Abstract
The mouse, as a model organism to study the brain, gives us unprecedented experimental access to the mammalian cerebral cortex. By determining the cortex’s cellular composition, revealing the interaction between its different components, and systematically perturbing these components, we are obtaining mechanistic insight into some of the most basic properties of cortical function. In this review, we describe recent advances in our understanding of how circuits of cortical neurons implement computations, as revealed by the study of mouse primary visual cortex. Further, we discuss how studying the mouse has broadened our understanding of the range of computations performed by visual cortex. Finally, we address how future approaches will fulfill the promise of the mouse in elucidating fundamental operations of cortex.
Keywords: receptive field, orientation selectivity, direction selectivity, contextual modulation, behavioral state, perception
INTRODUCTION
What chiefly distinguishes cerebral cortex from other parts of the central nervous system is the great diversity of its cell types and interconnexions. It would be astonishing if such a structure did not profoundly modify the response patterns of fibres coming into it.
—Hubel & Wiesel (1962, p. 106)
These two prescient sentences at the beginning of Hubel & Wiesel’s (1962) landmark publication encapsulate a key question in understanding visual cortex: How do diverse cell types and their connectivity sort out and make sense of the incoming stream of visual information? The study of mouse primary visual cortex (V1) has advanced our understanding of the relationship between cortical structure and function. How so? Progress in biology relies on the discovery of a phenomenon, its quantification and parameterization, and its mechanistic understanding. Research on primates and carnivores led to the discovery of many fundamental response properties of visual cortex to visual stimuli and allowed investigators to precisely quantify these responses relative to the stimulus parameters (Gilbert & Wiesel 1990, Hubel & Wiesel 1968, Movshon et al. 1978, Reid et al. 1991). Furthermore, this quantitative effort has led to models of cortical function that made mechanistic predictions (e.g., Carandini & Heeger 1995, Ferster & Miller 2000). However, due to the complexity of the mammalian cortex, experimental verification of these predictions has been slow and required heroic effort (Malpeli et al. 1981, Priebe & Ferster 2008, Reid & Alonso 1995, Sillito 1975). As a consequence, our mechanistic understanding of cortical vision has lagged substantially relative to the progress made on discovering and parameterizing phenomena. How do the different types of cortical neurons each contribute to the response of V1 to visual stimuli? What is the role of local recurrent connectivity among cortical neurons? How are the results of computations distributed to downstream targets to enable visually guided behavior? By using the mouse, we can harness the power of molecular biology to selectively record and perturb the activity of the individual cellular components of the cortex and provide insight into the cellular mechanisms that enable V1 to see.
How appropriate is mouse V1 for studying cortical computations? A number of response properties of mouse V1 to visual stimuli and nonvisual variables have been well established and parameterized (Ayaz et al. 2013; Niell & Stryker 2008, 2010; Self et al. 2014; Van den Bergh et al. 2010). A mechanistic understanding of how the underlying computations are implemented provides profound insight into the biology of the mammalian cortex in general. Can these findings generalize to V1 of other species? Many fundamental properties of visual cortical function originally reported in primates and carnivores are similar in the mouse (Niell & Stryker 2008, Van den Bergh et al. 2010). The question of whether those computations are implemented by the same mechanisms in mice as they are in the cortex of carnivores and primates will have to wait until we can experimentally access the cortex of other model organisms as thoroughly as we can access that of the mouse today. However, some examples already suggest that at least some of these mechanisms are indeed shared across species (Lien & Scanziani 2013, Liu et al. 2011, Priebe & Ferster 2008, Reid & Alonso 1995). Clearly, the mouse visual system differs from that of the primate in several ways, including low acuity, lack of a fovea, the natural visual environment within which it operates, and the repertoire of visually guided behaviors it subserves (Huberman & Niell 2011, Seabrook et al. 2017). However, even in foveate animals, much of the cortex is dedicated to processing vision outside the fovea, and mouse vision shares a strong similarity with primate peripheral vision, from the low acuity and rod dominance to behavioral roles such as detecting salient stimuli and guiding navigation. We expect that studies of mouse V1 will reveal canonical principles of visual cortical function and may demonstrate that primate specializations represent detailed implementation rather than fundamental differences.
Finally, the striking similarity between the microcircuit organization across cortical areas has long suggested the possibility that the cortical circuit performs a canonical computation (Douglas et al. 1989, Miller 2016) shared across modalities and species but that differs based on the nature of the input received and the detailed local connectivity of individual neurons. Thus, determining how the circuits of mouse V1 implement visual processing could lead to a general understanding of how cortex computes.
COMPUTATION OF VISUAL RESPONSE PROPERTIES BY THE CORTICAL CIRCUIT
The amount of available data in mouse visual cortex on cell types, including morphology, electrophysiological properties, molecular identity, and local and long-range connectivity patterns, exceeds that of any other area of mammalian cortex (Gouwens et al. 2019, Harris et al. 2019, Jiang et al. 2015, Pfeffer et al. 2013, Tasic et al. 2016). This list of parts and their wiring provides the basis for understanding the cellular mechanisms of cortical computations and constraining models. In the Supplemental Appendix, we present a very brief overview of the anatomical organization of visual cortex (Figure 1), including cell types and their connectivity, to provide context for understanding the computations performed. Below, we describe how these circuit elements generate a range of visual response properties, from orientation selectivity to contextual modulation, beginning with the transformation that occurs from the thalamic input to cortical responses in layer 4 (L4).
Figure 1.
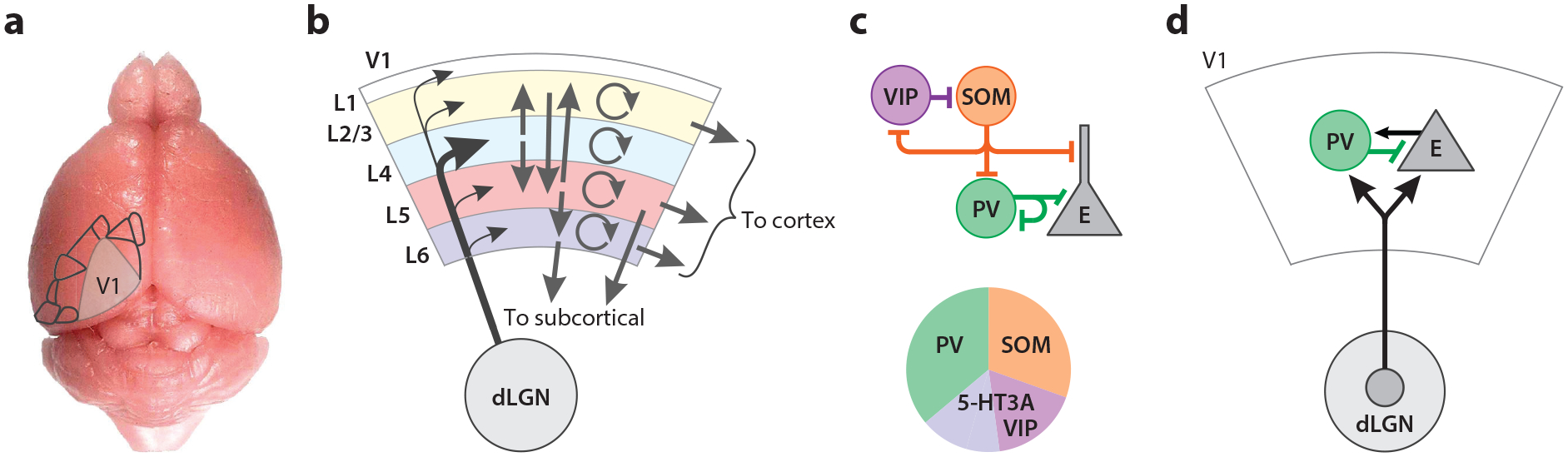
Canonical circuits of mouse visual cortex. (a) Overlay of V1 and higher visual areas on the mouse brain showing location and relative size. (b) Summary of layer-specific excitatory connectivity. V1 outputs project to higher visual areas and other cortical regions as well as many subcortical targets, including structures involved in behavioral output such as superior colliculus and basal ganglia. Panel b adapted from Ji et al. (2016), Jiang et al. (2015), Morgenstern et al. (2016), and Seeman et al. (2018). (c) Summary of inhibitory connectivity motifs. Panel c adapted from Karnani et al. (2016), Lee et al. (2010), and Pfeffer et al. (2013). (d) Thalamocortical input targets both excitatory neurons and PV-positive inhibitory neurons. Panel d adapted from Ji et al. (2016), Jiang et al. (2015), and Seeman et al. (2018). See the Supplemental Appendix for further overview of anatomical circuit organization. Abbreviations: dLGN, dorsal lateral geniculate nucleus; E, excitatory; L, layer; PV, parvalbumin; SOM, somatostatin; V1, primary visual cortex; VIP, vasoactive intestinal peptide.
The Thalamocortical Transformation
As in other mammalian species, most dorsal lateral geniculate nucleus (dLGN) neurons have a standard center-surround organization (Figure 2a), with either ON or OFF polarity (i.e., responding to increments or decrements of light, respectively) and transient or sustained temporal dynamics. A minority of neurons have more diverse response properties (Marshel et al. 2012, Piscopo et al. 2013, Zhao et al. 2013), including neurons that prefer stimuli moving in a certain direction (direction selectivity). Although such noncanonical responses are present in the dLGN of other species, including cat and primate, their proportion is greater in the mouse, though still a small fraction of the total dLGN population (Scholl et al. 2013). Since the discovery that receptive field (RF) structure in V1 differs from that in dLGN, a large effort has gone into understanding the logic of this transformation.
Figure 2.
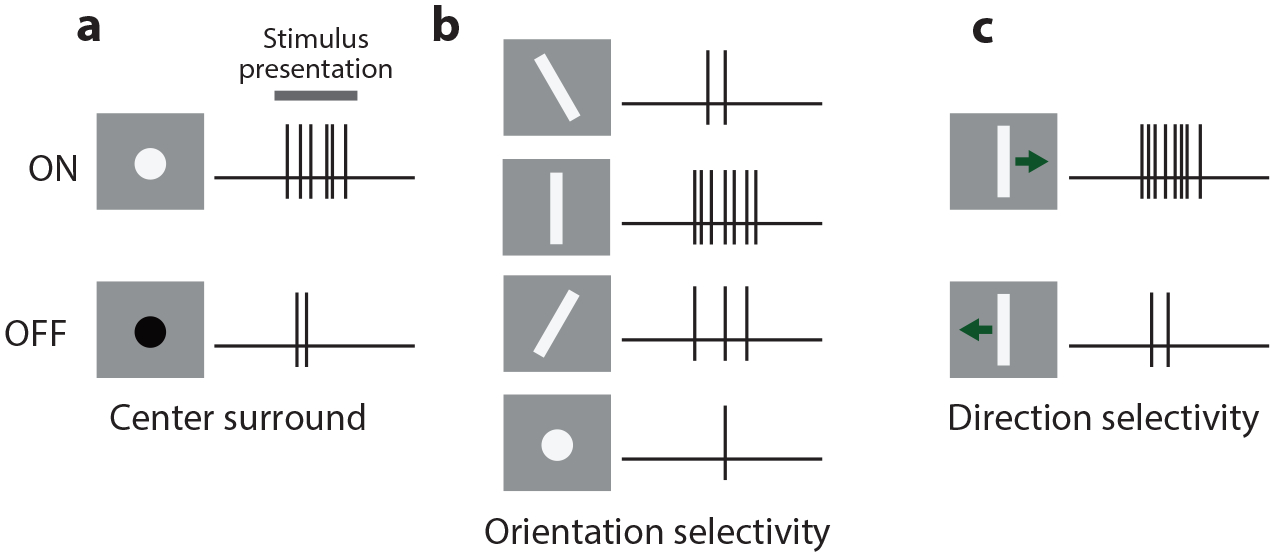
Overview of classical visual response properties. (a) Response of ON sustained (top) and OFF transient (bottom) center-surround neurons. (b) Response of an orientation-selective neuron preferring vertical orientation. (c) Response of a direction-selective neuron preferring rightward motion.
Thalamic convergence and unitary amplitude.
It takes many thalamic afferents to fire a cortical neuron. A single thalamic afferent impinging onto L4 excitatory neurons triggers a so-called unitary excitatory postsynaptic potential averaging 0.8 mV (Lien & Scanziani 2018) [ranging from0.1 to 3.4 mV; similar to that observed in rat somatosensory cortex (Bruno & Sakmann 2006) and cat visual cortex (Sedigh-Sarvestani et al. 2017)], thus too small to depolarize the membrane enough to reach threshold for action potential generation. As a consequence, the activation of L4 excitatory neurons by a visual stimulus must rely on the summed activity of many dLGN neurons. This is an important property because it means that a L4 neuron can selectively respond to features of the visual environment that are not represented by any of its individual dLGN afferents but are instead captured by a conjunction of features in the activity of the dLGN afferents from which it receives input. In other words, L4 neurons can extract features of the visual environment that are not explicitly represented by individual neurons in upstream stages of visual processing. It has been estimated that, in response to a visual stimulus, L4 excitatory neurons receive the convergent activity of approximately 80 dLGN inputs (Lien & Scanziani 2018, Bruno & Sakmann 2006). However, a much smaller number of dLGN inputs, as low as two to six, may be sufficient to account for the majority of a cortical neuron’s response (Ringach 2021).
Orientation selectivity.
Neurons in V1 preferentially respond to edges of luminance of a particular orientation, a property referred to as orientation selectivity (OS) (Figure 2b). This property, originally discovered in cat (Ferster & Miller 2000, Hubel & Wiesel 1962), has been confirmed across mammalian species, including mouse V1 (Niell & Stryker 2008), and represents one of the most salient differences between the response of dLGN and cortical neurons. Because most dLGN neurons do not show a preferential response to edges of any particular orientation (Piscopo et al. 2013), most OS responses are most likely generated in cortex. Is OS in mouse V1 a property that emerges through the interaction between cortical neurons or through the convergence of multiple dLGN afferents onto cortical neurons? A number of models have been proposed (Ferster & Miller 2000, Hubel & Wiesel 1962), and evidence for the convergence of appropriately aligned dLGN inputs was provided by heroic paired recordings in dLGN and cortex of cat (Reid & Alonso 1995). However, to directly address this question, one needs to isolate thalamic excitation from recurrent cortical excitation, an approach that was pioneered in the cat (Ferster et al. 1996) and recently optimized using genetic approaches in the mouse. By performing whole-cell recordings from L4 excitatory neurons while optogenetically silencing V1, it is possible to directly record thalamic synaptic excitation in isolation (Li et al. 2013, Lien & Scanziani 2013). This approach revealed that the RF structure generated by the ensemble of dLGN afferents converging onto individual L4 excitatory neurons is made of spatially separated yet overlapping ON and OFF subregions (Lien & Scanziani 2013). This RF structure likely results from the fact that ON- and OFF-centered dLGN neurons with spatially offset RFs converge onto individual L4 neurons (Figure 3a), thereby imparting OS (Figure 3b,c). Thus, the convergence of dLGN neurons with distinct polarities (either ON or OFF) and distinct RF location imparts L4 neurons with the ability to detect a feature of the visual environment that is not necessarily captured by any individual dLGN neuron from which they receive input.
Figure 3.
