Abstract
Objective
This study aimed to determine whether the intra-articular injection of human adipose-derived mesenchymal stem cells (ADSCs) protects against the progression of murine post-traumatic osteoarthritis.
Design
ADSCs were isolated from human abdomen or buttock adipose tissues. In in vitro study, ADSCs conditioned medium was added to human chondrocytes pre-treated with interleukin-1β (IL-1β), and resultant gene expression of target inflammatory genes was measured by real-time quantitative polymerase chain reaction. A mouse model of knee osteoarthritis was generated by unilaterally transecting the medial meniscus in the right hind limb of 20 female C57BL/6 mice. Mice were randomly assigned to 2 treatment groups that received 6 µl intra-articular injections of either phosphate-buffered saline (control) or 2 × 104 cells/μl of ADSCs 14, 28, and 42 days post-surgery. Mice were euthanized 84 days post-surgery and histological and micro-computed tomography evaluation of knee joints were analyzed. Hind limb weight-bearing distribution was measured pre-surgery and 28 and 84 days post-surgery.
Results
Conditioned medium from cultured human adipose-derived mesenchymal stem cells suppressed the expression of target inflammatory genes in chondrocytes pre-treated with IL-1β, suggesting anti-inflammatory properties (P < 0.01). Histological analyses indicated that the progression of destabilization of medial meniscus-induced knee osteoarthritis was suppressed by the administration of ADSCs compared with control group at medial femorotibial joint in vivo. This protective effect was related to a reduction in articular cartilage loss.
Conclusion
The intra-articular injection of ADSCs suppressed articular cartilage loss in a mouse model of knee osteoarthritis, possible through anti-inflammatory mechanisms.
Keywords: knee osteoarthritis, adipose-derived mesenchymal stem cells, mouse model, adipose-derived mesenchymal stem cells conditioned medium, post-traumatic osteoarthritis
Introduction
Knee osteoarthritis (OA) is increasing in aging societies and is a major public health concern worldwide. Disability caused by joint diseases results in the need for long-term care. Symptomatic knee OA occurs in 10% of men and 13% of women aged 60 years or older.1 Several types of treatment are available for osteoarthritis, such as steroids and nonsteroidal anti-inflammatory drugs. These drugs can relieve symptoms to some extent but are not disease-modifying effect. Therefore, the development and assessment of novel disease-modifying therapies and suppression of the progression of OA are required.
Biotherapies, the use of biological substances to help healing of musculoskeletal injuries, become an area of interest as potential treatments to modify disease symptoms and heal injured tissues. There are several sources of biotherapies, such as peripheral blood, bone marrow-derived mesenchymal stem cells (MSCs), adipose-derived MSCs (ADSCs), amniotic membrane-derived MSCs, umbilical cord-derived MSCs, and stem cell-derived extracellular vesicles (EVs). EVs have become an area of intense interest in regenerative medicine because stem cell-derived EVs theoretically have similar biological functions to stem cells.2 -4 The use of autologous ADSCs for clinical applications has increased because they are safe autologous cells and relatively easy to harvest compared with other stromal cells such as those derived from bone marrow and synovial cell, and lipoaspirates were shown to result in higher progenitor cell yields than bone marrow aspirates.5 Although many clinical trials investigating MSC-based therapies have demonstrated promising outcomes, there are some problems, including lack of understanding of the mechanism of action or biological function to support their clinical use, and insufficient data from in vitro assays, animal models, and clinical studies regarding their safety profile for use in patients.6,7
A post-traumatic osteoarthritis (PTOA) is one of the major causes of secondary knee osteoarthritis.8 Since PTOA occurs following knee injury, the onset of PTOA can predict. Therefore, development of the treatment strategy to prevent PTOA is very important issue. In mice, a PTOA model induced by destabilization of the medial meniscus (DMM) caused mild degenerative changes by 4 to 8 weeks post-surgery, progressing to more severe degenerative changes by 10 weeks.9,10 This disease course is similar to the pathogenesis of human OA. The DMM model has been used in many basic science studies. In the present study, we assessed whether the intra-articular injection of human ADSCs protected against the progression of murine PTOA. We hypothesized that the intra-articular injection of ADSC would suppress the progression of knee OA.
Methods
Ethics Statement
All experimental procedures and protocols in this study were approved by the Institutional Animal Care and Use Ethics Committee of Juntendo University (approval number 300263).
