Abstract
Aim: Cardiovascular disease (CVD) is the second largest cause of death in Japanese women. Pregnancy and childbirth are events that put a strain on the cardiovascular system. When postpartum weight retention is insufficient, weight gain due to fat deposition during pregnancy might lead to obesity. Thus, we examined the effects of body mass index (BMI) in middle and older ages and the number of children on CVD and metabolic disorders.
Methods: From the Tohoku Medical Megabank database, we used data from 32,000 women aged ≥ 50 years. This database contains obstetrical history, medical history, and laboratory data obtained once from 2013 to 2015.
Results: The mean age of participants was 64.2 years, and 47.7% of women had two children. Compared with nulliparous women, those who had a higher number of children had higher BMI and systolic blood pressure. The prevalence of CVD was highest in obese class I (30 kg/m2 ≤ BMI) women with three or more children and the prevalence of hypertension was high in pre-obese (25 kg/m2 ≤ BMI <30 kg/m2) and obese class I women with children. Conversely, the prevalence of diabetes and proportion of women whose HbA1c values were >6.5% was highest in obese class I women with no children.
Conclusion: In this study, we found that not only BMI but also the number of children influenced the health status of middle- and older-aged women, suggesting the importance of childbirth history in the health management of women.
Keywords: Cardiovascular risk in women, BMI, Number of children
See editorial vol. 30: 107-109
1. Introduction
Obesity is strongly associated with cardiovascular diseases (CVD). For Japanese people, although high body mass index (BMI) is associated with an increased risk of CVD in men, it is unclear to what extent BMI contributes to the risk of CVD in women 1) . Pregnancy and childbirth are events that necessitate substantial physiological changes and impose various burdens on women during a limited period of 10 months. Mechanisms such as an increase in circulating blood volume, changes in lipid composition, and glucose metabolism promote the efficient development of the fetus. Nevertheless, they cause vascular endothelial damage to the mother 2 - 4) . Thus, as the number of childbirths increases, there is a concern regarding the accumulation of damage to the maternal cardiovascular system. Additionally, we must consider bodyweight increase during pregnancy due to fat accumulation, which can lead to obesity in women if postpartum weight loss is insufficient 5) . Without proper weight control, excess fat accumulation during pregnancy may increase future CVD risk.
Several reports documented a potential relationship between the number of childbirths and subsequent CVD 6 - 8) . A meta-analysis using 10 cohort studies from China indicated that there is a significant association between parity and CVD risk 8) . Another study from Japan found a relationship between reproductive history and CVD mortality 9) . Nonetheless, these studies did not evaluate changes in real laboratory data.
Presently, CVD is the second largest cause of death after cancer in Japanese women 10) . Although the incidence of CVD is lower than that in other developed countries, CVD eradication is an important medical issue in Japan. Hence, in order to analyze how obesity and childbirth affect CVD, using a database including over 30,000 women from the Tohoku Medical Megabank Community-Based Cohort Study, we decided to examine the effects of BMI in middle and older ages and the number of children on CVD and underlying conditions.
2. Aim
The present study aims to elucidate the impact of the number of children and BMI in middle and older ages on subsequent CVD and underlying conditions including hypertension (HT), dyslipidemia (DL), and diabetes mellitus (DM).
3. Methods
3.1 Study Design
The design of this study is cross-sectional.
3.2 Approval and Ethics
This study was approved by the Medical Research Ethics Committee of Tokyo Medical and Dental University (M2019-190).
3.3 Study Population
The database from the Tohoku Medical Megabank Community-Based Cohort Study version 2.3.0 (TMM CommCohort Study, available at http://www.dist.megabank.tohoku.ac.jp/about/data/_2.3.0/index.html [Accessed 1 November-2021]) was utilized. This database released by the Tohoku Medical Megabank Organization, Tohoku University, on July 31, 2019, contained the data of 67,000 local residents with active consents, who were ≥ 20 years of age and lived in Miyagi and Iwate Prefectures in Northeastern Japan 11) . Type 1 survey was conducted following baseline assessment, underwent a health checkup conducted by local governments once during 3 years, i.e., from 2013 to 2015, and biospecimens, including serum and plasma, were collected. At the same time, participants completed questionnaires regarding their medical history and lifestyle. Thus, this database contains family information, obstetric history in women, and medical history including conditions such as CVD and HT. Additionally, we used laboratory data values obtained at health checkups. In this study, approximately 32,000 women in their 50s and older who had finished giving birth were surveyed to investigate the effect of pregnancy and childbirth. We analyzed 32,189 women, excluding cases with missing data or obvious input errors.
