Background:
Myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease (MOGAD) is an acquired inflammatory demyelinating disease with optic neuritis (ON) as the most frequent clinical symptom. The hallmark of the disease is the presence of autoantibodies against MOG (MOG-IgG) in the serum of patients. Whereas the role of MOG in the experimental autoimmune encephalomyelitis animal model is well-established, the pathogenesis of the human disease and the role of human MOG-IgG is still not fully clear.
Evidence Acquisition:
PubMed was searched for the terms “MOGAD,” “optic neuritis,” “MOG antibodies,” and “experimental autoimmune encephalomyelitis” alone or in combination, to find articles of interest for this review. Only articles written in English language were included and reference lists were searched for further relevant papers.
Results:
B and T cells play a role in the pathogenesis of human MOGAD. The distribution of lesions and their development toward the optic pathway is influenced by the genetic background in animal models. Moreover, MOGAD-associated ON is frequently bilateral and often relapsing with generally favorable visual outcome. Activated T-cell subsets create an inflammatory environment and B cells are necessary to produce autoantibodies directed against the MOG protein. Here, pathologic mechanisms of MOG-IgG are discussed, and histopathologic findings are presented.
Conclusions:
MOGAD patients often present with ON and harbor antibodies against MOG. Furthermore, pathogenesis is most likely a synergy between encephalitogenic T and antibody producing B cells. However, to which extent MOG-IgG are pathogenic and the exact pathologic mechanism is still not well understood.
Myelin oligodendrocyte glycoprotein (MOG), a minor component of myelin in the central nervous system (CNS), is expressed in the outermost layer of myelin (1). It is a Type 1 integral membrane glycoprotein of 26–28 kDa, only found in mammals and is highly conserved between species (2,3). Up to 15 splice variants have been described in humans and nonhuman primates, but not in rodents, that mainly differ in their cytoplasmatic domains (4). Despite intensive research, the function of MOG still remains to be fully determined. Postulated biological roles include an adhesion molecule, a compactor of myelin, or a stabilizer of microtubules (5,6). Furthermore, it has been shown to interact with C1q, nerve growth factor, dendritic-cell (DC)-specific intercellular adhesion molecule-3 grabbing nonintegrin, and to be a cellular receptor for rubella virus (7,8,9,10). The extracellular site is composed of an immunoglobulin (Ig)-V-like domain that is highly immunogenic and can evoke inflammatory demyelinating immune responses. It was used extensively to induce inflammation in experimental autoimmune encephalomyelitis (EAE), a proposed animal model of multiple sclerosis (MS). However, the use of cell-based assays with full-length natively-folded MOG for the detection of human MOG immunoglobulin G antibodies (MOG-IgG) in patients with acquired demyelinating diseases (ADS) showed that MOG-IgG-associated disease (MOGAD) represents a disease distinct from MS (1,11).
MOGAD is a rare disease with an incidence of 0.16/100,000 people (12), but the spectrum of clinical symptoms is ever expanding. The most common presentations are optic neuritis (ON), acute disseminated encephalomyelitis (ADEM), transverse myelitis, aquaporin 4 (AQP4)-IgG negative neuromyelitis optica spectrum disorders (NMOSD), brainstem syndrome, and cortical encephalitis (13). Moreover, there is a correlation between age and clinical presentation, with ADEM being more common in children and optico-spinal lesions being more present in adults (12,14,15,16,17,18,19,20,21,22).
Despite the increasing knowledge of clinical MOGAD presentations, the pathophysiology and importantly, the pathogenic role of human MOG-IgG, remains to be fully determined. This review aims to summarize present studies on MOG-IgG pathology and pathogenesis of this rare inflammatory demyelinating disease with a focus on optic pathway involvement.
HUMAN MYELIN OLIGODENDROCYTE GLYCOPROTEIN-IgG—DETECTION AND BINDING TO MYELIN OLIGODENDROCYTE GLYCOPROTEIN
The introduction of state-of-the-art cell-based assays for the detection of human MOG-IgG resulted in the characterization of a novel subset of ADS different from MS and NMOSD (1,11,23). Importantly, only antibodies recognizing conformational epitopes present on the full-length protein were found to be of clinical interest (11,24). Therefore, the use of linear peptides or unfolded proteins in ELISA and immunoblots is not suitable for detection of MOG-IgG in human serum samples (11,25). The epitopes recognized most frequently in human MOG are located within the extracellular IgV-like domain and are heterogenic. Proline 42 is the most important amino acid for antibody recognition, located in the CC′ loop, followed by histidine 103 and serine 104 (26,27). The latter constitutes the main binding site of the monoclonal antibody 8-18-C5 (28). Most human MOG-IgGs are not or only weakly cross-reactive with rodent MOG, with the important P42S mutation, which hampers investigation in rodent models (26,29,30). Moreover, it has been shown that in patients with persisting MOG-IgG serostatus, the epitope remains constant (26).
Human MOG-IgG has a reduced binding to paraformaldehyde-treated MOG (27). This further supports the dependence on binding to natively-folded conformational epitopes. MOG has a glycosylation site at asparagine 31 and studies have shown conflicting results regarding MOG-IgG binding in the absence of the glycan. Using the mutant N31D, some serum samples revealed better recognition of MOG (23,26,31). Nevertheless, another study additionally using the mutant N31A found that 60% of MOG-IgG binding was altered (32). The human MOG gene undergoes alternative splicing and distinct MOG isoforms, that differ in their cytoplasmatic domain, have been described (33,34,35,36). Intriguingly, the hydrophobic cytoplasmatic membrane-associated domain was recently described to play a pivotal role for the recognition of human MOG-IgG and the authors propose that this domain generates a certain distance between distinct MOG proteins enabling bivalent binding of MOG-IgG (37). A recent study investigated the binding of MOG-IgG to 6 major MOG isoforms. A third of all patient samples only recognized MOGα1 and MOGβ1, both of which have this hydrophobic domain. However, most of the samples recognized all or most MOG isoforms tested, despite the lack of this domain (38). These findings reveal that human MOG-IgG has a complex and dynamic epitope specificity.
T- AND B-CELL MEDIATED PATHOGENESIS OF MYELIN OLIGODENDROCYTE GLYCOPROTEIN-ASSOCIATED DISEASE
The encephalitogenic role of MOG has been analyzed since decades because it is frequently used as an autoantigen in the EAE model of CNS demyelination (39,40,41,42). In this model, animals are actively immunized with different myelin proteins/peptides or are used for passive transfer experiments to study the underlying immunopathogenesis. Dependence on T and B cells and their orchestration is highly mediated by the type of antigen (i.e., the specific myelin protein, recombinant protein/peptide) and the genetic background of animals. MOG-IgG has been shown to enhance T-cell mediated disease in some animal models, whereas B cells were demonstrated to be unimportant for disease development in other animals (reviewed in Refs. 1,43).
In patients with MOGAD, genetic studies showed no strong correlation between human leukocyte antigen (HLA) genotype and MOGAD development; importantly, no cause for disease pathogenesis has been found. A recent study found a protective effect of the HLA-C*03:04 allele (44), whereas a Dutch study could not find any associations (45). In addition, in a Chinese cohort, there was an association between pediatric-onset MOGAD for DQB1*05:02-DRB1*16:02, but not for adult MOGAD (46). Moreover, in some cases, a viral infection preceded MOGAD diagnosis: Epstein–Barr virus, herpes simplex virus 1, rubella, varicella zoster virus, and severe acute respiratory syndrome–coronavirus-2 (47,48,49,50,51,52). A rare paraneoplastic incidence of MOGAD has also been described (53). A few patients developed MOGAD while given tumor necrosis factor-α (TNFα) inhibitors, yet this is an uncommon phenomenon (54).
Similar to the EAE animal, in human MOGAD, a synergy between encephalitogenic T cells and B cells is observed (Fig. 1). Under normal circumstances, the CNS parenchyma is free of lymphocytes. In recent years, however, it has become clear that neuroimmune interactions are important for CNS homeostasis (55,56,57). A pro-inflammatory environment that enables opening of the blood–brain barrier (BBB) for the entry of potentially pathogenic antibodies is crucial for the pathogenesis of MOGAD. There are 2 possible explanations for the generation of autoimmune responses against MOG: the “inside-out” hypothesis postulates primary damage of oligodendrocytes that leads to drainage of myelin antigens into lymph nodes (LN) via lymphatic vessels of the dura mater (58,59,60,61). These are transported as soluble antigens or by DC of the choroid plexus or the meninges into the deep cervical LN, where antigen presentation and priming of T cells takes place (60,62,63,64,65,66,67). However, drainage of foreign antigens via this pathway has also been shown to induce tolerance of immune cells toward self-antigens rather than autoimmune activation; therefore, it is possible that a certain threshold of drained CNS antigens has to be reached (68,69,70,71). The second possibility, called the “outside-in” hypothesis, posits an activation of lymphocytes in peripheral LN through molecular mimicry or pan-activation after a systemic viral infection (65). Cross-reactivity has been shown between MOG-IgG and butyrophilin, a milk protein (72). Furthermore, negative thymic selection of T cells toward MOG self-tolerance is believed to be incomplete (73,74). In line, a lack of immune tolerance toward MOG has been shown in knock-out mice (75) and immune tolerance was restored using mRNA-based vaccination with MOG peptides or transgenic expression of MOG within immune cells (76,77).
FIG. 1.
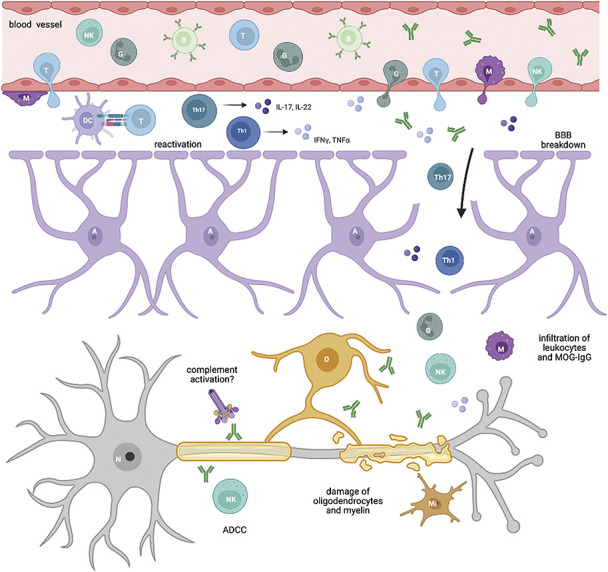
Pathogenesis of MOGAD. Encephalitogenic T cells gain access into the CNS (mostly through the meninges), where they seek contact with APC (DC) and get reactivated. After polarization toward Th1 and Th17 T-cell subsets, subsequent release of cytokines leads to a breakdown of the BBB and to a massive infiltration of other leukocytes and MOG-IgG, that were produced in the periphery. MOG-IgG bind to their target on the surface of oligodendrocytes and on myelin and can be directly pathogenic via ADCC, altering the cytoskeleton of oligodendrocytes or also complement activation. The synergy of activated immune cells and MOG-IgG eventually leads to damage of oligodendrocytes and to demyelination. A indicates astrocyte; ADCC, antibody-dependent cellular cytotoxicity; B, B-cell; BBB, blood–brain barrier; DC, dendritic cell; G, granulocyte; IFNγ, interferon-γ; IL, interleukin; M, macrophage; Mi, microglia; MOG, myelin oligodendrocyte glycoprotein; N, neuron; NK, natural killer cell; O, oligodendrocyte; T, T cell; Th1, T-helper 1 cell; Th17, T-helper 17 cell; TNFα, tumor necrosis factor-α. Created with BioRender.com.
