Abstract
Objective
Netrin-1 expression in articular cartilage is correlated with osteoarthritic changes. We aimed to investigate the contribution of Netrin-1 secreted by human osteoarthritic articular chondrocytes to angiogenesis process in vitro.
Design
Human articular chondrocytes were extracted from non-osteoarthritic (n = 10) and osteoarthritic (n = 22) joints obtained from surgical specimens and incubated for 24 hours. Medium conditioned by non-osteoarthritic and osteoarthritic articular chondrocytes were collected. Human umbilical vein endothelial cells (HUVEC) were treated with control and conditioned medium and assessed using assays for cell adherence, migration, and tube formation. Netrin-1 expression and secretion was compared between non-osteoarthritic and osteoarthritic chondrocytes by qPCR, Western blot, and ELISA. The role of chondrocyte-secreted Netrin-1 on HUVEC functions was assessed by immunological neutralization using an anti-Netrin-1 monoclonal antibody.
Results
As compared with medium conditioned by non-osteoarthritic chondrocytes, medium conditioned by osteoarthritic chondrocytes permitted tube formation by HUVEC. Both non-osteoarthritic and osteoarthritic chondrocytes expressed Netrin-1 at the RNA and protein levels. At the RNA level, Netrin-1 expression did not differ between non-osteoarthritic and osteoarthritic chondrocytes. At the protein level, Netrin-1 appeared as a full protein of 64 kDa in non-osteoarthritic chondrocytes and as two cleaved proteins of 55 kDa and 64 kDa in osteoarthritic chondrocytes. Immunological neutralization of endogenous Netrin-1 reduced the pro-angiogenic and pro-inflammatory transcriptional profile of HUVEC treated with the medium conditioned by osteoarthritic chondrocytes, as well as their capacities to form tubes.
Conclusions
Medium conditioned by osteoarthritic chondrocytes permits tube formation by HUVEC in vitro. This permissive effect is mediated by Netrin-1.
Keywords: osteoarthritis, articular cartilage, chondrocyte, angiogenesis, HUVEC, Netrin-1
Introduction
At adult age, normal articular cartilage displays no blood vessels and resists metastatic processes.1 This property is in part explained by the presence of anti-angiogenic factors and of protease inhibitors in the cartilage extracellular matrix.2-4 Articular cartilage vascularization can occur under pathological conditions such as osteoarthritis.5,6 In osteoarthritis, hypertrophic articular chondrocytes produce pro-angiogenic factors such as the fibroblast growth factor and the vascular endothelial growth factor A. The expression of these factors is greater in osteoarthritic than in normal cartilage.7,8 Under their influence, articular chondrocytes produce matrix metalloproteinases that contribute to cartilage degradation and vascular invasion. In mice, intra-articular injection of exogenous vascular endothelial growth factor in knee joint induces progressive cartilage degeneration.9
In recent years, axon guidance molecules such as Netrin-1 have been shown to be other key players in blood vessel guidance10 and to have a role in the regulation of arterial differentiation and vascular branching pattern. The truncated form of Netrin-1 (55 kDa) contributes to pathological vascularization throughout its receptor Unc5b.11 Netrin-1 has also been involved in inflammation12,13 and cancer progression.14-16 The exact role of Netrin-1 in joint disorders is unknown but has raised intense interest. In a model of aging cartilage in wild-type mice, our group showed a correlation of Netrin-1 expression in articular cartilage with OARSI score that reflects osteoarthritic changes.17 In a murine model of temporomandibular joint osteoarthritis, Netrin-1 was involved in subchondral bone changes.18 Further evidence suggested that Netrin-1 secreted by osteoclasts during subchondral bone remodeling was involved in inducing sensory innervation in osteoarthritis pain19 and in spinal pain.20 Netrin-1 also seems involved in inflammatory joint disorders. In the murine model of K/BxN serum transfer-induced arthritis, blockade of Netrin-1/Unc5b by monoclonal antibodies prevented bone destruction and reduced arthritis severity through a decrease in osteoclast number and activity.21
We hypothesized that changes in the expression of Netrin-1 in articular cartilage and its secretion could occur in osteoarthritis, and may promote functional changes in surrounding endothelial cells.
Method
Chondrocyte Isolation from Human Articular Cartilage
Human joint tissues were obtained from surgical specimens at the orthopedic department of Cochin Hospital (Paris, France). Cartilage samples were collected from osteoarthritic patients after total knee replacement and from non-osteoarthritic patients after tumoral surgery. Human articular chondrocytes were isolated by enzymatic digestion as described.22 They were seeded at high density (100,000 cells/cm2) and incubated in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum (Lonza) for 24 hours. The number of independent cell culture isolated from articular chondrocytes ranged from 3 to 6.
Human Umbilical Vein Endothelial Cells (HUVEC) Culture
HUVEC were obtained from Sigma Aldrich. HUVEC were cultured in endothelial cell growth medium supplemented with 10% fetal bovine serum (Lonza). HUVEC were used for specific assays between passages 2 and 6.
Medium Conditioning
Medium conditioned by human non-osteoarthritic or osteoarthritic articular chondrocytes were collected after 24 hours, divided into two samples and stored at –80°C. One sample was used for Netrin-1 quantification by enzyme-linked immunosorbent assay. The other sample was diluted v/v with serum-free endothelial cell growth medium and used to treat HUVEC. Nonconditioned medium served as a control medium and was a v/v mixture of complete Dulbecco’s Modified Eagle Medium and serum-free endothelial cell growth medium.
HUVEC Adherence and Viability Assays
Overall, 10,000 HUVEC per 16-well e-view plates were seeded in xCELLigence RTCxA system (Roche).23,24 HUVEC were treated in duplicate with 0.2 ml of the control medium or medium conditioned by human non-osteoarthritic or osteoarthritic articular chondrocytes. HUVEC proliferation and attachment were quantified in real time by monitoring electrical impedance. The cell index value reflecting HUVEC adherence was calculated after 5 hours of treatment using the RTCA software (Roche). HUVEC viability was quantified after 24 hours using trypan blue staining.
