Abstract
Aims
Cardiometabolic diseases (CMDs), including diabetes, heart disease, and stroke, are established risk factors for dementia, but their combined impact has been investigated only recently. This study aimed to examine the association between mid- and late-life cardiometabolic multimorbidity and dementia and explore the role of genetic background in this association.
Methods and results
Within the Swedish Twin Registry, 17 913 dementia-free individuals aged ≥60 were followed for 18 years. CMDs [including age of onset in mid (60) or late (≥60) life] and dementia were ascertained from medical records. Cardiometabolic multimorbidity was defined as having ≥2 CMDs. Cox regression was used to estimate the CMD–dementia association in (i) a classical cohort study design and (ii) a co-twin study design involving 356 monozygotic and dizygotic pairs. By comparing the strength of the association in the two designs, the contribution of genetic background was estimated. At baseline, 3,312 (18.5%) participants had 1 CMD and 839 (4.7%) had ≥2 CMDs. Over the follow-up period, 3,020 participants developed dementia. In the classic cohort design, the hazard ratio (95% confidence interval) of dementia was 1.42 (1.27–1.58) for 1 CMD and 2.10 (1.73–2.57) for ≥2 CMDs. Dementia risk was stronger with mid-life as opposed to late-life CMDs. In the co-twin design, the CMD–dementia association was attenuated among monozygotic [0.99 (0.50–1.98)] but not dizygotic [1.55 (1.15–2.09)] twins, suggesting that the association was in part due to genetic factors common to both CMDs and dementia.
Conclusion
Cardiometabolic multimorbidity, particularly in mid-life, is associated with an increased risk of dementia. Genetic background may underpin this association.
Keywords: Cardiometabolic disease, Dementia, Alzheimer's disease, Vascular dementia, Twin study, Life-course approach
Structured Graphical Abstract
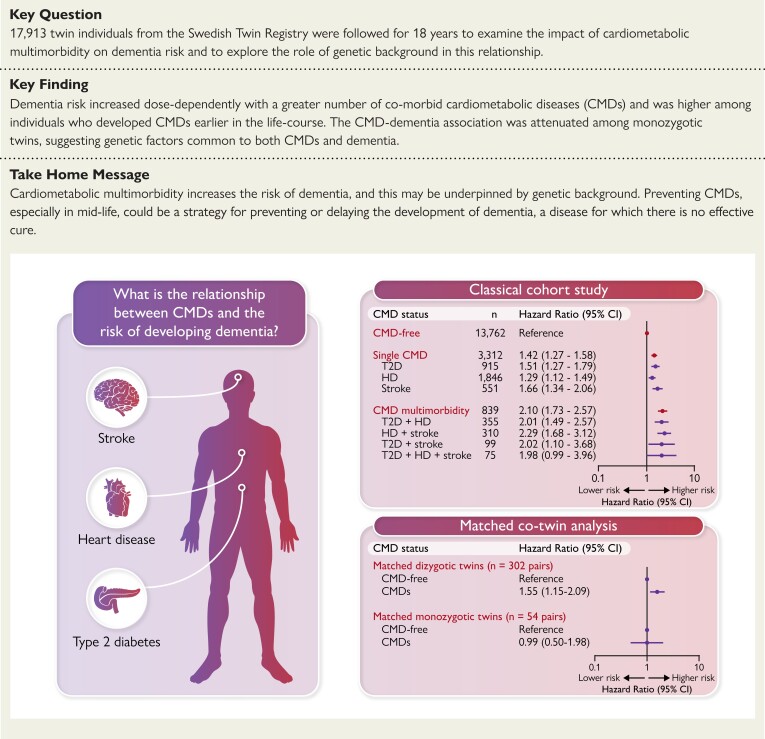
Structured Graphical Abstract.
Summary of the key findings of the study. CMD, cardiometabolic disease; DZ, dizygotic; HD, heart disease; MZ, monozygotic; T2D, Type 2 diabetes
See the editorial comment for this article ‘Deciphering dementia in the cardiometabolic continuum’, by G. P. Fadini and M. L. Morieri, https://doi.org/10.1093/eurheartj/ehac691.
Introduction
Cardiometabolic diseases (CMDs)—a cluster of diseases including type 2 diabetes (T2D), heart disease (HD), and stroke1,2—are a growing challenge in our ageing society. With gains in life expectancy and continued advances in the management of cardiovascular disease and diabetes, people are living longer with CMDs and are increasingly likely to accumulate more than one of these conditions over a lifetime.3 Cardiometabolic multimorbidity—that is, the coexistence of two or more CMDs—has been associated with mortality and other negative health outcomes1,4 and affects an estimated 30% of older adults.5
T2D, HD, and stroke are well-established individual risk factors for dementia,6 but only a few studies have addressed relationship between cardiometabolic multimorbidity and dementia.7–9 It is currently unclear how dementia risk is impacted by the timing of CMD development across the adult lifespan, given the variability of CMDs as chronic diseases with a potentially decades-long time course. Additionally, although CMDs may be linked to dementia through several biologically plausible pathways, our understanding of the mechanisms underlying this association is still limited.
Genetic factors are involved in the development of both CMDs10 and dementia,11,12 but whether and to what extent genetic background contributes to the association between CMDs and dementia is uncertain. Twin studies provide a unique opportunity to address this question. Twins represent naturally matched pairs among whom the confounding effects of a large number of potentially causal factors (e.g. genetics and early-life environment) can be removed.13 It is therefore possible to elucidate the role of genetic background in the relationship between CMDs and dementia by comparing the CMD–dementia associations observed in twin individuals and among monozygotic (MZ) and dizygotic (DZ) twin pairs.14,15
In the present study, we aimed to (i) examine the impact of cardiometabolic multimorbidity on the risk of dementia, including Alzheimer’s disease (AD) and vascular dementia (VaD), (ii) assess how the timing of CMD onset over the life-course influences dementia risk, and (iii) explore the role of genetic background in the CMD–dementia association using 18-year follow-up data from nearly 18 000 twin individuals.