3.4 Variable Definitions
The number of children was self-reported on a questionnaire administered at entry and was categorized as 0, 1, 2, and ≥ 3. In a Japanese survey from 2015, couples with more than four children are only accounted for 3.3% of the whole sample 9) ; thus, we divided women into four groups to avoid extreme differences in the number of subjects between groups. Demographic information including age at first birth, age at last birth, age at menopause, educational level, history of hypertensive disorders of pregnancy (HDP), and smoking status were also determined by self-report. Menopausal status was defined as women who self-reported being postmenopausal. In terms of smoking history, we classified participants into two groups based on whether or not they had smoked more than 100 cigarettes in their lifetime. BMI was calculated on the basis of the height and weight measured at medical checkups. Using the standard from Japan Society for the Study of Obesity, we categorized BMI into four groups: underweight (BMI <18.5 kg/m2), normal range (18.5 kg/m2 ≤ BMI <25 kg/m2), pre-obese (25 kg/m2 ≤ BMI <30 kg/m2), and obese class I (BMI ≥ 30 kg/m2). Waist circumference (cm) was measured midway between the lowest rib bone and iliac crest. Weight change from age 20 years was calculated by subtracting self-reported body weight at 20 years of age from current weight. Blood pressure was measured in the sitting position. Blood was drawn following venipuncture, regardless of the time after a meal.
In terms of laboratory data values, the diagnostic criteria of each disease were used; systolic blood pressure of 140 mmHg and diastolic blood pressure of 90 mmHg were the diagnostic criteria for HT, HbA1c of 6.5% was the criterion for DM, and LDL cholesterol of 140 mg/dL, triglyceride of 150 mg/dL and high-density lipoprotein cholesterol (HDL-C) of 40 mg/dL was the criterion for DL.
The outcome was the presence of any of the following diseases: CVD, HT, DL, and DM. CVD was divided into the following six categories: 1) cerebral hemorrhage, 2) cerebral infarction, 3) subarachnoid hemorrhage, 4) angina/myocardial infarction, 5) aneurysm/aortic dissection, and 6) heart failure. The presence of these diseases was defined as currently being treated or the previous diagnosis.
3.5 Statistical Method
The baseline characteristics of the study population were compared on the basis of the number of children. Continuous variables are summarized using the median and interquartile interval, and analyzed using the Kruskal-Wallis test. Categorical variables are summarized using the number and percentage, and analyzed using χ2 test. The prevalences of CVD, HT, DL, and DM are shown as a heatmap to visualize the differences due to BMI and the number of children. Similarly, the ratio of women whose data values exceeded the diagnostic criteria is shown. Statistical analyses were performed using JMPⓇ 15 (SAS Institute Inc., Cary, NC, USA). All tests were two-sided, and p-values of <.05 were considered statistically significant.
4. Results
For participants, Table 1-1 shows the baseline characteristics of all women participating in the study by the number of children. The mean age of participants was 64.2±6.1 years. The modal number of children was two, and 47.7% of women had two children. Among parous women, those who had a higher number of children had a younger age at the birth of their first child, older age at the birth of their last child, and a lower percentage with a university/college degree or higher education history and were less likely to smoke. Age at menopause and percentage of menopausal women were almost the same in all groups. The percentage of women who developed HDP was highest in the group with one child (5.4%).
Table 1-1. Baseline characteristics of women by number of children.
