Abstract
Background:
The traditionally reported outcomes for patients with ischemic wounds have centered on amputation-free survival. However, that discounts the importance of other patient-centered outcomes such as the wound healing time (WHT) and wound-free period (WFP). We evaluated the long-term wound outcomes of patients treated for chronic limb-threatening ischemia at our institution.
Methods:
From 2014 to 2017, we identified all patients with chronic limb-threatening ischemia and ischemic wounds using symptomatic and hemodynamic criteria. The primary data included the wound size, wound location, WIfI (wound, ischemia, foot infection) grade, WHT, WFP, minor and major amputation, and death. Wounds were not considered healed if the patient had required a major amputation or had died before wound healing. The WHT was calculated as the interval in days between the diagnosis and determination of a healed wound. The WFP was calculated as the interval in days between a healed wound and wound recurrence, major amputation, death, or the end of the study period. A comparison of the wound healing parameters stratified by revascularization status was performed using the Student t test. A generalized linear model adjusted for age, sex, initial wound size, and WIfI grade was used to evaluate the risk of wound healing with and without revascularization.
Results:
A total of 256 patients had presented with 372 wounds. Of the 256 patients, 48% had undergone revascularization. During the study period, 97 minor amputations and 100 major amputations had been required, and 132 patients had died. The average wound size was 13.9 ± 52.0 cm2; however, for the 155 wounds that had healed, the average size was only 4.0 ± 9.6 cm2 (P = .002). No differences were found in the wound size when stratified by revascularization status (P = .727). Adjusted for the initial wound size, the risk of wound healing was not different when stratified by revascularization (risk ratio, 1.22; 95% confidence interval, 0.80–1.87; P = .354). For those whose wounds had healed, the average WHT and WFP were 173 ± 169 days and 775 ± 317 days, respectively. The WHT was not faster for the revascularized group (155 days vs 188 days; P = .221). When stratified by revascularization status, the rate of wound recurrence was 4.6 vs 8.9 wounds per 100 person-years for the revascularized and nonrevascularized groups, respectively (P = .125).
Conclusions:
In our study, we found that, except for patients who presented with severe ischemia, revascularization was not associated with improved rates of wound healing. Among the wounds that healed, regardless of the initial ischemia grade, revascularization was not associated with a faster WHT or longer WFPs.
Keywords: Peripheral arterial disease, Surgical outcomes, Wound healing
Chronic limb-threatening ischemia (CLTI) is associated with increased morbidity, including the risk of major amputation, and mortality. Tissue loss in patients with CLTI will lead to major lower extremity amputation in >20% of patients, and an estimated 25% of patients with CLTI will die within 1 year after the diagnosis.1,2 In addition, the limb loss and death rates have shown no signs of abatement.2–4 However, these metrics only describe a part of the issues that patients with CLTI must manage. The disability associated with the ongoing care of ischemic wounds and the durability of wound healing outcomes have not been well described. Patient-centered outcomes such as pain relief, wound healing time (WHT), and wound-free period (WFP), are needed to fully describe the complex limb-based outcomes and set expectations for patients as they embark on a care plan for ischemic wounds.5
The Society for Vascular Surgery WIfI (wound, ischemia, foot infection) classification and associated studies have described the extent to which components of the limb-based presentation can contribute to the risk of limb loss, and a variety of interventions are available to treat each of these limb-based issues.1,6–8 Little evidence is available, however, to support which sequences of treatment or treatment combinations will be the best for maximizing patient-centered outcomes such as the WHT and WFP.9 The success of revascularization, whether endovascular or open, is often measured via vessel patency rates rather than more patient-centered outcomes such as relief from pain or wound healing. To the best of our knowledge, only a few studies have focused on wound healing for CLTI patients, and whether revascularization for patients with mild to moderate ischemia will improve WHT and WFP remains unknown.5,10
We evaluated the long-term wound outcomes of patients treated for CLTI at our institution. The aims of the present study were (1) to compare the WHT and WFP for patients who had received wound care only vs those who had undergone revascularization, in addition to wound care; and (2) to estimate the association between revascularization and wound healing, modified by the WIfI ischemia grade. We hypothesized that WHT and WFP would be improved for patients who had received revascularization in addition to wound care and that this healing benefit would be greater for patients with increasingly severe WIfI ischemia grades.