Methods
Study population
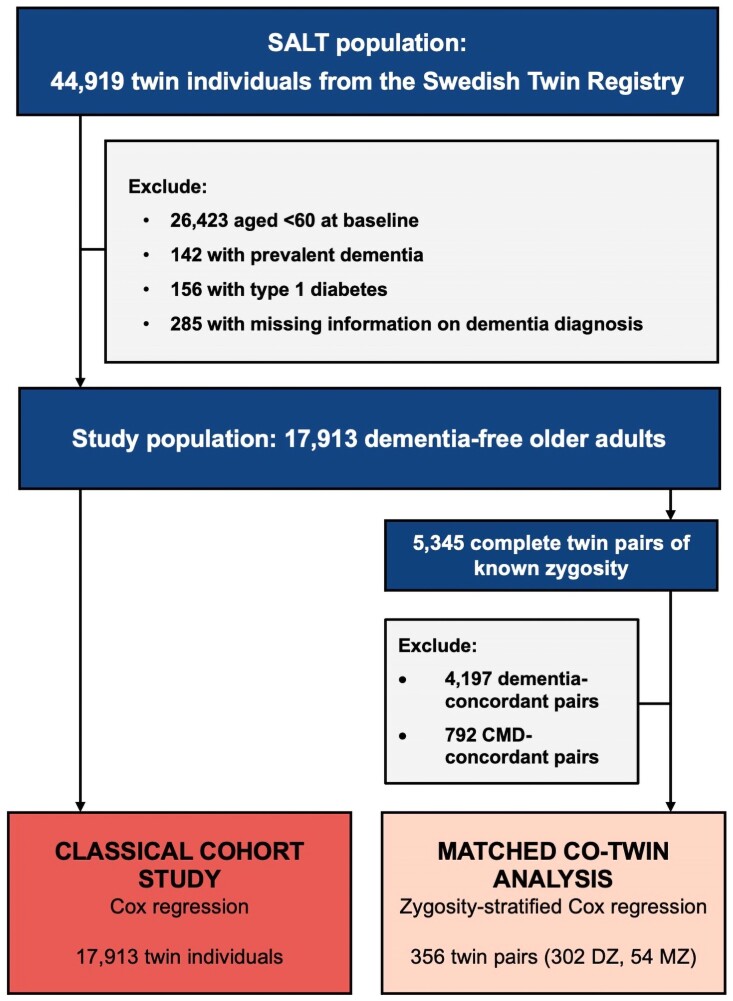
Study participants were drawn from the Swedish Twin Registry (STR), a nationwide database of all twins born in Sweden since the late 1800s and the largest population-based twin registry in the world.13,16 Between March 1998 and December 2002, all living twins in the registry aged >40 years (i.e. born in 1958 or earlier) were invited to participate in a computer-assisted telephone interview on demographics and health conditions as part of the Screening Across the Lifespan Twin (SALT) study (response rate: 72.6%).13,16 Changes in health status among these participants were monitored for a maximum of 18 years (i.e. until December 2016).
A total of 44 919 twin individuals participated in the SALT baseline assessment, 18 496 of whom were of older age (i.e. ≥ 60 years). From this group, we excluded individuals with prevalent dementia (n = 142) and, to avoid possible misclassification of the exposure, individuals with type 1 diabetes (n = 156). We further excluded individuals who were missing information on the timing of dementia diagnosis over follow-up (n = 285), leaving 17 913 participants for the current study (Figure 1).
Figure 1.
Study population and study design.
All participants provided informed consent, and data collection procedures were approved by the Regional Ethics Committee at Karolinska Institute.
Data collection
Information on participants’ zygosity status, socio-demographic characteristics (e.g. age, sex, educational background, and marital status), lifestyle factors (e.g. physical activity level, smoking history, and alcohol consumption), and anthropometrics (e.g. height and weight) was collected through SALT’s baseline interview.13
Zygosity was ascertained based on participants’ responses to the question, “During childhood, were you and your twin as alike as ‘two peas in a pod’ or not more alike than siblings in general?” Twin pairs were categorized as MZ, (i.e. identical) if both co-twins indicated that they were ‘as alike as two peas in a pod,’ DZ (i.e. fraternal) if both co-twins indicated that they were no more alike than typical siblings, and undetermined if the co-twins did not agree, or if only one member of the pair responded to the question.13 Validation studies using DNA genotyping have shown this method to be >98% accurate for distinguishing MZ and DZ twins.13
Education was defined as the total number of years of formal schooling and dichotomized as <8 years vs. ≥ 8 years.17 Marital status was categorized as married/cohabitating vs. single. Physical activity levels were ascertained through a survey question on annual exercise patterns and categorized as low (‘almost never’ or ‘much less than average’) or regular (‘less than average,’ ‘average,’ ‘more than average,’ ‘much more than average,’ or ‘maximum’).18 Smoking status was dichotomized as non-smokers vs. current/former smokers. Alcohol consumption was categorized as no/moderate drinking vs. heavy drinking.
Height and weight were collected by self-report. Previous investigations within the STR have shown self-reported height and weight to be highly consistent with the corresponding measured values (correlation coefficients of 0.97 and 0.95, respectively).19 From this information, body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2) and classified as underweight (<20 kg/m2), normal weight (≥20 to <25 kg/m2), overweight (≥25 to <30 kg/m2), or obese (≥30 kg/m2).20
Medical conditions including hypertension and depression were identified according to International Classification of Disease (ICD) codes in the Swedish National Patient Register (NPR), which covers all nationwide inpatient diagnoses since 1987 and outpatient diagnoses since 2001.21
Assessment of cardiometabolic diseases
CMDs were defined as T2D, HD, and stroke, following previous studies examining the impact of cardiometabolic multimorbidity on health outcomes.1,2,4,7,9,22–24 CMDs were assessed at baseline using data from multiple sources. T2D was identified based on self-reported histories of diabetes, records from the NPR, and use of glucose-lowering medications according to the Swedish Prescribed Drug Register. HD (including coronary heart disease, atrial fibrillation, and heart failure) and stroke (including ischaemic stroke and haemorrhagic stroke) were ascertained based on NPR records (see Supplementary material online, Appendix A for the full list of ICD codes).
CMD status was defined according to participants’ total number of CMDs at baseline and categorized as CMD-free, single CMD (i.e. T2D, HD, or stroke alone), or CMD multimorbidity (i.e. two or more co-morbid CMDs). We additionally grouped participants according to their specific profile of single and co-morbid CMDs (i.e. CMD-free, T2D alone, HD alone, stroke alone, T2D/HD, HD/stroke, T2D/stroke, and T2D/HD/stroke).The age of HD and stroke onset were defined based on the earliest recorded date of HD or stroke diagnosis in the NPR. The age of T2D onset was estimated according to the earliest recorded date of T2D diagnosis in the NPR or the earliest recorded date of glucose-lowering medication usage in the Swedish Prescribed Drug Register, whichever occurred first. Using this information, we determined the age at which participants were diagnosed with their first CMD and, in the case of CMD multimorbidity, their second CMD. The ages of first and second CMD diagnoses were dichotomized as occurring in mid-life (i.e. < 60 years) or late-life (i.e. ≥ 60 years).
Dementia diagnosis
Dementia was diagnosed based on records from the NPR. All recorded diagnoses were based on neurological examinations performed at neurology clinics. Dementia diagnoses were further categorized as AD or VaD according to ICD codes if information on dementia subtype was available (see Supplementary material online, Appendix A for the full list of ICD codes). For participants who died over follow-up without a dementia diagnosis, dementia status was verified using information from the Swedish Cause of Death Register, which contains information on underlying and contributing causes of death.
Statistical analysis
Baseline characteristics of the study participants by CMD status were assessed using χ2 tests for categorical variables and one-way ANOVA for continuous variables. Statistical analyses were then conducted according to two different strategies: (i) a classical cohort study design including all twin individuals and (ii) a matched co-twin analysis involving twin pairs discordant for both CMDs and dementia (Figure 1).