Adipose-Derived Mesenchymal Stem Cells and Conditioned Medium Preparation
Human abdomen or buttock adipose tissues were collected with informed consent from patients receiving regenerative medicine using ADSCs at Sunfield Clinic (Tokyo, Japan). ADSCs were isolated from samples, and the stromal vascular fraction was cultured with sf-DOT (BioMimetics Sympathies, Inc., Tokyo, Japan), a serum-free culture medium, at 37°C in an atmosphere containing 5% CO2. After reaching confluence, adherent cells were trypsinized using TrypLe Select (Thermo Fisher Scientific, Tokyo, Japan) and replated. Cells were passaged using sf-DOT 3 or 4 times, and the characteristics of ADSCs were verified by analysis of their differentiation, proliferation, and immunologic phenotypes.
These expanded cells were provided from BioMimetics Sympathies, Inc. The cells were frozen and delivered to Juntendo University. After thawing, the cells were immediately washed, counted, suspended in phosphate-buffered saline (PBS), and administered to mice.
Approximately 1.2 × 106 ADSCs of passage 3 were seeded in a T175 CellBIND culture flask (Corning, Arizona, USA) containing 30 ml of sf-DOT medium. After 5 days of incubation, the resulting ADSC-conditioned medium (CM) was collected, centrifuged at 400 ×g for 5 minutes to remove the cell debris, and filtered through a 0.22-μm filter. ADSC-CM was aliquoted and stored at −20°C until use.
Chondrocyte Culture and Real-Time Quantitative Polymerase Chain Reaction
Human chondrocytes (PromoCell, USA) were cultivated in Chondrocyte Growth Medium (PromoCell, USA) in a humidified incubator with 5% CO2 at 37°C. Chondrocytes (5 × 105 cells per well) were plated into CellBIND 6-well plates (Corning, USA) overnight in 2 ml of culture medium at 37°C. On the next day, 10 ng/ml interleukin-1β (IL-1β) was added and 50% ADSC-CM was used to replace the old media and cultured for 24 hours. After 24 hours culture, chondrocyte total RNA was isolated by a ReliaPrep RNA Miniprep System (Promega, USA). Then, 1 μg of total RNA was used to synthesize first-strand cDNA. The PrimeScript RT Master Mix (Takara, Japan) was used for this procedure. In addition, real-time quantitative polymerase chain reaction (qPCR) was performed according to the following conditions with the CFX Connect Real Time System (Bio-Rad Laboratories, USA): 5 minutes at 95°C, followed by 40 cycles of 15 s at 95°C and 30 s at 60°C. Then, 10 μl Thunderbird SYBR qPCR Mix (Toyobo, Japan), 0.4 μl of each 5 μM reverse primer and forward primer, and 9.2 μl diluted cDNA were included in the reaction system to a total volume of 20 μl. Fold change (2−ΔΔCt) was used to analyze the relative expression of the target gene. GAPDH was used as the internal standard to normalize the gene concentration of target mRNAs. Each gene analysis was performed in triplicate. The real-time qPCR primers were as follows: matrix metalloproteinase (MMP) 13, forward primer 5ʹ-CCAGTCTCCGAGGAGAAACA-3ʹ and reverse primer 5ʹ-AAAAACAGCTCCGCATCAAC-3ʹ; a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5), forward primer 5ʹ-GCTGGGAGATGACCATGAGG-3ʹ and reverse primer 5ʹ-AAATGTCCGATTTCGTGAGCC-3ʹ; and GAPDH, forward primer 5ʹ-AGCCACATCGCTCAGACAC-3ʹ and reverse primer 5ʹ-GCCCAATACGACCAAATCC-3ʹ.
Measuring the Concentration of Secreted Factors in ADSC-Conditioned Medium
The concentrations of tissue inhibitor of metalloproteinase (TIMP) 1 and TIMP2 in the 3 different conditioned media were measured by a Human TIMP1 and TIMP2 ELISA kit (Abcam, UK), respectively. The concentrations of interleukin (IL)-10 and IL1-receptor antagonist (IL-1Ra), anti-inflammatory cytokines, were measured with a Human Cytokine Magnetic Luminex Performance Assay 14-plex fixed panel by LUMINEX200 (Merck Millipore, USA).
Induction of OA and Treatment
Exclusion criterion for animals was death during the experiment. There were no exclusions. We established a humane endpoint after DMM surgery whereby if the walking ability deteriorated due to pain as the condition progressed, and if the animal became weak due to difficulty in eating food, it was euthanized as a human endpoint. All animal experiments were performed on 20 female C57BL/6 mice aged 8 weeks from the Jackson Laboratory Japan, Inc. (Yokohama, Kanagawa, Japan). The mice were housed in groups of 4 or 5 per cage, under a 12-hour light/dark cycle with food and water. Knee OA was induced unilaterally by DMM as described previously.6 Mice were anesthetized via the inhalation of isoflurane and oxygen under aseptic conditions. A small (3–5 mm) medial para-patellar skin incision was made in the right hind limb. The joint capsule immediately medial to the patellar tendon was incised and the joint capsule opened to expose the medial meniscotibial ligament (MMTL). The MMTL was transected to give DMM.