METHODS
Study cohort.
The study cohort included only those patients who had met both hemodynamic and symptomatic criteria for CLTI. All lower extremity arterial duplex ultrasound examinations performed at our institution from January 1, 2014 to December 31, 2017 were reviewed for hemodynamic criteria consistent with possible CLTI. The criteria were defined as follows: ankle pressure <70 mm Hg, toe pressure <50 mm Hg, ankle-brachial index (ABI) of ≤0.5, and pedal transcutaneous oxygen level <30 mm Hg. A total of 572 patients met the criteria. Of the 572 patients, 129 without an ischemic wound and 187 without complete follow-up information were excluded. The final analysis sample size was 256 patients with 372 wounds.
We performed a retrospective review of the medical records for the demographic data (ie, age, sex, race) and comorbidities (ie, anemia, cerebrovascular disease, chronic pulmonary disease, dementia, diabetes mellitus, hypertension, coronary artery disease, a history of myocardial infarction, hyperlipidemia, obesity, renal disease, smoking history [positive history vs never smoked], venous insufficiency, venous thromboembolism, weight loss, and a history of revascularization or limb amputation). The patients’ WIfI stage was determined by the degree of ischemia from the index peripheral vascular laboratory test and the best estimates of wound and infection severity from the review of the medical records.1 We specific reviewed and recorded the wound details and documented each patient’s wound healing journey for 3 years after the first documentation of their wound.
Study design.
The primary exposure was whether a patient had undergone revascularization during the study period. All the patients, revascularization and no revascularization groups, had received wound therapy at a multidisciplinary wound healing and limb preservation center with previously described comprehensive wound management protocols.11,12 In brief and consistent with our previously reported practice patterns, our therapy is centered on optimizing wound offloading, wound debridement, infection control, and the occasional use of hyperbaric oxygen and living cellular wound therapies.13 Podiatrists assisted in the evaluation and design of the offloading devices. The wound sizes were monitored at each visit by recording the maximum length and width. The healing rates were documented by digital photographs using a standard digital camera, which allowed for an accurate assignment of the wound grades and time stamped confirmation of complete healing. Furthermore, local infection was characterized at each visit. The use of photographs allowed for estimates of cellulitis >2 cm from the wound edge, which, in turn, allowed for estimations of the infection grade. The presence of systemic sepsis had not been reliably recorded and was not included in the dataset.
Because of the comorbidities patients with CLTI will often have, the practice at our institution has been initial wound therapy for patients with tissue loss but without significant rest pain and a toe pressure >30 mm Hg.14 If these patients’ wounds respond well to the first 3 to 6 weeks of wound therapy, measured by improvements in wound granulation and a reduction in wound size, we will continue this wound protocol and will not recommend revascularization. Patients with wounds that do not respond during the initial period of wound therapy and those with wounds that worsen at any point will be offered revascularization unless their medical comorbidities prohibit an invasive procedure. For patients with tissue loss who have well-controlled comorbidities and are relatively good surgical risk, revascularization could be offered without a wound care trial, regardless of toe pressure, based on individualized patient and surgeon shared decision-making.13
Primary revascularization was either open or endovascular, with all revascularizations performed by vascular surgeons. The decision for open vs endovascular intervention was determined by the operating surgeon, and standard considerations, such as location and extent of atherosclerosis, body habitus, and availability of an autogenous vein conduit contributed to the shared decision-making for determining the type of revascularization.13
The primary outcomes of interest during the 3-year study period were wound healing and wound recurrence, with an emphasis on the WHT and WFP. The secondary outcomes were minor amputations, major amputation, and death. Wounds were not considered healed if the patient had required a major amputation or had died before documentation of wound healing. The WHT was calculated as the difference in days between the first record of wound occurrence and the date the wound had been declared healed. The WFP was calculated as the difference in days between when the wound had been declared healed and a record of wound recurrence, major amputation, death, or the end of the study period. Additional covariates included age, sex, initial wound size, wound location, and WIfI ischemia grade.
Statistical analysis.