Classical cohort study design
In the classical cohort study design, we aimed to estimate the association between CMDs and dementia in the whole study population of twin individuals, taking into account the age of CMD onset (mid-life vs. late-life). Cox regression models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause dementia among participants with a single CMD or CMD multimorbidity in comparison to those who were CMD-free. Follow-up time (in years) was calculated as the time from study entry until dementia diagnosis, death, or the last available follow-up (31 December 2016). The proportional hazard assumption was tested using Schoenfeld residuals regressed against follow-up time. A violation of proportionality was observed for BMI. To account for this, BMI was treated as a stratified factor in the model, allowing the baseline hazard function to differ by BMI level. The model additionally included a sandwich estimator to correct the standard errors given the clustering of co-twins within a pair. To assess whether an increasing burden of CMDs impacts dementia risk in a dose-dependent manner, we repeated the analyses using the total CMD number as a continuous variable. The analysis was also repeated after grouping participants by their specific profiles of single and multiple CMDs to explore the dementia risk associated with specific constellations of co-morbid CMDs. To examine the impact of CMDs on the subtypes of dementia, we estimated the cause-specific hazards of AD and VaD while censoring the development of other forms of dementia. Finally, to assess how age of CMD onset impacts all-cause dementia risk, we reran the regression models after stratifying participants by the timing (i.e. mid-life vs. late-life) of the diagnosis of single and multiple CMDs. We also assessed dementia risk as a function of the decade of CMD diagnosis (<50, ≥ 50 to <60, ≥ 60 to <70, ≥ 70 to <80, or ≥80 years).
Matched co-twin study design
The aim of the matched co-twin analysis was to assess the role of genetic background in the CMD–dementia association. The analysis was restricted to twin pairs that were discordant for both CMD and dementia status. Therefore, from 5345 complete twin pairs of known zygosity in the study population, we excluded twin pairs in which (i) both co-twins developed dementia or both co-twins remained dementia-free, and (ii) both co-twins had baseline CMDs or both co-twins were CMD-free. This left 356 CMD- and dementia-discordant twin pairs (Figure 1); that is, each twin pair contained one CMD-free individual and one individual with baseline CMDs, only one of whom went on to develop dementia. Among these twin pairs, zygosity-stratified Cox regression models were used to assess the CMD–dementia association in DZ twin pairs (n = 302) and MZ twin pairs (n = 54) separately. We further examined whether the CMD–dementia association significantly differed as a function of zygosity by incorporating the cross-product term of these variables (zygosity × CMD status) into the model. Whereas DZ twins share 50% of their genetic background, MZ twins share 100%. Therefore, the confounding influence of genetic background can be fully controlled among MZ pairs. In this study design, if the association originally observed in the classical cohort study is attenuated in MZ pairs compared with DZ pairs, this would indicate that there are genetic factors common to both CMDs and dementia that contribute to the association.15,25
Cofounded adjustment and sensitivity analyses
All regression analyses were adjusted for potential confounders, including socio-demographic factors (age, sex, education level, marital status), cardiometabolic risk factors (BMI, hypertension), lifestyle factors (smoking status, alcohol consumption, physical activity level), and other medical conditions (depression). These were defined a priori and chosen based on a literature review. Missing values for education (n = 1118), marital status (n = 662), BMI (n = 1640), smoking (n = 1084), alcohol consumption (n = 1138), and physical activity level (n = 6131) were imputed using fully conditional specification. Estimates from 10 iterations were pooled according to Rubin’s rules.26
In sensitivity analyses, we (i) repeated the analysis after excluding individuals who received a dementia diagnosis within 1 (n = 63), 3 (n = 253), 5 (n = 526), and 7 (n = 826) years of baseline to minimize reverse causality; (ii) repeated the analysis after excluding 6462 participants with missing values for covariates; (iii) used Fine and Grey regression to estimate the CMD–dementia association while accounting for the competing risk of death.
All statistical analyses were performed using Stata SE 16.0 (StataCorp, College Station, TX, USA) and P-values <0.05 were considered statistically significant.
Results
Baseline characteristics of the study population
The baseline characteristics of the 17 913 study participants (mean age 70.1 ± 7.5 years; 55.0% female) are shown in Table 1. At baseline, 3312 (18.5%) had a single CMD, and 839 (4.7%) had CMD multimorbidity. Compared with CMD-free individuals, those with CMDs were more likely to be older, male, single, and have fewer years of formal education. People with CMDs also had a higher prevalence of overweight/obesity, depression, heavy drinking, current or former smoking, and physical inactivity.
Table 1.
Baseline characteristics of the SALT study population by baseline CMD status (n = 17 913)
| CMD-free (n = 13 762) | Single CMD (n = 3312) | CMD multimorbidity (n = 839) | P-value | |
|---|---|---|---|---|
| Age, years | 69.3 ± 7.3 | 72.5 ± 7.7a | 74.1 ± 7.3a | <0.001 |
| Sex | – | – | – | <0.001 |
| ȃMale | 5803 (42.2) | 1788 (54.0) | 470 (56.0) | |
| ȃFemale | 7959 (57.8) | 1524 (46.0) | 369 (44.0) | |
| Education | – | – | – | <0.001 |
| ȃ<8 years | 6965 (53.3) | 1746 (58.0) | 438 (61.1) | |
| ȃ≥8 years | 6100 (46.7) | 1267 (42.1) | 279 (38.9) | |
| Marital status | – | – | – | <0.001 |
| ȃMarried/cohabitating | 8860 (66.3) | 1938 (61.9) | 440 (58.7) | |
| ȃSingle | 4510 (33.7) | 1194 (38.1) | 309 (41.3) | |
| Zygosity | – | – | – | 0.057 |
| ȃMZ | 2767 (20.1) | 704 (21.3) | 162 (19.3) | |
| ȃDZ | 9444 (68.6) | 2279 (68.8) | 598 (71.3) | |
| ȃȃSame sex | 4629 (33.6) | 1231 (37.2) | 295 (35.2) | |
| ȃȃOpposite sex | 4815 (35.0) | 1048 (31.6) | 303 (36.1) | |
| ȃUndetermined | 1551 (11.3) | 329 (9.9) | 79 (9.4) | |
| BMI, kg/m2 | 25.0 ± 3.5 | 25.7 ± 3.7a | 26.3 ± 6.1a | <0.001 |
| ȃUnderweight (<20) | 681 (5.4) | 137 (4.7) | 28 (4.1) | <0.001 |
| ȃNormal weight (≥20 to <25) | 6062 (47.8) | 1212 (41.6) | 260 (38.1) | |
| ȃOverweight (≥25 to <30) | 4954 (39.1) | 1235 (42.4) | 293 (42.9) | |
| ȃObese (≥30) | 979 (7.7) | 330 (11.3) | 102 (14.9) | |
| Smoking | – | – | – | <0.001 |
| ȃNon-smokers | 7682 (58.7) | 1612 (53.4) | 365 (50.6) | |
| ȃCurrent/former smokers | 5408 (41.3) | 1406 (46.6) | 356 (49.4) | |
| Alcohol consumption | – | – | – | 0.005 |
| ȃNo/moderate drinking | 12 448 (95.4) | 2826 (94.1) | 676 (93.8) | |
| ȃHeavy drinking | 604 (4.6) | 176 (5.9) | 45 (6.2) | |
| Physical activity level | – | – | – | <0.001 |
| ȃPhysically inactive | 762 (7.9) | 190 (10.6) | 65 (18.1) | |
| ȃRegular physical activity | 8873 (92.1) | 1598 (89.4) | 294 (81.9) | |
| Hypertension | 1864 (13.5) | 518 (15.6) | 110 (13.1) | 0.006 |
| Depression | 317 (2.3) | 111 (3.4) | 34 (4.1) | <0.001 |
Data are presented as mean ± standard deviation, or n (%).