HUVEC Migration Assay (Wound-Healing Assay)
Overall, 40,000 HUVEC were seeded in a 2-well culture-insert (Ibidi system) and cultured in endothelial cell growth medium supplemented with 10% fetal bovine serum (Lonza) for 24 hours. After HUVEC attachment, the medium and culture-insert were removed, creating a cell-free gap in which the cell migration could be visualized in real time. HUVEC were treated in triplicate with 2 ml of the control medium or medium conditioned by human non-osteoarthritic or osteoarthritic articular chondrocytes and were filmed continuously (4 images/h) for 24 hours under videomicroscopy (Zeiss, objective at phase contrast, 10x magnification). The cell-free gap width was quantified every 2 hours using Zen imaging software.
HUVEC Tube Formation Assay
Overall, 40,000 HUVEC per 48-well were seeded on Matrigel coated plates (Matrigel no. 354234 ECM Gel Sigma). Gel was thawed overnight at 2° to 8°C before use and dispensed to a multiwall plate using a plate and pipettes that are pre-cooled to 2° to 8°C. The gel was diluted up to 2-fold with 2° to 8°C Dulbecco’s Modified Eagle Medium, and it was done before gel was added to the plate. The product gelled within 5 minutes at 20°C. Cells were plated on top of a thin gel layer of 0.5 mm or cultured inside a 1 mm layer. To dissociate cells from the gel, ethylenediaminetetraacetic acid (EDTA) was used at a concentration of 0.6 to 2.4 units per mL. HUVEC were treated in triplicate with 0.5 ml of the medium conditioned by human non-osteoarthritic or osteoarthritic articular chondrocytes and once with 0.5 ml of the control medium. HUVEC were filmed continuously (4 images/h) for 24 hours under videomicroscopy (Zeiss, objective at phase contrast, 10x magnification). Number of nodules and branches and tube length were semi-quantified at baseline, 3, 6, 9, 12, and 24 hours using the “angiogenesis analyzer” extension of ImageJ software. To assess the role of Netrin-1 and of vascular endothelial growth factor A in tube formation, HUVEC treated with the medium conditioned by osteoarthritic human articular chondrocytes were also treated in triplicate with 2 µg/ml 2F5 (Netrin-1 blocking monoclonal antibody, Adipogen) or 2.5 mg/ml bevacizumab (vascular endothelial growth factor A blocking monoclonal antibody, Roche).
Quantitative Polymerase Chain Reaction
Total RNA was extracted from human non-osteoarthritic and osteoarthritic articular chondrocytes and HUVEC using Trizol reagent (Invitrogen). Overall, 1 μg of total RNA was reverse-transcribed using the high capacity cDNA reverse transcription kit (Applied Biosystems). Levels of mRNA were normalized to those of the ribosomal protein L13. Primers used in the present study for quantitative polymerase chain reaction are listed in the appendix.
Western Blot
Proteins were extracted from human non-osteoarthritic and osteoarthritic articular chondrocytes using protein lysis buffer (Hepes 20 mM pH 7.6, NaCl 25 mM, EDTA 1 mM, 0.2% NP40, Glycerol 10%, DTT 1 mM and complete, protease Inhibitor cocktail Tablet 1x). Overall, 20 µg of proteins were size-separated by sodium dodecyl sulfate SDS-page 4% to 12% polyacrylamide gel electrophoresis. Gels were electro blotted on nitrocellulose membranes and incubated overnight at 4°C with anti-Netrin-1 (Abcam, ab122903; 1/500) and anti-actin (Santa Cruz, sc-69879; 1/5000). Blots were incubated for 1 hour at room temperature with a horseradish peroxidase conjugated secondary antibody anti-goat (Santa Cruz, sc2020; 1/10000) and anti-mice (Santa Cruz, sc2031; 1/5000), respectively. Bound Netrin-1 antibodies were visualized using Fusion Solo S imaging system (Vilber). Results are standardized by actin by quantifying the bands with ImageJ software.
Netrin-1 Enzyme-Linked Immunosorbent Assay
Netrin-1 secreted by human non-osteoarthritic and osteoarthritic articular chondrocytes in control medium was quantified using the human Netrin-1 Elisa kit (EK3998, Signalway antibody LLC), according to supplier’s instructions. This kit recognizes the N-terminal end of Netrin-1.
Statistical Analysis
Quantitative variables were expressed with mean (SD) of duplicates or triplicates and were compared with nonparametric Mann–Whitney U test. All analyses involved using GraphPad Prism 7.03 (GraphPad Software Inc., San Diego, CA, USA). A P value < 0.05 was considered statistically significant.
Role of the Funding Source and Ethical Consideration
Our work was funded by the Research on OsteoArthritis Diseases Network funded by the ARTHRITIS Foundation, by the Agence Universitaire de la Francophonie and by the CNRS-L. The funding sources were not involved in the study design, collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication. The study protocol was approved by the CPP Sud-Ouest et Outre-Mer 1 (No. ID-RCB: 2018-A02987-48). All participants were informed and gave written consent for the use of cartilage specimens.
Patient and Public Involvement Statement
It was not appropriate or possible to involve patients or the public in the design, or conduct, or reporting, or dissemination plans of our research.
Results
Participants
Cartilage specimens from 32 patients who underwent surgery at the orthopedic department of Cochin Hospital (Paris, France) were used: 10 patients were non-osteoarthritic (tumoral surgery), and 22 patients were osteoarthritic (total knee replacement). Mean age was 40.2 years (SD = 17.0 years) in the non-osteoarthritic group and 73.1 years (SD = 9.2 years) in the osteoarthritic group. Mean body mass index was 30.9 kg/m2 (SD = 5.3 kg/m2) in the non-osteoarthritic group and 28.9 kg/m2 (SD = 6.8 kg/m2) in the osteoarthritic group. Overall, 16/32 (50%) participants were females: 3/10 (30%) in the non-osteoarthritic group and 10/22 (46%) in the osteoarthritic group.