Descriptive statistics were used for wounds that had and had not healed and for patients who had and had not undergone revascularization. The Student t test was used to compare age, initial wound size, and wound healing parameters (ie, WFP, WHT). Modification by ischemia grade was assessed by producing stratum-specific estimates to evaluate the joint effects of wound healing and ischemia grade and conducting a likelihood ratio test. Because of the presence of clustering for patients who had presented with multiple wounds (ie, multiple wounds on one person cannot be considered completely independent), log-binomial generalized estimating equations, a subset of generalized linear models, were used to calculate the risk ratios (RRs) to assess the association between revascularization and wound healing. The patients who had not undergone revascularization were considered the reference group. The final adjustment set included age, initial wound size, and WIfI ischemia grade. These were considered confounders of the association between the use of revascularization and wound healing. This final set was determined using our a priori directed acyclic graph (Supplementary Fig, online only), robust model selection, and relevant substantive knowledge.
Statistical analyses were performed using SAS, version 9.4, software (SAS Institute, Cary, NC). The institutional review board at the University of North Carolina at Chapel Hill approved the present study and waived the requirement for patient informed consent.
RESULTS
Our analysis cohort included 256 patients who had presented with 372 wounds. Their average age was 67 ± 13 years, 41% were women, 48% were White, non-Hispanic, 48% had undergone revascularization, and 42% of the wounds had healed (Table I). The average wound size was 13.9 ± 52.0 cm2. However, for the 155 wounds that had healed, the average size was only 4.0 ± 9.6 cm2 (P = .002). No differences were found in wound size between the revascularization and no revascularization groups (P = .727). During the study period, 97 minor amputations and 100 major amputations had been required, and 132 patients had died. Of the 97 minor amputations, 27 (28%) had later progressed to a major amputation. Of the 141 patients who had undergone revascularization, 36 (25.5%) had required reintervention during the 3-year study period.
Table I.
Descriptive statistics of cohort, stratified by wound healing and revascularization
| Wound healed | Revascularization | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Variable | Total wounds (N = 372; 100%) | Yes (n = 155; 41.7%) | No (n = 217; 58.3%) | P value | Yes (n = 177; 47.6%) | No (n = 195; 52.4%) | P value |
|
| |||||||
| Age, years | 66.7 ± 13.4 | 66.3 ± 12.2 | 67.0 ± 14.2 | .628 | 66.6 ± 12.3 | 66.8 ± 14.3 | .879 |
| Female sex | 149 (40.9) | 70 (44.9) | 79 (36.1) | .089 | 66 (37.3) | 83 (42.6) | .298 |
| Race | |||||||
| White, non-Hispanic | 122 (47.7) | 60 (52.2) | 62 (44.0) | .190 | 61 (50.0) | 61 (45.5) | .472 |
| Black, non-Hispanic | 105 (41.0) | 44 (38.3) | 61 (43.3) | .418 | 37 (38.5) | 58 (43.3) | .032 |