Pairwise means comparison using the Bonferroni correction: P < 0.05 (reference group: CMD-free).
Missing data: 1118 were missing data on education, 662 on marital status, 1640 on BMI, 1084 on smoking status, 1138 on alcohol consumption, and 6131 on physical activity.
Cardiometabolic diseases and the risk of dementia, Alzheimer’s disease, and vascular dementia
During the follow-up (median 15.4 years, accounting for 228 849 person-years), a total of 3020 (16.9%) participants developed dementia, including 1050 (5.9%) with AD and 638 (3.6%) with VaD. The presence of an increasing number of CMDs was dose-dependently associated with a greater risk of dementia and all dementia subtypes: with each additional CMD, the risk of all-cause dementia rose 42% (HR: 1.42, 95% CI: 1.31–1.53), the risk of AD rose 26% (HR: 1.26, 95% CI: 1.10–1.45), and the risk of VaD rose 64% (HR: 1.64, 95% CI: 1.42–1.88). Compared with CMD-free individuals, those with a single CMD had a 42% increased risk of all-cause dementia (HR: 1.42, 95% CI: 1.27–1.58), as well as a significantly increased risk of both AD (HR: 1.31, 95% CI: 1.08–1.59) and VaD (HR: 1.78, 95% CI: 1.44–2.21). CMD multimorbidity was associated with over double the risk of dementia (HR: 2.10, 95% CI: 1.73–2.57), including a significant risk of AD (HR: 1.49, 95% CI: 1.02–2.20) and VaD (HR: 2.65, 95% CI: 1.83–3.84) (Table 2).
Table 2.
HR and 95% CIs for the association between single and co-morbid CMDs and dementia subtypes
| CMD status | All-cause dementia (3020 cases) | Dementia subtypes | ||
|---|---|---|---|---|
| Alzheimer’s disease (1050 cases) | Vascular dementia (638 cases) | |||
| n | HR (95% CI)a | HR (95% CI)a | HR (95% CI)a | |
| CMD-free | 13 762 | Reference | Reference | Reference |
| Single CMD | 3312 | 1.42 (1.27–1.58) | 1.31 (1.08–1.59) | 1.78 (1.44–2.21) |
| ȃT2D alone | 915 | 1.51 (1.27–1.79) | 1.50 (1.12–2.02) | 1.52 (1.06–2.18) |
| ȃHD alone | 1846 | 1.29 (1.12–1.49) | 1.34 (1.05–1.72) | 1.45 (1.08–1.95) |
| ȃStroke alone | 551 | 1.66 (1.34–2.06) | 0.95 (0.60–1.49) | 3.55 (2.47–5.10) |
| CMD multimorbidity | 839 | 2.10 (1.73–2.57) | 1.49 (1.02–2.20) | 2.65 (1.83–3.84) |
| ȃT2D + HD | 355 | 2.01 (1.49–2.72) | 1.54 (0.87–2.71) | 1.25 (0.55–2.86) |
| ȃHD + stroke | 310 | 2.29 (1.68–3.12) | 1.86 (1.05–3.28) | 3.57 (2.11–6.04) |
| ȃT2D + stroke | 99 | 2.02 (1.10–3.68) | 0.38 (0.05–2.90) | 4.62 (1.69–12.68) |
| ȃT2D + HD + stroke | 75 | 1.98 (0.99–3.96) | 1.75 (0.47–6.48) | 3.07 (1.18–7.98) |
| Hazard ratio of dementia per each additional co-morbid CMD | – | 1.42 (1.31–1.53) | 1.26 (1.10–1.45) | 1.64 (1.42–1.88) |
Bold indicates statistical significance (p < 0.05).
Cox regression models adjusted for age, sex, education level, marital status, BMI, hypertension, smoking status, alcohol consumption, physical activity level, and depression.
We also examined the association between specific constellations of co-morbid CMDs and dementia, finding that every possible combination of T2D, HD, and stroke, whether alone or in combination, was associated with a significantly increased risk of either all-cause dementia, AD, or VaD (Table 2).
Mid- and late-life cardiometabolic diseases in relation to dementia risk
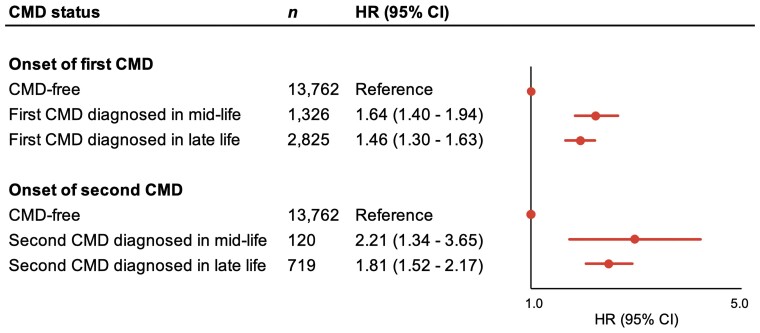
In analyses accounting for the age at CMD onset, we found that the risk effect of CMDs on dementia was attenuated the later in life CMDs developed. The risk of dementia was reduced by 11% for each decade of older age at the development of a first CMD (HR: 0.89, 95% CI: 0.86–0.92), and by 16% for each decade of older age at the development of a second CMD (HR: 0.84, 95% CI: 0.81–0.89).
From a life-course perspective, the risk of dementia was higher if an individual’s first CMD diagnosis occurred during mid-life (HR: 1.64, 95% CI: 1.40–1.94) as opposed to late-life (HR: 1.46, 95% CI: 1.30–1.63). Additionally, the risk of dementia seemed to be higher for those who went on to develop a second CMD in mid-life (HR: 2.21, 95% CI: 1.34–3.65) compared with late-life (HR: 1.81, 95% CI: 1.52–2.17) (Figure 2). We observed a similar pattern of results for VaD, but not for AD (see Supplementary material online, Table S1).
Figure 2.
All-cause dementia risk in relation to age of CMD onset. Hazard ratios with 95% confidence intervals of all-cause dementia from Cox regression models adjusted for age, sex, education level, marital status, body mass index, hypertension, smoking status, alcohol consumption, physical activity level, and depression.