Mice were randomly assigned to 2 treatment groups: 6 μl of PBS (control) or 2 × 104 cells/μl of ADSC. Each groups received 3 consecutive intra-articular injections. The first injection was given 14 days after DMM surgery, and consecutive injections were repeated at days 28 and 42. Mice were euthanized at day 84 after DMM surgery, and knee joints were harvested for histological evaluation. There were no interventions or steps taken to reduce pain in the experimental protocols.
Measurement of Hind Limb Weight Distribution
Weight-bearing distribution was measured with an incapacitance tester (Linton Instrumentation) to evaluate changes in the load of the surgical limb. The mice were placed in a prismatic plexiglass case with each hind limb resting on a separate force plate. Measurements were made on days 0, 28, and 84. At least 100 measurements per mouse were recorded at each time point. The measurer was blinded to group allocation, and the tester’s display was covered to blind them to the measured values. Percent weight distribution of the right limb (injection side) was calculated by the following formula11:
% weight distribution of right limb = right weight/(right weight + left weight) × 100.
The mean of these measurement values was used for statistical analysis.
Histological Analyses
Knees were fixed in 4% formalin for 72 hours, decalcified with EDTA solution (EDT-X, FALMA, Tokyo, Japan) for 28 days, and embedded in paraffin wax. Frontal sections of 6 μm thickness were cut for the analysis of cartilage damage. The sections were stained with Safranin O and fast green. Cartilage degeneration was scored by 3 blinded observers according to the Osteoarthritis Research Society International (OARSI) recommendations.12 Cartilage in the medial compartment including the medial femoral condyle (MFC), medial tibial plateau (MTP), and medial tibiofemoral joint (MTJ) of the knee was scored on all sections with 24-μm intervals between the sections. The maximum scores of the sections for each compartment were used for the analyses.
Micro-Computed Tomography Evaluation
Knee joint images were obtained from micro-computed tomography (μCT) equipment (Latheta LCT-200, Hitachi, Ltd., Tokyo, Japan). Three-dimensional joint morphology reconstruction was then performed using VGStudio MAX (Volume Graphics, Aichi, Japan). The region of interest (ROI) for the bone mineral density (BMD) measurement of MTP and lateral tibia plateau was set at 0.2 mm under the subchondral bone plate on the tibial plateau with a size that covered most of the subchondral bone in the compartment.
Statistical Analysis
The results of real-time qPCR were analyzed by 1-way analysis of variance (ANOVA) followed by post hoc Tukey’s multiple comparisons test. Analysis of weight-bearing distribution was performed by 2-way repeated-measures ANOVA with post hoc Tukey’s multiple comparisons test. μCT analysis was performed using a paired t test for MTP and lateral tibia plateau (LTP), and comparisons between groups and histological scores used a Student’s t test. Sections of MTP, LFC, LTP, and LFTJ were used for Mann-Whitney test because MTP, LFC, LTP, and LFTJ scores were non-normally distributed as shown by the Shapiro-Wilk test (P < 0.05). Correlation analysis between the BMD of MTP and histological scores was performed using Spearman’s correlation test. All analyses were performed using GraphPad Prism ver8.0 (GraphPad Software, Inc, La Jolla, USA). All P values were 2-sided and P values <0.05 were considered statistically significant.
Results
ADSC-CM Suppresses Inflammation-Induced Catabolic Effects in Human Chondrocytes
To investigate whether ADSC-CM possessed anti-catabolic effects, human chondrocytes were treated with IL-1β. The expression levels of MMP 13 and ADAMTS5 were increased after IL-1β stimulation. These effects were significantly suppressed after adding ADSC-CM (P < 0.01) (Fig. 1) suggesting that ADSC-CM has anti-catabolic effects on chondrocytes. Next, the levels of MSC-derived anti-inflammatory cytokines including TIMP1, TIMP2, and hematopoietic cell-derived anti-inflammatory cytokines including IL-10 and IL-1Ra were measured in ADSC-CM. The levels of TIMP1 and TIMP2 were 199,913 ± 24,901 and 14,914 ± 4130 pg/ml, respectively. IL-10 and IL-1Ra levels were below the detection limit (Fig. 2).