| Number of children | 0 | 1 | 2 | ≧3 | Number of respondents (n) | P |
|---|---|---|---|---|---|---|
| No. of subjects | 2685 (8.3%) | 2839 (8.8%) | 15381 (47.8%) | 11284 (35.1%) | 32,189 | N.A |
| Age, y Median (interquartile range) | 64 (58-68) | 65 (60-70) | 65 (61-69) | 65 (60-69) | 32,189 | <0.0001 |
| Age at first child, y Median (interquartile range) | NA | 27 (24-30) | 25 (23-27) | 24 (22-26) | 27,702 | <0.0001 |
| Age at last child, y Median (interquartile range) | NA | 28 (25-31) | 28 (26-30) | 30 (29-33) | 27,402 | <0.0001 |
| Age at menopause, y Median (interquartile range) | 50 (46-52) | 50 (47-52) | 50 (48-53) | 50 (58-43) | 28,033 | <0.0001 |
| Percentage of menopausal women, % | 93.5 | 95.4 | 96.4 | 95.8 | 29,936 | <0.0001 |
| (2110/2257) | (2536/2658) | (13943/14465) | (10109/10556) | |||
| Educational level University/collee degree, % | 6.9 | 4.0 | 2.8 | 3.1 | 31,739 | <0.0001 |
| (167/2426) | (112/2822) | (422/15279) | (346/11212) | |||
| Smoking status | 8.3 | 5.6 | 3.8 | 3.8 | 31,141 | <0.0001 |
| Percentage of women who have smoked more than 100 cigarettes in their lifetime, % | (216/2587) | (155/2752) | (563/14897) | (413/10905) | ||
| Percentage of women who developed hypertensive disorders of pregnancy, % | NA* | 5.4 | 5.1 | 5.1 | 28,842 | <0.0001 |
| (0/2366) | (138/2560) | (698/13725) | (517/10191) |
*Removed 12 women
Table 1-2 shows previous and present medical history. When the six CVD categories were combined, the prevalence rate was highest in the group with three or more children (4.9%). This tendency was particularly remarkable for cerebral infarction and angina/myocardial infarction. As the number of children increased, the prevalence of HT and type II DM increased and the rate of these diseases was highest in the group with three or more children (39.3% and 13.2%, respectively). The prevalence of DL was highest in the group with one child (28.8%).
Table 1-2. Medical history of women by number of children.
| Number of children | 0 | 1 | 2 | ≧3 | P |
|---|---|---|---|---|---|
| No. of subjects | 2685 | 2839 | 15381 | 11284 | N.A |
| Arteriosclerotic cardiovascular disease | 107 (4.0%) | 130 (4.6%) | 648 (4.2%) | 553 (4.9%) | 0.03 |
| cerebral hemorrhage | 11 (0.4%) | 8 (0.3%) | 47 (0.3%) | 38 (0.3%) | 0.80 |
| cerebral infarction | 25 (0.9%) | 40 (1.4%) | 159 (1.0%) | 162 (1.4%) | 0.01 |
| subarachnoid hemorrhage | 24 (0.9%) | 18 (0.6%) | 97 (0.6%) | 63 (0.6%) | 0.27 |
| angina / myocardial infarction | 39 (1.5%) | 56 (2.0%) | 264 (1.7%) | 213 (1.9%) | 0.34 |
| aneurysm / aortic dissection | 12 (0.5%) | 12 (0.4%) | 114 (0.7%) | 79 (0.7%) | 0.12 |
| heart failure | 3 (0.1%) | 6 (0.2%) | 37 (0.2%) | 28 (0.3%) | 0.59 |
| Hypertension | 812 (30.2%) | 1037 (36.5%) | 5807 (37.8%) | 4433 (39.3%) | <0.0001 |
| Dyslipidemia | 683 (25.4%) | 817 (28.8%) | 4401 (28.6%) | 2854 (25.3%) | <0.0001 |
| type II diabetes | 299 (11.1%) | 348 (12.3%) | 1994 (13.0%) | 1486 (13.2%) | 0.03 |
Data are n (%)
Table 2 shows the laboratory data obtained at health checkups via the number of children. Compared with nulliparous women, those who had a higher number of children had higher BMI, increased waist circumference, increased weight change from age 20 years, and higher systolic blood pressure. Blood glucose levels and triglyceride levels were highest, and HDL-C was lowest in the group with three or more children (93 mg/dL, 102 mg/dL, and 64 mg/dL, respectively). Conversely, total cholesterol levels were lowest in the group with three or more children. LDL levels showed a similar pattern to that of total cholesterol levels.
Table 2. Laboratory data of women by number of children.
