Abstract
Gastrointestinal dysfunction in cystic fibrosis (CF) is a prominent source of pain among patients with CF. Linaclotide, a guanylate cyclase C (GCC) receptor agonist, is a US Food and Drug Administration-approved drug prescribed for chronic constipation but has not been widely used in CF, as the cystic fibrosis transmembrane conductance regulator (CFTR) is the main mechanism of action. However, anecdotal clinical evidence suggests that linaclotide may be effective for treating some gastrointestinal symptoms in CF. The goal of this study was to determine the effectiveness and mechanism of linaclotide in treating CF gastrointestinal disorders using CF mouse models. Intestinal transit, chloride secretion, and intestinal lumen fluidity were assessed in wild-type and CF mouse models in response to linaclotide. CFTR and sodium/hydrogen exchanger 3 (NHE3) response to linaclotide was also evaluated. Linaclotide treatment improved intestinal transit in mice carrying either F508del or null Cftr mutations but did not induce detectable Cl− secretion. Linaclotide increased fluid retention and fluidity of CF intestinal contents, suggesting inhibition of fluid absorption. Targeted inhibition of sodium absorption by the NHE3 inhibitor tenapanor produced improvements in gastrointestinal transit similar to those produced by linaclotide treatment, suggesting that inhibition of fluid absorption by linaclotide contributes to improved gastrointestinal transit in CF. Our results demonstrate that linaclotide improves gastrointestinal transit in CF mouse models by increasing luminal fluidity through inhibiting NHE3-mediated sodium absorption. Further studies are necessary to assess whether linaclotide could improve CF intestinal pathologies in patients. GCC signaling and NHE3 inhibition may be therapeutic targets for CF intestinal manifestations.
NEW & NOTEWORTHY Linaclotide’s primary mechanism of action in alleviating chronic constipation is through cystic fibrosis transmembrane conductance regulator (CFTR), negating its use in patients with cystic fibrosis (CF). For the first time, our findings suggest that in the absence of CFTR, linaclotide can improve fluidity of the intestinal lumen through the inhibition of sodium/hydrogen exchanger 3. These findings suggest that linaclotide could improve CF intestinal pathologies in patients.
Keywords: cystic fibrosis, intestine, linaclotide
INTRODUCTION
Cystic fibrosis (CF) is an autosomal recessive disorder characterized by a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. CFTR is an anion channel that is highly expressed at the apical membrane of epithelial tissues and expressed at lower levels in other tissue types. Patients with CF suffer from a variety of intestinal maladies including pancreatic insufficiency, meconium ileus, distal intestine obstructive syndrome, and constipation (13, 62, 64, 65). Loss of CFTR reduces anion flow into the intestinal lumen, which in turn decreases luminal fluid content, leading to increased amounts of viscous mucus and slowed transit of intestinal contents (23). Treatments of intestinal disorders in patients with CF include laxatives, enema treatments, and surgical intervention. However, patients with CF still experience pain arising from intestinal manifestations despite administration of these current therapies, indicating that more effective treatments are necessary.
Linaclotide is a US Food and Drug Administration-approved drug frequently prescribed for the treatment of chronic idiopathic constipation and irritable bowel syndrome with chronic constipation. Linaclotide is an agonist for the guanylate cyclase C (GCC) receptor (18), an intestinally expressed receptor which increases the production of the second messenger cyclic guanosine monophosphate (cGMP). cGMP is involved in regulating a variety of cellular proteins, including cGMP-dependent protein kinase II (PKG II) and protein kinase A (PKA; 11, 30, 68). Both PKG II and PKA can activate CFTR, increasing luminal chloride and fluid transport, thereby increasing gastrointestinal (GI) transit and relieving intestinal pain (11). In addition, increased cellular cGMP can lead to inhibition of sodium absorption by the sodium/hydrogen exchanger 3 (NHE3), preventing absorption of fluid from the lumen (24).
Currently, linaclotide is not a widely used treatment for constipation in patients with CF due to CFTR being the primary mediator of its effects. However, anecdotal evidence from linaclotide-treated patients with CF at Rainbow Babies and Children’s Hospital indicates efficacy for linaclotide in treating CF intestinal manifestations. Patients with CF who have reported improvements in intestinal manifestations on linaclotide had various CFTR mutations including F508del, in which small amounts of CFTR may be present at the apical membrane, as well as CFTR null mutations, which have no CFTR present at the apical membrane. These findings suggest that linaclotide may have a CFTR-dependent and/or CFTR-independent mechanism of action that is effective for the treatment of CF-related GI disorders.
To examine the efficacy of linaclotide for improving CF intestinal manifestations, we used CF mouse models that display many of the same intestinal manifestations as patients with CF. Mouse models carrying either an F508del mutation or a null mutation in Cftr were evaluated to determine whether any potential benefit of linaclotide was dependent on residual CFTR function. In this study, we demonstrated that linaclotide increases intestinal lumen fluidity and improves small intestinal transit. We hypothesize that this improved luminal fluid content and transit occurs through inhibition of Na+ absorption by linaclotide. This study suggests that linaclotide may have therapeutic applications for the treatment of CF-based intestinal manifestations and that cGMP signaling and/or Na+ absorption inhibition may be therapeutic targets to alleviate CF manifestations in other tissue types.
MATERIALS AND METHODS
Mouse strains.
Two CF mouse models were used in these experiments, one carrying the F508del Cftr mutation (Cftrtm1kth; 71) and one carrying a S489X null Cftr mutation (Cftrtm1Unc; 60). Both mutations are congenic on the C57BL/6J background, and wild-type (WT) littermates were used as controls. All mice were allowed unrestricted access to water and solid chow (Harlan Teklad 7960; Harlan Teklad Global Diets). The Institutional Animal Care and Use Committee of Case Western Reserve University approved all animal protocols.
Measurement of GI transit.
GI transit was measured as previously described (22, 66). Briefly, the mice were fasted overnight with free access to water. The next day, drug or vehicle control (100 µl) was administered to mice by oral gavage. Following this treatment, the mice were also given rhodamine-labeled dextran (100 µl at 25 mg/ml; Sigma-Aldrich) by oral gavage. The mice were euthanized 40 min after the rhodamine dextran treatment, and the GI tract from the stomach to the cecum was removed. The small intestine was divided into 10 segments of equal length and flushed with 2 ml saline. The flushed contents were centrifuged at 500 rpm for 10 min, and 200 µl of the supernatant were placed in a 96-well plate. The fluorescent signal of each segment was quantified using a microplate reader (FLUOstar Omega microplate reader; BMG LabTech), which was used to calculate the geometric center of fluorescence (GCF), a measurement of GI transit. GCF was determined by calculating the fraction of fluorescence per segment multiplied by the segment number and adding all segments together. Linaclotide was administered at a concentration of 50 µg/kg, whereas tenapanor was administered at 1 mg/kg. Linaclotide and tenapanor doses were based on previous animal studies (12, 61, 73) and are similar to human doses when normalizing for body surface area (53).
Intestinal short-circuit measurements.
Short-circuit measurements on intestinal sections were obtained as previously described (33). Muscularis propria was not removed from intestinal segments, and all experiments were done under short-circuit conditions. The change in short-circuit current was calculated after the addition of 10 µM linaclotide to both the apical and basolateral sides of the intestinal sections.
Intestinal organoid harvest and culture from mouse small intestines.
Intestinal organoids were harvested from adult mice as previously described (46, 56). Briefly, the mouse was euthanized, and the small intestine was removed and flushed using PBS. The intestine was cut longitudinally, villi were removed with a razor blade, and the remaining intestine was cut into ~0.5-cm segments and suspended in Gentle Cell Dissociation Reagent (STEMCELL Technologies) in a 50-ml conical tube for 30 min under gentle agitation with a shaker. In a sterile tissue culture hood, the intestinal segments were vigorously shaken by hand for 30 s, and the supernatant was deposited in a 10-cm dish. The segments were resuspended in ice-cold PBS lacking Mg2+ and Ca2+ and vigorously shaken again for 30 s. This process was repeated until four fractions of supernatant were produced. The fraction that was most enriched for intestinal crypts was filtered using a 100-µm cell strainer and pelleted at 450 g for 10 min. The supernatant was discarded, and the pellet was resuspended in 500 µl of a 1:1 mixture of MatriGel (Corning) and IntestiCult Organoid Growth Media (OGM; STEMCELL Technologies). Crypts were diluted to a concentration of 10 crypts/µl and plated into non-tissue-culture-treated 24-well plates, with 35 µl of MatriGel-OGM mixture deposited in each well. The MatriGel-OGM was solidified at 37°C for 15 min, and then 500 µl of OGM were gently deposited in each well. The organoids were stored in a 37°C incubator with 5% CO2. The OGM was changed once every 3 days, and the organoids were passaged once every 7 days.
Quantification of intestinal organoid swelling.
Swelling of intestinal organoids was performed similarly to previously described methods (21, 46, 48). Briefly, intestinal organoids were cultured for 6–8 days, split into non-tissue-culture-treated 48-well plates (Genesee Scientific), and allowed to grow for 24 h before drug treatment (10 µM linaclotide or tenapanor). Swelling was assessed by treating organoids and imaging for 50 min under live cell conditions with bright-field microscopy on a Lionheart FX Automated Microscope (BioTek). Organoid swelling was quantified by identifying and normalizing organoid area to t = 0 with Gen5 ImagePrime software (BioTek). Area under the curve at t = 50 min was calculated to compare statistical significance between treatment groups. All organoids used for swelling quantifications were in culture for <1 mo.