In both cases, T-cells home to the brain after priming where they most likely enter the brain parenchyma through the meninges or the choroid plexus (55,65,78,79). After entry into the CNS border regions, T cells need to be reactivated by antigen-presenting cells (APC) to gain access to the CNS parenchyma across the BBB. Production of cytokines/chemokines and subsequent activation of nearby tissue including the blood–meningeal barrier and BBB enables the infiltration of more immune cells and MOG-IgG into the CNS parenchyma that directly damage neurons and glia (reviewed in Refs. 80,81,82,83,84). Resident DC or infiltrating myeloid cells likely contribute (78,85,86,87,88). Furthermore, CNS border-associated macrophages get highly activated in the course of EAE, which also includes upregulation of major histocompatibility complex (MHC) 2 (89,90).
As different T-cell subsets have diverse roles in immunopathogenesis, it is important to understand toward which lineages T cells are polarized. Different studies examining the cytokine/chemokine profiles in patients with MOGAD measured increased levels of T-helper (Th)17-related cytokines/chemokines (interleukin [IL]-6, IL-8, IL-17a), granulocyte-colony stimulating factor, Th1-related cytokines (interferon-γ, TNFα), and several B-cell associated factors (a-proliferation-induced ligand, B-cell activating factor, C-X-C motif chemokine ligand 13) in cerebrospinal fluid (CSF) and serum (91,92,93).
Tocilizumab (anti-IL-6 receptor antibody) is used off-label for the treatment of AQP4-IgG seropositive NMOSD and because of increased IL-6 levels in the CSF of MOGAD patients (92,93), off-label treatment was evaluated in several case series.
Increased neurofilament light chain levels were observed in the serum of MOGAD patients that also correlated with attack severity and could therefore serve as a potential biomarker (97). In addition, another study found increased CSF myelin basic protein levels in MOGAD and NMOSD patients compared with MS and controls, but glial fibrillary acidic protein levels were only increased in NMOSD (98).
In MOGAD, the detection of MOG-specific T cells is still challenging. One study stimulated patient-derived peripheral blood mononuclear cells of MOG-IgG-positive patients with different MOG peptides, but could not find any specific proliferation. The authors suggest that the use of peptides could be insufficient for T-cell stimulation (99). Another investigation used bead-coupled recombinant MOG for stimulation of T cells in MS patients and observed MOG reactivity in about half of them. However, all patients were treated with natalizumab and only one patient harbored MOG-IgG (100).
As MOGAD is associated with the presence of MOG-IgG, the question arises whether these antibodies are directly pathogenic, or the epiphenomena of a secondary immune response against MOG. Understanding this distinction can help to figure out the role of MOG-specific B cells in disease development. B cells can damage CNS tissue through diverse mechanisms including release of toxic exosomes and cytokines, antigen presentation to T cells, and antibody secretion (reviewed in Refs. 101,102). The importance and ability of B cells to sufficiently activate T cells through antigen presentation in EAE mouse studies has revealed contrasting results (103,104). In human MOGAD, one study identified MOG-specific B cells in 60% of patients; still, this did not correlate with MOG-IgG serum titers (105). In contrast, B-cell activation associated with the production of IL-10 in EAE mice has been shown to exert a beneficial effect (106,107). Importantly, IL-10 producing regulatory B cells were reduced in the periphery of MOGAD patients, whereas pro-inflammatory memory B cells, and follicular T cells, that drive B-cell differentiation toward memory cells and long-lived plasma cells, were observed at higher levels (108).
Several T- and B-cell targeting drugs are used off-label in the treatment of MOGAD, including azathioprine and mycophenolate mofetil (109,110,111). One of the most frequently used drugs is rituximab, targeting CD20+ B cells (84,109,110,112). However, despite efficient B-cell depletion, only 55% of patients were relapse free in the first and 33% in the second year (113,114). Thus, B-cell depletion was less effective as in AQP4-IgG-positive NMOSD, indicating that B cells may be less important in MOGAD.
THE ROLE OF HUMAN MYELIN OLIGODENDROCYTE GLYCOPROTEIN-IgG AND NEUROPATHOLOGICAL FINDINGS
As mentioned above, the investigation of the pathogenic potential of human MOG-IgG is hampered by the fact that not all human MOG-IgG cross-react with rodent MOG (26,29,115). Different possible mechanisms for MOG-IgG-derived pathogenicity have been described in the literature. Most MOG-IgG production is believed to take place in the periphery as oligoclonal CSF bands are missing in 90% of MOGAD patients (116). Nonetheless, isolated CSF MOG-IgG positivity was observed in rare cases (117,118). MOG-IgG are primarily IgG1 isotype, but IgG2, IgG3, and IgG4 are sometimes present (119). The role of complement activation in MOGAD is still under debate and not well-established. Only a portion of monoclonal MOG-antibodies was able to activate complement in vivo (120) and injection of human MOG-IgG together with human complement resulted in only low amounts of complement deposition (121). In addition, an ex vivo study found complement activation in only one of 10 samples (29). In contrast, increased serum levels of complement products were found in MOGAD compared with MS, and NMOSD (122). Interestingly, after the transfer of human MOG-IgG cross-reactive to rodent MOG into different rat models, increased T-cell infiltration or complement deposition, together with MOG- or MBP-specific T cells, respectively, was observed (115).
MOG-IgG has shown a direct pathogenic effect on oligodendrocytes: changing the cytoskeleton, repartitioning of MOG into lipid rafts, altering the phosphorylation pattern of different proteins (6,123,124), and furthermore, changing the expression of axonal proteins (121). Moreover, human MOG-IgG induced natural killer-cell-mediated killing of MOG expressing cells in vitro (125) and enhanced antigen presentation through opsonization by APC (126,127).
Systematic neuropathological examinations of patients with MOGAD are rare and include several case reports and 2 larger studies (128,129). The neuropathological examinations of autopsies and biopsies from patients revealed a pattern of perivenous and confluent demyelination present in white matter, the cortex, and in deep gray matter structures (128,129,130). Importantly, confluent lesions were the result of fusion of perivenous lesions rather than MS-like radial expanding lesions. Moreover, slowly expanding plaques, as observed in MS, were missing, and in only one case, a rim of macrophages was present (128). Meningeal inflammation was observed in 86% of a biopsy cohort and furthermore, subpial lesions were present and myelin-laden macrophages/microglial cells were abundantly found within active demyelinating areas (128). In contrast to MS, infiltrating lymphocytes were mainly of the CD4+ type with only few B cells and CD8+ T cells (128,129,131). Eosinophils and neutrophils were observed in low-to-moderate numbers. Axons were relatively preserved, but reactive astrogliosis was observed without loss of AQP4 staining (128,132). Creutzfeldt-Peter cells were observed in one study (128), but absent in another cohort (129). Complement activation was demonstrated in active lesions, resembling a Pattern II lesion type in some studies (31,128,132,133), yet was largely absent in another investigation of 11 biopsies (129). In addition, destruction of oligodendrocytes was variable, and selective loss of MOG was missing (31,128,132); however, described in another study (129). Premyelinating oligodendrocytes were found in lesions without evidence of active remyelination (31,128). Interestingly, in a study describing the MRI lesion resolution in patients with MOGAD, NMOSD, and MS, MOGAD lesions were found to be resolving completely more frequently compared with the other groups, suggesting better repair capacities (i.e., remyelination and better axonal preservation) (134).
MYELIN OLIGODENDROCYTE GLYCOPROTEIN-ASSOCIATED DISEASE LESIONS OF THE OPTIC NERVE AND THE VISUAL PATHWAY
The predominant phenotype in adult MOGAD patients is ON, but it is also frequently found in pediatric cases. Studies reported between 44% and 61% onset presentations with ON in adult MOGAD patients (12,14,16,135) and in up to 38% of children (17,18,19,22). Moreover, studies examining the prevalence of MOG-IgG in ON patients found MOG-IgG in 4%–31% of ON cases (136,137,138,139,140,141). MOG-IgG-positive ON was associated with bilateral ON in 24%–45% of patients (15,16,17,136,137,138,139,142) and pain and optic disc swelling were observed frequently (138,141,143) (Fig. 2). Around half of patients followed a relapsing disease course (15,16,143). Interestingly, of those, around 88% developed isolated ON as relapse, whereas the remaining patients developed NMOSD-like relapses, transverse myelitis, or an optico-spinal phenotype (15,16,143). ON at follow-up was observed in 47% of children and 63% of adult patients with MOGAD (15). Studies reported between 4% and 16% chronic relapsing inflammatory optic neuritis (CRION) patients within MOGAD-ON cohorts and found that CRION patients positive for MOG-IgG were younger, showed bilateral involvement more often and had more relapses compared with seronegative patients (15,143,144). The spectrum of ophthalmic manifestations associated with MOG-IgG is however expanding and therefore, we would like to refer to a recent review (145).
FIG. 2.
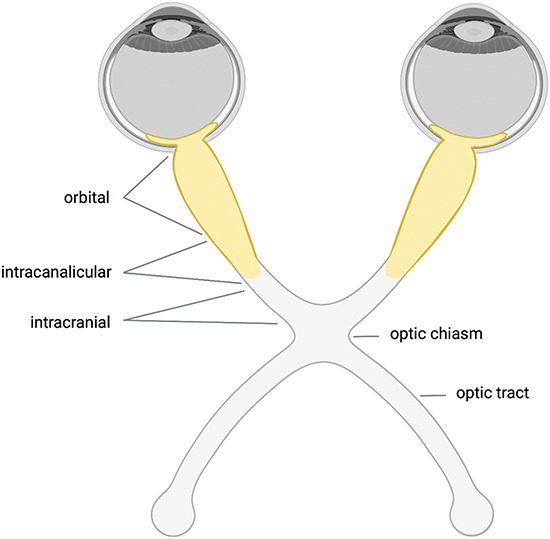
Optic nerve lesions in MOGAD. In MOGAD, bilateral ON is observed in up to 45% of patients and optic disc edema is common. MRI shows enhancement of the optic nerve and perineural abnormalities including optic nerve sheath enhancements in half of the patients. Furthermore, lesions are usually longitudinal extensive and affect predominantly the prechiasmic optic nerve (highlighted in yellow). Involvement of the optic chiasm and the optic tract is only observed in 12% and 2% of patients, respectively. Data from (Refs. 141,143). Created with BioRender.com. MOGAD indicates myelin oligodendrocyte glycoprotein-associated disease; ON, optic neuritis
Optical coherence tomography measurements of the peripapillary retinal nerve-fiber-layer (pRNFL) thickness revealed higher values in acute MOGAD-ON compared with MS because of optic disc edema. PRNFL thickening could serve as an indicator to distinguish MOGAD and MS in acute ON (146). After thickening in the acute phase the RNFL and ganglion-cell and inner-plexiform-layer (GCIPL) undergo degeneration (136,147,148), but visual outcome was generally favorable with only 6%–8% showing a poor visual acuity at last follow-up (16,136,143). In MRI of the optic nerve, enhancement was observed in all patients and 50%–88% also showed perineural enhancement. Lesions are usually long, affect the orbital portion more, and can also extend into the orbital fat (141,143,149). Only about 2%–5% developed optic tract abnormalities and 12%–16% showed involvement of the optic chiasm, that was linked to longitudinally extensive lesions in 54% (139,143,150). Prechiasmal and chiasmal lesions were associated with a bad visual prognosis (151).
