Summary
Background
Breakthrough SARS-CoV-2 infections following vaccination against COVID-19 are of international concern. Patients with cancer have been observed to have worse outcomes associated with COVID-19 during the pandemic. We sought to evaluate the clinical characteristics and outcomes of patients with cancer who developed breakthrough SARS-CoV-2 infections after 2 or 3 doses of mRNA vaccines.
Methods
We evaluated the clinical characteristics of patients with cancer who developed breakthrough infections using data from the multi-institutional COVID-19 and Cancer Consortium (CCC19; NCT04354701). Analysis was restricted to patients with laboratory-confirmed SARS-CoV-2 diagnosed in 2021 or 2022, to allow for a contemporary unvaccinated control population; potential differences were evaluated using a multivariable logistic regression model after inverse probability of treatment weighting to adjust for potential baseline confounding variables. Adjusted odds ratios (aOR) and 95% confidence intervals (CI) are reported. The primary endpoint was 30-day mortality, with key secondary endpoints of hospitalization and ICU and/or mechanical ventilation (ICU/MV).
Findings
The analysis included 2486 patients, of which 564 and 385 had received 2 or 3 doses of an mRNA vaccine prior to infection, respectively. Hematologic malignancies and recent receipt of systemic anti-neoplastic therapy were more frequent among vaccinated patients. Vaccination was associated with improved outcomes: in the primary analysis, 2 doses (aOR: 0.62, 95% CI: 0.44–0.88) and 3 doses (aOR: 0.20, 95% CI: 0.11–0.36) were associated with decreased 30-day mortality. There were similar findings for the key secondary endpoints of ICU/MV (aOR: 0.60, 95% CI: 0.45–0.82 and 0.37, 95% CI: 0.24–0.58) and hospitalization (aOR: 0.60, 95% CI: 0.48–0.75 and 0.35, 95% CI: 0.26–0.46) for 2 and 3 doses, respectively. Importantly, Black patients had higher rates of hospitalization (aOR: 1.47, 95% CI: 1.12–1.92), and Hispanic patients presented with higher rates of ICU/MV (aOR: 1.61, 95% CI: 1.06–2.44).
Interpretation
Vaccination against COVID-19, especially with additional doses, is a fundamental strategy in the prevention of adverse outcomes including death, among patients with cancer.
Funding
This study was partly supported by grants from the National Cancer Institute grant number P30 CA068485 to C-YH, YS, SM, JLW; T32-CA236621 and P30-CA046592 to C.R.F; CTSA 2UL1TR001425-05A1 to TMW-D; ACS/FHI Real-World Data Impact Award, P50 MD017341-01, R21 CA242044-01A1, Susan G. Komen Leadership Grant Hunt to MKA. REDCap is developed and supported by Vanderbilt Institute for Clinical and Translational Research grant support (UL1 TR000445 from NCATS/NIH).
Keywords: Covid-19, Vaccination, SARS-CoV-2, Cancer, Breakthrough infection, mRNA vaccine
Research in context.
Evidence before this study
Patients with cancer are a highly vulnerable population susceptible to poor outcomes during SARS-CoV-2 infection. While a 3-dose COVID-19 vaccination series is recommended in patients with cancer, their immunological response to COVID-19 vaccines may be lessened, notably among those with hematologic malignancies. Breakthrough SARS-CoV-2 infections are increasingly being reported and are thought to be due to a waning immune response and the emergence of novel SARS-CoV-2 variants of concern. We searched PubMed for research articles published in English between November 1, 2020 and August 20, 2022, analyzing breakthrough COVID-19 infections in patients with cancer, using search terms “COVID-19”, “vaccination”, “tumor”, “cancer”, and “breakthrough”. The findings of the search revealed insufficient evidence to comprehensively characterize breakthrough SARS-CoV-2 infections among patients with cancer following 2 or 3 doses of authorized vaccines.
Added value of this study
Patients with cancer who developed breakthrough SARS-CoV-2 infections following the receipt of 2 or 3 doses of approved mRNA vaccines had better clinical outcomes compared to an unvaccinated weighted population, with lower rates of death, ICU admission, and hospitalization. Moreover, a higher number of administered COVID-19 vaccines appeared to be associated with an additional protective benefit. Patients with hematologic malignancies and those recently treated with systemic anti-neoplastic regimens were more highly represented among patients with breakthrough infections, compared to the unvaccinated group. Clinical factors associated with worse outcomes among vaccinated and unvaccinated patients with cancer and COVID-19 included increasing age, active and progressing cancer, poor ECOG performance status, hematologic malignancies, and the presence of lymphopenia.
Implications of all the available evidence
As patients with cancer were largely excluded from the landmark clinical trials evaluating COVID-19 vaccines, our current findings help to identify vaccination against COVID-19 as an essential strategy to improve outcomes in this high-risk population. Our results also support guidelines that patients with cancer should receive at least 3 COVID-19 vaccine doses. Additionally, we identify risk factors of poor outcomes among vaccinated patients with COVID-19, helping to better inform clinical decision-making about the prognosis of these patients.