Measurement of intestinal fluid absorption.
The rate of fluid absorption from the intestinal lumen was measured using an in vitro gravimetric method as previously described (19). WT or null mice were euthanized with CO2 asphyxiation, and the small intestine was removed without disturbing the mesentery by cutting immediately below the stomach and immediately above the cecum. The entire intestine was placed into ice-cold HEPES-buffered Ringer solution (HBR, in mM: 138 NaCl, 10 HEPES, 5 KCl, 2.5 Na2HPO4, 1.8 CaCl2, and 1 MgSO4) supplemented with 10 mM glucose. The mesentery was carefully cut away with surgical scissors to untangle the intestine. The jejunum and ileum were removed and discarded, whereas the duodenum was cannulated with a rubber-tipped gavage needle attached to a 50-ml syringe. The syringe was used to flush HBR supplemented with 10 mM glucose and 1 µM indomethacin through intestine to clear any fecal matter. The syringe was then filled with Krebs bicarbonate Ringer solution (KBR, in mM: 115 NaCl, 25 NaHCO3, 5 KCl, 2.5 Na2HPO4, 1.8 CaCl2, 1 MgSO4) supplemented with 10 mM mannitol, 0.1 µM tetrodotoxin, and 1 µM indomethacin. For linaclotide-treated segments, 10 µM linaclotide was included in the KBR solution. The KBR was flushed through the duodenum, and during flushing, the distal end of the duodenum was tied off with surgical suture to inflate the duodenum with KBR. Any remaining mesentery tissue was cut away after inflating the duodenum. The duodenum was divided into 2-cm segments by sliding a suture thread knot ~2 cm up the duodenum and securing the knot tightly. A second suture thread knot was tied ~0.5 cm past the previous knot, and the small segment between the two knots was severed to separate two fluid-filled segments. This process was repeated to produce up to four segments per duodenum. The segments were placed into scintillation vials with 5 ml of KBR supplemented with 10 mM glucose, 0.1 µM tetrodotoxin, and 1 µM indomethacin, with one segment per vial. The segments were incubated in a 37°C water bath for 10 min before beginning measurements. To weigh each segment, the segment was removed from the vial, blotted on filter paper, weighed, and then returned to the vial. The vial was gassed with 95%O2-5% CO2 for 10 s and returned to the 37°C water bath. Segments were weighed at 10-min intervals for 2 h. Tissue wet weight for each segment was recorded by piercing, draining, and weighing the segment following the experiment. Segment weights were normalized by dividing by tissue wet weight.
Luminal fluidity measurements.
Fluidity of intestinal contents was measured as previously described (9). The luminal contents of the small intestine of null mice were collected 1 h after treatment with 50 µg/kg linaclotide or water (100 µl) by oral gavage. The luminal contents were weighed immediately after collection, placed overnight in a 55°C oven, and weighed again in the morning. Fluidity was normalized by dividing wet weight by dry weight.
Cell culture.
Human Caco-2 cells with CFTR deletion were used to test NHE3 inhibition. A subclonal line of Caco-2 was generated by CRISPR/Cas9-mediated mutagenesis, in which a guide RNA recognizing exon 11 was used. In this line, there were two alleles of CFTR created, each carrying frame-shifting insertions (c.1432insCC and c1433.insAA), which resulted in no detectable CFTR protein or function as assessed by Western blot or short-circuit currents, respectively. Five days before performing fluorescence recordings, these nonpolarized human Caco-2 cells with CFTR deletion were seeded onto 18-mm poly-d-lysine-coated coverslips (no. 1 thickness, cat. no. GG-18-PDL; Neuvitro, Vancouver, WA), each placed in a 3.5-cm culture dish, at a density of 1 × 105 cells per dish and cultured at 37°C in a 5% CO2 incubator. Caco-2 cells were grown in Eagle’s minimum essential medium without l-glutamine (Lonza) supplemented with 10% fetal bovine serum, 1% GlutaMAX, and 1% penicillin-streptomycin in a 37°C cell culture incubator with 5% CO2-95% O2.
Solutions.
The standard HEPES-buffered solution (HBS) contained (in mM) 113.6 NaCl, 5 KCl, 2 NaH2PO4, 1 CaCl2, 1.2 MgSO4, 32.5 HEPES, and 10.5 glucose titrated to pH 7.4 at 37°C with NaOH. Twenty millimolar NH4Cl was substituted for 20 mM NaCl in the NH3/-containing solution. Solution osmolalities were measured using a vapor pressure osmometer (no. 5520; Wescor, Logan, UT) and were adjusted to an osmolality of 300 ± 5 (mean ± SE) mosmol/kg.
Compounds.
Stocks of cariporide (4-isopropyl-3-methylsulfonylbenzoylguanidine methanesulfonate, cat. no. 5358; Tocris Bioscience, Bristol, United Kingdom) and S3226 [3-(54)-N-isopropylidene-2-methyl-acrylamide dihydrochloride; Sigma-Aldrich, St. Louis, MO] were dissolved in 100% dimethyl sulfoxide (DMSO), and the stock was diluted so that the working solutions contained ≤0.1% DMSO. A working concentration of 20 μM cariporide was applied to block both NHE1 and NHE2 (4, 44), whereas 10 μM S3226 selectively inhibits NHE3 (59). Linaclotide stocks were dissolved in sterile H2O.
Intracellular pH recovery assay.
NHE activity was assayed as the maximum rate of intracellular pH (pHi) recovery after an acid load [d(pHi/dt)max] imposed by a 20 mM NH4Cl prepulse followed by return to HBS buffer (7, 54). pHi recordings were performed using a previously described imaging setup (8, 55). Briefly, test compounds were added to the culture media of the Caco-2 cell coverslips 30 min before commencing recording. For the final 10 min of the incubation, media were exchanged for HBS containing 10 μM of the pH-sensitive dye precursor 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM, B-1170; Thermo Fisher Scientific, Waltham, MA) and any appropriate test compound(s). The coverslip was then removed from the incubator and assembled to form the floor of the perfusion chamber, which was then mounted on the automated stage of an Olympus IX-81 microscope equipped with epifluorescence imaging. Flow of HBS at 37°C (plus or minus S3226 or cariporide, as appropriate) commenced immediately, along with the excitation of the BCECF, alternately at 440 and 490 nm. Emitted light was captured at wavelengths >530 nm, to record changes in pHi. The exposure time for each of the two excitation wavelengths was 100 ms, separated by ~20 ms. A 440-nm (I440) and 490-nm (I490) intensity data pair was acquired every 5 s. Between the collection of the data pairs, incident light was obstructed. Solutions were delivered to the experimental chamber at 3 ml/min using syringe pumps (model 33; Harvard Apparatus, Holliston, MA). Solutions were selected using a computerized valve system and maintained at a temperature of 37°C by means of a water jacket system placed between the valves and the chamber. Slidebook 6.0.14 software (Intelligent Imaging Innovation, Denver, CO) provided data acquisition.
For each cell analyzed, an area of interest was selected by using the outline tool in Slidebook to encompass the cell body. The rate constant describing the rate of change of I440 (−k440) was calculated continuously throughout experiments as an index of membrane integrity. Examples of −k440 time courses are shown in Fig. 5A, bottom. If the absolute value of −k440 is <5% min–1 (5), we regarded the cells as healthy, and we only included healthy cells in the analyses.
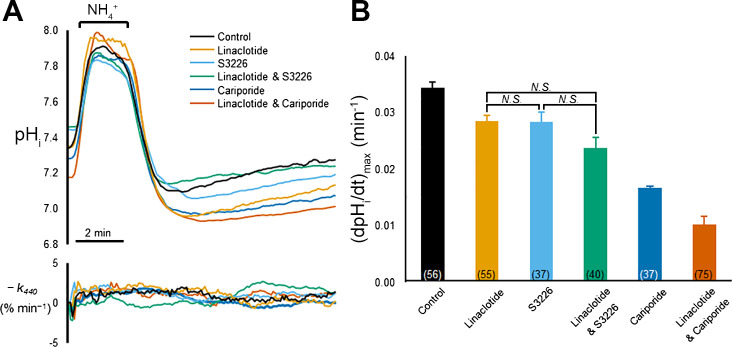
Fig. 5.