In EAE animal models immunized with MOG, the lesion distribution was determined by different influences such as gender, the genetic background and the immunization method used (152,153,154). Double transgenic mice (MOG-specific T and B cells, called 2D2/Th) developed spontaneous optico-spinal phenotypes (155,156,157,158). However, single transgenic mice (2D2) also developed ON, although at lower frequencies, suggesting an enhancing role of antibodies (157,159).
Histopathologic examinations of animals revealed infiltration of inflammatory cells, demyelination with axonal loss, and reactive gliosis in retina and optic nerves (152,156,157,160). Besides, complement activation was found in one study (161). The retinal ganglion cell layer was also shown to undergo degeneration in mice after inflammatory responses and activation of microglia cells in later stages of EAE and may be the product of secondary degenerative mechanisms, because there are no MOG-expressing oligodendrocytes present in the retina (157,162,163). As a result, authors observed reduced neuritic density in the inner plexiform layer in mice (157). In contrast, a study examining the pRNFL and GCIPL in MOGAD patients found no evidence for attack-independent degeneration (164). Activation of microglia was furthermore linked to optokinetic tracking threshold decline in functional examinations in experimental autoimmune ON mice (165). In addition, visual evoked potential recordings in dark agouti rats immunized with MOG showed latency delay, a decrease in amplitude, and MOG dose-dependent lack of flash evoked response suggestive of axonal conduction block (160,166). Intriguingly, investigations showed that MOG expression is higher in the optic nerves than in the spinal cord and brain on protein and mRNA levels in mice (155,159). However, the vulnerability of the optic nerve head is likely the result of a lack of microvessels with BBB characteristics and nonspecific permeability in this region (167,168,169).
CONCLUSION
To summarize, the spectrum of MOGAD-associated symptoms is broad, but most patients present with ON, that is usually associated with a good visual recovery. Histopathology revealed perivenous demyelinating lesions and infiltration of leukocytes. Nevertheless, the role of human MOG-IgG is less clear and different pathogenic mechanisms are discussed. Future studies that aim to define the exact pathogenesis, are needed to further identify targets for efficient treatment strategies.
STATEMENT OF AUTHORSHIP
Conception and design: M. Lerch, M. Reindl; Acquisition of data: Not applicable; Analysis and interpretation of data: Not applicable. Drafting the manuscript: M. Lerch, A. Bauer, M. Reindl; Revising the manuscript for intellectual content: M. Lerch, A. Bauer, M. Reindl. Final approval of the completed manuscript: M. Lerch, A. Bauer, M. Reindl.
Footnotes
Supported by a research grant from the Austrian Science Fund (FWF project P32699).
A. Bauer has participated in meetings sponsored by or received travel funding from Novartis, Sanofi-Genzyme, Merck, Almirall and Biogen. M. Reindl was supported by research support from Euroimmun and Roche (to institution). The University Hospital and Medical University of Innsbruck (Austria, employer of M. Reindl) receives payments for antibody assays (MOG, AQP4, and other autoantibodies) and for MOG and AQP4 antibody validation experiments organized by Euroimmun (Lübeck, Germany). M. Lerch has no conflict of interest to declare.
Contributor Information
Magdalena Lerch, Email: magdalena.lerch@i-med.ac.at.
Angelika Bauer, Email: angelika.bauer@i-med.ac.at.
REFERENCES
- 1.Peschl P, Bradl M, Höftberger R, Berger T, Reindl M. Myelin oligodendrocyte glycoprotein: deciphering a target in inflammatory demyelinating diseases. Front Immunol. 2017;8:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clements CS, Reid HH, Beddoe T, Tynan FE, Perugini MA, Johns TG, Bernard CCA, Rossjohn J. The crystal structure of myelin oligodendrocyte glycoprotein, a key autoantigen in multiple sclerosis. Proc Natl Acad Sci U S A. 2003;100:11059–11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birling MC, Roussel G, Nussbaum F, Nussbaum JL. Biochemical and immunohistochemical studies with specific polyclonal antibodies directed against bovine myelin/oligodendrocyte glycoprotein. Neurochem Res. 1993;18:937–945. [DOI] [PubMed] [Google Scholar]
- 4.Delarasse C, Della Gaspera B, Lu CW, Lachapelle F, Gelot A, Rodriguez D, Dautigny A, Genain C, Pham-Dinh D. Complex alternative splicing of the myelin oligodendrocyte glycoprotein gene is unique to human and non-human primates. J Neurochem. 2006;98:1707–1717. [DOI] [PubMed] [Google Scholar]
- 5.Johns TG, Bernard CC. The structure and function of myelin oligodendrocyte glycoprotein. J Neurochem. 1999;72:1–9. [DOI] [PubMed] [Google Scholar]
- 6.Dyer CA, Matthieu JM. Antibodies to myelin/oligodendrocyte-specific protein and myelin/oligodendrocyte glycoprotein signal distinct changes in the organization of cultured oligodendroglial membrane sheets. J Neurochem. 1994;62:777–787. [DOI] [PubMed] [Google Scholar]
- 7.Johns TG, Bernard CCA. Binding of complement component Clq to myelin oligodendrocyte glycoprotein: a novel mechanism for regulating CNS inflammation. Mol Immunol. 1997;34:33–38. [DOI] [PubMed] [Google Scholar]
- 8.von Büdingen HC, Mei F, Greenfield A, Jahn S, Shen Y-AA, Reid HH, McKemy DD, Chan JR. The myelin oligodendrocyte glycoprotein directly binds nerve growth factor to modulate central axon circuitry. J Cell Biol. 2015;210:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Vallejo JJ, Ilarregui JM, Kalay H, Chamorro S, Koning N, Unger WW, Ambrosini M, Montserrat V, Fernandes RJ, Bruijns SCM, van Weering JRT, Paauw NJ, O'Toole T, van Horssen J, van der Valk P, Nazmi K, Bolscher JGM, Bajramovic J, Dijkstra CD, 't Hart BA, van Kooyk Y. CNS myelin induces regulatory functions of DC-SIGN-expressing, antigen-presenting cells via cognate interaction with MOG. J Exp Med. 2014;211:1465–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cong H, Jiang Y, Tien P. Identification of the myelin oligodendrocyte glycoprotein as a cellular receptor for rubella virus. J Virol. 2011;85:11038–11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol. 2019;15:89–102. [DOI] [PubMed] [Google Scholar]
- 12.de Mol CL, Wong Y, van Pelt ED, Wokke B, Siepman T, Neuteboom RF, Hamann D, Hintzen RQ. The clinical spectrum and incidence of anti-MOG-associated acquired demyelinating syndromes in children and adults. Mult Scler. 2020;26:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartels F, Lu A, Oertel FC, Finke C, Paul F, Chien C. Clinical and neuroimaging findings in MOGAD-MRI and OCT. Clin Exp Immunol. 2021;206:266–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brill L, Ganelin-Cohen E, Dabby R, Rabinowicz S, Zohar-Dayan E, Rein N, Aloni E, Karmon Y, Vaknin-Dembinsky A. Age-related clinical presentation of MOG-IgG seropositivity in Israel. Front Neurol. 2020;11:612304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jurynczyk M, Messina S, Woodhall MR, Raza N, Everett R, Roca-Fernandez A, Tackley G, Hamid S, Sheard A, Reynolds G, Chandratre S, Hemingway C, Jacob A, Vincent A, Leite MI, Waters P, Palace J. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140:3128–3138. [DOI] [PubMed] [Google Scholar]
- 16.Cobo-Calvo A, Ruiz A, Maillart E, Audoin B, Zephir H, Bourre B, Ciron J, Collongues N, Brassat D, Cotton F, Papeix C, Durand-Dubief F, Laplaud D, Deschamps R, Cohen M, Biotti D, Ayrignac X, Tilikete C, Thouvenot E, Brochet B, Dulau C, Moreau T, Tourbah A, Lebranchu P, Michel L, Lebrun-Frenay C, Montcuquet A, Mathey G, Debouverie M, Pelletier J, Labauge P, Derache N, Coustans M, Rollot F, De Seze J, Vukusic S, Marignier R; OFSEP and NOMADMUS Study Group. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology. 2018;90:e1858–e1869. [DOI] [PubMed] [Google Scholar]
- 17.Armangue T, Olivé-Cirera G, Martínez-Hernandez E, Sepulveda M, Ruiz-Garcia R, Muñoz-Batista M, Ariño H, González-Álvarez V, Felipe-Rucián A, Jesús Martínez-González M, Cantarín-Extremera V, Concepción Miranda-Herrero M, Monge-Galindo L, Tomás-Vila M, Miravet E, Málaga I, Arrambide G, Auger C, Tintoré M, Montalban X, Vanderver A, Graus F, Saiz A, Dalmau J, Spanish Pediatric anti-MOG Study Group, Aguilera-Albesa S, Alvarez Demanuel D, Alvarez Molinero M, Aquino Fariña L, Arrabal L, Arriola-Pereda G, Aznar-Laín G, Benavides-Medina M, Bermejo T, Blanco-Lago R, Caballero E, Calvo R, Camacho Salas A, Conejo-Moreno D, Delgadillo-Chilavert V, Elosegi-Castellanos A, Esteban Canto V, Fernández-Ramos J, Garcia-Puig M, García-Ribes A, Gómez-Martín H, Gonzalez-Barrios D, González-Gutiérrez-Solana L, Jimena-Garcia S, Jiménez-Legido M, Juliá-Palacios N, López-Laso E, Martí-Carrera I, Martínez González M, Martín-Viota L, Mattozi S, Maqueda-Castellote E, Mendibe MDM, Mora-Ramírez MD, Muñoz-Cabello B, Navarro-Morón J, Nunes-Cabrera T, Orellana G, Pujol-Soler B, Querol L, Ramírez A, Rodriguez-Lucenilla MI, Ruiz C, Soto-Insuga V, Toledo Bravo de Laguna L, Turon-Viñas E, Vázquez-López M, Villar-Vera C. Associations of paediatric demyelinating and encephalitic syndromes with myelin oligodendrocyte glycoprotein antibodies: a multicentre observational study. Lancet Neurol. 2020;19:234–246. [DOI] [PubMed] [Google Scholar]