| Native American/Alaskan Native | 1 (0.4) | 0 (0.0) | 1 (0.7) | .368 | 0 (0.0) | 1 (0.8) | .337 |
| Asian | 1 (0.4) | 0 (0.0) | 1 (0.7) | .368 | 1 (0.8) | 0 (0.0) | .294 |
| Other | 18 (7.0) | 7 (6.1) | 11 (7.8) | .596 | 11 (9.0) | 7 (5.2) | .234 |
| Unknown | 9 (3.5) | 4 (3.5) | 5 (3.6) | .976 | 2 (1.6) | 7 (5.2) | .119 |
| WIfI | |||||||
| Overall stage | |||||||
| 1 | 87 (23.4) | 44 (28.4) | 43 (19.8) | .054 | 35 (19.8) | 52 (26.7) | .116 |
| 2 | 70 (18.8) | 32 (20.7) | 38 (17.5) | .447 | 35 (19.8) | 35 (18.0) | .653 |
| 3 | 167 (44.9) | 74 (47.7) | 93 (42.9) | .352 | 79 (44.6) | 88 (45.1) | .920 |
| 4 | 48 (12.9) | 5 (3.2) | 43 (19.8) | <.001 | 28 (15.8) | 20 (10.3) | .110 |
| Ischemia grade | |||||||
| 1 | 80 (21.5) | 44 (28.4) | 36 (16.6) | .006 | 22 (12.4) | 58 (29.7) | <.001 |
| 2 | 85 (22.9) | 42 (27.1) | 43 (19.8) | .099 | 39 (22.0) | 46 (23.6) | .719 |
| 3 | 207 (55.7) | 69 (44.5) | 138 (63.6) | <.001 | 116 (65.5) | 91 (46.7) | <.001 |
| Wound size, cm2 | 13.9 ± 52.0 | 4.0 ± 9.6 | 22.4 ± 69.1 | .002 | 13.0 ± 57.2 | 15.0 ± 46.6 | .727 |
| Wound location | |||||||
| Toe | 184 (49.5) | 84 (54.2) | 100 (46.1) | .124 | 105 (59.3) | 79 (40.5) | <.001 |
| Foot | 78 (21.0) | 31 (20.0) | 47 (21.7) | .697 | 31 (17.5) | 47 (24.1) | .119 |
| Heel | 31 (8.3) | 10 (6.5) | 21 (9.7) | .267 | 9 (5.1) | 22 (11.3) | .031 |
| Ankle | 19 (5.1) | 4 (2.6) | 15 (6.9) | .061 | 8 (4.5) | 11 (5.6) | .624 |
| Leg | 60 (16.1) | 26 (16.8) | 34 (15.7) | .772 | 24 (13.6) | 36 (18.5) | .201 |
| Revascularization | 177 (47.6) | 80 (51.6) | 97 (44.7) | .187 | NA | NA | NA |
| Healed | 155 (41.7) | NA | NA | NA | 80 (45.2) | 75 (38.5) | .189 |
| Wound recurrence | 25 (13.7) | NA | NA | NA | 9 (10.3) | 16 (16.7) | .230 |
| Minor amputation | 97 (26.7) | NA | NA | NA | 58 (33.9) | 39 (20.3) | .005 |
| Major amputation | 100 (27.2) | NA | NA | NA | 58 (33.0) | 42 (21.9) | .015 |
| Death | 132 (35.6) | NA | NA | NA | 55 (31.3) | 77 (39.5) | .091 |
| Comorbidity | |||||||
| Hypertension | 235 (91.8) | 105 (91.3) | 130 (92.2) | .795 | 115 (94.3) | 120 (89.6) | .171 |
| Diabetes mellitus | 180 (70.3) | 78 (67.8) | 102 (72.3) | .430 | 88 (72.1) | 92 (68.7) | .542 |
| Coronary artery disease | 146 (57.0) | 57 (49.6) | 89 (63.1) | .029 | 75 (61.5) | 71 (53.0) | .171 |
| Hyperlipidemia | 181 (70.7) | 84 (73.0) | 97 (68.8) | .459 | 94 (77.1) | 87 (64.9) | .033 |
| Obesity | 72 (28.1) | 32 (27.8) | 40 (28.4) | .920 | 33 (27.1) | 39 (29.1) | .711 |
| Smoking history | 173 (67.6) | 76 (66.1) | 97 (68.8) | .646 | 93 (76.2) | 80 (59.7) | .005 |
| Cerebrovascular disease | 112 (43.8) | 42 (36.5) | 70 (49.7) | .035 | 54 (44.3) | 58 (43.3) | .873 |
| Chronic obstructive pulmonary disease | 85 (33.2) | 33 (28.7) | 52 (36.9) | .168 | 41 (33.6) | 44 (32.8) | .897 |
| Dementia | 33 (12.9) | 10 (8.7) | 23 (16.3) | .070 | 11 (9.0) | 22 (16.4) | .077 |
| Myocardial infarction | 72 (28.1) | 29 (25.2) | 43 (30.5) | .352 | 37 (30.3) | 35 (26.1) | .453 |
| Renal disease | 58 (22.7) | 18 (15.7) | 40 (28.4) | .016 | 27 (22.1) | 31 (23.1) | .849 |
NA, Not applicable; WIfI, wound, ischemia, foot infection.
Data presented as mean ± standard deviation or number (%).