Linaclotide treatment inhibits the S3226 [3-(54)-N-isopropylidene-2-methyl-acrylamide dihydrochloride]-sensitive component intracellular pH (pHi) recovery after an acid load in Caco-2 cells. A, top: representative traces of pHi recordings from Caco-2 cells pretreated with different combinations of linaclotide and NHE inhibitors and then subjected to the application and withdrawal of , which imposes an intracellular acid load. Bottom: rate constant describing the loss of the 440-nm-wavelength intensity (I440) signal (−k440) for each of the cells displayed in the pHi recordings. Cells are considered healthy if the absolute value of −k440 is <5% min–1. Linaclotide, cariporide, and S3226 were preincubated with the cultures at 10, 20, and 10 μM, respectively. Cariporide and S3226 were perfused throughout the experiment at the same concentrations at the preincubations. B: summary of maximal rates of pHi recovery from intracellular acid loads [d(pHi/dt)max], imposed by prepulses, for each of the conditions in A. Bars represent means ± SE; number of replicates is in parentheses at the bottom of each bar. All six mean values are significantly different from each other, except for the three comparisons indicated by “N.S.” (not significant) above the brackets. Significant differences were determined by one-way ANOVA, with a Holm-Bonferroni correction (see materials and methods).
pHi values were calculated by using the high-K+-nigericin technique (63). At the end of each recording, Na-free, high-K+ solutions containing 10 μM nigericin (N-1495; Thermo Fisher Scientific) and 135 mM K+, buffered at five pH values of 5.8, 6.4, 7.0, 7.6, and 8.5 were applied for each coverslip. The I490-to-I440 ratio data for each cell are described by a pH titration curve
| (1) |
Equation 1 was used to calculate pHi from I490-to-I440 ratios and the fitted values for the dissociation constant (pKa), the minimum I490-to-I440 ratio (a), and scaling factor (b) for each cell. The initial rate of pHi recoveries from acid loads was analyzed by fitting the pHi-versus-time record with a straight line.
Statistics.
Results are expressed as the means ± SE. Differences between groups were determined using either an ANOVA with post hoc Tukey test or a two-tailed unpaired t-test. A P value of <0.05 was considered significant. The mean d(pHi/dt)max data (Fig. 5B) were analyzed by one-way analysis of variance (ANOVA), and we controlled for type I errors across multiple means comparisons with a Holm-Bonferroni correction (34), setting the family-wise error rate to α = 0.05. Briefly, the unadjusted P values for all n comparisons in each data set were ordered from lowest to highest. For the first test, the lowest unadjusted P value was compared with the first adjusted α-value, α/n. If the null hypothesis is rejected, then the second-lowest P value was compared with the second adjusted α-value, α/(n – 1), and so on, until if at any point the unadjusted P value is greater than or equal to the adjusted α-value, the null hypothesis is accepted, and all subsequent hypotheses in the test group are considered null.
RESULTS
Linaclotide does not activate F508del CFTR.
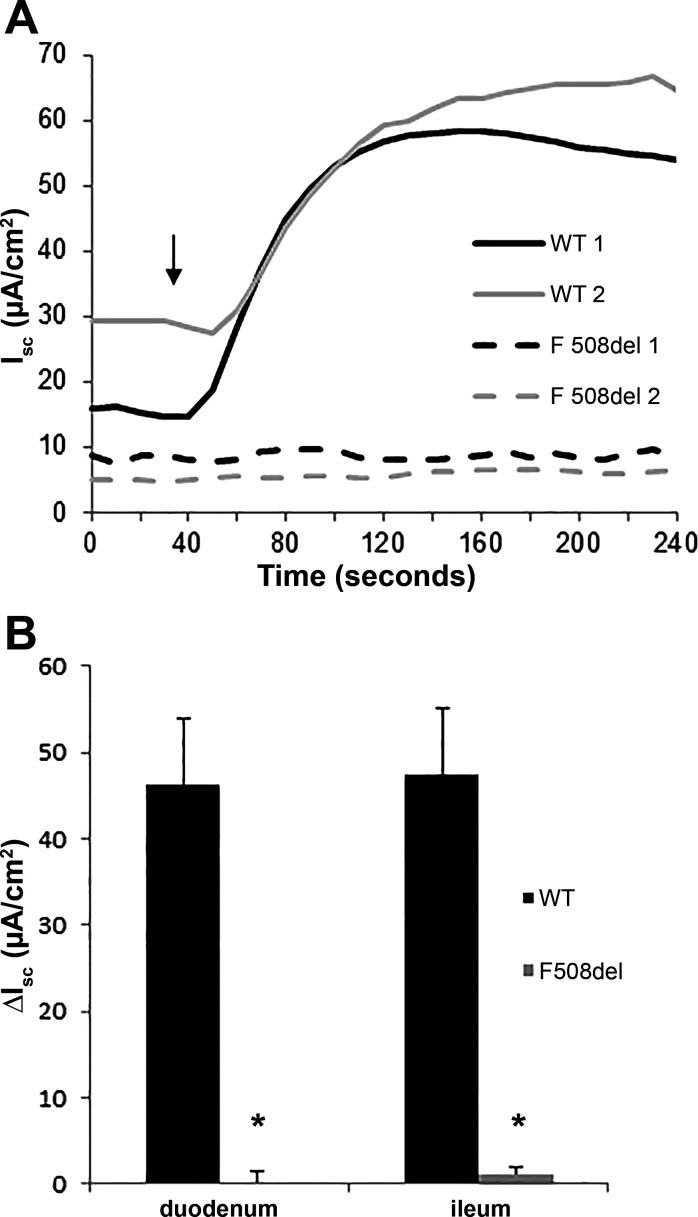
Clinical anecdotal reports suggest that linaclotide may decrease intestinal manifestations in patients who carry the F508del mutation. Improvement of CF intestinal manifestations may be attributable to activation of mutant F508del CFTR by linaclotide. To determine whether anion secretion through F508del CFTR occurs because of linaclotide treatment, we measured short-circuit currents across intestinal sections before and after linaclotide treatment. Duodenum and ileum sections of WT mice displayed a robust change of short-circuit current after the addition of linaclotide, 46.2 and 47.5 µA/cm2, respectively (Fig. 1). However, the duodenum and ileum sections from F08del mice displayed no change in short-circuit current after linaclotide addition, 0.1 and 1.2 µA/cm2, respectively, indicating a lack of CFTR activation (Fig. 1). This result suggests that activation of F508del CFTR is not responsible for the improvement in CF intestinal symptoms mediated by linaclotide that has been reported by patients.
Fig. 1.
Linaclotide does not induce a change in short-circuit current (Isc) in F508del intestinal tissue. A: representative Isc tracings from two wild-type (WT) and F508del duodenums treated with 10 µM linaclotide (indicated by ↓). B: average change in Isc measurements from the duodenum and ileum of WT and F508del mice. (Isc was averaged from a minimum of 4 mice per group ± SE; *P < 0.05.)
Linaclotide increases GI transit in CF mice.
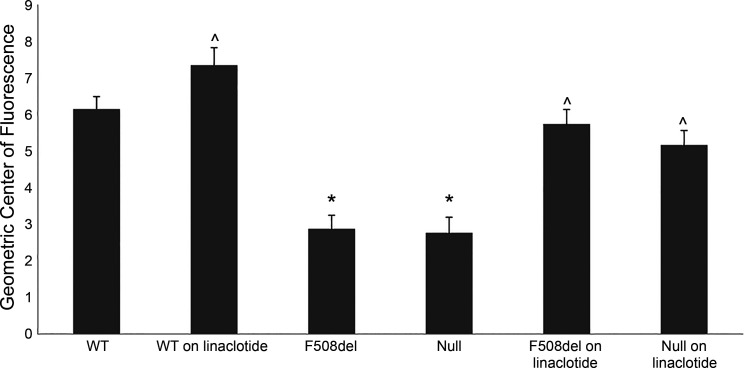
Transit of intestinal contents is significantly slower in the small bowel of patients with CF compared with non-CF individuals (27). Some treatments for CF GI manifestations, such as osmotic laxatives, are aimed at improving intestinal transit. To test whether observations by patients with CF experiencing reduced intestinal pain and improved bowel movement while on linaclotide originate from improved intestinal transit, we assessed rates of GI transit in CF mice carrying the F508del allele or a null allele in response to linaclotide treatment. The effect of linaclotide on intestinal transit in F508del and WT mice was assessed by administering 50 µg/kg linaclotide or vehicle control by oral gavage and assessing the GCF. In this assay, a higher GCF indicates faster transit of intestinal contents. Consistent with previous reports (22, 23, 66), CF mice had significantly slower GI transit than control mice (GCF: F508del, 2.87 ± 0.39; null, 2.77 ± 0.42; control, 6.16 ± 0.32; P < 0.001; Fig. 2). Linaclotide treatment in both the F508del and null mice increased intestinal transit compared with nontreated F508del and null mice (GCF: treated F508del, 5.73 ± 0.41; treated null, 5.16 ± 0.4; P < 0.001 compared with untreated groups, Fig. 2). Additionally, there was a significant increase in transit between linaclotide-treated and nontreated WT mice (GCF: nontreated 6.16 ± 0.32 vs. treated 7.35 ± 0.48; P < 0.05); however, the magnitude of transit increase was less than that in linaclotide-treated CF mice due to the fluorescent dye reaching the end of the small intestine. These data highlight two points regarding linaclotide treatment in CF: 1) linaclotide increases GI transit in CF mice, and 2) this improved transit occurs independently of CFTR protein.
Fig. 2.
Linaclotide treatment improves gastrointestinal transit in F508del and Cftr null mice. Adult wild-type (WT), null, and F508del mice were treated with either 50 µg/kg linaclotide or vehicle control. (Bars represent means ± SE; *P < 0.01 vs. WT, ^P < 0.05 vs. vehicle group of the same genotype, n ≥ 7.)
Linaclotide does not induce fluid secretion in the intestine.