Introduction
Vaccination against COVID-19, the disease caused by SARS-CoV-2, has shown substantial efficacy in preventing symptomatic infection and associated severe outcomes1,2 and was identified as an essential measure in controlling viral transmission.3 Breakthrough SARS-CoV-2 infections were identified among vaccinated patients,4 owing to a time-dependent waning immunity5,6 and the emergence of novel SARS-CoV-2 variants of concern, such as the B.1.617.2 (Delta) and B.1.1.529 (Omicron) variants. Evaluation of data related to viral activity and duration of immunity subsequently led to the authorization of a third mRNA vaccine dose in the general population.7,8
Patients with cancer are a highly vulnerable population during COVID-19, with substantially worse outcomes and higher mortality compared to the general population.9, 10, 11 In these patients, baseline immunosuppression and recent receipt of anti-neoplastic regimens were associated with severe COVID-19,12 and might hinder the efficacy of vaccines.13 Following a 2-dose vaccination schedule against COVID-19, patients with cancer displayed lower seroconversion rates compared to healthy individuals.13,14 Additionally, a poorer immunological response following vaccination was identified against variants of concern, and among patients with hematologic malignancies compared to those with solid cancers.15 The receipt of a third vaccine dose was found to induce improved neutralizing responses in patients with cancer, as opposed to 2 doses only.16
From a clinical perspective, the landmark trials investigating COVID-19 vaccines largely excluded patients with underlying malignancies,1,2 leading to minimal understanding about their clinical efficacy in this patient population. Our group, the COVID-19 and Cancer Consortium (CCC19), analyzed the characteristics and outcomes of patients with cancer who developed breakthrough infections following 2 doses of mRNA vaccines (BNT162b2 or mRNA-1273) or one dose of the Ad.26.COV2.S vaccine.17 In this preliminary analysis, vaccinated patients with cancer were found to have comparable rates of severe outcomes, including death, in relation to an unvaccinated population, but presented with a higher frequency of hematologic malignancies and/or lymphopenia.17 The number of fully vaccinated patients in this preliminary report (n = 54) precluded extensive adjustment for potential confounding variables. Thus, stronger evidence is still needed to better understand the clinical attributes of patients with cancer who develop breakthrough infections following a 2 doses of mRNA vaccines. Additionally, while previous studies evaluated the risk of breakthrough infections and associated severe outcomes among patients with cancer (as compared to patients without cancer) who received 2 or 3 doses of COVID-19 vaccines,18, 19, 20 they did not fully account for potential demographic and clinical confounding factors, were not able to assess the most recent time periods and did not evaluate the determinants of worse outcomes in patients with cancer specifically.
Here, we sought to evaluate in a larger cohort that encompasses the most recent infection waves the clinical features of patients with cancer who developed breakthrough infections following 2 or 3 doses of mRNA vaccines, and their outcomes compared to unvaccinated patients, using updated data from the multi-institutional CCC19 registry. We hypothesized that both the primary endpoint of 30-day all-cause mortality and key secondary endpoints of hospitalization and intensive care unit and/or mechanical ventilation (ICU/MV) requirement would be improved in the vaccinated groups with more doses of the vaccines being associated with further improvement in outcomes.
Methods
The CCC19 maintains a multi-institutional registry of patients diagnosed with COVID-19 with a current or previous history of cancer. Data are captured and managed using REDCap21 at Vanderbilt University Medical Center, with a methodology outlined previously.22 Deidentified data are collected, with a comprehensive set of variables for each patient, related to demographics, cancer status, anti-neoplastic regimens, COVID-19 (either presumed or laboratory-confirmed SARS-CoV-2 infection), and COVID-19 vaccination schedule. Data collection on COVID-19 vaccination for each newly entered case started with the first international approval in November 2020. Eligible cases included patients with cancer diagnosed with COVID-19 in 2021 or 2022 (to match the dates of widespread vaccine deployment), with laboratory-confirmed SARS-CoV-2 infection and current or previous history of invasive cancer. Cases with unknown vaccination status, an unknown primary outcome (30-day mortality), or poor data quality (i.e., quality score ≥5 using our previously defined metric) were excluded.22
Breakthrough infections were defined in the current study as laboratory-confirmed SARS-CoV-2 infection following the receipt of 2 or 3 doses of mRNA vaccines (BNT162b2 or mRNA-1273). Patients with cancer who were administered 3 doses of mRNA vaccines were not included in the group of 2 doses of mRNA vaccines, such as the two groups of vaccines patients (2 and 3 doses of mRNA vaccines) were mutually exclusive. Patients who received a single dose of mRNA vaccine prior to SARS-CoV-2 infection were excluded, as were patients who were diagnosed with COVID-19 before 2021. Patients who were administered COVID-19 vaccines following a laboratory-confirmed SARS-CoV-2 infection were excluded. By consensus, patients vaccinated with adenovirus vector vaccines (Ad.26.COV2.S) were excluded, even if they were administered a dose of mRNA vaccine in subsequent time periods.
The primary endpoint was defined as 30-day all-cause mortality among patients vaccinated with 2 or 3 doses of mRNA vaccines, compared to unvaccinated patients, and was evaluated using a multivariable logistic regression model after Inverse Probability of Treatment Weighting (IPTW) to adjust for potential baseline confounding variables. Secondary endpoints were defined as the rates of Intensive Care Unit admission and/or Mechanical Ventilation (ICU/MV), and hospitalization rates in a multivariable logistic analysis after IPTW to adjust for baseline variables.