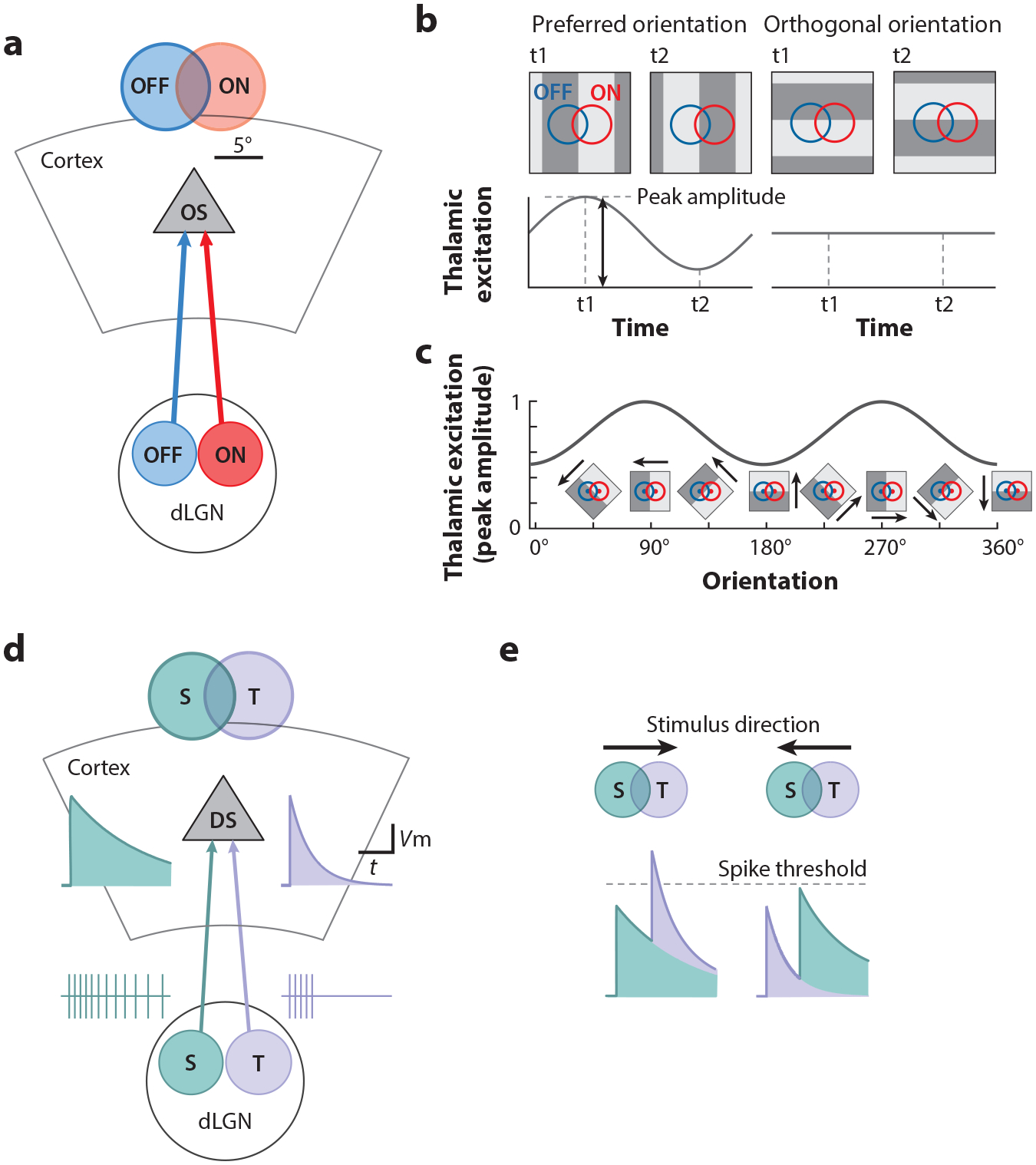
The emergence of orientation and direction selectivity in L4. (a) L4 neurons receive spatially offset ON versus OFF input from dLGN, which imparts OS. (b) To optimally activate a L4 neuron, the dark portion of an edge needs to cover the OFF region and the bright portion the ON region, a configuration that occurs if the main axis of the edge is perpendicular to the axis that connects the center of the ON and the OFF subregions of the RF. Any other orientation of the edge would produce a suboptimal excitation of the L4 neuron. As a grating drifts across the RF, it produces alternating optimal (t1) and suboptimal (t2) excitation. (c) Examination of the peak excitation across orientations reveals an orientation tuning curve. Panels a–c adapted from Lien & Scanziani (2013). (d) Direction-selective L4 neurons receive spatially offset transient versus sustained input from dLGN. (e, left) A visual stimulus moving in the preferred direction will first cross the RF of the dLGN neuron with a sustained response and then the RF of the dLGN neuron with a transient response. By doing so, the excitation produced by these two inputs will sum optimally. (Right) When the stimulus moves in the opposite direction, the transient response will have already largely subsided by the time the stimulus crosses the RF of the dLGN neuron with a sustained response, leading to less summation. Panels d and e adapted from Lien & Scanziani (2018). Abbreviations: dLGN, dorsal lateral geniculate nucleus; DS, direction selectivity; L, layer; OS, orientation selectivity; RF, receptive field; S, sustained; T, transient.
Pharmacological and genetic manipulations in several mammals, including mice (Sarnaik et al. 2014), demonstrate that OS can persist even in the absence of ON inputs from the retina, implying that other mechanisms could also contribute to OS, for example, the convergence of aligned dLGN inputs of like polarity along the axis of preferred orientation (Chapman et al. 1991, Li et al. 2013).
Direction selectivity.
Moving stimuli are particularly salient. The ability of V1 neurons to preferentially respond not only to edges of luminance of a specific orientation but also to the motion of those edges in a specific direction is another prominent feature of cortical responses to visual stimuli (Hubel & Wiesel 1959, 1962). This property is referred to as direction selectivity (DS) (Figure 2c), and the underlying mechanisms have intrigued scores of scholars. Like V1 neurons of many mammals, neurons in mouse V1 also display DS (Niell & Stryker 2008). How does DS emerge in V1? Because motion is a process that occurs across space and time, DS requires a comparison across those two dimensions (Albrecht & Geisler 1991, DeAngelis et al. 1993, Livingstone 1998, McLean & Palmer 1989, Reid et al. 1987, Saul & Humphrey 1992). Using the approach described above, namely the optogenetic silencing of V1 to isolate thalamic excitation, it could be demonstrated that, in L4 neurons, DS emerges through the convergence of dLGN neurons with distinct spatial and temporal RFs (Lien & Scanziani 2018) (Figure 3d). The RF of dLGN neurons is characterized not only by its spatial coordinates and polarity (ON or OFF) but also by its temporal properties. dLGN neurons can have different response dynamics, with some responding transiently to a visual stimulus while others respond in a more sustained manner (Lien & Scanziani 2018, Piscopo et al. 2013). The convergence of dLGN neurons with spatially offset RFs and with different response dynamics to visual stimuli onto individual L4 neurons imparts DS (Figure 3e). This is another clear example of how, by combining the response diversity of dLGN neurons, L4 neurons can extract features of the environment (e.g., direction of motion) that are not necessarily represented in the activity of any of the individual dLGN neurons from which they receive input. DS is further enhanced in V1 through the spatial separation of the RFs of excitatory and inhibitory synaptic conductances. Electrophysiological recordings from L4 neurons show that, in direction-selective neurons, the spatial position of a stimulus that triggers maximal excitation is offset relative to the position that triggers maximal inhibition (Li et al. 2015). This offset results in a delay of inhibition relative to excitation specifically for stimuli moving in the preferred direction, thus contributing to the DS of the neuron. Similarly, the spatial position (in terms of retinotopic coordinates) of inhibitory neurons that are presynaptic to a direction-selective neuron is offset relative to that of its presynaptic excitatory neurons. This spatial offset predicts the preferred direction of the direction-selective neuron (Rossi et al. 2020).
A small fraction of dLGN neurons inherit DS from the retina (Piscopo et al. 2013) and project across cortical layers (Sun et al. 2016). Do they contribute to DS in V1? Disruption of DS in the retina does not affect the overall distribution of direction-selective neurons in V1, implying that most DS in V1 is not inherited from the retina (Hillier et al. 2017). However, after impairing retinal DS, one observes a reduction in the fraction of L2/3 neurons whose preferred direction is posterior motion (i.e., the direction of lateral visual flow experienced by the mouse as it moves forward in its environment) (Hillier et al. 2017). Thus, while the direction preference for most motion directions is computed de novo in V1, the sensitivity to posterior motion in L2/3 is in part inherited from the retina (Cruz-Martín et al. 2014, Rasmussen et al. 2020).
Computations by Recurrent Excitation
Even though dLGN afferents are the main input from the visual periphery into V1, they represent only a minority of the excitatory drive within cortex. Recurrent synaptic connections among excitatory neurons are a defining characteristic of the cortical circuit. Below we discuss how recurrent excitation shapes the stimulus selectivity and response dynamics.
Functional connectivity among V1 excitatory neurons.
Beyond the general intra- and interlaminar connectivity principles (Figure 1b; Supplemental Appendix), what are the rules that guide specific connectivity among excitatory neurons in V1, and how do they relate to their functional properties? A general principle of connectivity that has emerged through many studies is like-to-like (Figure 4a). In L2/3, for example, the probability of connection (Cossell et al. 2015, Ko et al. 2011, Lee et al. 2016, Wertz et al. 2015), the synapse size (Lee et al. 2016), and the unitary amplitude of connections (Cossell et al. 2015, Ko et al. 2011) are all greater among excitatory neurons with similar orientation preference. Consistent with this finding, a L2/3 excitatory neuron that receives excitatory input from another L2/3 excitatory neuron is more likely to project back to that L2/3 excitatory neuron than chance, and these two neurons are more likely than chance to receive a common input from a L4 excitatory neuron (Yoshimura et al. 2005). Like-to-like connectivity also occurs across layers, with L2/3 neurons being more likely to receive input from L4 neurons tuned to the same orientation (Rossi et al. 2020), although some interlaminar input populations are tuned for mismatched orientations (Wertz et al. 2015). This rule of connectivity is also reflected at the entry point of visual information into V1 where the tuning of the dLGN input matches that of recurrent excitation in L4 neurons (Li et al. 2013, Lien & Scanziani 2013), and connected L4 neurons are more likely to receive shared input from dLGN (Morgenstern et al. 2016). The rules of connectivity also obey general principles across cortical space, such that neurons with spatially offset RFs tend to be connected if their preferred orientations align with the axis connecting their RFs (Iacaruso et al. 2017, Rossi et al. 2020) (Figure 4b), consistent with tuning for extended contours. Thus, the visual response properties of V1 excitatory neurons are a key variable in predicting who talks to who.
Figure 4.
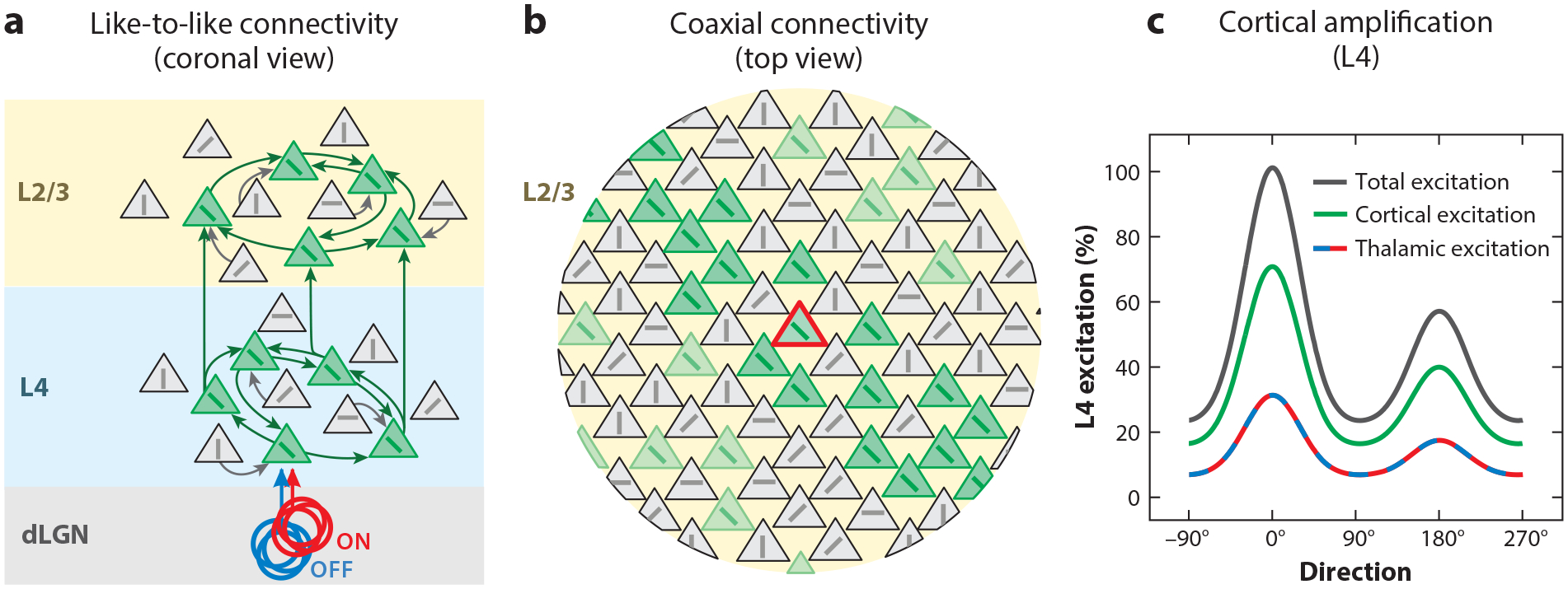
Recurrent excitatory connectivity. (a) Connectivity within the cortical circuit is primarily like to like, with preferential (though not exclusive) connections between neurons tuned to similar orientations. This applies to both feedforward connectivity from L4 to L2/3 and recurrent connectivity within L2/3. Panel a adapted from Ko et al. (2011), Lee et al. (2016), Lien & Scanziani (2013), Morgenstern et al. (2016), Rossi et al. (2020), Wertz et al. (2015), and Yoshimura et al. (2005). (b) The spatial organization of connectivity is coaxial, whereby a neuron (red) tends to receive input from neurons (green) with similar orientation preference and whose location, in retinotopic coordinates, is along the axis of their preferred orientation. Panel b adapted from Iacaruso et al. (2017) and Rossi et al. (2020). (c) Recurrent cortical excitation is larger than incoming thalamic excitation but is matched in tuning, resulting in cortical amplification (total excitation). Panel c adapted from Li et al. (2013) and Lien & Scanziani (2013, 2018). Abbreviations: dLGN, dorsal lateral geniculate nucleus; L, layer.
Dynamics of the cortical response: amplification.
While there are no exact numbers for the mouse, in other mammals only about 5–20% of excitatory synapses onto L4 neurons are of dLGN origin (Ahmed et al. 1994, Garcia-Marin et al. 2019). Consistent with this, in mice only 30% or so of visually evoked synaptic excitation of L4 excitatory neurons directly originates from dLGN afferents (Li et al. 2013, Lien & Scanziani 2013). The remainder of visually evoked excitation (~70%) is mediated by other cortical neurons (Li et al. 2013, Lien & Scanziani 2013), most likely L4 neurons, since they excite one another through recurrent connections (Seeman et al. 2018) (Figure 4c). The relative fraction of afferent thalamic versus recurrent excitation evolves in time after the onset of the visual stimulus (Reinhold et al. 2015). Excitation is predominantly thalamic at the beginning of the stimulus and shifts toward recurrent after a few tens of milliseconds as L4 excitatory neurons begin to fire action potentials in response to the stimulus (Reinhold et al. 2015). Visually evoked responses are thus amplified by recurrent connections. Importantly, this cortical amplification does not degrade the orientation and direction preference imparted by the dLGN input onto L4 neurons (Li et al. 2013, Lien & Scanziani 2013) because, as described above, the connectivity pattern among V1 neurons is biased toward neurons with similar orientation and direction preferences. Such amplification was one of the first proposed canonical cortical computations (Douglas et al. 1989).