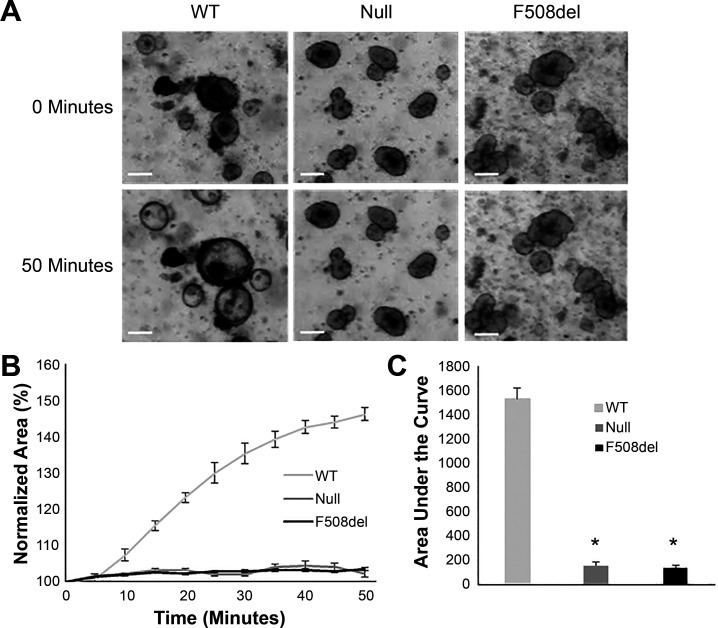
The increased intestinal transit observed in null mice treated with linaclotide suggests a CFTR-independent mechanism of action. CFTR is one of many downstream targets of cGMP signaling, making it possible that a non-CFTR Cl− channel or any other ion channel that can induce fluid secretion could be activated by linaclotide (39, 40, 47, 57). We sought to assess whether fluid secretion through a non-CFTR ion channel is activated by linaclotide. Therefore, we generated intestinal organoids from WT, F508del, and Cftr null mouse small intestines. Activation of CFTR in intestinal organoids increases the salt content in the central organoid lumen, drawing fluid into the lumen. The increased fluid content of the lumen results in a prominent swelling phenotype (Fig. 3A). Activation of Cl− secretion by an alternate Cl− channel or an ion channel that induces fluid secretion is likely to create a similar swelling phenotype. Swelling was observed only in WT organoids following treatment with 10 µM linaclotide, with null and F508del organoids displaying no swelling (Fig. 3A). The organoid swelling was quantified as a change in area with a significant increase in area of WT organoids versus no change in area of CF organoids (normalized percent area at 50 min: WT, 146 ± 1.9; F508del, 103 ± 0.4; null, 102 ± 0.9; Fig. 3B). In an additional analysis, area under the curve demonstrated significant differences between WT and both CF intestinal organoid lines as well (WT, 1,537.5 ± 85.5; F508del, 138.9 ± 17.1; null, 156.9 ± 29.5; P < 0.0001, Fig. 3C). The lack of swelling in F508del and null organoids indicates that linaclotide does not induce fluid secretion in the CF intestine.
Fig. 3.
Linaclotide does not induce swelling of F508del or null intestinal organoids. A: representative images at t = 0 and t = 50 min of intestinal organoids harvested from wild-type (WT), F508del, or null mice following treatment with 10 µM linaclotide. Scale bar = 100 µm. B: total change in area was measured over 50 min and normalized to area at t = 0. Each curve represents the average of at least four wells. C: area under the curve for normalized measurements for each treatment group (baseline set to 100%; *P < 0.01, n ≥ 4 wells).
Linaclotide inhibits fluid absorption in the CF intestine.
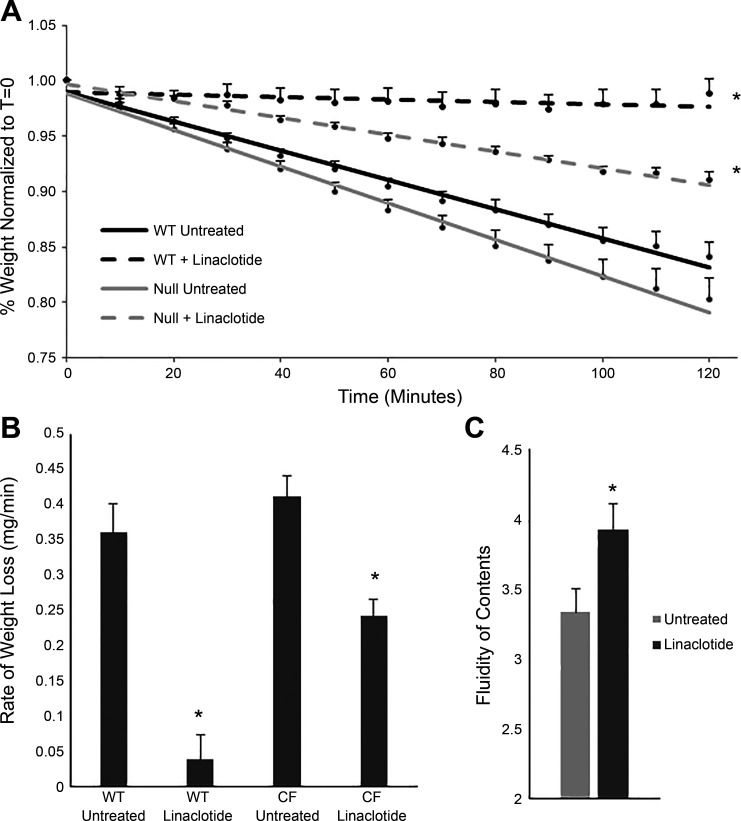
Another mechanism by which linaclotide could improve GI transit is inhibition of fluid absorption. Decreased absorption of fluids may improve fluidity of the intestinal lumen, increasing GI transit. To determine whether inhibition of fluid absorption by linaclotide can improve fluid content in the CF intestine, we assessed the rate of fluid absorption from intestinal segments using an in vitro intestinal fluid absorption assay. In this assay, increased retention of segment weight is indicative of an increased lumen solute content. The weight gain or loss of the segment over time reflects net fluid flux, which includes CFTR-dependent fluid secretion and parallel Na+/H+ and Cl−/ exchanger-dependent fluid absorption. Untreated WT segments retained slightly more fluid than untreated CF segments likely because of CFTR-dependent basal fluid secretion, but the difference between the two groups was not significant (P = 0.074). The weight of WT segments treated with linaclotide stayed consistent during the experiment, indicating activation of CFTR and increased fluid content of the intestinal lumen compared with untreated WT segments (percent final weight normalized to starting weight: WT untreated, 84 ± 1.4; WT + linaclotide, 98 ± 1.4; Fig. 4A, P < 0.001). CF intestinal segments treated with linaclotide retained more weight than untreated CF intestinal segments (CF untreated, 80 ± 1.9; CF + linaclotide, 91 ± 0.6; Fig. 4A, P < 0.001). The retention of fluid in linaclotide-treated CF segments was also greater than WT untreated segments (WT untreated, 84 ± 1.4; CF + linaclotide, 91 ± 0.6; Fig. 4A, P < 0.05). When assessing the weight lost per minute over the entire experiment, untreated segments had a greater rate of loss than linaclotide-treated WT segments [WT untreated, 0.36 ± 0.04 (mg/min); WT + linaclotide, 0.04 ± 0.03, P < 0.005], and untreated CF segments had a greater rate of weight loss than linaclotide-treated CF segments (CF untreated, 0.41 ± 0.03; CF + linaclotide, 0.24 ± 0.02; P < 0.005, Fig. 4B). In WT segments, linaclotide activates CFTR, and fluid secretion occurs. In CF segments, CFTR is absent, so no fluid secretion occurs, but linaclotide still leads to increased fluid retention, likely due to inhibition of fluid absorption. The loss of weight in CF segments in the presence of linaclotide may be due to incomplete inhibition of NHE3 by linaclotide or other sodium coupled absorptive processes not affected by linaclotide. To assess whether these in vitro results were representative of the in vivo intestine, we measured fluidity of intestinal contents of CF mice treated with either linaclotide or water. Linaclotide treatment was sufficient to increase the luminal fluid content in the intestine of CF mice compared with water treatment (wet weight-to-dry weight ratios: untreated, 3.33 ± 0.19; linaclotide-treated, 3.92 ± 0.16; Fig. 4C; P < 0.05). These results suggest that linaclotide increases the fluidity of the CF intestinal lumen, possibly by inhibiting fluid absorption in the absence of CFTR.
Fig. 4.
Linaclotide-treated cystic fibrosis (CF) intestinal segments retain more fluid than untreated segments. A: change in weight over time (t) of duodenal segments from adult null and wild-type (WT) mice that were linaclotide treated or untreated (*P < 0.001 compared with untreated controls for both WT and CF, n ≥ 6 segments from at least 3 mice per group). Treatment groups are represented by best-fit lines. B: rate of weight loss (mg/min) in each treatment group pictured in A (*P < 0.01 vs. untreated groups for both WT and CF). C: fluidity of the intestinal contents in linaclotide-treated and untreated null animals (*P < 0.05, n ≥ 5).
Linaclotide inhibits intestinal sodium absorption.
