IMPORTANCE AND OBJECTIVES:
The primary objective of this study was to determine whether expression of the multifunctional and adherens junction-regulating protein, annexin A2 (A2), is altered following cardiopulmonary bypass (CPB). A secondary objective was to determine whether depletion of A2 is associated with post-CPB organ dysfunction in children.
DESIGN:
In a prospective, observational study conducted over a 1-year period in children undergoing cardiac surgery requiring CPB, we analyzed A2 expression in peripheral blood mononuclear cells at different time points. We then assessed the relationship of A2 expression with organ function at each time point in the early postoperative period.
SETTING:
Twenty-three-bed mixed PICU in a tertiary academic center.
PARTICIPANTS:
Patients 1 month to 18 years old undergoing cardiac surgery requiring CPB.
MEAN OUTCOME MEASUREMENTS AND RESULTS:
We analyzed A2 expression in 22 enrolled subjects (n = 9, 1–23 mo old; n = 13, 2–18 yr old) and found a proteolysis-mediated decline in intact A2 immediately after bypass (p = 0.0009), reaching a median of 4% of baseline at 6 hours after bypass (p < 0.0001), and recovery by postoperative day 1. The degree of A2 depletion immediately after bypass in 1–23-month-olds correlated strongly with the extent of organ dysfunction, as measured by PICU admission Vasoactive-Ventilation-Renal (p = 0.004) and PEdiatric Logistic Organ Dysfunction-2 (p = 0.039) scores on postoperative day 1. A2 depletion immediately after bypass also correlated with more protracted requirement for both respiratory support (p = 0.007) and invasive ventilation (p = 0.013) in the 1–23-month-olds.
CONCLUSIONS AND RELEVANCE:
The degree of depletion of A2 following CPB correlates with more severe organ dysfunction, especially acute respiratory compromise in children under 2 years. These findings suggest that loss of A2 may contribute to pulmonary microvascular leak in young children following CPB.
Keywords: annexin A2, cardiopulmonary bypass, endothelium, hypoxia, inflammation, pulmonary edema
RESEARCH IN CONTEXT
Cardiopulmonary bypass often leads to postoperative organ dysfunction, especially acute lung dysfunction, due to systemic inflammation-induced endothelial dysfunction.
The plasma membrane protein annexin A2 maintains pulmonary microvascular integrity in the hypoxic murine lung by preventing phosphorylation of vascular endothelial cadherin at the endothelial-endothelial adherence junction.
This prospective observational study reports the modulation of cellular annexin A2 expression after cardiopulmonary bypass and how it impacts the development of acute postoperative lung dysfunction in children.
AT THE BEDSIDE
This study demonstrates for the first time that annexin A2 becomes degraded after cardiopulmonary bypass and its degree of degradation is associated with worse respiratory dysfunction in young children under 2 years old.
This finding introduces annexin A2 as a potential new target for the investigation of molecular pathology underlying cardiopulmonary bypass-induced acute lung dysfunction in children.
Measures to preserve cellular annexin A2 may ameliorate development of acute postoperative respiratory dysfunction after cardiopulmonary bypass in children.
Acute respiratory dysfunction (ARD) is a common complication following cardiopulmonary bypass (CPB) due to CPB-induced acute lung injury (CPB-ALI) (1). ARD can be mild, with transient hypoxemia, or severe, with need for prolonged mechanical ventilation (1). Both CPB-induced systemic inflammation and ischemia/reperfusion (I/R) injury are implicated in the pathogenesis of CPB-ALI (2). Initiation of CPB triggers an intense systemic inflammatory response (SIR) through sequential activation of serine proteases in the coagulation (3) and complement cascades (4), with increased thrombin generation accompanied by enhanced fibrinolysis (3). The lungs remain relatively ischemic throughout CPB (5), with hypoxia exacerbated by the application of aortic cross-clamp (AXC) (5, 6). I/R injury perpetuates SIR (6) upon removal of the AXC and restoration of blood flow to the heart and lungs through generation of reactive oxygen species and activation of cysteine protease caspases (7). The ensuing severe persistent endothelial cell dysfunction, characterized by capillary leak and microthrombosis (8), can lead to CPB-ALI (2). Therapeutic focus has shifted to targeting endothelial pathways that may mitigate the development of CPB-ALI (9).
Annexin A2 (A2) is a phospholipid-binding protein that promotes cell surface-based fibrinolysis (10) and supports pulmonary microvascular integrity (11). A2 maintains vessel patency and organ perfusion by promoting cell surface-oriented, tissue plasminogen activator-dependent fibrinolysis of microthrombi (12). More recently, we found that A2 protects against hypoxia-induced pulmonary edema by linking vascular endothelial cadherin (VEC) and tyrosine phosphatase at the endothelial-endothelial adherence junction, thereby maintaining pulmonary microvascular integrity in mice (11). A2 up-regulation in response to increased thrombin generation or during hypoxia may be a necessary adaptive response to vascular injury and/or hypoxia, especially in the lungs. We hypothesize that inappropriate activation of proteases directed at A2 can occur during CPB and that maladaptive A2 modulation following CPB can cause disruption of microvascular integrity with ensuing postoperative organ dysfunction, especially in the lungs.