- 18.Waters P, Fadda G, Woodhall M, O'Mahony J, Brown RA, Castro DA, Longoni G, Irani SR, Sun B, Yeh EA, Marrie RA, Arnold DL, Banwell B, Bar-Or A; Canadian Pediatric Demyelinating Disease Network. Serial anti-myelin oligodendrocyte glycoprotein antibody analyses and outcomes in children with demyelinating syndromes. JAMA Neurol. 2020;77:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Mol CL, Wong YYM, van Pelt ED, Ketelslegers IA, Bakker DP, Boon M, Braun KPJ, van Dijk KGJ, Eikelenboom MJ, Engelen M, Geleijns K, Haaxma CA, Niermeijer JMF, Niks EH, Peeters EAJ, Peeters-Scholte CMPCD, Poll-The BT, Portier RP, de Rijk-van Andel JF, Samijn JPA, Schippers HM, Snoeck IN, Stroink H, Vermeulen RJ, Verrips A, Visscher F, Vles JSH, Willemsen MAAP, Catsman-Berrevoets CE, Hintzen RQ, Neuteboom RF. Incidence and outcome of acquired demyelinating syndromes in Dutch children: update of a nationwide and prospective study. J Neurol. 2018;265:1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senanayake B, Jitprapaikulsan J, Aravinthan M, Wijesekera JC, Ranawaka UK, Riffsy MT, Paramanathan T, Sagen J, Fryer JP, Schmeling J, Majed M, Flanagan EP, Pittock SJ. Seroprevalence and clinical phenotype of MOG-IgG-associated disorders in Sri Lanka. J Neurol Neurosurg Psychiatry. 2019;90:1381–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossor T, Benetou C, Wright S, Duignan S, Lascelles K, Robinson R, Das K, Ciccarelli O, Wassmer E, Hemingway C, Lim M, Hacohen Y. Early predictors of epilepsy and subsequent relapse in children with acute disseminated encephalomyelitis. Mult Scler. 2020;26:333–342. [DOI] [PubMed] [Google Scholar]
- 22.Hennes E-M, Baumann M, Schanda K, Anlar B, Bajer-Kornek B, Blaschek A, Brantner-Inthaler S, Diepold K, Eisenkölbl A, Gotwald T, Kuchukhidze G, Gruber-Sedlmayr U, Häusler M, Höftberger R, Karenfort M, Klein A, Koch J, Kraus V, Lechner C, Leiz S, Leypoldt F, Mader S, Marquard K, Poggenburg I, Pohl D, Pritsch M, Raucherzauner M, Schimmel M, Thiels C, Tibussek D, Vieker S, Zeches C, Berger T, Reindl M, Rostásy K; BIOMARKER Study Group. Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology. 2017;89:900–908. [DOI] [PubMed] [Google Scholar]
- 23.O'Connor KC, McLaughlin KA, De Jager PL, Chitnis T, Bettelli E, Xu C, Robinson WH, Cherry SV, Bar-Or A, Banwell B, Fukaura H, Fukazawa T, Tenembaum S, Wong SJ, Tavakoli NP, Idrissova Z, Viglietta V, Rostasy K, Pohl D, Dale RC, Freedman M, Steinman L, Buckle GJ, Kuchroo VK, Hafler DA, Wucherpfennig KW. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat Med. 2007;13:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brehm U, Piddlesden SJ, Gardinier MV, Linington C. Epitope specificity of demyelinating monoclonal autoantibodies directed against the human myelin oligodendrocyte glycoprotein (MOG). J Neuroimmunol. 1999;97:9–15. [DOI] [PubMed] [Google Scholar]
- 25.Menge T, Lalive PH, von Büdingen HC, Genain CP. Conformational epitopes of myelin oligodendrocyte glycoprotein are targets of potentially pathogenic antibody responses in multiple sclerosis. J Neuroinflammation. 2011;8:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer MC, Breithaupt C, Reindl M, Schanda K, Rostásy K, Berger T, Dale RC, Brilot F, Olsson T, Jenne D, Pröbstel A-K, Dornmair K, Wekerle H, Hohlfeld R, Banwell B, Bar-Or A, Meinl E. Distinction and temporal stability of conformational epitopes on myelin oligodendrocyte glycoprotein recognized by patients with different inflammatory central nervous system diseases. J Immunol. 2013;191:3594–3604. [DOI] [PubMed] [Google Scholar]
- 27.Tea F, Lopez JA, Ramanathan S, Merheb V, Lee FXZ, Zou A, Pilli D, Patrick E, van der Walt A, Monif M, Tantsis EM, Yiu EM, Vucic S, Henderson APD, Fok A, Fraser CL, Lechner-Scott J, Reddel SW, Broadley S, Barnett MH, Brown DA, Lunemann JD, Dale RC, Brilot F; Australasian and New Zealand MOG Study Group. Characterization of the human myelin oligodendrocyte glycoprotein antibody response in demyelination. Acta Neuropathol Commun. 2019;7:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breithaupt C, Schäfer B, Pellkofer H, Huber R, Linington C, Jacob U. Demyelinating myelin oligodendrocyte glycoprotein-specific autoantibody response is focused on one dominant conformational epitope region in rodents. J Immunol. 2008;181:1255–1263. [DOI] [PubMed] [Google Scholar]
- 29.Peschl P, Schanda K, Zeka B, Given K, Böhm D, Ruprecht K, Saiz A, Lutterotti A, Rostásy K, Höftberger R, Berger T, Macklin W, Lassmann H, Bradl M, Bennett JL, Reindl M. Human antibodies against the myelin oligodendrocyte glycoprotein can cause complement-dependent demyelination. J Neuroinflammation. 2017;14:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sepúlveda M, Armangue T, Martinez-Hernandez E, Arrambide G, Sola-Valls N, Sabater L, Téllez N, Midaglia L, Ariño H, Peschl P, Reindl M, Rovira A, Montalban X, Blanco Y, Dalmau J, Graus F, Saiz A. Clinical spectrum associated with MOG autoimmunity in adults: significance of sharing rodent MOG epitopes. J Neurol. 2016;263:1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spadaro M, Gerdes LA, Mayer MC, Ertl-Wagner B, Laurent S, Krumbholz M, Breithaupt C, Högen T, Straube A, Giese A, Hohlfeld R, Lassmann H, Meinl E, Kümpfel T. Histopathology and clinical course of MOG-antibody-associated encephalomyelitis. Ann Clin Transl Neurol. 2015;2:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marti Fernandez I, Macrini C, Krumbholz M, Hensbergen PJ, Hipgrave Ederveen AL, Winklmeier S, Vural A, Kurne A, Jenne D, Kamp F, Gerdes LA, Hohlfeld R, Wuhrer M, Kümpfel T, Meinl E. The glycosylation site of myelin oligodendrocyte glycoprotein affects autoantibody recognition in a large proportion of patients. Front Immunol. 2019;10:1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allamargot C, Gardinier MV. Alternative isoforms of myelin/oligodendrocyte glycoprotein with variable cytoplasmic domains are expressed in human brain. J Neurochem. 2007;101:298–312. [DOI] [PubMed] [Google Scholar]
- 34.Ballenthin PA, Gardinier MV. Myelin/oligodendrocyte glycoprotein is alternatively spliced in humans but not mice. J Neurosci Res. 1996;46:271–281. [DOI] [PubMed] [Google Scholar]
- 35.Pham-Dinh D, Della Gaspera B, Kerlero de Rosbo N, Dautigny A. Structure of the human myelin/oligodendrocyte glycoprotein gene and multiple alternative spliced isoforms. Genomics. 1995;29:345–352. [DOI] [PubMed] [Google Scholar]
- 36.Boyle LH, Traherne JA, Plotnek G, Ward R, Trowsdale J. Splice variation in the cytoplasmic domains of myelin oligodendrocyte glycoprotein affects its cellular localisation and transport. J Neurochem. 2007;102:1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macrini C, Gerhards R, Winklmeier S, Bergmann L, Mader S, Spadaro M, Vural A, Smolle M, Hohlfeld R, Kümpfel T, Lichtenthaler SF, Franquelim HG, Jenne D, Meinl E. Features of MOG required for recognition by patients with MOG antibody-associated disorders. Brain. 2021;144:2375–2389. [DOI] [PubMed] [Google Scholar]
- 38.Schanda K, Peschl P, Lerch M, Seebacher B, Mindorf S, Ritter N, Probst M, Hegen H, Di Pauli F, Wendel E-M, Lechner C, Baumann M, Mariotto S, Ferrari S, Saiz A, Farrell M, Leite MIS, Irani SR, Palace J, Lutterotti A, Kümpfel T, Vukusic S, Marignier R, Waters P, Rostasy K, Berger T, Probst C, Höftberger R, Reindl M. Differential binding of autoantibodies to MOG isoforms in inflammatory demyelinating diseases. Neurol Neuroimmunol Neuroinflamm. 2021;8:e1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linington C, Lassmann H. Antibody responses in chronic relapsing experimental allergic encephalomyelitis: correlation of serum demyelinating activity with antibody titre to the myelin/oligodendrocyte glycoprotein (MOG). J Neuroimmunol. 1987;17:61–69. [DOI] [PubMed] [Google Scholar]
- 40.Linington C, Berger T, Perry L, Weerth S, Hinze-Selch D, Zhang Y, Lu HC, Lassmann H, Wekerle H. T cells specific for the myelin oligodendrocyte glycoprotein mediate an unusual autoimmune inflammatory response in the central nervous system. Eur J Immunol. 1993;23:1364–1372. [DOI] [PubMed] [Google Scholar]
- 41.Lassmann H, Brunner C, Bradl M, Linington C. Experimental allergic encephalomyelitis: the balance between encephalitogenic T lymphocytes and demyelinating antibodies determines size and structure of demyelinated lesions. Acta Neuropathol. 1988;75:566–576. [DOI] [PubMed] [Google Scholar]
- 42.Amor S, Groome N, Linington C, Morris MM, Dornmair K, Gardinier MV, Matthieu JM, Baker D. Identification of epitopes of myelin oligodendrocyte glycoprotein for the induction of experimental allergic encephalomyelitis in SJL and Biozzi AB/H mice. J Immunol. 1994;153:4349–4356. [PubMed] [Google Scholar]
- 43.Krishnamoorthy G, Wekerle H. EAE: an immunologist's magic eye. Eur J Immunol. 2009;39:2031–2035. [DOI] [PubMed] [Google Scholar]