As would be expected, in the overall cohort, the patients who had presented with a less severe WIfI clinical stage were more likely to have experienced wound healing (Fig 1). Considering the component grades of the WIfI staging system, patients with less severe ischemia at initial presentation were more likely to have experienced wound healing (55%, 49%, and 33% of wounds with ischemia grade 1, 2, and 3, respectively). For those whose wounds had healed, the average WHT was 173 ± 169 days. Although the average WHT was shorter for the no revascularization group than that for the revascularization group (155 vs 188 days, respectively), the difference between the two groups was not statistically significant (P = .221; Table II). The distribution of WHT by WIfI clinical stage revealed that no wounds of patients who had presented with WIfI stage 4 disease had healed if the patient had not undergone revascularization (Fig 2). The modification analysis produced a likelihood ratio test result of 3.95 (P = .047), confirming an interaction between the ischemia grade and wound healing. However, after adjustment for age, initial wound size, and WIfI ischemia grade, the overall risk of wound healing was not different when stratified by revascularization status (RR 1.22; 95% confidence interval, 0.80–1.87; P = .354; Table III). Stratum-specific RRs of 3-year wound healing stratified by revascularization were not significant for ischemia grades 1 and 2. However, the RR for ischemia grade 3 was 1.64 (95% confidence interval, 1.06–2.54; P = .027), indicating increased healing for those with the most severe ischemia if they had undergone revascularization (Table IV).
Fig 1.
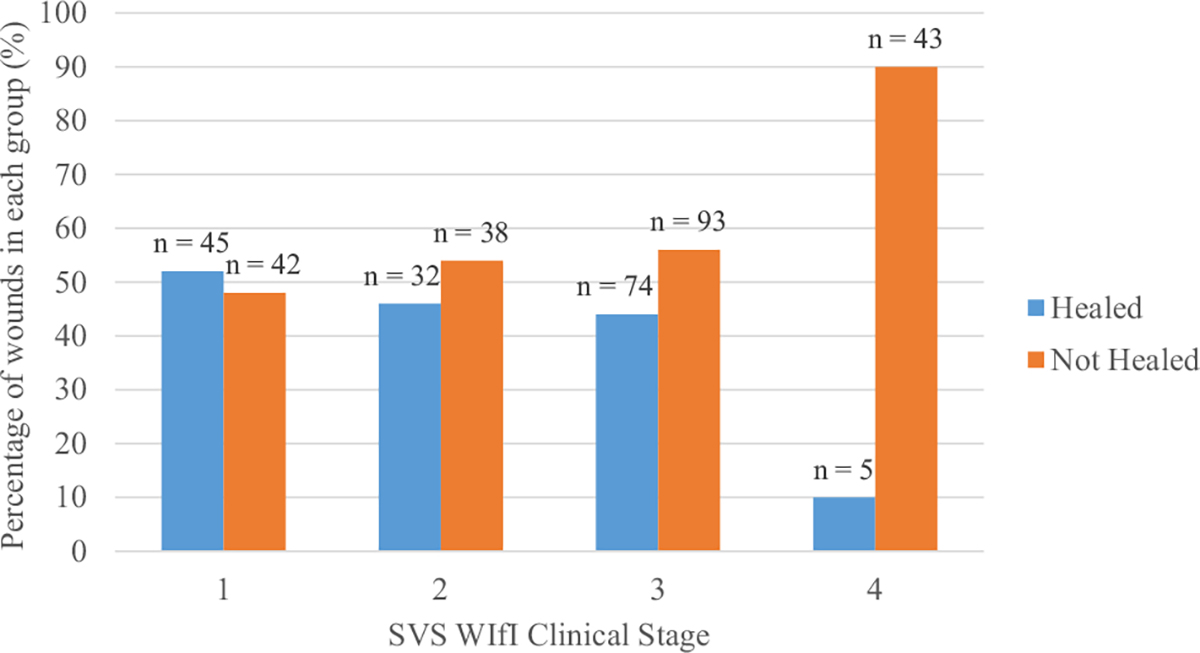
Distribution of WIfI (wound, ischemia, foot infection) clinical stages stratified by wound healing. SVS, Society for Vascular Surgery.
Table II.
Average wound healing time (WHT) and wound-free period (WFP) stratified by revascularization
| Revascularization |
||||
|---|---|---|---|---|
| Variable | Total | Yes | No | P value |
|
| ||||
| WHT, days | 173 ± 169 | 188 ± 190 | 155 ± 143 | .221 |
| WFP, days | 775 ± 317 | 798 ± 295 | 754 ± 338 | .392 |
Data presented as mean ± standard deviation.