Figure 1.
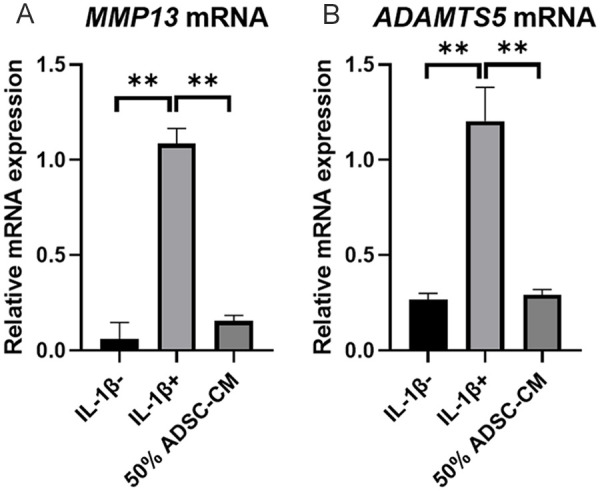
The relative expressions of MMP13 and ADAMTS5 in 50% ADSC-CM. (A) The relative expression of MMP13 in IL-1β stimulated mRNA compared to with and without ADSC-CM. (B) The relative expression of ADAMTS5 in IL-1β stimulated mRNA compared to with and without ADSC-CM. Data are presented as the mean ± SD (*P < 0.05, **P < 0.01). MMP = matrix metalloproteinase; ADAMST5 = a disintegrin and metalloproteinase with thrombospondin motifs 5; IL = interleukin; ADSC = adipose-derived mesenchymal stem cells; CM = conditioned medium.
Figure 2.
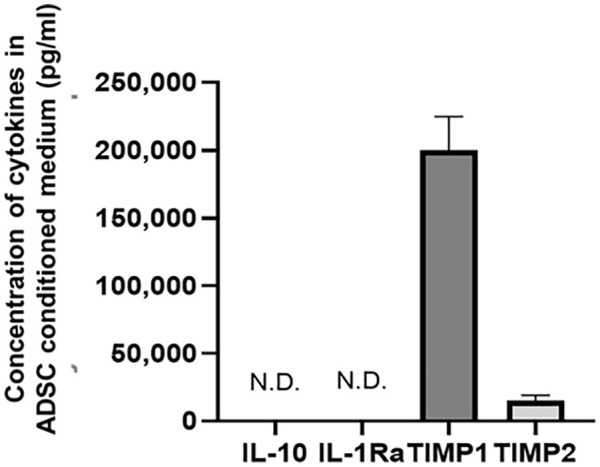
Concentration of anti-inflammatory cytokines in ADSC-CM: results for IL-10, IL-1Ra, TIMP 1, and TIMP 2. Data are presented as the mean ± SD. ADSC = adipose-derived mesenchymal stem cells; CM = conditioned medium; IL = interleukin; Ra = receptor antagonist; TIMP = tissue inhibitor of metalloproteinase; N.D. = not detected.
Mouse Knee OA Model and Weight-Bearing Post-Surgery
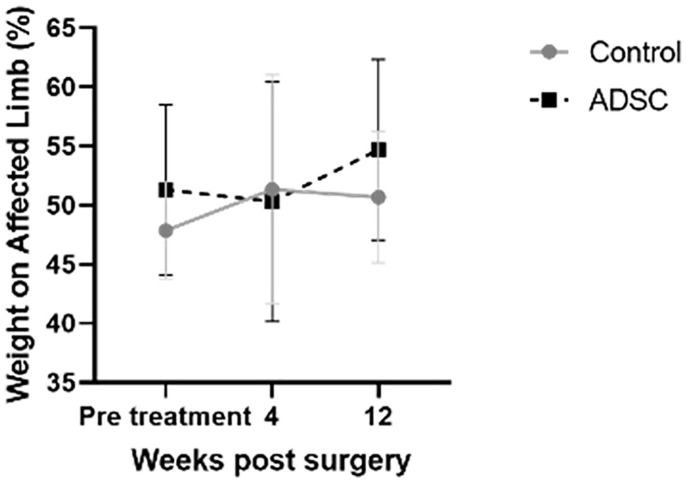
To assess whether ADSC protects against the progression of articular cartilage destruction in knee OA in vivo, an instability-induced mouse knee OA model (DMM model) was induced in the right hind limb, and ADSC was administrated 2, 4, and 6 weeks post-surgery. Figure 3 shows the change of weight distribution of the right hind limb and left hind limb post-surgery. There were no differences between the affected and non-affected hind limbs in each group at any time point. Therefore, both knees of these mice could bear their weight after surgery without limping to avoid pain.