Dynamics of the cortical response: decay time course.
Can recurrent excitation sustain visually evoked activity without ongoing thalamic input? Given that shortly after the onset of the visual stimulus, recurrent excitation makes up most of the excitation received by L4 neurons, one could assume that visually evoked activity may persist for some time even without ongoing dLGN input. This is, however, not the case. No matter how strong the visual stimulus is and, accordingly, how large the response of V1 to that visual stimulus is, silencing of the dLGN input to V1 leads to a fast decay (~10 ms) of the visual response in V1 (Reinhold et al. 2015). This fast decay is on the order of magnitude of the membrane time constant of a neuron. Thus, despite the strong amplification by recurrent excitation, the response of V1 remains tightly linked to the dLGN input. This ensures that temporal fluctuations in the response of dLGN neurons to visual stimuli are closely followed by temporal fluctuations in V1 activity (Reinhold et al. 2015). Selective amplification thereby achieves two functions: amplifying specific features and allowing high temporal fidelity (Murphy & Miller 2009). Intracortical inhibition likely plays a key role in the rapid decay of visually evoked activity in V1 following the interruption of dLGN input.
Computations Through Cortical Inhibition
Approximately 20% of cortical neurons are inhibitory (Meinecke & Peters 1987), and their integration into the cortical circuit ensures that during normal cortical function, excitation and inhibition are inseparable—they walk hand in hand. As discussed below, the combination of synaptic excitation and inhibition underlies several fundamental cortical computations. For an overview of inhibitory circuitry, see the Supplemental Appendix.
Computing with two opposing forces.
Visually evoked activity elicits both excitation and inhibition in V1. The recruitment of these two opposing forces already occurs in the first steps of cortical processing, because dLGN afferents contact both excitatory and PV-expressing inhibitory neurons in V1 (Ji et al. 2016) (Figure 1d). Furthermore, excitation by dLGN afferents is stronger onto PV neurons than onto excitatory neurons (Ji et al. 2016). As a consequence, even the weakest visual stimuli generate, on average, both excitation and inhibition in V1 neurons (Adesnik 2017) (Figure 5a), and as stimulus contrast is increased, excitation and inhibition grow approximately proportionally (Adesnik 2017) (Figure 5b). Why should the dLGN input to V1 push on both the accelerator and the brake at the same time? The functional consequences of the proportionality between excitation and inhibition in cortex have been the focus of extensive modeling (Ahmadian et al. 2013, Brunel 2000, Sadeh & Clopath 2021, van Vreeswijk & Sompolinsky 1996) and have been discussed in more detail elsewhere (Isaacson & Scanziani 2011, Miller 2016). Briefly, a proportional increase of inhibition with excitation enables V1 to respond over a wide range of stimulus intensities (Liu et al. 2011), remaining sensitive to weak stimuli (in which weak excitation is counteracted by only weak inhibition) yet not saturating in response to strong stimuli (because strong excitation is counteracted by strong inhibition). Through this proportional increase, for example, orientation tuning of a V1 neuron changes little or becomes even sharper as stimulus contrast increases (Li et al. 2012) and the inputs of the two eyes sum sublinearly in binocular neurons (Longordo et al. 2013). Furthermore, the ability to operate with strong excitation without the risk that recurrent excitatory synapses could lead to runaway excitation enables cortex to have fast dynamics (Reinhold et al. 2015); to use the analogy of a car, if you want to go fast, you need brakes to stay in control. The divergence of feedforward excitation onto excitatory and PV neurons also occurs at the next stage of processing, from L4 to L2/3, and here, too, L4 afferents provide stronger excitation onto PV neurons as compared to L2/3 excitatory neurons (Adesnik et al. 2012). Furthermore, L2/3 excitatory neurons homeostatically regulate the strength of PV-mediated inhibition as a function of the magnitude of excitation they receive from L4 (Xue et al. 2014). Thus, the divergence of afferent axons onto excitatory and inhibitory neurons ensures proportionality between excitation and inhibition and may be a general property of each stage of cortical processing (Miller 2016).
Figure 5.
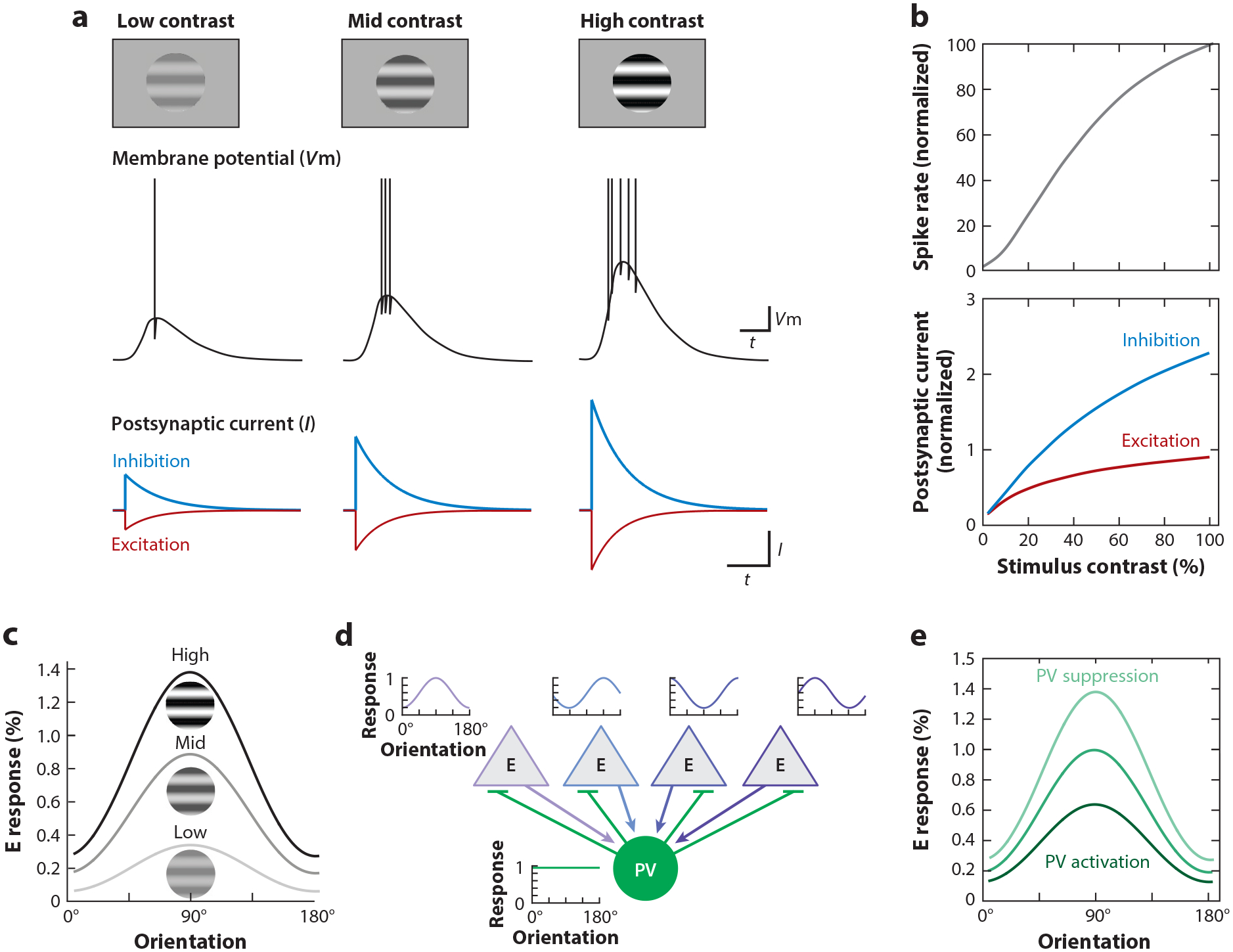
Gain control through feedforward inhibition. (a) Stimuli of increasing contrast elicit larger synaptic depolarization of the membrane potential (Vm) and higher spike frequencies, yet the underlying synaptic excitatory (E) and inhibitory (I) currents increase proportionally, and even the weakest contrasts elicit both excitation and inhibition. (b) The change in spike rate (top) and excitatory and inhibitory currents (bottom) as a function of contrast. The proportional increase of excitation and inhibition prevents runaway excitation as stimulus contrast increases. Panels a and b adapted from Adesnik (2017). (c) Increasing stimulus contrast results in a multiplicative increase in response of excitatory neurons (E response) across the orientation tuning curve while leaving selectivity unchanged, i.e., a gain change. (d) Parvalbumin (PV) inhibitory neurons pool excitatory inputs tuned to different orientations, resulting in nonselective orientation tuning. Panel d adapted from Bock et al. (2011). (e) The response of excitatory neurons to stimuli of different orientations is modulated by the activity of PV neurons. Because PV neurons are reciprocally connected with the local excitatory population, their activity can provide multiplicative gain control that mimics the effects of varying contrast. Panel e adapted from Atallah et al. (2012).
In contrast to excitatory neurons, in mouse V1, PV inhibitory neurons tend to be broadly tuned for orientation (Kerlin et al. 2010, Liu et al. 2009, Niell & Stryker 2008, Runyan et al. 2010), reflecting the fact that they pool inputs relatively nonselectively from the local excitatory populations (Bock et al. 2011, Hofer et al. 2011). The result is that PV neurons provide untuned inhibition to their targets. PV-mediated inhibition thus reflects population activity rather than specific features of the stimulus, going up and down in tandem with excitatory neurons. This untuned inhibition is ideally suited to control the gain of V1 responses relative to the stimulus (Atallah et al. 2012, Lee et al. 2012) (Figure 5c–e), that is, to change the magnitude of the response of excitatory neurons without impacting their tuning preferences, like turning the volume knob on a stereo. Some PV neurons located in L6 extend their axons throughout the depth of the cortex (Bortone et al. 2014), which enables them to control the gain of V1 responses across all cortical layers (Atallah et al. 2012, Lee et al. 2012, Olsen et al. 2012). Untuned inhibition may also mediate divisive normalization (Wilson et al. 2012), a proposed canonical computation of cortex (Carandini & Heeger 2011). In contrast to PV neurons, other inhibitory neurons are more selective to the orientation of the stimulus (Ayzenshtat et al. 2016, Lee et al. 2012, Ma et al. 2010), but the mechanism and implications of this are less well understood.
Contextual modulation.
Context is a fundamental attribute to our perception of any sensory stimulus, providing meaning in the sensory scene. In the visual world, context refers to the visual environment surrounding a stimulus. Psychophysical experiments demonstrate that the context of a stimulus influences our perception of that stimulus, including its size, color, or contrast (Albright & Stoner 2002). Indeed, much of the computational power of cortex in visual processing may arise from such contextual modulation.
Several laboratories have found clear physiological signatures of such perceptual phenomena in V1 (Nurminen & Angelucci 2014), and experiments in mouse are contributing to our understanding of some of the underlying cellular mechanisms. Stimuli outside of the RF of a neuron, i.e., stimuli presented in its surround, while generally unable to elicit a response alone (but see below), can, however, modulate the response of the neuron to a stimulus placed in its RF. The nature of this modulation is often suppressive, as shown when the size of a stimulus is increased to cover both the RF and the surrounding regions, and is referred to as surround suppression (Nurminen & Angelucci 2014, Van den Bergh et al. 2010) (Figure 6a). What accounts for this suppression? Recordings in L2/3 of mouse V1 have shown that the relationship between excitation and inhibition changes with the size of the stimulus or with the position of the stimulus relative to the center of the RF. Inhibition becomes progressively more prominent relative to excitation as the stimulus size increases or as the stimulus is shifted toward the periphery of the RF (Adesnik 2017, Haider et al. 2013). What is the cellular basis for this phenomenon? Surround suppression is not only observed in excitatory neurons but also in two types of inhibitory neurons: the PV and vasoactive intestinal peptide (VIP) cells (Adesnik et al. 2012, Keller et al. 2020a). Remarkably, the other main type of inhibitory neuron, the somatostatin (SOM) cell, shows much less surround suppression, and the responses of these neurons instead often continue to increase with increasing stimulus size (Adesnik et al. 2012, Keller et al. 2020a) (Figure 6a). This property of SOM cells, and the fact that SOM cells inhibit all other cell types (Figure 1c), suggests that SOM cells may actually be a key contributor to surround suppression. Indeed, optogenetic silencing of SOM cells strongly diminishes surround suppression, at least in L2/3 excitatory neurons (Adesnik et al. 2012). Thus, SOM cells, by lacking surround suppression and hence robustly responding to large stimuli, suppress neighboring neurons, contributing to their surround suppression (Figure 6b).
Figure 6.
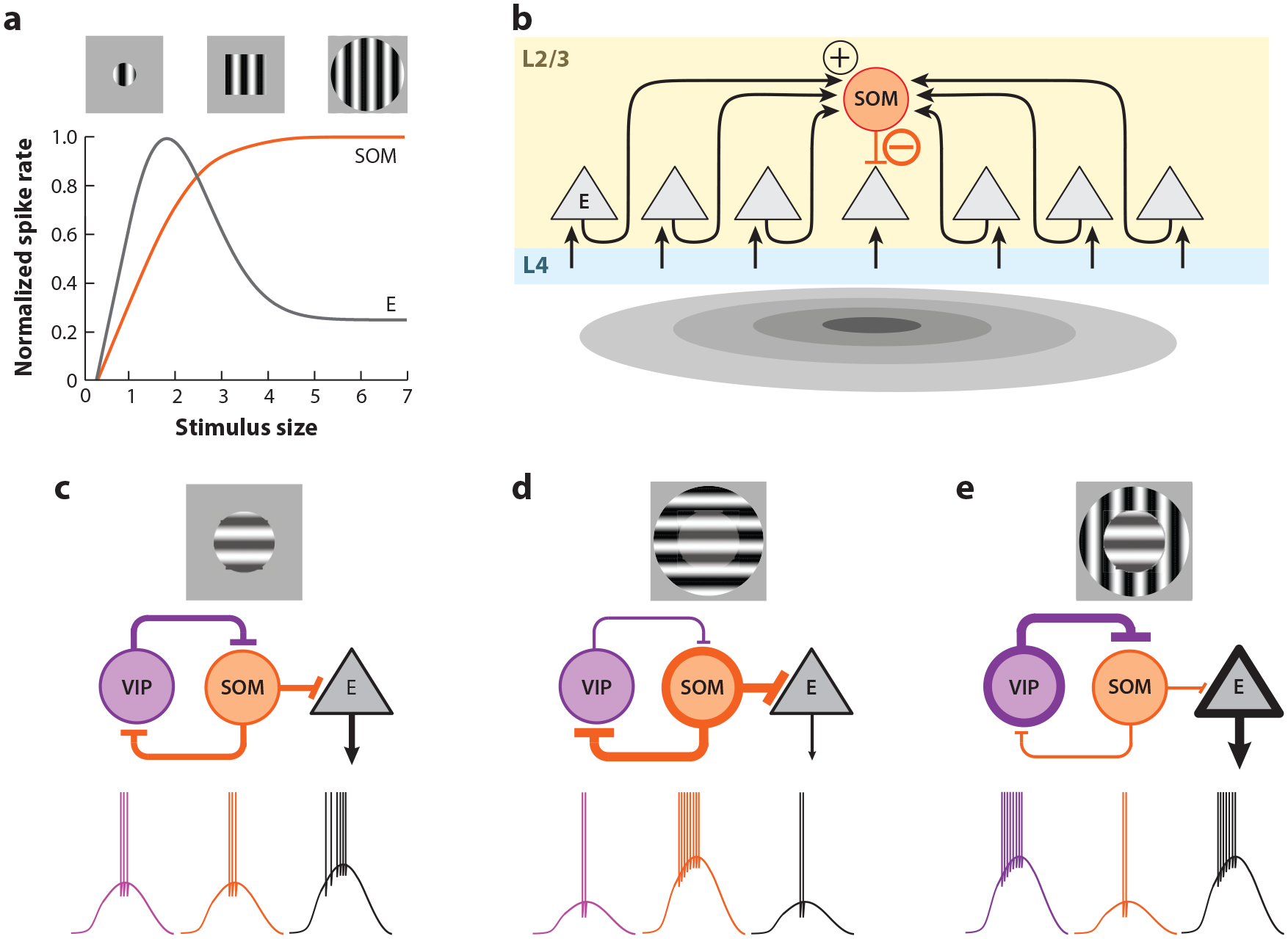
Inhibitory circuits for contextual modulation. (a) In contrast to excitatory (E) neurons, the response of somatostatin (SOM) neurons continues to increase up to a plateau with stimulus size. (b) SOM neurons receive input across a large area and do not inhibit each other. As a consequence, their response increases, leading to increasing responses with stimulus size. By inhibiting nearby excitatory neurons when large stimuli are presented, SOM neurons mediate surround suppression. Panels a and b adapted from Adesnik et al. (2012). (c–e) Contextual modulation depends on properties of the stimulus in the surround. An iso-oriented surround suppresses the response, while a cross-oriented surround increases the response. This gives rise to the illusory perception that the central grating contrast in panel d is greater than in panel c. The disinhibitory circuit from vasoactive intestinal peptide (VIP) neurons onto SOM neurons mediates this context dependence, as the activation of VIP neurons in the cross-oriented condition inhibits the surround suppression that would otherwise be provided by SOM neurons. Panels c–e adapted from Keller et al. (2020a).