Fig 2.

Wound healing time (WHT) and WIfI (wound, ischemia, foot infection) clinical stages stratified by revascularization status. No wounds with WIfI stage 4 in patients who had not undergone revascularization had healed. SVS, Society for Vascular Surgery.
Table III.
Overall risk of wound healing stratified by revascularization
| Wound healed, No. |
|||||||
|---|---|---|---|---|---|---|---|
| Revascularization | Yes | No | Total | Risk of wound healing | Modela | RR (95% CI) | P value |
|
| |||||||
| Yes | 80 | 97 | 177 | 0.452 | Crude | 1.30 (1.00–1.70) | .051 |
| No | 75 | 120 | 195 | 0.385 | Full | 1.22 (0.80–1.87) | .354 |
| Total | 155 | 217 | 372 | NA | NA | NA | NA |
CI, Confidence interval; NA, not applicable; RR, risk ratio.
Model was adjusted for age, sex, initial wound size, and WIfI ischemia grade.
Table IV.
Risk of wound healing by ischemia grade
| Healed, No. |
||||||||
|---|---|---|---|---|---|---|---|---|
| Ischemia grade | Revascularization | Yes | No | Total | Risk | Modela | RR (95% CI) | P value |
|
| ||||||||
| 1 | Yes | 14 | 8 | 22 | 0.636 | Crude | 1.20 (0.78–1.83) | .404 |
| No | 30 | 28 | 58 | 0.517 | Full | 1.22 (0.80–1.87) | .354 | |
| 2 | Yes | 19 | 20 | 39 | 0.487 | Crude | 1.26 (0.75–2.10) | .386 |
| No | 23 | 23 | 46 | 0.500 | Full | 1.24 (0.75–2.05) | .409 | |
| 3 | Yes | 47 | 69 | 116 | 0.405 | Crude | 1.75 (1.11–2.77) | .016 |
| No | 22 | 69 | 91 | 0.242 | Full | 1.64 (1.06–2.54) | .027 | |
CI, Confidence interval; RR, risk ratio.
Model was adjusted for age, sex, initial wound size, and WIfI ischemia grade.
For those patients whose wounds had healed, the average WFP was 775 ± 317 days. The women had required a longer WHT (195.5 vs 153.3 days; P = .136) but had had a shorter WFP (752.1 vs 796.3 days; P = .338) than the men. However, these differences were not statistically significant. Although the average WFP was longer for the patients who had undergone revascularization than for those who had not (798 vs 754 days, respectively), the difference between the two groups was not statistically significant (P = .392; Table II). The rate of wound recurrence was 6.6 wounds/100 person-years for the overall study group. When stratified by revascularization status, the rate of wound recurrence was 8.9 wounds/100 person-years for the no revascularization group compared with 4.6 wounds/100 person-years for the revascularization group (P = .125).
DISCUSSION
We were surprised to find that a combined treatment strategy of wound care and revascularization did not significantly improve the WHT, lengthen the WFP, or reduce the incidence of ischemic wound recurrence compared with wound care alone for most patients. Regardless of revascularization status, we found a trend toward healing when stratified by ischemia grade, with the proportion of healing greater for grade 1 vs grade 2 vs grade 3. However, within our cohort, patients with ischemia grade 1 (toe pressure, 40–59 mm Hg or ABI, 0.6–0.79) were significantly less likely to have undergone revascularization, and patients with ischemia grade 3 (toe pressure, <30 mm Hg or ABI, ≤0.39) were significantly more likely to have undergone revascularization. The wounds of patients with WIfI grade ischemia 3 were significantly more likely to heal if the patient had undergone revascularization, as would be expected by previously reported studies supporting the WIfI staging system. However, the association between revascularization and improved wound healing was not present for patients presenting with WIfI ischemia grade 1 and 2 disease.