Our study aimed to establish a pattern of A2 modulation in response to CPB and its potential association with clinical parameters of postoperative organ dysfunction in children. We performed an exploratory study analyzing A2 expression on peripheral blood mononuclear cells (PBMCs) from timed blood samples and investigated its correlation to markers of organ dysfunction. Here, we report that depletion of A2 is associated with accelerated organ dysfunction, especially ARD, in the immediate post-CPB period in children.
MATERIALS AND METHODS
In this prospective, observational study, we enrolled 22 consecutive subjects who underwent cardiac surgery at New York Presbyterian Hospital-Weill Cornell Medical Center (WCM) between September 2018 and December 2019. Patients between the ages of 30 days and 18 years who underwent Congenital Heart Disease surgery requiring CPB were included in the study. We obtained informed parental consent for all subjects, as well as assent from subjects between 7 and 18 years old. Patients less than 30 days old or under 3.5 kg in weight were excluded (13). The study was approved by the Institutional Review Board at Weill Cornell Medicine (No. 1709018583) on March 13, 2018. Procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975. Both intraoperative and postoperative management were provided according to institutional standards, including early extubation and diuresis, as detailed in Supplemental Expanded Methods (http://links.lww.com/CCX/B142).
For the primary endpoint, we performed immunoblot analysis of A2 expression on timed PMBC samples. Blood samples (3 mL) were collected in citrate-treated collection tubes following induction of anesthesia (baseline), immediately and 6 hours following completion of CPB, and at 5 am on postoperative day (POD) 1. All samples were stored at 4°C and processed within 24 hours. One mL of whole blood was centrifuged twice at 400g (4°C) to collect platelet poor plasma for biochemical assays. As previously described (14), PBMCs, used as a surrogate for endothelial cells, were isolated from the remaining 2 mL of whole blood using FicollPaque Plus (GE Healthcare Life Sciences, Chicago, IL) density gradient centrifugation. PBMC expression of A2 was analyzed as previously described, with minor modifications detailed in Supplemental Expanded Methods (http://links.lww.com/CCX/B142) (14). All samples were stored immediately in aliquots at –80°C and assayed within 1–3 months of collection. For each sample, the level of intact A2, the highest molecular mass band on western blot, was evaluated by standard densitometry and normalized to PBMC number and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) band density and expressed as A2/104 PBMC/GAPDH. For each subject, timed A2 levels were expressed as a ratio in relation to the baseline densitometry value, which was set at 1.
Assays of plasma interleukin (IL)-6 (V-Plex Plus Human Pro-inflammatory I Kit, Meso Scale Discovery, Rockville, MD) and d-dimer (Enzyme-linked immunosorbent assays; IMUCLONE, Biomedica, Windsor, NS, Canada) were performed in the WCM Clinical & Translational Science Center General Core Laboratory.
For the secondary endpoint, we assessed the association of timed A2 expression to clinical outcome parameters. For all subjects, we collected baseline demographic information, Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery (STAT) mortality categories and noted any preexisting conditions. Intraoperative variables including CPB duration, AXC time, and blood product administration were analyzed. We collected global clinical outcome data including duration of PICU stay, composite organ dysfunction scores, such as the PICU admit/peak/48-hr Vasoactive-Ventilation-Renal (VVR) scores (15), daily PEdiatric Logistic Organ Dysfunction-2 (PELOD-2) score (16), and hospital mortality. For respiratory function, we evaluated the duration of invasive mechanical ventilation (IMV), noninvasive positive pressure ventilation (NIPPV), positive pressure ventilation (PPV), which includes IMV and NIPPV. We also evaluated duration of PPV-free supplemental oxygen, as well as indices of oxygenation. Other organ-specific outcome data collected over the first 48 postoperative hours include duration of inotropic support, peak Vasoactive-Inotrope Score (17), and peak to preoperative creatinine ratio.
