. Carbohydrate loading and female endurance athletes: effect of menstrual-cycle phase. Int J Sport Nutr Exerc Metab. 2007;17(2):189–205. [DOI] [PubMed] [Google Scholar]
- 34.Nicklas BJ, Hackney AC, Sharp RL. The menstrual cycle and exercise: performance, muscle glycogen, and substrate responses. Int J Sports Med. 1989;10(4):264–9. [DOI] [PubMed] [Google Scholar]
- 35.Jeukendrup AE. Carbohydrate and exercise performance: the role of multiple transportable carbohydrates. Curr Opin Clin Nutr Metab Care. 2010;13(4):452–7. [DOI] [PubMed] [Google Scholar]
- 36.Gaskell SK, Rauch CE, Costa RJS. Gastrointestinal assessment and therapeutic intervention for the management of exercise-associated gastrointestinal symptoms: a case series translational and professional practice approach. Front Physiol. 2021;12:719142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Datz FL, Christian PE, Moore J. Gender-related differences in gastric emptying. J Nucl Med. 1987;28(7):1204–7. [PubMed] [Google Scholar]
- 38.Bruinvels G Goldsmith E Blagrove R, et al. Prevalence and frequency of menstrual cycle symptoms are associated with availability to train and compete: a study of 6812 exercising women recruited using the Strava exercise app. Br J Sports Med. 2021;55(8):438–43. [DOI] [PubMed] [Google Scholar]
- 39.Burke LM. Nutritional approaches to counter performance constraints in high-level sports competition. Exp Physiol. 2021;106(12):2304–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burke LM, Maughan RJ. The governor has a sweet tooth—mouth sensing of nutrients to enhance sports performance. Eur J Sport Sci. 2015;15(1):29–40. [DOI] [PubMed] [Google Scholar]
- 41.Hawkins KR, Krishnan S, Ringos L, Garcia V, Cooper JA. Running performance with nutritive and non-nutritive sweetened mouth rinses. Int J Sports Physiol Perform. 2017;12(8):1105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pereira PEA, Azevedo P, Azevedo K, Azevedo W, Machado M. Caffeine supplementation or carbohydrate mouth rinse improves performance. Int J Sports Med. 2021;42(2):147–52. [DOI] [PubMed] [Google Scholar]
- 43.Tiller NB, Elliott-Sale KJ, Knechtle B, Wilson PB, Roberts JD, Millet GY. Do sex differences in physiology confer a female advantage in ultra-endurance sport? Sports Med. 2021;51(5):895–915. [DOI] [PubMed] [Google Scholar]
- 44.Geliebter A, Pantazatos SP, McOuatt H, Puma L, Gibson CD, Atalayer D. Sex-based fMRI differences in obese humans in response to high vs. low energy food cues. Behav Brain Res. 2013;243(1):91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kirwan JP, O’Gorman D, Evans WJ. A moderate glycemic meal before endurance exercise can enhance performance. J Appl Physiol (1985). 1998;84(1):53–9. [DOI] [PubMed] [Google Scholar]
- 46.Lee CL, Cheng CF, Astorino TA, Lee CJ, Huang HW, Chang WD. Effects of carbohydrate combined with caffeine on repeated sprint cycling and agility performance in female athletes. J Int Soc Sports Nutr. 2014;11(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flynn S, Rosales A, Hailes W, Ruby B. Males and females exhibit similar muscle glycogen recovery with varied recovery food sources. Eur J Appl Physiol. 2020;120(5):1131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mock MG, Hirsch KR, Blue MNM, Trexler ET, Roelofs EJ, Smith-Ryan AE. Post-exercise ingestion of low or high molecular weight glucose polymer solution does not improve cycle performance in female athletes. J Strength Cond Res. 2021;35(1):124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fallowfield JL, Williams C. The influence of a high carbohydrate intake during recovery from prolonged, constant-pace running. Int J Sport Nutr. 1997;7(1):10–25. [DOI] [PubMed] [Google Scholar]
- 50.Tarnopolsky MA, Bosman M, MacDonald JR, Vandeputte D, Martin J, Roy BD. Postexercise protein–carbohydrate and carbohydrate supplements increase muscle glycogen in men and women. J Appl Physiol (1985). 1997;83(6):1877–83. [DOI] [PubMed] [Google Scholar]
- 51.Betts JA, Williams C. Short-term recovery from prolonged exercise: exploring the potential for protein ingestion to accentuate the benefits of carbohydrate supplements. Sports Med. 2010;40(11):941–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