The role of genetic background in the cardiometabolic disease–dementia association
In matched co-twin analysis, the significant association between CMDs and dementia uncovered in the classical cohort study design (HR: 1.51, 95% CI: 1.37–1.66) remained present among CMD- and dementia-discordant DZ twin pairs (n = 302 pairs; HR: 1.55, 95% CI: 1.15–2.09) but was attenuated among CMD- and dementia-discordant MZ twin pairs (n = 54 pairs; HR: 0.99, 95% CI: 0.50–1.98) (Table 3). Since the confounding influence of genetic background can be controlled for among MZ twins (who are genetically identical), our findings suggest that the genetic background common to CMDs and dementia may underlie the CMD–dementia association. As a further indication that the strength of the CMD–dementia association differed between MZ and DZ twin pairs, we detected a significant interaction between zygosity and CMD status on dementia risk (P = 0.005).
Table 3.
HRs and 95% CIs for the association between CMDs and all-cause dementia in the classical cohort study design and the matched co-twin analysis
| Classical cohort study design 17 913 twin individuals |
Matched co-twin analysisa 356 twin pairs |
|||
|---|---|---|---|---|
| DZ twin pairs (n = 302 pairs) | MZ twin pairs (n = 54 pairs) | Zygosity–CMD interaction | ||
| HR (95% CI)b | HR (95% CI)c | HR (95% CI)c | P-value | |
| CMD-free | Reference | Reference | Reference | – |
| Any CMD | 1.51 (1.37–1.66) | 1.55 (1.15–2.09) | 0.99 (0.50–1.98) | 0.005 |
Bold indicates statistical significance (p < 0.05).
Includes only twin pairs that were discordant for both CMD status and dementia status.
Cox regression models adjusted for age, sex, education level, marital status, BMI, hypertension, smoking status, alcohol consumption, physical activity level, and depression.
Cox regression models adjusted for sex, education level, marital status, BMI, hypertension, smoking status, alcohol consumption, physical activity level, and depression.
Sensitivity analyses
In sensitivity analyses, we obtained results consistent with those from the main analyses after (i) excluding individuals diagnosed with dementia within the first 1, 3, 5, and 7 years of follow-up and (ii) excluding 6462 participants with missing values for covariates (see Supplementary material online, Tables S2 and S3). The associations were attenuated when we used Fine and Grey regression models to account for the competing risk of death in the CMD–dementia association (see Supplementary material online, Table S4).
Discussion
In this nationwide twin study, we found that (i) cardiometabolic multimorbidity increases the risk of dementia, including both AD and VaD; (ii) CMDs that develop in mid-life seem to confer the greatest risk of dementia; and (iii) genetic background may underpin the CMD–dementia association (Structured Graphical Abstract).
T2D,27 HD,28,29 and stroke30 are widely recognized dementia risk factors, but only recently has the combined impact of multiple CMDs on dementia risk been explored. Previous studies from our group have shown that the risk of both cognitive impairment8 and dementia7 increases with a growing number of co-morbid CMDs (including T2D, HD, and stroke), using data from the Swedish National Study on Aging and Care—Kungsholmen (SNAC-K). In line with this, a recent study using data from the UK Biobank also reported a monotonic increase in dementia risk with one, two, and three co-morbid CMDs (including T2D, myocardial infarction, and stroke).9
In this large, nationally representative sample of Swedish older adults, we found that the risk of dementia increased by 42% with each additional co-morbid CMD, and further that the risk effect of cardiometabolic multimorbidity on dementia applies to both AD and VaD. Our study takes the additional steps of examining how the timing of CMD onset across the life-course impacts dementia risk and the contribution of genetic background to the CMD–dementia association.
We found that the development of any CMD in mid-life (<60 years) as opposed to late life (≥60 years), was associated with a higher risk of dementia, and further that the risk of dementia decreased by 11% for each additional decade of age at the onset of a first CMD. For individuals with CMD multimorbidity, the risk of dementia decreased by 16% for each additional decade of older age at the development of their second CMD. Together, these results suggest that the earlier CMDs set in, the more damaging they may be to cognitive health. One possible explanation for the apparently greater risk effect of mid-life CMDs is that they could represent a more aggressive form of disease than those that appears in late life. Moreover, the development of CMDs earlier in life could entail more years of exposure to processes that are damaging to the brain. From a clinical perspective, preventing or delaying the development of CMDs, particularly in mid-life, could be a strategy for avoiding dementia in older age.
Consistent with our findings, previous reports from the STR and the Whitehall II study have demonstrated a stronger risk effect of mid-life T2D as opposed to late-life T2D on dementia.31,32 Similarly, the presence of other vascular risk factors such as hypertension, dyslipidemia, and overweight/obesity in mid-life has been related to an increased risk of dementia, but their presence in late life may not be.29,33,34 However, on the contrary, results from the Finnish Cardiovascular Risk Factors, Aging, and Dementia (CAIDE) study reported an increased risk of dementia with atrial fibrillation and heart failure that developed in late-life but not in mid-life.35 Further research is needed to better understand the interplay between CMDs and cognitive health over the life-course, given the heterogeneity of CMDs as chronic diseases that individuals can live with for multiple decades.
Both CMDs and dementia are complex disorders with multifactorial aetiologies involving environmental, lifestyle-related, and genetic factors.10–12 Since CMDs and dementia are both partially heritable, it has been proposed that genetic factors may play a role in the CMD–dementia association. Twin data allowed us to address this question. In the matched co-twin analysis using CMD- and dementia-discordant twin pairs, the CMD–dementia association originally observed in the classical cohort study design was attenuated among MZ twin pairs (who are genetically identical) but not among DZ twin pairs (who are as related as typical siblings). This indicates that genetic background contributes to the CMD–dementia association, suggesting that common genes may underlie the risk of both CMDs and dementia.
So far, some CMD-relevant genes involved in lipid metabolism, insulin signalling, and blood pressure [including Apolipoprotein E (APOE),36 fat mass and obesity-associated protein (FTO),37 insulin-degrading enzymes (IDE),38 and the angiotensin-converting enzyme (ACE)39] have been shown to impact dementia risk. However, only a few studies to date have addressed the role of genetic background in the association between CMDs and dementia. Previous work from our group indicates that genetic variations in IDE40 and APOE41 may underlie the increased risk of dementia associated with T2D. Additionally, genetic susceptibility to coronary artery disease has been found to modify the association between cardiovascular disease and dementia, particularly VaD.42 Recent studies have examined the interplay between dementia-related genes and CMDs, but with conflicting results. A UK Biobank study showed a strong association between cardiometabolic multimorbidity and dementia, regardless of an individual’s AD-related polygenic risk.9 On the other hand, a Danish population-based study reported that 10-year dementia risk among people with vascular risk factors like T2D or hypertension can vary substantially according to their APOE genotype and dementia-related polygenic risk score.43 Future studies are needed to identify which genes are involved in the CMD–dementia association and whether these could point to common biological pathways implicated in both CMDs and dementia.