All continuous variables are reported as medians with 25th–75th interquartile range. Values for categorical variables are expressed as number with the percentage of the subject population. The one-sampled, two-tailed Wilcoxon signed-rank test was used to compare median ratios of intact A2 to baseline, set as a hypothetical median of 1. The Wilcoxon matched-pairs signed rank test was used to compare medians of cytokine and d-dimer levels at different time points. To assess correlation to median ratios of intact A2 to baseline at each time point, the two-tailed Spearman correlation test was performed for continuous baseline characteristics and organ function metrics, whereas Wilcoxon rank-sum tests or Kruskal-Wallis tests were performed for categorical baseline characteristics, as appropriate. A p value of less than 0.05 was considered statistically significant. The analyses were performed on the whole cohort as well as the age-defined subgroups. Given the exploratory nature of this study, adjustment of multiplicity was not performed and nonsignificant (p ≥ 0.05 to < 0.1) correlations were reported to show the trend (GraphPad Prism v. 9.0.2 Software for Windows, San Diego, CA).
RESULTS
Table 1 shows preoperative and operative clinical variables, as well as major postoperative outcomes for all 22 subjects. Operative procedures performed for each STAT category and organ-specific outcome data are provided in Supplemental Tables 1 and 2 (http://links.lww.com/CCX/B142). We found a statistically significant difference in preoperative body weight but not in other preoperative clinical variables or operative variables, including STAT category, CPB and AXC time, or frequency of steroid administration, between the two age-defined subgroups (n = 9, 1–23 mo; n = 13, 2–18 yr). However, intraoperative blood products were administered more often to the 1–23-month-old group reflecting our institutional practice of adding washed, leukocyte-reduced packed RBCs to the CPB priming solution for most patients under 10 kg (Table 1). The duration of PICU stay did not differ between the two subgroups, and there was no in-hospital mortality or need for reoperation among either subgroup. For all subjects, the median CPB and AXC durations were 90 and 51.5 minutes, respectively (Table 1). None of the study subjects required circulatory arrest.
TABLE 1.
Subject Characteristics and Outcomes
| Characteristics | Entire Cohort (n = 22) | 1–23-mo-olds (n = 9) | 2–18-yr-olds (n = 13) | p a |
|---|---|---|---|---|
| Median age (IQR, mo) | 78.5 (7.8–112) | 7 (4.5–12) | 106 (48–146) | < 0.001 |
| Median weight (IQR, kg) | 18.0 (7.9–33.1) | 6.5 (5.4–9.4) | 30.8 (20.3–53.2) | < 0.001 |
| Gender (male/female) | 12/10 | 4/5 | 8/5 | 0.666 |
| Previous cardiac surgery requiring CPB, n (%) | 5 (22.7) | 1 (11.1) | 5 (38.5) | 0.333 |
| History of chronic lung disease, n (%) | 4 (18.2) | 2 (22.2) | 2 (15.4) | > 0.999 |
| Single ventricle physiology, n (%) | 2 (9.1) | 2 (22.2) | 0 (0) | 0.156 |
| Pediatric Risk of Mortality III score, median (IQR) | 3 (2–5.3) | 3 (1.5–8) | 3 (2–5) | 0.7 |
| Cardiopulmonary bypass time, min, median (IQR) | 90 (60.8–109) | 90 (67–102) | 70 (52.5–110.5) | 0.68 |
| Aortic cross-clamp time, min, median (IQR) | 51.5 (39.5–69) | 48 (44.5–61.5) | 54 (32.5–88) | 0.86 |
| Intraoperative blood products, n (%)b | 11 (50) | 8 (88.9) | 3 (23.1) | 0.008 |
| Intraoperative steroid administration before CPB, n (%)c | 18 (81.8) | 7 (77.8) | 11 (84.6) | > 0.999 |
| Hospital mortality, n (%) | 0 (0) | 0 (0) | 0 (0) | > 0.999 |
| Duration of PICU stay, d, median (IQR) | 4.5 (3–7) | 5 (3–6) | 4 (3–7.5) | 0.96 |
| Need for reoperation within same hospitalization, n (%) | 0 (0) | 0 (0) | 0 (0) | > 0.999 |
CPB = cardiopulmonary bypass, IQR = interquartile range.
Bold entries indicate statistically significant differences between two subgroups.
p values comparing differences between two age groups.
Refers to any blood product that is not subject’s own, i.e., autologous blood or cell saver.
Steroid in the forms of hydrocortisone or dexamethasone at the discretion of anesthesia.
Depletion of Intact A2 Following CPB
Similar to previous reports, we found evidence of robust systemic inflammation and fibrinolysis after CPB (18, 19). There was a 23-fold increase in plasma IL-6 (Supplemental Fig. 1, http://links.lww.com/CCX/B142) and a 2.3-fold increase in d-dimer (Supplemental Fig. 2, http://links.lww.com/CCX/B142) above baseline by POD1. There was no correlation between levels of IL-6 or d-dimer and intact A2 at any of the corresponding time points (Supplemental Table 3, http://links.lww.com/CCX/B142).