Figure 3.
Change of weight distribution on the surgical side by an incapacitance tester. Data are presented as the mean ± SD. ADSC = adipose-derived mesenchymal stem cells.
ADSC Suppressed DMM-Induced Knee OA by Histological Analyses
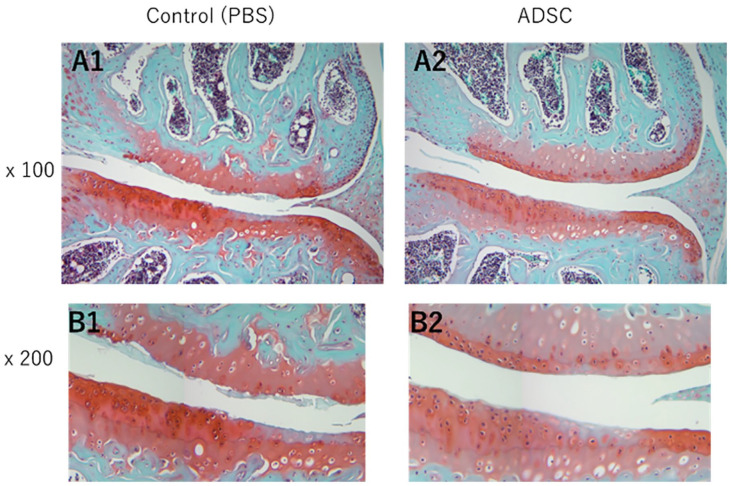
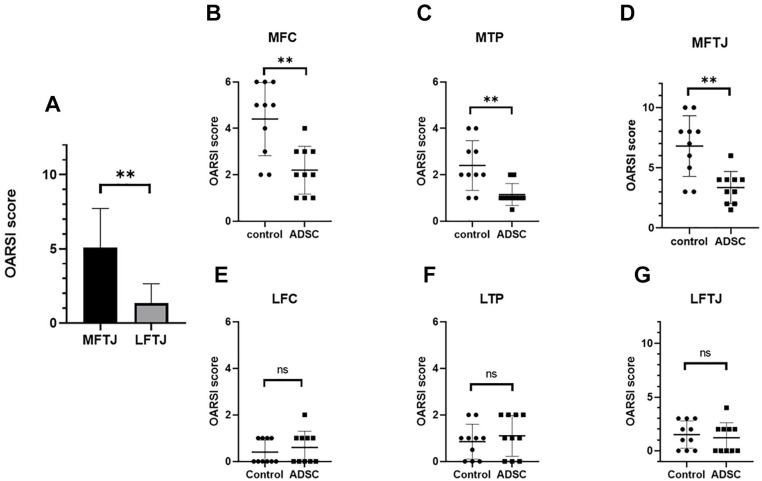
Histological analysis of knee joints was performed at 12 weeks post-surgery. Figure 4 shows a coronal section of medial femorotibial joint (MFTJ) stained with Safranin O and fast green. First, the OARSI score between MFTJ and lateral femorotibial joint (LFTJ) was compared regardless of the treatment group (Fig. 5A). The OARSI score at MFTJ was significantly higher compared with that for LFTJ. The DMM-induced destruction of articular cartilage was severe at MFTJ. Then, the OARSI score was compared between each treatment group. The OARSI score was significantly lower in the ADSC group compared with controls at the MTP and MFC (Fig. 5B and C). In contrast to MFTJ, there were no differences in OARSI score at LFTJ (Fig. 5E, F, and G). These results indicate that the progression of DMM-induced knee OA was suppressed by the administration of ADSCs. Power analysis was performed using G*Power version 3.1.9.4. software (Christian-Albrechts-Universität Kiel, Kiel, Germany). The effect size calculated from the result of OARSI scoring at MFTJ was 0.826 and β was 0.97 when setting the α as 0.05.
Figure 4.
Histological analysis of cartilage from images. Representative images of medial compartment stained with Safranin-O. Magnification = 100x (A1-3) and 200x (B1-3). PBS = phosphate-buffered saline; ADSC = adipose-derived mesenchymal stem cells.
Figure 5.