- 44.Grant-Peters M, Passos GRD, Yeung H-Y, Jacob A, Huda S, Leite MI, Dendrou CA, Palace J. No strong HLA association with MOG antibody disease in the UK population. Ann Clin Transl Neurol. 2021;8:1502–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruijstens AL, Wong YYM, van Pelt DE, van der Linden PJE, Haasnoot GW, Hintzen RQ, Claas FHJ, Neuteboom RF, Wokke BHA. HLA association in MOG-IgG- and AQP4-IgG-related disorders of the CNS in the Dutch population. Neurol Neuroimmunol Neuroinflamm. 2020;7:e702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun X, Qiu W, Wang J, Wang S, Wang Y, Zhong X, Liu C, Cui C, Hong H, Yang H, Li X-J, Lu Z, Hu X, Kermode AG, Peng L. Myelin oligodendrocyte glycoprotein-associated disorders are associated with HLA subtypes in a Chinese paediatric-onset cohort. J Neurol Neurosurg Psychiatry. 2020;91:733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakamura Y, Nakajima H, Tani H, Hosokawa T, Ishida S, Kimura F, Kaneko K, Takahashi T, Nakashima I. Anti-MOG antibody-positive ADEM following infectious mononucleosis due to a primary EBV infection: a case report. BMC Neurol. 2017;17:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato R, Okanari K, Maeda T, Kaneko K, Takahashi T, Kenji I. Postinfectious acute disseminated encephalomyelitis associated with antimyelin oligodendrocyte glycoprotein antibody. Child Neurol Open. 2020;7:2329048X20942442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakamura M, Iwasaki Y, Takahashi T, Kaneko K, Nakashima I, Kunieda T, Kaneko S, Kusaka H. A case of MOG antibody-positive bilateral optic neuritis and meningoganglionitis following a genital herpes simplex virus infection. Mult Scler Relat Disord. 2017;17:148–150. [DOI] [PubMed] [Google Scholar]
- 50.Choi SJ, Oh DA, Chun W, Kim SM. The relationship between anti-myelin oligodendrocyte glycoprotein antibody-associated disease and the rubella virus. J Clin Neurol. 2018;14:598–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou S, Jones-Lopez EC, Soneji DJ, Azevedo CJ, Patel VR. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis and myelitis in COVID-19. J Neuroophthalmol. 2020;40:398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Pauli F, Morschewsky P, Berek K, Auer M, Bauer A, Berger T, Bsteh G, Rhomberg P, Schanda K, Zinganell A, Deisenhammer F, Reindl M, Hegen H. Myelin oligodendrocyte glycoprotein antibody-associated disease and varicella zoster virus infection—frequency of an association. Front Immunol. 2021;12:769653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wildemann B, Jarius S, Franz J, Ruprecht K, Reindl M, Stadelmann C. MOG-expressing teratoma followed by MOG-IgG-positive optic neuritis. Acta Neuropathol. 2021;141:127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Redenbaugh V, Flanagan EP, Floris V, Zara P, Bhatti MT, Sanchez F, Koster M, Mariotto S, Pittock SJ, Chen JJ, Cauli A, Solla P, Sechi E. Exposure to TNF inhibitors is rare at MOGAD presentation. J Neurol Sci. 2022;432:120044. [DOI] [PubMed] [Google Scholar]
- 55.Kipnis J. Multifaceted interactions between adaptive immunity and the central nervous system. Science. 2016;353:766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wekerle H. Breaking ignorance: the case of the brain. Curr Top Microbiol Immunol. 2006;305:25–50. [DOI] [PubMed] [Google Scholar]
- 57.Louveau A, Harris TH, Kipnis J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 2015;36:569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Traka M, Podojil JR, McCarthy DP, Miller SD, Popko B. Oligodendrocyte death results in immune-mediated CNS demyelination. Nat Neurosci. 2016;19:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lopez JA, Denkova M, Ramanathan S, Dale RC, Brilot F. Pathogenesis of autoimmune demyelination: from multiple sclerosis to neuromyelitis optica spectrum disorders and myelin oligodendrocyte glycoprotein antibody-associated disease. Clin Transl Immunol. 2021;10:e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, Herod SG, Knopp J, Setliff JC, Lupi AL, Da Mesquita S, Frost EL, Gaultier A, Harris TH, Cao R, Hu S, Lukens JR, Smirnov I, Overall CC, Oliver G, Kipnis J. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci. 2018;21:1380–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alves de Lima K, Rustenhoven J, Kipnis J. Meningeal immunity and its function in maintenance of the central nervous system in health and disease. Annu Rev Immunol. 2020;38:597–620. [DOI] [PubMed] [Google Scholar]
- 63.Norris GT, Kipnis J. Immune cells and CNS physiology: microglia and beyond. J Exp Med. 2019;216:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest. 2017;127:3210–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Korn T, Kallies A. T cell responses in the central nervous system. Nat Rev Immunol. 2017;17:179–194. [DOI] [PubMed] [Google Scholar]
- 66.Schetters STT, Gomez-Nicola D, Garcia-Vallejo JJ, van Kooyk Y. Neuroinflammation: microglia and T cells get ready to Tango. Front Immunol. 2017;8:1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Vos AF, van Meurs M, Brok HP, Boven LA, Hintzen RQ, van der Valk P, Ravid R, Rensing S, Boon L, 't Hart BA, Laman JD. Transfer of central nervous system autoantigens and presentation in secondary lymphoid organs. J Immunol. 2002;169:5415–5423. [DOI] [PubMed] [Google Scholar]
- 68.Na S-Y, Hermann A, Sanchez-Ruiz M, Storch A, Deckert M, Hünig T. Oligodendrocytes enforce immune tolerance of the uninfected brain by purging the peripheral repertoire of autoreactive CD8+ T cells. Immunity. 2012;37:134–146. [DOI] [PubMed] [Google Scholar]
- 69.Fletcher AL, Malhotra D, Turley SJ. Lymph node stroma broaden the peripheral tolerance paradigm. Trends Immunol. 2011;32:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rouhani SJ, Eccles JD, Riccardi P, Peske JD, Tewalt EF, Cohen JN, Liblau R, Mäkinen T, Engelhard VH. Roles of lymphatic endothelial cells expressing peripheral tissue antigens in CD4 T-cell tolerance induction. Nat Commun. 2015;6:6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Louveau A, Da Mesquita S, Kipnis J. Lymphatics in neurological disorders: a neuro-lympho-vascular component of multiple sclerosis and Alzheimer's disease? Neuron. 2016;91:957–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guggenmos J, Schubart AS, Ogg S, Andersson M, Olsson T, Mather IH, Linington C. Antibody cross-reactivity between myelin oligodendrocyte glycoprotein and the milk protein butyrophilin in multiple sclerosis. J Immunol. 2004;172:661–668. [DOI] [PubMed] [Google Scholar]
- 73.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. [DOI] [PubMed] [Google Scholar]
- 74.Pagany M, Jagodic M, Schubart A, Pham-Dinh D, Bachelin C, Baron van Evercooren A, Lachapelle F, Olsson T, Linington C. Myelin oligodendrocyte glycoprotein is expressed in the peripheral nervous system of rodents and primates. Neurosci Lett. 2003;350:165–168. [DOI] [PubMed] [Google Scholar]
- 75.Delarasse C, Daubas P, Mars LT, Vizler C, Litzenburger T, Iglesias A, Bauer J, Della Gaspera B, Schubart A, Decker L, Dimitri D, Roussel G, Dierich A, Amor S, Dautigny A, Liblau R, Pham-Dinh D. Myelin/oligodendrocyte glycoprotein–deficient (MOG-deficient) mice reveal lack of immune tolerance to MOG in wild-type mice. J Clin Invest. 2003;112:544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Na S-Y, Krishnamoorthy G. Targeted expression of myelin autoantigen in the periphery induces antigen-specific T and B cell tolerance and ameliorates autoimmune disease. Front Immunol. 2021;12:668487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krienke C, Kolb L, Diken E, Streuber M, Kirchhoff S, Bukur T, Akilli-Öztürk Ö, Kranz LM, Berger H, Petschenka J, Diken M, Kreiter S, Yogev N, Waisman A, Karikó K, Türeci Ö, Sahin U. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science. 2021;371:145–153. [DOI] [PubMed] [Google Scholar]
- 78.Mundt S, Greter M, Flügel A, Becher B. The CNS immune landscape from the viewpoint of a T cell. Trends Neurosci. 2019;42:667–679. [DOI] [PubMed] [Google Scholar]
- 79.Kyratsous NI, Bauer IJ, Zhang G, Pesic M, Bartholomäus I, Mues M, Fang P, Wörner M, Everts S, Ellwart JW, Watt JM, Potter BVL, Hohlfeld R, Wekerle H, Kawakami N. Visualizing context-dependent calcium signaling in encephalitogenic T cells in vivo by two-photon microscopy. Proc Natl Acad Sci U S A. 2017;114:E6381–E6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 2012;33:579–589. [DOI] [PubMed] [Google Scholar]
- 81.Flügel A, Berkowicz T, Ritter T, Labeur M, Jenne DE, Li Z, Ellwart JW, Willem M, Lassmann H, Wekerle H. Migratory activity and functional changes of green fluorescent effector cells before and during experimental autoimmune encephalomyelitis. Immunity. 2001;14:547–560. [DOI] [PubMed] [Google Scholar]
- 82.Kunkl M, Frascolla S, Amormino C, Volpe E, Tuosto L. T helper cells: the modulators of inflammation in multiple sclerosis. Cells. 2020;9:E482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang X, Hussain B, Chang J. Peripheral inflammation and blood-brain barrier disruption: effects and mechanisms. CNS Neurosci Ther. 2021;27:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fujihara K, Bennett JL, de Seze J, Haramura M, Kleiter I, Weinshenker BG, Kang D, Mughal T, Yamamura T. Interleukin-6 in neuromyelitis optica spectrum disorder pathophysiology. Neurol Neuroimmunol Neuroinflamm. 2020;7:e841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. [DOI] [PubMed] [Google Scholar]
- 86.Dong Y, Yong VW. When encephalitogenic T cells collaborate with microglia in multiple sclerosis. Nat Rev Neurol. 2019;15:704–717. [DOI] [PubMed] [Google Scholar]
- 87.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. [DOI] [PubMed] [Google Scholar]