In every subject, immunoblot analysis revealed a reproducible pattern of proteolytic breakdown of intact A2 immediately after cessation of CPB, with the disappearance of the parent protein and the appearance of multiple smaller, immunoreactive bands with apparent masses of 10–35 kilodaltons (kDa) (Fig. 1A). This presumptive proteolysis progressed with the appearance of additional fragments by 6 hours after CPB. In the same samples, we noted no degradation of annexin A5 or loading control protein GAPDH, suggesting that the observed event was specific for A2. The subjects’ baseline A2 expression served as its own control with subsequent A2 levels expressed as a fraction of the baseline value. In addition, by analyzing A2 expression in two healthy adult samples processed and stored in parallel for the same duration as study samples, we determined that observed proteolysis was not a storage artifact since we found no breakdown of A2 in the storage control samples (Fig. 1A).
Figure 1.
Proteolytic degradation of annexin A2 (A2) following cardiopulmonary bypass (CPB). A, Representative immunoblots of peripheral blood mononuclear cell (PBMC) A2, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and annexin A5 (A5) over a range of time points in two different subjects (left and right). PBMC lysates were probed with immunoglobulin G directed against A2, GAPDH, and A5. B, Levels of intact A2 following CPB as quantified by densitometry. A2 levels (n = 22) are shown relative to each subject’s baseline value, set at 1. The data were analyzed using a one-sampled two-tailed Wilcoxon signed-rank test comparing median (median with interquartile range) ratios of intact A2 to baseline for statistical significance (GraphPad Prism v. 9.0.2 Software for Windows, San Diego, CA). 6hr p CPB = 6 hr post-cardiopulmonary bypass, Imm p CPB = immediately post-cardiopulmonary bypass, NS = not significant, POD1 = postoperative day 1.
Quantitative analysis revealed that intact A2 declined immediately after CPB to 56% of baseline (p = 0.0009; median) and reached 4% at 6 hours after CPB (p < 0.0001; median) (Fig. 1B). Variable degrees of recovery of A2 occurred by POD1. The degree of depletion or recovery of A2 was not associated with any of the evaluated baseline characteristics including age, weight, operative variables, STAT categories, CPB or AXC time, steroid use, or blood product administration at any study time points. These data reveal that selective proteolysis of A2 is triggered by CPB with the most profound depletion occurring at 6 hours following bypass in all subjects.
Loss of A2 and Organ Function in Younger Patients
To test whether the degradation of A2 might correlate with the postoperative course in individual subjects, we examined its relationship to overall organ function using the PELOD-2 score (16). We also used the VVR score (15), which is more specific for organ dysfunction in children after CPB (15), focusing on the cardiovascular, pulmonary, and renal systems. Subgroup analyses revealed a strong correlation between the degree of A2 depletion after CPB and the severity of postoperative organ dysfunction scores in subjects 1–23 months old. Lower A2 correlated significantly with higher PELOD-2 scores on POD1 (Rho = –0.69; p = 0.045, Fig. 2A), indicating worse overall organ function. Furthermore, lower A2 correlated highly significantly with PICU admission VVR scores (Rho = –0.87; p = 0.004; Fig. 2B), especially elevated ventilation score, reflecting profound respiratory dysfunction, in six of the nine subjects. At 6 hours, however, A2 levels no longer correlated with organ dysfunction scores for the whole cohort, although the older 2–18-year-old group showed a trend toward significance between loss of A2 and VVR score (Rho = –0.54; p = 0.061; Supplemental Table 4, http://links.lww.com/CCX/B142). Together, these data indicate that the degree of A2 loss following CPB correlates with early organ dysfunction in the immediate postoperative period in patients under 2 years old.
Figure 2.
Annexin A2 (A2) levels post-cardiopulmonary bypass and organ dysfunction scores in subjects 1–23 mo old. A, PEdiatric Logistic Organ Dysfunction-2 (PELOD-2) score (16) on postoperative day 1 (POD 1) as a function of A2 level (n = 9). B, Postoperative Vasoactive-Ventilation-Renal (VVR) score (15) upon admission to the PICU as a function of A2 level (n = 9). Statistical significance was evaluated using the two-tailed Spearman (Rho, p) test (GraphPad Prism v. 9.0.2 Software for Windows, San Diego, CA). GAPDH = glyceraldehyde-3-phosphate dehydrogenase, Imm p CPB = immediately post-cardiopulmonary bypass.