It takes time for surround suppression to kick in. The presentation of a large stimulus, which covers both the RF and its surround, initially triggers a response in V1 excitatory neurons that is similar in magnitude to that of a stimulus that covers just the RF. Several tens of milliseconds later, however, the response starts to get suppressed (Self et al. 2014). Consistent with the role of SOM cells in surround suppression, their activation by a visual stimulus is also delayed (Ma et al. 2010). This delay likely results from the fact that SOM cells need repeated activity from their excitatory synaptic input in order to respond because their excitatory inputs are facilitating; that is, synaptic excitation starts small and increases progressively with repeated activity of the afferents (Karnani et al. 2016). Thus, synaptic dynamics of a specific component of the cortical circuit can affect the time course of the sensory response in V1.
Why do L2/3 SOM cells have little or no surround suppression? Unlike other L2/3 cell types, SOM cells do not receive afferent excitatory input from L4, the main input layer to L2/3 (Adesnik et al. 2012). The excitation of SOM cells is in large part provided by recurrent axons within L2/3. This property, together with the fact that SOM cells do not inhibit one another (Adesnik et al. 2012, Pfeffer et al. 2013) (Figure 1c; see also the section on inhibitory circuitry in the Supplemental Appendix), may underlie their ability to integrate stimuli that cover large portions of visual space (Figure 6b).
Not all stimuli in the surround are suppressive (Nurminen & Angelucci 2014). A grating in the surround of a neuron’s RF whose orientation is orthogonal relative to the orientation of the grating in the neuron’s RF (cross-oriented surround) triggers much less surround suppression than if its orientation were the same as that in the neuron’s RF (iso-oriented surround; as discussed above). This phenomenon may represent the physiological basis for the fact that the perception of a stimulus with a cross-oriented surround is more salient than the same stimulus with an iso-oriented surround, as demonstrated by many visual illusions (Figure 6c). Work in L2/3 of mouse V1 suggests that this contextual modulation relies, at least in part, on the reciprocal interaction between two types of inhibitory neurons, namely VIP and SOM cells (Keller et al. 2020a). These two neurons have complementary responses to iso- and cross-oriented surrounds. On one hand, iso-oriented stimuli elicit strong responses in SOM cells (as mentioned above) but only weak ones in VIP cells (Keller et al. 2020a) (Figure 6c). On the other hand, cross-oriented stimuli trigger strong responses in VIP cells but poorly stimulate SOM cells (Keller et al. 2020a). Importantly, VIP cells are integrated in the cortical circuit in a very particular manner: They preferentially inhibit inhibitory neurons rather than excitatory neurons (Pfeffer et al. 2013) (Figure 1c; see also the section on cell types and circuit organization in the Supplemental Appendix). Their activity thus has a disinhibitory impact on excitatory neurons in V1. Recent experimental and modeling approaches suggest that the activation of VIP cells by a cross-oriented surround suppresses SOM cells, thereby leading to the relief of excitatory neurons from suppression (Keller et al. 2020a). This same disinhibitory circuit has been shown to regulate the degree of surround suppression based on the contrast of the stimulus (Millman et al. 2019) as well as to enhance visual responses based on attentional inputs from cingulate cortex (Zhang et al. 2014). Thus, this canonical disinhibitory circuit may represent one of the key mechanisms for how context impacts the perception of a stimulus.
Finally, stimuli presented in the surround that would otherwise suppress the response to a stimulus in the RF can become excitatory when presented in the absence of a stimulus in the RF (Fiorani Júnior et al. 1992, Jones et al. 2001, Rossi et al. 2001, Schnabel et al. 2018, von der Heydt et al. 1984). These responses to the surround stimulus alone may represent the physiological signature of perceptual completion, that is, they may allow the visual system to use context to estimate the nature of a stimulus in the RF when the latter is poorly visible or occluded. Work in mouse has shown that the excitation of V1 neurons to stimuli presented in the surround alone is mediated by feedback projections originating in higher visual areas (HVAs) (Keller et al. 2020b). This is a clear example of how the response of V1 neurons is shaped not only by the feedforward pathway ascending from the retina and the local V1 circuitry but also by feedback projections descending from HVAs.
Sharpening the Receptive Field
Recurrent synaptic excitation among V1 neurons is biased toward neurons with similar orientation preferences (see the section titled Functional Connectivity Among V1 Excitatory Neurons), yet this bias is by no means absolute. Excitatory neurons in V1 also receive many synaptic inputs from excitatory neurons that have different orientation preferences. As a consequence, the orientation tuning of synaptic excitation is very broad, in fact much broader than the spiking response of the neurons themselves. What accounts for this sharpening of the spike response? Whole-cell recordings from mouse V1 neurons have demonstrated that at least two factors, spike threshold and broad inhibition, contribute to the sharpening (Figure 7). Due to the membrane potential threshold for action potential generation, there is a supra-linear relationship between the amplitude of the postsynaptic potential and spike frequency. As was shown originally in the cat (Priebe & Ferster 2008), this supralinear input-output relationship enables the largest visually evoked excitatory postsynaptic potentials to trigger spikes while the smaller ones remain sub-threshold, thereby sharpening the orientation tuning of the spike response (Liu et al. 2011) (Figure 7a). Another important factor in the sharpening of the spike response is the fact that the orientation tuning of synaptic inhibition is even broader than that of synaptic excitation. This is likely a consequence of the fact that, as discussed above, PV neurons, a main source of inhibition to excitatory neurons, are largely untuned to orientation. With synaptic inhibition more broadly tuned than synaptic excitation, the relationship between excitation and inhibition changes with the orientation of the stimulus, being biased toward excitation for the preferred orientation as compared to flanking orientations (Liu et al. 2011) (Figure 7b). Finally, active dendritic conductances also contribute to the sharpening of the orientation preferences of V1 neurons. Bursts of local dendritic sodium spikes generated in response to stimuli presented at the preferred orientation, but not at flanking orientations, sharpen the orientation tuning of the somatic depolarization (Smith et al. 2013) (Figure 7c). Local dendritic spikes may be a consequence of the correlated activity of synaptic inputs clustering on the dendrites of L2/3 neurons (Iacaruso et al. 2017, Lee et al. 2019). Indeed, inputs with overlapping RFs are more likely than chance to be close neighbors on a dendritic branch (Iacaruso et al. 2017). Furthermore, while the preferred orientation of inputs does not predict their spatial relationship on a dendrite (Chen et al. 2013, Iacaruso et al. 2017), in neurons receiving callosal projection from contralateral V1, the orientation preference of those callosal inputs correlates with the orientation preference of their noncallosal neighboring inputs (Lee et al. 2019). Thus, both synaptic and intrinsic voltage-dependent conductances profoundly shape the orientation tuning of the neuron.
Figure 7.
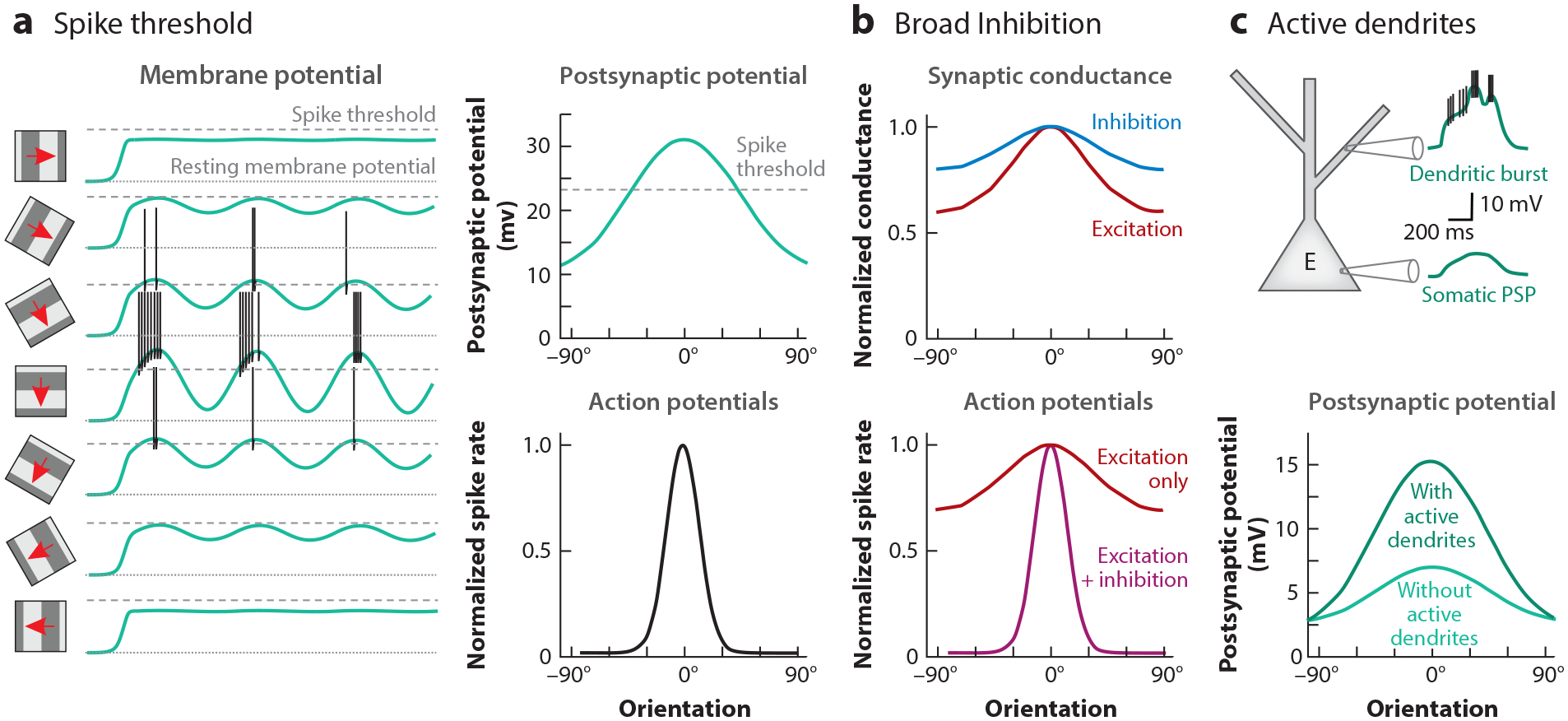
Mechanisms for sharpening response selectivity. (a) The membrane potential threshold for spike generation implies that even stimuli that elicit a significant depolarization may not elicit spiking, thereby sharpening the response to optimal stimuli. This is demonstrated in the temporal response to drifting gratings of varying orientation (left) and the corresponding orientation tuning curves (right) for membrane potential (top) and spikes (bottom). Panel a adapted from Liu et al. (2011). (b) The tuning of inhibitory synaptic conductances is broader than that of excitatory (E) synaptic conductances (top). The relative dominance of inhibition at nonpreferred orientations suppresses responses at these orientations and sharpens selectivity (bottom). Panel b adapted from Liu et al. (2011). (c) Visual input at the preferred orientation elicits dendritic bursts (top). This leads to greater orientation selectivity relative to when active conductances underlying dendritic bursts are suppressed (bottom). Panel c adapted from Smith et al. (2013).
Representation of Cortical Computations at the Population Level
The cortical response to visual stimuli has classically been characterized by tuning curves or RFs of individual neurons, as discussed in this section. However, the firing of one neuron cannot unambiguously convey information about the stimulus. As a simple example, a change in firing rate of an orientation-selective cell could result from either a change in stimulus contrast or a change in stimulus orientation, thus precluding readout of either parameter independently. Information encoding in individual neurons is further confounded by the fact that neuronal responses to a given stimulus can be highly variable. However, these challenges can be resolved by considering the representation across many neurons in the population code. Recent methods for recording large numbers of neurons have thus begun to reveal how sensory information is encoded across large populations of neurons (Fairhall 2014, Panzeri et al. 2015, Whiteway & Butts 2019).
One important aspect of a population code is how information is distributed across the activity of many neurons, in terms of the diversity and overlap of response properties. Stringer et al. (2019b) quantitatively assessed the response of tens of thousands of neurons to a large battery of natural scene images using two-photon calcium imaging in mouse V1. The results demonstrated that responses are distributed across the population according to a power law that optimizes efficiency (ability to represent as many stimuli as possible) while maintaining smoothness (similar stimuli evoke similar patterns of neural activity). Another key question in population coding is the impact of correlations on pooling population activity to overcome variability inherent to individual neurons. It has been known for some time that the variability in response to a stimulus is often correlated across neurons, as a result of multiple factors, including shared input that is not directly related to the stimulus (such as behavioral state variables) or fluctuations resulting from local network dynamics (Kohn et al. 2016, Engel & Steinmetz 2019, Zohary et al. 1994). These noise correlations may or may not limit the amount of information that large populations of neurons can encode, depending on how the correlations are aligned with stimulus coding. From a simple perspective, if the correlated variability between neurons results in a pattern of population activity that resembles the pattern evoked by a visual feature, then this creates a confound in pooling information from those neurons to decode that feature. However, determining whether this is in fact the case depends on recording from large numbers of neurons, in order to determine how decoding accuracy increases with the number of neurons. This is now feasible with two-photon calcium imaging. Rumyantsev et al. (2020) used this approach to demonstrate that the ability to decode stimulus orientation increases with pooling neurons up to at least 1,000 neurons or beyond, as only 10% of the noise correlation overlapped with stimulus coding. Stringer et al. (2019a) used a similar approach and found that information does not saturate even up to 20,000 neurons, with the consequence that readout from sufficiently large populations in mouse V1 could allow estimation of stimulus orientation to significantly less than one degree. Thus, it appears that correlations are distributed across neurons in a manner that minimizes the impact on the encoding of visual information and that limits on the accuracy of orientation discrimination as measured behaviorally [approximately 5 degrees (Glickfeld et al. 2013b)] likely arise downstream of V1.