In addition to having a stimulatory effect on CFTR, cGMP signaling has been shown to inhibit sodium absorption by blocking intestinal NHE3 (14, 15, 24). NHE3 has been implicated as the primary regulator of sodium absorption in the intestinal epithelium (31). Knockout of NHE3 in CF mice has been shown to reduce incidence of obstruction (9), indicating that blockage of NHE3-mediated Na+ absorption is sufficient to improve GI pathology. Inhibition of NHE3 by cGMP signaling may be responsible for reduced fluid absorption during linaclotide treatment. The effect of linaclotide on Na+/H+ exchange was assessed by measuring d(pHi/dt)max in CFTR null Caco-2 cells, following an -induced acid load in nominally CO2/-free HBS. Caco-2 cells express endogenous NHE1, NHE2, and NHE3 (43). Thus, in this assay, a reduction in d(pHi/dt)max would primarily reflect decreased NHE activity, which would be accompanied by a decrease in Na+ uptake. The data show that linaclotide preincubation significantly reduced d(pHi/dt)max compared with nontreated controls (Fig. 5, A, top, and B). The magnitude of the linaclotide-mediated inhibition was equivalent to that of S3226, a specific inhibitor of NHE3 (59). Cotreatment with linaclotide and S3226 caused no significant additional reduction d(pHi/dt)max compared with linaclotide or S3226 alone. Twenty micromolar cariporide caused a substantial reduction in d(pHi/dt)max. Cariporide is a specific inhibitor of NHE1 in the submicromolar range that inhibits NHE2 at doses 20 times those required to inhibit NHE1 (4, 44), Moreover, cotreatment with linaclotide and cariporide significantly reduced d(pHi/dt)max beyond the effect of cariporide alone, which inhibits NHE1 and NHE2 but does not target NHE3. For all experiments, the absolute value of −k440 was <5% min–1, confirming cell viability (Fig. 5A, bottom). The results of Fig. 5, A and B, suggest that linaclotide primarily inhibits the NHE3-mediated component of Na+/H+ exchange. Collectively, the results in Fig. 5 are consistent with the hypothesis that linaclotide-mediated cGMP signaling inhibits NHE3, leading to reduced Na+/H+ exchange and reduced Na+ absorption.
NHE3 inhibition is sufficient to improve GI transit in CF mice.
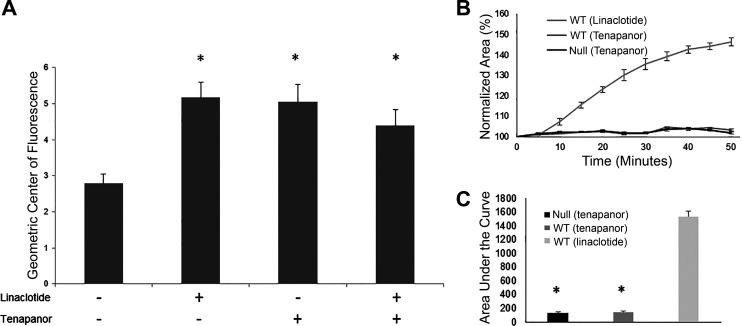
We assessed the effects of NHE3 inhibition on GI transit in the CF mouse intestine using tenapanor, a highly specific NHE3 inhibitor (37, 61). We hypothesized that if NHE3 inhibition is sufficient to increase GI transit in the CF intestine, tenapanor and linaclotide would increase GI transit in a similar manner. We measured transit of intestinal contents in response to 1 mg/kg tenapanor treatment in null mice. Tenapanor significantly increased the rate of intestinal transit compared with nontreated CF mice (GCF: no treatment 2.79 ± 0.25 vs. tenapanor 5.05 ± 0.48, P = 0.0003; Fig. 6A). The increase in GI transit in response to tenapanor was not significantly different from the increase in response to treatment with 50 µg/kg linaclotide (GCF: linaclotide 5.15 ± 0.42 vs. tenapanor 5.05 ± 0.48, P = 0.1). In addition, CF mice treated with both linaclotide and tenapanor displayed a similar GI transit to those treated with either drug alone (4.39 ± 0.42). This result suggests that NHE3 inhibition by linaclotide is sufficient to improve GI transit in CF mice. Finally, WT and null intestinal organoids were treated with tenapanor and displayed no CFTR-dependent swelling (Fig. 6, B and C) confirming the CFTR-independent mechanism of tenapanor.
Fig. 6.
Targeted inhibition of sodium/hydrogen exchanger 3 improves gastrointestinal transit in null mice. A: rates of gastrointestinal transit were measured in adult null mice treated with water, 1 mg/kg tenapanor, 50 µg/kg linaclotide, or both tenapanor and linaclotide. (*P < 0.005, n ≥ 5). B: intestinal organoids were harvested from wild-type (WT) or null mice and treated with either 10 µM linaclotide or 10 µM tenapanor to assess cystic fibrosis transmembrane conductance regulator-dependent swelling. Total change in area was measured over 50 min and normalized to area at t = 0. Each curve represents the average of at least four wells. C: area under the curve for normalized measurements for each treatment group (baseline set to 100%; *P < 0.01, n ≥ 4 wells).
DISCUSSION
The intestinal manifestations of CF are a common source of pain and morbidity among patients with CF. Current treatments for CF intestinal manifestations do not sufficiently prevent or reduce the symptoms of CF intestinal disorders, as indicated by continued discomfort despite the use of these currently available treatments. Thus, new therapies are necessary to increase the quality of life of patients with CF. Linaclotide is a GCC agonist which activates CFTR through cGMP signaling to increase luminal fluidity in the intestine (18). In non-CF patients, linaclotide has been demonstrated to be effective at decreasing symptoms of irritable bowel syndrome with chronic constipation (52). As the primary mechanism of action is through CFTR, linaclotide was not predicted to be effective for patients with CF and thus was not widely prescribed. However, anecdotal reports from patients with CF who were prescribed linaclotide at Rainbow Babies and Children’s Hospital and reported GI improvement suggest that linaclotide may be beneficial in CF. The goal of this study was to examine this observed improvement in CF-related intestinal manifestations by using mouse models with CF that develop GI manifestations similar to those of patients with CF.
To test the effectiveness of linaclotide, we used two independent CF mouse models, one carrying a null Cftr allele and one carrying an F508del allele. Surprisingly, both CF models displayed improved intestinal transit upon treatment with linaclotide. Our data suggest that this improvement was not due to activation of mutant CFTR (in the F508del model) or activation of other ion channels that would induce fluid secretion. This result is in agreement with that of Pattison et al., who did not observe intestinal organoid swelling following linaclotide treatment with CFTR inhibition (48). Rather, our data support a CFTR-independent mechanism for linaclotide in CF.
Increasing fluid content in the CF intestine has proven to have significant benefits for decreasing CF intestinal pathologies. Patients with CF are commonly treated with osmotic laxatives to treat constipation and distal intestine obstructive syndrome. Similarly, osmotic laxatives prevent intestinal obstruction in CF mice (17). Talniflumate, an inhibitor of intestinal Cl−/ exchange, has increased survival in CF mice (67). Inhibition of Cl−/ exchange likely leads to inhibition of Na+/H+ exchange through NHE3 (67). NHE3 is one of several members of the NHE family, displays exclusive expression in the intestine and kidney (70), and has been demonstrated to have a significant absorptive function in these organs (26). NHE3 and CFTR work in conjunction with one another to regulate salt and fluid balance in the intestine, as both proteins colocalize at the apical membrane in the same regulatory complex (28). Interestingly, NHE3 expression is reduced in the absence of CFTR, and CFTR expression is reduced in the absence of NHE3, suggesting a response of the cell to balance electrolyte and water absorption (25). Deletion of NHE3 in the mouse leads to a significant reduction in Na+ absorption in the intestine, increased intestinal luminal fluidity, and severe diarrhea (26, 58). Inhibiting NHE3 in CF has been hypothesized as a possible therapeutic role for CF intestinal pathologies given the contrasting roles they serve in intestinal fluid regulation (9, 25). Bradford et al. tested this hypothesis by creating a double knockout mouse of CFTR and NHE3 (9). NHE3 knockout in CFTR null mice restored hydration of the intestinal lumen, which decreased the incidence of intestinal obstruction and thus increased survival compared with CF mice with functional NHE3 (9). These data suggest that hydration of the intestinal lumen can be preserved by blocking intestinal fluid absorption in the absence of fluid secretion and are consistent with what we observed in the present study. Treating CF mice with linaclotide increased intestinal fluid retention hydrating the intestinal lumen and improving intestinal transit. The absence of CFTR, the main reported mechanism of action for this drug, did not prevent linaclotide from producing significant improvements in CF mice. Although this result was initially surprising, studies have suggested that the water accumulation in the intestine through the GCC receptor and cGMP signaling is more of a consequence of impaired fluid absorption through NHE rather than the result of secretion of electrolytes through CFTR (41, 42). This is supported by our treatment of CF mice with tenapanor, a selective NHE3 inhibitor (37, 61, 73), which displayed intestinal transit that had improved similarly to that of CF mice on linaclotide independent of CFTR. Though tenapanor is not the focus of this study, the data presented here suggest that tenapanor may also be effective at improving GI transit in patients with CF through NHE3 inhibition, but further study is necessary to ensure its safety and efficacy. Whether through linaclotide or tenapanor, blockage of Na+ absorption by inhibition of NHE3 may be sufficient for treating certain CF small intestinal pathologies. In addition, our data using colon cells suggest that linaclotide-mediated NHE3 inhibition occurs in the CF large intestine as well, but further studies are necessary to establish whether linaclotide improves transit in the CF large intestine as it does in non-CF patients (2, 12). Although our studies suggest NHE3 as the main target of linaclotide, a potential direct effect on other transporters in the small intestine such as Slc26a3 [downregulated in adenoma (DRA)] or Slc26a6 [putative anion transporter 1 (PAT1)], which also effect in fluid absorption (69), cannot be ruled out.