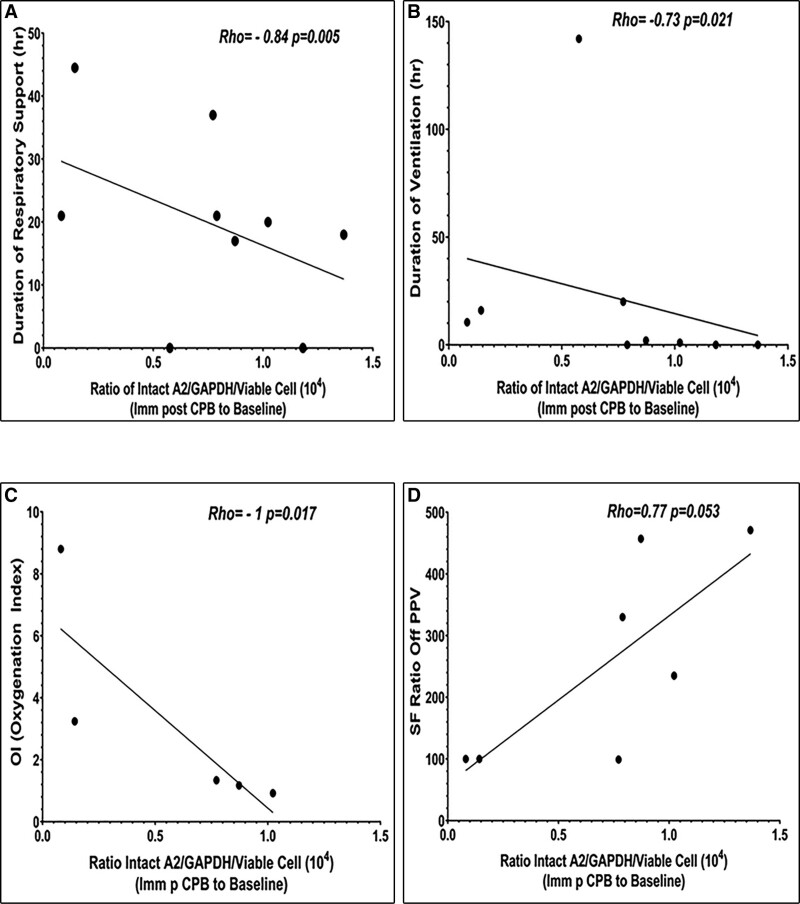
A2 Depletion Correlates With Pronounced Respiratory Dysfunction in Younger Patients
To assess the impact of A2 on postoperative respiratory function, we evaluated the duration of IMV, as well as total duration of respiratory support (DRS), including duration of IMV, NIPPV, and PPV-free oxygen requirement (Fig. 3). We also assessed the oxygenation index (OI) (20) in subjects receiving IMV, as well as the oxygen saturation/Fio2 (SF) (21) ratio in those not receiving PPV (Supplemental Table 4, http://links.lww.com/CCX/B142). Among subjects in the 1–23-month age group, a lower level of intact A2 immediately after CPB correlated significantly with longer DRS (Rho = –0.84; p = 0.005; Fig. 3A) and IMV (Rho = –0.73; p = 0.021; Fig. 3B). In addition, lower intact A2 immediately after bypass in this younger age group, but not in the older 2–18-year-old subjects, correlated with higher OI (Rho = –1; p = 0.017; Fig. 3C) in patients on IMV, and with a lower SF ratio (Rho = 0.77; p = 0.053; Fig. 3D) in those off PPV, reflecting impaired lung function. Of note, data from two subjects with unrepaired cyanotic heart disease were excluded from OI and SF assessments (20, 21). While longer CPB duration and more blood product administration also correlated with a lower SF ratio (Supplemental Table 5, http://links.lww.com/CCX/B142) in the younger age group, it did not correlate with longer DRS, IMV duration or OI. This suggests the potential contribution of A2 to postoperative ARD may be independent of CPB duration or blood product exposure. We found no correlation between A2 and markers of respiratory dysfunction immediately after CPB in 2–18-year-old subjects; at 6 hours, however, there was a trend toward association between lower A2 and total DRS in the older subjects (Rho = –0.52; p = 0.07; Supplemental Table 4, http://links.lww.com/CCX/B142). These data indicate that bypass-associated loss of A2 correlates with profound postoperative respiratory impairment in children under 2 years old.
Figure 3.
Annexin A2 (A2) levels and indices of respiratory dysfunction immediately after cardiopulmonary bypass in 1–23-mo-old subjects. Total duration of respiratory support (n = 9) (A), duration of mechanical ventilation (n = 9) (B), worst oxygenation index (OI) (20) on the ventilator (n = 5) (C), and oxygen saturation/Fio2 (SF) ratio (21) off positive pressure ventilation (PPV) (n = 7) (D) were compared with intact A2. Two-tailed Spearman correlation analysis (Rho, p) was used to evaluate statistical significance (GraphPad Prism v. 9.0.2 Software for Windows, San Diego, CA). GAPDH = glyceraldehyde-3-phosphate dehydrogenase, Imm p CPB = immediately post-cardiopulmonary bypass.