These large-scale aspects of the cortical computation almost certainly depend on specific circuit mechanisms. For example, the distribution of information across neurons in a power law described above (Stringer et al. 2019b) likely results from patterns of synaptic connectivity that determine the diversity and width of tuning properties. Likewise, the correlation structure of population activity may be determined by cell type–specific circuit motifs. Indeed, modeling studies have suggested that local inhibition could play an important role in limiting correlations generated by local network activity, and in fact inhibitory neuron activity is increased in brain states characterized by lower correlations (Huang et al. 2019, Stringer et al. 2016). However, we still have little understanding of how layer- or cell type–specific interactions shape population codes relative to our understanding of single-neuron response properties. Applying the tools available for studying cortical computations in mouse at the level of population dynamics is therefore an important research direction.
NONVISUAL COMPUTATIONS IN V1
In addition to revealing circuit mechanisms underlying classical aspects of visual processing, studies of mouse visual cortex have also led to the discovery of novel aspects of neural coding in V1 and particularly the contribution of a wide range of nonvisual factors, including movement, navigation, arousal, and vestibular signals (Figure 8). These findings suggest that an important property of V1 is the ability to integrate different sources of sensory and nonsensory information in order to generate flexible representations of the sensory environment to drive appropriate behaviors.
Figure 8.
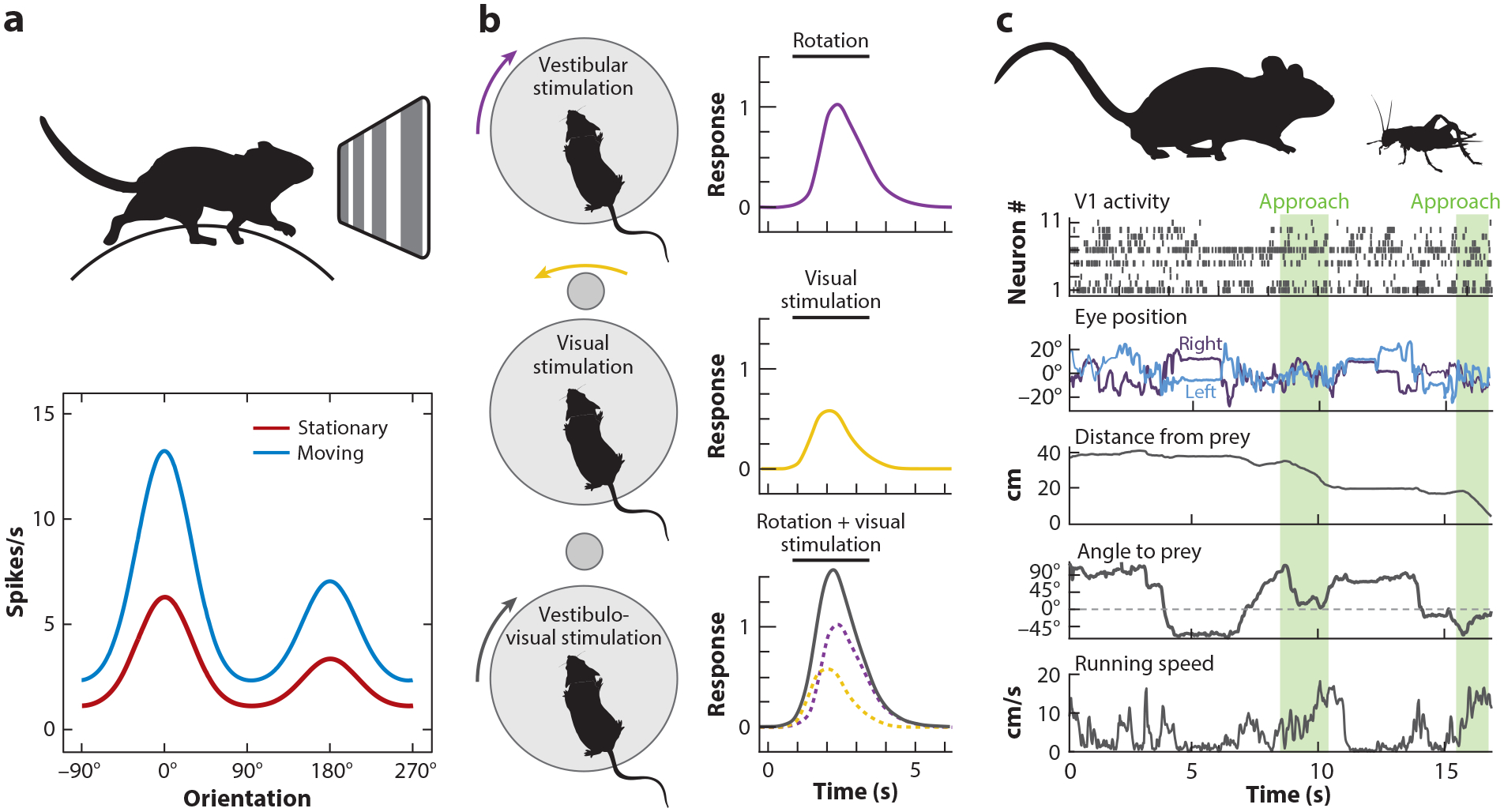
Nonvisual signals in primary visual cortex (V1). (a) The orientation tuning curve measured in head-fixed mice on a spherical treadmill shows a multiplicative increase (gain modulation) when the animal is moving versus stationary. Panel a adapted from Niell & Stryker (2010). (b) L6 neurons respond to vestibular input from rotation (top) and the corresponding rotation of the visual scene (middle), which summate and result in a signal representing head direction (bottom). Panel b adapted from Vélez-Fort et al. (2018). (c) Combining rich quantification of eye and body movements with neural recordings during ethological behavior, for example, cricket hunting, may allow investigation of visual and nonvisual signals in a natural context. Panel c adapted from Meyer et al. (2018) and Michaiel et al. (2020).
Locomotion and Arousal
Locomotion has a profound impact on mouse V1 (Niell & Stryker 2010), roughly doubling the responses to visual stimuli compared to when the animal is stationary (Figure 8a). This effect includes a shift in spatial integration by increasing the drive in the classical RF and decreasing the suppressive surround (Ayaz et al. 2013). Interestingly, locomotion alone can drive neural activity in V1 even in the absence of a visual stimulus (Keller et al. 2012), with a subset of neurons tuned to specific locomotion speeds, as well as differentially combining locomotion speed with visual stimuli (Saleem et al. 2013). Besides locomotion, a wide range of movements, from facial twitches to operant responses, generate activity in V1 (Musall et al. 2019, Stringer et al. 2019c). In fact, the effects of locomotion on V1 are often paralleled by general changes in arousal, although the effects are dissociable as well (Vinck et al. 2015).
At least some of the mechanisms by which locomotion exerts its impact rely on cholinergic neurons in the basal forebrain that project to V1 (Lee et al. 2014). By receiving input from the mesencephalic motor region, which encodes running speed, the basal forebrain is well poised to send locomotion signals to V1. Through the release of acetylcholine onto VIP neurons, locomotion likely engages a disinhibitory circuit involving the suppression of SOM neurons, as described above in the section titled Contextual Modulation (Fu et al. 2014). Measurement of the activity of three inhibitory neurons subtypes, however, along with computational modeling (Dipoppa et al. 2018), suggests that locomotion likely modulates multiple aspects of the cortical circuit rather than acting through a single mechanism.
Movement-Based Visual Computations
Locomotor signals represent at least two aspects of behavior: (a) the overall behavioral state (e.g., stationary versus moving, unalert versus aroused), which may serve as a global control and act through neuromodulatory mechanisms, and (b) the detailed structure of the movements themselves. The latter may serve to account for the effect of movement on the visual input itself. Indeed, the largest source of visual motion on the retina is generated by self-motion, i.e., the motion of the head and eyes relative to the visual scene, rather than by objects in the visual scene. Incorporating movement information can enable an organism to more accurately reconstruct the visual scene by correcting for self-motion, as well as by enabling the extraction of additional features such as depth through motion parallax (Leopold & Park 2020, Parker et al. 2020). Consistent with these roles, neurons in rat V1 encode a representation of the three-dimensional rotation of the head in space (Guitchounts et al. 2020). Likewise, the speed and direction of movement play predictive roles in processing visual inputs in V1, resulting in mismatch signals when the visual input does not correspond to that expected during locomotion (Leinweber et al. 2017). These predictive signals arise from anterior cingulate and secondary motor cortex, and their impact on V1 is retinotopically specific. Contextual signals, including locomotion and visuo-motor mismatch, are delivered to V1 from pulvinar as well (Roth et al. 2016).
Vestibular signals are another important source of information about head movements relative to the visual scene, and they strongly modulate V1 activity across all layers (Bouvier et al. 2020, Vélez-Fort et al. 2018) (Figure 8b). Furthermore, L6 excitatory neurons sum vestibular activity with motion of the visual scene to create a representation that integrates internal and external motion signals (Vélez-Fort et al. 2018). The impact of vestibular signals on V1 neurons across layers switches polarity depending on ambient luminance (Bouvier et al. 2020), suppressing activity in the dark and increasing activity in the light. Notably, this suppression in darkness is dependent on SOM inhibitory neurons, again implicating this neuron type in subtractively regulating V1 activity (Wilson et al. 2012) depending on context. Although some aspects of these movement and head orientation signals can be probed in head-fixed conditions as described here, fully elucidating their role in vision is likely to require studies under freely moving conditions. This is now facilitated by methods to measure head and eye movements, together with neural activity, in naturally behaving mice (Meyer et al. 2018, Michaiel et al. 2020) (Figure 8c).
Additional Behavioral Context Representations
Multiple other aspects of behavioral context are also represented in mouse V1. These include responses to the timing of an anticipated reward (Shuler & Bear 2006), increased response to task-relevant visual stimuli (Poort et al. 2015), responses to other sensory modalities (Ibrahim et al. 2016, Iurilli et al. 2012), and firing in specific locations of a virtual environment independent of the specific visual input, similar to hippocampal place cells (Fiser et al. 2016, Saleem et al. 2018). The specific source of many of these signals and the circuit mechanisms that integrate them into V1 processing are beginning to be identified (Makino & Komiyama 2015, Roth et al. 2016, Zhang et al. 2014). Furthermore, this diversity suggests that their role extends beyond improving the representation of the visual scene (as in compensating for self-motion) to include, for example, amplifying specific features that are relevant for ongoing behavior.
THE RESULTS OF CORTICAL COMPUTATION: V1 OUTPUTS AND BEHAVIOR
The computations performed in V1 only have an impact to the extent their results get conveyed to other brain regions. Studying the outputs from V1 can therefore provide key insight into its essential functions. V1 neurons project to several cortical and subcortical areas. What visual features are encoded in different output cells of V1, and how do these relate to their downstream targets and to the behavior these targets mediate?
V1 Output to Higher Visual Areas
In primates, cortical visual processing is organized in a parallel, hierarchical structure: V1 projects to a series of higher visual cortical areas that respond successively to more complex visual stimuli such as objects in inferior temporal cortex (Nassi & Callaway 2009). Similarly, mouse V1 projects to approximately ten retinotopically organized HVAs (Wang & Burkhalter 2007). Although these HVAs are not arranged in as clear a hierarchy as they are in primates (Nassi & Callaway 2009, Harris et al. 2019), they show some degree of specialization for certain properties of visual stimuli (Andermann et al. 2011, Glickfeld & Olsen 2017, Juavinett & Callaway 2015, La Chioma et al. 2019, Marshel et al. 2011, Murgas et al. 2020, Roth et al. 2012, Sit & Goard 2020). Projections from V1 are often schematized as dedicated pathways, yet among all V1 neurons projecting to HVAs, only 25% target a single HVA, while 60% target two to three HVAs, and 15% target four or more (Han et al. 2018). Still, for neurons targeting multiple HVAs, certain motifs of shared connectivity are more frequent than others. Thus, given that information from a given V1 neuron is not unspecifically broadcast across all HVAs, we can address how distinct computations performed by distinct V1 neurons contribute to the distinct response properties of their target areas.
Two HVAs, one lateral and the other medial relative to V1, called AL (anterolateral) and PM (posteromedial), respectively, represent a good example of this approach. The populations of V1 neurons that project to AL versus PM are largely distinct, and, interestingly, neurons within each population are connected to each other but not to the other population (Kim et al. 2018). Furthermore, these two populations differ in their transcriptional profile (Kim et al. 2020). Importantly, the response properties of AL- and PM-projecting V1 neurons are also different, with neurons projecting to AL preferring high speeds and neurons projecting to PM preferring lower-speed visual stimuli (Glickfeld et al. 2013a) (Figure 9a). Because the target areas AL and PM as a whole also preferentially respond to high- and low-speed visual stimuli, respectively, the unique tuning properties of these areas may, at least in part, be inherited from distinct populations of V1 output neurons (Glickfeld et al. 2013a). A similar conclusion was made regarding the rostrolateral HVA called RL, which inherits its response to directional visual stimuli from a specific set of L2/3 V1 neurons who, themselves, inherit this property from directionally selective retinal ganglion cells (Rasmussen et al. 2020). Thus, for some response properties, pathway specificity may be maintained all the way from the retina to HVAs.
Figure 9.
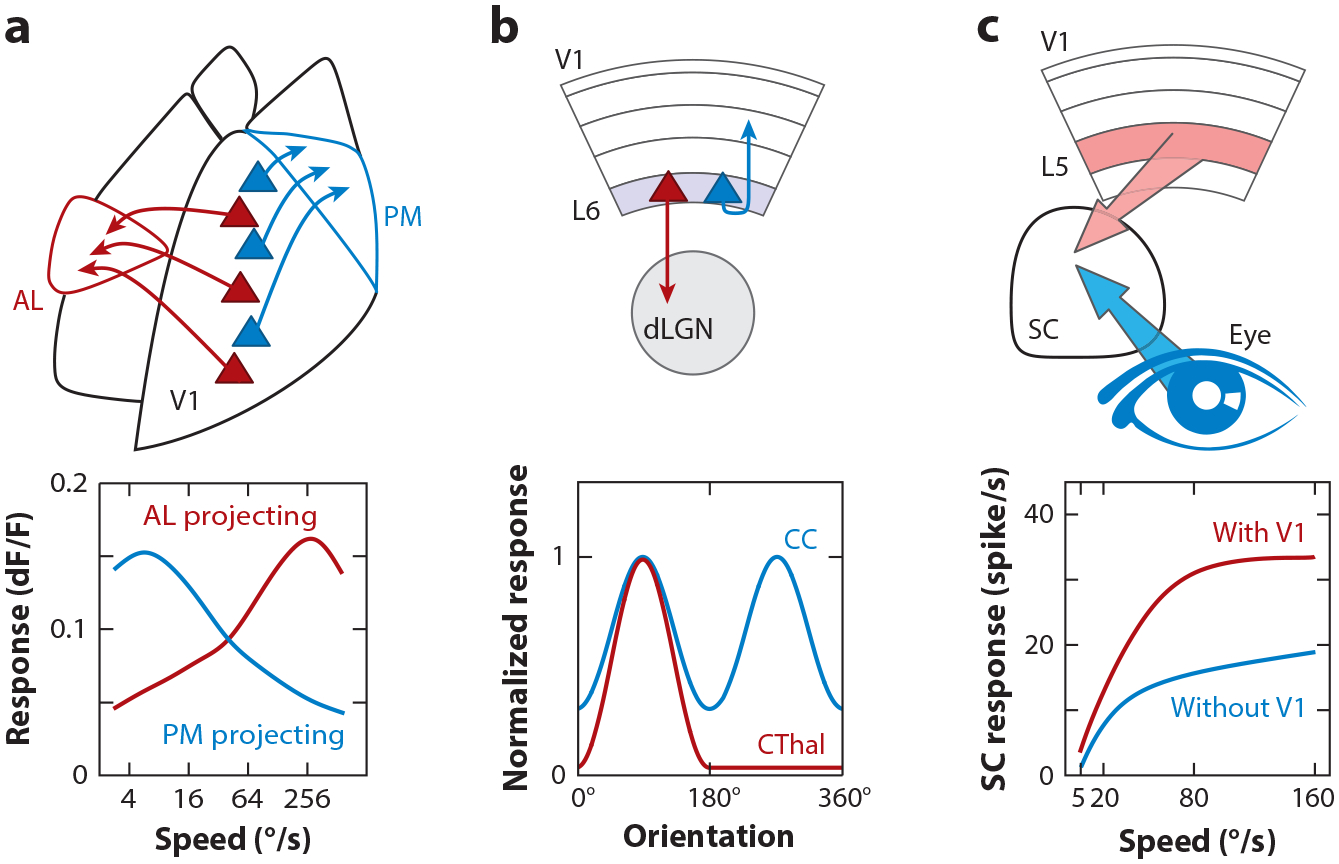
Selectivity and impact of V1 outputs. (a) Two populations of V1 neurons (red, blue) projecting to different higher visual areas, AL and PM, have different speed tuning. Panel a adapted from Glickfeld et al. (2013a). (b) L6 neurons projecting to dLGN (CThal) have sparse and highly selective responses to orientation relative to L6 neurons that project within cortex (CC). Panel b adapted from Vélez-Fort et al. (2014). (c) L5 neurons that project to SC boost the amplitude of response to a looming visual stimulus relative to the retinal input alone, suggesting a role in modulating innate behavior. Panel c adapted from Zhao et al. (2014). Abbreviations: AL, anterolateral; CC, corticocortical; CThal, corticothalamic; dLGN, dorsal lateral geniculate nucleus; L, layer; PM, posteromedial; SC, superior colliculus; V1, primary visual cortex.