Although our data clearly support a CFTR-independent mechanism for increasing intestinal luminal fluid, further studies are necessary to explore possible activation of mutant CFTR by linaclotide through the guanylate cyclase pathway. Previous work has shown that mutant F508del CFTR has small levels of activity in the nasal epithelium when stimulated with C-type natriuretic peptide and forskolin (35). C-type natriuretic peptide increases cellular cGMP levels through interactions with guanylate cyclases, a pathway similar to that used by linaclotide in the intestine. Forskolin increases cAMP levels, also contributing to CFTR activation. This suggests that cGMP signaling alone may be inadequate to activate F508del CFTR, but a combination of cAMP and cGMP signaling may allow F508del CFTR activation. In addition, reduced Cftr expression has been noted in the F508del mouse model (72), most likely producing less F508del CFTR to activate at the apical membrane. Interestingly, linaclotide has been shown to increase WT CFTR trafficking to the cell membrane through the PKG II and possibly PKA pathways (1), raising the possibility that mutant CFTR trafficking could also be modified. Work from Arora et al. supports this suggestion as they observed a small but significant increase of forskolin-induced swelling in F508del patient-derived organoids after 24-h exposure to a GCC agonist (3).
Our results highlight the utility of cGMP signaling to increase intestinal lumen fluid content for the treatment of CF intestinal manifestations. cGMP signaling can be activated in a wide range of tissue types, including the airway, where absence of Cl− secretion leads to Na+ hyperabsorption, contributing to airway dehydration (16, 32). Dehydration in the airway leads to infection, inflammation, reduced lung function, and mortality in patients with CF (20, 38, 45, 49). For many patients with CF, therapies that restore function to CFTR are not available; thus restoration of Cl− secretion is not a viable therapy. However, our data indicate that blockage of Na+ absorption may increase the fluidity of the airway lumen, helping to alleviate the epithelial tissue dehydration found in patients with CF. Whereas the GCC receptor is only expressed specifically in the intestine (10, 51), other guanylate cyclase receptors are present in other tissues, including the airway epithelium (36). Activation of cGMP signaling has been shown to inhibit Na+ absorption through inhibition of epithelial sodium channels (ENaC; 29, 35). Similar to NHE3, ENaC has been proposed to be a therapeutic target for CF, and ENaC inhibition through cGMP signaling in the airway could have hydrating effects on the airway lumen (32, 50). Although the pharmacological effects of linaclotide are restricted to the intestine, the effect of linaclotide on cGMP signaling in CF demonstrates that blocking Na+ absorption may be effective for hydrating the epithelial surface of the airway, which may alleviate certain CF airway pathologies.
In conclusion, the study presented here indicates that linaclotide may restore luminal fluidity to the dehydrated CF intestine leading to improved GI transit. The mechanism of action in CF is CFTR independent and relies on inhibition of Na+ absorption through NHE3. Constitutive inactivation of NHE3 in the CF mouse has been previously studied and led to similar increased intestinal lumen fluidity, decreased formation of obstructions, and improved survival (9). These results indicate that chronic treatment of patients with CF with linaclotide, or NHE3 inhibitors, could result in improvement of CF intestinal manifestations. Identifying which CF intestinal pathologies, such as goblet cell hyperplasia, bacterial overgrowth, and inflammation, are alleviated is still necessary. For instance, the clearance of mucus from the lower villus region and crypt region through NHE3 inhibition may not be as effective, as NHE3 is present in higher amounts in the upper villus compared with the lower villus (6, 9). A combination of linaclotide and osmotic laxative may be necessary in patients with CF to achieve the highest effectiveness in alleviating intestinal discomfort caused by reduced GI transit. A well-designed clinical study to assess specific improvements in GI manifestations in patients with CF prescribed linaclotide is necessary. These studies suggest that the US Food and Drug Administration-approved drug linaclotide may be effective in treating CF intestinal pathologies since an identified mechanism of action is CFTR independent. In addition, these studies suggest that cGMP signaling leading to inhibition of Na+ absorption is a therapeutic target in the CF intestine and perhaps in other organs as well.
GRANTS
This work was supported by Cystic Fibrosis Foundation Grant DRUMM15R0 (to M. L. Drumm).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.R.M., F.J.M., W.F.B., R.C.S., K.M., and C.A.H. conceived and designed research; D.R.M., C.U.C., F.J.M., M.V., D.M.V., S.H., A.J., and C.A.H. performed experiments; D.R.M., F.J.M., M.V., S.H., M.L.D., and C.A.H. analyzed data; D.R.M., C.U.C., F.J.M., T.J.K., M.L.D., W.F.B., R.C.S., K.M., and C.A.H. interpreted results of experiments; D.R.M., F.J.M., and C.A.H. prepared figures; D.R.M. and C.A.H. drafted manuscript; D.R.M., C.U.C., F.J.M., M.V., D.M.V., T.J.K., S.H., A.J., M.L.D., W.F.B., R.C.S., K.M., and C.A.H. edited and revised manuscript; D.R.M., F.J.M., S.H., M.L.D., W.F.B., and C.A.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Alma Wilson, Molly Halligan, and Amanda Barabas of the CF Mouse Models Core at Case Western Reserve University for assistance in maintaining the mouse colony. We thank Dr. Scott Howell of the Case Western Reserve Microscopy and Digital Imaging Core for assistance in imaging organoid swelling. We thank Dr. Patricia Conrad of the Case Western Reserve School of Medicine Light Microscopy Core for assistance in quantifying organoid swelling.
REFERENCES
- 1.Ahsan MK, Tchernychev B, Kessler MM, Solinga RM, Arthur D, Linde CI, Silos-Santiago I, Hannig G, Ameen NA. Linaclotide activates guanylate cyclase-C/cGMP/protein kinase-II-dependent trafficking of CFTR in the intestine. Physiol Rep 5: e13299, 2017. doi: 10.14814/phy2.13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andresen V, Camilleri M, Busciglio IA, Grudell A, Burton D, McKinzie S, Foxx-Orenstein A, Kurtz CB, Sharma V, Johnston JM, Currie MG, Zinsmeister AR. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology 133: 761–768, 2007. doi: 10.1053/j.gastro.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 3.Arora K, Huang Y, Mun K, Yarlagadda S, Sundaram N, Kessler MM, Hannig G, Kurtz CB, Silos-Santiago I, Helmrath M, Palermo JJ, Clancy JP, Steinbrecher KA, Naren AP. Guanylate cyclase 2C agonism corrects CFTR mutants. JCI Insight 2: e93686, 2017. doi: 10.1172/jci.insight.93686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann O, Sonnentag T, Siegel WK, Lamprecht G, Weichert A, Gregor M, Seidler U. Different acid secretagogues activate different Na+/H+ exchanger isoforms in rabbit parietal cells. Am J Physiol Gastrointest Liver Physiol 275: G1085–G1093, 1998. doi: 10.1152/ajpgi.1998.275.5.G1085. [DOI] [PubMed] [Google Scholar]
- 5.Bevensee MO, Schwiening CJ, Boron WF. Use of BCECF and propidium iodide to assess membrane integrity of acutely isolated CA1 neurons from rat hippocampus. J Neurosci Methods 58: 61–75, 1995. doi: 10.1016/0165-0270(94)00159-E. [DOI] [PubMed] [Google Scholar]