| Number of children | 0 | 1 | 2 | ≧3 | Number of respondents (n) | P |
|---|---|---|---|---|---|---|
| BMI, Kg/m2 | 22.4 (20.2-25) | 22.6 (20.4-25) | 22.7 (20.6-25) | 23.4 (21.3-25.8) | 32,171 | <0.0001 |
| Waist circumference, cm | 80.7 (74.2-87.2) | 81 (75-87.8) | 81.3 (75.5-87.3) | 83 (77-88.8) | 32,147 | <0.0001 |
| Weight change since age 20y, Kg | 1.7 (-3-7.6) | 2.4 (-2.5-8.1) | 2.4 (-2.6-7.6) | 3.2 (-2.1-8.5) | 29,504 | <0.0001 |
| BMI at age 20y, Kg/m2 | 21.3 (19.8-23.1) | 21.4 (19.9-23.1) | 21.6 (20.1-23.2) | 21.9 (20.4-23.6) | 29,504 | <0.0001 |
| Systolic BP, mmHg | 125 (115-137) | 125 (115-137) | 126 (116-137) | 126 (116-137) | 32,169 | 0.0149 |
| Diastolic BP, mmHg | 74 (68-81) | 74 (68-81) | 75 (68-81) | 75 (68-81) | 32,169 | 0.0536 |
| Glucose, mg/dl | 92 (85-102) | 92 (85-103) | 92 (85-102) | 93 (85-105) | 32,160 | <0.0001 |
| HgbA1C, % | 5.6 (5.4-5.8) | 5.6 (5.4-5.8) | 5.6 (5.4-5.8) | 5.6 (5.4-5.9) | 32,185 | <0.0001 |
| T-chol, mg/dl | 216 (195-240) | 213 (193-238) | 215 (194-237) | 212 (191-234) | 17,176 | <0.0001 |
| LDL, mg/dl | 127 (107-147) | 124 (106-146) | 125 (107-146) | 124 (106-144) | 28,822 | 0.0008 |
| HDL-C, mg/dl | 67 (57-79) | 65 (55-77) | 66 (56-77) | 64 (54-75) | 17,176 | <0.0001 |
| Triglycerides, mg/dl | 99 (72-142) | 100 (72-144) | 99 (73-138) | 102 (74-143) | 32,171 | 0.0002 |
Data are Median (interquartile range).
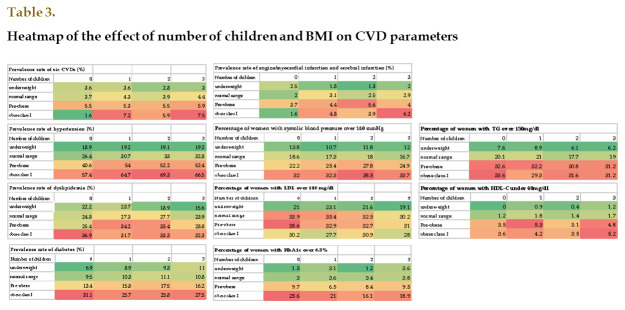
Since BMI tended to increase as the number of children increased, and BMI was a known risk factor for metabolic disorders and CVD, we performed additional analysis regarding the prevalence of CVD and metabolic disorders according to BMI and the number of children ( Table 3 ) . The prevalence of HT was high in pre-obese and obese class I women with children. The prevalence of DM was highest in the obese class I women with no children. The prevalence of CVD was highest in obese class I women with three or more children and lowest in obese class I women with no children. Similarly, regarding laboratory data, the ratio of women with systolic blood pressure values of >140 mmHg was high in pre-obese and obese class I women with children. The ratio of women whose HbA1c values were >6.5% was highest among obese class I women with no children.
5. Discussion
5.1 Number of children and current BMI was Associated with CVD and Metabolic Disorders
In this study of women aged ≥ 50 years living in the Tohoku region, we showed that the prevalence of combined six CVDs was the highest at 4.9% in the group with three or more children. The number of children was positively associated with middle and older age BMI, waist circumference, and weight change from age 20 years. It is also associated with the prevalence of HT and type II DM. The levels of systolic blood pressure and blood glucose also increased with the number of children. Since BMI is a well-known and obvious risk factor, we attempted to isolate the effects of current BMI and the number of children on the prevalence of CVD or metabolic disorders.
5.2 Differential Impact of the Number of Children on HT and DM
As previously reported, the prevalences of HT, DM, and DL were naturally higher in women with high BMI. We found that the prevalence of HT in women with children was higher than that in women without children. By contrast, the prevalence of DM was higher in obese class I women without children than in those with children. These data are in good agreement with measured systolic blood pressure and HbA1c levels. Nonetheless, we failed to detect any trend between the prevalence of DL and the number of children.