These outputs to HVAs are reciprocal. Neurons in V1 receive feedback from the areas to which they project to form loops with cellular, laminar, and functional specificity (D’Souza et al. 2016, Marques et al. 2018, Young et al. 2021). These feedback projections modulate responses in V1, including amplification of tuning properties and mediating contextual modulations (Vangeneugden et al. 2019, Huh et al. 2018, Keller et al. 2020b).
V1 Output to Subcortical Areas and Impact on Behavior
Despite the emphasis on V1 output to the cortical hierarchy, as illustrated by the iconic diagram of Felleman & Van Essen (1991), a large fraction of V1 output actually goes to subcortical areas as well. This includes projections from L6 neurons to the dLGN and the reticular thalamic nucleus (TRN), as well as projections from L5 neurons to higher-order thalamus, which may serve as an alternate pathway to the HVAs (Guillery & Sherman 2002), and to structures associated with behavioral output such as superior colliculus (SC), striatum, accessory optic system (AOS), and amygdala. Therefore, even the low-level visual representations of V1 may directly impact behavior.
L6 neurons projecting back to the dLGN and the TRN form a functionally distinct population, with very sparse firing and high stimulus selectivity, while cortically projecting L6 neurons are more broadly responsive (Vélez-Fort et al. 2014) (Figure 9b). Intriguingly, these thalamic-projecting neurons also receive most of their input not from the local cortical circuit but from higher cortical areas. The exact role of this feedback projection to the primary thalamic nucleus from which V1 receives ascending visual information still needs to be elucidated. Recent studies show that it contributes to the sharpening of the RF of dLGN through surround suppression (Born et al. 2021), most likely through the strong disynaptic inhibition mediated by TRN neurons (Olsen et al. 2012).
Little is known regarding what information is conveyed from V1 to the different subcortical targets and how this compares to projections to cortical areas. In L5, neurons projecting to SC have, on average, broader tuning and higher contrast sensitivity to drifting gratings than do neurons projecting to the striatum, while cortically projecting neurons are intermediate between these two (Lur et al. 2016). This suggests that outputs to SC are specialized for detection, while those to striatum are specialized for discrimination. However, these differences represent more of a bias than a true segregation. Most likely, we have not yet determined the stimuli or contexts that best differentiate the response properties between these populations of L5 neurons.
SC thus receives two sources of visual information: one directly ascending from the retina and the other descending from cortex. Projections from V1 strongly impact visual responses in the SC by increasing their gain (Zhao et al. 2014) (Figure 9c). As a consequence, this projection potentiates SC-mediated behavior, like the animal freezing in response to a flashed stimulus (Liang et al. 2015). The impact that V1 exerts on the SC may also explain the impairment in performance of basic tasks, such as detecting the presence of a stimulus or detecting the change in orientation of a grating, upon V1 inactivation or lesion (Glickfeld et al. 2013b, Prusky & Douglas 2004, Ruediger & Scanziani 2020). This is especially the case for low-contrast or high–spatial frequency stimuli, consistent with the modulatory impact of V1 on SC.
Through its corticofugal projections to the AOS in the midbrain, a structure that generates the optokinetic reflex (OKR) and that, like the SC, receives direct retinal input, V1 can directly impact image stabilizing reflexes (Liu et al. 2016). Indeed, V1 not only modulates the gain of the OKR but also contributes to its plasticity (Liu et al. 2016). Thus, through the projections to both SC and AOS, V1 provides a signal that boosts the ongoing function of a retinorecipient subcortical region, allowing for fine-tuning and top-down control of innate behaviors.
The functional role of the corticofugal pathway out of V1 to the striatum (Khibnik et al. 2014) is only beginning to be elucidated. V1 provides a major drive for visual responses in this structure (Peters et al. 2021), and specific lesions of this corticofugal pathway show that it contributes to the learning speed of simple detection tasks (Ruediger & Scanziani 2020).
In addition to modulating the function of subcortical structures and the behaviors encoded in those structures, V1 also likely provides instructive information for behavior. This is the case when the animal is trained to discriminate between stimuli rather than to simply detect the presence of a stimulus or a sudden change in the visual environment. The ability of a mouse to, for example, discriminate between dots moving in different directions (Marques et al. 2018) or gratings of different orientations (Poort et al. 2015, Resulaj et al. 2018, Wekselblatt et al. 2016) is brought down to chance levels upon V1 inactivation. Interestingly, for relatively simple discriminations, like the ability to discriminate between gratings with large differences in orientation, even brief time windows of activity in V1, during which most neurons fire either no or one action potential, are sufficient for the animal to perform well above chance (Resulaj et al. 2018). In addition, activation of appropriate ensembles of neurons in V1, using holographic optogenetic stimulation, is sufficient to elicit appropriate behavioral responses in such orientation tasks (Carrillo-Reid et al. 2019, Marshel et al. 2019). Some of these behavioral roles may be mediated through V1’s projections to higher visual areas in the cortical visual pathway. However, at least in one behavioral paradigm, a specific subcortical output pathway from V1 providing instructive information to downstream targets has been identified: L5 neurons projecting to the pons, but not striatum, are necessary for eyeblink conditioning (Tang & Higley 2020). In contrast to the SC- and AOS-dependent behaviors described above, V1 is the primary source of visual input for eyeblink conditioning, and the L5 neurons encode both the behavior output and sensory information. Thus, V1 subcortical output can actually directly mediate, rather than just modulate, visually driven behavior.
The most essential role of V1 in behavior may be to enable learning and flexible processing of visual information based on experience. Training on visual behaviors induces changes in responses of V1 neurons, including stimulus selectivity (Poort et al. 2015), and association with reward and location (Pakan et al. 2018). Furthermore, training in a reaching task increases glutamate receptor levels in V1 (Roth et al. 2020), particularly when the training was performed in the light and, therefore, was presumably visually guided. These changes may refine the representation of the visual scene to better discriminate relevant stimuli or represent the engram of learned visual stimuli. Alternately, these changes may not encode visual information, per se, but the association of visual stimuli with the context that evoked them.
OUTLOOK
The techniques available for use in mouse have enabled cellular and molecular approaches to be applied to system-level phenomena. This research has been extremely successful in answering long-standing questions and has led to the discovery of general principles, such as how diverse inputs are combined to generate new representations, canonical excitatory and inhibitory circuit motifs, and how information is selectively routed to downstream targets.
However, there are still major gaps in our understanding of cortical function as highlighted by the fact that many structural properties of cortex remain unaccounted for relative to the function they may implement: Why do cortical neurons receive such a large number of synapses? Why does cortex need multiple layers and so many cell types? Why are cortical areas that perform apparently different functions such as sensation, motor actions, or executive functions structurally so similar? Or are they? In these respects, it seems that the potential power of the mouse in addressing the circuit basis of cortical computation has not yet been fulfilled.
Beyond technical limitations, there are also conceptual reasons why progress on the big picture may feel limited. Is there a canonical computation performed by cortex, such as predictive coding or Bayesian inference? We still lack a compelling framework regarding cortical function that can guide future research. Without guiding principles, even the most accurate descriptions of cortical properties, while captivating to a biologist’s mind, lack explanatory power. As stated by Barlow, “A wing would be a most mystifying structure if one did not know that birds flew…. Without knowing this, and without understanding something of the principles of flight, a more detailed examination of the wing itself would probably be unrewarding” (Barlow 1961, p. 217).
The discoveries of Hubel & Wiesel (1962), demonstrating that V1 extracts specific features of the visual scene, e.g., the orientation of edges, have led to the influential hypothesis that subsequent stages iterate this process, leading to high-level representations of ethologically relevant stimuli, e.g., faces. However, visual cortex function is likely more than extracting specific features of the visual world. First, as mentioned above, simple circuits of neurons can extract these features, leaving the role of many elements of the cortical network unaccounted for. Second, neurons receive a large number of inputs that are tuned for features that are not matched to their preferred features (Cossell et al. 2015), and it is not clear how these would contribute in a simple feature extraction framework. Critically, even our knowledge of the specific visual features captured by a given neuron does not predict its responses to a range of visual scenes (de Vries et al. 2020). Finally, repeated presentation of the same visual scene leads to responses that vary from trial to trial due to unaccounted variables beyond the visual stimulus (Busse et al. 2017, Engel & Steinmetz 2019).
The explanatory gap between the feature extraction framework and the organization of cortical circuits may result from the fact that we are only probing a narrow region of the parameter space within which cortex generally operates. A key feature that is missing in simplified stimulus paradigms is context, within both the visual scene and the animal’s interaction with the scene. As discussed above, cortical circuits not only compute the classical RF but also embed that response within the ongoing visual and behavioral context. In this view, the classic RF is a low-level representation of a higher-dimensional response to a range of image structures and behavioral associations. Two neurons may respond similarly when probed by gratings (their classical RF) but dramatically differently when probed by images with that same feature in different contexts, like visual scenes in a natural environment. Furthermore, even passive viewing of natural stimuli lacks much of the richness of what visual cortex experiences when an animal moves through its environment, including motion, vestibular and other sensory cues, and predictive signals based on the animal’s experience and intentions. Current approaches in mouse to study cortical processing in rich visual environments, with both virtual reality and natural behavioral paradigms such as hunting or escape behaviors (Hoy et al. 2016, Vale et al. 2017), while maintaining a detailed characterization of behavioral variables (Meyer et al. 2018, Michaiel et al. 2020) (Figure 8c), may allow us to address these issues and lead to a broader understanding of cortical function.
An alternative way to understand what V1 contributes to brain function may be to determine its impact on subcortical targets. Many vertebrates go on with their lives without much cortex to speak of and have been successful at it for eons, yet the brain of mammals dedicates a lot of space to this structure. What does cortex add to the subcortical lizard brain? The cortex, through its exquisite ability to extract specific features of the sensory world, to learn and to predict, may modulate innate behaviors according to prevailing conditions and experience, as discussed above in the section titled V1 Output to Subcortical Areas and Impact on Behavior. This would expand the behavioral repertoire encoded in subcortical structures, a possibility already recognized more than a century ago by Edinger (1908, pp. 453–54): “In mammals we meet a brain which has so large a neoencephalon (neocortex) that we may well expect a subordination of reflexes and instincts to associative and intelligent actions.” Techniques that are available for use in the mouse to study multiple brain areas, and the cell type–specific connections between them, are providing us with the tools to test this hypothesis directly.
CONCLUSION
A tremendous benefit of studying mouse visual cortex has been a shift in how we approach cortical processing, from spike trains and tuning curves in isolation to the cell types and interconnexions raised in Hubel & Wiesel’s original paper quoted in the Introduction. At the same time, there has been a broadening in the scope of study, from examining V1 and the cortical hierarchy in isolation to exploring connectivity and functional interactions with other brain regions, and from studying low-level visual features in isolation to incorporating a range of contextual factors. As a result of these studies, we have gained detailed insight into how cortical circuits mediate a range of visual computations, but this has also revealed the gaps in our knowledge and opened new directions of inquiry. We are now looking forward to the next decade where the mechanistic understanding of computations performed in V1 can be extended into an integrated view of how cortex functions.
Supplementary Material
FUTURE ISSUES.
What are the similarities and differences in anatomical and functional organization between primary visual cortex (V1) and other cortical areas, as well as between mouse V1 and primate V1? Are there fundamental differences in the computations being performed or primarily specializations within a shared architecture?
Why are there so many types of excitatory and inhibitory neurons, even within the broad categories described here? Does each have its own role, such as in the wide diversity of retinal ganglion cells? Or do subtypes represent variations on a theme, such as a violin and a viola, which are functionally similar but tuned to a different parameter range?
What is the role of layers? Do layers represent multiple stages of processing, or do they primarily serve to segregate different input and output pathways?
Does thalamic input only ignite layer 4? Or is the input to other layers also sufficient to drive activity that can propagate through the circuit?
What does recurrent amplification do? Can it be tuned based on context?
To what extent do visual context and behavioral (nonvisual) context share common circuit mechanisms?
Do nonvisual signals change the tuning of neurons for visual features (e.g., preferred orientation) or simply act as multiplicative or additive factors to dial the response to these features up or down?
How separate are the circuits within V1 that eventually lead to distinct output pathways? Are there dedicated pathways through the cortical circuit, or do output neurons selectively integrate responses from a pool of multipurpose representations?
V1 receives many inputs beyond the dorsal lateral geniculate nucleus, including higher-order visual thalamus, higher visual cortical areas, and nonvisual cortical areas such as other sensory and associational cortices. How do these inputs contribute to the computations performed by V1?