The IPTW analysis was used to adjust for differences in baseline clinical variables between vaccinated and unvaccinated (Supplementary Methods) and included the following variables: age (as a continuous variable truncated at 90 years due to Health Insurance Portability and Accountability Act requirements), sex (female or male), race/ethnicity (non-Hispanic white, non-Hispanic Black, Hispanic, other), smoking status (current or former smoker, never smoker), Eastern Cooperative Oncology Group performance status (ECOG PS 0, 1 or ≥2), baseline systemic corticosteroid use (none, ≤10 mg/day prednisone dose equivalent [PDE], >10 mg/day PDE), lymphopenia (absolute lymphocyte count [ALC] ≤ 1000 cells/μl, >1000 cells/μl), modified Charlson comorbidity index (mCCI: 0, 1 or ≥2) (Supplementary Table S1), cancer status (active and progressing at time of COVID-19 diagnosis versus not active and progressing), cancer type (solid organ malignancy, hematologic neoplasm, or both), and recent systemic anti-neoplastic therapy (cytotoxic chemotherapy, immunotherapy, targeted therapy, and/or endocrine therapy, as single therapeutic agents or in combination, in the 3 months before COVID-19 diagnosis, or not). Multiple imputation was performed for missing covariates (Supplementary Methods), except for Eastern Cooperative Oncology Group (ECOG) performance status. To better understand the clinical characteristics of patients with cancer who developed breakthrough infections, the ORs are reported as explanatory modeling for the associations between these variables and the outcomes of interest.23 Such associations are interpreted as descriptive risk factors.
Sensitivity analyses are fully described in the Supplementary Methods, and included the following: the removal of cases with a “possible” vaccination status, the addition of cancer stage as a covariate in the regression model, the implementation of cluster-robust standard errors to adjust the estimates for the participating institution, the evaluation of recent time periods ranging from October 2021 to March 2022 only to account for temporal variations of the pandemic, the evaluation of patients who received 2 or 3 doses of mRNA vaccines only, and a regression analysis where IPTW was not used to balance covariate distributions.
The data dictionary employed for the current analysis, in addition to instructions on accessing the full CCC19 data dictionary and code used to generate all derived variables are found in Supplementary Table S2. Data analysis was performed using R v4.0.3, along with the R packages Hmisc 4.4.2, MatchIt 4.2.0, ipw 1.0–11, survey 4.0, sandwich 3.0–1, and glmnet 4.1–1.
Ethical statement
This study was exempt from Institutional Review Board (IRB) review (VUMC IRB#200467) and approved by IRBs at participating sites. This ongoing study is registered on ClinicalTrials.gov (NCT04354701).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Based on eligibility criteria, 2486 patients with cancer and COVID-19 were included in the present study (Supplementary Table S3). Of these, 564 (23%) and 385 (15%) patients were administered 2 and 3 doses of mRNA vaccines prior to COVID-19, respectively, while 1537 patients (62%) had no recorded receipt of any vaccination prior to COVID-19. Baseline clinical characteristics stratified by vaccination status are shown in Table 1. Among all patients included, 1400 (56%) were female, and 1580 (64%) were non-Hispanic white.
Table 1.
Baseline demographic and clinical variables among patients with cancer and COVID-19 according to vaccination status.
| Unvaccinated (n = 1537) | 2 doses of mRNA vaccines (n = 564) | 3 doses of mRNA vaccines (n = 385) | |
|---|---|---|---|
| Age, median years (IQR) | 64.0 (53.0–74.0) | 66.0 (57.0–75.0) | 67.0 (59.0–76.0) |
| Sex | |||
| Male | 656 (42%) | 252 (47%) | 209 (49%) |
| Female | 880 (58%) | 311 (53%) | 176 (51%) |
| Missing | 1 (<1%) | 1 (<1%) | 0 |
| Race & ethnicity | |||
| Non-Hispanic White | 896 (58%) | 400 (71%) | 284 (74%) |
| Non-Hispanic Black | 287 (19%) | 71 (13%) | 46 (12%) |
| Hispanic or Latino | 176 (11%) | 38 (7%) | 25 (6%) |
| Other | 149 (10%) | 46 (8%) | 19 (5%) |
| Unknown or missing | 29 (2%) | 9 (2%) | 11 (3%) |
| ECOG PS | |||
| 0 | 464 (30%) | 175 (31%) | 160 (42%) |
| 1 | 424 (28%) | 177 (31%) | 116 (30%) |
| ≥2 | 211 (14%) | 96 (17%) | 55 (14%) |
| Unknown or missing | 438 (28%) | 116 (21%) | 54 (14%) |
| mCCI | |||
| 0 | 748 (49%) | 266 (47%) | 202 (52%) |
| 1 | 368 (24%) | 134 (24%) | 80 (21%) |
| ≥2 | 406 (26%) | 159 (28%) | 99 (26%) |
| Unknown or missing | 15 (1%) | 5 (<1%) | 4 (1%) |
| Smoking status | |||
| Never (non-smoker) | 743 (48%) | 249 (44%) | 189 (49%) |
| Current or former smoker | 734 (48%) | 300 (53%) | 182 (47%) |
| Unknown or missing | 60 (4%) | 15 (3%) | 14 (4%) |
| Cancer type | |||
| Solid organ tumour | 1174 (76%) | 390 (69%) | 226 (59%) |
| Hematologic malignancy | 312 (20%) | 149 (26%) | 128 (33%) |
| Both | 51 (3%) | 25 (4%) | 31 (8%) |
| Cancer status | |||
| Active and progressing | 240 (15%) | 93 (16%) | 52 (14%) |
| Not active or progressing | 1130 (74%) | 406 (72%) | 300 (78%) |
| Unknown or missing | 167 (11%) | 65 (12%) | 33 (9%) |
| Recent systemic anti-cancer therapy | |||
| Yes | 663 (43%) | 287 (51%) | 221 (57%) |
| No | 861 (56%) | 269 (48%) | 162 (42%) |
| Unknown or missing | 13 (1%) | 8 (1%) | <5 (1%) |
| Baseline corticosteroids | |||
| None | 1291 (84%) | 454 (81%) | 305 (79%) |
| ≤10 mg/day PDE | 82 (5%) | 35 (6%) | 28 (7%) |
| >10 mg/day PDE | 121 (8%) | 56 (10%) | 38 (10%) |
| Unknown or missing | 43 (3%) | 19 (3%) | 14 (4%) |
| Lymphopenia | |||
| Yes | 549 (36%) | 197 (35%) | 92 (24%) |
| No | 322 (21%) | 94 (17%) | 57 (17%) |
| Unknown or missinga | 666 (43%) | 273 (48%) | 236 (61%) |
| Type of vaccine received | |||
| BNT162b2 | 0 | 366 (65%) | 234 (61%) |
| mRNA-1273 | 0 | 193 (34%) | 142 (37%) |
| Unspecified, including multiple types (“mix and match”) | 0 | 5 (1%) | 9 (2%) |
ECOG PS: Eastern Cooperative Oncology Group performance status; mCCI: modified Charlson comorbidity index; PDE: prednisone dose equivalent; US: United States.