Okazaki et al5 introduced WHT and WFP as useful measures to evaluate wound healing and recurrence in CLTI patients as an alternative to more the traditional end points such as amputation-free survival. The comparisons they reported had focused on differences in the WHT and WFP for CLTI patients who had undergone endovascular therapy vs those who had undergone surgical revascularization.5 They found that surgical revascularization was associated with a shorter WHT, an increased rate of wound healing, and a longer WFP compared with endovascular revascularization.5 In contrast, in our study, we focused on the difference in WHT and WFP for patients with CLTI who had received wound care only vs those who had received wound care and had undergone any type of infrainguinal arterial revascularization. We found that the use of revascularization, overall, did not significantly improve the WHT, lengthen the WFP, or reduce the incidence of ischemic wound recurrence. The results from both the study by Okazaki et al5 and ours are limited because relief from pain (including neuropathic, ischemic, and wound related) were not measured or reported.
As expected, the initial wound size was associated with the healing of an ischemic wound. Thus, the care of patients with CLTI should focus on medical optimization and wound and podiatric care, just as much as on the vascular intervention, especially for patients with only mild or moderate ischemia. In our cohort, the wounds that had healed had been significantly smaller at initial presentation than the wounds that had not healed. However, the wound size did not differ when stratified by revascularization in our practice. A 2006 retrospective cohort study of patients with ischemic wounds showed that limb salvage is attainable with wound care alone for most patients and that only patients with an ABI <0.5 had required revascularization.15 Similar to our findings, they also determined that wound closure was associated with the initial wound size. However, in their study, the average wound size was 8.4 ± 20.3 cm2, which was smaller than the average wound size (13.9 ± 52.0 cm2) in our study performed 15 years later.15 At present, even with wound care and revascularization performed at a multidisciplinary limb salvage center, the wound healing rates for these patients were low (only 42% in our cohort). Overall, we found no significant differences in healing between wound care alone vs both wound care and revascularization, a finding supported by Crowner et al.13 This information indicates that wounds and comorbidities are worsening and more clarity on the benefits offered by the current treatments and interventions is required.
Although the present study had several strengths, it also had some limitations. Our study was a retrospective, single-institution cohort study, and although designed to minimize biases, the threat of bias was still present. The revascularization decision and the type of revascularization were at surgeon discretion, which is inherently biased. However, until the results from the BEST-CLI (best endovascular vs. best surgical therapy in patients with critical limb ischemia) trial are available, all surgeons and interventionalists will be required to make these same biased decisions. To produce true estimates of the association between revascularization and wound healing and wound recurrence and to identify which patients will benefit from which sequences and combinations of care, a larger, nationally representative study is required. We could not report on revascularization efficacy or how that might be associated with healing, because we did not have postprocedural WIfI grades or a sufficient sample size to draw these conclusions. Theoretically, each patient had received the best possible revascularization, and the reinvention rate throughout the 3-year study period was only 25%. Wound healing was determined from a review of the medical records. However, the exact date of wound healing and wound recurrence had not always been explicitly stated. Thus, a patients’ wound could have healed in the time between visits. This would have also affected our ability to accurately calculate the WHT and WFP. The precise status of the relevant comorbidities (eg, controlled vs un-controlled diabetes or changes in diabetes control during the 3-year study period), compliance with offloading in unmonitored settings, and adherence to scheduled follow-up appointments vs “no show” rates were not available from the dataset. Thus, these were un-controlled confounders that had the potential to affect the relationship between revascularization and wound healing. In addition, it was difficult to delineate why some patients with large, nonhealing wounds that had not improved after a trial of wound care alone were not offered revascularization. Thus, we could not determine whether this would have altered their ability to heal.
CONCLUSIONS
Except for patients who had presented with severe ischemia, revascularization was not associated with improved rates of wound healing. Among the wounds that had healed, regardless of the initial ischemia grade, revascularization was not associated with a faster WHT or a longer WFP. In particular, for patients who have present with large wounds, it is important to counsel them regarding the WHT and WFP and to engage patients in their wound and podiatric care just as much as with the shared decision-making regarding vascular intervention. Although robust CLTI staging systems have been tested, the results from the present study have highlighted the need to better define which patients have end-stage disease to determine the most appropriate course of treatment for each patient.9
Supplementary Material
ARTICLE HIGHLIGHTS.
Type of Research: A single-center, retrospective cohort study
Key Findings: In 256 patients with 372 ischemic wounds, only patients presenting with an ankle-brachial index of <0.4 or toe pressure of <30 mm Hg had experienced a healing benefit after revascularization (risk ratio, 1.64; 95% confidence interval, 1.06–2.54; P = .027). Of those with healing, the wound healing time was not faster for the patients who had undergone revascularization (155 vs 188 days; P = .221).