ACKNOWLEDGMENTS
We thank members of the Niell and Scanziani labs for many useful discussions. This work was supported by National Institutes of Health grants R01NS118461 and R34NS111669 (to C.M.N.) and R01EY025668 and U19NS107613 (to M.S.).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Adesnik H 2017. Synaptic mechanisms of feature coding in the visual cortex of awake mice. Neuron 95(5):1147–59.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adesnik H, Bruns W, Taniguchi H, Huang ZJ, Scanziani M. 2012. A neural circuit for spatial summation in visual cortex. Nature 490(7419):226–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadian Y, Rubin DB, Miller KD. 2013. Analysis of the stabilized supralinear network. Neural Comput 25(8):1994–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed B, Anderson JC, Douglas RJ, Martin KA, Nelson JC. 1994. Polyneuronal innervation of spiny stellate neurons in cat visual cortex. J. Comp. Neurol 341(1):39–49 [DOI] [PubMed] [Google Scholar]
- Albrecht DG, Geisler WS. 1991. Motion selectivity and the contrast-response function of simple cells in the visual cortex. Vis. Neurosci 7(6):531–46 [DOI] [PubMed] [Google Scholar]
- Albright TD, Stoner GR. 2002. Contextual influences on visual processing. Annu. Rev. Neurosci 25:339–79 [DOI] [PubMed] [Google Scholar]
- Andermann ML, Kerlin AM, Roumis DK, Glickfeld LL, Reid RC. 2011. Functional specialization of mouse higher visual cortical areas. Neuron 72(6):1025–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah BV, Bruns W, Carandini M, Scanziani M. 2012. Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron 73(1):159–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz A, Saleem AB, Schölvinck ML, Carandini M. 2013. Locomotion controls spatial integration in mouse visual cortex. Curr. Biol 23(10):890–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayzenshtat I, Karnani MM, Jackson J, Yuste R. 2016. Cortical control of spatial resolution by VIP+ interneurons. J. Neurosci 36(45):11498–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB. 1961. Possible principles underlying the transformations of sensory messages. In Sensory Communication, ed. WA Rosenblith, pp. 217–34. Cambridge, MA: MIT Press [Google Scholar]
- Bock DD, Lee W-CA, Kerlin AM, Andermann ML, Hood G, et al. 2011. Network anatomy and in vivo physiology of visual cortical neurons. Nature 471(7337):177–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born G, Schneider FA, Erisken S, Klein A, Lao CL, et al. 2021. Corticothalamic feedback sculpts visual spatial integration in mouse thalamus. bioRxiv 104000. 10.1101/2020.05.19.104000 [DOI] [PubMed] [Google Scholar]
- Bortone DS, Olsen SR, Scanziani M. 2014. Translaminar inhibitory cells recruited by layer 6 corticothalamic neurons suppress visual cortex. Neuron 82(2):474–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier G, Senzai Y, Scanziani M. 2020. Head movements control the activity of primary visual cortex in a luminance-dependent manner. Neuron 108:500–11.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunel N 2000. Dynamics of sparsely connected networks of excitatory and inhibitory spiking neurons. J. Comput. Neurosci 8(3):183–208 [DOI] [PubMed] [Google Scholar]
- Bruno RM, Sakmann B. 2006. Cortex is driven by weak but synchronously active thalamocortical synapses. Science 312(5780):1622–27 [DOI] [PubMed] [Google Scholar]
- Busse L, Cardin JA, Chiappe ME, Halassa MM, McGinley MJ, et al. 2017. Sensation during active behaviors. J. Neurosci 37(45):10826–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ. 1995. Summation and division in V1 simple cells. In The Neurobiology of Computation, ed. Bower JM, pp. 59–65. Boston, MA: Springer [Google Scholar]
- Carandini M, Heeger DJ. 2011. Normalization as a canonical neural computation. Nat. Rev. Neurosci 13(1):51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Reid L, Han S, Yang W, Akrouh A, Yuste R. 2019. Controlling visually guided behavior by holographic recalling of cortical ensembles. Cell 178(2):447–57.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Zahs KR, Stryker MP. 1991. Relation of cortical cell orientation selectivity to alignment of receptive fields of the geniculocortical afferents that arborize within a single orientation column in ferret visual cortex. J. Neurosci 11(5):1347–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, et al. 2013. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499(7458):295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossell L, Iacaruso MF, Muir DR, Houlton R, Sader EN, et al. 2015. Functional organization of excitatory synaptic strength in primary visual cortex. Nature 518(7539):399–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Martín A, El-Danaf RN, Osakada F, Sriram B, Dhande OS, et al. 2014. A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex. Nature 507(7492):358–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries SEJ, Lecoq JA, Buice MA, Groblewski PA, Ocker GK, et al. 2020. A large-scale standardized physiological survey reveals functional organization of the mouse visual cortex. Nat. Neurosci 23(1):138–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis GC, Ohzawa I, Freeman RD. 1993. Spatiotemporal organization of simple-cell receptive fields in the cat’s striate cortex. II. Linearity of temporal and spatial summation. J. Neurophysiol 69(4):1118–35 [DOI] [PubMed] [Google Scholar]
- Dipoppa M, Ranson A, Krumin M, Pachitariu M, Carandini M, Harris KD. 2018. Vision and locomotion shape the interactions between neuron types in mouse visual cortex. Neuron 98(3):602–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RJ, Martin KAC, Whitteridge D. 1989. A canonical microcircuit for neocortex. Neural Comput 1:480–88 [Google Scholar]
- D’Souza RD, Meier AM, Bista P, Wang Q, Burkhalter A. 2016. Recruitment of inhibition and excitation across mouse visual cortex depends on the hierarchy of interconnecting areas. eLife 5:e19332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger L 1908. The relations of comparative anatomy to comparative psychology. J. Comp. Neurol. Psychol 18:437–57 [Google Scholar]
- Engel TA, Steinmetz NA. 2019. New perspectives on dimensionality and variability from large-scale cortical dynamics. Curr. Opin. Neurobiol 58:181–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhall A 2014. The receptive field is dead. Long live the receptive field? Curr. Opin. Neurobiol 25:ix–xii [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. 1991. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1(1):1–47 [DOI] [PubMed] [Google Scholar]
- Ferster D, Chung S, Wheat H. 1996. Orientation selectivity of thalamic input to simple cells of cat visual cortex. Nature 380(6571):249–52 [DOI] [PubMed] [Google Scholar]
- Ferster D, Miller KD. 2000. Neural mechanisms of orientation selectivity in the visual cortex. Annu. Rev. Neurosci 23:441–71 [DOI] [PubMed] [Google Scholar]
- Fiorani Júnior M, Rosa MG, Gattass R, Rocha-Miranda CE. 1992. Dynamic surrounds of receptive fields in primate striate cortex: a physiological basis for perceptual completion? PNAS 89(18):8547–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiser A, Mahringer D, Oyibo HK, Petersen AV, Leinweber M, Keller GB. 2016. Experience-dependent spatial expectations in mouse visual cortex. Nat. Neurosci 19(12):1658–64 [DOI] [PubMed] [Google Scholar]
- Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, et al. 2014. A cortical circuit for gain control by behavioral state. Cell 156(6):1139–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Marin V, Kelly JG, Hawken MJ. 2019. Major feedforward thalamic input into layer 4C of primary visual cortex in primate. Cereb. Cortex 29(1):134–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. 1990. The influence of contextual stimuli on the orientation selectivity of cells in primary visual cortex of the cat. Vision Res 30(11):1689–701 [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Andermann ML, Bonin V, Reid RC. 2013a. Cortico-cortical projections in mouse visual cortex are functionally target specific. Nat. Neurosci 16(2):219–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickfeld LL, Histed MH, Maunsell JHR. 2013b. Mouse primary visual cortex is used to detect both orientation and contrast changes. J. Neurosci 33(50):19416–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickfeld LL, Olsen SR. 2017. Higher-order areas of the mouse visual cortex. Annu. Rev. Vis. Sci 3:251–73 [DOI] [PubMed] [Google Scholar]
- Gouwens NW, Sorensen SA, Berg J, Lee C, Jarsky T, et al. 2019. Classification of electrophysiological and morphological neuron types in the mouse visual cortex. Nat. Neurosci 22(7):1182–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery RW, Sherman SM. 2002. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron 33(2):163–75 [DOI] [PubMed] [Google Scholar]
- Guitchounts G, Masís J, Wolff SBE, Cox D. 2020. Encoding of 3D head orienting movements in the primary visual cortex. Neuron 108:512–25.e4 [DOI] [PubMed] [Google Scholar]
- Haider B, Häusser M, Carandini M. 2013. Inhibition dominates sensory responses in the awake cortex. Nature 493(7430):97–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Kebschull JM, Campbell RAA, Cowan D, Imhof F, et al. 2018. The logic of single-cell projections from visual cortex. Nature 556(7699):51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Mihalas S, Hirokawa KE, Whitesell JD, Choi H, et al. 2019. Hierarchical organization of cortical and thalamic connectivity. Nature 575(7781):195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier D, Fiscella M, Drinnenberg A, Trenholm S, Rompani SB, et al. 2017. Causal evidence for retina-dependent and -independent visual motion computations in mouse cortex. Nat. Neurosci 20(7):960–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer SB, Ko H, Pichler B, Vogelstein J, Ros H, et al. 2011. Differential connectivity and response dynamics of excitatory and inhibitory neurons in visual cortex. Nat. Neurosci 14(8):1045–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy JL, Yavorska I, Wehr M, Niell CM. 2016. Vision drives accurate approach behavior during prey capture in laboratory mice. Curr. Biol 26(22):3046–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Ruff DA, Pyle R, Rosenbaum R, Cohen MR, Doiron B. 2019. Circuit models of low-dimensional shared variability in cortical networks. Neuron 101(2):337–48.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. 1959. Receptive fields of single neurones in the cat’s striate cortex. J. Physiol 148:574–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. 1962. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J. Physiol 160(1):106–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. 1968. Receptive fields and functional architecture of monkey striate cortex. J. Physiol 195(1):215–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Niell CM. 2011. What can mice tell us about how vision works? Trends Neurosci 34(9):464–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh CYL, Peach JP, Bennett C, Vega RM, Hestrin S. 2018. Feature-specific organization of feedback pathways in mouse visual cortex. Curr. Biol 28(1):114–20.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacaruso MF, Gasler IT, Hofer SB. 2017. Synaptic organization of visual space in primary visual cortex. Nature 547(7664):449–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim LA, Mesik L, Ji X-Y, Fang Q, Li H-F, et al. 2016. Cross-modality sharpening of visual cortical processing through layer-1-mediated inhibition and disinhibition. Neuron 89(5):1031–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. 2011. How inhibition shapes cortical activity. Neuron 72(2):231–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iurilli G, Ghezzi D, Olcese U, Lassi G, Nazzaro C, et al. 2012. Sound-driven synaptic inhibition in primary visual cortex. Neuron 73(4):814–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X-Y, Zingg B, Mesik L, Xiao Z, Zhang LI, Tao HW. 2016. Thalamocortical innervation pattern in mouse auditory and visual cortex: laminar and cell-type specificity. Cereb. Cortex 26(6):2612–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Shen S, Cadwell CR, Berens P, Sinz F, et al. 2015. Principles of connectivity among morphologically defined cell types in adult neocortex. Science 350(6264):aac9462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Grieve KL, Wang W, Sillito AM. 2001. Surround suppression in primate V1. J. Neurophysiol 86(4):2011–28 [DOI] [PubMed] [Google Scholar]
- Juavinett AL, Callaway EM. 2015. Pattern and component motion responses in mouse visual cortical areas. Curr. Biol 25(13):1759–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnani MM, Jackson J, Ayzenshtat I, Tucciarone J, Manoocheri K, et al. 2016. Cooperative subnetworks of molecularly similar interneurons in mouse neocortex. Neuron 90(1):86–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller AJ, Dipoppa M, Roth MM, Caudill MS, Ingrosso A, et al. 2020a. A disinhibitory circuit for contextual modulation in primary visual cortex. Neuron 108:1181–93.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller AJ, Roth MM, Scanziani M. 2020b. Feedback generates a second receptive field in neurons of the visual cortex. Nature 582(7813):545–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller GB, Bonhoeffer T, Hübener M. 2012. Sensorimotor mismatch signals in primary visual cortex of the behaving mouse. Neuron 74(5):809–15 [DOI] [PubMed] [Google Scholar]
- Kerlin AM, Andermann ML, Berezovskii VK, Reid RC. 2010. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron 67(5):858–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khibnik LA, Tritsch NX, Sabatini BL. 2014. A direct projection from mouse primary visual cortex to dorsomedial striatum. PLoS One 9(8):e104501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Zhang Z, Huang L, Ito-Cole T, Jacobs MW, et al. 2020. Extraction of distinct neuronal cell types from within a genetically continuous population. Neuron 107(2):274–82.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M-H, Znamenskiy P, Iacaruso MF, Mrsic-Flogel TD. 2018. Segregated subnetworks of intracortical projection neurons in primary visual cortex. Neuron 100(6):1313–21.e6 [DOI] [PubMed] [Google Scholar]
- Ko H, Hofer SB, Pichler B, Buchanan KA, Sjöström PJ, Mrsic-Flogel TD. 2011. Functional specificity of local synaptic connections in neocortical networks. Nature 473(7345):87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn A, Coen-Cagli R, Kanitscheider I, Pouget A. 2016. Correlations and neuronal population information. Annu. Rev. Neurosci 39:237–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Chioma A, Bonhoeffer T, Hübener M. 2019. Area-specific mapping of binocular disparity across mouse visual cortex. Curr. Biol 29(17):2954–60.e5 [DOI] [PubMed] [Google Scholar]
- Lee AM, Hoy JL, Bonci A, Wilbrecht L, Stryker MP, Niell CM. 2014. Identification of a brainstem circuit regulating visual cortical state in parallel with locomotion. Neuron 83(2):455–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K-S, Vandemark K, Mezey D, Shultz N, Fitzpatrick D. 2019. Functional synaptic architecture of callosal inputs in mouse primary visual cortex. Neuron 101(3):421–28.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. 2010. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J. Neurosci 30(50):16796–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-H, Kwan AC, Zhang S, Phoumthipphavong V, Flannery JG, et al. 2012. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature 488:379–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W-CA, Bonin V, Reed M, Graham BJ, Hood G, et al. 2016. Anatomy and function of an excitatory network in the visual cortex. Nature 532:370–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinweber M, Ward DR, Sobczak JM, Attinger A, Keller GB. 2017. A sensorimotor circuit in mouse cortex for visual flow predictions. Neuron 95:1420–32.e5 [DOI] [PubMed] [Google Scholar]
- Leopold DA, Park SH. 2020. Studying the visual brain in its natural rhythm. Neuroimage 216:116790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-T, Ibrahim LA, Liu B-H, Zhang LI, Tao HW. 2013. Linear transformation of thalamocortical input by intracortical excitation. Nat. Neurosci 16(9):1324–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-T, Liu B-H, Chou X-L, Zhang LI, Tao HW. 2015. Strengthening of direction selectivity by broadly tuned and spatiotemporally slightly offset inhibition in mouse visual cortex. Cereb. Cortex 25(9):2466–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-T, Ma W-P, Li L-Y, Ibrahim LA, Wang S-Z, Tao HW. 2012. Broadening of inhibitory tuning underlies contrast-dependent sharpening of orientation selectivity in mouse visual cortex. J. Neurosci 32(46):16466–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, Xiong XR, Zingg B, Ji X-Y, Zhang LI, Tao HW. 2015. Sensory cortical control of a visually induced arrest behavior via corticotectal projections. Neuron 86(3):755–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien AD, Scanziani M. 2013. Tuned thalamic excitation is amplified by visual cortical circuits. Nat. Neurosci 16(9):1315–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien AD, Scanziani M. 2018. Cortical direction selectivity emerges at convergence of thalamic synapses. Nature 558(7708):80–86 [DOI] [PubMed] [Google Scholar]