This includes a substantial number of cases where labs were not drawn, as is typically the case for milder cases of COVID-19.
Patients who received 2 or 3 doses of mRNA vaccines were older, with a median age of 66 (interquartile range [IQR]: 57–75) and 67 years (IQR: 59–76), respectively, compared to 64 years (IQR: 53–74 years) in the unvaccinated group. More patients in the vaccinated groups were non-Hispanic white, 400 (71%) and 284 (74%), compared to 880 (57%) in the unvaccinated group. The rates of smoking, baseline systemic corticosteroids, comorbidities, and progression of cancer at baseline were relatively similar across the groups. Hematologic malignancies were reported in 149 (26%) and 128 (33%) patients who received 2 and 3 doses of mRNA vaccines prior to COVID-19 infection, respectively, while only 312 (20%) unvaccinated patients had a hematologic malignancy. Systemic anti-neoplastic therapy within 3 months prior to COVID-19 diagnosis was received in 287 (51%) and 221 (57%) patients who developed breakthrough infections following 2 and 3 mRNA vaccines, respectively, as compared to 663 (43%) in the unvaccinated population.
As shown in Table 2, the 30-day mortality rate was 10% (n = 55) and 4% (n = 17) among patients who received 2 and 3 doses of mRNA vaccines, respectively, compared to 12% (n = 189) in the unvaccinated group. Similarly, rates of ICU/MV and hospitalization were lower among patients in the vaccinated groups [2 mRNA vaccines: 12% (n = 67) and 50% (n = 281), respectively; and 3 mRNA vaccines: 8% (n = 32) and 38% (n = 145), respectively], in relation to patients without prior vaccination [16% (n = 241) and 56% (n = 864), respectively].
Table 2.
Rates of clinical outcomes among patients with cancer and COVID-19 according to vaccination status.
| Incidence, % (95%CI) | Unvaccinated (n = 1537) | 2 doses of mRNA vaccines (n = 564) | 3 doses of mRNA vaccines (n = 385) |
|---|---|---|---|
| 30-day mortality | 12% (11–14) | 10% (7–12) | 4% (3–6) |
| ICU/MV | 16% (15–18) | 12% (9–15) | 8% (6–11) |
| Hospitalization | 57% (54–59) | 50% (46–54) | 38% (33–43) |
CI: confidence interval; ICU: intensive care unit; MV: mechanical ventilation.
Following IPTW (Supplementary Table S5), receipt of vaccination (versus not) was associated with better outcomes for the primary endpoint of 30-day all-cause mortality, with a stronger association identified for 3 doses of mRNA vaccines [adjusted odds ratio (aOR): 0.20, 95% confidence interval (95% CI): 0.11–0.36], as compared to 2 doses (aOR: 0.62, 95% CI: 0.44–0.88) (Fig. 1, Supplementary Table S6). We report absolute vaccine effectiveness as a 66% and 21% lower risk of 30-day all-cause mortality for patients vaccinated with 3 and 2 doses of mRNA vaccines, respectively, as compared to unvaccinated patients with cancer. Similar associations between vaccination status and outcomes were also found in regard to the secondary endpoints of ICU/MV (2 mRNA doses: aOR: 0.60, 95% CI: 0.45–0.82 and 3 mRNA doses: aOR: 0.37, 95% CI: 0.24–0.58), and hospitalization (2 mRNA doses: aOR: 0.60, 95% CI: 0.48–0.75 and 3 mRNA doses: aOR: 0.35, 95% CI: 0.26–0.46) (Fig. 2, Supplementary Table S6).
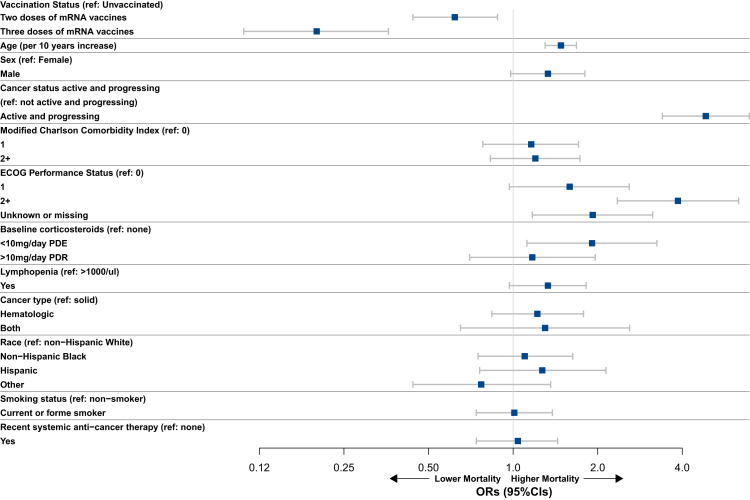
Fig. 1.