Medium Conditioned by Human Non-Osteoarthritic and Osteoarthritic Articular Chondrocytes Have Similar Effects on HUVEC Adherence, Viability, and Migration in 2D Cultures
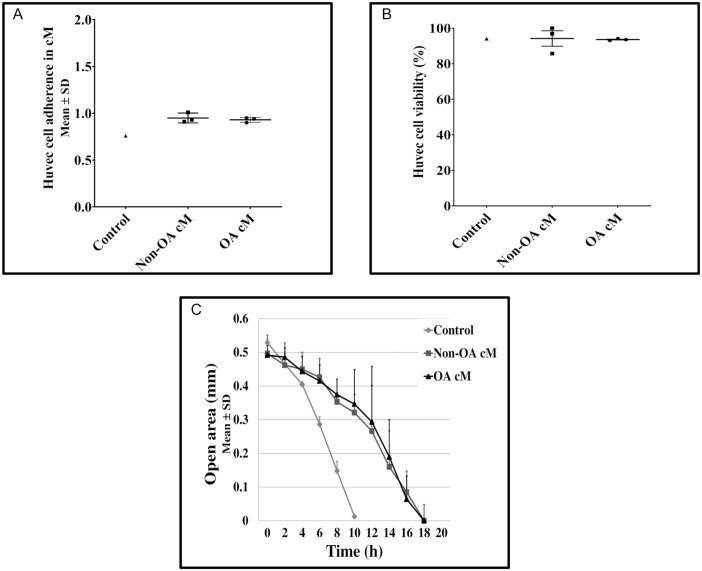
We compared the potential effects of the medium conditioned by human non-osteoarthritic and osteoarthritic articular chondrocytes on adherence, viability, and migration of HUVEC cultured in 2D on plastic flasks. We found no difference in adhesion level of HUVEC treated with the medium conditioned by human non-osteoarthritic or osteoarthritic articular chondrocytes for 24 hours (Fig. 1A). HUVEC viability was greater than 90% in control medium and medium conditioned by human non-osteoarthritic and osteoarthritic articular chondrocytes (Fig. 1B). Finally, we found that migration of HUVEC treated with the medium conditioned by human non-osteoarthritic or osteoarthritic articular chondrocytes was slower as compared with HUVEC treated with the control medium (26.9 [1.1] μm/h and 22.3 [5.3] μm/h vs. 31.7 [3.7] μm/h, respectively). However, we found no difference between HUVEC treated with the medium conditioned by human non-osteoarthritic and osteoarthritic articular chondrocytes on the time necessary to complete wound healing (Fig. 1C).
Figure 1.
Effects of medium conditioned by human non-osteoarthritic and osteoarthritic articular chondrocytes on HUVEC adherence, viability, and migration in 2D cultures. HUVEC adherence and viability were assessed using real-time impedance analysis (xCELLigence RTCA software system). Briefly, 10,000 HUVEC per well were seeded in duplicate in 16-well e-plates and treated with control medium (n = 1), medium conditioned by human non-osteoarthritic articular chondrocytes (n = 3), or medium conditioned by human osteoarthritic articular chondrocytes (n = 3). (A) The cell index, which reflects cell adherence, was assessed by continuous monitoring. Results observed after 5-hour incubation (steady state) were expressed as mean (SD) number of adherent HUVEC. Results between two conditions were compared using the nonparametric Mann–Whitney U test. All between-group comparisons yielded nonsignificant results. (B) HUVEC viability was assessed by counting the number of positive cells for trypan blue reported to the total number of HUVEC. Results observed after 24 hours were expressed as the percentage of viable HUVEC. No between-group comparisons were performed. (C) The time necessary to complete wound healing was assessed. Results were expressed as the mean (SD) cell-free gap width (mm) for each time point until wound healing was completed. No between-group comparisons were performed. HUVEC = human umbilical vein endothelial cells; cM = conditioned medium; OA = osteoarthritis.
Medium Conditioned by Human Osteoarthritic Articular Chondrocytes Permits HUVEC Tube Formation in 3D Cultures
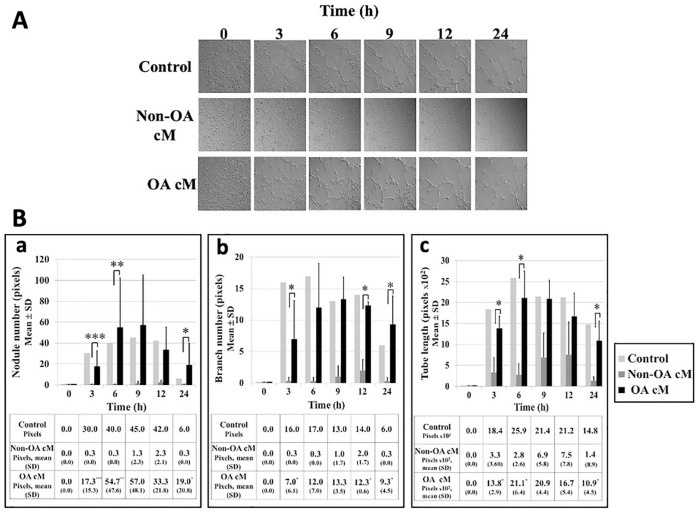
We compared the potential effects of the medium conditioned by human non-osteoarthritic and osteoarthritic articular chondrocytes on tube formation by HUVEC cultured in 3D on Matrigel (Sigma Aldrich) for 24 hours. When HUVEC were treated with the control medium, HUVEC tube formation occurred after 3 hours, increased to a maximum between 6 and 12 hours and decreased at 24 hours (Fig. 2A). When HUVEC were treated with the medium conditioned by human non-osteoarthritic articular chondrocytes, HUVEC tube formation did not occur. Activated HUVEC spheroid sprouting in Matrigel was inhibited and HUVEC remained dispersed. Conversely, when HUVEC were treated with the medium conditioned by human osteoarthritic articular chondrocytes, HUVEC tube formation occurred following the same pattern as HUVEC treated with the control medium. At each time point assessed, number of nodules (Fig. 2Ba) and branches (Fig. 2Bb) and tube length (Fig. 2Bc) were similar in HUVEC treated with the control medium or the medium conditioned by human osteoarthritic articular chondrocytes.
Figure 2.