Histological analysis of cartilage by OARSI score. (A) Comparison of the average OARSI histological scores of 2 groups between MFTJ and LFTJ. Average of OARSI histological scores for MFC and MTP at (B) and (C), LFC and LTP at (E) and (F), and MFTJ and LFTJ at (D) and (G). Bars represent the mean and SD. *P < 0.05, **P < 0.01. OARSI = Osteoarthritis Research Society International; MFTJ = medial and lateral femorotibial joint; LFTJ = lateral and lateral femorotibial joint; MFC = medial femoral condyle; MTP = medial tibia plateau; LFC = lateral femoral condyle; LTP = lateral tibia plateau; ADSC = adipose-derived mesenchymal stem cells; N.S. = not significant.
Evaluation of Sclerosis of the Subchondral Bone in DMM-Induced Knee OA
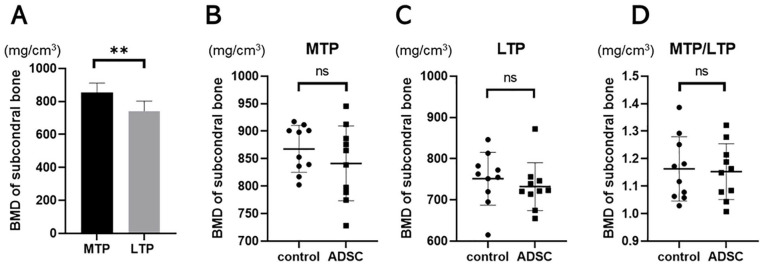
Histological analysis confirmed DMM-induced articular cartilage loss at MFTJ. Sclerosis of the subchondral bone, another feature of knee OA, was evaluated based on 3-dimensional μCT analysis. BMD at the subchondral trabecular bone was compared between the medial and lateral tibial plateau regardless of the treatment group (Fig. 6A). The BMD at MTP was significantly higher compared with that at LTP (854.5 ± 56.8 and 741.7 ± 60.3, respectively, P < .01). The mean BMD at the MTP in the control and ADSC groups was 867.7 ± 42.6 and 841.3 ± 68.0, respectively. Although sclerosis of subchondral bone was observed in the MTP, there were no differences in BMD between the control and ADSC groups (Fig. 6B, C, D). There was a significantly correlation between OARSI score and BMD in the MTP (r = 0.48, P = 0.02). Therefore, bone density of subchondral bone was similar in the each group.
Figure 6.
μCT analysis for BMD of subchondral bone. (A) Comparison of the BMD of 2 groups between MTP and LTP. Average BMD for MFC and MTP at (B) and (C), and MTP/LTP at (D). Bars represent the mean and SD. *P < 0.05, **P < 0.01. BMD = bone mineral density; MTP = medial tibia plateau; LTP = lateral tibia plateau; ADSC = adipose-derived mesenchymal stem cells; N.S. = not significant.
Discussion
The use of biologic therapies for the treatment of knee OA has increased, despite an insufficient understanding of their mechanisms. In this study, we demonstrated that the intra-articular injection of ADSCs suppressed articular cartilage loss in a mouse model of PTOA (Fig. 5). ADSC-CM contained anti-inflammatory cytokines including TIMPs, and the administration of ADSC-CM suppressed IL-1β-induced inflammatory effects on human chondrocytes (Figs. 1 and 2). These results suggest that ADSC-based biologic therapies have chondroprotective effects through suppressing the inflammation-induced catabolic effect of articular cartilage. These mechanistic findings indicate how ADSCs might protect against knee OA in vivo.
MSCs are multipotent stromal cells that can differentiate into mesenchymal lineage cells such as fibroblasts, osteoblasts, and chondrocytes. In addition to their multi-lineage potential, they have immunomodulatory and anti-inflammatory properties. In 2010, Caplan asked the cell-therapy industry to change the term mesenchymal stem cells to medicinal signaling cells.13 This was because, although MSCs can differentiate into mesenchymal lineage cells in vitro, this has not been proven in vivo. He proposed that MSCs exert their actions mainly via paracrine mechanisms. Therefore, it is possible that ADSC-CM, containing bioactive factors secreted from ADSCs, has the similar effect as ADSCs.
MSCs might exert their effects via paracrine mechanisms as well as direct actions such as cell migration to injured tissues and cell-cell interactions. In addition, MSCs can continue to release bioactive substances as long as they are alive in vivo.13 Further study is needed to elucidate the mechanisms related to how MSCs exert their actions after administration into mice.