Forest plot showing results of the multivariable logistic regression analysis following IPTW for the primary endpoint of 30-day mortality among patients with cancer and COVID-19. CI: confidence interval; ECOG PS: Eastern Cooperative Oncology Group performance status; OR: odds ratio; PDE: prednisone dose equivalent; ref: reference.
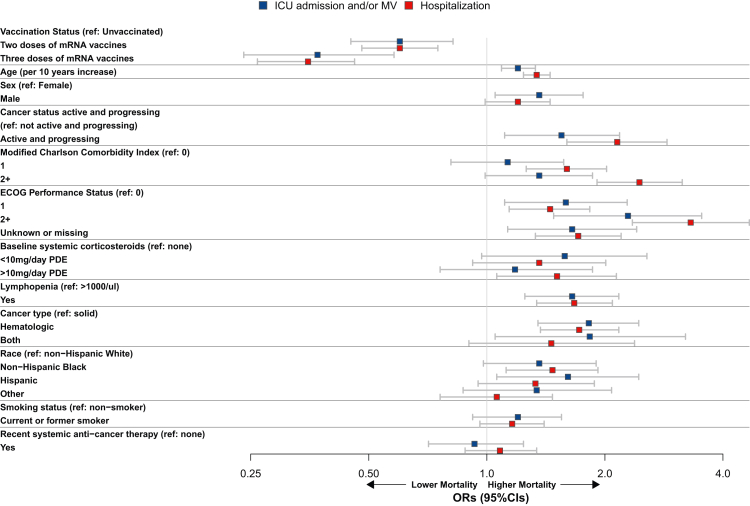
Fig. 2.
Forest plot showing results of the multivariable logistic regression analysis following IPTW for the secondary endpoints of ICU admission and/or MV, and hospitalization among patients with cancer and COVID-19. CI: confidence interval; ECOG PS: Eastern Cooperative Oncology Group performance status; ICU: intensive care unit; MV: mechanical ventilation; OR: odds ratio; PDE: prednisone dose equivalent; ref: reference.
In the multivariable analysis (Fig. 1, Fig. 2, Supplementary Table S6), increasing age, active and progressing malignancies (versus not), and a poor ECOG PS (≥2 versus 0) were associated with worse outcomes in regard to the primary endpoint of 30-day mortality (aOR: 1.48, 95% CI: 1.30–1.68; aOR: 4.85, 95% CI: 3.40–6.93; and aOR: 3.86, 95% CI: 2.35–6.35, respectively), and were also linked to higher rates of ICU/MV (aOR: 1.20, 95% CI: 1.09–1.33; aOR: 1.55, 95% CI: 1.11–2.18; and aOR: 2.29, 95% CI: 1.48–3.53, respectively), and hospitalization (aOR: 1.34, 95% CI: 1.24–1.45; aOR: 2.15, 95% CI: 1.60–2.88; and aOR: 3.31, 95% CI: 2.35–4.67, respectively). Hematologic malignancies (versus solid organ tumors) and the presence of lymphopenia (versus not) were associated with an increased risk of ICU/MV (aOR: 1.82, 95% CI: 1.35–2.44; and aOR: 1.65, 95% CI: 1.25–2.17, respectively), and hospitalization (aOR: 1.72, 95% CI: 1.37–2.17; and aOR: 1.67, 95% CI: 1.34–2.09, respectively). Similarly, Hispanic patients (versus non-Hispanic white) were presented with an increased risk of ICU/MV (aOR: 1.61, 95% CI: 1.06–2.44), while non-Hispanic Black patients appeared to have a higher risk of hospitalization (aOR: 1.47, 95% CI: 1.12–1.92).
Results from the sensitivity analyses are described in Supplementary Tables S7–S13. In summary, vaccination against COVID-19 remained strongly associated with better outcomes, both for the primary and secondary endpoints, with an additional benefit identified for patients who received 3 doses of mRNA vaccines. Risk factors linked to worse outcomes in the primary analysis showed overall very similar associations across the different sensitivity analyses computed.
Discussion
In this study, we provide a comprehensive clinical characterization of breakthrough SARS-CoV-2 infections in patients with cancer following the receipt of 2 or 3 doses of mRNA vaccines and analyze their outcomes in relation to a contemporary unvaccinated population. To our knowledge, this is the first and largest study examining outcomes for vaccinated and boosted patients with cancer in a North American setting, primarily the United States. After controlling for baseline potential adverse clinical factors, vaccination against COVID-19 was associated with improved outcomes in relation to all evaluated endpoints, including mortality. Moreover, a higher number of mRNA vaccine doses administered (3 versus 2) appeared to confer additional protection against severe COVID-19 among this population of patients with cancer, supporting our hypothesis of additional benefit from a third dose of mRNA vaccines. This aligns with previous evidence showing improved immune responses among patients with cancer after the administration of a third dose of an mRNA vaccine, including against variants of concern of SARS-CoV-2, in addition to a lower risk to develop breakthrough infections identified among patients with cancer16,18, 19, 20 Our current work presents with meaningful clinical implications, as it helps to characterize the protective benefit of mRNA vaccines in relation to clinical outcomes among patients with cancer who develop breakthrough infections in recent times, and identifies determinants of worse prognosis. This aims to ensure an optimal assessment and management of this vulnerable population during breakthrough COVID-19.
Patients with hematologic malignancies and those who received recent systemic anti-cancer therapies were more highly represented in the vaccinated (versus unvaccinated) groups. This is concordant with prior studies identifying poorer immune responses to COVID-19 vaccines among patients with hematologic (versus solid) cancers, across various vaccination schedules13,24, 25, 26 and lower seroconversion rates following vaccination against COVID-19 among patients with cancer recently treated with cytotoxic chemotherapy or other anti-neoplastic immunosuppressive regimens.13 There were high rates of non-Hispanic white patients in the vaccinated subgroups. Whether these differences are due to an underlying disparity in access, systematic differences in clinical care, or geographic uptake of vaccination, versus differential presentation of breakthrough infections, is unclear due to a limited sample size and the general limitations of the retrospective study design.