- 6.Bookstein C, DePaoli AM, Xie Y, Niu P, Musch MW, Rao MC, Chang EB. Na+/H+ exchangers, NHE-1 and NHE-3, of rat intestine. Expression and localization. J Clin Invest 93: 106–113, 1994. doi: 10.1172/JCI116933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boron WF, De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol 67: 91–112, 1976. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouyer P, Bradley SR, Zhao J, Wang W, Richerson GB, Boron WF. Effect of extracellular acid-base disturbances on the intracellular pH of neurones cultured from rat medullary raphe or hippocampus. J Physiol 559: 85–101, 2004. doi: 10.1113/jphysiol.2004.067793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradford EM, Sartor MA, Gawenis LR, Clarke LL, Shull GE. Reduced NHE3-mediated Na+ absorption increases survival and decreases the incidence of intestinal obstructions in cystic fibrosis mice. Am J Physiol Gastrointest Liver Physiol 296: G886–G898, 2009. doi: 10.1152/ajpgi.90520.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brierley SM. Guanylate cyclase-C receptor activation: unexpected biology. Curr Opin Pharmacol 12: 632–640, 2012. doi: 10.1016/j.coph.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Bryant AP, Busby RW, Bartolini WP, Cordero EA, Hannig G, Kessler MM, Pierce CM, Solinga RM, Tobin JV, Mahajan-Miklos S, Cohen MB, Kurtz CB, Currie MG. Linaclotide is a potent and selective guanylate cyclase C agonist that elicits pharmacological effects locally in the gastrointestinal tract. Life Sci 86: 760–765, 2010. doi: 10.1016/j.lfs.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Busby RW, Kessler MM, Bartolini WP, Bryant AP, Hannig G, Higgins CS, Solinga RM, Tobin JV, Wakefield JD, Kurtz CB, Currie MG. Pharmacologic properties, metabolism, and disposition of linaclotide, a novel therapeutic peptide approved for the treatment of irritable bowel syndrome with constipation and chronic idiopathic constipation. J Pharmacol Exp Ther 344: 196–206, 2013. doi: 10.1124/jpet.112.199430. [DOI] [PubMed] [Google Scholar]
- 13.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 303: G775–G785, 2012. doi: 10.1152/ajpgi.00155.2012. [DOI] [PubMed] [Google Scholar]
- 14.Cha B, Kim JH, Hut H, Hogema BM, Nadarja J, Zizak M, Cavet M, Lee-Kwon W, Lohmann SM, Smolenski A, Tse CM, Yun C, de Jonge HR, Donowitz M. cGMP inhibition of Na+/H+ antiporter 3 (NHE3) requires PDZ domain adapter NHERF2, a broad specificity protein kinase G-anchoring protein. J Biol Chem 280: 16642–16650, 2005. doi: 10.1074/jbc.M500505200. [DOI] [PubMed] [Google Scholar]
- 15.Chen T, Kocinsky HS, Cha B, Murtazina R, Yang J, Tse CM, Singh V, Cole R, Aronson PS, de Jonge H, Sarker R, Donowitz M. Cyclic GMP kinase II (cGKII) inhibits NHE3 by altering its trafficking and phosphorylating NHE3 at three required sites: identification of a multifunctional phosphorylation site. J Biol Chem 290: 1952–1965, 2015. doi: 10.1074/jbc.M114.590174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chinet TC, Fullton JM, Yankaskas JR, Boucher RC, Stutts MJ. Mechanism of sodium hyperabsorption in cultured cystic fibrosis nasal epithelium: a patch-clamp study. Am J Physiol Cell Physiol 266: C1061–C1068, 1994. doi: 10.1152/ajpcell.1994.266.4.C1061. [DOI] [PubMed] [Google Scholar]
- 17.Clarke LL, Gawenis LR, Franklin CL, Harline MC. Increased survival of CFTR knockout mice with an oral osmotic laxative. Lab Anim Sci 46: 612–618, 1996. [PubMed] [Google Scholar]
- 18.Corsetti M, Tack J. Linaclotide: a new drug for the treatment of chronic constipation and irritable bowel syndrome with constipation. United European Gastroenterol J 1: 7–20, 2013. doi: 10.1177/2050640612474446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotton CU, Reuss L. Characterization of epithelial ion transport. In: Epithelial Transport: A Guide to Methods and Experimental Analysis, edited by Wills NK, Reuss L, Lewis SA. London: Chapman and Hall, 1996, p. 70–92. doi: 10.1007/978-94-009-1495-7_4. [DOI] [Google Scholar]
- 20.Davis SD, Ratjen F. Reduced lung function in cystic fibrosis: a primary or secondary phenotype? Am J Respir Crit Care Med 178: 2–3, 2008. doi: 10.1164/rccm.200804-502ED. [DOI] [PubMed] [Google Scholar]
- 21.Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NW, Bijvelds MJ, Scholte BJ, Nieuwenhuis EE, van den Brink S, Clevers H, van der Ent CK, Middendorp S, Beekman JM. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 19: 939–945, 2013. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 22.De Lisle RC. Altered transit and bacterial overgrowth in the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol 293: G104–G111, 2007. doi: 10.1152/ajpgi.00548.2006. [DOI] [PubMed] [Google Scholar]
- 23.De Lisle RC, Borowitz D. The cystic fibrosis intestine. Cold Spring Harb Perspect Med 3: a009753, 2013. doi: 10.1101/cshperspect.a009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donowitz M, Cha B, Zachos NC, Brett CL, Sharma A, Tse CM, Li X. NHERF family and NHE3 regulation. J Physiol 567: 3–11, 2005. doi: 10.1113/jphysiol.2005.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gawenis LR, Hut H, Bot AG, Shull GE, de Jonge HR, Stien X, Miller ML, Clarke LL. Electroneutral sodium absorption and electrogenic anion secretion across murine small intestine are regulated in parallel. Am J Physiol Gastrointest Liver Physiol 287: G1140–G1149, 2004. doi: 10.1152/ajpgi.00177.2004. [DOI] [PubMed] [Google Scholar]
- 26.Gawenis LR, Stien X, Shull GE, Schultheis PJ, Woo AL, Walker NM, Clarke LL. Intestinal NaCl transport in NHE2 and NHE3 knockout mice. Am J Physiol Gastrointest Liver Physiol 282: G776–G784, 2002. doi: 10.1152/ajpgi.00297.2001. [DOI] [PubMed] [Google Scholar]
- 27.Gelfond D, Ma C, Semler J, Borowitz D. Intestinal pH and gastrointestinal transit profiles in cystic fibrosis patients measured by wireless motility capsule. Dig Dis Sci 58: 2275–2281, 2013. doi: 10.1007/s10620-012-2209-1. [DOI] [PubMed] [Google Scholar]
- 28.Guerra L, Fanelli T, Favia M, Riccardi SM, Busco G, Cardone RA, Carrabino S, Weinman EJ, Reshkin SJ, Conese M, Casavola V. Na+/H+ exchanger regulatory factor isoform 1 overexpression modulates cystic fibrosis transmembrane conductance regulator (CFTR) expression and activity in human airway 16HBE14o- cells and rescues ΔF508 CFTR functional expression in cystic fibrosis cells. J Biol Chem 280: 40925–40933, 2005. doi: 10.1074/jbc.M505103200. [DOI] [PubMed] [Google Scholar]
- 29.Guo LJ, Alli AA, Eaton DC, Bao HF. ENaC is regulated by natriuretic peptide receptor-dependent cGMP signaling. Am J Physiol Renal Physiol 304: F930–F937, 2013. doi: 10.1152/ajprenal.00638.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hannig G, Tchernychev B, Kurtz CB, Bryant AP, Currie MG, Silos-Santiago I. Guanylate cyclase-C/cGMP: an emerging pathway in the regulation of visceral pain. Front Mol Neurosci 7: 31, 2014. doi: 10.3389/fnmol.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He P, Yun CC. Mechanisms of the regulation of the intestinal Na+/H+ exchanger NHE3. J Biomed Biotechnol 2010: 238080, 2010. doi: 10.1155/2010/238080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hobbs CA, Da Tan C, Tarran R. Does epithelial sodium channel hyperactivity contribute to cystic fibrosis lung disease? J Physiol 591: 4377–4387, 2013. doi: 10.1113/jphysiol.2012.240861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodges CA, Cotton CU, Palmert MR, Drumm ML. Generation of a conditional null allele for Cftr in mice. Genesis 46: 546–552, 2008. doi: 10.1002/dvg.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 6: 65–70, 1979. [Google Scholar]
- 35.Kelley TJ, Cotton CU, Drumm ML. In vivo activation of CFTR-dependent chloride transport in murine airway epithelium by CNP. Am J Physiol Lung Cell Mol Physiol 273: L1065–L1072, 1997. doi: 10.1152/ajplung.1997.273.5.L1065. [DOI] [PubMed] [Google Scholar]
- 36.Kelley TJ, Drumm ML. Inducible nitric oxide synthase expression is reduced in cystic fibrosis murine and human airway epithelial cells. J Clin Invest 102: 1200–1207, 1998. doi: 10.1172/JCI2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Labonté ED, Carreras CW, Leadbetter MR, Kozuka K, Kohler J, Koo-McCoy S, He L, Dy E, Black D, Zhong Z, Langsetmo I, Spencer AG, Bell N, Deshpande D, Navre M, Lewis JG, Jacobs JW, Charmot D. Gastrointestinal inhibition of sodium-hydrogen exchanger 3 reduces phosphorus absorption and protects against vascular calcification in CKD. J Am Soc Nephrol 26: 1138–1149, 2015. doi: 10.1681/ASN.2014030317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liou TG, Adler FR, Cahill BC, FitzSimmons SC, Huang D, Hibbs JR, Marshall BC. Survival effect of lung transplantation among patients with cystic fibrosis. JAMA 286: 2683–2689, 2001. doi: 10.1001/jama.286.21.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipecka J, Bali M, Thomas A, Fanen P, Edelman A, Fritsch J. Distribution of ClC-2 chloride channel in rat and human epithelial tissues. Am J Physiol Cell Physiol 282: C805–C816, 2002. doi: 10.1152/ajpcell.00291.2001. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Li T, Riederer B, Lenzen H, Ludolph L, Yeruva S, Tuo B, Soleimani M, Seidler U. Loss of Slc26a9 anion transporter alters intestinal electrolyte and transport and reduces survival in CFTR-deficient mice. Pflügers Arch 467: 1261–1275, 2015. doi: 10.1007/s00424-014-1543-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lucas ML. A reconsideration of the evidence for Escherichia coli STa (heat stable) enterotoxin-driven fluid secretion: a new view of STa action and a new paradigm for fluid absorption. J Appl Microbiol 90: 7–26, 2001. doi: 10.1046/j.1365-2672.2001.01225.x. [DOI] [PubMed] [Google Scholar]