DISCUSSION
To our knowledge, this is the first study to examine the proteolytic processing of A2 in the setting of the sterile SIR associated with CPB. Proteolysis of A2 was a universal finding among all subjects in our study cohort. In addition, we show for the first time that reduction in A2 on PBMCs correlates significantly with overall organ dysfunction, especially pulmonary impairment in the early postoperative period in children under 2 years old.
A2 is a multifunctional protein that is expressed by vascular endothelial cells (22), tumor cells, and macrophages (23). Human monocytes are the major A2-expressing cells in circulating blood (24). A2 consists of a core domain that contains four membrane-binding “annexin repeats” and an amino-terminal domain that interacts with protein S100A10 to form the (A2-S100A10)2 heterotetramer, its primary membrane-associated configuration. Translocation of cytosolic A2 to the cell surface is inducible by thrombin (25) and requires Sarcoma (Src) kinase-mediated phosphorylation of tyrosine 23 (26) within the amino-terminus. The current study revealed a striking and reproducible pattern of proteolysis of A2 following CPB. Using an antibody that recognizes phenylalanine 307 and surrounding residues within its carboxy-terminus of A2 (Supplemental Fig. 3, http://links.lww.com/CCX/B142), we followed progressive degradation of A2 after CPB and the release of fragments containing the protein’s carboxy-terminus. We found the same pattern of proteolysis of A2 immediately following CPB in all subjects studied; degradation of A2 did not appear to be a storage artifact. Depletion of A2 persisted through the first 6 hours following CPB and recovered partially by POD1. We suspect that these smaller A2 segments are less functional due to their lack of an amino-terminus. Interestingly, d-dimer analysis did not demonstrate a decrease in systemic fibrinolysis (Supplemental Fig. 2, http://links.lww.com/CCX/B142) activity, despite the loss of intact A2. This may be attributable to the fact that d-dimer is a better marker for the fluid phase of fibrinolysis but not a sensitive marker for A2-mediated cell surface-based fibrinolysis (14, 26, 27).
A2 is also one of the most abundantly expressed plasma membrane proteins in the lung endothelium (11, 28) and is upregulated under hypoxic conditions through the interaction of hypoxia-inducible-factor-1 transcription factor with a hypoxia-responsive element within its promoter region (29). Recently, we observed that Anxa2–/– mice were highly susceptible to pulmonary edema under hypoxia (11). We demonstrated that A2 forms a complex with VEC and its key phosphatases, VEC tyrosine phosphatase and Src homology phosphatase-2 (11). In the absence of A2, hyperphosphorylation of VEC at tyrosine 731 prevents homotypic VEC-VEC interactions that support endothelial cell adherens junctions in the lung microvasculature, leading to vascular leak. Thus, A2 is instrumental in preventing hypoxia-induced pulmonary edema in the murine lung.
Throughout CPB, lung perfusion is reliant on the bronchial circulation, which is not sufficient to prevent pulmonary tissue hypoxia (5, 30). Upon reperfusion after CPB, oxygen-free radicals augment systemic inflammation and can further exacerbate CPB-ALI (31). In the murine lung, activation of Src-kinase with phosphorylation of VEC is known to engender pulmonary microvascular permeability in the post-CPB period (32). The ensuing pulmonary edema and leukocyte infiltration are likely proximal causes of ARD (2). Our study noted a strong association between A2 depletion and organ function impairment after CPB, especially respiratory dysfunction. Extreme loss of A2 correlated with longer duration of all forms of respiratory support, including assisted ventilation and supplemental oxygen requirement in children under 2 years old. These findings may reflect increased pulmonary vascular leak and pulmonary edema, as previously demonstrated in A2-deficient hypoxic murine lung (11).
We propose that compromised pulmonary function after CPB may result from loss of A2 and compromised vascular integrity in the lung (11). In our most vulnerable, younger patients, we found that the level of A2 was inversely proportional to the intensity of needed respiratory support. This could reflect increased pulmonary vascular leak, or possibly reduced surfactant production by type II alveolar cells, to which A2 has been linked (33). Clinically, disturbed pulmonary microvascular integrity manifests itself as the need for prolonged respiratory dysfunction and impaired oxygenation.
The current study provides the framework for a larger prospective examination of A2 in CPB. The limitations of this study include the small sample size, which precluded co-variant analyses to exclude potential confounding factors. Blood sampling limitations did not allow us to include neonates (13), a subpopulation whose postoperative course is associated with greater morbidity and mortality (34). Also, while a control group was not needed to perform the primary analysis of changes in A2 in individual subjects pre- and post-CPB; the absence of a control group limits our ability to determine whether the proteolytic process observed is unique to CPB or may be seen in other systemic inflammatory processes. In addition, we used PBMCs as a surrogate for pulmonary microvascular endothelial cells, which cannot be feasibly obtained. We intend to conduct additional studies to demonstrate the extent to which PBMCs are faithful surrogates for endothelial cell-based functions (14, 24) beyond fibrinolysis. Finally, we would hope to examine the mechanisms whereby A2 is selectively proteolyzed in CPB and the pathophysiologic pathways by which its loss may compromise organ function.