5.3 Weight Gain due to Pregnancy and Future CVD
There are several reports on the relationship between parity and the future risk of CVD 6 - 8) . The results of these studies have been conflicting, but most indicate that the risk of CVD demonstrated a U- or J-shaped relationship with parity, with the nadir of risk at two or three children. A report from Sweden documented that CVD risk in high parity women was mainly mediated by weight gain, which increases the risk of CVD through diabetes 7) . In our study, as the number of children increased, BMI, waist circumference, and weight change from age 20 years increased. The prevalence of the six CVDs was highest at 7.5% in the obese class I group with three or more children. Similar observations were obtained when only two CVDs, angina/myocardial infarction and cerebral infarction, were considered. The prevalence of these two diseases was the highest at 6.2% in the obese class I group with three or more children. This obese condition may be due to excess weight gain during pregnancy or insufficient postpartum weight loss. It is intriguing that the second highest prevalence of CVD was found in obese class I women with one child and not with two children. To critically examine whether high BMI plays a role in CVD development in women with multiple children, this inconsistent relationship between the number of children and CVD will be examined in a future prospective study.
5.4 Number of Children and Future DM
Previous reports showed that having multiple children is positively associated with the subsequent risk of maternal diabetes 12) . It is known that the risk of gestational diabetes mellitus (GDM) increases with the number of children 13) . Since GDM is a well-known risk factor for subsequent type II DM 14) , pregnancy and childbirth itself can increase the future risk of DM. By contrast, the prevalence of DM was highest at 31.3% in obese class I women with no children in our study. This implies the possibility that there was a group of women who were already obese and became diabetic during their reproductive age, which was inappropriate condition for pregnancy. This notion suggests that the relationship between the number of children and DM can be considered in cases of a successful pregnancy.
5.5 Limitations
There are several limitations to this study. Since our data are cross-sectional data, and no prospective analysis was conducted, the causal relationship between factors remains unknown. Moreover, samples from participants in the Tohoku Medical Megabank Project were collected at medical checkups rather than medical hospitals visits, suggesting that slightly “healthy” or “health-conscious” individuals were involved. It is well known that the daily intake of salt in the Tohoku region is higher than that of the Japanese average, although it has been decreasing for the past 10 years 10) ; however, we did not include this analysis in our study. Moreover, we could not collect detailed information during and after pregnancy such as that regarding miscarriage, stillbirth, and lactation, which may impact future CVD risk in women 15 , 16) . We failed to control the timing of blood sample collection so that both fasting and nonfasting plasma specimens were included, and lack of data collection such as lipoprotein(a) and remnant-like particle-cholesterol.
6. Conclusion
Our findings suggest that the health management of middle- and older-aged women must consider not only BMI but also pregnancy and childbirth history. It is necessary to raise the awareness of physicians regarding the importance of pregnancy and childbirth in later life. Thus, it is important to provide guidance on maintaining optimal body weight, measuring home blood pressure, and annual medical checkups for multiparous women. A prospective study to confirm when BMI increases and blood pressure elevates should be conducted in the near future.
Declaration of Interests
None.
Funding
This research was supported in part by a grant from the Japan Atherosclerosis Research Foundation.
References
- 1).Renzhe Cui, Hiroyasu Iso, Hideaki Toyoshima, Chigusa Date, Akio Yamamoto, Shogo Kikuchi, Takaaki Kondo, Yoshiyuki Watanabe, Akio Koizumi, Yasuhiko Wada, Yutaka Inaba, Akiko Tamakoshi, JACC Study Group. Body mass index and mortality from cardiovascular disease among Japanese men and women: the JACC study. Stroke, 2005; 36: 1377-1382 [DOI] [PubMed] [Google Scholar]