Multiple variables were found to be associated with worse outcomes among vaccinated and unvaccinated patients with cancer and COVID-19, including increasing age, poor performance status, active and progressing malignancy, hematologic malignancies, and presence of lymphopenia. These represent previously established associations in patients with cancer for severe COVID-19 disease.9,12,27 Notably, due to exclusion of 2020 cases, there is little overlap in the current analysis and our previously published work establishing these general associations. Non-Hispanic Black and Hispanic patients also presented with more severe outcomes as compared to non-Hispanic white patients, in agreement with earlier reports showing similar results, all suggesting that the pandemic has exacerbated existing health disparities.12,28,29
The current study presents with limitations, primarily based on the design being a retrospective observational study which is subject to the inherent potential for bias and confounding. Longitudinal follow-up is reliant on registry data entry, and therefore the outcome data may be subject to measurement biases, in addition to a non-exhaustive identification of patients with cancer and COVID-19 across participating institutions; however, there was very limited missing data. Data accuracy was reliant on survey respondents and did not include independent monitoring per study design. While patients analyzed derive from various medical centers, distributed across most geographic areas in North America, smaller centers with limited resources might be less represented in the current dataset. Based on the collected variables, it was not possible to assess the difference in clinical outcomes between early versus late breakthrough infections, as well as a potential de-escalation of COVID-19 care among patients with advanced malignancies. Additionally, there was an inability to analyze specific SARS-CoV-2 variants of concern, as these are not tested for clinically. However, a sensitivity analysis evaluating specifically the most recent time periods corresponding to the spread of novel variants of concern, showed overall similar results to those identified in the main analysis, but with a potentially reduced benefit of three doses of mRNA vaccines as compared to the main analysis. The evaluation of clinical outcomes across subgroups of solid malignancies (e.g. breast cancer, thoracic malignancies) was restricted by a limited number of events. As only patients with cancer with a diagnosis of COVID-19 were captured, the investigation of vaccinated patients who did not develop breakthrough infections could not be performed. Patients who were vaccinated after developing COVID-19 were excluded to avoid the introduction of survivorship bias; however, omission of these patients could make outcomes for the unvaccinated cohort appear to be relatively worse. Although we cannot exclude the possibility of immortal time bias due to the exclusion of patients who died prior to receiving the third dose of mRNA vaccine, this possibility is unlikely because had these patients survived, they would also be excluded due to ineligibility to receive the third dose. In addition, because of this consideration there could be additional bias related to selection or disease severity particularly related to temporal differences (e.g. SARS-CoV-2 variants) or overall patient health status by the number of vaccine doses administered. Although not evaluated in the current work, the protective benefit of a single dose of mRNA vaccine among patients with cancer should be investigated in future efforts and would be highly relevant to areas with limited resources. After exclusions to partially address time-varying confounding, the sample size is relatively modest such that some of the confidence intervals in the primary analysis and especially in the sensitivity analyses are wide. There may be residual confounding due to regional and center-level factors which are incompletely captured in the CCC19 data model.30 Additionally, the exact timing of breakthrough infections in relation to COVID-19 vaccination were not precisely determined, as our current analysis relied on time intervals instead. Despite such limitations, this represents one of the largest cohorts with comprehensive clinical and biological data on vaccinated patients with cancer and breakthrough COVID-19 reported to date. Moreover, to our knowledge, this is one of the first studies to evaluate breakthrough infections following the receipt of 3 doses of mRNA vaccines among patients with cancer.
Future studies should aim to investigate duration of immunity and the associated immunological characteristics of breakthrough infections through dedicated laboratory evaluations. Also, the precise timing of breakthrough infections from the last dose of vaccination in relation to COVID-19 outcomes among patients with cancer, especially in specific population subgroups, should be investigated to enhance provision of clinical care. This is difficult in a retrospective study design, given that post-vaccination titers and cellular responses are not routinely captured in clinical care. Additionally, the effects of a 4th dose of vaccination, second boosters, and whether certain vaccines or “mix and match” strategies are effective, will need to be investigated.
In conclusion, vaccination is an essential strategy to prevent symptomatic and severe COVID-19 across all patient groups, including those with cancer. Although this was not a causal analysis, vaccination appears to protect from severe outcomes, including death, among patients with cancer who develop breakthrough infections. With the emergence of novel SARS-CoV-2 variants of concern, vaccination according to recommended schedules, judicious social distancing, and mask wearing represent important measures to help control the spread of the virus and ensure optimal outcomes across the patient population.
Contributors
TKC, CL, ZB, YS, DF, and JLW: Conceptualization, study design, methodology, original draft writing, review and editing.
DRR: study design, methodology, review and editing.
YS, CH, and JW: data analyses performed by using R 4.0.3 and the R packages Hmisc 4.4.2, MatchIt 4.2.0, ipw 1.0–11, survey 4.0, sandwich 3.0–1, and glmnet 4.1–1.
SM: Project administration, coordination, review and editing.
ALS, CH, SR-KS, CJ, LBW, EAG, SH, UW, SB, TEO'C, TMW-D, OAP, EJK, MJ, FY, MSD, NTNG, SB, HS, RN, RRM, TKN, RQ, SMR, MP, BHM, PV, BF, HAZ, SADP, KR, DYR, MKA, HS, and CRF: contributed substantial patient cases to the CCC19; reading and editing.