Take Home Message: Ischemic wound healing is associated with the initial wound size and is achievable without revascularization in patients with only mild to moderate ischemia. The wound healing time and wound-free period did not differ with revascularization.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Presented at the Forty-sixth Annual Meeting of the Southern Association for Vascular Surgery, Manalapan, FL, January 19-22, 2022.
Additional material for this article may be found online at www.jvascsurg.org.
REFERENCES
- 1.Mills JL, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, et al. The Society for Vascular Surgery lower extremity threatened limb classification system: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg 2014;59:220–34.e2. [DOI] [PubMed] [Google Scholar]
- 2.Sampson UKA, Fowkes FGR, McDermott MM, Criqui MH, Aboyans V, Norman PE, et al. Global and regional burden of death and disability from peripheral artery disease: 21 world regions, 1990 to 2010. Glob Heart 2014;9:145–58.e21. [DOI] [PubMed] [Google Scholar]
- 3.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007;45:S5–67. [DOI] [PubMed] [Google Scholar]
- 4.Davies MG. Critical limb ischemia: epidemiology. Methodist DeBakey Cardiovasc J 2012;8:10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okazaki J, Matsuda D, Tanaka K, Masaru I, Kuma S, Morisaki K, et al. Analysis of wound healing time and wound-free period as outcomes after surgical and endovascular revascularization for critical lower limb ischemia. J Vasc Surg 2018;67:817–25. [DOI] [PubMed] [Google Scholar]
- 6.Zhan LX, Branco BC, Armstrong DG, Mills JL. The Society for Vascular Surgery lower extremity threatened limb classification system based on wound, ischemia, and foot infection (WIfI) correlates with risk of major amputation and time to wound healing. J Vasc Surg 2015;61:939–44. [DOI] [PubMed] [Google Scholar]
- 7.Mills JL. The application of the Society for Vascular Surgery wound, ischemia, and foot infection (WIfI) classification to stratify amputation risk. J Vasc Surg 2017;65:591–3. [DOI] [PubMed] [Google Scholar]
- 8.Mayor J, Chung J, Zhang Q, Montero-Baker M, Schanzer A, Conte MS, et al. Using the Society for Vascular Surgery wound, ischemia, and foot infection classification to identify patients most likely to benefit from revascularization. J Vasc Surg 2019;70:776–85.e1. [DOI] [PubMed] [Google Scholar]
- 9.McGinigle KL, Freeman NLB, Marston WA, Farber A, Conte MS, Kosorok MR, et al. Precision medicine enables more TNM-like staging in patients with chronic limb threatening ischemia. Front Cardiovasc Med 2021;8:709904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman M, Monroe DM. The multiple roles of tissue factor in wound healing. Front Biosci (Schol Ed) 2012;4:713–21. [DOI] [PubMed] [Google Scholar]
- 11.Gottrup F, Holstein P, Jørgensen B, Lohmann M, Karlsmar T. A new concept of a multidisciplinary wound healing center and a national expert function of wound healing. Arch Surg 2001;136:765–72. [DOI] [PubMed] [Google Scholar]
- 12.Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11(Suppl 1):S1–28. [DOI] [PubMed] [Google Scholar]
- 13.Crowner JR, Marston WA, Freeman NLB, Farber MA, Chung J, McGinigle KL, et al. The Society for Vascular Surgery objective performance goals for critical limb ischemia are attainable in select patients with ischemic wounds managed with wound care alone. Ann Vasc Surg 2022;78:28–35. [DOI] [PubMed] [Google Scholar]
- 14.Vallabhaneni R, Kalbaugh CA, Kouri A, Farber MA, Marston WA. Current accepted hemodynamic criteria for critical limb ischemia do not accurately stratify patients at high risk for limb loss. J Vasc Surg 2016;63:105–13. [DOI] [PubMed] [Google Scholar]
- 15.Marston WA, Davies SW, Armstrong B, Farber MA, Mendes RC, Fulton JJ, et al. Natural history of limbs with arterial insufficiency and chronic ulceration treated without revascularization. J Vasc Surg 2006;44:108–14.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