CONCLUSIONS
This novel observational study demonstrates that A2 is reproducibly and specifically depleted in response to CPB and that loss of functional A2 in PBMCs correlates with early organ injury, especially impaired lung function in young children. Measures to preserve A2 may ameliorate CPB-induced ARD through A2’s ability to support vascular patency and integrity, particularly in children under 2 years old.
ACKNOWLEDGMENTS
We thank Sujit Sheth, MD, for his support and insightful discussions concerning this study.
Supplementary Material
Footnotes
This work was performed at New York Presbyterian Hospital-Weill Cornell Medical Center.
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000457 as well as the Weill Cornell Medicine Department of Pediatrics Pilot Grant 87000482.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Prockop has consulted for ADMA Biotherapeutics, Cell Evolve, and Smart Immune. Dr. Dayton receives consulting fees as a member of Data Safety Monitoring Board at Zogenix. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Drs. Hsing and Hajjar were responsible for all aspects of the work, including conceptualization and design of the study; acquisition, analysis, and interpretation of data for the work; drafting the work and revising it critically for important intellectual content; and final approval of the version to be published. Drs. Stock, Greenwald, Bacha, Flynn, Carroll, Dayton, and Prockop were responsible for analysis and interpretation of data; revising the work critically for intellectual content and providing final approval of version to be published. Ms. Qiu, Ms. Almeida, and Mr. Tamura were responsible for acquisition and analysis of data for the work, revising it critically and providing final approval of version to be published.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Huffmyer JL, Groves DS: Pulmonary complications of cardiopulmonary bypass. Best Pract Res Clin Anaesthesiol 2015; 29:163–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apostolakis E, Filos KS, Dougenis D, et al. : Lung dysfunction following cardiopulmonary bypass. J Card Surg 2010; 25:47–55 [DOI] [PubMed] [Google Scholar]
- 3.Sniecinski RM, Chandler WL: Activation of the hemostatic system during cardiopulmonary bypass. Anesth Analg 2011; 113:1319–1333 [DOI] [PubMed] [Google Scholar]
- 4.Al-Fares A, Pettenuzzo T, Del Sorbo L: Extracorporeal life support and systemic inflammation. Intensive Care Med Exp 2019; 7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chai PJ, Williamson JA, Ungerleider RM, et al. : Effects of ischemia on pulmonary dysfunction after cardiopulmonary bypass. Ann Thorac Surg 1999; 67:731–735 [DOI] [PubMed] [Google Scholar]
- 6.Warren OJ, Smith AJ, Athanasiou T, et al. : The inflammatory response to cardiopulmonary bypass: Part 1--mechanisms of pathogenesis. J Cardiothorac Vasc Anesth 2009; 23:223–231 [DOI] [PubMed] [Google Scholar]
- 7.Lv S, Liu H, Wang H: The interplay between autophagy and NLRP3 inflammasome in ischemia/reperfusion injury. Int J Mol Sci 2021; 22:8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brix-Christensen V: The systemic inflammatory response after cardiac surgery with cardiopulmonary bypass in children. Acta Anaesthesiol Scand 2001; 45:671–679 [DOI] [PubMed] [Google Scholar]
- 9.Giacinto O, Satriano U, Chello M, et al. : Inflammatory response and endothelial dysfunction following cardiopulmonary bypass: Pathophysiology and pharmacological targets. Recent Pat Inflamm Allergy Drug Discov 2019; 13:158–173 [DOI] [PubMed] [Google Scholar]
- 10.Hajjar KA: The biology of annexin A2: From vascular fibrinolysis to innate immunity. Trans Am Clin Climatol Assoc 2015; 126:144–155 [PMC free article] [PubMed] [Google Scholar]
- 11.Luo M, Flood EC, Hajjar KA, et al. : Annexin A2 supports pulmonary microvascular integrity by linking vascular endothelial cadherin and protein tyrosine phosphatases. J Exp Med 2017; 214:2535–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajjar KA, Menell JS: Annexin II: A novel mediator of cell surface plasmin generation. Ann N Y Acad Sci 1997; 811:337–349 [DOI] [PubMed] [Google Scholar]