- 88.Jordão MJC, Sankowski R, Brendecke SM, Sagar, Locatelli G, Tai Y-H, Tay TL, Schramm E, Armbruster S, Hagemeyer N, Groß O, Mai D, Çiçek Ö, Falk T, Kerschensteiner M, Grün D, Prinz M. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science. 2019;363:eaat7554. [DOI] [PubMed] [Google Scholar]
- 89.Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP, Lelios I, Heppner FL, Kipnis J, Merkler D, Greter M, Becher B. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity. 2018;48:380–395.e6. [DOI] [PubMed] [Google Scholar]
- 90.Goldmann T, Wieghofer P, Jordão MJC, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, Locatelli G, Hochgerner H, Zeiser R, Epelman S, Geissmann F, Priller J, Rossi FMV, Bechmann I, Kerschensteiner M, Linnarsson S, Jung S, Prinz M. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016;17:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kothur K, Wienholt L, Tantsis EM, Earl J, Bandodkar S, Prelog K, Tea F, Ramanathan S, Brilot F, Dale RC. B cell, Th17, and neutrophil related cerebrospinal fluid cytokine/chemokines are elevated in MOG antibody associated demyelination. PLoS One. 2016;11:e0149411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaneko K, Sato DK, Nakashima I, Ogawa R, Akaishi T, Takai Y, Nishiyama S, Takahashi T, Misu T, Kuroda H, Tanaka S, Nomura K, Hashimoto Y, Callegaro D, Steinman L, Fujihara K, Aoki M. CSF cytokine profile in MOG-IgG+ neurological disease is similar to AQP4-IgG+ NMOSD but distinct from MS: a cross-sectional study and potential therapeutic implications. J Neurol Neurosurg Psychiatry. 2018;89:927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hofer LS, Mariotto S, Wurth S, Ferrari S, Mancinelli CR, Delogu R, Monaco S, Gajofatto A, Schwaiger C, Rostasy K, Deisenhammer F, Höftberger R, Berger T, Reindl M. Distinct serum and cerebrospinal fluid cytokine and chemokine profiles in autoantibody-associated demyelinating diseases. Mult Scler J Exp Transl Clin. 2019;5:2055217319848463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Elsbernd PM, Hoffman WR, Carter JL, Wingerchuk DM. Interleukin-6 inhibition with tocilizumab for relapsing MOG-IgG associated disorder (MOGAD): a case-series and review. Mult Scler Relat Disord. 2021;48:102696. [DOI] [PubMed] [Google Scholar]
- 95.Rigal J, Pugnet G, Ciron J, Lépine Z, Biotti D. Off-label use of tocilizumab in neuromyelitis optica spectrum disorders and MOG-antibody-associated diseases: a case-series. Mult Scler Relat Disord. 2020;46:102483. [DOI] [PubMed] [Google Scholar]
- 96.Ringelstein M, Ayzenberg I, Lindenblatt G, Fischer K, Gahlen A, Novi G, Hayward-Könnecke H, Schippling S, Rommer PS, Kornek B, Zrzavy T, Biotti D, Ciron J, Audoin B, Berthele A, Giglhuber K, Zephir H, Kümpfel T, Berger R, Röther J, Häußler V, Stellmann J-P, Whittam D, Jacob A, Kraemer M, Gueguen A, Deschamps R, Bayas A, Hümmert MW, Trebst C, Haarmann A, Jarius S, Wildemann B, Grothe M, Siebert N, Ruprecht K, Paul F, Collongues N, Marignier R, Levy M, Karenfort M, Deppe M, Albrecht P, Hellwig K, Gold R, Hartung H-P, Meuth SG, Kleiter I, Aktas O; Neuromyelitis Optica Study Group NEMOS. Interleukin-6 receptor blockade in treatment-refractory MOG-IgG-associated disease and neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm. 2022;9:e1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mariotto S, Ferrari S, Gastaldi M, Franciotta D, Sechi E, Capra R, Mancinelli C, Schanda K, Alberti D, Orlandi R, Bombardi R, Zuliani L, Zoccarato M, Benedetti MD, Tanel R, Calabria F, Rossi F, Pavone A, Grazian L, Sechi G, Batzu L, Murdeu N, Janes F, Fetoni V, Fulitano D, Stenta G, Federle L, Cantalupo G, Reindl M, Monaco S, Gajofatto A. Neurofilament light chain serum levels reflect disease severity in MOG-Ab associated disorders. J Neurol Neurosurg Psychiatry. 2019;90:1293–1296. [DOI] [PubMed] [Google Scholar]
- 98.Kaneko K, Sato DK, Nakashima I, Nishiyama S, Tanaka S, Marignier R, Hyun J-W, Oliveira LMd, Reindl M, Seifert-Held T, Sepulveda M, Siritho S, Waters PJ, Kurosawa K, Akaishi T, Kuroda H, Misu T, Prayoonwiwat N, Berger T, Saiz A, Kim HJ, Nomura K, Callegaro D, Fujihara K, Aoki M. Myelin injury without astrocytopathy in neuroinflammatory disorders with MOG antibodies. J Neurol Neurosurg Psychiatry. 2016;87:1257–1259. [DOI] [PubMed] [Google Scholar]
- 99.Hofer LS, Ramberger M, Gredler V, Pescoller AS, Rostásy K, Sospedra M, Hegen H, Berger T, Lutterotti A, Reindl M. Comparative analysis of T-cell responses to aquaporin-4 and myelin oligodendrocyte glycoprotein in inflammatory demyelinating central nervous system diseases. Front Immunol. 2020;11:1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bronge M, Ruhrmann S, Carvalho-Queiroz C, Nilsson OB, Kaiser A, Holmgren E, Macrini C, Winklmeier S, Meinl E, Brundin L, Khademi M, Olsson T, Gafvelin G, Grönlund H. Myelin oligodendrocyte glycoprotein revisited-sensitive detection of MOG-specific T-cells in multiple sclerosis. J Autoimmun. 2019;102:38–49. [DOI] [PubMed] [Google Scholar]
- 101.Jain RW, Yong VW. B cells in central nervous system disease: diversity, locations and pathophysiology. Nat Rev Immunol. 2022;22:513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jelcic I, Al Nimer F, Wang J, Lentsch V, Planas R, Jelcic I, Madjovski A, Ruhrmann S, Faigle W, Frauenknecht K, Pinilla C, Santos R, Hammer C, Ortiz Y, Opitz L, Grönlund H, Rogler G, Boyman O, Reynolds R, Lutterotti A, Khademi M, Olsson T, Piehl F, Sospedra M, Martin R. Memory B cells activate brain-homing, autoreactive CD4+ T cells in multiple sclerosis. Cell. 2018;175:85–100.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Archambault AS, Carrero JA, Barnett LG, McGee NG, Sim J, Wright JO, Raabe T, Chen P, Ding H, Allenspach EJ, Dragatsis I, Laufer TM, Wu GF. Cutting edge: conditional MHC class II expression reveals a limited role for B cell antigen presentation in primary and secondary CD4 T cell responses. J Immunol. 2013;191:545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Molnarfi N, Schulze-Topphoff U, Weber MS, Patarroyo JC, Prod'homme T, Varrin-Doyer M, Shetty A, Linington C, Slavin AJ, Hidalgo J, Jenne DE, Wekerle H, Sobel RA, Bernard CCA, Shlomchik MJ, Zamvil SS. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J Exp Med. 2013;210:2921–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Winklmeier S, Schlüter M, Spadaro M, Thaler FS, Vural A, Gerhards R, Macrini C, Mader S, Kurne A, Inan B, Karabudak R, Özbay FG, Esendagli G, Hohlfeld R, Kümpfel T, Meinl E. Identification of circulating MOG-specific B cells in patients with MOG antibodies. Neurol Neuroimmunol Neuroinflamm. 2019;6:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. [DOI] [PubMed] [Google Scholar]
- 107.Rojas OL, Pröbstel A-K, Porfilio EA, Wang AA, Charabati M, Sun T, Lee DSW, Galicia G, Ramaglia V, Ward LA, Leung LYT, Najafi G, Khaleghi K, Garcillán B, Li A, Besla R, Naouar I, Cao EY, Chiaranunt P, Burrows K, Robinson HG, Allanach JR, Yam J, Luck H, Campbell DJ, Allman D, Brooks DG, Tomura M, Baumann R, Zamvil SS, Bar-Or A, Horwitz MS, Winer DA, Mortha A, Mackay F, Prat A, Osborne LC, Robbins C, Baranzini SE, Gommerman JL. Recirculating intestinal IgA-producing cells regulate neuroinflammation via IL-10. Cell. 2019;177:492–493. e18. [DOI] [PubMed] [Google Scholar]
- 108.Li X, Wang L, Zhou L, ZhangBao J, Miao MZ, Lu C, Lu J, Quan C. The imbalance between regulatory and memory B cells accompanied by an increased number of circulating T-follicular helper cells in MOG-antibody-associated demyelination. Mult Scler Relat Disord. 2019;36:101397. [DOI] [PubMed] [Google Scholar]
- 109.Cobo-Calvo A, Sepúlveda M, Rollot F, Armangué T, Ruiz A, Maillart E, Papeix C, Audoin B, Zephir H, Biotti D, Ciron J, Durand-Dubief F, Collongues N, Ayrignac X, Labauge P, Thouvenot E, Bourre B, Montcuquet A, Cohen M, Deschamps R, Solà-Valls N, Llufriu S, De Seze J, Blanco Y, Vukusic S, Saiz A, Marignier R. Evaluation of treatment response in adults with relapsing MOG-Ab-associated disease. J Neuroinflammation. 2019;16:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen JJ, Flanagan EP, Bhatti MT, Jitprapaikulsan J, Dubey D, Lopez Chiriboga ASS, Fryer JP, Weinshenker BG, McKeon A, Tillema J-M, Lennon VA, Lucchinetti CF, Kunchok A, McClelland CM, Lee MS, Bennett JL, Pelak VS, van Stavern G, Adesina O-OO, Eggenberger ER, Acierno MD, Wingerchuk DM, Lam BL, Moss H, Beres S, Gilbert AL, Shah V, Armstrong G, Heidary G, Cestari DM, Stiebel-Kalish H, Pittock SJ. Steroid-sparing maintenance immunotherapy for MOG-IgG associated disorder. Neurology. 2020;95:e111–e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Montcuquet A, Collongues N, Papeix C, Zephir H, Audoin B, Laplaud D, Bourre B, Brochet B, Camdessanche J-P, Labauge P, Moreau T, Brassat D, Stankoff B, de Seze J, Vukusic S, Marignier R; NOMADMUS Study Group and the Observatoire Francais de la Sclerose en Plaques OFSEP. Effectiveness of mycophenolate mofetil as first-line therapy in AQP4-IgG, MOG-IgG, and seronegative neuromyelitis optica spectrum disorders. Mult Scler. 2017;23:1377–1384. [DOI] [PubMed] [Google Scholar]
- 112.Nepal G, Kharel S, Coghlan MA, Rayamajhi P, Ojha R. Safety and efficacy of rituximab for relapse prevention in myelin oligodendrocyte glycoprotein immunoglobulin G (MOG-IgG)-associated disorders (MOGAD): a systematic review and meta-analysis. J Neuroimmunol. 2022;364:577812. [DOI] [PubMed] [Google Scholar]