A growing body of literature suggests that T2D, HD, and stroke can contribute, to different extents, to neurodegenerative AD-related pathologies in addition to their well-established role in the development of cerebrovascular pathology and VaD.44,45 However, the combined impact of multiple CMDs on brain pathology has not been widely examined. We found that cardiometabolic multimorbidity was related to a 50% increased risk of AD (HR: 1.49, 95% CI: 1.02–2.20) and more than double the risk of VaD (HR: 2.65, 95% CI: 1.83–3.84), supporting the notion that mixed pathologies (both neurodegenerative and vascular) may be involved in the development of dementia in people with CMDs.
Cardiometabolic multimorbidity could contribute to vascular and neurodegenerative brain pathologies through several overlapping mechanisms. The chronic hyperglycaemia that characterizes T2D contributes to oxidative stress—a process underlying both cerebral atherosclerosis and neurodegeneration—and can also lead directly to neuronal death through its toxic effect on the myelin sheath.46 Another pathophysiological hallmark of T2D, cerebral insulin resistance, has been linked to tau hyperphosphorylation and increased generation of amyloid-β.46 Additionally, chronic cerebral hypoperfusion—a consequence of stroke or reduced cardiac output from HD—can alter cerebral blood flow velocity, contributing to the development of vascular brain lesions.47 Cerebral hypoperfusion could also trigger brain hypoxia, which can impair peptide clearance and promote the deposition of amyloid-β.48 Moreover, the endothelial dysfunction that characterizes CMDs can disrupt the integrity of the blood–brain barrier, leading to impaired amyloid-β clearance.49 At the intersection of many of these mechanisms is inflammation, which plays a well-established role in the pathogenesis of CMDs50 and may accelerate the progression of both neurodegenerative and vascular brain pathologies.51,52 Elucidating the mechanisms by which cardiometabolic multimorbidity impacts dementia will require future studies integrating longitudinal measures of cognitive function with neuropathological, genetic, and biomarker data.
Strengths and limitations
Strengths of this study include the use of a large, nationally representative cohort with a long follow-up, as well as the unique, genetically informative twin study design, which allowed us to explore the role of genetic background in the CMD–dementia association through comparisons of MZ and DZ twin pairs. Additionally, the ascertainment of dementia and vital status through records in Swedish national registries ensured the follow-up of all study participants, eliminating the possibility of attrition. Finally, the availability of information on AD and VaD diagnoses provided insight on the potential mechanisms by which CMDs affect the brain.
However, the study has some limitations that should be acknowledged. First, the true proportion of individuals in our study who had baseline CMDs or developed incident dementia may be higher than what we estimated using medical records data, resulting in a possible underestimation of the associations reported here. The Swedish NPR includes only records from inpatient and outpatient care,53 so we could not capture diagnoses of CMDs or dementia that occurred in a primary care setting, nor could we identify individuals with undiagnosed CMDs or dementia. In particular, the present method can identify dementia cases in the STR with 98% specificity but only 63% sensitivity,54 so there is a high likelihood that some participants had undiagnosed dementia at baseline, raising the possibility of reverse causality. We attempted to compensate for this by performing sensitivity analyses excluding people with possible prodromal or undiagnosed dementia at baseline (i.e. participants who received a formal diagnosis of dementia within the first 1, 3, 5, or 7 years of follow-up), finding the results consistent with the original analysis. Second, there is a risk of differential outcome misclassification insofar as people with CMDs may interact with the healthcare system more often than those without CMDs and could therefore be more likely to receive a dementia diagnosis, possibly resulting in an overestimation of the associations reported here. However, both the proportion of participants who developed dementia over follow-up and the strength of the CMD–dementia association reported here are comparable to what we found in a previous study using a different Swedish population-based cohort, SNAC-K, in which dementia was identified based on regular and comprehensive cognitive assessments rather than via registry data.7
Another limitation is the relatively low sample size of twin pairs available for the matched co-twin analysis. Specifically, the CMD- and dementia-discordant twin pairs constitute a younger and relatively healthier sample than the study population as a whole, potentially leading to an underestimation of the CMD–dementia association in the matched co-twin analysis. Additionally, diagnoses of AD and VaD were not confirmed through post-mortem or neuroimaging studies. This may have resulted in misclassification of the dementia subtypes, particularly given the high prevalence of mixed AD and VaD pathology, especially among older adults.55,56 Furthermore, information on socio-demographic and lifestyle-related covariates, including education level, marital status, BMI, smoking history, alcohol consumption, and physical activity, were collected via self-report and could be subject to possible recall bias. Finally, we cannot rule out the influence of potential residual confounding due to unmeasured factors.
Conclusion
To our knowledge, this study is the first to demonstrate that CMDs, particularly when developed in mid-life, increase the risk of dementia, including both AD and VaD. These findings add to the growing evidence of a connection between cardiometabolic multimorbidity and both vascular and neurodegenerative forms of dementia and highlight the need for special monitoring of individuals who develop T2D, HD, or stroke in mid-life in order to reduce their risk of developing dementia in older age. Using a twin study design, we also provide evidence that genetic background may underpin the association between cardiometabolic disease and dementia. Our findings call for the identification of these common genes for both CMDs and dementia in future studies.
Supplementary Material
Acknowledgements
The authors would like to express their gratitude to the SALT study participants and the staff involved in the Swedish Twin Registry data collection and management.
Contributor Information
Abigail Dove, Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Tomtebodavägen 18A, Solna SE-17165, Sweden.
Jie Guo, Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Tomtebodavägen 18A, Solna SE-17165, Sweden.
Anna Marseglia, Division of Clinical Geriatrics, Department of Neurobiology, Care Sciences, and Society, Karolinska Institutet, Blickagången 16, Huddinge SE-14183, Sweden.
Johan Fastbom, Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Tomtebodavägen 18A, Solna SE-17165, Sweden.
Davide Liborio Vetrano, Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Tomtebodavägen 18A, Solna SE-17165, Sweden; Stockholm Gerontology Research Center, Sveavägen 115, Stockholm SE-11346, Sweden.
Laura Fratiglioni, Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Tomtebodavägen 18A, Solna SE-17165, Sweden; Stockholm Gerontology Research Center, Sveavägen 115, Stockholm SE-11346, Sweden.
Nancy L Pedersen, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Nobel väg 12A, Solna SE-17165, Sweden.
Weili Xu, Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Tomtebodavägen 18A, Solna SE-17165, Sweden; School of Public Health, Department of Epidemiology and Biostatistics, Tianjin Medical University, 22 Qi Xiang Tai Road, Tianjin, China.
Authors’ contributions
A.D., J.G., and W.X. contributed to the conception and design of the study. A.D. conducted the statistical analyses, performed the literature search, and drafted the manuscript. J.G., A.M., J.F., D.L.V., L.F., N.L.P., and W.X. reviewed and edited the manuscript. N.L.P. was the principal investigator of the SALT study. All authors critically revised the manuscript for important intellectual content. All authors made a significant contribution to finalize the manuscript and approved the final version for publication.
Supplementary data
Supplementary data is available at European Heart Journal online.