Extracellular matrix homeostasis is partly controlled by the activity of MMPs and their counterparts, TIMPs. Patients with joint injury have a persistent increase in proMMPs in the synovial fluid and an increase in activated MMPs.14,15 Lozito et al.16 demonstrated that bone marrow-derived MSCs inhibited endogenous and exogenous MMPs via secreted TIMPs and MSCs inhibited extracellular matrix degradation by increasing the binding of MMPs at the cell surface and secreting TIMPs. They also demonstrated that adipose-derived MSCs exhibited higher cell-associated MMP activity levels than bone marrow-derived MSCs.16 In this study, ADSC-CM suppressed IL-1β-induced MMP-13 and ADAMTS5 expressions in human chondrocytes (Fig. 1) in vitro. ADSCs suppressed mouse OA progression in vivo. These observations suggest that ADSCs exert trophic and anti-inflammatory actions efficiently via secreted factors and by interacting with extracellular matrix molecules and local cells in injured tissues.
Subchondral sclerosis is one of the clinical features observed in patients with knee OA. Subchondral bone sclerosis, together with progressive cartilage degradation, is widely considered a hallmark of OA.17 In the mouse DMM-induced knee OA model, subchondral bone sclerosis is predominant at the MTP, and this model develops instability of the medial meniscus, which results in a medial type of knee OA. Indeed, the BMD at the MTP was significantly higher compared with that at the LTP (Fig. 6). Articular cartilage loss (OARSI score) significantly correlated with subchondral sclerosis (BMD) (Fig. 7). Although ADSCs administration suppressed articular cartilage loss (Fig. 5), there were no differences in BMD at the MTP between the control and treatment groups (Fig. 6). This suggests that the intra-articular injection of ADSCs suppressed cartilage degradation within knee joints but failed to control the instability of the meniscus resulting in the progression of subchondral bone sclerosis. Conversely, intra-articular ADSCs administration might partly suppress articular cartilage degeneration, even in unstable environments. The importance of the subchondral bone has been recognized for its contribution to the development and progression of OA.18 Further study will elucidate the interactions between articular cartilage and subchondral bone on the progression of knee OA.
Figure 7.
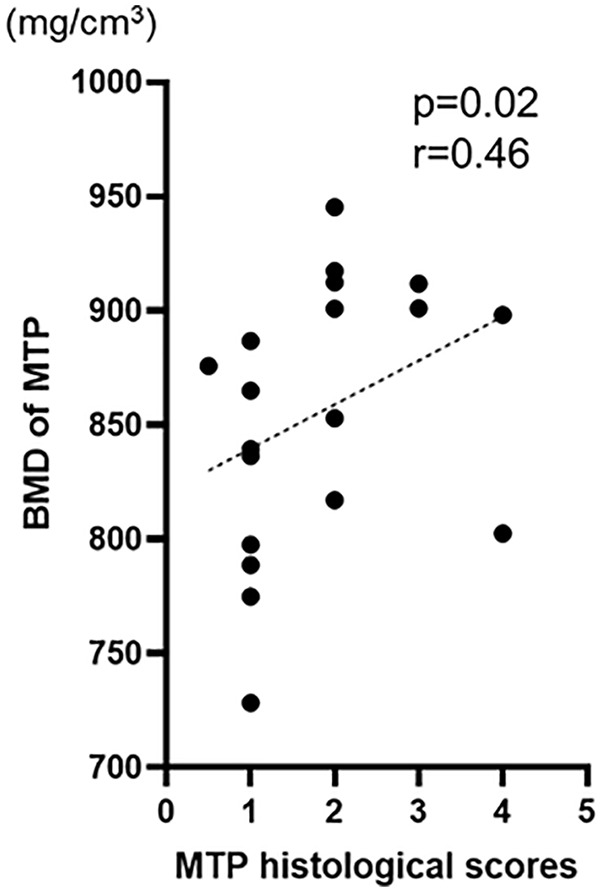
Correlation between the BMD and histological score at MTP. Distribution of the BMD and histological score at MTP. BMD = bone mineral density; MTP = medial tibia plateau.
This study had some limitations. First, the mechanism of action of how ADSCs injection suppresses progression of PTOA was only partly elucidated. Further studies on the actions of stem cells and factors secreted by them in vivo are needed. Second, MMP activity after the administration of ADSCs in knee joint was not measured. Therefore, it is unknown whether ADSCs exerts anti-inflammatory effects in vivo.
In conclusion, this study demonstrated that intra-articular injections of ADSCs suppressed DMM-induced OA in vivo and ADSC-CM suppressed IL-1β-induced inflammatory responses in vitro. These results suggest that the intra-articular injection of ADSCs might suppress the progression of OA. Our findings add to the knowledge of the mechanisms underlying cartilage protective effects and support the appropriate use of ADSCs for the treatment of post-traumatic arthritis and PTOA.