- 2).Sanghavi M, Kulinski J, Ayers CR, Nelson D, Stewart R, Parikh N, Lemos JA, Khera A. Association between number of live births and markers of subclinical atherosclerosis: The Dallas Heart Study. Eur J Prev Cardiol, 2016; 23: 391-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Skilton MR, Serusclat A, Begg LM, Moulin P, Bonnet F. Parity and carotid atherosclerosis in men and women: insights into the roles of childbearing and child-rearing. Stroke, 2009; 40: 1152-1157 [DOI] [PubMed] [Google Scholar]
- 4).Wang XY, Ye F, Zeng LX, Tu S, Luo WZ, Deng X, Zhang ZH. Parity and carotid atherosclerosis in elderly Chinese women. J Geriatr Cardiol, 2020; 17: 759-765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Rooney BL, Schauberger CW, Mathiason MA. Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstet Gynecol, 2005; 106: 1349-1356 [DOI] [PubMed] [Google Scholar]
- 6).Oliver-Williams C, Vladutiu CJ, Loehr LR, Rosamond WD, Stuebe AM. The Association Between Parity and Subsequent Cardiovascular Disease in Women: The Atherosclerosis Risk in Communities Study. J Womens Health (Larchmt), 2019; 28: 721-727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Klingberg S, Brekke HK, Winkvist A, Engstrom G, Hedblad B, Drake I. Parity, weight change, and maternal risk of cardiovascular events. Am J Obstet Gynecol, 2017; 216: 172.e1-172.e15 [DOI] [PubMed] [Google Scholar]
- 8).Li W, Ruan W, Lu Z, Wang D. Parity and risk of maternal cardiovascular disease: A dose-response meta-analysis of cohort studies. Eur J Prev Cardiol, 2019; 26: 592-602 [DOI] [PubMed] [Google Scholar]
- 9).Tanigawa K, Ikehara S, Kimura T, Imano H, Muraki I, Shirai K, Tamakoshi A, Iso H, JACC study group. Relationships between reproductive history and mortality from cardiovascular diseases among Japanese women: The Japan Collaborative Cohort Study for evaluation of cancer risk (JACC) study. J Epidemiol, 2020; 30: 509-515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Vital Statistics. Ministry of Health, Labor and Welfare. https://www.mhlw.go.jp/toukei/list/81-1.html (Accessed August1, 2021) [Google Scholar]
- 11).Hozawa A, Tanno K, Nakaya N, Nakamura T, Tsuchiya N, Hirata T, Narita A, Kogure M, Nochioka K, Sasaki R, Takanashi N, Otsuka K, Sakata K, Kuriyama S, Kikuya M, Tanabe O, Sugawara J, Suzuki K, Suzuki Y, Kodama EN, Fuse N, Kiyomoto H, Tomita H, Uruno A, Hamanaka Y, Metoki H, Ishikuro M, Obara T, Kobayashi T, Kitatani K, Igarashi KT, Ogishima S, Satoh M, Ohmomo H, Tsuboi A, Egawa S, Ishii T, Ito K, Ito S, Taki Y, Minegishi N, Ishii N, Nagasaki M, Igarashi K, Koshiba S, Shimizu R, Tamiya G, Nakayama K, Motohashi H, Yasuda J, Shimizu A, Hachiya T, Shiwa Y, Tominaga T, Tanaka H, Oyama K, Tanaka R, Kawame H, Fukushima A, Ishigaki Y, Tokutomi T, Osumi N, Kobayashi T, Nagami F, Hashizume H, Arai T, Kawaguchi Y, Higuchi S, Sakaida M, Endo R, Nishizuka S, Tsuji I, Hitomi J, Nakamura M, Ogasawara K, Yaegashi N, Kinoshita K, Kure, Sakai A, Kobayashi S, Sobue K, Sasaki M, and Yamamoto M. Study Profile of the Tohoku Medical Megabank Community-Based Cohort Study. J Epidemiol, 2021; 31: 65-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Mueller NT, Mueller NJ, Odegaard AO, Gross MD, Koh WP, Yuan JM, Pereira MA. Higher parity is associated with an increased risk of type-II diabetes in Chinese women: the Singapore Chinese Health Study. BJOG, 2013; 120: 1483-1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet Med, 2004; 21: 103-113 [DOI] [PubMed] [Google Scholar]
- 14).Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet, 2009; 373: 1773-1779 [DOI] [PubMed] [Google Scholar]
- 15).Peters SA, Woodward M. Women's reproductive factors and incident cardiovascular disease in the UK Biobank. Heart, 2018; 104: 1069-1075 [DOI] [PubMed] [Google Scholar]
- 16).Peters SA, van der Schouw YT, Wood AM, Sweeting MJ, Moons KG, Weiderpass E, Arriola L, Benetou V, Boeing H, Bonnet F, Butt ST, Clavel-Chapelon F, Drake I, Gavrila D, Key TJ, Klinaki E, Krogh V, Kuhn T, Lassale C, Masala G, Matullo G, Merritt M, Molina-Portillo E, Moreno-Iribas C, Nest TH, Olsen A, Onland-Moret NC, Overvad K, Panico S, Redondo ML, Tjonneland A, Trichopoulou A, Tumino R, Turzanski-Fortner R, Tzoulaki I, Wennberg P, Winkvist A, Thompson SG, Angelantonio ED, Riboli E, Wareham NJ, Danesh J, Butterworth AS. Parity, breastfeeding and risk of coronary heart disease: A pan-European case-cohort study. Eur J Prev Cardiol, 2016; 23: 1755-1765 [DOI] [PMC free article] [PubMed] [Google Scholar]