- 113.Whittam DH, Cobo-Calvo A, Lopez-Chiriboga AS, Pardo S, Gornall M, Cicconi S, Brandt A, Berek K, Berger T, Jelcic I, Gombolay G, Oliveira LM, Callegaro D, Kaneko K, Misu T, Capobianco M, Gibbons E, Karthikeayan V, Brochet B, Audoin B, Mathey G, Laplaud D, Thouvenot E, Cohen M, Tourbah A, Maillart E, Ciron J, Deschamps R, Biotti D, Rostasy K, Neuteboom R, Hemingway C, Forsyth R, Matiello M, Webb S, Hunt D, Murray K, Hacohen Y, Lim M, Leite MI, Palace J, Solomon T, Lutterotti A, Fujihara K, Nakashima I, Bennett JL, Pandit L, Chitnis T, Weinshenker BG, Wildemann B, Sato DK, Kim SH, Huda S, Kim HJ, Reindl M, Levy M, Jarius S, Tenembaum S, Paul F, Pittock S, Marignier R, Jacob A. Treatment of MOG-IgG-associated disorder with rituximab: an international study of 121 patients. Mult Scler Relat Disord. 2020;44:102251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Graf J, Mares J, Barnett M, Aktas O, Albrecht P, Zamvil SS, Hartung H-P. Targeting B cells to modify MS, NMOSD, and MOGAD: part 2. Neurol Neuroimmunol Neuroinflamm. 2021;8:e919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Spadaro M, Winklmeier S, Beltrán E, Macrini C, Höftberger R, Schuh E, Thaler FS, Gerdes LA, Laurent S, Gerhards R, Brändle S, Dornmair K, Breithaupt C, Krumbholz M, Moser M, Krishnamoorthy G, Kamp F, Jenne D, Hohlfeld R, Kümpfel T, Lassmann H, Kawakami N, Meinl E. Pathogenicity of human antibodies against myelin oligodendrocyte glycoprotein. Ann Neurol. 2018;84:315–328. [DOI] [PubMed] [Google Scholar]
- 116.Jarius S, Pellkofer H, Siebert N, Korporal-Kuhnke M, Hümmert MW, Ringelstein M, Rommer PS, Ayzenberg I, Ruprecht K, Klotz L, Asgari N, Zrzavy T, Höftberger R, Tobia R, Buttmann M, Fechner K, Schanda K, Weber M, Asseyer S, Haas J, Lechner C, Kleiter I, Aktas O, Trebst C, Rostasy K, Reindl M, Kümpfel T, Paul F, Wildemann B; In Cooperation With the Neuromyelitis Optica Study Group NEMOS. Cerebrospinal fluid findings in patients with myelin oligodendrocyte glycoprotein (MOG) antibodies. Part 1: results from 163 lumbar punctures in 100 adult patients. J Neuroinflammation. 2020;17:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mariotto S, Gajofatto A, Batzu L, Delogu R, Sechi G, Leoni S, Pirastru MI, Bonetti B, Zanoni M, Alberti D, Schanda K, Monaco S, Reindl M, Ferrari S. Relevance of antibodies to myelin oligodendrocyte glycoprotein in CSF of seronegative cases. Neurology. 2019;93:e1867–e1872. [DOI] [PubMed] [Google Scholar]
- 118.Kwon YN, Kim B, Kim J-S, Mo H, Choi K, Oh S-I, Kim J-E, Nam T-S, Sohn EH, Heo SH, Kim SB, Park K-C, Yoon SS, Oh J, Baek S-H, Kim B-J, Park KS, Sung J-J, Jung JH, Kim S-J, Park S-H, Waters P, Kim S-M. Myelin oligodendrocyte glycoprotein-immunoglobulin G in the CSF: clinical implication of testing and association with disability. Neurol Neuroimmunol Neuroinflamm. 2022;9:e1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mariotto S, Ferrari S, Monaco S, Benedetti MD, Schanda K, Alberti D, Farinazzo A, Capra R, Mancinelli C, De Rossi N, Bombardi R, Zuliani L, Zoccarato M, Tanel R, Bonora A, Turatti M, Calabrese M, Polo A, Pavone A, Grazian L, Sechi G, Sechi E, Urso D, Delogu R, Janes F, Deotto L, Cadaldini M, Bianchi MR, Cantalupo G, Reindl M, Gajofatto A. Clinical spectrum and IgG subclass analysis of anti-myelin oligodendrocyte glycoprotein antibody-associated syndromes: a multicenter study. J Neurol. 2017;264:2420–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Piddlesden SJ, Lassmann H, Zimprich F, Morgan BP, Linington C. The demyelinating potential of antibodies to myelin oligodendrocyte glycoprotein is related to their ability to fix complement. Am J Pathol. 1993;143:555–564. [PMC free article] [PubMed] [Google Scholar]
- 121.Saadoun S, Waters P, Owens GP, Bennett JL, Vincent A, Papadopoulos MC. Neuromyelitis optica MOG-IgG causes reversible lesions in mouse brain. Acta Neuropathol Commun. 2014;2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Keller CW, Lopez JA, Wendel E-M, Ramanathan S, Gross CC, Klotz L, Reindl M, Dale RC, Wiendl H, Rostásy K, Brilot F, Lünemann JD. Complement activation is a prominent feature of MOGAD. Ann Neurol. 2021;90:976–982. [DOI] [PubMed] [Google Scholar]
- 123.Dale RC, Tantsis EM, Merheb V, Kumaran R-YA, Sinmaz N, Pathmanandavel K, Ramanathan S, Booth DR, Wienholt LA, Prelog K, Clark DR, Guillemin GJ, Lim CK, Mathey EK, Brilot F. Antibodies to MOG have a demyelination phenotype and affect oligodendrocyte cytoskeleton. Neurol Neuroimmunol Neuroinflamm. 2014;1:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marta CB, Taylor CM, Coetzee T, Kim T, Winkler S, Bansal R, Pfeiffer SE. Antibody cross-linking of myelin oligodendrocyte glycoprotein leads to its rapid repartitioning into detergent-insoluble fractions, and altered protein phosphorylation and cell morphology. J Neurosci. 2003;23:5461–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brilot F, Dale RC, Selter RC, Grummel V, Kalluri SR, Aslam M, Busch V, Zhou D, Cepok S, Hemmer B. Antibodies to native myelin oligodendrocyte glycoprotein in children with inflammatory demyelinating central nervous system disease. Ann Neurol. 2009;66:833–842. [DOI] [PubMed] [Google Scholar]
- 126.Flach A-C, Litke T, Strauss J, Haberl M, Gómez CC, Reindl M, Saiz A, Fehling H-J, Wienands J, Odoardi F, Lühder F, Flügel A. Autoantibody-boosted T-cell reactivation in the target organ triggers manifestation of autoimmune CNS disease. Proc Natl Acad Sci U S A. 2016;113:3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kinzel S, Lehmann-Horn K, Torke S, Häusler D, Winkler A, Stadelmann C, Payne N, Feldmann L, Saiz A, Reindl M, Lalive PH, Bernard CC, Brück W, Weber MS. Myelin-reactive antibodies initiate T cell-mediated CNS autoimmune disease by opsonization of endogenous antigen. Acta Neuropathol. 2016;132:43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Höftberger R, Guo Y, Flanagan EP, Lopez-Chiriboga AS, Endmayr V, Hochmeister S, Joldic D, Pittock SJ, Tillema JM, Gorman M, Lassmann H, Lucchinetti CF. The pathology of central nervous system inflammatory demyelinating disease accompanying myelin oligodendrocyte glycoprotein autoantibody. Acta Neuropathol. 2020;139:875–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Takai Y, Misu T, Kaneko K, Chihara N, Narikawa K, Tsuchida S, Nishida H, Komori T, Seki M, Komatsu T, Nakamagoe K, Ikeda T, Yoshida M, Takahashi T, Ono H, Nishiyama S, Kuroda H, Nakashima I, Suzuki H, Bradl M, Lassmann H, Fujihara K, Aoki M; Japan MOG-antibody Disease Consortium. Myelin oligodendrocyte glycoprotein antibody-associated disease: an immunopathological study. Brain. 2020;143:1431–1446. [DOI] [PubMed] [Google Scholar]
- 130.Shu Y, Long Y, Wang S, Hu W, Zhou J, Xu H, Chen C, Ou Y, Lu Z, Lau AY, Yu X, Kermode AG, Qiu W. Brain histopathological study and prognosis in MOG antibody-associated demyelinating pseudotumor. Ann Clin Transl Neurol. 2019;6:392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhou L, Huang Y, Li H, Fan J, ZhangBao J, Yu H, Li Y, Lu J, Zhao C, Lu C, Wang M, Quan C. MOG-antibody associated demyelinating disease of the CNS: a clinical and pathological study in Chinese Han patients. J Neuroimmunol. 2017;305:19–28. [DOI] [PubMed] [Google Scholar]
- 132.Jarius S, Metz I, König FB, Ruprecht K, Reindl M, Paul F, Brück W, Wildemann B. Screening for MOG-IgG and 27 other anti-glial and anti-neuronal autoantibodies in 'pattern II multiple sclerosis' and brain biopsy findings in a MOG-IgG-positive case. Mult Scler. 2016;22:1541–1549. [DOI] [PubMed] [Google Scholar]
- 133.Körtvélyessy P, Breu M, Pawlitzki M, Metz I, Heinze H-J, Matzke M, Mawrin C, Rommer P, Kovacs GG, Mitter C, Reindl M, Brück W, Wandinger K-P, Lassmann H, Höftberger R, Leypoldt F. ADEM-like presentation, anti-MOG antibodies, and MS pathology: two case reports. Neurol Neuroimmunol Neuroinflamm. 2017;4:e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sechi E, Krecke KN, Messina SA, Buciuc M, Pittock SJ, Chen JJ, Weinshenker BG, Lopez-Chiriboga AS, Lucchinetti CF, Zalewski NL, Tillema JM, Kunchok A, Monaco S, Morris PP, Fryer JP, Nguyen A, Greenwood T, Syc-Mazurek SB, Keegan BM, Flanagan EP. Comparison of MRI lesion evolution in different central nervous system demyelinating disorders. Neurology. 2021;97:e1097–e1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.ZhangBao J, Huang W, Zhou L, Wang L, Chang X, Lu C, Zhao C, Lu J, Quan C. Myelitis in inflammatory disorders associated with myelin oligodendrocyte glycoprotein antibody and aquaporin-4 antibody: a comparative study in Chinese Han patients. Eur J Neurol. 2021;28:1308–1315. [DOI] [PubMed] [Google Scholar]
- 136.Zhao G, Chen Q, Huang Y, Li Z, Sun X, Lu P, Yan S, Wang M, Tian G. Clinical characteristics of myelin oligodendrocyte glycoprotein seropositive optic neuritis: a cohort study in Shanghai, China. J Neurol. 2018;265:33–40. [DOI] [PubMed] [Google Scholar]
- 137.Liu H, Zhou H, Wang J, Sun M, Teng D, Song H, Xu Q, Wei S. The prevalence and prognostic value of myelin oligodendrocyte glycoprotein antibody in adult optic neuritis. J Neurol Sci. 2019;396:225–231. [DOI] [PubMed] [Google Scholar]
- 138.Deschamps R, Lecler A, Lamirel C, Aboab J, Gueguen A, Bensa C, Vignal C, Gout O. Etiologies of acute demyelinating optic neuritis: an observational study of 110 patients. Eur J Neurol. 2017;24:875–879. [DOI] [PubMed] [Google Scholar]