Effects of medium conditioned by human non-osteoarthritic and osteoarthritic articular chondrocytes on HUVEC tube formation in 3D cultures. Briefly, 40,000 HUVEC were seeded on Matrigel (Sigma Aldrich) and treated with control medium (n = 1), medium conditioned by human non-osteoarthritic articular chondrocytes (n = 3), or medium conditioned by human osteoarthritic articular chondrocytes (n = 3) and observed in real time using videomicroscopy (Zeiss, objective at phase contrast, 10x magnification). (A) Images were acquired at baseline, 3, 6, 9, 12, and 24 hours. (B) (a) Number of nodules, (b) number of branches, and (c) tube length were semi-quantified at each time point using the “angiogenesis analyzer” extension of ImageJ software. Results were expressed as mean (SD) pixels. For each variable assessed, results between two conditions were compared using the nonparametric Mann–Whitney U test with *P < 0.05, **P < 0.02, and ***P < 0.007. HUVEC = human umbilical vein endothelial cells; cM = conditioned medium; OA = osteoarthritis.
Netrin-1 Is Differently Expressed and Secreted by Human Non-Osteoarthritic and Osteoarthritic Articular Chondrocytes
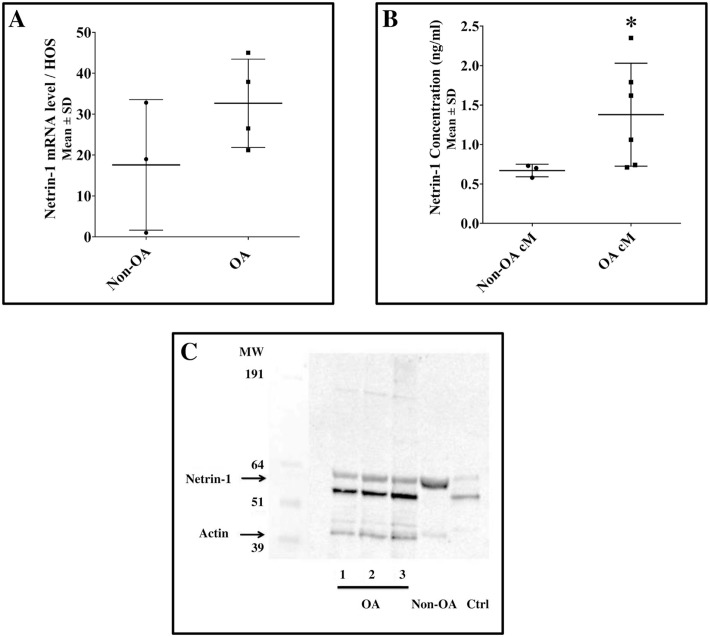
We found that both human non-osteoarthritic and osteoarthritic articular chondrocytes expressed Netrin-1 at the RNA and protein levels. At the RNA level, Netrin-1 expression did not differ between human non-osteoarthritic and osteoarthritic articular chondrocytes (Fig. 3A). At the protein level, levels of Netrin-1 secreted in the medium conditioned by human osteoarthritic articular chondrocytes were greater than in the medium conditioned by human non-osteoarthritic articular chondrocytes (1.3 [0.8] ng/ml vs. 0.7 [0.1] ng/ml, respectively; Fig. 3B). To compare the molecular forms of Netrin-1 expressed by human non-osteoarthritic and osteoarthritic articular chondrocytes, total proteins were extracted from chondrocytes and analyzed by Western blot. We found that Netrin-1 was expressed as a full protein of 64 kDa in human non-osteoarthritic articular chondrocytes and as a cleaved protein of 55 kDa and 64 kDa in human osteoarthritic articular chondrocytes, with a predominant band at 55 kDa, like in the positive control (human brain lysates; Fig. 3C).
Figure 3.
Netrine-1 expression in human articular non-osteoarthritic and osteoarthritic chondrocytes and Netrin-1 secretion in conditioned media. Human non-osteoarthritic and osteoarthritic chondrocytes articular chondrocytes were isolated by enzymatic digestion. They were seeded at high density (100,000 cells/cm2) and incubated in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum (Lonza) for 24 hours. (A) Netrin-1 mRNA was quantified by quantitative polymerase chain reaction in human articular non-osteoarthritic (n = 3) and osteoarthritic chondrocyte (n = 4). Briefly, total RNA was extracted from chondrocyte cell pellets. RNA was reverse-transcribed twice, and quantitative polymerase chain reaction was performed in triplicate on each reverse transcription. mRNA levels were standardized to those of human osteosarcoma (HOS) cell line as internal control. Results were expressed as mean (SD) fold-change of the internal control. Results between two conditions were compared using the nonparametric Mann–Whitney U test. All between-group comparisons yielded nonsignificant results. (B) Netrin-1 secreted by human articular non-osteoarthritic (n = 3) and osteoarthritic chondrocyte (n = 6) in the conditioned media was quantified by enzyme-linked immunosorbent assay (Signalway antibody LLC). According to the manufacturer, antibodies used recognize the N-terminal part of Netrin-1, which includes the 55-kDa V-VI fragment. Results were expressed as mean (SD) ng/ml. Results between two conditions were compared using the nonparametric Mann–Whitney U test with P < 0.05. (C) Molecular forms of Netrin-1 expressed by human non-osteoarthritic and osteoarthritic articular chondrocytes were assessed by Western blot. Total proteins (intra- and extracellular proteins attached to the cell membrane) from human non-osteoarthritic (n = 1) and osteoarthritic articular chondrocytes (n = 3) were extracted and separated on nitrocellulose membrane. Anti-Netrin-1 (Abcam, ab122903; 2/1000) was used as primary antibody, and anti-Actin (Santa Cruz, sc-69879; 1/5000) antibodies were used as internal control (42 kDa). According to the manufacturer, antibodies used recognize the C-terminal part of Netrin-1. Human brain lysates served as a positive control for Netrin-1. No between-group comparisons were performed. HUVEC = human umbilical vein endothelial cells; cM = conditioned medium; OA = osteoarthritis.