Footnotes
Acknowledgments and Funding: This work was a joint research study between Juntendo University and BioMimetics Sympathies. The authors are grateful to Yuji Takazawa, Masataka Nagayama, Haruka Kaneko, Jun Takeda, and other physicians and staff working at Juntendo Hospital for supporting our work. We thank J. Ludovic Croxford, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this article. The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was funded by BioMimetics Sympathies.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: This study was funded by BioMimetics Sympathies.
Ethical Approval: All experimental procedures and protocols in this study were approved by the Institutional Animal Care and Use Ethics Committee of Juntendo University (approval number 300263).
The Institution Where the Work Reported Was Done: We performed in vivo research at Juntendo University and in vitro experiments at BioMimetics Sympathies.
ORCID iD: Yoshitomo Saita  https://orcid.org/0000-0003-1614-2575
https://orcid.org/0000-0003-1614-2575
References
- 1. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li JJ, Hosseini-Beheshti E, Grau GE, Zreiqat H, Little CB. Stem cell-derived extracellular vesicles for treating joint injury and osteoarthritis. Nanomaterials (Basel). 2019;9:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen P, Tang S, Gao H, Zhang H, Chen C, Fang Z, et al. Wharton’s jelly mesenchymal stem cell-derived small extracellular vesicles as natural nanoparticles to attenuate cartilage injury via microRNA regulation. Int J Pharm. 2022;623:121952. [DOI] [PubMed] [Google Scholar]
- 4. Lee Y, Park YS, Choi NY, Kim YI, Koh YG. Proteomic analysis reveals commonly secreted proteins of mesenchymal stem cells derived from bone marrow, adipose tissue, and synovial membrane to show potential for cartilage regeneration in knee osteoarthritis. Stem Cells Int. 2021;2021:6694299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di Matteo B, El Araby MM, D’Angelo A, Iacono F, Nannini A, Vitale ND, et al. Adipose-derived stem cell treatments and formulations. Clin Sports Med. 2019;38:61-78. [DOI] [PubMed] [Google Scholar]
- 6. Murray IR, Chahla J, Frank RM, Piuzzi NS, Mandelbaum BR, Dragoo JL, et al. Rogue stem cell clinics. Bone Joint J. 2020;102-B(2): 148-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hall M, McCafferty J, Agarwalla A, Nwachuku I, Liu JN, Amin NH. Safety and efficacy of cultured/noncultured mesenchymal stromal cells without concurrent surgery for knee osteoarthritis: a systematic review of randomized controlled trials. J Long Term Eff Med Implants. 2020;30(1): 31-47. [DOI] [PubMed] [Google Scholar]
- 8. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30(2): 160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15(9): 1061-9. [DOI] [PubMed] [Google Scholar]
- 10. Halpern B, Chaudhury S, Rodeo SA, Hayter C, Bogner E, Potter HG, et al. Clinical and MRI outcomes after platelet-rich plasma treatment for knee osteoarthritis. Clin J Sport Med. 2013;23(3): 238-9. [DOI] [PubMed] [Google Scholar]
- 11. Pomonis JD, Boulet JM, Gottshall SL, Phillips S, Sellers R, Bunton T, et al. Development and pharmacological characterization of a rat model of osteoarthritis pain. Pain. 2005;114(3): 339-46. [DOI] [PubMed] [Google Scholar]
- 12. Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17-23. [DOI] [PubMed] [Google Scholar]
- 13. Caplan AI. Medicinal signalling cells: they work, so use them. Nature. 2019;566(7742): 39. [DOI] [PubMed] [Google Scholar]
- 14. Tchetverikov I, Lohmander LS, Verzijl N, Huizinga TW, TeKoppele JM, Hanemaaijer R, et al. MMP protein and activity levels in synovial fluid from patients with joint injury, inflammatory arthritis, and osteoarthritis. Ann Rheum Dis. 2005;64(5): 694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lozito TP, Tuan RS. Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J Cell Physiol. 2011;226(2): 385-96. [DOI] [PubMed] [Google Scholar]
- 16. Lozito TP, Jackson WM, Nesti LJ, Tuan RS. Human mesenchymal stem cells generate a distinct pericellular zone of MMP activities via binding of MMPs and secretion of high levels of TIMPs. Matrix Biol. 2014;34:132-43. [DOI] [PubMed] [Google Scholar]
- 17. Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol. 2012;8:665-73. [DOI] [PubMed] [Google Scholar]
- 18. Felson DT, McLaughlin S, Goggins J, LaValley MP, Gale ME, Totterman S, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139:330-6. [DOI] [PubMed] [Google Scholar]