- 139.Zhao Y, Tan S, Chan TCY, Xu Q, Zhao J, Teng D, Fu H, Wei S. Clinical features of demyelinating optic neuritis with seropositive myelin oligodendrocyte glycoprotein antibody in Chinese patients. Br J Ophthalmol. 2018;102:1372–1377. [DOI] [PubMed] [Google Scholar]
- 140.Soelberg K, Jarius S, Skejoe H, Engberg H, Mehlsen JJ, Nilsson AC, Madsen JS, Reindl M, Wildemann B, Grauslund J, Kyvik KO, Smith TJ, Lillevang ST, Paul F, Weinshenker BG, Asgari N. A population-based prospective study of optic neuritis. Mult Scler. 2017;23:1893–1901. [DOI] [PubMed] [Google Scholar]
- 141.Ducloyer J-B, Caignard A, Aidaoui R, Ollivier Y, Plubeau G, Santos-Moskalyk S, Porphyre L, Le Jeune C, Bihl L, Alamine S, Marignier R, Bourcier R, Ducloyer M, Weber M, Le Meur G, Wiertlewski S, Lebranchu P. MOG-Ab prevalence in optic neuritis and clinical predictive factors for diagnosis. Br J Ophthalmol. 2020;104:842–845. [DOI] [PubMed] [Google Scholar]
- 142.Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, Pache F, Stich O, Beume L-A, Hümmert MW, Ringelstein M, Trebst C, Winkelmann A, Schwarz A, Buttmann M, Zimmermann H, Kuchling J, Franciotta D, Capobianco M, Siebert E, Lukas C, Korporal-Kuhnke M, Haas J, Fechner K, Brandt AU, Schanda K, Aktas O, Paul F, Reindl M, Wildemann B; In cooperation With the Neuromyelitis Optica Study Group NEMOS. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen JJ, Flanagan EP, Jitprapaikulsan J, López-Chiriboga ASS, Fryer JP, Leavitt JA, Weinshenker BG, McKeon A, Tillema J-M, Lennon VA, Tobin WO, Keegan BM, Lucchinetti CF, Kantarci OH, McClelland CM, Lee MS, Bennett JL, Pelak VS, Chen Y, VanStavern G, Adesina O-OO, Eggenberger ER, Acierno MD, Wingerchuk DM, Brazis PW, Sagen J, Pittock SJ. Myelin oligodendrocyte glycoprotein antibody-positive optic neuritis: clinical characteristics, radiologic clues, and outcome. Am J Ophthalmol. 2018;195:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu H, Zhou H, Wang J, Xu Q, Wei S. Antibodies to myelin oligodendrocyte glycoprotein in chronic relapsing inflammatory optic neuropathy. Br J Ophthalmol. 2019;103:1423–1428. [DOI] [PubMed] [Google Scholar]
- 145.Vosoughi AR, Ling J, Tam KT, Blackwood J, Micieli JA. Ophthalmic manifestations of myelin oligodendrocyte glycoprotein-IgG-associated disorder other than optic neuritis: a systematic review. Br J Ophthalmol. 2021;105:1591–1598. [DOI] [PubMed] [Google Scholar]
- 146.Chen JJ, Sotirchos ES, Henderson AD, Vasileiou ES, Flanagan EP, Bhatti MT, Jamali S, Eggenberger ER, Dinome M, Frohman LP, Arnold AC, Bonelli L, Seleme N, Mejia-Vergara AJ, Moss HE, Padungkiatsagul T, Stiebel-Kalish H, Lotan I, Hellmann MA, Hodge D, Oertel FC, Paul F, Saidha S, Calabresi PA, Pittock SJ. OCT retinal nerve fiber layer thickness differentiates acute optic neuritis from MOG antibody-associated disease and multiple sclerosis: RNFL thickening in acute optic neuritis from MOGAD vs MS. Mult Scler Relat Disord. 2022;58:103525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sotirchos ES, Filippatou A, Fitzgerald KC, Salama S, Pardo S, Wang J, Ogbuokiri E, Cowley NJ, Pellegrini N, Murphy OC, Mealy MA, Prince JL, Levy M, Calabresi PA, Saidha S. Aquaporin-4 IgG seropositivity is associated with worse visual outcomes after optic neuritis than MOG-IgG seropositivity and multiple sclerosis, independent of macular ganglion cell layer thinning. Mult Scler. 2020;26:1360–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yu J, Huang Y, Quan C, Zhou L, ZhangBao J, Wu K, Zong Y, Zhou X, Wang M. Alterations in the retinal vascular network and structure in MOG antibody-associated disease: an optical coherence tomography angiography study. J Neuroophthalmol. 2021;41:e424–e432. [DOI] [PubMed] [Google Scholar]
- 149.Ramanathan S, Prelog K, Barnes EH, Tantsis EM, Reddel SW, Henderson APD, Vucic S, Gorman MP, Benson LA, Alper G, Riney CJ, Barnett M, Parratt JDE, Hardy TA, Leventer RJ, Merheb V, Nosadini M, Fung VSC, Brilot F, Dale RC. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler. 2016;22:470–482. [DOI] [PubMed] [Google Scholar]
- 150.Tajfirouz D, Padungkiatsagul T, Beres S, Moss HE, Pittock S, Flanagan E, Kunchok A, Shah S, Bhatti MT, Chen JJ. Optic chiasm involvement in AQP-4 antibody-positive NMO and MOG antibody-associated disorder. Mult Scler. 2022;28:149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Akaishi T, Himori N, Takeshita T, Misu T, Takahashi T, Takai Y, Nishiyama S, Fujimori J, Ishii T, Aoki M, Fujihara K, Nakazawa T, Nakashima I. Five-year visual outcomes after optic neuritis in anti-MOG antibody-associated disease. Mult Scler Relat Disord. 2021;56:103222. [DOI] [PubMed] [Google Scholar]
- 152.Storch MK, Stefferl A, Brehm U, Weissert R, Wallström E, Kerschensteiner M, Olsson T, Linington C, Lassmann H. Autoimmunity to myelin oligodendrocyte glycoprotein in rats mimics the spectrum of multiple sclerosis pathology. Brain Pathol. 1998;8:681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Weissert R, de Graaf KL, Storch MK, Barth S, Linington C, Lassmann H, Olsson T. MHC class II-regulated central nervous system autoaggression and T cell responses in peripheral lymphoid tissues are dissociated in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis. J Immunol. 2001;166:7588–7599. [DOI] [PubMed] [Google Scholar]
- 154.Weissert R, Wallström E, Storch MK, Stefferl A, Lorentzen J, Lassmann H, Linington C, Olsson T. MHC haplotype-dependent regulation of MOG-induced EAE in rats. J Clin Invest. 1998;102:1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bettelli E, Baeten D, Jäger A, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J Clin Invest. 2006;116:2393–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Krishnamoorthy G, Lassmann H, Wekerle H, Holz A. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J Clin Invest. 2006;116:2385–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jin J, Shneyderman M, Smith MD, Gharagozloo M, Sotirchos ES, Calabresi PA. Retinal pathology in spontaneous opticospinal experimental autoimmune encephalitis mice. J Neuroimmunol. 2022;367:577859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dietrich M, Aktas O, Hartung H-P, Albrecht P. Assessing the anterior visual pathway in optic neuritis: recent experimental and clinical aspects. Curr Opin Neurol. 2019;32:346–357. [DOI] [PubMed] [Google Scholar]
- 159.Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Castoldi V, Marenna S, Huang S-C, d'Isa R, Chaabane L, Comi G, Leocani L. Dose-dependent effect of myelin oligodendrocyte glycoprotein on visual function and optic nerve damage in experimental autoimmune encephalomyelitis. J Neurosci Res. 2022;100:855–868. [DOI] [PubMed] [Google Scholar]
- 161.Petrikowski L, Reinehr S, Haupeltshofer S, Deppe L, Graz F, Kleiter I, Dick HB, Gold R, Faissner S, Joachim SC. Progressive retinal and optic nerve damage in a mouse model of spontaneous opticospinal encephalomyelitis. Front Immunol. 2021;12:759389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Horstmann L, Kuehn S, Pedreiturria X, Haak K, Pfarrer C, Dick HB, Kleiter I, Joachim SC. Microglia response in retina and optic nerve in chronic experimental autoimmune encephalomyelitis. J Neuroimmunol. 2016;298:32–41. [DOI] [PubMed] [Google Scholar]
- 163.Bradl M, Reindl M, Lassmann H. Mechanisms for lesion localization in neuromyelitis optica spectrum disorders. Curr Opin Neurol. 2018;31:325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Oertel FC, Sotirchos ES, Zimmermann HG, Motamedi S, Specovius S, Asseyer ES, Chien C, Cook L, Vasileiou E, Filippatou A, Calabresi PA, Saidha S, Pandit L, D'Cunha A, Outteryck O, Zéphir H, Pittock S, Flanagan EP, Bhatti MT, Rommer PS, Bsteh G, Zrzavy T, Kuempfel T, Aktas O, Ringelstein M, Albrecht P, Ayzenberg I, Pakeerathan T, Knier B, Aly L, Asgari N, Soelberg K, Marignier R, Tilikete CF, Cobo Calvo A, Villoslada P, Sanchez-Dalmau B, Martinez-Lapiscina EH, Llufriu S, Green AJ, Yeaman MR, Smith TJ, Brandt AU, Chen J, Paul F, Havla J; With the GJCF International Clinical Consortium for NMOSD and the CROCTINO Study Group. Longitudinal retinal changes in MOGAD. Ann Neurol. 2022;92:476–485. [DOI] [PubMed] [Google Scholar]
- 165.Matsunaga Y, Kezuka T, An X, Fujita K, Matsuyama N, Matsuda R, Usui Y, Yamakawa N, Kuroda M, Goto H. Visual functional and histopathological correlation in experimental autoimmune optic neuritis. Invest Ophthalmol Vis Sci. 2012;53:6964–6971. [DOI] [PubMed] [Google Scholar]
- 166.Castoldi V, Marenna S, d'Isa R, Huang S-C, De Battista D, Chirizzi C, Chaabane L, Kumar D, Boschert U, Comi G, Leocani L. Non-invasive visual evoked potentials to assess optic nerve involvement in the dark agouti rat model of experimental autoimmune encephalomyelitis induced by myelin oligodendrocyte glycoprotein. Brain Pathol. 2020;30:137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kawachi I. Clinical characteristics of autoimmune optic neuritis. Clin Exp Neuroimmunol. 2017;8:8–16. [Google Scholar]
- 168.Hofman P, Hoyng P, vanderWerf F, Vrensen GF, Schlingemann RO. Lack of blood-brain barrier properties in microvessels of the prelaminar optic nerve head. Invest Ophthalmol Vis Sci. 2001;42:895–901. [PubMed] [Google Scholar]
- 169.Guy J, Rao NA. Acute and chronic experimental optic neuritis. Alteration in the blood-optic nerve barrier. Arch Ophthalmol. 1984;102:450–454. [DOI] [PubMed] [Google Scholar]