Funding
The Swedish Twin Registry is managed by Karolinska Institutet and receives funding from the Swedish Research Council (No. 2017–00641). W.X. received grants from the Swedish Research Council (No. 2017–00981 and No. 2021–01647) and the Swedish Council for Health Working Life and Welfare (No. 2021–01826). This study was accomplished within the context of the Swedish National Graduate School on Aging and Health (SWEAH).
Data availability
Requests for access to the Swedish Twin Registry data can be made here: https://ki.se/en/research/swedish-twin-registry-for-researchers.
References
- 1. DiAngelantonio E, Kaptoge S, Wormser D, Willeit P, Butterworth AS, Bansal N, et al. Association of cardiometabolic multimorbidity with mortality. JAMA 2015;314:52–60. 10.1001/jama.2015.7008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keenan T, Zhao W, Rasheed A, Ho WK, Malik R, Felix JF, et al. Causal assessment of serum urate levels in cardiometabolic diseases through a Mendelian randomization study. J Am Coll Cardiol 2016;67:407–416. 10.1016/j.jacc.2015.10.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sattar N, Gill JMR, Alazawi W. Improving prevention strategies for cardiometabolic disease. Nat Med 2020;26:320–325. 10.1038/s41591-020-0786-7 [DOI] [PubMed] [Google Scholar]
- 4. Xu X, Mishra GD, Dobson AJ, Jones M. Progression of diabetes, heart disease, and stroke multimorbidity in middle-aged women: a 20-year cohort study. PLoS Med 2018;15:e1002516. 10.1371/journal.pmed.1002516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gerdts E, Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med 2019;25:1657–1666. 10.1038/s41591-019-0643-8 [DOI] [PubMed] [Google Scholar]
- 6. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020;396:413–446. 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Z, Marseglia A, Shang Y, Dintica C, Patrone C, Xu W. Leisure activity and social integration mitigate the risk of dementia related to cardiometabolic diseases: a population-based longitudinal study. Alzheimer’s Dement 2020;16:316–325. 10.1016/j.jalz.2019.09.003 [DOI] [PubMed] [Google Scholar]
- 8. Dove A, Marseglia A, Shang Y, Grande G, Vetrano DL, Laukka EJ, et al. Cardiometabolic multimorbidity accelerates cognitive decline and dementia progression. Alzheimer’s Dement 2022:1–10. 10.1002/alz.12708 [DOI] [PubMed] [Google Scholar]
- 9. Tai XY, Veldsman M, Lyall DM, Littlejohns TJ, Langa KM, Husain M, et al. Cardiometabolic multimorbidity, genetic risk, and dementia: a prospective cohort study. Lancet Healthy Longev 2022;3:e428–e436. 10.1016/S2666-7568(22)00117-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernández-Rhodes L, Young KL, Lilly AG, Raffield LM, Highland HM, Wojcik GL, et al. Importance of genetic studies of cardiometabolic disease in diverse populations. Circ Res 2020;126:1816–1840. 10.1161/CIRCRESAHA.120.315893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fan J, Tao W, Li X, Li H, Zhang J, Wei D, et al. The contribution of genetic factors to cognitive impairment and dementia: apolipoprotein E gene, gene interactions, and polygenic risk. Int J Mol Sci 2019;20:1177. 10.3390/ijms20051177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jansen IE, Savage JE, Watanabe K, Bryois J, Williams DM, Steinberg S, et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat Genet 2019;51:404–413. 10.1038/s41588-018-0311-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lichtenstein P, de Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish twin registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med 2002;252:184–205. 10.1046/j.1365-2796.2002.01032.x [DOI] [PubMed] [Google Scholar]
- 14. Kato K, Sullivan PF, Evengård B, Pedersen NL. Premorbid predictors of chronic fatigue. Arch Gen Psychiatry 2006;63:1267–1272. 10.1001/archpsyc.63.11.1267 [DOI] [PubMed] [Google Scholar]
- 15. Eriksson UK, Bennet AM, Gatz M, Dickman PW, Pedersen NL. Non-stroke cardiovascular disease and risk of Alzheimer’s disease and dementia. Alzheimer Dis Assoc Disord 2010;24:213–219. 10.1097/WAD.0b013e3181d1b99b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lichtenstein P, Sullivan PF, Cnattingius S, Gatz M, Johansson S, Carlström E, et al. The Swedish twin registry in the third millennium: an update. Twin Res Hum Genet 2006;9:875–882. 10.1375/twin.9.6.875 [DOI] [PubMed] [Google Scholar]
- 17. Xu WL, Caracciolo B, Wang HX, Santoni G, Winblad B, Fratiglioni L. Accelerated progression from mild cognitive impairment to dementia among APOE ε4ε4 carriers. J Alzheimer’s Dis 2012;33:507–515. 10.3233/JAD-2012-121369 [DOI] [PubMed] [Google Scholar]
- 18. Tomata Y, Li X, Karlsson IK, Mosing MA, Pedersen NL, Hägg S. Joint impact of common risk factors on incident dementia: a cohort study of the Swedish twin registry. J Intern Med 2020;288:234–247. 10.1111/joim.13071 [DOI] [PubMed] [Google Scholar]
- 19. Stunkard AJ, Harris JR, Pedersen NL, McClearn GE. The body-mass index of twins who have been reared apart. N Engl J Med 1990;322:1483–1487. 10.1056/NEJM199005243222102 [DOI] [PubMed] [Google Scholar]
- 20. Atti AR, Palmer K, Volpato S, Winblad B, DeRonchi D, Fratiglioni L. Late-life body mass index and dementia incidence: nine-year follow-up data from the Kungsholmen project. J Am Geriatr Soc 2008;56:111–116. 10.1111/j.1532-5415.2007.01458.x [DOI] [PubMed] [Google Scholar]
- 21. Patientregistret—Socialstyrelsen. https://www.socialstyrelsen.se/statistik-och-data/register/alla-register/patientregistret/(23 September 2021)
- 22. Kivimäki M, Kuosma E, Ferrie JE, Luukkonen R, Nyberg ST, Alfredsson L, et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health 2017;2:e277–e285. 10.1016/S2468-2667(17)30074-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singh-Manoux A, Fayosse A, Sabia S, Tabak A, Shipley M, Dugravot A, et al. Clinical, socioeconomic, and behavioural factors at age 50 years and risk of cardiometabolic multimorbidity and mortality: a cohort study. PLoS Med 2018;15:e1002571. 10.1371/journal.pmed.1002571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Han Y, Hu Y, Yu C, Guo Y, Pei P, Yang L, et al. Lifestyle, cardiometabolic disease, and multimorbidity in a prospective Chinese study. Eur Heart J 2021;42:3374–3384. 10.1093/eurheartj/ehab413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Song C, Chang Z, Magnusson PKE, Ingelsson E, Pedersen NL. Genetic factors may play a prominent role in the development of coronary heart disease dependent on important environmental factors. J Intern Med 2014;275:631–639. 10.1111/joim.12177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: C. J. Wiley & Sons, Inc., 1987. [Google Scholar]