- 42.Lucas ML, Thom MM, Bradley JM, O’Reilly NF, McIlvenny TJ, Nelson YB. Escherichia coli heat stable (STa) enterotoxin and the upper small intestine: lack of evidence in vivo for net fluid secretion. J Membr Biol 206: 29–42, 2005. doi: 10.1007/s00232-005-0771-6. [DOI] [PubMed] [Google Scholar]
- 43.Magro F, Fraga S, Soares-da-Silva P. Signaling of short- and long-term regulation of intestinal epithelial type 1 Na+/H+ exchanger by interferon-γ. Br J Pharmacol 145: 93–103, 2005. doi: 10.1038/sj.bjp.0706167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masereel B, Pochet L, Laeckmann D. An overview of inhibitors of Na+/H+ exchanger. Eur J Med Chem 38: 547–554, 2003. doi: 10.1016/S0223-5234(03)00100-4. [DOI] [PubMed] [Google Scholar]
- 45.Matsui H, Wagner VE, Hill DB, Schwab UE, Rogers TD, Button B, Taylor RM II, Superfine R, Rubinstein M, Iglewski BH, Boucher RC. A physical linkage between cystic fibrosis airway surface dehydration and Pseudomonas aeruginosa biofilms. Proc Natl Acad Sci USA 103: 18131–18136, 2006. doi: 10.1073/pnas.0606428103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McHugh DR, Steele MS, Valerio DM, Miron A, Mann RJ, LePage DF, Conlon RA, Cotton CU, Drumm ML, Hodges CA. A G542X cystic fibrosis mouse model for examining nonsense mutation directed therapies. PLoS One 13: e0199573, 2018. doi: 10.1371/journal.pone.0199573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murek M, Kopic S, Geibel J. Evidence for intestinal chloride secretion. Exp Physiol 95: 471–478, 2010. doi: 10.1113/expphysiol.2009.049445. [DOI] [PubMed] [Google Scholar]
- 48.Pattison AM, Blomain ES, Merlino DJ, Wang F, Crissey MA, Kraft CL, Rappaport JA, Snook AE, Lynch JP, Waldman SA. Intestinal enteroids model guanylate cyclase C-dependent secretion induced by heat-stable enterotoxins. Infect Immun 84: 3083–3091, 2016. doi: 10.1128/IAI.00639-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pillarisetti N, Williamson E, Linnane B, Skoric B, Robertson CF, Robinson P, Massie J, Hall GL, Sly P, Stick S, Ranganathan S; Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) . Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am J Respir Crit Care Med 184: 75–81, 2011. doi: 10.1164/rccm.201011-1892OC. [DOI] [PubMed] [Google Scholar]
- 50.Poschet JF, Timmins GS, Taylor-Cousar JL, Ornatowski W, Fazio J, Perkett E, Wilson KR, Yu HD, de Jonge HR, Deretic V. Pharmacological modulation of cGMP levels by phosphodiesterase 5 inhibitors as a therapeutic strategy for treatment of respiratory pathology in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 293: L712–L719, 2007. doi: 10.1152/ajplung.00314.2006. [DOI] [PubMed] [Google Scholar]
- 51.Qian X, Prabhakar S, Nandi A, Visweswariah SS, Goy MF. Expression of GC-C, a receptor-guanylate cyclase, and its endogenous ligands uroguanylin and guanylin along the rostrocaudal axis of the intestine. Endocrinology 141: 3210–3224, 2000. doi: 10.1210/endo.141.9.7644. [DOI] [PubMed] [Google Scholar]
- 52.Quigley EM, Tack J, Chey WD, Rao SS, Fortea J, Falques M, Diaz C, Shiff SJ, Currie MG, Johnston JM. Randomised clinical trials: linaclotide phase 3 studies in IBS-C - a prespecified further analysis based on European Medicines Agency-specified endpoints. Aliment Pharmacol Ther 37: 49–61, 2013. doi: 10.1111/apt.12123. [DOI] [PubMed] [Google Scholar]
- 53.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 22: 659–661, 2008. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 54.Roos A, Boron WF. Intracellular pH. Physiol Rev 61: 296–434, 1981. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- 55.Salameh AI, Ruffin VA, Boron WF. Effects of metabolic acidosis on intracellular pH responses in multiple cell types. Am J Physiol Regul Integr Comp Physiol 307: R1413–R1427, 2014. doi: 10.1152/ajpregu.00154.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141: 1762–1772, 2011. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 57.Schreiber R, Faria D, Skryabin BV, Wanitchakool P, Rock JR, Kunzelmann K. Anoctamins support calcium-dependent chloride secretion by facilitating calcium signaling in adult mouse intestine. Pflügers Arch 467: 1203–1213, 2015. doi: 10.1007/s00424-014-1559-2. [DOI] [PubMed] [Google Scholar]
- 58.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 59.Schwark JR, Jansen HW, Lang HJ, Krick W, Burckhardt G, Hropot M. S3226, a novel inhibitor of Na+/H+ exchanger subtype 3 in various cell types. Pflügers Arch 436: 797–800, 1998. doi: 10.1007/s004240050704. [DOI] [PubMed] [Google Scholar]
- 60.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science 257: 1083–1088, 1992. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 61.Spencer AG, Labonte ED, Rosenbaum DP, Plato CF, Carreras CW, Leadbetter MR, Kozuka K, Kohler J, Koo-McCoy S, He L, Bell N, Tabora J, Joly KM, Navre M, Jacobs JW, Charmot D. Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med 6: 227ra36, 2014. doi: 10.1126/scitranslmed.3007790. [DOI] [PubMed] [Google Scholar]
- 62.Sun L, Rommens JM, Corvol H, Li W, Li X, Chiang TA, Lin F, Dorfman R, Busson PF, Parekh RV, Zelenika D, Blackman SM, Corey M, Doshi VK, Henderson L, Naughton KM, O’Neal WK, Pace RG, Stonebraker JR, Wood SD, Wright FA, Zielenski J, Clement A, Drumm ML, Boëlle PY, Cutting GR, Knowles MR, Durie PR, Strug LJ. Multiple apical plasma membrane constituents are associated with susceptibility to meconium ileus in individuals with cystic fibrosis. Nat Genet 44: 562–569, 2012. doi: 10.1038/ng.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry 18: 2210–2218, 1979. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- 64.van der Doef HP, Kokke FT, Beek FJ, Woestenenk JW, Froeling SP, Houwen RH. Constipation in pediatric cystic fibrosis patients: an underestimated medical condition. J Cyst Fibros 9: 59–63, 2010. doi: 10.1016/j.jcf.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 65.van der Doef HP, Kokke FT, van der Ent CK, Houwen RH. Intestinal obstruction syndromes in cystic fibrosis: meconium ileus, distal intestinal obstruction syndrome, and constipation. Curr Gastroenterol Rep 13: 265–270, 2011. doi: 10.1007/s11894-011-0185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vitko M, Valerio DM, Rye PD, Onsøyen E, Myrset AH, Dessen A, Drumm ML, Hodges CA. A novel guluronate oligomer improves intestinal transit and survival in cystic fibrosis mice. J Cyst Fibros 15: 745–751, 2016. doi: 10.1016/j.jcf.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 67.Walker NM, Simpson JE, Levitt RC, Boyle KT, Clarke LL. Talniflumate increases survival in a cystic fibrosis mouse model of distal intestinal obstructive syndrome. J Pharmacol Exp Ther 317: 275–283, 2006. doi: 10.1124/jpet.105.094847. [DOI] [PubMed] [Google Scholar]
- 68.Weiglmeier PR, Rösch P, Berkner H. Cure and curse: E. coli heat-stable enterotoxin and its receptor guanylyl cyclase C. Toxins (Basel) 2: 2213–2229, 2010. doi: 10.3390/toxins2092213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Whittamore JM, Hatch M. Loss of the anion exchanger DRA (Slc26a3), or PAT1 (Slc26a6), alters sulfate transport by the distal ileum and overall sulfate homeostasis. Am J Physiol Gastrointest Liver Physiol 313: G166–G179, 2017. doi: 10.1152/ajpgi.00079.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yun CH, Tse CM, Nath SK, Levine SA, Brant SR, Donowitz M. Mammalian Na+/H+ exchanger gene family: structure and function studies. Am J Physiol Gastrointest Liver Physiol 269: G1–G11, 1995. doi: 10.1113/jphysiol.1995.sp020558. [DOI] [PubMed] [Google Scholar]
- 71.Zeiher BG, Eichwald E, Zabner J, Smith JJ, Puga AP, McCray PB Jr, Capecchi MR, Welsh MJ, Thomas KR. A mouse model for the delta F508 allele of cystic fibrosis. J Clin Invest 96: 2051–2064, 1995. doi: 10.1172/JCI118253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang S, Ranganath NK, Skinner D, Bedwell DM, Buckley-Lanier JA, Sorscher EJ, Woodworth BA. Marked repression of CFTR mRNA in the transgenic Cftrtm1kth mouse model. J Cyst Fibros 13: 351–352, 2014. doi: 10.1016/j.jcf.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zielińska M, Wasilewski A, Fichna J. Tenapanor hydrochloride for the treatment of constipation-predominant irritable bowel syndrome. Expert Opin Investig Drugs 24: 1093–1099, 2015. doi: 10.1517/13543784.2015.1054480. [DOI] [PubMed] [Google Scholar]