Immunological Neutralization of Netrin-1 Reduces the Permissive Effects of the Medium Conditioned by Human Osteoarthritic Articular Chondrocyte on HUVEC Tube Formation in 3D Cultures
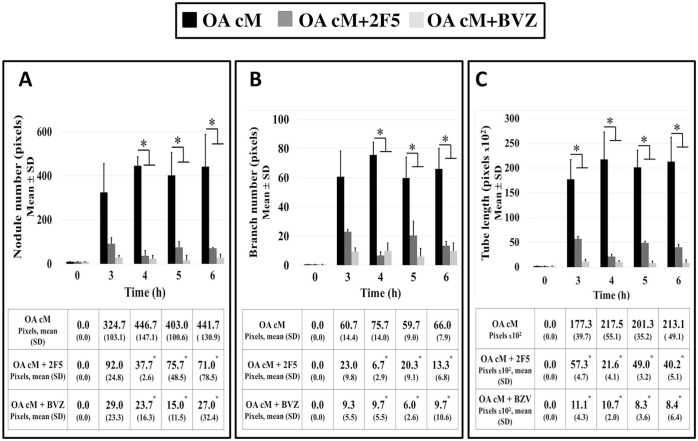
To assess whether Netrin-1 secreted in the medium conditioned by human osteoarthritic articular chondrocyte could promote HUVEC tube formation and to compare these effects with those of vascular endothelial growth factor A, HUVEC treated with the medium conditioned by osteoarthritic human articular chondrocytes were also treated with Netrin-1 or vascular endothelial growth factor A blocking monoclonal antibodies (Fig. 4). After 6 hours, immunological neutralization of Netrin-1 reduced HUVEC tube formation as reflected by a reduction in the number of nodules (Fig. 4A) and branches (Fig. 4B) and tube length (Fig. 4C), as compared with untreated HUVEC. These effects were of the same magnitude as those observed with immunological neutralization of vascular endothelial growth factor A.
Figure 4.
Effects of immunological neutralization of Netrin-1 and vascular endothelial growth factor A on HUVEC tube formation in 3D cultures. HUVEC treated with the medium conditioned by osteoarthritic human articular chondrocytes were also treated with 2 µg/ml 2F5 (Netrin-1 blocking monoclonal antibody, Adipogen, n = 3) or 2.5 mg/ml bevacizumab (vascular endothelial growth factor A blocking monoclonal antibody, Roche, n = 3) for 6 hours and observed in real time. HUVEC treated only with the medium conditioned by osteoarthritic human articular chondrocytes served as controls (n = 3). (A) Number of nodules, (B) number of branches, and (C) tube length were semi-quantified at each time point using the “angiogenesis analyzer” extension of ImageJ software. Results were expressed as mean (SD) pixels. For each variable assessed, results between two conditions were compared using the nonparametric Mann–Whitney U test with *P < 0.05, **P < 0.02, and ***P < 0.007. HUVEC = human umbilical vein endothelial cells; BVZ = bevacizumab; cM = conditioned medium; OA = osteoarthritis.
Immunological Neutralization of Netrin-1 Reduces the Pro-Angiogenic and Pro-Inflammatory Transcriptional Profile of HUVEC
We examined whether Netrin-1 could promote pro-angiogenic and pro-inflammatory transcriptional profile of HUVEC. In HUVEC treated with the medium conditioned by human osteoarthritic articular chondrocytes, we found that immunological neutralization of Netrin-1 using blocking monoclonal antibodies reduced mRNA expression of pro-angiogenic markers including vascular endothelial growth factor A, vascular endothelial growth factor receptor 2, fibroblast growth factor 1 and fibroblast growth factor receptor 1, as well as of pro-inflammatory markers including peroxisome proliferator-activated receptor γ, cyclooxygenase 2, and nuclear factor-κB (Table 1).
Table 1.
Pro-Angiogenic and Pro-Inflammatory Transcriptional Profile of Human Umbilical Vein Endothelial Cells.
| HUVEC treated with OA cM | HUVEC treated with OA cM + 2F5 | |
|---|---|---|
| Cox-2 | 12.2 (11.0) | 0.3 (0.2) |
| FGF-1 | 3.2 (2.6) | 1.1 (0.7) |
| FGFR-1 | 0.9 (0.4) | 0.8 (0.4) |
| NF-κβ | 1.7 (0.4) | 0.3 (0.0) |
| PPAR-α | 2.0 (0.3) | 0.8 (0.2) |
| PPAR-γ | 2.2 (1.0) | 0.5 (0.2) |
| VEGF-A | 1.6 (0.2) | 0.5 (0.1) |
| VEFG-R2 | 1.3 (0.5) | 0.5 (0.2) |
Levels of mRNA were normalized to those of the ribosomal protein L13. All results were expressed as mean (SD) fold-change of the internal control. HUVEC = human umbilical vein endothelial cells; OA = osteoarthritis; cM = conditioned medium; Cox-2 = cyclooxygenase 2; FGF-1 = fibroblast growth factor 1; FGFR-1 = fibroblast growth factor receptor 1; NF-κβ = nuclear factor-κB; PPAR = peroxisome proliferator-activated receptor; VEGF-A = vascular endothelial growth factor A; VEGF-R2 = vascular endothelial growth factor receptor 2.
Discussion
In the present study, we found that medium conditioned by human osteoarthritic articular chondrocytes permitted tube formation by HUVEC as compared with medium conditioned by human non-osteoarthritic articular chondrocytes. Both human non-osteoarthritic and osteoarthritic articular chondrocytes expressed Netrin-1 at the RNA and protein levels. At the protein level, Netrin-1 appeared as a full protein of 64 kDa in human non-osteoarthritic articular chondrocytes, but as a cleaved protein of 55 kDa and 64 kDa in human osteoarthritic articular chondrocytes. Immunological neutralization of Netrin-1 reduced the pro-angiogenic and pro-inflammatory transcriptional profile of HUVEC treated with the medium conditioned by human osteoarthritic articular chondrocytes, and their capacities to form tubes.
We first compared the effects of control and conditioned media on HUVEC core functions including adherence, migration, and tube formation. Pesesse and colleagues previously reported increased HUVEC adhesion when treated with a medium conditioned by hypertrophic chondrocytes.25 Medium conditioned by dental pulp stromal cells and osteoblasts26 have also been shown to modulate angiogenic properties of endothelial cells including adhesion and migration. Interestingly, we found differences between medium conditioned by non-osteoarthritic and osteoarthritic chondrocytes, but not on HUVEC adherence and migration. Our results suggest that osteoarthritic changes may predominantly impact tube formation functions over other endothelial functions. Our results are consistent with those of Camaj and colleagues, who reported that HUVEC germination could be inhibited when HUVEC were treated with medium conditioned by human primary chondrocytes.