- Liu B-H, Huberman AD, Scanziani M. 2016. Cortico-fugal output from visual cortex promotes plasticity of innate motor behaviour. Nature 538(7625):383–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B-H, Li P, Li Y-T, Sun YJ, Yanagawa Y, et al. 2009. Visual receptive field structure of cortical inhibitory neurons revealed by two-photon imaging guided recording. J. Neurosci 29(34):10520–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B-H, Li Y-T, Ma W-P, Pan C-J, Zhang LI, Tao HW. 2011. Broad inhibition sharpens orientation selectivity by expanding input dynamic range in mouse simple cells. Neuron 71(3):542–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS. 1998. Mechanisms of direction selectivity in macaque V1. Neuron 20(3):509–26 [DOI] [PubMed] [Google Scholar]
- Longordo F, To M-S, Ikeda K, Stuart GJ. 2013. Sublinear integration underlies binocular processing in primary visual cortex. Nat. Neurosci 16(6):714–23 [DOI] [PubMed] [Google Scholar]
- Lur G, Vinck MA, Tang L, Cardin JA, Higley MJ. 2016. Projection-specific visual feature encoding by layer 5 cortical subnetworks. Cell Rep 14(11):2538–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W-P, Liu B-H, Li Y-T, Huang ZJ, Zhang LI, Tao HW. 2010. Visual representations by cortical somatostatin inhibitory neurons—selective but with weak and delayed responses. J. Neurosci 30(43):14371–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino H, Komiyama T. 2015. Learning enhances the relative impact of top-down processing in the visual cortex. Nat. Neurosci 18(8):1116–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpeli JG, Schiller PH, Colby CL. 1981. Response properties of single cells in monkey striate cortex during reversible inactivation of individual lateral geniculate laminae. J. Neurophysiol 46(5):1102–19 [DOI] [PubMed] [Google Scholar]
- Marques T, Summers MT, Fioreze G, Fridman M, Dias RF, et al. 2018. A role for mouse primary visual cortex in motion perception. Curr. Biol 28(11):1703–13.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshel JH, Garrett ME, Nauhaus I, Callaway EM. 2011. Functional specialization of seven mouse visual cortical areas. Neuron 72(6):1040–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshel JH, Kaye AP, Nauhaus I, Callaway EM. 2012. Anterior-posterior direction opponency in the superficial mouse lateral geniculate nucleus. Neuron 76(4):713–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshel JH, Kim YS, Machado TA, Quirin S, Benson B, et al. 2019. Cortical layer-specific critical dynamics triggering perception. Science 365(6453):eaaw5202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean J, Palmer LA. 1989. Contribution of linear spatiotemporal receptive field structure to velocity selectivity of simple cells in area 17 of cat. Vision Res 29(6):675–79 [DOI] [PubMed] [Google Scholar]
- Meinecke DL, Peters A. 1987. GABA immunoreactive neurons in rat visual cortex. J. Comp. Neurol 261(3):388–404 [DOI] [PubMed] [Google Scholar]
- Meyer AF, Poort J, O’Keefe J, Sahani M, Linden JF. 2018. A head-mounted camera system integrates detailed behavioral monitoring with multichannel electrophysiology in freely moving mice. Neuron 100(1):46–60.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaiel AM, Abe ET, Niell CM. 2020. Dynamics of gaze control during prey capture in freely moving mice. eLife 9:e57458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD. 2016. Canonical computations of cerebral cortex. Curr. Opin. Neurobiol 37:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman DJ, Ocker GK, Caldejon S, Kato I, Larkin JD, et al. 2019. VIP interneurons selectively enhance weak but behaviorally-relevant stimuli. bioRxiv 858001. 10.1101/858001 [DOI] [Google Scholar]
- Morgenstern NA, Bourg J, Petreanu L. 2016. Multilaminar networks of cortical neurons integrate common inputs from sensory thalamus. Nat. Neurosci 19(8):1034–40 [DOI] [PubMed] [Google Scholar]
- Movshon JA, Thompson ID, Tolhurst DJ. 1978. Spatial summation in the receptive fields of simple cells in the cat’s striate cortex. J. Physiol 283:53–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgas KA, Wilson AM, Michael V, Glickfeld LL. 2020. Unique spatial integration in mouse primary visual cortex and higher visual areas. J. Neurosci 40(9):1862–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BK, Miller KD. 2009. Balanced amplification: a new mechanism of selective amplification of neural activity patterns. Neuron 61(4):635–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musall S, Kaufman MT, Juavinett AL, Gluf S, Churchland AK. 2019. Single-trial neural dynamics are dominated by richly varied movements. Nat. Neurosci 22(10):1677–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassi JJ, Callaway EM. 2009. Parallel processing strategies of the primate visual system. Nat. Rev. Neurosci 10(5):360–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. 2008. Highly selective receptive fields in mouse visual cortex. J. Neurosci 28(30):7520–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. 2010. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65(4):472–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminen L, Angelucci A. 2014. Multiple components of surround modulation in primary visual cortex: multiple neural circuits with multiple functions? Vision Res 104:47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Bortone DS, Adesnik H, Scanziani M. 2012. Gain control by layer six in cortical circuits of vision. Nature 483(7387):47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakan JMP, Currie SP, Fischer L, Rochefort NL. 2018. The impact of visual cues, reward, and motor feedback on the representation of behaviorally relevant spatial locations in primary visual cortex. Cell Rep 24(10):2521–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzeri S, Macke JH, Gross J, Kayser C. 2015. Neural population coding: combining insights from microscopic and mass signals. Trends Cogn. Sci 19(3):162–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker PRL, Brown MA, Smear MC, Niell CM. 2020. Movement-related signals in sensory areas: roles in natural behavior. Trends Neurosci 43(8):581–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AJ, Fabre JMJ, Steinmetz NA, Harris KD, Carandini M. 2021. Striatal activity topographically reflects cortical activity. Nature 591(7850):420–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. 2013. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat. Neurosci 16(8):1068–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscopo DM, El-Danaf RN, Huberman AD, Niell CM. 2013. Diverse visual features encoded in mouse lateral geniculate nucleus. J. Neurosci 33(11):4642–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poort J, Khan AG, Pachitariu M, Nemri A, Orsolic I, et al. 2015. Learning enhances sensory and multiple non-sensory representations in primary visual cortex. Neuron 86(6):1478–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe NJ, Ferster D. 2008. Inhibition, spike threshold, and stimulus selectivity in primary visual cortex. Neuron 57(4):482–97 [DOI] [PubMed] [Google Scholar]
- Prusky GT, Douglas RM. 2004. Characterization of mouse cortical spatial vision. Vision Res (28):3411–18 [DOI] [PubMed] [Google Scholar]
- Rasmussen R, Matsumoto A, Dahlstrup Sietam M, Yonehara K. 2020. A segregated cortical stream for retinal direction selectivity. Nat. Commun 11(1):831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RC, Alonso JM. 1995. Specificity of monosynaptic connections from thalamus to visual cortex. Nature 378(6554):281–84 [DOI] [PubMed] [Google Scholar]
- Reid RC, Soodak RE, Shapley RM. 1987. Linear mechanisms of directional selectivity in simple cells of cat striate cortex. PNAS 84(23):8740–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RC, Soodak RE, Shapley RM. 1991. Directional selectivity and spatiotemporal structure of receptive fields of simple cells in cat striate cortex. J. Neurophysiol 66(2):505–29 [DOI] [PubMed] [Google Scholar]
- Reinhold K, Lien AD, Scanziani M. 2015. Distinct recurrent versus afferent dynamics in cortical visual processing. Nat. Neurosci 18(12):1789–97 [DOI] [PubMed] [Google Scholar]
- Resulaj A, Ruediger S, Olsen SR, Scanziani M. 2018. First spikes in visual cortex enable perceptual discrimination. eLife 7:e34044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringach DL. 2021. Sparse thalamocortical convergence. Curr. Biol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AF, Desimone R, Ungerleider LG. 2001. Contextual modulation in primary visual cortex of macaques. J. Neurosci 21(5):1698–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi LF, Harris KD, Carandini M. 2020. Spatial connectivity matches direction selectivity in visual cortex. Nature 588(7839):648–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MM, Dahmen JC, Muir DR, Imhof F, Martini FJ, Hofer SB. 2016. Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex. Nat. Neurosci 19(2):299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MM, Helmchen F, Kampa BM. 2012. Distinct functional properties of primary and posteromedial visual area of mouse neocortex. J. Neurosci 32(28):9716–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RH, Cudmore RH, Tan HL, Hong I, Zhang Y, Huganir RL. 2020. Cortical synaptic AMPA receptor plasticity during motor learning. Neuron 105(5):895–908.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruediger S, Scanziani M. 2020. Learning speed and detection sensitivity controlled by distinct cortico-fugal neurons in visual cortex. eLife 9:e59247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumyantsev OI, Lecoq JA, Hernandez O, Zhang Y, Savall J, et al. 2020. Fundamental bounds on the fidelity of sensory cortical coding. Nature 580(7801):100–105 [DOI] [PubMed] [Google Scholar]
- Runyan CA, Schummers J, Van Wart A, Kuhlman SJ, Wilson NR, et al. 2010. Response features of parvalbumin-expressing interneurons suggest precise roles for subtypes of inhibition in visual cortex. Neuron 67(5):847–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh S, Clopath C. 2021. Inhibitory stabilization and cortical computation. Nat. Rev. Neurosci 22(1):21–37 [DOI] [PubMed] [Google Scholar]
- Saleem AB, Ayaz A, Jeffery KJ, Harris KD, Carandini M. 2013. Integration of visual motion and locomotion in mouse visual cortex. Nat. Neurosci 16(12):1864–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem AB, Diamanti EM, Fournier J, Harris KD, Carandini M. 2018. Coherent encoding of subjective spatial position in visual cortex and hippocampus. Nature 562(7725):124–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnaik R, Chen H, Liu X, Cang J. 2014. Genetic disruption of the On visual pathway affects cortical orientation selectivity and contrast sensitivity in mice. J. Neurophysiol 111(11):2276–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul AB, Humphrey AL. 1992. Evidence of input from lagged cells in the lateral geniculate nucleus to simple cells in cortical area 17 of the cat. J. Neurophysiol 68(4):1190–208 [DOI] [PubMed] [Google Scholar]
- Schnabel UH, Bossens C, Lorteije JAM, Self MW, Op de Beeck H, Roelfsema PR. 2018. Figure-ground perception in the awake mouse and neuronal activity elicited by figure-ground stimuli in primary visual cortex. Sci. Rep 8(1):17800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl B, Tan AYY, Corey J, Priebe NJ. 2013. Emergence of orientation selectivity in the mammalian visual pathway. J. Neurosci 33(26):10616–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook TA, Burbridge TJ, Crair MC, Huberman AD. 2017. Architecture, function, and assembly of the mouse visual system. Annu. Rev. Neurosci 40:499–538 [DOI] [PubMed] [Google Scholar]
- Sedigh-Sarvestani M, Vigeland L, Fernandez-Lamo I, Taylor MM, Palmer LA, Contreras D. 2017. Intracellular, in vivo, dynamics of thalamocortical synapses in visual cortex. J. Neurosci 37(21):5250–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman SC, Campagnola L, Davoudian PA, Hoggarth A, Hage TA, et al. 2018. Sparse recurrent excitatory connectivity in the microcircuit of the adult mouse and human cortex. eLife 7:e37349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self MW, Lorteije JAM, Vangeneugden J, van Beest EH, Grigore ME, et al. 2014. Orientation-tuned surround suppression in mouse visual cortex. J. Neurosci 34(28):9290–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuler MG, Bear MF. 2006. Reward timing in the primary visual cortex. Science 311(5767):1606–9 [DOI] [PubMed] [Google Scholar]
- Sillito AM. 1975. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J. Physiol 250(2):305–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit KK, Goard MJ. 2020. Distributed and retinotopically asymmetric processing of coherent motion in mouse visual cortex. Nat. Commun 11(1):3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SL, Smith IT, Branco T, Häusser M. 2013. Dendritic spikes enhance stimulus selectivity in cortical neurons in vivo. Nature 503(7474):115–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer C, Michaelos M, Pachitariu M. 2019a. High precision coding in visual cortex. bioRxiv 679324. 10.1101/679324 [DOI] [PubMed] [Google Scholar]
- Stringer C, Pachitariu M, Steinmetz N, Carandini M, Harris KD. 2019b. High-dimensional geometry of population responses in visual cortex. Nature 571(7765):361–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer C, Pachitariu M, Steinmetz N, Okun M, Bartho P, et al. 2016. Inhibitory control of correlated intrinsic variability in cortical networks. eLife 5:e19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer C, Pachitariu M, Steinmetz N, Reddy CB, Carandini M, Harris KD. 2019c. Spontaneous behaviors drive multidimensional, brainwide activity. Science 364(6437):eaav7893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Tan Z, Mensh BD, Ji N. 2016. Thalamus provides layer 4 of primary visual cortex with orientation- and direction-tuned inputs. Nat. Neurosci 19(2):308–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Higley MJ. 2020. Layer 5 circuits in V1 differentially control visuomotor behavior. Neuron 105(2):346–54.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasic B, Menon V, Nguyen TN, Kim TK, Jarsky T, et al. 2016. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat. Neurosci 19(2):335–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R, Evans DA, Branco T. 2017. Rapid spatial learning controls instinctive defensive behavior in mice. Curr. Biol 27(9):1342–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh G, Zhang B, Arckens L, Chino YM. 2010. Receptive-field properties of V1 and V2 neurons in mice and macaque monkeys. J. Comp. Neurol 518(11):2051–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vreeswijk C, Sompolinsky H. 1996. Chaos in neuronal networks with balanced excitatory and inhibitory activity. Science 274(5293):1724–26 [DOI] [PubMed] [Google Scholar]
- Vangeneugden J, van Beest EH, Cohen MX, Lorteije JAM, Mukherjee S, et al. 2019. Activity in lateral visual areas contributes to surround suppression in awake mouse V1. Curr. Biol 29(24):4268–75.e7 [DOI] [PubMed] [Google Scholar]
- Vélez-Fort M, Bracey EF, Keshavarzi S, Rousseau CV, Cossell L, et al. 2018. A circuit for integration of head- and visual-motion signals in layer 6 of mouse primary visual cortex. Neuron 98(1):179–91.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez-Fort M, Rousseau CV, Niedworok CJ, Wickersham IR, Rancz EA, et al. 2014. The stimulus selectivity and connectivity of layer six principal cells reveals cortical microcircuits underlying visual processing. Neuron 83(6):1431–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M, Batista-Brito R, Knoblich U, Cardin JA. 2015. Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 86(3):740–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Heydt R, Peterhans E, Baumgartner G. 1984. Illusory contours and cortical neuron responses. Science 224(4654):1260–62 [DOI] [PubMed] [Google Scholar]
- Wang Q, Burkhalter A. 2007. Area map of mouse visual cortex. J. Comp. Neurol 502(3):339–57 [DOI] [PubMed] [Google Scholar]
- Wekselblatt JB, Flister ED, Piscopo DM, Niell CM. 2016. Large-scale imaging of cortical dynamics during sensory perception and behavior. J. Neurophysiol 115(6):2852–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz A, Trenholm S, Yonehara K, Hillier D, Raics Z, et al. 2015. Single-cell-initiated monosynaptic tracing reveals layer-specific cortical network modules. Science 349(6243):70–74 [DOI] [PubMed] [Google Scholar]
- Whiteway MR, Butts DA. 2019. The quest for interpretable models of neural population activity. Curr. Opin. Neurobiol 58:86–93 [DOI] [PubMed] [Google Scholar]
- Wilson NR, Runyan CA, Wang FL, Sur M. 2012. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature 488(7411):343–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Atallah BV, Scanziani M. 2014. Equalizing excitation-inhibition ratios across visual cortical neurons. Nature 511(7511):596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y, Dantzker JLM, Callaway EM. 2005. Excitatory cortical neurons form fine-scale functional networks. Nature 433(7028):868–73 [DOI] [PubMed] [Google Scholar]
- Young H, Belbut B, Baeta M, Petreanu L. 2021. Laminar-specific cortico-cortical loops in mouse visual cortex. eLife 10:e59551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Xu M, Kamigaki T, Hoang Do JP, Chang W-C, et al. 2014. Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science 345(6197):660–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Chen H, Liu X, Cang J. 2013. Orientation-selective responses in the mouse lateral geniculate nucleus. J. Neurosci 33(31):12751–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Liu M, Cang J. 2014. Visual cortex modulates the magnitude but not the selectivity of looming-evoked responses in the superior colliculus of awake mice. Neuron 84(1):202–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohary E, Shadlen MN, Newsome WT. 1994. Correlated neuronal discharge rate and its implications for psychophysical performance. Nature 370(6485):140–43 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