GdLL and HS: reading and editing.
C-YH and JLW accessed and verified the data.
A full list of contributors and institutions is provided in the Supplementary Material.
Declaration of interests
TKC reports grants, personal fees and non-financial support from Merck, BMS, Exelixis, Astra Zeneca, Eli Lilly, Eisai, Novartis, GSK, Pfizer, EMD Serono; stocks in Pionyr, Tempest, outside the submitted work; In addition, TKC reports patent: pending International Patent Application No. PCT/US2018/12209, entitled “PBRM1 Biomarkers Predictive of Anti-Immune Checkpoint Response,” filed January 3, 2018, claiming priority to U.S. Provisional Patent Application No. 62/445,094, filed January 11, 2017; pending International Patent Application No. PCT/US2018/058430, entitled “Biomarkers of Clinical Response and Benefit to Immune Checkpoint Inhibitor Therapy,” filed October 31, 2018, claiming priority to U.S. Provisional Patent Application No. 62/581,175, filed November 3, 2017; TKC sits on National Comprehensive Cancer Network kidney panel. CL reports grants from Genentech/ImCore. ZB reports non-financial support from Bristol Myers Squibb, grants from Genentech/ImCore, personal fees from UpToDate, outside the submitted work. ALS reports non-financial support from Astellas and Pfizer outside the submitted work. GdLL reports personal fees from Boehringer Ingelheim, Pfizer, AstraZeneca; grants from AstraZeneca, Merck Sharp & Dohme, EMD Serono, AstraZeneca, Blueprint Medicines, Tesaro, Bavarian Nordic, Novartis, G1 Therapeutics, Adaptimmune, BMS, GSK, Abbvie, Rgenix, Pfizer, Roche, Genentech, Eli Lilly, Janssen; personal fees from Boehringer Ingelheim, Pfizer, E.R. Squibb Sons, LLC, Janssen; all outside the submitted work. CH reports grants from Merck, Bayer, Genentech, AstraZeneca, Bausch Health; Consulting fees from Tempus, Genzyme, EMD Sorono, payment or honoraria from OncLive/MJH Life Sciences, support for attending meetings and/or travel from Merck, participation on a data safety monitoring or advisory board of Henry Ford Cancer Institute, Hoosier Cancer Research Network; Leadership or fiduciary role in Wayne County Medical Society of Southeast Michigan; Stock or stock options in Johnson and Johnson, all outside the submitted work. EAG reports Consulting fees from Alexion Inc, Picnic Health, AbbVie, CTI Biopharma, Genentech Inc., Novartis, Celgene/Bristol Myers-Squibb, Takeda oncology, Taiho Oncology and Research Funding from Genentech Inc, Astex Pharmaceuticals, and BluePrint Medicines, outside the submitted work. SH reports grants/research supports from ASCO TAPUR, Astellas; honoraria or consultation fees from Sanofi, Aveo Oncology, outside the submitted work. SB reports Consulting fees from BMS, Exelexis, Eisai, Pfizer, Myovant, SeaGen; Payment or honoraria from Exelexis, Eisai, BMS; Participation on a Data Safety Monitoring Board or Advisory Board from SeaGen, Pfizer, Myovant; Stock or stock options in Natera; outside the submitted work. MJ reports grants from AstraZeneca, Pfizer; Eisai, personal fees from Seagen, Sanofi, outside the submitted work. NTNG reports personal fees from Novocure, outside the submitted work. RRM reports Advisory board/consultant—Aveo, AstraZeneca, Bayer, Bristol Myers Squib, Calithera, Caris, Dendreon, Exelixis, Janssen, Merck, Myovant, Novartis, Pfizer, Sanofi, Sorrento therapeutics, Pfizer, Tempus, Vividion, unrelated to this work. SMR reports advisory for Roche, Janssen, Sanofi, and EUSA Pharma, unrelated to this work. PV reports institutional research funding from Sanofi; stocks or stock options in Novavax, Biontech. HAZ acknowledges support from Georgia NCORP. CRF reports grants from Merck Foundation, grants from NCCN/Pfizer, grants from National Cancer Institute, other from National Cancer Institute, other from Patient-Centered Outcomes Research Institute, outside the submitted work. SM reports support from National Cancer Institute, and Intl Assoc. for the Study of Lung Cancer during the conduct of the study; and personal fees from National Geographic outside the submitted work. DF reports Grants or contracts from Merck, Viracor, Astellas; Support for attending meetings and/or travel from Viracor; outside the submitted work. JLW reports grants from NIH during the conduct of the study; personal fees from Roche, Westat, Flatiron Health, Melax Tech, IBM Watson Health, ownership of HemOnc.org LLC, grants from AACR; outside the submitted work. TMW-D reports grants from BMS, Merck & Co, GSK/Tesaro, Janssen; personal fees from Exicure, Shattuck Labs, SITC, Merck & Co, Caris Life Sciences, outside the submitted work.
C-YH, SRKS, CJ, LBW, UW, TEO'C, OAP, EJK, HS, RN, TKN, RQ, MP, BHM, SADP, KR, BF, DYR, MKA, HS, DRR, YS, and SB have nothing to disclose.
The content is solely the responsibility of the authors and does not necessarily represent the US Food and Drug Administration official views or policies.
Acknowledgements
We thank all members of the CCC19 steering committee for their invaluable guidance of the CCC19 consortium.