- 27. Srikanth V, Sinclair AJ, Hill-Briggs F, Moran C, Biessels GJ. Type 2 diabetes and cognitive dysfunction—towards effective management of both comorbidities. Lancet Diabetes Endocrinol 2020;8:535–545. 10.1016/S2213-8587(20)30118-2 [DOI] [PubMed] [Google Scholar]
- 28. Wolters FJ, Segufa RA, Darweesh SKL, Bos D, Ikram MA, Sabayan B, et al. Coronary heart disease, heart failure, and the risk of dementia: a systematic review and meta-analysis. Alzheimer’s Dement 2018;14:1493–1504. 10.1016/j.jalz.2018.01.007 [DOI] [PubMed] [Google Scholar]
- 29. Singh-Manoux A, Fayosse A, Sabia S, Canonico M, Bobak M, Elbaz A, et al. Atrial fibrillation as a risk factor for cognitive decline and dementia. Eur Heart J 2017;38:2612–2618. 10.1093/eurheartj/ehx208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Surawan J, Areemit S, Tiamkao S, Sirithanawuthichai T, Saensak S. Risk factors associated with post-stroke dementia: a systematic review and meta-analysis. Neurol Int 2017;9:7216. 10.4081/ni.2017.7216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes 2009;58:71–77. 10.2337/db08-0586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barbiellini AC, Fayosse A, Dumurgier J, MacHado-Fragua MD, Tabak AG, Van Sloten T, et al. Association between age at diabetes onset and subsequent risk of dementia. JAMA 2021;325:1640–1649. 10.1001/jama.2021.4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 2014;13:788–794. 10.1016/S1474-4422(14)70136-X [DOI] [PubMed] [Google Scholar]
- 34. Qiu C, De Ronchi D, Fratiglioni L. The epidemiology of the dementias: an update. Curr Opin Psychiatry 2007;20:380–385. 10.1097/YCO.0b013e32816ebc7b [DOI] [PubMed] [Google Scholar]
- 35. Rusanen M, Kivipelto M, Levälahti E, Laatikainen T, Tuomilehto J, Soininen H, et al. Heart diseases and long-term risk of dementia and Alzheimer’s disease: a population-based CAIDE study. J Alzheimers Dis 2014;42:183–191. 10.3233/JAD-132363 [DOI] [PubMed] [Google Scholar]
- 36. Martins IJ, Hone E, Foster JK, Sünram-Lea SI, Gnjec A, Fuller SJ, et al. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer’s disease and cardiovascular disease. Mol Psychiatry 2006;11:721–736. 10.1038/sj.mp.4001854 [DOI] [PubMed] [Google Scholar]
- 37. Keller L, Xu W, Wang HX, Winblad B, Fratiglioni L, Graff C. The obesity related gene, FTO, interacts with APOE, and is associated with Alzheimer’s disease risk: a prospective cohort study. J Alzheimer’s Dis 2011;23:461–469. 10.3233/JAD-2010-101068 [DOI] [PubMed] [Google Scholar]
- 38. Qiu WQ, Folstein MF. Insulin, insulin-degrading enzyme and amyloid-β peptide in Alzheimer’s disease: review and hypothesis. Neurobiol Aging 2006;27:190–198. 10.1016/j.neurobiolaging.2005.01.004 [DOI] [PubMed] [Google Scholar]
- 39. Narain Y, Furlong RA, Robinsztein DC, Yip A, Murphy T, Brayne C, et al. The ACE gene and Alzheimer’s disease susceptibility. J Med Genet 2000;37:695–697. 10.1136/jmg.37.9.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu WL, Pedersen NL, Keller L, Kalpouzos G, Wang HX, Caroline G, et al. HHEX_23 AA genotype exacerbates effect of diabetes on dementia and Alzheimer disease: a population-based longitudinal study. PLoS Med 2015;12:e1001853. 10.1371/journal.pmed.1001853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu W, Caracciolo B, Wang H-X, Winblad B, Bäckman L, Qiu C, et al. Accelerated progression from mild cognitive impairment to dementia in people with diabetes. Diabetes 2010;59:2928–2935. 10.2337/db10-0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Karlsson IK, Ploner A, Song C, Gatz M, Pedersen NL, Hägg S. Genetic susceptibility to cardiovascular disease and risk of dementia. Transl Psychiatry 2017;7:e1142. 10.1038/tp.2017.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rasmussen IJ, Rasmussen KL, Nordestgaard BG, Tybjaerg-Hansen A, Frikke-Schmidt R. Impact of cardiovascular risk factors and genetics on 10-year absolute risk of dementia: risk charts for targeted prevention. Eur Heart J 2020;41:4024–4033. 10.1093/eurheartj/ehaa695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Winblad B, Amouyel P, Andrieu S, Ballard C, Brayne C, Brodaty H, et al. Defeating Alzheimer’s disease and other dementias: a priority for European Science and Society. Lancet Neurol 2016;15:455–532. 10.1016/S1474-4422(16)00062-4 [DOI] [PubMed] [Google Scholar]
- 45. Duron E, Hanon O. Vascular risk factors, cognitve decline, and dementia. Vasc Health Risk Manag 2008;4:363. 10.2147/VHRM.S1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol 2018;14:591–604. 10.1038/s41574-018-0048-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pase MP, Grima NA, Stough CK, Scholey A, Pipingas A. Cardiovascular disease risk and cerebral blood flow velocity. Stroke 2012;43:2803–2805. 10.1161/STROKEAHA.112.666727 [DOI] [PubMed] [Google Scholar]
- 48. Dotti CG, De Strooper B. Alzheimer’s dementia by circulation disorders: when trees hide the forest. Nat Cell Biol 2009;11:114–116. 10.1038/ncb0209-114 [DOI] [PubMed] [Google Scholar]
- 49. Zuo W, Wu J. The interaction and pathogenesis between cognitive impairment and common cardiovascular diseases in the elderly. Ther Adv Chronic Dis 2022;13:204062232110630. 10.1177/20406223211063020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Donath MY, Meier DT, Böni-Schnetzler M. Inflammation in the pathophysiology and therapy of cardiometabolic disease. Endocr Rev 2019;40:1080–1091. 10.1210/er.2019-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sankowski R, Mader S, Valdés-Ferrer SI. Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front Cell Neurosci 2015;9:28. 10.3389/fncel.2015.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol 2015;14:388–405. 10.1016/S1474-4422(15)70016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jin YP, Gatz M, Johansson B, Pedersen NL. Sensitivity and specificity of dementia coding in two Swedish disease registries. Neurology 2004;63:739–741. 10.1212/01.WNL.0000134604.48018.97 [DOI] [PubMed] [Google Scholar]
- 55. Qiu C, Fratiglioni L. A major role for cardiovascular burden in age-related cognitive decline. Nat Rev Cardiol 2015;12:267–277. 10.1038/nrcardio.2014.223 [DOI] [PubMed] [Google Scholar]
- 56. Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–2204. 10.1212/01.wnl.0000271090.28148.24 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for access to the Swedish Twin Registry data can be made here: https://ki.se/en/research/swedish-twin-registry-for-researchers.