Remarkably, we found that HUVEC could form tubes with control and osteoarthritic human articular chondrocyte-conditioned media, but not with non-osteoarthritic human articular chondrocyte-conditioned medium. Several hypotheses may explain our results. First, non-osteoarthritic human articular chondrocytes may produce specific matrix components able to inhibit HUVEC tube formation. In the field of bioengineering, Choi and colleagues found that chondrocyte-derived extracellular matrix could inhibit blood vessel invasion in vitro and in vivo as compared with Matrigel.27 Consistently, articular cartilage has been shown to contain intrinsic angiogenesis inhibitors such as chondromodulin-I, endostatin, and thrombospondin-1.28 These inhibitors could have played a role in our model; in the control and osteoarthritic human articular chondrocyte-conditioned media, the absence of these inhibitors could have allowed tube formation by HUVEC on Matrigel. Second, osteoarthritic human articular chondrocyte-conditioned medium could also contain pro-angiogenic molecules. A relevant candidate was Netrin-1 because Netrin-1 is involved in axon guidance and angiogenesis,10 and its expression in articular cartilage is correlated with OARSI score in a model of aging cartilage in wild-type mice.17
To identify molecules involved in the effects observed on HUVEC, we used a candidate approach and chose to explore the role of a chemotactic guiding factor Netrin-1. We found expression of Netrin-1 in both human non-osteoarthritic and osteoarthritic articular cartilage at the mRNA and protein levels. We also found higher levels of secreted Netrin-1 in medium conditioned by osteoarthritic chondrocytes than non-osteoarthritic chondrocytes. Interestingly, osteoarthritic chondrocytes mainly expressed Netrin-1 in its cleaved form and non-osteoarthritic human chondrocytes in its full form. Recent evidence has suggested specific biological effects for cleaved forms of Netrin-1. In a model of diabetic retinopathy, Miloudi and colleagues have shown that Netrin-1 can be metabolized into a bioactive fragment corresponding to the amino-terminal domains VI and V that promote vascular permeability by binding to Unc5b receptor.11 In our study, the fragment at 55 kDa mainly expressed by osteoarthritic chondrocytes could correspond to this bioactive form described by Miloudi and colleagues. The cleavage mechanisms of Netrin-1 in osteoarthritis remain to be elucidated but may involve activated matrix metalloproteinases.
Finally, we showed that immunological neutralization of Netrin-1 decreased HUVEC tube formation induced by osteoarthritic conditioned medium, and the expression of some pro-angiogenic and pro-inflammatory markers. Our results suggest that in our model Netrin-1 could promote angiogenic processes, directly by regulating the expression of pro-angiogenic factors, and indirectly by regulating the expression of pro-inflammatory factors, according to mechanisms that remain to be elucidated. Angiogenesis and inflammation are closely related in osteoarthritis. Several studies have demonstrated the angiogenic role of Netrin-1 in vitro.29-33 Prieto and colleagues reported that the medium conditioned by Wharton’s jelly mesenchymal stem cells promote angiogenesis and that the neutralization of Netrin-1 by 2F5 inhibited the HUVEC angiogenic, like in our model.34 In in vivo inflammatory models, Netrin-1 could promote inflammation.12 However, some authors reported anti-inflammatory of Netrin-1 in vitro.35 These apparent inconsistencies may be in part explained by differential bioactivity of Netrin-1 in its cleaved and full forms.
Our study has limitations. Our approach could be enriched by an unbiased analysis of the secretomes of non-osteoarthritic and osteoarthritic articular chondrocytes. Netrin-1 concentration could be elaborated by immunoprecipitation assay. The immunological neutralization approach could be complemented by Netrin-1 loss and gain of function, using genomic editing tools, to confirm the specificity of the effects we observed. Mechanisms leading to Netrin-1 cleavage need to be further explored as well as the bioactivity of the fragments observed and their interactions with targeted receptors. Target strategies of MMP-9 to inhibit Netrin-1 cleavage may be relevant. Netrin-1 functions should be confirmed in in vivo models of osteoarthritis. Because Netrin-1 is an axonal guidance molecule, it would be relevant to carry out further studies to assess whether Netrin-1 has a role in osteoarthritic pain. Finally, extending the study of the relation between Netrin-1 and VEGF would be of interest. Netrine-1 may act upstream of VEGF.32 In vivo, Netrin-1 stimulates angiogenesis and augments the response to VEGF.10 Other data show that Netrin-1 and VEGF act independently on angiogenesis.34
In summary, Netrin-1 secreted by human osteoarthritic articular chondrocytes contributes to angiogenesis in vitro. This effect may be promoted by a switch to a pro-angiogenic and pro-inflammatory phenotype of endothelial cells. Altogether, our in vitro results suggest that Netrin-1 may play a role in abnormal vascularization in osteoarthritic cartilage.
Appendix
Primers Used for Quantitative Polymerase Chain Reaction.
| Gene | Lower | Upper |
|---|---|---|
| Cox-2 | TAG TCC TGT ATG CCC TTT TCC | AGA TTA GTC CGC CGT AGT CG |
| FGF-1 | TCA AAG GAG TGT GTG CTA AC | TTT CAG TGC CAC ATA CCA AC |
| FGFR-1 | GTC TGC TGA CTC CAG TGC AT | ACG GTT GGG TTT GTC CTT GT |
| Netrin-1 | GTGGATCTGGACGGCATAGT | GTGGAGGAGCCTGAAGACTG |
| NF-κβ | TTC CTT ACC CCG TTT TCC | TCA GCA CAC ACC CAA TG |
| PPAR-α | TCA TCA CGG ACA CGC TTT CA | CGC GTG GAC TCC GTA ATG AT |
| PPAR-γ | GCA ATC AAA GTG GAG CCT GC | TCT CCG GAA GAA ACC CTT GC |
| RPL13 | AAG GTC GTG CGT CTG AAG | GAG TCC GTG GGT CTT GAG |
| VEGF-A | CCT GGT GGA CAT CTT CCA GGA GTA | CTC ACC GCC TCG GCT TGT CAC A |
| VEFG-R2 | CAG ACA CCA CCG TGT ACT CC | CTG CAG TCC GAG GTC CTT TT |
Cox-2 = cyclooxygenase 2; FGF-1 = fibroblast growth factor 1; FGFR-1 = fibroblast growth factor receptor 1; NF-κβ = nuclear factor-κB; PPAR = peroxisome proliferator-activated receptor; RPL13 = Ribosomal Protein L13; VEGF-A = vascular endothelial growth factor A; VEGF-R2 = vascular endothelial growth factor receptor 2.