Funding: This study was partly supported by grants from the National Cancer Institute grant number P30 CA068485 to C-YH, YS, SM, JLW; T32-CA236621 and P30-CA046592 to C.R.F; CTSA 2UL1TR001425-05A1 to TMW-D; ACS/FHI Real-World Data Impact Award, P50 MD017341-01, R21 CA242044-01A1, Susan G. Komen Leadership Grant Hunt to MKA. REDCap is developed and supported by Vanderbilt Institute for Clinical and Translational Research grant support (UL1 TR000445 from NCATS/NIH).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100445.
Appendix A. Supplementary data
References
- 1.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384(5):1–14. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris R.J., Hall J.A., Zaidi A., Andrews N.J., Dunbar J.K., Dabrera G. Effect of vaccination on household transmission of SARS-CoV-2 in England. N Engl J Med. 2021;385:759–760. doi: 10.1056/NEJMc2107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergwerk M., Gonen T., Lustig Y., et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg Y., Mandel M., Bar-On Y.M., et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abu-Raddad L.J., Chemaitelly H., Bertollini R. Waning mRNA-1273 vaccine effectiveness against SARS-CoV-2 infection in Qatar. N Engl J Med. 2022;386:1091–1093. doi: 10.1056/NEJMc2119432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Accorsi E.K., Britton A., Fleming-Dutra K.E., et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA. 2022;327:639–651. doi: 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bar-On Y.M., Goldberg Y., Mandel M., et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakouny Z., Hawley J.E., Choueiri T.K., et al. COVID-19 and cancer: current challenges and perspectives. Cancer Cell. 2020;38:629–646. doi: 10.1016/j.ccell.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elkrief A., Wu J.T., Jani C., et al. Learning through a pandemic: the current state of knowledge on COVID-19 and cancer. Cancer Discov. 2022;12:303–330. doi: 10.1158/2159-8290.CD-21-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grivas P., Khaki A.R., Wise-Draper T.M., et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and cancer consortium. Ann Oncol. 2021;32:787–800. doi: 10.1016/j.annonc.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thakkar A., Gonzalez-Lugo J.D., Goradia N., et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021;39:1081–1090.e2. doi: 10.1016/j.ccell.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goshen-Lago T., Waldhorn I., Holland R., et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021;7:1507–1513. doi: 10.1001/jamaoncol.2021.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fendler A., Shepherd S.T.C., Au L., et al. Adaptive immunity and neutralizing antibodies against SARS-CoV-2 variants of concern following vaccination in patients with cancer: the CAPTURE study. Nat Cancer. 2021;2:1305–1320. doi: 10.1038/s43018-021-00274-w. [DOI] [PubMed] [Google Scholar]
- 16.Fendler A., Shepherd S.T.C., Au L., et al. Omicron neutralising antibodies after third COVID-19 vaccine dose in patients with cancer. Lancet. 2022;399:905–907. doi: 10.1016/S0140-6736(22)00147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt A.L., Labaki C., Hsu C.Y., et al. COVID-19 vaccination and breakthrough infections in patients with cancer. Ann Oncol. 2022;33:340–346. doi: 10.1016/j.annonc.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W., Kaelber D.C., Xu R., Berger N.A. Breakthrough SARS-CoV-2 infections, hospitalizations, and mortality in vaccinated patients with cancer in the US between December 2020 and November 2021. JAMA Oncol. 2022;8:1027–1034. doi: 10.1001/jamaoncol.2022.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee L.Y.W., Starkey T., Ionescu M.C., et al. Vaccine effectiveness against COVID-19 breakthrough infections in patients with cancer (UKCCEP): a population-based test-negative case-control study. Lancet Oncol. 2022;23:748–757. doi: 10.1016/S1470-2045(22)00202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song Q., Bates B., Shao Y.R., et al. Risk and outcome of breakthrough COVID-19 infections in vaccinated patients with cancer: real-world evidence from the national COVID cohort collaborative. J Clin Oncol. 2022;40:1414–1427. doi: 10.1200/JCO.21.02419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.A systematic framework to rapidly obtain data on patients with cancer and COVID-19: CCC19 governance, protocol, and quality assurance. Cancer Cell. 2020;38:761–766. doi: 10.1016/j.ccell.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shmueli G. To explain or to predict? Stat Sci. 2010;25:289–310. [Google Scholar]
- 24.Fendler A., Au L., Shepherd S.T.C., et al. Functional antibody and T cell immunity following SARS-CoV-2 infection, including by variants of concern, in patients with cancer: the CAPTURE study. Nat Cancer. 2021;2:1321–1337. doi: 10.1038/s43018-021-00275-9. [DOI] [PubMed] [Google Scholar]
- 25.Fendler A., Shepherd S.T.C., Au L., et al. Immune responses following third COVID-19 vaccination are reduced in patients with hematological malignancies compared to patients with solid cancer. Cancer Cell. 2022;40:114–116. doi: 10.1016/j.ccell.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monin L., Laing A.G., Muñoz-Ruiz M., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lièvre A., Turpin A., Ray-Coquard I., et al. Risk factors for Coronavirus Disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: a French nationwide cohort study (GCO-002 CACOVID-19) Eur J Cancer. 2020;141:62–81. doi: 10.1016/j.ejca.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q.Q., Berger N.A., Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021;7:220–227. doi: 10.1001/jamaoncol.2020.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu J., Reid S.A., French B., et al. Racial disparities in COVID-19 outcomes among Black and white patients with cancer. JAMA Netw Open. 2022;5:e224304. doi: 10.1001/jamanetworkopen.2022.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawley J.E., Sun T., Chism D.D., et al. Assessment of regional variability in COVID-19 outcomes among patients with cancer in the United States. JAMA Netw Open. 2022;5:e2142046. doi: 10.1001/jamanetworkopen.2021.42046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.