- 13.Howie SR: Blood sample volumes in child health research: Review of safe limits. Bull World Health Organ 2011; 89:46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fassel H, Chen H, Hajjar KA, et al. : Reduced expression of annexin A2 is associated with impaired cell surface fibrinolysis and venous thromboembolism. Blood 2021; 137:2221–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miletic KG, Delius RE, Mastropietro CW, et al. : Prospective validation of a novel vasoactive-ventilation-renal score as a predictor of outcomes after pediatric cardiac surgery. Ann Thorac Surg 2016; 101:1558–1563 [DOI] [PubMed] [Google Scholar]
- 16.Leteurtre S, Duhamel A, Salleron J, et al. ; Groupe Francophone de Réanimation et d’Urgences Pédiatriques (GFRUP): PELOD-2: An update of the PEdiatric logistic organ dysfunction score. Crit Care Med 2013; 41:1761–1773 [DOI] [PubMed] [Google Scholar]
- 17.Gaies MG, Jeffries HE, Thiagarajan RR, et al. : Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: An analysis from the Pediatric Cardiac Critical Care Consortium and Virtual PICU System Registries. Pediatr Crit Care Med 2014; 15:529–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chew MS, Brandslund I, Tonnesen E, et al. : Tissue injury and the inflammatory response to pediatric cardiac surgery with cardiopulmonary bypass: A descriptive study. Anesthesiology 2001; 94:745–753; discussion 745A [DOI] [PubMed] [Google Scholar]
- 19.Hunt BJ, Parratt RN, Yacoub M, et al. : Activation of coagulation and fibrinolysis during cardiothoracic operations. Ann Thorac Surg 1998; 65:712–718 [DOI] [PubMed] [Google Scholar]
- 20.Ortiz RM, Cilley RE, Bartlett RH: Extracorporeal membrane oxygenation in pediatric respiratory failure. Pediatr Clin North Am 1987; 34:39–46 [DOI] [PubMed] [Google Scholar]
- 21.Pandharipande PP, Shintani AK, Ely EW, et al. : Derivation and validation of Spo2/Fio2 ratio to impute for Pao2/Fio2 ratio in the respiratory component of the Sequential Organ Failure Assessment score. Crit Care Med 2009; 37:1317–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dallacasagrande V, Hajjar KA: Annexin A2 in inflammation and host defense. Cells 2020; 9:1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajjar KA, Krishnan S: Annexin II: A mediator of the plasmin/plasminogen activator system. Trends Cardiovasc Med 1999; 9:128–138 [DOI] [PubMed] [Google Scholar]
- 24.Brownstein C, Deora AB, Hajjar KA, et al. : Annexin II mediates plasminogen-dependent matrix invasion by human monocytes: Enhanced expression by macrophages. Blood 2004; 103:317–324 [DOI] [PubMed] [Google Scholar]
- 25.Peterson EA, Sutherland MR, Pryzdial EL, et al. : Thrombin induces endothelial cell-surface exposure of the plasminogen receptor annexin 2. J Cell Sci 2003; 116:2399–2408 [DOI] [PubMed] [Google Scholar]
- 26.Deora AB, Kreitzer G, Hajjar KA, et al. : An annexin 2 phosphorylation switch mediates p11-dependent translocation of annexin 2 to the cell surface. J Biol Chem 2004; 279:43411–43418 [DOI] [PubMed] [Google Scholar]
- 27.Flood EC, Hajjar KA: The annexin A2 system and vascular homeostasis. Vascul Pharmacol 2011; 54:59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo M, Hajjar KA: Annexin A2 system in human biology: Cell surface and beyond. Semin Thromb Hemost 2013; 39:338–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang B, Deora AB, Hajjar KA, et al. : Hypoxia-inducible factor-1 drives annexin A2 system-mediated perivascular fibrin clearance in oxygen-induced retinopathy in mice. Blood 2011; 118:2918–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlensak C, Doenst T, Beyersdorf F, et al. : Bronchial artery perfusion during cardiopulmonary bypass does not prevent ischemia of the lung in piglets: Assessment of bronchial artery blood flow with fluorescent microspheres. Eur J Cardiothorac Surg 2001; 19:326–331; disciussion 331–322 [DOI] [PubMed] [Google Scholar]
- 31.Friedman M, Sellke FW, Johnson RG, et al. : Parameters of pulmonary injury after total or partial cardiopulmonary bypass. Circulation 1994; 90(5 Pt 2):II262–II268 [PubMed] [Google Scholar]
- 32.Zhang J, Jiang Z, Zhu J, et al. : Cardiopulmonary bypass increases pulmonary microvascular permeability through the Src kinase pathway: Involvement of caveolin-1 and vascular endothelial cadherin. Mol Med Rep 2016; 13:2918–2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh TK, Abonyo B, Liu L, et al. : Reorganization of cytoskeleton during surfactant secretion in lung type II cells: A role of annexin II. Cell Signal 2004; 16:63–70 [DOI] [PubMed] [Google Scholar]
- 34.DeWitt AG, Rossano JW, Zhang W, et al. : Predicting and surviving prolonged critical illness after congenital heart surgery. Crit Care Med 2020; 48:e557–e564 [DOI] [PMC free article] [PubMed] [Google Scholar]