Footnotes
Author Contributions: Conception and design of the study: J.A., M-T.C., K.T., F.R., C.N.
Drafting of the original protocol: CN.
Obtaining of funding: J.A., C.N.
Coordination of the study: C.N.
Acquisition of data: J.A., M-T.C., K.T., P.A., D.B., D.B., F.É.
Design of the statistical analysis plan: C.N.
Analysis and interpretation of the data: J.A., M-T.C., K.T., D.B., F.R., C.N.
Drafting of the present manuscript: J.A., M-T.C., F.R., C.N.
Reviewing and providing comments on manuscript: K.T., P.A., D.B., D.B., F.É.
Final approval: J.A., M-T.C., K.T., P.A., D.B., D.B., F.É., F.R., C.N.
The last author (christelle.nguyen2@aphp.fr) takes responsibility for the integrity of the work as a whole, from inception to finished article.
Acknowledgments and Funding: The authors thank Associate Professor Olivier Biondi, Mrs. Delphine Sapaly, and Dr. Céline Tomkiewicz-Raulet for assistance with the xCELLigence RTCxA system and the videomicroscope and staff members of the BioMedTech Facilities (INSERM US36—CNRS UMS2009—Université Paris Cité, Paris, France). The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Research on OsteoArthritis Diseases Network funded by the ARTHRITIS Foundation, by the Agence Universitaire de la Francophonie and by the CNRS-L. The funding sources were not involved in the study design, collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study protocol was approved by the Comité de Protection des Personnes Sud-Ouest et Outre-Mer 1 (No ID-RCB: 2018-A02987-48).
ORCID iD: Christelle Nguyen  https://orcid.org/0000-0001-7141-3230
https://orcid.org/0000-0001-7141-3230
Data Statement: The full data set can be accessed by academic researchers by contacting professor Christelle Nguyen (christelle.nguyen2@aphp.fr).
References
- 1. Moses MA, Sudhalter J, Langer R. Identification of an inhibitor of neovascularization from cartilage. Science. 1990;248:1408-10. doi: 10.1126/science.1694043. [DOI] [PubMed] [Google Scholar]
- 2. Hiraki Y, Shukunami C. Chondromodulin-I as a novel cartilage-specific growth-modulating factor. Pediatr Nephrol. 2000;14(7):602-5. doi: 10.1007/s004670000339. [DOI] [PubMed] [Google Scholar]
- 3. Pfander D, Cramer T, Deuerling D, Weseloh G, Swoboda B. Expression of thrombospondin-1 and its receptor CD36 in human osteoarthritic cartilage. Ann Rheum Dis. 2000;59(6):448-54. doi: 10.1136/ard.59.6.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Troeberg L, Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta. 2012;1824(1):133-45. doi: 10.1016/j.bbapap.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ashraf S, Walsh DA. Angiogenesis in osteoarthritis. Curr Opin Rheumatol. 2008;20:573-80. doi: 10.1097/BOR.0b013e3283103d12. [DOI] [PubMed] [Google Scholar]
- 6. Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8:390-8. doi: 10.1038/nrrheum.2012.80. [DOI] [PubMed] [Google Scholar]
- 7. Pufe T, Petersen W, Tillmann B, Mentlein R. The splice variants VEGF121 and VEGF189 of the angiogenic peptide vascular endothelial growth factor are expressed in osteoarthritic cartilage. Arthritis Rheum. 2001;44(5):1082-8. doi:. [DOI] [PubMed] [Google Scholar]
- 8. Pfander D, Kortje D, Zimmermann R, Weseloh G, Kirsch T, Gesslein M, et al. Vascular endothelial growth factor in articular cartilage of healthy and osteoarthritic human knee joints. Ann Rheum Dis. 2001;60(11):1070-3. doi: 10.1136/ard.60.11.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shen P, Jiao Z, Zheng JS, Xu WF, Zhang SY, Qin A, et al. Injecting vascular endothelial growth factor into the temporomandibular joint induces osteoarthritis in mice. Sci Rep. 2015;5:16244. doi: 10.1038/srep16244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park KW, Crouse D, Lee M, Karnik SK, Sorensen LK, Murphy KJ, et al. The axonal attractant Netrin-1 is an angiogenic factor. Proc Natl Acad Sci U S A. 2004;101:16210-5. doi: 10.1073/pnas.0405984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miloudi K, Binet F, Wilson A, Cerani A, Oubaha M, Menard C, et al. Truncated netrin-1 contributes to pathological vascular permeability in diabetic retinopathy. J Clin Invest. 2016;126:3006-22. doi: 10.1172/JCI84767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mediero A, Ramkhelawon B, Wilder T, Purdue PE, Goldring SR, Dewan MZ, et al. Netrin-1 is highly expressed and required in inflammatory infiltrates in wear particle-induced osteolysis. Ann Rheum Dis. 2016;75(9):1706-13. doi: 10.1136/annrheumdis-2015-207593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ranganathan P, Mohamed R, Jayakumar C, Ramesh G. Guidance cue netrin-1 and the regulation of inflammation in acute and chronic kidney disease. Mediators Inflamm. 2014;2014:525891. doi: 10.1155/2014/525891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yin K, Wang L, Zhang X, He Z, Xia Y, Xu J, et al. Netrin-1 promotes gastric cancer cell proliferation and invasion via the receptor neogenin through PI3K/AKT signaling pathway. Oncotarget. 2017;8:51177-89. doi: 10.18632/oncotarget.17750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harter PN, Zinke J, Scholz A, Tichy J, Zachskorn C, Kvasnicka HM, et al. Netrin-1 expression is an independent prognostic factor for poor patient survival in brain metastases. PLoS ONE. 2014;9(3):e92311. doi: 10.1371/journal.pone.0092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kefeli U, Ucuncu Kefeli A, Cabuk D, Isik U, Sonkaya A, Acikgoz O, et al. Netrin-1 in cancer: potential biomarker and therapeutic target. Tumour Biol. 2017;39(4):1010428317698388. doi: 10.1177/1010428317698388. [DOI] [PubMed] [Google Scholar]
- 17. Akoum J, Tahiri K, Corvol MT, Borderie D, Étienne F, Rannou F, et al. Aging cartilage in wild-type mice: an observational study. Cartilage. 2020; 13:1407S-1411S. doi: 10.1177/1947603520926713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xiao M, Hu Z, Jiang H, Li C, Guo H, Fang W, et al. The expression of Netrin-1 in the MIA-induced osteoarthritic temporomandibular joint in mice. Sci Rep. 2021;11:15695. doi: 10.1038/s41598-021-95251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu S, Zhu J, Zhen G, Hu Y, An S, Li Y, et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J Clin Invest. 2019;129:1076-93. doi: 10.1172/jci121561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ni S, Ling Z, Wang X, Cao Y, Wu T, Deng R, et al. Sensory innervation in porous endplates by Netrin-1 from osteoclasts mediates PGE2-induced spinal hypersensitivity in mice. Nat Commun. 2019;10:5643. doi: 10.1038/s41467-019-13476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mediero A, Wilder T, Ramkhelawon B, Moore KJ, Cronstein BN. Netrin-1 and its receptor Unc5b are novel targets for the treatment of inflammatory arthritis. FASEB J. 2016;30(11):3835-44. doi: 10.1096/fj.201600615R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Montembault A, Tahiri K, Korwin-Zmijowska C, Chevalier X, Corvol MT, Domard A. A material decoy of biological media based on chitosan physical hydrogels: application to cartilage tissue engineering. Biochimie. 2006;88(5):551-64. [DOI] [PubMed] [Google Scholar]
- 23. Hamidi H, Lilja J, Ivaska J. Using xCELLigence RTCA instrument to measure cell adhesion. Bio Protoc. 2017;7:24. doi: 10.21769/BioProtoc.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bernardo L, Corallo L, Caterini J, Su J, Gisonni-Lex L, Gajewska B. Application of xCELLigence real-time cell analysis to the microplate assay for pertussis toxin induced clustering in CHO cells. PLoS ONE. 2021;16(3):e0248491. doi: 10.1371/journal.pone.0248491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pesesse L, Sanchez C, Delcour JP, Bellahcène A, Baudouin C, Msika P, et al. Consequences of chondrocyte hypertrophy on osteoarthritic cartilage: potential effect on angiogenesis. Osteoarthritis Cartilage. 2013;21(12):1913-23. doi: 10.1016/j.joca.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 26. Athanasopoulos AN, Schneider D, Keiper T, Alt V, Pendurthi UR, Liegibel UM, et al. Vascular endothelial growth factor (VEGF)-induced up-regulation of CCN1 in osteoblasts mediates proangiogenic activities in endothelial cells and promotes fracture healing. J Biol Chem. 2007;282:26746-53. doi: 10.1074/jbc.M705200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Choi BH, Choi KH, Lee HS, Song BR, Park SR, Yang JW, et al. Inhibition of blood vessel formation by a chondrocyte-derived extracellular matrix. Biomaterials. 2014;35(22):5711-20. doi: 10.1016/j.biomaterials.2014.03.083. [DOI] [PubMed] [Google Scholar]
- 28. Patra D, Sandell LJ. Antiangiogenic and anticancer molecules in cartilage. Expert Rev Mol Med. 2012;14:e10. doi: 10.1017/erm.2012.3. [DOI] [PubMed] [Google Scholar]
- 29. Delloye-Bourgeois C, Fitamant J, Paradisi A, Cappellen D, Douc-Rasy S, Raquin M-A, et al. Netrin-1 acts as a survival factor for aggressive neuroblastoma. J Exp Med. 2009;206:833-47. doi: 10.1084/jem.20082299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Akino T, Han X, Nakayama H, McNeish B, Zurakowski D, Mammoto A, et al. Netrin-1 promotes medulloblastoma cell invasiveness and angiogenesis, and demonstrates elevated expression in tumor tissue and urine of patients with pediatric medulloblastoma. Cancer Res. 2014;74:3716-26. doi: 10.1158/0008-5472.CAN-13-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prieto CP, Casas BS, Falcon P, Villanueva A, Lois P, Lattus J, et al. Downregulation of the Netrin-1 receptor UNC5b underlies increased placental angiogenesis in human gestational diabetes mellitus. Int J Mol Sci. 2019;20:1408. doi: 10.3390/ijms20061408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tu T, Zhang C, Yan H, Luo Y, Kong R, Wen P, et al. CD146 acts as a novel receptor for netrin-1 in promoting angiogenesis and vascular development. Cell Res. 2015;25(3):275-87. doi: 10.1038/cr.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ding Q, Liao SJ, Yu J. Axon guidance factor netrin-1 and its receptors regulate angiogenesis after cerebral ischemia. Neurosci Bull. 2014;30(4):683-91. doi: 10.1007/s12264-013-1441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prieto CP, Ortiz MC, Villanueva A, Villarroel C, Edwards SS, Elliott M, et al. Netrin-1 acts as a non-canonical angiogenic factor produced by human Wharton’s jelly mesenchymal stem cells (WJ-MSC). Stem Cell Res Ther. 2017;8:43. doi: 10.1186/s13287-017-0494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin Z, Jin J, Bai W, Li J, Shan X. Netrin-1 prevents the attachment of monocytes to endothelial cells via an anti-inflammatory effect. Mol Immunol. 2018;103:166-72. doi: 10.1016/j.molimm.2018.08.021. [DOI] [PubMed] [Google Scholar]